Abstract
During sporulation, Bacillus thuringiensis produces crystalline inclusions comprised of a mixture of δ-endotoxins. Following ingestion by insect larvae, these inclusion proteins are solubilized, and the protoxins are converted to toxins. These bind specifically to receptors on the surfaces of midgut apical cells and are then incorporated into the membrane to form ion channels. The steps required for toxin insertion into the membrane and possible oligomerization to form a channel have been examined. When bound to vesicles from the midguts of Manduca sexta larvae, the Cry1Ac toxin was largely resistant to digestion with protease K. Only about 60 amino acids were removed from the Cry1Ac amino terminus, which included primarily helix α1. Following incubation of the Cry1Ab or Cry1Ac toxins with vesicles, the preparations were solubilized by relatively mild conditions, and the toxin antigens were analyzed by immunoblotting. In both cases, most of the toxin formed a large, antigenic aggregate of ca. 200 kDa. These toxin aggregates did not include the toxin receptor aminopeptidase N, but interactions with other vesicle components were not excluded. No oligomerization occurred when inactive toxins with mutations in amphipathic helices (α5) and known to insert into the membrane were tested. Active toxins with other mutations in this helix did form oligomers. There was one exception; a very active helix α5 mutant toxin bound very well to membranes, but no oligomers were detected. Toxins with mutations in the loop connecting helices α2 and α3, which affected the irreversible binding to vesicles, also did not oligomerize. There was a greater extent of oligomerization of the Cry1Ac toxin with vesicles from the Heliothis virescens midgut than with those from the M. sexta midgut, which correlated with observed differences in toxicity. Tight binding of virtually the entire toxin molecule to the membrane and the subsequent oligomerization are both important steps in toxicity.
Bacillus thuringiensis, primarily during sporulation, produces large intracellular inclusions which are comprised of a mixture of protoxins (δ-endotoxins) (1, 12, 29). Following ingestion by insect larvae, the inclusions are solubilized, and the protoxins are converted to toxins of about 60 kDa. Initially, the toxins bind reversibly to receptors on the surfaces of larval midgut cells. At least two such receptors have been identified (15, 28, 31), and there may even be more than one toxin binding site on one of these, aminopeptidase N (25). There is some correlation between the specificity of this binding and toxicity (32, 33), but there are exceptions (14, 24, 35).
Subsequently, the toxin inserts into the membrane in an irreversible step which, at least in some cases, appears to be more specific (14, 20). The factors involved in this toxin insertion step are not known. A receptor complex purified from Manduca sexta brush border membrane vesicles (BBMV) (including the aminopeptidase N receptor) formed functional ion channels (28, 30), as did a receptor complex from Heliothis virescens (21). These ion channels are presumably formed in the membrane by an oligomerization of toxin monomers (9, 16), but the nature of this process (i.e., whether it occurs at the membrane surface or within the membrane, the number of toxin monomers involved, and whether there are any interactions with membrane components) is not known.
There is a report that the cytolytic protein (CytA) produced by Bacillus thuringiensis subsp. israelensis oligomerizes in membrane vesicles (5). This protein is structurally and functionally very different from the δ-endotoxins (11, 18, 19). δ-Endotoxins are processed from the amino ends of the protoxins and are comprised of three structural domains (11, 18). Domain I consists of seven amphipathic α-helices and is believed to be the portion of the toxin which inserts into the membrane to form the ion channel. Evidence for this contention is based on the high frequency of losses of toxicity due to mutations in certain helices, especially the very hydrophobic α4-loop-α5 region (17, 29, 37). There are also studies of the binding of synthetic peptides to these helices which show that only helices α4 and α5 insert into the membrane, while the other helices appear to be localized at the membrane surface (7–10). The kinetics of binding of peptide helix α5 indicates cooperative interactions, suggesting oligomerization (7–10). A mutation in the α5 synthetic peptide, which is known to reduce toxicity, also resulted in a decrease of binding to and insertion into the membrane (7, 8). In addition, the α5 peptide formed ion channels in phospholipid vesicles (8). Domains II and III of the δ-endotoxins are comprised of β sheets, and selected regions, especially certain loops, are important in toxin binding and specificity (29).
While these studies are very helpful for defining the functional regions of these toxins, little is known about the steps between the initial, reversible binding to the receptor and the ultimate formation of an ion channel. We have exploited the availability of mutant toxins with known defects in reversible or irreversible binding and in toxicity to help define some of the steps which follow toxin binding. We have found that, after binding, about 90% of the toxin molecule is protected from protease, and much of the toxin is present as a large aggregate, due to oligomerization and/or interaction with membrane components. The relevance of these processes to the mode of action of the toxin is discussed.
MATERIALS AND METHODS
Toxin purification and vesicle preparation.
Clones of the Bacillus thuringiensis subsp. kurstaki HD1 cry1Ab and cry1Ac genes and mutant cry1Ac genes were electroporated into the acrystalliferous strain B. thuringiensis CryB (2, 37). The Cry1Ac protoxin was also purified from B. thuringiensis subsp. kurstaki HD73. Cells were grown and sporulated at 30°C on G-Tris agar plates containing 25 μg of erythromycin ml−1 (2). The spores plus inclusions were harvested in 1 M KCl–0.005 M EDTA, pH 8.0, and washed three times with deionized water. The inclusions were purified on Renografin gradients (2), and the protoxins were solubilized in 0.03 M Na2CO3–1% β-mercaptoethanol, pH 9.6, by three extractions of 20 min each at 37°C. The protoxins were then dialyzed at 4°C against a 2,000× volume (relative to the extract) of 0.01 M Tris, pH 8.5, and digested with tolylsulfonyl phenylalanyl chloromethyl ketone-treated trypsin (1:50) (Sigma) at 37°C for 90 min, followed by a second addition of trypsin and a further 90-min incubation. The digests were dialyzed at 4°C in 50-cut dialysis tubing (Spectropore) against two 1,000-volume changes of 0.01 M NaHCO3–0.25 M NaCl, pH 9.5. Final purification was done with a Mono Q cartridge (Pharmacia) eluting with a NaCl step gradient from 0.2 to 0.4 M in 0.02 M Tris, pH 8.7. The toxins were eluted at 0.3 to 0.4 M NaCl and, following dialysis against 0.03 M NaHCO3–0.25 M NaCl, pH 9.5, were stored at −70°C (23). All of the toxins used were present as monomers in solution except for wild-type Cry1Ac. This purified toxin contained variable amounts of what appeared to be dimers and trimers (see Fig. 3 to 5).
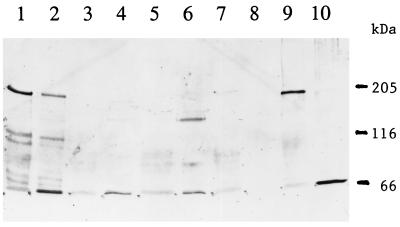
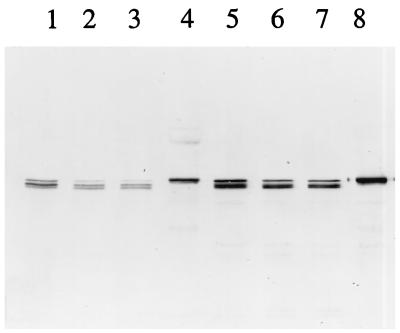
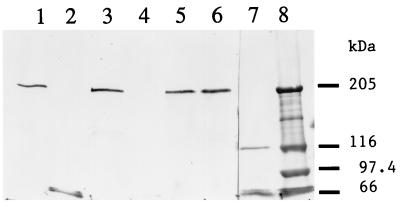
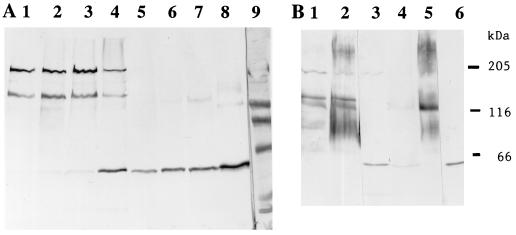
FIG. 3.
Immunoblot of toxin antigens extracted from M. sexta BBMV resolved by SDS–6% PAGE. Lane 1, extraction of the Cry1Ac antigen from BBMV; lane 2, 50 ng of Cry1Ac toxin; lane 3, extraction of the Cry1Ac A164P toxin from BBMV; lane 4, 30 ng of Cry1Ac A164P toxin; lane 5, extraction of the Cry1Ac L167F toxin from BBMV; lane 6, 50 ng of Cry1Ac L167F toxin; lane 7, extraction of the Cry1Ac A92D toxin from BBMV; lane 8, extraction of the Cry1Ac R93F toxin from BBMV; lane 9, extraction of the Cry1Ab toxin from BBMV; lane 10, 50 ng of Cry1Ab toxin.
FIG. 5.
Immunoblot of toxin antigens and aminopeptidase N extracted from M. sexta BBMV and resolved by SDS–6% PAGE. Lane 1, incubation of 50 ng of Cry1Ac toxin with BBMV; lane 2, 50 ng of Cry1Ac toxin; lane 3, incubation of the Cry1Ac W210C toxin with BBMV; lane 4, 50 ng of Cry1Ac W210C toxin; lane 5, 50 ng of Cry1C toxin; lane 6, same as lane 1 but treated with aminopeptidase N antibody; lane 7, M. sexta BBMV extract treated with aminopeptidase N antibody; lane 8 (from top to bottom), thyroglobulin, myosin, β-galactosidase, and bovine serum albumin standards.
Mutant Cry1Ac toxins A164D, Q163P, L167F, A164P, H168R, and S170C all had mutations in helix α5 and have been described (37) (Table 1). Among these, the S170C toxin retained full activity and the H168R toxin was slightly more active, whereas all of the others were at least 100 times less active than the wild type in three test insects (37). The mutation W210C is in helix α6, and the resulting mutant toxin is fully toxic (2). All of these mutant toxins bound to an M. sexta larval midgut vesicle protein in immunoblots or to BBMV (2, 37), so the initial receptor binding was not markedly altered. Mutant toxins A92D and R93F have substitutions in the loop connecting helices α2 and α3 and are nontoxic (37). They both bind to the receptor in immunoblots, but the A92D toxin did not insert into membrane vesicles (R93F was not tested) (4, 13).
TABLE 1.
Summary of the properties of the toxins and their aggregation within M. sexta BBMV
| Toxina | Site of mutation | Toxicityb | Size of aggregate extracted from BBMV | Reference |
|---|---|---|---|---|
| Cry 1Ab | NAc | + | 200 kDa (Fig. 2 and 3) | |
| Cry 1Ac | NAc | + | 200 kDa (Fig. 3 to 5) | |
| Cry1Ac S170C | α5 | + | 200 kDa (Fig. 2) | 17 |
| Cry1Ac A164D | α5 | − | Not present (Fig. 2) | 37 |
| Cry1Ac W210C | α6 | + | 200 kDa (Fig. 2) | 2 |
| Cry1Ac A164P | α5 | − | Not present (Fig. 3) | 37 |
| Cry1Ac L167F | α5 | − | Not present (Fig. 3) | 37 |
| Cry1Ac A92D | α2-α3 loop | − | Not present (Fig. 3) | 37 |
| Cry1Ac R93F | α2-α3 loop | − | Not present (Fig. 3) | 37 |
| Cry1Ac H168R | α5 | +d | 65-kDa monomer (Fig. 4) | 37 |
−, no detectable toxicity for M. sexta larvae; +, toxic to M. sexta as wild type (2, 37). Many were also tested on Trichoplusia ni and H. virescens larvae with the same results (37).
NA, not applicable.
The 50% lethal dose of this mutant toxin for the three test larvae was consistently two- to threefold lower than the 50% lethal dose of wild-type Cry1Ac toxin (37).
BBMV were prepared from the midguts of fifth-instar larvae of M. sexta and H. virescens, essentially as described previously (2, 27). The protein contents of the vesicles were determined with the bicinchoninic acid reagent (Pierce Chemical Co.), and suspensions were aliquoted and stored at −70°C. M. sexta eggs were obtained from either Purdue University or Carolina Biological Supplies. H. virescens eggs were supplied by the U.S. Department of Agriculture, Stoneville, Miss.
Vesicle binding and protease K digestion.
Fifty to one hundred nanograms of purified toxin was incubated with vesicles (10 to 20 μg of protein) in 50 μl of 0.1 M NaHCO3–0.1 M NaCl, pH 9.5, at 27°C for 30 min. The suspensions were pelleted in a microcentrifuge (8 min) and washed twice with 0.5 ml (in each wash) of the incubation buffer, followed by a wash with either 1 ml of 0.01 M Tris, pH 7.8, or 1 ml of 0.03 M NaHCO3–0.25 M NaCl, pH 9.5 (identical results). The pellets were suspended either in 20 μl of 0.05 M Tris–0.5% sodium dodecyl sulfate (SDS), pH 8.5, with incubation at 65°C for 5 to 15 min, or in 0.05 M Tris–1% octyl-β-d-glucopyranoside (Calbiochem), pH 8.5. The former protocol was based on one used in studies of Staphylococcus α-hemolysin oligomerization (34), where the oligomers were stable at 65°C in SDS but dissociated at 70°C. The δ-endotoxins retained their activity at 65°C but not at 70°C in 0.5% SDS, so the former temperature was selected for extraction. A mutant toxin, A92D, which did not bind irreversibly to vesicles (4, 37) was used to control adventitious toxin binding.
After the 65°C incubation or octylglucoside extraction, the suspensions underwent SDS–6% polyacrylamide gel electrophoresis (PAGE). Following electrophoresis and transfer to polyvinylidene difluoride (PVDF) membranes, the membranes were treated with various antibodies (2, 27): Cry1Ac rabbit polyclonal, Cry1Ab monoclonal, or anti-gypsy moth aminopeptidase N antibody, kindly provided by D. Dean.
For protease digestion, BBMV plus toxin was incubated as described above, and the washed vesicles were suspended in 0.01 M MOPS [3(N-morpholino)propanesulfonic acid]–5 mM CaCl2, pH 7.0. Protease K (Sigma) at 0.2 to 0.5 μg ml−1 was added, and the suspensions were incubated at 37°C for 45 min. Controls were 50 to 100 ng of toxin plus protease K with no vesicles and the vesicle-toxin complex incubated without protease K. These samples were boiled in loading buffer and fractionated by SDS–8% PAGE. Following transfer to PVDF (26), the two closely migrating bands of ca. 65 and 60 kDa in the vesicle-protease K lane were cut out, and 18 to 20 residues of each were sequenced. Gels were also stained with Coomassie blue, and photographs of the gels were scanned in a General Dynamics ImageQuant.
RESULTS
Toxin bound to BBMV is largely protected from protease K.
As shown in Fig. 1, >95% of the Cry1Ac toxin bound to M. sexta BBMV was protected from complete digestion by 0.1 to 0.5 μg of protease K and was present in two bands, one of which was the same size as the intact toxin and the other slightly smaller. In contrast, >70% of the toxin in solution was completely digested by 0.2 to 0.5 μg of protease K. The lack of digestion of the toxin bound to BBMV was not due to the inactivation of protease K, since after the incubation, the supernatant was capable of digesting toxin (17).
FIG. 1.
Digestion of the Cry1Ac toxin either in solution (lanes 1 to 4) or bound to M. sexta BBMV (lanes 5 to 8) with various concentrations of protease K at 37°C for 45 min. Following incubation, the samples were boiled in loading buffer for 3 min. Fractionation was achieved by SDS–10% PAGE, and staining was done with Coomassie blue. Lanes 1 and 5, treatment with 0.1 μg; lanes 2 and 6, treatment with 0.2 μg; lanes 3 and 7, treatment with 0.5 μg; lanes 4 and 8, no treatment. The stained bands were quantitated in a General Dynamics ImageQuant with the following percentages of control values: lane 1, 80%; lane 2, 28%; lane 3, 25%; and lanes 5 to 7, 95 to 100%.
The two bands protected from complete protease K digestion by BBMV were excised and sequenced. The lower band gave the sequence LVDIIWGIFG-S-DAFLV (where the dashes indicate the lack of definitive identification of a residue), indicating cleavage at about residue 59, which is just after helix α1 and perhaps into helix α2A (11). The upper band sequence was TGYTPIDSLSLTQFLL, which starts at about residue 29, as was expected for the intact toxin. Cleavage by protease K had reduced the mass of the toxin by about 3,100 Da, and there was no evidence of further degradation with longer incubation of the vesicle-toxin complex. Similar results were obtained with the Cry1Ab toxin (17). Pronase (type VI protease) (Sigma) was also tested, but it was more difficult to find conditions under which the addition of this protease resulted in nearly complete digestion of the toxin in solution without also digesting the BBMV-bound toxin (and probably membrane proteins as well).
Higher-molecular-weight forms of toxin extracted from BBMV.
Following incubation of the Cry1Ab or Cry1Ac toxins with M. sexta BBMV, the toxins were solubilized by incubation at 65°C, as described in Materials and Methods, and were resolved by SDS–6% PAGE. In both cases, the major toxin antigen extracted from the vesicles had a molecular mass of 190 to 200 kDa with the virtual absence of toxin monomers (Fig. 2 and 3). In solution, the Cry1Ac toxin contained some oligomers of the same sizes as those extracted from BBMV (Fig. 3, lane 2, and Fig. 4 and 5), suggesting that vesicle binding enhanced oligomerization. There were no such aggregates present in the Cry1Ab toxin preparations (Fig. 2, lane 2) or among all of the Cry1Ac mutant toxins. Yet the active mutants (S170C and W210C) could also be extracted from BBMV as ca. 200-kDa aggregates (Fig. 2). The presence of some aggregated toxin in the initial preparation was not essential, therefore, for this oligomerization.
FIG. 2.
Immunoblot of toxin antigens extracted from M. sexta BBMV (see Materials and Methods). Lane 1, extraction of the Cry1Ab antigen from BBMV; lane 2, 50 ng of Cry1Ab; lanes 3 and 6, extraction of the Cry1Ac S170C toxin from BBMV; lane 4, extraction of the Cry1Ac A164D toxin from BBMV; lane 5, extraction of the Cry1Ac W210C toxin from BBMV; lane 7, standards of β-galactosidase and bovine serum albumin; lane 8 (from top to bottom), myosin, β-galactosidase, phosphorylase b, and bovine serum albumin standards.
FIG. 4.
Immunoblot of Cry1Ac and H168R, following incubation with M. sexta BBMV. (A) Lanes 1 to 3, 20 μg of BBMV incubated with 20, 40, and 60 ng of Cry1Ac toxin, respectively; lane 4, 60 ng of Cry1Ac toxin; lanes 5 to 7, 20 μg of BBMV incubated with 20, 40, and 60 ng of H168R toxin, respectively; lane 8, 60 ng of H168R toxin; lane 9, standards as in Fig. 2 plus ovalbumin (45 kDa) and carbonic anhydrase (29 kDa). (B) Toxin samples (20 ng) were incubated with 20 μg of BBMV and then extracted for different times at 65°C. Lane 1, Cry1Ac toxin extracted at 65°C for 15 min; lane 2, Cry1Ac toxin extracted at 65°C for 5 min; lane 3, 20 ng of Cry1Ac toxin; lane 4, H168R toxin extracted at 65°C for 15 min; lane 5, H168R toxin extracted at 65°C for 5 min; lane 6, 20 ng of H168R toxin. Markers on the right indicate (from top to bottom) positions of myosin, β-galactosidase, and bovine serum albumin.
In contrast, mutant Cry1Ac toxins with low or no activity (in three insect species), such as A164D, A164P, L167F, A92D, and R93F, did not remain associated with vesicles in any form (Fig. 2 and 3). The first three of these toxins have mutations in helix α5, and the latter two have mutations in a loop connecting helices α2 and α3 (Table 1). The A92D toxin, which has no detectable toxicity, can bind reversibly to BBMV but cannot insert into the membrane (4, 13).
The one exception to a total correlation between activity and the formation of a ca. 200-kDa oligomer was the Cry1Ac mutant toxin H168R (Fig. 4). This toxin is at least as active as the wild type (37), and yet no oligomers were found. Instead, a relatively large amount of the monomer could be extracted from BBMV after washing. Trimers may have formed, but they were unstable under the extraction conditions used. There was no ca. 200-kDa band even when heating at 65°C was reduced to 5 min (Fig. 4B). Instead, there was a band of >200 kDa, which was also present with the wild-type Cry1Ac toxin and when other active toxins were incubated with BBMV (17). The possible significance of this larger aggregate is discussed below.
Toxin was not bound to aminopeptidase N in BBMV.
Extracts of BBMV incubated with either the wild-type Cry1Ac toxin or the fully active mutant Cry1Ac toxin W210C were electrophoresed, blotted to PVDF, and treated either with anti-Cry1Ac antibody or with antibody to aminopeptidase N (Fig. 5). Aggregates of ca. 200 kDa were present for the two toxins. The aminopeptidase N antibody reacted with a major band of ca. 120 kDa, the expected size of this enzyme, as well as with a minor band of >200 kDa and some bands of <120 kDa, but the antibody reacted to no band of the same size as the toxin aggregate. The profile was the same for BBMV incubated with or without toxin, with no detectable shifts in the size of aminopeptidase N after incubation with toxin.
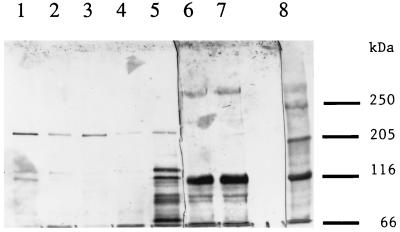
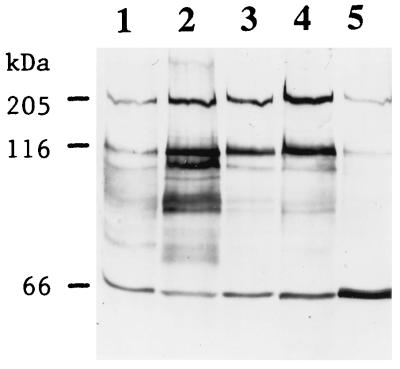
Binding of the Cry1Ac toxin to BBMV from different insects.
BBMV were prepared from late-instar larvae of M. sexta and H. virescens and incubated with the Cry1Ac toxin (Fig. 6). In all cases, there were bands of ca. 130 and 200 kDa as well as monomers. At both concentrations of toxin, the 200-kDa band extracted from H. virescens BBMV was about twice as intense as the band from M. sexta BBMV. The total amount of Cry1Ac antigen recovered from the BBMV at higher toxin concentrations (Fig. 6, lanes 2 and 4) is somewhat greater (50 and 20%, respectively) than the amount of toxin added (lane 5). The Cry1Ac toxin is about three to four times more active in H. virescens than in M. sexta (12).
FIG. 6.
Immunoblot of the Cry1Ac toxin antigen extracted from BBMV prepared from M. sexta and H. virescens. Lane 1, 10 μg of M. sexta BBMV plus 50 ng of Cry1Ac toxin; lane 2, 20 μg of M. sexta BBMV plus 50 ng of Cry1Ac toxin; lane 3, 10 μg of H. virescens BBMV plus 50 ng of Cry1Ac toxin; lane 4, 20 μg of H. virescens BBMV plus 50 ng of Cry1Ac toxin; lane 5, 50 ng of Cry1Ac toxin.
DISCUSSION
Following incubation with BBMV, most of the Cry1Ac toxin molecule was protected from digestion by protease K. In an earlier study, it was found that only intact Cry1B toxin was extracted from Pieris brassicae BBMV following a prolonged incubation (36). In this study, the extracted toxin was intact, and only the ca. 30 N-terminal amino acids, which primarily comprise helix α1 (11), were susceptible to protease K. Interestingly, the synthetic α1 peptide was the only one of the seven amphipathic α-helical peptides which did not bind to synthetic vesicles (10). The rest of the toxin was protected, perhaps with a surface location for all parts except α4-loop-α5, which is very hydrophobic (11). Mutations in these particular helices, but not in the loop, resulted in loss of toxicity (17, 29, 37), whereas mutations in presumed surface helices α2, α3, and α6 had no effect (2, 37). The α4 and α5 synthetic peptides were the only ones to insert into phospholipid vesicles, and the kinetics of insertion for peptide α5 indicated aggregation within the membrane (8–10). There was no evidence of cleavage within domain I following binding to BBMV, as has been reported for the CryIIIA toxin (3).
Under the particular conditions used (i.e., either mild heating or solubilization with octylglucoside [17]), toxin bound to the membrane was extracted primarily as an aggregate or oligomer of about 200 kDa. In some cases, a band of ca. 130 kDa was also present (Fig. 4 and 6), but this was variable. No toxin aggregates were found after incubation of the Cry1Ac toxin with BBMV pretreated with octylglucoside or with the lipid fraction extracted from BBMV (6) or by addition of deoxycholate at concentrations which formed micelles (17). Oligomerization appears to require more than just a hydrophobic environment and very likely requires an intact membrane.
Based on size, the toxin is present either as a trimer or as toxin monomers (or dimers) bound to some component of BBMV. The latter is unlikely to be aminopeptidase N, which can function as a receptor for initial, reversible toxin binding (15, 28) and perhaps for toxin insertion (28, 30). There is evidence for a BBMV component affecting the properties of the ion channel (22, 30), so such an interaction is possible. Since the size of the ca. 200-kDa aggregate is identical to that of a small fraction of the Cry1Ac toxin found in solution (Fig. 3 to 5), it is likely that the aggregates are toxin trimers. These soluble aggregates may reflect the oligomerization required to form channels, or they may be stable intermediates, either in the formation of the pore or in its degradation during extraction.
There was a correlation between the extent of formation of aggregates by the Cry1Ac toxin and its effectiveness in two insect species (Fig. 6). There was also a good correlation between the presence of these toxin aggregates and the activity of the Cry1Ac mutant toxins. Two such mutants with full activity formed aggregates, whereas five others with low or no toxicity due to mutations either in helix α5 or in the loop between helices α2 and α3 failed to do so. The one exception was the very active Cry1Ac toxin mutant H168R, which bound well to the membrane, but no band of ca. 200 kDa was found (Fig. 4). The tighter binding of the monomer to BBMV must be due to the H168R substitution in the hydrophobic face of helix α5 (37), which also appears to alter the stability of oligomers, at least the relatively stable molecule of ca. 200 kDa. If this oligomer is important to toxin function, it must form transiently in this mutant. Alternatively, the aggregate of >200 kDa seen with both the wild-type and H168R mutant toxins (Fig. 4B) could be more indicative of toxin function. If so, the ca. 200-kDa band may be a stable intermediate or a degradation by-product.
Recently, it has been found that toxins inactive due to mutations in helix α4 form aggregates of 200 kDa following incubation with BBMV (17). Overall, the results are consistent with helix α5 being critical for aggregation (perhaps among other functions), whereas helix α4 would be essential for the formation of a functional channel.
It is not known whether toxin aggregation occurs at the surface of the membrane following binding to the receptor or only after portions of the toxin insert into the membrane. All of the nontoxic Cry1Ac mutants bind to the receptor (2, 37), but those lacking a positive charge in the loop between helices α2 and α3 do not insert irreversibly (4, 13), and those with mutations in helix α5 do not aggregate. The loop between helices α2 and α3 may be part of the face of the toxin which interacts with the phospholipids in the membrane (13), whereas helix α5 appears to be pivotal for aggregation. In solution, this helix is surrounded by the other helices of domain I (11) and thus is probably not accessible for interaction until it inserts into the membrane as part of the hydrophobic helix α4-loop-α5 segment of the toxin.
ACKNOWLEDGMENTS
This research was supported by a grant from the Binational Agricultural Research and Development Fund (Bard project IS-2629-95).
The Purdue Laboratory for Macromolecular Structure did the protein sequencing. M. sexta larvae were kindly provided by the Department of Entomology at Purdue. Michael Wolfersberger kindly provided critical comments.
REFERENCES
- 1.Aronson A I. Flexibility in the protoxin composition of Bacillus thuringiensis. FEMS Microbiol Lett. 1994;117:21–28. doi: 10.1111/j.1574-6968.1994.tb06737.x. [DOI] [PubMed] [Google Scholar]
- 2.Aronson A I, Wu D, Zhang C. Mutagenesis of specificity and toxicity regions of a Bacillus thuringiensis protoxin gene. J Bacteriol. 1995;177:4059–4065. doi: 10.1128/jb.177.14.4059-4065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll J, Convents D, Van Damme J, Boets A, Van Rie J, Ellar D J. Intramolecular proteolytic cleavage of Bacillus thuringiensis Cry3A δ-endotoxin may facilitate its coleopteran toxicity. J Invertebr Pathol. 1997;70:41–49. doi: 10.1006/jipa.1997.4656. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Curtiss A, Alcantra E, Dean D. Mutations in domain 1 of the Bacillus thuringiensis δ-endotoxin CryIAb reduce the irreversible binding of toxin to Manduca sexta brush border membrane vesicles. J Biol Chem. 1995;270:6412–6419. doi: 10.1074/jbc.270.11.6412. [DOI] [PubMed] [Google Scholar]
- 5.Chow E, Singh G J P, Gill S S. Binding and aggregation of the 25-kilodalton toxin of Bacillus thuringiensis subsp. israelensis to cell membranes and alteration by monoclonal antibodies and amino acid modifiers. Appl Environ Microbiol. 1989;55:2779–2788. doi: 10.1128/aem.55.11.2779-2788.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garczynski, S. Personal communication.
- 7.Gazit E, Shai Y. Structural and functional characterization of the alpha5 segment of Bacillus thuringiensis endotoxin. Biochemistry. 1993;32:3429–3436. doi: 10.1021/bi00064a029. [DOI] [PubMed] [Google Scholar]
- 8.Gazit E, Bach D, Kerr I D, Sansom M S P, Chejanovsky N, Shai Y. The alpha 5 segment of Bacillus thuringiensis endotoxins: in vitro activity, ion channel formation and molecular modeling. Biochem J. 1994;304:895–902. doi: 10.1042/bj3040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazit E, Shai Y. The assembly and organization of the α5 and α7 helices from the pore forming domain of Bacillus thuringiensis δ-endotoxins: relevance to a functional model. J Biol Chem. 1995;270:2571–2578. doi: 10.1074/jbc.270.6.2571. [DOI] [PubMed] [Google Scholar]
- 10.Gazit E, La Rocca P, Sansom M S P, Shai Y. The structure and organization within the membrane of the helices composing the pore-forming domain of Bacillus thuringiensis δ-endotoxins are consistent with an “umbrella-like” structure of the pore. Proc Natl Acad Sci USA. 1998;95:12289–12294. doi: 10.1073/pnas.95.21.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz J-L, Brousseau R, Cygler M. Bacillus thuringiensis Cry1A(a) insecticidal toxin: crystal structure and channel formation. J Mol Biol. 1995;254:447–464. doi: 10.1006/jmbi.1995.0630. [DOI] [PubMed] [Google Scholar]
- 12.Höfte H, Whiteley H R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain S-R A, Aronson A I, Dean D H. Substitution of residues on the proximal side of Cry1A Bacillus thuringiensis δ-endotoxins affects irreversible binding to Manduca sexta midgut membrane. Biochem Biophys Res Commun. 1996;226:8–14. doi: 10.1006/bbrc.1996.1303. [DOI] [PubMed] [Google Scholar]
- 14.Ihara H, Kuroda E, Wadano A, Himino M. Specific toxicity of endotoxins from Bacillus thuringiensis to Bombyx mori. Biosci Biotechnol Biochem. 1993;57:200–204. doi: 10.1271/bbb.57.200. [DOI] [PubMed] [Google Scholar]
- 15.Knight P J K, Crickmore N, Ellar D J. The receptor for Bacillus thuringiensis CryIAc delta-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol Microbiol. 1994;11:429–436. doi: 10.1111/j.1365-2958.1994.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles B H, Dow J A T. The crystal δ-endotoxins of Bacillus thuringiensis: models for their mechanism of action on the insect gut. Bioessays. 1993;15:469–476. [Google Scholar]
- 17.Kumar, M., and A. I. Aronson. Unpublished results.
- 18.Li J, Carroll J, Ellar D J. Crystal structure of an insecticidal protein. The δ-endotoxin from Bacillus thuringiensis subsp. tenebrionis at 2.5 Å resolution. Nature. 1991;353:815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Koni P A, Ellar D J. Structure of the mosquitocidal δ-endotoxin CytB from Bacillus thuringiensis sp. kyushuensis and implications for membrane pore formation. J Mol Biol. 1996;257:129–152. doi: 10.1006/jmbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Patel S, Dean D H. Irreversible binding kinetics of Bacillus thuringiensis δ-endotoxin to gypsy moth brush border membrane vesicles is directly correlated to toxicity. J Biol Chem. 1995;270:24719–24724. doi: 10.1074/jbc.270.42.24719. [DOI] [PubMed] [Google Scholar]
- 21.Luo K, Sangadala S, Masson L, Mazza A, Brousseau R, Adang M J. The Heliothis virescens 170kDa aminopeptidase functions as “receptor A” by mediating specific Bacillus thuringiensis Cry1A delta-endotoxin binding and pore formation. Insect Biochem Mol Biol. 1997;27:735–743. doi: 10.1016/s0965-1748(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 22.Martin F G, Wolfersberger M G. Bacillus thuringiensis δ-endotoxin and Manduca sexta brush-border membrane vesicles act synergistically to cause very large increases in the conductance of planar lipid bilayers. J Exp Biol. 1995;198:91–96. doi: 10.1242/jeb.198.1.91. [DOI] [PubMed] [Google Scholar]
- 23.Masson, L. Personal communication.
- 24.Masson L, Mazza A, Brousseau R, Tabashnik B. Kinetics of Bacillus thuringiensis toxin binding with brush border membrane vesicles from susceptible and resistant larvae of Plutella xylostella. J Biol Chem. 1995;270:11887–11896. doi: 10.1074/jbc.270.20.11887. [DOI] [PubMed] [Google Scholar]
- 25.Masson L, Lu Y-J, Mazza A, Brousseau R, Adang M J. The Cry1A(c) receptor purified from Manduca sexta displays multiple specificities. J Biol Chem. 1995;270:20309–20315. doi: 10.1074/jbc.270.35.20309. [DOI] [PubMed] [Google Scholar]
- 26.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 27.Mohammed S I, Johnson D E, Aronson A I. Altered binding of the Cry1Ac toxin to larval membranes but not to the toxin-binding protein in Plodia interpunctella selected for resistance to different Bacillus thuringiensis isolates. Appl Environ Microbiol. 1996;62:4168–4173. doi: 10.1128/aem.62.11.4168-4173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sangadala S, Walters F S, English L H, Adang M J. A mixture of Manduca sexta aminopeptidase and phosphatase enhances Bacillus thuringiensis insecticidal CryIAc toxin binding and 86Rb+-K+ efflux in vitro. J Biol Chem. 1994;269:10088–10092. [PubMed] [Google Scholar]
- 29.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz J-L, Lu Y-J, Sohnlein P, Brousseau R, Laprade R, Masson L, Adang M J. Ion channels formed in planar lipid bilayers by Bacillus thuringiensis toxins in the presence of Manduca sexta midgut receptors. FEBS Lett. 1997;412:270–276. doi: 10.1016/s0014-5793(97)00801-6. [DOI] [PubMed] [Google Scholar]
- 31.Vadlamudi R K, Ji R H, Bulla L A. A specific binding protein from Manduca sexta for the insecticidal toxin of Bacillus thuringiensis subsp. berliner. J Biol Chem. 1993;268:12334–12340. [PubMed] [Google Scholar]
- 32.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Specificity of Bacillus thuringiensis δ-endotoxins. Importance of specific receptors on the brush border membrane of the midgut of target insects. Eur J Biochem. 1989;186:239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- 33.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxins. Appl Environ Microbiol. 1990;56:1378–1385. doi: 10.1128/aem.56.5.1378-1385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker B, Bayley H. Key residues for membrane binding, oligomerization, and pore forming activity of staphylococcal α-hemolysin identified by cysteine scanning mutagenesis and targeted chemical modification. J Biol Chem. 1995;270:23065–23071. doi: 10.1074/jbc.270.39.23065. [DOI] [PubMed] [Google Scholar]
- 35.Wolfersberger M. The toxicity of two Bacillus thuringiensis δ-endotoxins to gypsy moth larvae is inversely related to the affinity of the binding sites on midgut brush border membranes for the toxins. Experientia. 1990;46:475–477. doi: 10.1007/BF01954236. [DOI] [PubMed] [Google Scholar]
- 36.Wolfersberger, M. G. Personal communication.
- 37.Wu D, Aronson A I. Localized mutagenesis defines regions of the Bacillus thuringiensis δ-endotoxin involved in toxicity and specificity. J Biol Chem. 1992;267:2311–2317. [PubMed] [Google Scholar]