Abstract
Living a healthy lifestyle is one of the safest and most cost-effective ways to improve one’s quality of life and prevent and/or manage chronic disease. As such, current CKD management guidelines recommend that patients adhere to a healthy diet, perform ≥150 minutes per week of physical activity, manage their body weight, abstain from tobacco use, and limit alcohol. However, there are limited studies that investigate the relationship between these lifestyle factors and the progression of CKD among people with established CKD. In this narrative review, we examine the reported frequencies of health lifestyle behavior engagement among individuals with non–dialysis-dependent CKD and the existing literature that examines the influences of diet, physical activity, weight management, alcohol consumption, and tobacco use on the progression of CKD, as measured by decline in GFR, incident ESKD, or elevated proteinuria or albuminuria in individuals with CKD. Many of the available studies are limited by length of follow-up and small sample sizes, and meta-analyses were limited because the studies were sparse and had heterogeneous classifications of behaviors and/or referent groups and of CKD progression. Further research should be done to determine optimal methods to assess behaviors to better understand the levels at which healthy lifestyle behaviors are needed to slow CKD progression, to investigate the effect of combining multiple lifestyle behaviors on important clinical outcomes in CKD, and to develop effective techniques for behavior change. Despite the lack of evidence of efficacy from large trials on the ability of lifestyle behaviors to slow CKD progression, maintaining a healthy lifestyle remains a cornerstone of CKD management given the undisputed benefits of healthy lifestyle behaviors on cardiovascular health, BP control, and survival.
Keywords: chronic kidney disease, CKD, diet, ESKD, ESRD, exercise, kidney disease, lifestyle, obesity, physical activity, smoking
Introduction
CKD is an epidemic, affecting more than one in ten American adults (1). Patients with CKD have a high burden of risk factors for cardiovascular disease (CVD) and experience high rates of CVD events. Maintaining a healthy lifestyle offers a crosscutting approach to reduce the risk of CVD. Lifestyle behaviors are similarly recommended across relevant guidelines, including the Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for the Evaluation and Management of CKD, the KDIGO 2020 Guidelines for Diabetes Management in CKD, and the American College of Cardiology/American Heart Association guidelines on the primary prevention of CVD (Table 1) (2,3). In the CKD population, lifestyle behaviors, such as diet, physical activity, weight management, alcohol consumption, and tobacco use, have mainly been studied in relation to reducing CVD events and mortality risk (4–6), and less is known about limiting CKD progression. The purpose of this narrative review is to describe the current evidence of how select lifestyle behaviors relate to CKD progression, defined as progressive decline in GFR, development of ESKD, or increase in proteinuria or albuminuria among people with established CKD without kidney failure requiring transplant or dialysis. Using PubMed, we selected observational studies, randomized controlled trials (RCTs), systematic reviews, and meta-analyses that focused on the relationship of lifestyle behaviors and CKD progression. The search terms included the following: CKD progression, end-stage renal disease, ESRD, end-stage kidney disease, ESKD, glomerular filtration rate decline, GFR decline, lifestyle factors, diet, dietary pattern, dietary acid load, potential renal acid load, sodium, potassium, protein, phosphorous, smoking, smoking cessation, marijuana, alcohol, physical activity, exercise, obesity, body mass index, and BMI. We did not include studies that investigated the effect of multiple lifestyle behaviors and, therefore, this review does not cover how lifestyle behaviors in combination relate to CKD progression. As a narrative review, we were not inclusive of all published studies assessing the relationship between lifestyle behaviors and CKD progression, which limits our ability to assess all published results, but we have focused on including high-quality studies and limiting inclusion of studies that reported very similar results.
Table 1.
Lifestyle behavior recommendations across clinical practice guidelines
| Lifestyle Behavior (Definition/Measures) | Guideline Recommendations | ||
|---|---|---|---|
| General CKD Management (89) | CKD Management with Diabetes (17) | Cardiovascular Disease Primary Prevention for General Population (90) | |
| Diet (defined by components consumed, including macronutrients and micronutrients) | Individuals should receive expert dietary advice and information in the context of an education program, tailored to severity of CKD and the need to intervene on salt, phosphate, potassium, and protein intake where indicated | Individuals should consume an individualized diet high in vegetables, fruits, whole grains, fiber, legumes, plant-based proteins, unsaturated fats, and nuts; and lower in processed meats, refined carbohydrates, and sweetened beverages | Individuals should consume a healthy diet that emphasizes the intake of vegetables, fruits, nuts, whole grains, and fish; eliminate trans fats and minimize the intake of processed meats, refined carbohydrates, and sweetened beverages; replace saturated fat with monounsaturated and polyunsaturated fats, and reduce dietary cholesterol and sodium |
| Physical activity (bodily movement produced by skeletal muscle that requires energy expenditure) | Individuals should be encouraged to undertake physical activity compatible with cardiovascular health and tolerance (aiming for at least 30 minutes five times per week) | Individuals should undertake moderate-intensity physical activity for a cumulative duration of at least 150 minutes per week, or to a level compatible with their cardiovascular and physical tolerance | Individuals should engage in at least 150 minutes per week of accumulated moderate-intensity physical activity or 75 minutes per week of vigorous-intensity physical activity; for those unable to reach this minimum, engaging in at least some moderate-to-vigorous activity can still be beneficial |
| Healthy weight management (BMI is the predominant index to establish body habitus in clinical practice) | Individuals should be encouraged to achieve a healthy weight (BMI 20–25 kg/m2, according to country-specific demographics) | Physicians should consider advising/encouraging patients with obesity, diabetes, and CKD to lose weight, particularly patients with eGFR ≥30 ml/min per 1.73 m2 | For adults with overweight and obesity, counseling and caloric restriction are recommended for achieving and maintaining weight loss |
| Tobacco use (most commonly cigarette smoking) | Individuals should be encouraged to stop smoking | People who use tobacco should be advised to quit using tobacco products | Adults should be assessed at health-care visits for tobacco use, and those who use tobacco should be assisted and strongly advised to quit |
| Alcohol use (measured by standard alcohol drink per occasion and frequency per week) | No specific recommendations | No specific recommendations | For individuals with hypertension and who drink alcohol, reduce daily alcohol intake to: two or less drinks for men, one or less drink for women |
BMI, body mass index.
Lifestyle Behavior Prevalence in CKD
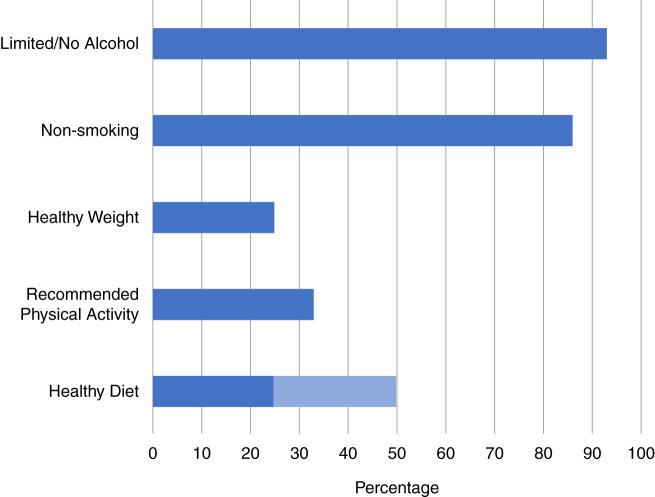
The majority of people with CKD do not adhere to several of the recommended levels of lifestyle behaviors (Figure 1). Two thirds of adults with CKD in the United States do not meet the recommended physical activity levels and are sedentary (7,8). More than three quarters of people with CKD are overweight/obese (9,10). Nearly a half to three quarters of people with CKD do not consume a healthy diet, depending on the definition of the recommended diet (4,6,9). Nonsmoking ranged between 71%–92% from CKD cohorts in Europe, North America, and Japan (11), and, among individuals with CKD in the United States, reports of nonsmoking is similar to that of the general population (approximately 86%) (4,6,9,12,13). Nearly all individuals with CKD (93%) meet alcohol recommendations (6).
Figure 1.
The majority of people with CKD do not adhere to several of the recommended levels of lifestyle behaviors. Estimates from the following published reports: alcohol (6), smoking (4,6,9,12,13), weight (9,10), physical activity (7,8), and diet (4,6,9).
Diet and CKD
The role of diet in progression of kidney disease has been of great interest for many decades. There are several guideline recommendations for diet and CKD (Table 2), and numerous studies examining the relationship of dietary components and patterns with clinical outcomes in CKD (Tables 3 and 4). By far, the most studied dietary intervention has been protein restriction. and ). The Brenner hyperfiltration theory posits that dietary protein restriction reduces progressive glomerular injury by reducing glomerular hyperfiltration (14). Although protein restriction has been shown to be effective in animal models, the data are mixed in human trials. A 2018 meta-analysis of low protein diets in adults with nondiabetic CKD reported that a low protein intake (0.5–0.6 g/kg per day) versus normal protein intake (≥0.8 g/kg per day) had no effect on risk of ESKD. A very low protein intake (0.3–0.4 g/kg per day) probably reduced the risk of ESKD (relative risk [RR], 0.65; 95% confidence interval [95% CI], 0.49 to 0.85) versus a low or normal protein intake, although there were limited data on adverse effects and no data on quality of life (15) (Figure 2) . As such, the Kidney Disease Outcomes Quality Initiative (KDOQI) 2020 nutrition guidelines recommend, under close clinical supervision, protein restriction with or without keto acid analogues to reduce risk of ESKD/death due to high quality evidence (Table 2). In patients with diabetic CKD, benefits are even less certain (16), with the KDIGO diabetes guideline reporting inconclusive evidence of protein restriction on change in eGFR, ESKD, or other health outcomes (17). The type of protein source may matter because animal-based protein seems to induce higher glomerular hyperfiltration than vegetable-based proteins (18), and a vegetarian diet with equivalent nutrients was shown to result in lower levels of serum phosphate, urine phosphate excretion, and fibroblast growth factor-23 levels (19). Studies in animals have shown that excessive phosphate intake can cause renal parenchymal calcification and proximal tubular injury (20). However, scarce evidence exists that current levels of phosphate intake in humans play a significant role in CKD progression, or that reduction of phosphate intake reduces kidney injury (21–23).
Table 2.
Diet recommendations for individuals with CKD
| Dietary Component | Recommendation | Source |
|---|---|---|
| Dietary pattern | Adults with CKD 1–5 not on dialysis or post-transplantation, with or without dyslipidemia: suggest prescribing a Mediterranean diet may improve lipid profiles (2C) | KDOQI Nutrition 2020 (87) |
| Protein intake | Adults with CKD 3–5 who are metabolically stable: recommend, under close clinical supervision, protein restriction with or without ketoacid analogues, to reduce risk for ESKD/death (1A) and improve quality of life (2C) | KDOQI Nutrition 2020 (87) |
| Adults with CKD 1–5D (1B) or those post-transplantation (OPINION): there is insufficient evidence to recommend a particular protein type (plant versus animal) in terms of the effects on nutritional status, calcium or phosphate levels, or the blood lipid profile | ||
| Patients with diabetes with CKD 1–5: suggest maintaining a protein intake of 0.8 g protein/kg (weight) per day (2C) | KDIGO Diabetes 2020 (17) | |
| Dietary acid load | Adults with CKD 1–4: suggest reducing NEAP through increased dietary intake of fruits and vegetables (2C) to reduce the rate of decline of residual kidney function | KDOQI Nutrition 2020 (87) |
| Sodium intake | Adults with CKD 3–5: recommend limiting sodium intake to <2.3 g/d to reduce BP and improve volume control (1B) | KDOQI Nutrition 2020 (87) |
| Adults with CKD 3–5: suggest limiting sodium intake to <2.3 g/d to reduce proteinuria synergistically with available pharmacologic interventions (2A) | ||
| Patients with diabetes with CKD: suggest limiting sodium intake to <2 g/d in patients with diabetes and CKD (2C) | KDIGO Diabetes 2020 (17) | |
| Patients with high BP and CKD: suggest targeting a sodium intake <2 g/d in patients with high BP and CKD (2C) | KDIGO Blood pressure 2020 (86) | |
| Potassium intake | Adults with CKD 3–5: reasonable to adjust dietary potassium intake to maintain serum potassium within the normal range (OPINION) | KDOQI Nutrition 2020 (87) |
| Calcium intake | Adults with CKD 3–4 not taking active vitamin D analogues: suggest total elemental calcium intake of 800–1000 mg/d (including dietary calcium, calcium supplementation, and calcium-based phosphate binders) to maintain a neutral calcium balance (2B) | KDOQI Nutrition 2020 (87) |
| Phosphorus intake | Adults with CKD 3–5: recommend adjusting dietary phosphate intake to maintain serum phosphate levels in the normal range (1B) | KDOQI Nutrition 2020 (87) |
| Adults with CKD 1–5: reasonable when making decisions about phosphate restriction treatment to consider the bioavailability of phosphate sources (e.g., animal, vegetable, additives) (OPINION) |
The strength of each recommendation is indicated as Level 1 ("recommended") or Level 2 ("suggested"), and the quality of the supporting evidence is shown as A, B, C, or D: high, moderate, low, and very low, respectively. Opinion is expert opinion without strong evidence in CKD population. KDOQI, Kidney Disease Outcomes Quality Initiative; KDIGO, Kidney Disease Improving Global Outcomes; NEAP, net endogenous acid production.
Table 3.
Summary of RCTs of meta-analyses and select large, randomized studies examining diet and CKD progression with at least 100 participants and 12 months of follow-up
| Publication Details | Comparators | Sample Description and Follow-Up | Kidney Function (CKD Stages, Mean eGFR: Other Key Inclusion/Exclusion Criteria) | Intervention Characteristics | CKD Progression Measure(s) | Key Findings/Conclusions |
|---|---|---|---|---|---|---|
| Hahn et al. 2018 (15), meta-analysis | LPD versus normal protein | Six trials with N=1814 with ESKD as outcome; eight trials with N=1680 with final or change in GFR as outcome | Stage 3–5 CKD | Included RCTs or quasi-RCTs in which adults with nondiabetic stage 3–5 CKD not on dialysis randomized to LPD versus normal protein intake for ≥12 months | ESKD; end or change in GFR | Compared with normal protein diet, LPD had no or little effect on ESKD (low certainty), with HR, 1.05 (95% CI, 0.73 to 1.53), and uncertain effect on final or change in GFR (very low certainty), with SMD −0.18 (95% CI, −0.75 to 0.38) |
| Hahn et al. 2018 (15), meta-analysis | VLPD versus LPD or normal protein diet | Ten trials with N=1010 with ESKD as outcome; six trials with N=456 with end or change in GFR as outcome; six trials with N=681 with death as outcome | Stage 3–5 CKD | Included RCTs or quasi-RCTs in which adults with nondiabetic stage 3–5 CKD not on dialysis randomized to VLPD versus LPD/normal protein intake for ≥12 months | ESKD; end or change in GFR | Compared with LPD or normal protein diet, VLPD probably reduced risk of ESKD (moderate certainty; RR, 0.64; 95% CI, 0.49 to 0.85) and uncertain effect on final or change in GFR (very low certainty; SMD, 0.12; 95% CI, −0.27 to 0.52) |
| KDIGO Diabetes Guideline 2020 (17) | LPD versus normal protein diet | One trial with N=82 for ESKD as outcome (follow-up 4 yr); one trial with N=88 for doubling of creatinine (follow-up 5 yr); two trials with N=170 (follow-up 4.5 yr) | Stage 1–5 CKD and type 1 or 2 diabetes | Included RCTs of patients with stage 1–5 CKD and T1D or T2D comparing low protein diets versus usual diets with kidney outcomes | ESKD; doubling of creatinine | Compared with normal protein diet, LPD had uncertain effect on ESKD (low certainty; RR, 0.47; 95% CI, 0.08 to 2.75) or doubling of serum creatinine (very low certainty; RR, 0.89; 95% CI, 0.37 to 2.15) |
| Navaneethan et al. 2019 (33), meta-analysis | Treatment of metabolic acidosis with dietary acid intake versus control | Six trials with N=1383 with eGFR decline outcome;two trials with N=434 with ESKD outcome | Stage 3–5 CKD; metabolic acidosis (TCO2 <22) or low-normal bicarbonate (22–24) | Included studies that used dietary intervention (two ketoanalogue-supplemented VLPD, one VLPD, one LPD, one “six-point diet”, one fruits/vegetables) versus control diets (usual diet or LPD) | eGFR decline ESKD | Treatment of metabolic acidosis with dietary acid intake probably reduces risk of eGFR decline (moderate): mean difference, −3.28 (95% CI, −4.42 to −2.14) ml/min per 1.73 m2 per year and might reduce risk of ESKD (low certainty; RR, 0.32; 95% CI, 0.18 to 0.56) |
| Garneata et al. 2016 (91), Romania | VLPD and KAA versus LPD | N=207 Mean age: 54 yr With diabetes: 0% Follow-up: 15 mo | CKD 4–5; mean eGFR: 18 ml/min per 1.73 m2; had to agree to follow vegetarian VLPD and KAA during run-in period, proteinuria <1 g/d, and good nutritional status; exclusion criteria included poor compliance, heart/liver failure | VLPD (0.3 g protein/kg ideal body weight per day) and KAA (Ketosteril 0.125 g/kg ideal dry body weight per day); conventional LPD (0.6 g protein/kg per day);both arms received intensive nutritional counseling and monitoring q2wk for 1 month, monthly for 3 months, and then q3 months | RRT or >50% reduction in eGFR | Vegetarian VLPD KAA group had lower risk of RRT or >50% reduction in GFR (13% versus 42%); no safety concerns in terms of anthropometric or biochemical nutritional parameters; only 42% of patients meeting all selection criteria accepted potentially following vegetarian diet |
| Goraya et al. 2014 (34), United States | Fruits/vegetables to reduce dietary acid load by 50% versus sodium bicarbonate 0.3 mEq/kg per day versus usual care | N=108 Mean age: 54 yr With diabetes: 0% Follow-up: 3 y | Stage 3 CKD; mean eGFR: 39.5 ml/min per 1.73 m2; TCO2 <25 mmol/L; exclusion criteria included K >4.6 mEq/L, primary kidney disease, heart/liver failure, nephrotic syndrome | Fruits and vegetables prescribed by dietitian and distributed from community food bank (sufficient for patient and each of their household member) | eGFR using cystatin C at 3 years | Both the fruit/vegetable (−7.8 ml/min per 1.73 m2; 95% CI, −12.15 to −3.45 ml/min per 1.73 m2) and bicarbonate (−6.0 ml/min per 1.73 m2; 95% CI, −10.44 to −1.56 ml/min per 1.73 m2) groups slowed eGFR decline at 3 years versus control |
| Cianciaruso et al. 2008 (92), Italy | LPD versus normal protein diet | N=423 Mean age: 61 yr With diabetes: 12% | Stage 4–5 CKD; mean eGFR 18 ml/min per 1.73 m2; exclusion criteria included unstable kidney function, malignancy, immunosuppression, proteinuria >5 g/d | Prescribed low protein diet (0.55 g/kg per d) versus normal protein diet (0.80 g/kg per d); 24-hour urine urea nitrogen every 3 months with dietary guidance by dietitian for all patients | eGFR | No difference in change in eGFR; BUN (primary outcome) lower in low protein group; compliance was 27% in the 0.55 g/kg per day group and 53% in the 0.8 g/kg per day group |
| Locatelli 1989 (93), Italy | LPD versus normal protein diet | N=456 Mean age: 48.5 yr With diabetes: 0% Follow-up: 2 y from start of study | CKD stage 3–5; mean eGFR not reported; exclusion criteria included nephrotic syndrome, systemic diseases, obstructive uropathy | LPD (0.6 g/kg per day); normal protein diet (1 g/kg per day) | Slope of 1/SCr; ESKD or doubling of creatinine | No difference in ESKD or decline in CrCl; 32% lost by final follow-up |
| Klahr et al. 1994 (94), MDRD study I, United States | LPD versus normal protein diet | N=585Mean age: 52 yr With diabetes: 3% Mean follow-up: 2.2 y (mean reported for MDRD Study 1 and 2) | CKD stage 2–3; mean eGFR 38 ml/min per 1.73 m2; exclusion criteria included bodyweight <80% or >160% of standard body weight, DM requiring insulin, kidney transplant, chronic medical conditions | LPD (0.58 g/kg per day);normal protein diet (1.3 g/kg per day); protein intake assessed monthly with 24-hour urine urea nitrogen | Slope of GFR decline measured by 125I iothalamate; ESKD | LPD had no effect on GFR decline at 3 years; no effect on ESKD |
| Klahr et al. 1994 (94), MDRD study II, United States | VLPD and KAA supplement versus LPD diet | N=255 Mean age: 52 yrWith diabetes: 3% Follow-up: 2.2 yr (mean reported for MDRD Study 1 and 2) | CKD stage 4–5; mean eGFR 19 ml/min per 1.73 m2; exclusion criteria included bodyweight <80% or >160% of standard body weight, DM requiring insulin, kidney transplant, chronic medical conditions | VLPD (0.28 g/kg per day) plus KAA supplement; LPD (0.58 g/kg per day);protein intake assessed monthly with 24-hour urine urea nitrogen | Slope of GFR decline measured by 125I iothalamate; ESKD | VLPD and KAA tended to slow GFR decline GFR (0.8 ml/min per year less; 95% CI, −0.1 to 1.8 ml/min); no effect on ESKD |
| Rosman et al. 1984 (95), study I, Netherlands | LPD (0.6 g/kg per day) versus normal protein diet | N=228 Mean age: 48 yr X% diabetes Follow-up: maximum 18 mo | CKD stage 3; mean eGFR not reported; exclusion criteria included immunologic diseases or cancer, NSAIDs | LPD (0.6 g/kg per day); 24-hour urine urea excretion used to guide therapy by dietitian; normal protein (free diet) | Slope of 1/SCr; ESKD | No effect on ESKD; LPD slowed rate of rate of 1/SCr decline |
| Rosman et al. 1984 (95), study II, Netherlands | VLPD (0.4 g/kg per day) versus normal protein | Netherlands Mean age: 48 yrX% diabetes Follow-up: maximum 18 mo | CKD stage 4–5; mean eGFR not reported; exclusion criteria included immunologic diseases or cancer, NSAIDs | LPD (0.6 g/kg per day); normal protein (free diet) | Slope of 1/SCr; ESKD | Decreased risk of ESKD (RR, 0.52; 95% CI, 0.31 to 0.88); VLPD slowed rate of rate of 1/SCr decline |
RCT, randomized controlled trial; LPD, low protein diet; HR, hazard ratio; 95% CI, 95% confidence interval; SMD, standardized mean difference; VLPD, very low protein diet; RR, risk ratio; KDIGO, Kidney Disease Improving Global Outcomes; T1D, type 1 diabetes; T2D, type 2 diabetes; TCO2, total carbon dioxide; KAA, ketoacid analogue; q2wk, every 2 weeks; q3 months, every 3 months; K, potassium; SCr, serum creatinine; CrCl, creatinine clearance; MDRD, Modification of Diet in Renal Disease; DM, diabetes mellitus; 125I, iodine-125; NSAID, nonsteroidal anti-inflammatory drug.
Table 4.
Summary of selected observational studies examining diet and CKD progression
| Publication Details | Sample Description (Size, Age, Location, % diabetes) | Kidney Function | Dietary Assessment | Key Findings/Conclusions |
|---|---|---|---|---|
| Dietary patterns | ||||
| Hu et al. 2021 (26), United States | N=2403 Age: 21–74 yr With diabetes: 43% Median follow-up: 12 yr | CKD stages 1–4; mean eGFR: 47 ml/min per 1.73 m2 | Dietary scores (HEI-2015, aHEI-2010, aMed, DASH) calculated using data from FFQ (NCI DHQ) at up to three time points (baseline, year 2, year 4) | Adherence to healthy dietary patterns associated with decreased risk of ESKD or 50% eGFR decline (highest adherence tertile versus lowest adherence tertile for aHEI-2010 [aHR, 0.83; 95% CI, 0.69 to 0.99], aMed [aHR, 0.75; 95% CI, 0.62 to 0.90], DASH [aHR, 0.83; 95% CI, 0.69 to 0.99]; no association for HEI-2015 score [aHR, 0.91; 95% CI, 0.77 to 1.09]) |
| Banerjee et al. 2019 (25), United States | N=1110 Mean age: 70.2 yr With diabetes: 28% Median follow-up: 7.8 yr | CKD stage 3; mean eGFR: 48.4 ml/min per 1.73 m2 | DASH score calculated using single 24-hour dietary recall | Lowest adherence quintile versus highest adherence quintile associated with higher risk of ESKD (aHR, 2.6; 95% CI, 1.9 to 3.8) |
| Ricardo et al. 2015 (4), United States | N=3006 Mean age: 58 yr With diabetes: 45% Median follow-up: 4 yr | CKD stages 1–4; mean eGFR: 43 ml/min per 1.73 m2 | “Healthy diet score” based on five dietary factors from AHA recommendations for CVD health promotion using data from FFQ (NCI DHQ) at baseline | No association between healthy diet score and CKD progression |
| Gutierrez et al. 2014 (96), United States | N=3972 Mean age: 68.2 yr With diabetes: 33% | CKD stages 1–5; mean eGFR: 68 ml/min per 1.73 m2 | Empirically derived dietary pattern scores using data from FFQ (block 98 FFQ): “convenience,” “plant-based,” “Southern,” “alcohol/salads” | There were no associations between five empirically derived dietary patterns with incident ESKD |
| Dietary acid load/net endogenous acid production | ||||
| Scialla et al. 2012 (31), United States | N=632 Mean age: 55 yr With diabetes: not reported Median follow-up: 3.2 yr | CKD stages 2–4; mean eGFR: 46.5 ml/min per 1.73 m2 | NEAP calculated using 24-hour urine urea nitrogen and potassium measured up to four times in first 2 years | Higher NEAP associated with faster decline in iothalamate-measured GFR (Q4 versus Q1, 1.01 ml/min per 1.73 m2 per year faster decline); ESKD outcome not significant (per 25 mEq/d, HR, 1.10; 95% CI, 0.96 to 1.26) |
| Banerjee et al. 2015 (97), United States | N=1486 Mean age: 73 yr With diabetes: 30% Median follow-up: 14.2 yr | CKD stages 3–4; eGFR 15–40 ml/min per 1.73 m2, 26%; eGFR 40–60 ml/min per 1.73 m2, 74% | Dietary acid load calculated using single 24-hour dietary recall | Higher dietary acid load associated increased risk of ESKD (tertile 3 versus tertile 1, HR, 3.04; 95% CI, 1.58 to 5.86; tertile 2 versus tertile 1, HR, 1.81; 95% CI, 0.89 to 3.68); risk of ESKD associated with DAL tertiles increased as eGFR decreased (trend P=0.001) |
| Scialla et al. 2017 (32), United States | N=980 Mean age: 58 yrWith diabetes: 51% Median follow-up: 6 yr | CKD stages 1–4; mean eGFR: 44 ml/min per 1.73 m2 | NAE (sum of urinary ammonium and titratable acidity calculated from urinary pH, phosphate, and creatinine); NEAP calculated using estimated protein and potassium intake on the basis of: (1) a single FFQ, and (2) a single 24-hour urine collection for urea nitrogen and potassium | Higher NAE associated with decreased risk of composite of ESKD or halving of eGFR in diabetics (aHR, 0.88 per 10 mEq/day higher NAE; 95% CI, 0.80 to 0.98), but not in those without diabetes (aHR, 1.04 per 10 mEq/day higher NAE; 95% CI, 0.89 to 1.22). In secondary analyses, higher NEAPurine was associated with the composite outcome (per 10 mEq/d, 1.03; 95% CI, 1.00 to 1.06) in those without diabetes but not in those with diabetes (per 10 mEq/d, 1.00; 95% CI, 0.97 to 1.02). No association between NEAP and ESKD using FFQ |
| Sodium and potassium | ||||
| He et al. 2016 (38), United States | N=3757 Mean age: 58 yr With diabetes: 48% Total cohort follow-up: 15,807 yr | CKD stages 2–4; mean eGFR: 44.5 ml/min per 1.73 m2 | Mean sodium and potassium of up to three 24-hour urine collections (N=2165 with three measurements; N=983 with two measurements; N=609 with one measurement) | Compared with the lowest quartile of urinary sodium excretion (<116.8 mmol per 24 h), highest quartile of urinary sodium excretion associated with increased risk of CKD progression (HR, 1.54; 95% CI, 1.23 to 1.92); compared with the lowest quartile of urinary potassium excretion, highest quartile of urinary potassium excretion associated with increased risk of CKD progression (HR, 1.59; 95% CI, 1.25 to 2.03) |
| Leonberg-Yoo et al. 2017 (98), United States | N=840 Mean age: 52 yr With diabetes: 5% Median follow-up: 19.2 yr | CKD stages 3–5; mean eGFR: 32.6 ml/min per 1.73 m2 | 24-Hour urine potassium measured at baseline and in sensitivity analysis every month (median number of urine collections, 24) | No association between baseline urine potassium and kidney failure (per 1-SD increase, aHR, 0.95; 95% CI, 0.87 to 1.04); similar findings using time-averaged potassium |
| Koo et al. 2018 (39), South Korea | N=2238 Age: 55 yr With diabetes: 25% Median follow-up: not reported | CKD stages 1–5; mean eGFR: 51.1 ml/min per 1.73 m2 | Single 24-hour urine sodium and potassium | Compared with lowest quartile of Na/K ratio, there was increased risk of 50% eGFR decline or ESKD in the highest quartile of Na/K ratio (aHR, 2.95; 95% CI, 1.56 to 5.81) and the second highest quartile of Na/K ratio (aHR, 2.48; 95% CI, 1.30 to 4.90) |
| Kim et al. 2019 (99), South Korea | N=1821 Mean age: 55 yr With diabetes: 34% Total cohort follow-up: 5326 person-years | CKD stages 1–5; mean eGFR: 47 ml/min per 1.73 m2 | Single urine potassium/creatinine ratio (n=1821); 24-hour urine potassium (n=855) | Compared with the highest quartile of each potassium measure, low potassium excretion was associated with increased risk of ESKD or 50% eGFR decline measured by spot urine potassium/creatinine ratio (aHR, 1.47; 95% CI, 1.01 to 2.12) and by 24-hour urine potassium (aHR, 3.05; 95% CI, 1.54 to 6.04) |
HEI-2014, Healthy Eating Index 2014; aHEI-2010, Alternative Healthy Eating Index 2010; aMed, alternate Mediterranean diet; DASH, Dietary Approaches to Stop Hypertension; FFQ, food frequency questionnaire; NCI, National Cancer Institute; DHQ, Diet History Questionnaire; aHR, adjusted hazard ratio; AHA, American Heart Association; CVD, cardiovascular disease; NEAP, net endogenous acid production; Q4, quartile 4; HR, hazard ratio; DAL, dietary acid load; NAE, net acid excretion; NAEPurine, net acid excretion of purine; Na, sodium; K, potassium.
Figure 2.
Among nondiabetics with CKD, low protein intake had no effect on risk of ESKD and very low protein intake probably reduced risk of ESKD. Reprinted from ref. 15, with permission. IV, inverse variance; M–H, Mantel–Haenszel; MDRD, Modification of Diet in Renal Disease; VLP, very low protein.
Dietary patterns may be a better way to capture the relationship between diet and chronic disease than studies examining individual nutrients. In addition, dietary patterns may make it easier to provide nutritional advice to patients by focusing on a combination of healthful food groups, rather than focusing on restricting specific nutrients. A systematic review and meta-analysis found that healthy dietary patterns were associated with 27% reduced mortality (n=7 studies; 13,930 participants) but not ESKD (RR, 1.04; 95% CI, 0.68 to 1.40; n=3 studies; 10,071 participants) (24). A study using data from the National Health and Nutrition Examination Survey (NHANES; 1988–1994) found that a low Dietary Approaches to Stop Hypertension (DASH) dietary score was associated with ESKD (quintile 1 relative hazard, 1.7; 95% CI, 1.1 to 2.7; quintile 2 relative hazard, 2.2; 95% CI, 1.1 to 4.1) (25). Hu et al. (26) examined the relationship between dietary intake and risk of CKD progression in the Chronic Renal Insufficiency Cohort (CRIC) Study. Compared with participants with the lowest tertile of adherence, the participants in the most adherent tertile of the Alternative Healthy Eating Index-2010 (AHEI-2010), alternate Mediterranean diet (aMed), and DASH diet were associated with lower risk of CKD progression (17% for AHEI-2010, 25% for aMed, 17% for DASH).
Observational studies examining associations between specific foods and risk of ESKD have often found conflicting findings and can be challenging to interpret given the different methods of estimating intake and differing approaches to adjustment. In the Singapore Chinese Health Study, red meat intake was associated with ESKD (highest quartile versus lowest quartile, hazard ratio, 1.40; 95% CI, 1.15 to 1.71), whereas the consumption of poultry, fish, eggs, or dairy products was not associated with ESKD (27). In the CRIC Study, the benefits observed with the aMed study were largely driven by vegetable and nut intake, whereas no association was seen with red/processed meat intake (26). In the NHANES study, the association between DASH dietary score and risk of ESKD appeared to be mediated, in part, by dietary potassium and magnesium, and, to a lesser extent, to dietary acid load (25).
High net endogenous acid production has been theorized to contribute to kidney disease progression by causing net acid retention, which is associated with increased kidney levels of endothelin-1, and markers of kidney and bone injury (28). The most acid-producing foods tend to be sulfur-rich foods, such as hard/processed cheeses, egg yolks, meat, poultry, and fish, because sulfur is oxidized to inorganic sulfate (29). By contrast, fruits and vegetables are rich in citrate and malate, which undergo combustion internally to produce bicarbonate.
Wesson et al. (30) have shown that metabolic acidosis could lead to inflammation and fibrosis. Observational studies have shown an association between dietary acid load and risk of ESKD in NHANES (25), and eGFR decline in the African American Study of Kidney Disease and Hypertension (31). However, these studies used estimates of acid load or net acid excretion. In a recent analysis of the CRIC Study, net acid excretion was measured as the sum of urine ammonium and titratable acidity in 24-hour urine samples of a 980-participant subcohort (32). Surprisingly, higher net acid excretion was associated with decreased risk of a composite outcome of ESKD or 50% eGFR decline in patients with diabetes, whereas no association was found in those without diabetes (Table 4). Reasons for this discrepancy are unclear, but the authors hypothesized there may be diet-independent changes in acid production in diabetes (32). Regardless, interventions reducing net endogenous acid production appear to be beneficial for slowing CKD progression. A systematic review of interventions that treat metabolic acidosis (oral alkali therapy or dietary acid reduction) in stage 3–5 CKD found evidence of low-to-moderate certainty that treatment of metabolic acidosis with oral alkali therapy or dietary acid reduction may slow the rate of eGFR decline or reduce the risk of ESKD (33) (Figure 3) . In a small RCT of 108 patients with stage 3 CKD and bicarbonate levels of 22–24 mmol/L, randomization to alkali therapy, whether delivered as sodium bicarbonate or by providing fruits and vegetables, slowed cystatin C–based eGFR decline over 3 years (34). The KDOQI 2020 nutrition guidelines suggest, based on limited evidence, that increasing intake of fruits and vegetables can reduce net endogenous acid production to slow CKD progression (Table 2).
Figure 3.
Among studies of people with stage 3–5 CKD, there was low-to-moderate evidence that treatment of metabolic acidosis with oral alkali therapy or dietary acid reduction slowed the rate of eGFR decline or reduced the risk of ESKD. Reprinted from ref. 33, with permission. IV, inverse variance.
Limited data exist on sodium and potassium intake and CKD progression. Capturing dietary sodium and potassium intake is challenging because collecting multiple-day, 24-hour urine collections or diet records can be burdensome and food frequency questionnaires can be limited if questions are not applicable to local dietary practices; both food frequency questionnaires and 24-hour diet recalls are limited by recall bias. Multiple 24-hour urine collections are the gold standard for estimating sodium intake (35), although there have not been validation studies of 24-hour urine sodium and potassium versus measured dietary intake specifically in patients with CKD. RCTs comparing different levels of sodium intake suggest that sodium reduction lowers albuminuria (36,37), and observational studies (CRIC Study, KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease [KNOW-CKD]) have reported an association between high 24-hour urine sodium and increased risk of CKD progression (Table 4). Data on potassium are mixed, with high 24-hour urine potassium excretion being associated with CKD progression in CRIC, whereas low 24-hour urine potassium excretion was associated with CKD progression in KNOW-CKD (38,39). Unfortunately, there are not yet any long-term RCT data to determine whether modulating either sodium or potassium intake affects CKD progression. A multicenter, double-blind, placebo-controlled RCT testing the effects of 40 mEq of potassium (delivered as potassium chloride or potassium citrate) versus placebo on eGFR decline in patients with hypertension and stage 3b/4 CKD is currently underway (https://clinicaltrials.gov/ct2/show/NCT03253172) (40).
Specific types of CKD may have particular benefits to dietary modification. For example, although an RCT of coaching to increase water intake in patients with stage 3 CKD and albuminuria over 1 year reported no significant effect on kidney function decline (41), there may be benefits in patients with adult autosomal dominant polycystic kidney disease (ADPKD) (42). Increased antidiuretic hormone (arginine vasopressin) stimulates cell proliferation through the vasopressin V2 receptor, resulting in increased intracellular cAMP. Interestingly, high water intake decreases urinary vasopressin, whereas high sodium intake stimulates urinary vasopressin (43). A recent study found that every 1 g/d of sodium was associated with increased eGFR decline (−0.11 ml/min per 1.73 m2 per gram of salt), whereas protein intake was not associated with CKD progression in patients with ADPKD. The association between sodium intake and CKD progression was substantially mediated by plasma copeptin, supporting the possible role of sodium increasing vasopressin and resulting in CKD progression (42). Further randomized trials are needed to determine whether sodium restriction or increased fluid intake can slow progression in ADPKD.
Physical Activity and CKD
There is little controversy regarding the beneficial effects of physical activity on overall health and, thus, the Physical Activity Guidelines for Americans recommends adults should move more and sit less throughout the day and participate in any amount of moderate-to-vigorous physical activity to gain some health benefits. For substantial health benefits, adults should do at least 150 minutes of moderate-intensity activity per week or 75 minutes of vigorous-intensity activity per week. Adults should also do strengthening activities of moderate or greater intensity on ≥2 days a week because these activities provide additional health benefits (44). The term exercise is typically applied to moderate or vigorous physical activities (44).
Depending on how physical activity is defined (see Box 1), many adults with CKD have self-reported sedentary behavior and most do not reach recommended levels of physical activity (4,5,8,9, 45). Reduced physical activity is associated with increased mortality (5) and more CVD events (6), but less is known about its relationship to CKD progression, with much of the research stemming from single-center observational studies and very few clinical trials (Table 5) (46). Robinson-Cohen, et al. (45) described the relationship between self-reported leisure-time physical activity and kidney function decline in 256 outpatients with stage 3–4 CKD (mean age, 61 years). Nearly a quarter of the patients reported no leisure-time activity at all, and they had a greater decline in eGFR per year compared with those who met the guideline-recommended levels of physical activity. Further, each 60 minute–greater duration of leisure-time physical activity was associated with a 0.5% per year slower decline in eGFR, but leisure-time activity was not associated with incident ESKD (45). Leisure-time physical activity was also not associated with rapid kidney function decline (>3 ml/min per year) among 558 older adults with an eGFR of <60 ml/min per 1.73 m2 (47). Walking and participation in physical activity more than one time per week in older adults (mean age, >70 years) has been reported to be associated with reduced risk of incident ESKD (48), but, in a younger cohort of people with CKD, recommended physical activity, compared with inactivity, was not associated with a composite CKD progression outcome (50% decline in eGFR from baseline or incident ESKD) (4). Together, these findings suggest that greater leisure-time physical activity may slow the rate of kidney function decline, and that any physical activity may be beneficial for lowering the risk of ESKD, particularly among older adults. However, due to the observational design and heterogeneous definitions for CKD progression, these studies cannot establish causality, and it is possible that physical activity is a marker of overall morbidity and could be confounded by survival bias or competing risk of CVD events in younger individuals. More data are needed to further assess the relationship of physical activity and, particularly the minimum amount needed to slow CKD progression.
Box 1.
Physical Activity Intensity and Classifications of Activity
Intensity: Effort required to perform an activity. Aerobic intensity is typically expressed as rate of energy expenditure using kilocalories per minute or metabolic equivalent of task (MET). One MET is equivalent to sitting at rest.
Sedentary: Activity ≤1.5 METs while sitting, reclining or lying down.
Light intensity: Nonsedentary activities while awake, 1.6 to <3.0 METs (e.g., walking at leisurely pace).
Moderate intensity: Activities 3 to <6 METs (e.g., walking briskly, swimming).
Vigorous intensity: Activities ≥6 METs (e.g., walking very fast, running).
Inactive: No moderate- or vigorous-intensity physical activity beyond daily life activities per week.
Insufficiently active: One to <150 min/wk of moderate-intensity physical activity or 1 to <75 min/wk of vigorous-intensity activity per week, or equivalent combination.
Active: Moderate-intensity activity for ≥150 min/week, or equivalent combination of moderate and vigorous (meets guidelines at this level).
Table 5.
Summary of select epidemiologic studies of the association between physical activity and CKD progression among individuals with established CKD
| Publication Details | Sample Description and Follow-Up | Kidney Function (CKD Stages, Mean eGFR) | Definition of PA | CKD Progression Measure(s) | Key Findings of Adjusted Analyses |
|---|---|---|---|---|---|
| Observational studies | |||||
| Robinson-Cohen et al.2009 (47), United States | N=558 Age: all >65 yr Median follow-up: 7 yr | Kidney function only reported <60 ml/min per 1.73 m2 | Self-report of leisure-time PA, converted to kilocalories per week | Rapid kidney function decline (>3 ml/min per year) using cystatin C–eGFR | PA was not significantly associated with rapid decline in kidney function |
| Muntner et al. 2013 (9), United States | N=3093 Age: mean 72.2 yr With diabetes: 35% Median follow-up: 4 yr | eGFR 47 ml/min per 1.73 m2 (CKD-EPI) | Self-report of PA and categorized into levels: no PA per week, intermediate (1–3 times per week), ideal (≥4 times per week) | Incident ESKD (dialysis or kidney transplant) | Compared with no PA, intermediate and ideal PA were associated with reduced risk of ESKD (aHR, 0.66 [95% CI, 0.46 to 0.94] and 0.50 [95% CI, 0.32 to 0.78], respectively) |
| Chen et al. 2014 (48), China | N=6363 Age: mean 70 yr With diabetes: 42% Median follow-up: 1.3 yr | Stage 3 (36%); stage 4 (20%); stage 5 (44%) | Self-report of walking in last 3 months; categorized into frequency (0, 1–2, 3–4, 5–6, ≥7 times) and duration of activity | Incident ESKD (dialysis or kidney transplant) | Walking was associated with lower ESKD risk (aHR, 0.79; 95% CI, 0.73 to 0.85), and significant trend with lower risk with more frequent walking |
| Robinson-Cohen et al. 2014 (45), United States | N=256 Mean age: 61 yr With diabetes: 54% Median follow-up: 3.7 yr | CKD 3–5; mean eGFR range: 37.6–43.9 ml/min per 1.73 m2 | Self-report of leisure-time PA in past 4 weeks (converted to minutes per week) and categorized into none, 1–60, 60–150 and >150 min/wk | Cystatin C–eGFR change per year; incident ESKD | Each 60-minute greater duration of weekly PA was associated with 0.5% per year slower decline in eGFR (P=0.04);PA was not associated with incident ESKD |
| Ricardo et al. 2015 (4), United States | N=3006 Mean age: 58 yr With diabetes: 45% Median follow-up: 4 yr | Mean eGFR: 43 ml/min per 1.73 m2 (Cr and cystatin C CRIC-specific equation) | Self-reported moderate and vigorous PA (converted to minutes/week) and categorized as ideal (moderate ≥150 min/wk, vigorous >75 min/wk, or moderate plus vigorous ≥150 min/wk), less than ideal (>0 min/wk but not ideal), and inactive (0 min/wk) | Composite of 50% decrease in eGFR from baseline or incident ESKD | Ideal PA, compared with inactivity, was not associated with CKD progression |
| Meta-analyses of exercise RCTs on kidney function | Included Trials | Comparators | CKD Progression Measure(s) | Key Findings | |
| Nakamura et al. 2020 (49) | Nine trials with N=459 with eGFR as outcome;five trials with N=231 with serum creatinine as outcome | Included RCTs comparing interventions of physical exercise (center based, home based, combined exercise) with usual care | eGFR; serum Cr | Effect of exercise training on eGFR was not significant compared to usual care (SMD, −0.34; P=0.67; moderate-low certainty); effect of exercise training on serum creatinine was not significant compared to usual care (SMD, 1.48; P=0.75; low certainty) | |
| Villanego et al. 2020 (50) | 11 trials with N=429 with eGFR as outcome; six trials with N=182 with proteinuria as outcome | Included RCTs comparing intervention that included an exercise component (aerobic and strength training) with a control group without physical exercise | eGFRproteinuria (24-h urine protein and albumin-creatinine ratio) | No differences in eGFR between intervention and control groups (SMD, −0.14; P=0.90; moderate-low certainty); no differences in proteinuria between intervention and control groups (SMD, 26.2; P=0.82; low certainty) | |
PA, physical activity; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; aHR, adjusted hazard ratio; Cr, creatinine; CRIC, Chronic Renal Insufficiency Cohort; RCT, randomized controlled trial; SMD, standardized mean difference.
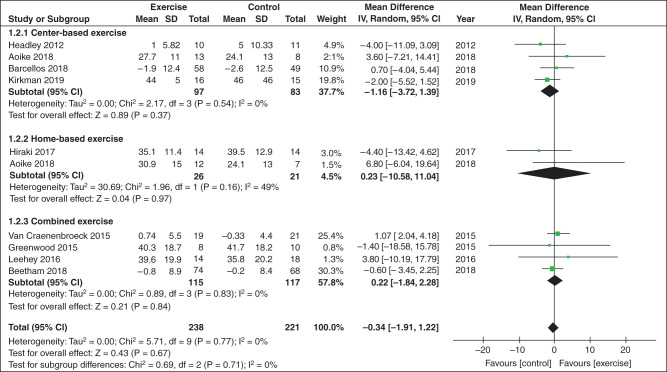
Directly assessing the effects of physical activity on CKD progression in patients with established CKD requires RCTs of physical activity or exercise training programs. Two recent meta-analyses investigated the effect of RCTs testing physical activity and exercise interventions on CKD progression among people with CKD, and both meta-analyses found that the interventions were not associated with reduced CKD progression, in terms of no difference in eGFR (Figure 4) (49,50), serum creatinine (49), or proteinuria (50) between intervention and control groups. However, RCTs included in these meta-analyses consisted of small sample sizes, were of short duration, and did not have adequate observation time to evaluate the effect of risk of disease progression. In addition, serum creatinine correlates with muscle mass, which poses a challenge in interpreting small increases in creatinine during an exercise trial due to increased physical activity and increased muscle metabolism, leading to an underestimation of eGFR and, therefore, kidney function.
Figure 4.
A meta-analysis of physical activity and exercise interventions tested in randomized controlled trials among people with CKD found there was no difference in eGFR among intervention and control groups. Reprinted from ref. 49, with permission.
Weight Management and CKD
In clinical practice, weight management is typically assessed with body mass index (BMI), determined by body weight and height (in kilograms per meter squared). The World Health Organization considers a BMI of between 18.5 and 25 kg/m2 as normal weight, a BMI between 25 and 30 kg/m2 as overweight, and a BMI of >30 kg/m2 as obese. The prevalence of obesity continues to increase worldwide, and a consistent, graded relationship between obesity and incident CKD has been demonstrated (11,51,52). A recent UK Biobank study using Mendelian randomization suggests that the obesity-CKD relationship is causal and mostly mediated by diabetes and elevated BP (53). However, the relationship between obesity and CKD progression is less clear in patients with established CKD (Table 6).
Table 6.
Summary of select epidemiologic studies of the association of BMI and CKD progression among individuals with established CKD
| Publication Details | Sample Description and Follow-Up | Kidney Function | Obesity Measure | CKD Progression Measure(s) | Key Findings |
|---|---|---|---|---|---|
| Hsu et al. 2006 (52), United States | N=unclear; source population 320,252 with and without CKD; unclear how many with eGFR <60 ml/min per 1.73 m2Age: 31–43 yr (across categories of weight)With diabetes: 13%–21% (across categories of weight)Follow-up: from 1964 to 1985 until 2000 | Individuals with eGFR <60 ml/min per 1.73 m2 (MDRD) or with presence of proteinuria or hematuria on urinalysis | BMI categories: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), class 1 obesity (30–34.9 kg/m2), class 2 obesity (35–39.9 kg/m2), and extreme obesity (≥40 kg/m2) | Incidence of ESKD (dialysis or transplant) | Compared with normal weight, overweight/obesity associated with increased risk of ESKD in models adjusted for demographics, smoking, myocardial infarction, cholesterol, creatinine, proteinuria, hematuria: BMI 25–29.9 kg/m2 (RR, 1.5; 95% CI, 1.2 to 2.0), BMI 30–34.9 kg/m2 (RR, 2.7; 95% CI, 2.0 to 3.6), BMI 35–39.9 kg/m2 (RR, 4.7; 95% CI, 3.3 to 6.8), BMI ≥40 kg/m2 (RR, 3.1; 95% CI, 1.8 to 5.3) |
| Babayev et al. 2013 (100), United States | N=12,534 participants of National Kidney Foundation Kidney Early Evaluation ProgramMean age: 66.8 yrWith diabetes: 42%Follow-up: 8 yr | CKD stages 3–4;mean eGFR: 48 ml/min per 1.73 m2 (CKD-EPI) | BMI categories: <30 kg/m2, 30–34.9 kg/m2, ≥35 kg/m2 | Incident ESKD | Compared with BMI <30 kg/m2, obesity not associated with ESKD in models adjusted for demographics, hypertension, diabetes, eGFR, and albuminuria: BMI 30–34.9 kg/m2 (HR, 0.90; 95% CI, 0.65 to 1.25); BMI ≥35 kg/m2 (HR, 1.09; 95% CI, 0.78 to 1.52) |
| Lu et al. 2014 (10), United States | N=453,946 US veterans with eGFR <60 ml/min per 1.73 m2Age: 73.9 yrWith diabetes: 41%Follow-up: from 2004 to 2006 until 2012 | Mean eGFR: 47.9 ml/min per 1.73 m2 (CKD-EPI) | BMI categories: <20, 20 to <25, 25 to <30, 30 to <35, 35 to <40, 40 to <45, 45 to <50, and ≥50 kg/m2 | Incident ESKD; doubling of serum creatinine; slopes of eGFR | BMI between 25–35 kg/m2, corresponding to overweight/mild obesity, had lowest risk of CKD progression. In models adjusted for demographics, comorbidities (except for hypertension, diabetes), medications, BMI ≥35 kg/m2 associated with worse outcomes in patients with stage 3 CKD, but not in stage 4–5 CKD; additional adjustment for hypertension and diabetes attenuated associations |
| Yun et al. 2018 (101), South Korea | N=1940 participants of KNOW-CKDAge: 53.5 yrWith diabetes: 34%Median follow-up: 3.1 yr | CKD 1–5; mean eGFR: 54.1 ml/min per 1.73 m2 (CKD-EPI) | Obesity defined as BMI ≥25 kg/m2 | Composite of 50% decline in eGFR from baseline or onset of ESKD (dialysis or kidney transplant) | Compared with nonobesity, obesity was associated with increased risk of CKD progression. In model adjusted for demographics, smoking, laboratory measures, left ventricular mass index, eGFR, proteinuria: obesity was associated with HR of 1.41 (95% CI, 1.08 of 1.83) |
| Chang et al. 2019 (11), individual-level meta-analysis | 39 general population cohort studies (N=5,459,014)Mean follow-up: 6 yr | Individuals with eGFR <60 ml/min per 1.73 m2 | Continuous BMI with linear splines | GFR decline determined by estimated eGFR decline ≥40%, initiation of RRT or eGFR <10 ml/min per 1.73 m2 | Among patients with eGFR <60 ml/min per 1.73 m2 in general population cohorts, J-shaped association between BMI and risk for GFR decline with significant heterogeneity across cohorts; in models adjusted for demographics and smoking, compared with a BMI of 25 kg/m2, HRs at 35 kg/m2: eGFR 30–59 ml/min per 1.73 m2 (HR, 1.78; 95% CI, 1.36 to 2.34); eGFR <30 ml/min per 1.73 m2 (HR, 1.88; 95% CI, 1.61 to 2.18);adjustment for potential mediators attenuated association (moderate certainty) |
| Chang et al. 2019 (11), individual-level meta-analysis | 18 cohort studies with participants with CKD (N=91,607)Mean follow-up: 4 yr | Mean eGFR range: 22–75 ml/min per 1.73 m2 across cohorts | BMI categories: 20, 25, 30, 35, 40, 45 kg/m2 | GFR decline determined by estimated eGFR decline ≥40%, initiation of RRT or eGFR <10 ml/min per 1.73 m2 | J-shaped association between BMI and risk for GFR decline with significant heterogeneity across cohorts; in model adjusted for demographics and smoking, compared with BMI 25 kg/m2, HR at 35 kg/m2 was 1.17 (95% CI, 1.04 to 1.31); excluding first 3 years of follow-up, magnified association (HR, 1.75; 95% CI, 1.30 to 2.37), suggesting reverse causation; adjustment for potential mediators attenuated association (moderate certainty) |
BMI, body mass index; MDRD, Modification of Diet in Renal Disease; RR, relative risk; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; HR, hazard ratio; KNOW-CKD, KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease.
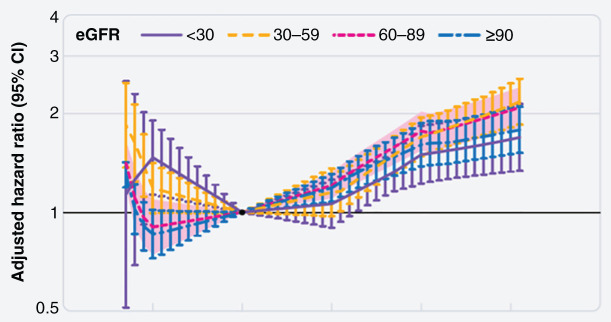
In a large cohort of 453,946 US veterans with an eGFR of <60 ml/min per 1.73 m2, a BMI corresponding to overweight/mild obesity (25–35 kg/m2) had the lowest risk for CKD progression, and a BMI <25 kg/m2 was consistently associated with worsening of kidney function (10). However, the generalizability of this study’s findings may be limited because the cohort was older (>70 years), and most participants were men. Individual-level meta-analyses were conducted by the CKD Prognosis Consortium in 5.5 million adults in 39 general population cohorts and 91,607 adults in 18 CKD cohorts (11). In models stratified by baseline eGFR, hazard ratios for risk of eGFR decline for a BMI of 35 kg/m2 versus a reference BMI of 25 kg/m2 were 1.88 (95% CI, 1.69 to 2.08) for those with an eGFR of 30–59 ml/min per 1.73 m2 were 1.78 (95% CI, 1.36 to 2.34) and 1.88 (95% CI, 1.61 to 2.18) for those with an eGFR of <30 ml/min per 1.73 m2 (Figure 5). Adjustment for potential mediators (e.g., hypertension, diabetes, albuminuria) attenuated results. Meta-analysis of participants in the CKD cohorts found smaller effect sizes, with sensitivity analysis excluding the first 3 years of follow-up suggesting some role of reverse causation (Figure 5, Table 6). Risks associated with obesity may vary by type of kidney disease. An elevated BMI among individuals with IgA nephropathy and ADPKD has been noted as a risk factor for CKD progression (54,55), whereas no association between elevated BMI and risk of CKD progression was noted in a cohort of patients with biopsy sample–proven glomerulonephritides (56).
Figure 5.
Individual-level meta-analyses found the risk of eGFR decline was higher among those with BMI 35 kg/m2, particularly with eGFR <60 ml/min per 1.73 m2. Meta-analyzed hazard ratios and 95% CIs are related to body mass index, modeled by linear splines with knots at body mass indices of 20, 25, 30, and 35 kg/m2 (reference is body mass index of 25 kg/m2 in each category). Reprinted from ref. 11, with permission.
CKD management guidelines recommend weight reduction for adults with CKD and obesity. However, intentional weight loss among these individuals has shown mixed results, and largely depends on the type of weight-loss intervention (e.g., diet, exercise, surgery, medications). The current evidence of the effects of weight loss on the progression of CKD is mainly on the basis of observational reports and a few small randomized trials of low-to-moderate quality. Bariatric surgery has most consistently suggested a beneficial effect of weight loss on kidney function (57), with a recent RCT of patients with type 2 diabetes and albuminuria demonstrating greater resolution of albuminuria in those undergoing Roux-en-Y gastric bypass versus best medical treatment (82% versus 48% remission) (58). At this time, there are no convincing data to support a specific diet for weight loss to slow CKD progression (59). Additionally, some controversy exists in more advanced CKD (i.e., eGFR of <30 ml/min per 1.73 m2) because malnutrition and muscle wasting are potential concerns and observational studies have found an association between weight loss and risk of death in patients with ESKD (60–62). However, much of this weight loss is unintentional, and recent studies have found that bariatric surgery is associated with reduced risk of death in patients with CKD and ESKD (63,64). It is beyond the scope of this review to describe in detail each of the weight-loss strategies and CKD progression; others have provided in-depth reviews of weight-loss strategies that have been used in CKD studies (65,66).
Tobacco Use and CKD
Tobacco use is the leading preventable cause of death and disability in the United States and is a major contributor to CVD (67), which is more common among those with CKD than those without kidney disease. Smoking is associated with increased risk of progression of CKD in some, but not all, studies, and the beneficial effects of smoking cessation on delaying CKD progression is unclear (Table 7) (68,69). Current cigarette smokers, compared with nonsmokers, have been found to have greater eGFR decline per year among those with early-stage CKD and type 2 diabetes (70), or biopsy sample–proven glomerular disease (71), but smoking has not been associated with rapid eGFR decline (>1 ml/min per 1.73 m2 per year) among those with stage 3 CKD and ADPKD (72). In terms of incident ESKD, smoking was observed to have a higher risk in a study of 1722 people with CKD stage 3 (73), but, among larger cohorts (N=3093 and N=6245) of people with similar levels of kidney function (9,13), smoking was not found to be associated with ESKD.
Table 7.
Summary of select epidemiologic studies of the association of smoking and CKD progression among people with established CKD
| Publication Details | Sample Description and Follow-Up | Kidney Function | Definition of Smoking | CKD Progression Measure(s) | Key Findings of Adjusted Analyses |
|---|---|---|---|---|---|
| Orth et al. 1998 (102) | Matched pairs: IgA-GN (N=54), ADPKD (N=48) Mean age: 47 (patients) and 49 (controls) yr among men, 48 (patients) and 51 (controls) yr among women | Mean serum creatinine was 1.4±0.5 mg/dl in patients with IgA-GN and 1.8±0.6 mg/dl in patients with ADPKD | Self-report of cigarette smoking; calculated pack-years | ESKD (hemodialysis or kidney transplant) | The risk of ESKD in men with >5 pack-years, compared with <5 pack-years, was increased among those without ACE-inhibitor treatment (OR, 10.1; 95% CI, 2.3 to 4.5) but not those on ACE-inhibitor therapy (OR, 1.4; 95% CI, 0.3 to 7.1) |
| Chuahirun and Wesson 2002 (70), United States | N=33 Mean age: mean 46 yr With diabetes: 100% with type 2 Mean follow-up: 5.3 yr | Mean eGFR: 98 ml/min (Cockcroft–Gault) | Self-reported current cigarette smoker or nonsmoker | Slope of eGFR (ml/min over 5 years) | Greater eGFR decline among smokers compared with nonsmokers (−0.59 to −0.09 ml/min; P=0.009) |
| Yoshida et al. 2008 (71), Japan | N=485 Mean age: 41.5 yr With diabetes: not reported Follow-up: 2002–2007 | CKD stage 1 and 2; mean eGFR: 86 ml/min per 1.73 m2 (MDRD) | Self-reported current cigarette smoker or nonsmoker | Slope of eGFR (ml/min per 1.73 m2 per year) | Smokers had greater eGFR decline than nonsmokers (−2.06 versus −1.58 ml/min per 1.73 m2 per year; P=0.01) |
| Grams et al. 2012 (73), United States | N=1722 Mean age: 49 yr in no vascular disease group and 60 yr in vascular disease group With diabetes: 6% Follow-up: 17.6 yr | Mean eGFR: 38.8 ml/min per 1.73 m2 (no vascular disease group);mean eGFR: 33.9 ml/min per 1.73 m2 (vascular disease group)(CKD-EPI) | Self-reported current cigarette smoker or nonsmoker | Incident ESKD (dialysis or transplant) | Compared with nonsmokers, smokers were associated with higher risk of ESKD (aHR, 1.30; 95% CI, 1.09 to 1.55) |
| Tanaka et al. 2013 (103), Japan | N=416 Mean age: 64 yr With diabetes: 32% Median follow-up: 3.3 yr | Mean eGFR: 55 ml/min per 1.73 m2 (Japanese-specific, creatinine-based equation) | Self-reported current cigarette smoker or nonsmoker | Composite of ESKD or doubling in creatinine | Compared with nonsmokers, smokers were not associated with ESKD (aHR, 1.25; 95% CI, 0.41 to 3.84) |
| Ozkok et al. 2013 (72), Turkey | N=323 with ADPKD Mean age: 53 yr With diabetes: not reported Mean follow-up: 8.3 yr | Mean eGFR: 60 ml/min per 1.73 m2 (MDRD) | Self-reported current cigarette smoker or nonsmoker | Rapid progressor defined as >1 ml/min per year decline in eGFR | Smoking not associated with rapid progression (aHR, 0.78; 95% CI, 0.28 to 2.16) |
| Muntner et al. 2013 (9), United States | N=3093 Mean age: 72.2 yr With diabetes: 35% Median follow-up: 4 yr | Mean eGFR: 47 ml/min per 1.73 m2 (CKD-EPI) | Self-reported cigarette use, and categorized as poor (current smoker), intermediate (former smoker, quit ≤12 mo), ideal (never smoker or quit >12 mo ago) | Incident ESKD | Compared with current smoking, nonsmoking was not associated with ESKD (aHR, 0.79; 95% CI, 0.50 to 1.23) |
| Ricardo et al. 2015 (4), United States | N=3006 Mean age: 58 yr With diabetes: 45% Median follow-up: 4 yr | Mean eGFR: 43 ml/min per 1.73 m2(cystatin C and Cr CRIC-specific equation) | Self-reported smoking (≥100 cigarettes in lifetime) and classified as current, past, or never smoker | Composite of 50% decrease in eGFR from baseline or incident ESKD | Compared with current smokers, past and never smokers had reduced risk of CKD progression (aHR, 0.79 [95% CI, 0.64 to 0.98] and 0.68 [95% CI, 0.55 to 0.84], respectively) |
| Staplin et al. 2016 (13), United States | N=6245 (part of RCT for simvastatin and ezetimibe versus placebo) Mean age: 61 yr With diabetes: 34% Median follow-up: 4.9 yr | CKD stages 2–5 (MDRD) | Self-reported current cigarette smoker, or previously been a regular smoker of cigarettes (former), or never smoker | Incident ESKD and composite of incident ESKD or doubling in creatinine | Compared with never smokers, former and current smokers had a similar rate of progression to ESKD and for the composite of ESKD or doubling in serum creatinine |
| Roehm et al. 2017 (79), United States | N=108 smokers and 108 nonsmokers with hypertension; all treated with ACE-inhibitorMean age: 50 yrDiabetes excluded Follow-up: 5 yr | Stage 2 CKD (eGFR 60–80 ml/min per 1.73 m2) with spot UACR >200 mg/g(CKD-EPI using cystatin C) | Smokers consumed ≥10 cigarettes per day for ≥1 yr;all smokers underwent smoking-cessation intervention program | Change in eGFR | 5-year eGFR was higher in quitters than in continued smokers (62.0±5.4 versus 52.9±5.6 ml/min per 1.73 m2, P<0.001); this value was lower in quitters than in nonsmokers (64.7±5.6 ml/min per 1.73 m2, P=0.02) |
| Bundy et al. 2018 (74), United States | N=3939 Mean age: 58 yr With diabetes: 48% Median follow-up: 5.5 yr | Mean eGFR: 44–51 ml/min per 1.73 m2 (cystatin C and Cr CRIC-specific equation) | Self-reported smoking (≥100 cigarettes in lifetime) and classified as current, past, or never smoker; modeled as cumulative average exposure | Composite of 50% decrease in eGFR from baseline or incident ESKD | Compared with nonsmoking throughout follow-up, persistent smoking was not associated with CKD progression (aHR, 1.02; 95% CI, 0.86 to 1.21) |
| Lee et al. 2021 (75), South Korea | N=1951 Mean age: 54 yr With diabetes: 33% Mean follow-up: 3 yr | Mean eGFR: 53 ml/min per 1.73 m2 | Self-report smoking classified as nonsmoker, former, or current smoker; and calculated as pack-years | Composite 50% decrease in eGFR from baseline or incident ESKD | Compared with never smokers, former and current smokers were associated with increased risk of CKD progression (aHR, 1.54 [95% CI, 1.11 to 2.15] and 1.56 [95% CI, 1.09 to 2.22], respectively); compared with never smokers, smokers with ≥30 pack-years had increased risk of CKD progression (aHR, 1.94; 95% CI, 1.35 to 2.77) |
ADPKD, autosomal dominant polycystic kidney disease; ACE, angiotensin-converting enzyme; OR, odds ratio; MDRD, modification of diet in renal disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; aHR, adjusted hazard ratio; Cr, creatinine; CRIC, chronic renal insufficiency cohort; RCT, randomized controlled trial; UACR, urinary albumin-creatinine ratio.
Former and/or current smoking has been associated with increased risk of a composite outcome of incident ESKD and 50% decline in eGFR in some, but not all, studies—depending on how the smoking exposure was modeled (Table 7). This is exemplified in two studies, using the same data from 3939 adults with an eGFR of 20–70 ml/min per 1.73 m2 in the CRIC Study, that observed current smoking did and did not have an increased risk of CKD progression when modeled from baseline exposure or as cumulative average exposure, respectively (4,74). The inconsistencies of smoking and CKD progression risk may be attributed to the observational nature of most of the smoking research in CKD, including the study design and population, such as differences in age (which could introduce survival bias), proportion with diabetes, and length of follow-up time to observe the outcome of interest.
There appears to be a dose-response relationship of greater smoking exposure and CKD progression in that higher smoking pack-years were associated with increased CKD progression. In a cohort of 1951 middle-aged adults with predominantly CKD stage 3 (Mean eGFR: 53 ml/min per 1.73 m2) from the KNOW-CKD Study, ≥15 pack-year smokers and ≥30 pack-year smokers, compared with never smokers, were each associated with increased risk of CKD progression over 3 years of follow-up (75).
The benefits of smoking cessation on CKD progression have not been well studied but appear to be promising. Several studies have shown that the risk for CKD progression of former smokers was lower than that of current smokers (76,77), and there was a graded decrease in risk as time since smoking cessation increased (78). There has been one clinical trial of 108 smokers and 108 nonsmokers with stage 2 CKD and proteinuria without diabetes treated with angiotensin-converting enzyme inhibitor therapy where the current smokers underwent a smoking-cessation intervention program. After 5 years of follow-up, eGFR was higher in nonsmokers and quitters than in continued smokers (79). The use of electronic cigarettes as a means to quit cigarette smoking is not currently supported because the data on the use of electronic cigarettes in those with kidney disease are sparse.
Alcohol Use and CKD
In the general population, alcohol use is associated with poorer health overall, but moderate drinking appears to be protective for some CVD events (80–84). It is proposed that alcohol consumption has cardioprotective effects on insulin sensitivity, thrombotic activity, and inflammation (85). However, among people with established CKD, no or moderate alcohol intake, compared with excessive intake, has been associated with increased risk of major coronary events (6). Similarly, the epidemiologic literature on alcohol and kidney function, which has focused on both the development and progression of kidney disease, has reported mixed observations, largely due to very few studies specifically investigating the relationship of alcohol consumption and the effect on disease progression among individuals with established CKD (Table 8). According to these studies, only binge alcohol drinking was associated with increased risk of CKD progression, and other levels of drinking (i.e., any, occasional, or moderate) were not associated with CKD progression. In the general population, reducing alcohol consumption among those who drink heavily has been demonstrated in RCTs to lower BP, but there are insufficient data on the risks or benefits of these interventions on BP in CKD populations, which precluded specific recommendations in the most recent KDIGO 2021 Guidelines for Blood Pressure and CKD (86).
Table 8.
Summary of select epidemiologic studies of the association of alcohol use and CKD progression among people with established CKD
| Publication Details | Sample Description and Follow-Up | Kidney Function | Definition of Alcohol | CKD Progression Measure(s) | Key Findings of Adjusted Analyses |
|---|---|---|---|---|---|
| Menon et al. 2010 (104), United States | N=618 Age: >65 yr With diabetes: 14% Mean follow-up: 5.6 yr | Reported as <60 ml/min per 1.73 m2 | Self-report alcohol consumption, categorized as none, former, current | Cystatin C–eGFR decline (>3 ml/min per 1.73 m2 per year) dichotomous outcome | Compared with no alcohol consumption, former or current alcohol consumption was not associated with rapid kidney function decline (OR, 0.38 [95% CI, 0.14 to 1.01] and 0.73 [95% CI, 0.45 to 1.19], respectively) |
| Bundy et al. 2018 (74), United States | N=3939 Mean age: 58 yr With diabetes: 48% Median follow-up: 5.5 yr | Mean eGFR: 44–51 ml/min per 1.73 m2 (cystatin C and Cr CRIC-specific equation) | Self-report alcohol consumption, categorized as drinkers if have one or more alcoholic beverage(s) per week; modeled as average persistent exposure over time | Composite of 50% decrease in eGFR from baseline or incident ESKD | Compared with nondrinking throughout follow-up, persistent alcohol drinking was not associated with CKD progression |
| Joo et al. 2020 (105), Republic of Korea | N=1883 in CKD cohort Mean age: 54 yr With diabetes: 33% Median follow-up: 2.95 yr | Mean eGFR 50 ml/min per 1.73 m2 (CKD-EPI) | Self-report alcohol consumption, categorized as nondrinking, occasional drinking (<6 drinks/wk), regular drinking (≥6 drinks/wk); binge drinking ≥5 drinks per occasion | Composite of 50% decrease in eGFR from baseline or incident ESKD | Compared with nondrinkers, occasional and moderate drinking were not associated with CKD progression; compared with nondrinkers, occasional binge drinking, and regular binge drinking were associated with increased risk of CKD progression (aHR, 1.99 [95% CI, 1.33 to 2.98] and 2.19 [95% CI, 1.38 to 3.46], respectively) |
OR, odds ratio; Cr, creatinine; CRIC, chronic renal insufficiency cohort; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; aHR, adjusted hazard ratio.
Future Research Directions
Published KDOQI and KDIGO clinical practice guidelines that address lifestyle behaviors for the management of CKD in adults have identified research directions to address gaps in the evidence for delaying CKD progression (17,86,87). We summarize some of these recommendations here, including establishing the best methods to assess dietary intake and to support dietary change, such as nutritional education and dietary modification through shared decision making, behavior-modification techniques, and motivational interviewing. More research is needed to understand the role and potential benefits of using technology to assess dietary intake and to support behavior change. Clinical trials are needed to evaluate different strategies for sodium reduction, including identifying the amount of dietary sodium intake that is associated with improved outcomes among people with CKD. Further studies should compare the benefits of various intensity levels and types of physical activity, including the incorporation of resistance training in those with CKD. This research could also identify individual factors for patients with CKD that have the greatest benefit in terms of clinical outcomes, including BP control, that could inform personalized physical-activity recommendations. In terms of weight-management research, future efforts could determine the relationship of weight loss and renoprotection and compare the effect of various weight-loss strategies on clinical outcomes in CKD. Additional lifestyle behavior research should include the effect of behaviors in combination on CKD progression and other important clinical and patient-reported outcomes.
Lifestyle Behaviors in Practice
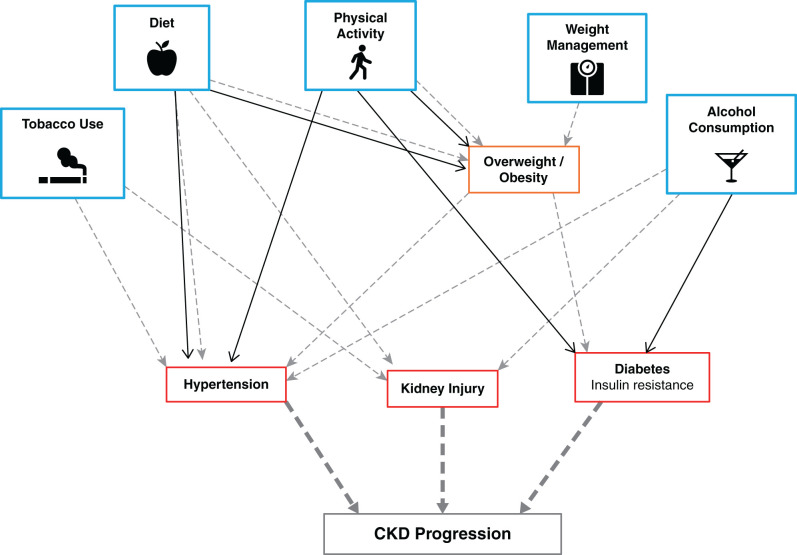
Despite lack of evidence of efficacy from large trials, maintaining a healthy lifestyle remains a cornerstone of CKD management because healthy lifestyle behaviors are largely modifiable and have been shown to improve cardiovascular health and BP control and are thought to indirectly or directly affect CKD progression (Figure 6). It is reasonable that all adults with CKD receive counseling on the benefits of a healthy lifestyle, including diet, physical activity, smoking cessation, weight management, and moderation of alcohol intake, and it is equally important to address suboptimal lifestyle behavior adherence during routine clinic visits despite competing issues. Health-care providers can refer patients with CKD to medical nutrition therapy–trained dieticians who specialize in behavioral change plans that include exercise, nutrition, and stress control, and this service is covered by most health insurance plans (88). Lifestyle recommendations are of relevance in all countries, with limited to no public health costs, and have the potential to make far-reaching public health gains. The importance of lifestyle behaviors in CKD is underscored in the international CKD management guidelines with the following statement: “for the practicing clinician, ideally working with a team of health-care professionals, it is important to institute general lifestyle modifications practices in people with CKD so that they may gain the benefit of these in addition to more kidney-specific strategies” (89).
Figure 6.
Healthy lifestyle behaviors indirectly and/or directly influence cardiovascular health and CKD progression. Gray dashed arrows indicate a harmful effect, and a solid black line reflects a beneficial effect.
Disclosures
A.R. Chang reports having other interests in/relationships with National Kidney Foundation (grant support for NKF Patient Network); having consultancy agreements with Novartis (as consultant); receiving research funding from Novo Nordisk (investigator-sponsored study); receiving honoraria from Reata; and serving as a scientific advisor for, or member of, Reata and Relypsa. S.J. Schrauben reports receiving honoraria from Cowen and Company, LLC. The remaining author has nothing to disclose.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants K23 DK 118198-01A1 (to S.J. Schrauben) and K23 DK106515 (to A.R. Chang).
Acknowledgments
The funders had no role in the study design, data collection, analysis and interpretation of data, in the writing of report, or in the decision to submit the article for publication.
Author Contributions
B.J. Apple, A.R. Chang, and S.J. Schrauben reviewed and edited the manuscript and were responsible for data curation; A.R. Chang and S.J. Schrauben were responsible for funding acquisition; A.R. Chang and S.J. Schrauben conceptualized the study and were responsible for formal analysis, investigation, and methodology; and S.J. Schrauben wrote the original draft.
References
- 1.Centers for Disease Control and Prevention : Chronic Kidney Disease in the United States. Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention, 2021 [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee : Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121: 586–613, 2010. 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010. 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricardo AC, Anderson CA, Yang W, Zhang X, Fischer MJ, Dember LM, Fink JC, Frydrych A, Jensvold NG, Lustigova E, Nessel LC, Porter AC, Rahman M, Wright Nunes JA, Daviglus ML, Lash JP; CRIC Study Investigators : Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 65: 412–424, 2015. 10.1053/j.ajkd.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T: Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 4: 1901–1906, 2009. 10.2215/CJN.01970309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrauben SJ, Hsu JY, Amaral S, Anderson AH, Feldman HI, Dember LM: Effect of kidney function on relationships between lifestyle behaviors and mortality or cardiovascular outcomes: A pooled cohort analysis. J Am Soc Nephrol 32: 663–675, 2021. 10.1681/ASN.2020040394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navaneethan SD, Kirwan JP, Arrigain S, Schreiber MJ, Sehgal AR, Schold JD: Overweight, obesity and intentional weight loss in chronic kidney disease: NHANES 1999-2006. Int J Obes 36: 1585–1590, 2012. 10.1038/ijo.2012.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beddhu S, Wei G, Marcus RL, Chonchol M, Greene T: Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin J Am Soc Nephrol 10: 1145–1153, 2015. 10.2215/CJN.08410814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muntner P, Judd SE, Gao L, Gutiérrez OM, Rizk DV, McClellan W, Cushman M, Warnock DG: Cardiovascular risk factors in CKD associate with both ESRD and mortality. J Am Soc Nephrol 24: 1159–1165, 2013. 10.1681/ASN.2012070642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP: Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol 25: 2088–2096, 2014. 10.1681/ASN.2013070754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang AR, Grams ME, Ballew SH, Bilo H, Correa A, Evans M, Gutierrez OM, Hosseinpanah F, Iseki K, Kenealy T, Klein B, Kronenberg F, Lee BJ, Li Y, Miura K, Navaneethan SD, Roderick PJ, Valdivielso JM, Visseren FLJ, Zhang L, Gansevoort RT, Hallan SI, Levey AS, Matsushita K, Shalev V, Woodward M; CKD Prognosis Consortium (CKD-PC) : Adiposity and risk of decline in glomerular filtration rate: Meta-analysis of individual participant data in a global consortium. BMJ 364: k5301, 2019. 10.1136/bmj.k5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention : Current cigarette smoking among adults in the United States, 2020. Available at: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm#nation. Accessed November 1, 2021
- 13.Staplin N, Haynes R, Herrington WG, Reith C, Cass A, Fellström B, Jiang L, Kasiske BL, Krane V, Levin A, Walker R, Wanner C, Wheeler DC, Landray MJ, Baigent C, Emberson J; SHARP Collaborative Group : Smoking and adverse outcomes in patients with CKD: The Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 68: 371–380, 2016. 10.1053/j.ajkd.2016.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner BM, Lawler EV, Mackenzie HS: The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996. 10.1038/ki.1996.265 [DOI] [PubMed] [Google Scholar]
- 15.Hahn D, Hodson EM, Fouque D: Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst Rev 10: CD001892, 2018. 10.1002/14651858.CD001892.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X-F, Xu J, Liu L-J, Wang F, He S-L, Su Y, Dong CP: Efficacy of low-protein diet in diabetic nephropathy: A meta-analysis of randomized controlled trials. Lipids Health Dis 18: 82, 2019. 10.1186/s12944-019-1007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group , 2020. Available at: https://kdigo.org/wp-content/uploads/2020/10/KDIGO-2020-Diabetes-in-CKD-GL.pdf. Accessed November 1, 2021 [Google Scholar]
- 18.Kontessis P, Jones S, Dodds R, Trevisan R, Nosadini R, Fioretto P, Borsato M, Sacerdoti D, Viberti G: Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int 38: 136–144, 1990. 10.1038/ki.1990.178 [DOI] [PubMed] [Google Scholar]
- 19.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011. 10.2215/CJN.05040610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay EM, Oliver J: Renal damage following the ingestion of a diet containing an excess of inorganic phosphate. J Exp Med 61: 319–334, 1935. 10.1084/jem.61.3.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang AR, Anderson C: Dietary phosphorus intake and the kidney. Annu Rev Nutr 37: 321–346, 2017. 10.1146/annurev-nutr-071816-064607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang AR, Miller ER 3rd, Anderson CA, Juraschek SP, Moser M, White K, Henry B, Krekel C, Oh S, Charleston J, Appel LJ: Phosphorus additives and albuminuria in early stages of CKD: A randomized controlled trial. Am J Kidney Dis 69: 200–209, 2017. 10.1053/j.ajkd.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruggiero B, Trillini M, Tartaglione L, Rotondi S, Perticucci E, Tripepi R, Aparicio C, Lecchi V, Perna A, Peraro F, Villa D, Ferrari S, Cannata A, Mazzaferro S, Mallamaci F, Zoccali C, Bellasi A, Cozzolino M, Remuzzi G, Ruggenenti P, Kohan DE; ANSWER Study Organization : Effects of sevelamer carbonate in patients with CKD and proteinuria: The ANSWER Randomized Trial. Am J Kidney Dis 74: 338–350, 2019. 10.1053/j.ajkd.2019.01.029 [DOI] [PubMed] [Google Scholar]
- 24.Kelly JT, Palmer SC, Wai SN, Ruospo M, Carrero J-J, Campbell KL, Strippoli GF: Healthy dietary patterns and risk of mortality and ESRD in CKD: A meta-analysis of cohort studies. Clin J Am Soc Nephrol 12: 272–279, 2017. 10.2215/CJN.06190616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee T, Crews DC, Tuot DS, Pavkov ME, Burrows NR, Stack AG, Saran R, Bragg-Gresham J, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int 95: 1433–1442, 2019. 10.1016/j.kint.2018.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu EA, Coresh J, Anderson CAM, Appel LJ, Grams ME, Crews DC, Mills KT, He J, Scialla J, Rahman M, Navaneethan SD, Lash JP, Ricardo AC, Feldman HI, Weir MR, Shou H, Rebholz CM; CRIC Study Investigators : Adherence to healthy dietary patterns and risk of CKD progression and all-cause mortality: Findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis 77: 235–244, 2021. 10.1053/j.ajkd.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lew QJ, Jafar TH, Koh HW, Jin A, Chow KY, Yuan JM, Koh WP: Red meat intake and risk of ESRD. J Am Soc Nephrol 28: 304–312, 2017. 10.1681/ASN.2016030248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wesson DE, Pruszynski J, Cai W, Simoni J: Acid retention with reduced glomerular filtration rate increases urine biomarkers of kidney and bone injury. Kidney Int 91: 914–927, 2017. 10.1016/j.kint.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 29.Scialla JJ, Anderson CA: Dietary acid load: A novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 20: 141–149, 2013. 10.1053/j.ackd.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesson DE, Buysse JM, Bushinsky DA: Mechanisms of metabolic acidosis-induced kidney injury in chronic kidney disease. J Am Soc Nephrol 31: 469–482, 2020. 10.1681/ASN.2019070677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scialla JJ, Appel LJ, Astor BC, Miller ER 3rd, Beddhu S, Woodward M, Parekh RS, Anderson CA; African American Study of Kidney Disease and Hypertension Study Group : Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int 82: 106–112, 2012. 10.1038/ki.2012.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scialla JJ, Asplin J, Dobre M, Chang AR, Lash J, Hsu CY, Kallem RR, Hamm LL, Feldman HI, Chen J, Appel LJ, Anderson CA, Wolf M; Chronic Renal Insufficiency Cohort Study Investigators : Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int 91: 204–215, 2017. 10.1016/j.kint.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navaneethan SD, Shao J, Buysse J, Bushinsky DA: Effects of treatment of metabolic acidosis in CKD: A systematic review and meta-analysis. Clin J Am Soc Nephrol 14: 1011–1020, 2019. 10.2215/CJN.13091118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goraya N, Simoni J, Jo C-H, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014. 10.1038/ki.2014.83 [DOI] [PubMed] [Google Scholar]
- 35.Ginos BNR, Engberink RHGO: Estimation of sodium and potassium intake: Current limitations and future perspectives. Nutrients 12: 3275, 2020. 10.3390/nu12113275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Campbell KL: A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 24: 2096–2103, 2013. 10.1681/ASN.2013030285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang JH, Chin HJ, Kim S, Kim DK, Kim S, Park JH, Shin SJ, Lee SH, Choi BS, Lim CS: Effects of intensive low-salt diet education on albuminuria among nondiabetic patients with hypertension treated with olmesartan: A single-blinded randomized, controlled trial. Clin J Am Soc Nephrol 9: 2059–2069, 2014. 10.2215/CJN.01310214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, Rosas SE, Porter A, Makos G, Weir MR, Hamm LL, Kusek JW; Chronic Renal Insufficiency Cohort Study Investigators : Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 27: 1202–1212, 2016. 10.1681/ASN.2015010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koo H, Hwang S, Kim TH, Kang SW, Oh K-H, Ahn C, Kim YH: The ratio of urinary sodium and potassium and chronic kidney disease progression: Results from the KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). Medicine (Baltimore) 97: e12820, 2018. 10.1097/MD.0000000000012820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gritter M, Vogt L, Yeung SMH, Wouda RD, Ramakers CRB, de Borst MH, Rotmans JI, Hoorn EJ: Rationale and design of a randomized placebo-controlled clinical trial assessing the renoprotective effects of potassium supplementation in chronic kidney disease. Nephron 140: 48–57, 2018. 10.1159/000490261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark WF, Sontrop JM, Huang SH, Gallo K, Moist L, House AA, Cuerden MS, Weir MA, Bagga A, Brimble S, Burke A, Muirhead N, Pandeya S, Garg AX: Effect of coaching to increase water intake on kidney function decline in adults with chronic kidney disease: The CKD WIT Randomized Clinical Trial. JAMA 319: 1870–1879, 2018. 10.1001/jama.2018.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres VE, Abebe KZ, Schrier RW, Perrone RD, Chapman AB, Yu AS, Braun WE, Steinman TI, Brosnahan G, Hogan MC, Rahbari FF, Grantham JJ, Bae KT, Moore CG, Flessner MF: Dietary salt restriction is beneficial to the management of autosomal dominant polycystic kidney disease. Kidney Int 91: 493–500, 2017. 10.1016/j.kint.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramers BJ, Koorevaar IW, Drenth JPH, de Fijter JW, Neto AG, Peters DJM, Vart P, Wetzels JF, Zietse R, Gansevoort RT, Meijer E: Salt, but not protein intake, is associated with accelerated disease progression in autosomal dominant polycystic kidney disease. Kidney Int 98: 989–998, 2020. 10.1016/j.kint.2020.04.053 [DOI] [PubMed] [Google Scholar]
- 44.US Department of Health and Human Services : Physical Activity Guidelines for Americans, 2nd Ed., Washington, DC, US Department of Health and Human Services, 2018 [Google Scholar]
- 45.Robinson-Cohen C, Littman AJ, Duncan GE, Weiss NS, Sachs MC, Ruzinski J, Kundzins J, Rock D, de Boer IH, Ikizler TA, Himmelfarb J, Kestenbaum BR: Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol 25: 399–406, 2014. 10.1681/ASN.2013040392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansen KL, Painter P: Exercise in individuals with CKD. Am J Kidney Dis 59: 126–134, 2012. 10.1053/j.ajkd.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M, Shlipak M, Siscovick D, Kestenbaum B: Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 169: 2116–2123, 2009. 10.1001/archinternmed.2009.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen I-R, Wang S-M, Liang CC, Kuo H-L, Chang C-T, Liu J-H, Lin HH, Wang IK, Yang YF, Chou CY, Huang CC: Association of walking with survival and RRT among patients with CKD stages 3-5. Clin J Am Soc Nephrol 9: 1183–1189, 2014. 10.2215/CJN.09810913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura K, Sasaki T, Yamamoto S, Hayashi H, Ako S, Tanaka Y: Effects of exercise on kidney and physical function in patients with non-dialysis chronic kidney disease: A systematic review and meta-analysis. Sci Rep 10: 18195, 2020. 10.1038/s41598-020-75405-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villanego F, Naranjo J, Vigara LA, Cazorla JM, Montero ME, García T, Torrado J, Mazuecos A: Impact of physical exercise in patients with chronic kidney disease: Sistematic review and meta-analysis. Nefrologia (Engl Ed) 40: 237–252, 2020. 10.1016/j.nefroe.2020.06.012 [DOI] [PubMed] [Google Scholar]
- 51.Vivante A, Golan E, Tzur D, Leiba A, Tirosh A, Skorecki K, Calderon-Margalit R: Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med 172: 1644–1650, 2012. 10.1001/2013.jamainternmed.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006. 10.7326/0003-4819-144-1-200601030-00006 [DOI] [PubMed] [Google Scholar]
- 53.Zhu P, Herrington WG, Haynes R, Emberson J, Landray MJ, Sudlow CLM, Woodward M, Baigent C, Lewington S, Staplin N: Conventional and genetic evidence on the association between adiposity and CKD. J Am Soc Nephrol 32: 127–137, 2021. 10.1681/ASN.2020050679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berthoux F, Mariat C, Maillard N: Overweight/obesity revisited as a predictive risk factor in primary IgA nephropathy. Nephrol Dial Transplant 28[Suppl 4]: iv160–iv166, 2013. 10.1093/ndt/gft286 [DOI] [PubMed] [Google Scholar]
- 55.Nowak KL, You Z, Gitomer B, Brosnahan G, Torres VE, Chapman AB, Perrone RD, Steinman TI, Abebe KZ, Rahbari-Oskoui FF, Yu ASL, Harris PC, Bae KT, Hogan M, Miskulin D, Chonchol M: Overweight and obesity are preditors of progression in early autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29: 571–578, 2018. 10.1681/ASN.2017070819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elyan BMP, Lees JS, Gillis KA, Mackinnon B, Fox JG, Geddes CC, McQuarrie EP: Obesity is not associated with progression to end stage renal disease in patients with biopsy-proven glomerular diseases. BMC Nephrol 20: 237, 2019. 10.1186/s12882-019-1434-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolignano D, Zoccali C: Effects of weight loss on renal function in obese CKD patients: A systematic review. Nephrol Dial Transplant 28: iv82–iv98, 2013. 10.1093/ndt/gft302 [DOI] [PubMed] [Google Scholar]
- 58.Cohen RV, Pereira TV, Aboud CM, Petry TBZ, Lopes Correa JL, Schiavon CA, Pompílio CE, Pechy FNQ, da Costa Silva ACC, de Melo FLG, Cunha da Silveira LP, de Paris Caravatto PP, Halpern H, Monteiro FLJ, da Costa Martins B, Kuga R, Palumbo TMS, Docherty NG, le Roux CW: Effect of gastric bypass vs best medical treatment on early-stage chronic kidney disease in patients with type 2 diabetes and obesity: A randomized clinical trial. JAMA Surg 155: e200420, 2020. 10.1001/jamasurg.2020.0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambert K, Beer J, Dumont R, Hewitt K, Manley K, Meade A, Salamon K, Campbell K: Weight management strategies for those with chronic kidney disease: A consensus report from the Asia Pacific Society of Nephrology and Australia and New Zealand Society of Nephrology 2016 renal dietitians meeting. Nephrology (Carlton) 23: 912–920, 2018. 10.1111/nep.13118 [DOI] [PubMed] [Google Scholar]
- 60.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, Krishnan M, Nissenson AR, Danovitch GM, Kalantar-Zadeh K: Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant 11: 725–736, 2011. 10.1111/j.1600-6143.2011.03468.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harhay MN, Chen X, Chu NM, Norman SP, Segev DL, McAdams-DeMarco M: Pre-kidney transplant unintentional weight loss leads to worse post-kidney transplant outcomes. Nephrol Dial Transplant 36: 1927–1936, 2021. 10.1093/ndt/gfab164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ku E, Kopple JD, Johansen KL, McCulloch CE, Go AS, Xie D, Lin F, Hamm LL, He J, Kusek JW, Navaneethan SD, Ricardo AC, Rincon-Choles H, Smogorzewski M, Hsu CY; CRIC Study Investigators : Longitudinal weight change during CKD progression and its association with subsequent mortality. Am J Kidney Dis 71: 657–665, 2018. 10.1053/j.ajkd.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheetz KH, Woodside KJ, Shahinian VB, Dimick JB, Montgomery JR, Waits SA: Trends in bariatric surgery procedures among patients with ESKD in the United States. Clin J Am Soc Nephrol 14: 1193–1199, 2019. 10.2215/CJN.01480219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coleman KJ, Shu YH, Fischer H, Johnson E, Yoon TK, Taylor B, Imam T, DeRose S, Haneuse S, Herrinton LJ, Fisher D, Li RA, Theis MK, Liu L, Courcoulas AP, Smith DH, Arterburn DE, Friedman AN: Bariatric surgery and risk of death in persons with chronic kidney disease [published online ahead of print March 3, 2021]. Ann Surg 10.1097/SLA.0000000000004851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chintam K, Chang AR: Strategies to treat obesity in patients with CKD. Am J Kidney Dis 77: 427–439, 2021. 10.1053/j.ajkd.2020.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman AN, Kaplan LM, le Roux CW, Schauer PR: Management of obesity in adults with CKD. J Am Soc Nephrol 32: 777–790, 2021. 10.1681/ASN.2020101472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.U.S. Department of Health and Human Services : The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 [Google Scholar]
- 68.Halimi JM, Giraudeau B, Vol S, Cacès E, Nivet H, Lebranchu Y, Tichet J: Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int 58: 1285–1292, 2000. 10.1046/j.1523-1755.2000.00284.x [DOI] [PubMed] [Google Scholar]
- 69.Sawicki PT, Mühlhauser I, Bender R, Pethke W, Heinemann L, Berger M: Effects of smoking on blood pressure and proteinuria in patients with diabetic nephropathy. J Intern Med 239: 345–352, 1996. 10.1046/j.1365-2796.1996.468809000.x [DOI] [PubMed] [Google Scholar]
- 70.Chuahirun T, Wesson DE: Cigarette smoking predicts faster progression of type 2 established diabetic nephropathy despite ACE inhibition. Am J Kidney Dis 39: 376–382, 2002. 10.1053/ajkd.2002.30559 [DOI] [PubMed] [Google Scholar]
- 71.Yoshida T, Takei T, Shirota S, Tsukada M, Sugiura H, Itabashi M, Ogawa T, Uchida K, Tsuchiya K, Nitta K: Risk factors for progression in patients with early-stage chronic kidney disease in the Japanese population. Intern Med 47: 1859–1864, 2008. 10.2169/internalmedicine.47.1171 [DOI] [PubMed] [Google Scholar]
- 72.Ozkok A, Akpinar TS, Tufan F, Kanitez NA, Uysal M, Guzel M, Caliskan Y, Alisir S, Yazici H, Ecder T: Clinical characteristics and predictors of progression of chronic kidney disease in autosomal dominant polycystic kidney disease: A single center experience. Clin Exp Nephrol 17: 345–351, 2013. 10.1007/s10157-012-0706-3 [DOI] [PubMed] [Google Scholar]
- 73.Grams ME, Coresh J, Segev DL, Kucirka LM, Tighiouart H, Sarnak MJ: Vascular disease, ESRD, and death: Interpreting competing risk analyses. Clin J Am Soc Nephrol 7: 1606–1614, 2012. 10.2215/CJN.03460412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bundy JD, Bazzano LA, Xie D, Cohan J, Dolata J, Fink JC, Hsu CY, Jamerson K, Lash J, Makos G, Steigerwalt S, Wang X, Mills KT, Chen J, He J; CRIC Study Investigators : Self-reported tobacco, alcohol, and illicit drug use and progression of chronic kidney disease. Clin J Am Soc Nephrol 13: 993–1001, 2018. 10.2215/CJN.11121017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee S, Kang S, Joo YS, Lee C, Nam KH, Yun H-R, Park JT, Chang TI, Yoo TH, Kim SW, Oh KH, Kim YH, Park SK, Kang SW, Choi KH, Ahn C, Han SH: Smoking, smoking cessation, and progression of chronic kidney disease: Results from KNOW-CKD Study. Nicotine Tob Res 23: 92–98, 2021. 10.1093/ntr/ntaa071 [DOI] [PubMed] [Google Scholar]
- 76.Schiffl H, Lang SM, Fischer R: Stopping smoking slows accelerated progression of renal failure in primary renal disease. J Nephrol 15: 270–274, 2002 [PubMed] [Google Scholar]
- 77.Chuahirun T, Simoni J, Hudson C, Seipel T, Khanna A, Harrist RB, Wesson DE: Cigarette smoking exacerbates and its cessation ameliorates renal injury in type 2 diabetes. Am J Med Sci 327: 57–67, 2004. 10.1097/00000441-200402000-00001 [DOI] [PubMed] [Google Scholar]
- 78.Hallan SI, Orth SR: Smoking is a risk factor in the progression to kidney failure. Kidney Int 80: 516–523, 2011. 10.1038/ki.2011.157 [DOI] [PubMed] [Google Scholar]
- 79.Roehm B, Simoni J, Pruszynski J, Wesson DE: Cigarette smoking attenuates kidney protection by angiotensin-converting enzyme inhibition in nondiabetic chronic kidney disease. Am J Nephrol 46: 260–267, 2017. 10.1159/000481206 [DOI] [PubMed] [Google Scholar]
- 80.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G: Alcohol dosing and total mortality in men and women: An updated meta-analysis of 34 prospective studies. Arch Intern Med 166: 2437–2445, 2006. 10.1001/archinte.166.22.2437 [DOI] [PubMed] [Google Scholar]
- 81.Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren WMM, Sato S, Njølstad I, Woodward M, Salomaa V, Nordestgaard BG, Yeap BB, Fletcher A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, de la Cámara AG, Völzke H, Dahm CC, Dale CE, Bergmann MM, Crespo CJ, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno-Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Tumino R, Blazer DG 2nd, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca-Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundström J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kühn T, Grobbee DE, Barrett-Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Després JP, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjønneland A, Brenner H, Palmieri L, Dallongeville J, Brunner EJ, Assmann G, Trevisan M, Gillum RF, Ford I, Sattar N, Lazo M, Thompson SG, Ferrari P, Leon DA, Smith GD, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J; Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group : Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 391: 1513–1523, 2018. 10.1016/S0140-6736(18)30134-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mukamal KJ, Chen CM, Rao SR, Breslow RA: Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. J Am Coll Cardiol 55: 1328–1335, 2010. 10.1016/j.jacc.2009.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB: Combined impact of lifestyle factors on mortality: Prospective cohort study in US women. BMJ 337: a1440, 2008. 10.1136/bmj.a1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, van Staveren WA: Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: The HALE project. JAMA 292: 1433–1439, 2004. 10.1001/jama.292.12.1433 [DOI] [PubMed] [Google Scholar]
- 85.Haseeb S, Alexander B, Baranchuk A: Wine and cardiovascular health: A comprehensive review. Circulation 136: 1434–1448, 2017. 10.1161/CIRCULATIONAHA.117.030387 [DOI] [PubMed] [Google Scholar]
- 86.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group : Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2021-BP-GL.pdf. Accessed Novembe 1, 2021 [Google Scholar]
- 87.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero J-J, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, Kaysen GA, Kopple JD, Teta D, Yee-Moon Wang A, Cuppari L: KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update [published correction appears in Am J Kidney Dis 77: 308, 2021]. Am J Kidney Dis 76: S1–S107, 2020. 10.1053/j.ajkd.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 88.Kramer H, Jimenez EY, Brommage D, Vassalotti J, Montgomery E, Steiber A, Schofield M: Medical nutrition therapy for patients with non-dialysis-dependent chronic kidney disease: Barriers and solutions. J Acad Nutr Diet 118: 1958–1965, 2018. 10.1016/j.jand.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 89.Kidney Disease Improving Global Outcome : KIDGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 90.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B: 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140: e596–e646, 2019. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G: Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J Am Soc Nephrol 27: 2164–2176, 2016. 10.1681/ASN.2015040369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cianciaruso B, Pota A, Pisani A, Torraca S, Annecchini R, Lombardi P, Capuano A, Nazzaro P, Bellizzi V, Sabbatini M: Metabolic effects of two low protein diets in chronic kidney disease stage 4-5--a randomized controlled trial. Nephrol Dial Transplant 23: 636–644, 2008. 10.1093/ndt/gfm576 [DOI] [PubMed] [Google Scholar]
- 93.Locatelli F: Controlled study of protein-restricted diet in chronic renal failure. Contrib Nephrol 75: 141–146, 1989. 10.1159/000417740 [DOI] [PubMed] [Google Scholar]
- 94.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994. 10.1056/NEJM199403313301301 [DOI] [PubMed] [Google Scholar]
- 95.Rosman JB, ter Wee PM, Meijer S, Piers-Becht TP, Sluiter WJ, Donker AJ: Prospective randomised trial of early dietary protein restriction in chronic renal failure. Lancet 2: 1291–1296, 1984. 10.1016/S0140-6736(84)90818-3 [DOI] [PubMed] [Google Scholar]
- 96.Gutiérrez OM, Muntner P, Rizk DV, McClellan WM, Warnock DG, Newby PK, Judd SE: Dietary patterns and risk of death and progression to ESRD in individuals with CKD: A cohort study. Am J Kidney Dis 64: 204–213, 2014. 10.1053/j.ajkd.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Ríos-Burrows N, Williams DE, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol 26: 1693–1700, 2015. 10.1681/ASN.2014040332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leonberg-Yoo AK, Tighiouart H, Levey AS, Beck GJ, Sarnak MJ: Urine potassium excretion, kidney failure, and mortality in CKD. Am J Kidney Dis 69: 341–349, 2017. 10.1053/j.ajkd.2016.03.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HW, Park JT, Yoo T-H, Lee J, Chung W, Lee K-B, Chae DW, Ahn C, Kang SW, Choi KH, Han SH; KNOW-CKD Study Investigators : Urinary potassium excretion and prorgression of CKD. Clin J Am Soc Nephrol 14: 330–340, 2019. 10.2215/CJN.07820618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Babayev R, Whaley-Connell A, Kshirsagar A, Klemmer P, Navaneethan S, Chen S-C, Li S, McCullough PA, Bakris G, Bomback A; KEEP Investigators : Association of race and body mass index with ESRD and mortality in CKD stages 3-4: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 61: 404–412, 2013. 10.1053/j.ajkd.2012.11.038 [DOI] [PubMed] [Google Scholar]
- 101.Yun H-R, Kim H, Park JT, Chang TI, Yoo TH, Kang SW, Choi KH, Sung S, Kim SW, Lee J, Oh KH, Ahn C, Han SH; Korean Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) Investigators : Obesity, metabolic abnormality, and progression of CKD. Am J Kidney Dis 72: 400–410, 2018. 10.1053/j.ajkd.2018.02.362 [DOI] [PubMed] [Google Scholar]
- 102.Orth SR, Stöckmann A, Conradt C, Ritz E, Ferro M, Kreusser W, Piccoli G, Rambausek M, Roccatello D, Schäfer K, Sieberth HG, Wanner C, Watschinger B, Zucchelli P: Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int 54: 926–931, 1998. 10.1046/j.1523-1755.1998.00067.x [DOI] [PubMed] [Google Scholar]
- 103.Tanaka K, Nakayama M, Kanno M, Kimura H, Watanabe K, Tani Y, Kusano Y, Suzuki H, Hayashi Y, Asahi K, Sato K, Miyata T, Watanabe T: Skin autofluorescence is associated with the progression of chronic kidney disease: A prospective observational study. PLoS One 8: e83799, 2013. 10.1371/journal.pone.0083799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Menon V, Katz R, Mukamal K, Kestenbaum B, de Boer IH, Siscovick DS, Sarnak MJ, Shlipak MG: Alcohol consumption and kidney function decline in the elderly: Alcohol and kidney disease. Nephrol Dial Transplant 25: 3301–3307, 2010. 10.1093/ndt/gfq188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joo YS, Koh H, Nam KH, Lee S, Kim J, Lee C, Yun HR, Park JT, Kang EW, Chang TI, Yoo TH, Oh KH, Chae DW, Lee KB, Kim SW, Lee J, Kang SW, Choi KH, Ahn C, Han SH: Alcohol consumption and progression of chronic kidney disease: Results from the Korean Cohort Study for Outcome in Patients with Chronic Kidney Disease. Mayo Clin Proc 95: 293–305, 2020. 10.1016/j.mayocp.2019.06.014 [DOI] [PubMed] [Google Scholar]