Key Points
The coronavirus disease 2019 pandemic has had an unprecedented effect on health and health care and posed challenges to the conduct of clinical trials.
Targeted mitigating strategies, on the basis of early and continued data collection from site surveys, limited disruption to the ASCEND trials.
Flexibly allowing hemoglobin assessment at local laboratories to inform randomized treatment dosing was key to limiting the discontinuation of treatment.
Keywords: chronic kidney disease, clinical trial, COVID-19, dialysis, pandemics, SARS-CoV-2
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had an unprecedented effect on health and health care. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has strained health care systems and caused disruption to non-COVID-19-related health care. Although the premature interruption or disruption of clinical trials is less well recognized, it has the potential to threaten the development of new non-COVID-19-related therapies (1–3).
Study disruptions need to be rapidly identified and addressed to preserve the integrity of ongoing studies and the design of new ones. Here, we describe the challenges that arose in three global phase 3 trials and the strategies undertaken to maintain and adapt the conduct of the studies safely during the COVID-19 pandemic.
Materials and Methods
The Anemia Studies in Chronic Kidney Disease: Erythropoiesis via a Novel Prolyl Hydroxylase Inhibitor Daprodustat (ASCEND) program encompassed five phase 3 trials, of which three were sponsored by GlaxoSmithKline (GSK) and conducted by Pharmaceutical Product Development (PPD). Study details for the nondialysis (ASCEND-ND; NCT028768355), dialysis (ASCEND-D; NCT02879305), and incident dialysis (ASCEND-ID; NCT03029208) trials are provided in the Supplemental Material.
Survey of Study Sites about Operations during the SARS-CoV-2 Pandemic
The sponsor developed and administered a questionnaire in collaboration with PPD to assess the effect of the COVID-19 pandemic on ASCEND research activities at clinical sites (the complete survey is provided in the Supplemental Material). Questions covered whether sites were open or temporarily closed and whether study visits and monitoring activities could be completed as usual or with adaptations such as remote visits and/or use of local laboratories. Furthermore, dialysis-specific questions were used to determine if participants were treated in their usual or different dialysis facilities, and the challenges of the latter in conducting the trials.
Survey Distribution and Analysis
Surveys were disseminated to active study sites beginning April 13, 2020. Survey data were updated every 2 weeks, then monthly, and thereafter on a targeted basis, depending on COVID-19 rates in the region. For sites with more than one participant in a study, multiple responses could be provided to describe study activities for participants. For these reasons, denominators for survey questions vary slightly. Survey results were not part of the trial clinical database, and data were not reconciled to the electronic case report forms.
Other Study Adaptations
Beginning February 24, 2020, processes were implemented to ensure continuity of randomized treatment. Where HemoCue hemoglobin (Hb) could not be assessed, study sites were able to transmit locally obtained Hb values to PPD through a query platform. When local Hb values could not be obtained, participants were temporarily placed on standard of care or received no anemia treatment.
Results
Key survey questions and results are presented in Tables 1 and 2.
Table 1.
Results of the initial ASCEND site survey (April 13–May 6, 2020): Effect on conducting study visits by study site staff who responded to initial survey a
| Survey Responses | Overall | ASCEND-ND | ASCEND-ID | ASCEND-D |
|---|---|---|---|---|
| Are site staff able to complete patient visits as per protocol? | N=1113 | N=566 | N=108 | N=439 |
| Yes | 75% | 71% | 72% | 81% |
| Full study visits | 64% | 59% | 63% | 70% |
| Limited to a subset of procedures | 7% | 7% | 8% | 6% |
| Combination of full/limited study visits | 4% | 4% | 0.9% | 4% |
| No response given | 0.6% | 0.5% | 0% | 0.9% |
| No | 21% | 25% | 21% | 16% |
| Only by telephone or telehealth | 16% | 19% | 11% | 13% |
| Only at participants’ homes | 0.4% | 0.5% | 0% | 0% |
| No remote visits completed | 3% | 4% | 10% | 1% |
| Combinations of above | 1% | 1% | 0 | 0.7% |
| No response given | 0.6% | 0.9% | 0% | 0.5% |
| No response given | 4% | 4% | 7% | 3% |
| Is hemoglobin being checked? | N=1053 | N=531 | N=105 | N=417 |
| Yes, at the study site/participants’ homes | 66% | 63% | 62% | 71% |
| Yes, at a local laboratory | 6% | 7% | 7% | 4% |
| Combinations of “yes” responsesb | 10% | 10% | 6% | 10% |
| No | 10% | 11% | 17% | 7% |
| Combinations of “yes” and “no” responsesb | 3% | 3% | 2% | 2% |
| No response given | 6% | 6% | 7% | 6% |
| Is the research office able to accept randomized treatment supply samples as normal? | N=1053 | N=531 | N=105 | N=417 |
| Yes | 89% | 89% | 87% | 90% |
| No | 6% | 7% | 7% | 4% |
| No response given | 6% | 5% | 7% | 7% |
| Is the site able to ship lab samples to central lab? | N=1053 | N=531 | N=105 | N=417 |
| Yes | 84% | 83% | 81% | 86% |
| No | 11% | 13% | 13% | 8% |
| No response given | 5% | 5% | 6% | 6% |
| If site cannot ship lab samples to central lab, can the site store frozen samples? | N=116 | N=68 | N=14 | N=34 |
| Yes | 63% | 68% | 36% | 65% |
| No | 29% | 25% | 50% | 29% |
| No response given | 8% | 7% | 14% | 6% |
Response tallies may be >100% due to rounding. N, the number of site staff contacted. ASCEND, Anemia Studies in Chronic Kidney Disease: Erythropoiesis via a Novel Prolyl Hydroxylase Inhibitor Daprodustat.
Only Research sites that had patients on dialysis were included.
For “Combination of ‘yes’ and ‘no’ responses,” sites had participants that aligned with each of these responses.
Table 2.
Results of the initial ASCEND site survey (April 13–May 6, 2020): Effect on dialysis participants by study site staff who responded to initial survey a
| Are study participants being dialyzed at their regular dialysis facilities? | N=718 |
| Yes | 94% |
| No | 2% |
| Combination of “yes” and “no” responsesb | 4% |
| For sites where study participants have changed sites, are the study staff still able to have oversight of participants’ dialysis? | N=41 |
| Yes | 51% |
| No | 12% |
| Yes, for some participants but not all | 22% |
| No response given | 15% |
| For sites where “oversight” is in question, are the staff at the new units aware that the patients are participating in ASCEND? | N=20 |
| Yes | 30% |
| No | 30% |
| Combination of “yes” and “no” responsesb | 10% |
| No response given | 30% |
| For sites with study participants at new dialysis units, are the staff having difficulty obtaining information about participants, including AE/SAE? | N=41 |
| Yes | 15% |
| No | 59% |
| Combination of “yes” and “no” responsesb | 20% |
| No response given | 7% |
| For sites with study participants at new dialysis units: Are participants able to continue randomized treatment? | N=41 |
| Yes | 59% |
| No | 20% |
| Combination of “yes” and “no” responsesb | 15% |
| No response given | 7% |
| For sites with study participants at new dialysis units where randomized treatment could not be continued, is there a possibility that they will resume randomized treatment when they return to regular dialysis units? | N=14 |
| Yes | 57% |
| No | 21% |
| No response given | 21% |
Response tallies may be >100% due to rounding. ASCEND, Anemia Studies in Chronic Kidney Disease: Erythropoiesis via a Novel Prolyl Hydroxylase Inhibitor Daprodustat; N, the number of site staff contacted. AE, adverse event; SAE, serious adverse event.
Only research sites that had patients on dialysis were included.
For “Combination of ‘yes’ and ‘no’ responses,” sites had participants that aligned with each of these responses.
Site Capabilities on the Basis of Initial Survey (April 13–May 6, 2020)
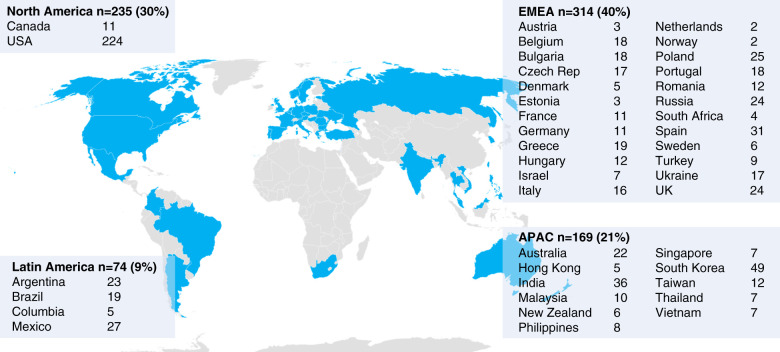
Across ASCEND-D, ASCEND-ID, and ASCEND-ND, 830 unique study sites in 41 countries received a survey between April 13 and May 6, 2020 (Figure 1). Responses to the initial survey were obtained from 792 sites (95% response rate) and reached 100% by July 13, 2020.
Figure 1.
Global distribution of study sites for the ASCEND-ND, ID, and D studies that participated in the initial survey. Asia Pacific (APAC): Australia, India, Hong Kong, Malaysia, New Zealand, Philippines, South Korea, Singapore, Taiwan, Thailand, Vietnam. Europe, Middle East, Africa (EMEA): Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, France, Germany, Greece, Hungary, Italy, Israel, Netherlands, Norway, Poland, Portugal, Romania, Russia, South Africa, Spain, Sweden, Turkey, Ukraine, United Kingdom. Latin America (LA): Argentina, Brazil, Columbia, Mexico. North America (NA): Canada, United States of America.
Because many sites participated in more than one ASCEND study, they contributed responses to each study; 75% reported that they were able to complete study visits per protocol, with 64% completing all procedures and 7% limited to a subset of procedures (Table 1). Twenty-one percent of study sites reported that study visits could not be completed in clinic. Sixteen percent were able to complete study visits by telephone or telehealth, and <1% were able to travel to participants’ homes. In 3% of sites, study visits could not be completed during temporary site closures. Hb testing was largely performed via HemoCue at the study site or at participants’ homes (66%) or at a local laboratory (6%) or some combination of these approaches (10%); 10% reported they were unable to monitor Hb at the time of the initial survey.
Ninety-four percent of sites reported that all patients receiving dialysis continued to dialyze in their usual facilities, 2% reported some patients dialyzing elsewhere, and 4% a combination of the two (Table 2). Overall, for the sites with participants at new dialysis units, 20% indicated that some patients could not continue randomized treatments.
Use of Local Hb Values between March 1 and May 6, 2020 and Overall
At the participant level, 4224 participants (across all three studies) were receiving randomized treatment and monitoring between March 1 and May 6, 2020. Although the majority received study treatment guided by Hb according to the protocol, approximately 3% received randomized treatment on the basis of Hb results obtained at local laboratories, with randomized treatment delivered to their homes; this rose to 5% when looking cumulatively from March 1 to August 24, 2020 (Table 3). Few participants (<2%) were temporarily converted to standard of care anemia treatment, and this outcome was more likely among nondialysis and incident dialysis participants (around 3% each) than for the D study (1%). A temporary switch to no anemia treatment was a rare occurrence (<1%).
Table 3.
Patient-level randomized treatment outcomes
| Expected Number of Dispensings of Randomized Treatment per Protocola | Continue Randomized Treatment, Dispensed on the Basis of Hb from Local Labb | |||
|---|---|---|---|---|
| Study | Initial | Cumulative | Initial, % | Cumulative, % |
| ASCEND-ND | 2861 | 7890 | 4 | 7 |
| ASCEND-ID | 111 | 162 | 2 | 3 |
| ASCEND-D | 2640 | 6785 | 1 | 2 |
| Total | 5612 | 14,837 | 3 | 5 |
Initial, March 1, 2020, through May 6, 2020; Cumulative, March 1, 2020, through August 24, 2020. ASCEND, Anemia Studies in Chronic Kidney Disease: Erythropoiesis via a Novel Prolyl Hydroxylase Inhibitor Daprodustat; D, dialysis study; Hb, hemoglobin; ID, incident dialysis study; ND, nondialysis study.
Data reported by Interactive Response Technology system.
Data reported via queries to Medical Monitors.
Site Status over Time
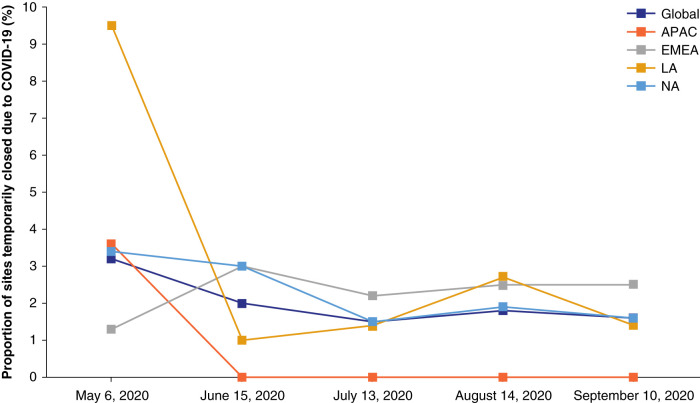
Figure 2 shows the percentage of study sites experiencing closures through September of 2020 overall and by region. Most regions were able to reopen sites between May and mid-June, despite varying levels of community spread of COVID-19. This pattern was particularly evident for Latin America, which had more site closures than other regions initially but similar percentages by mid-June.
Figure 2.
Proportion of sites that were temporarily closed due to COVID-19. Asia Pacific (APAC): Europe, Middle East, Africa (EMEA): Latin America (LA): Argentina, Brazil, Columbia, and Mexico; North America (NA): Canada and the United States.
Discussion
Despite widespread disruption during the COVID-19 pandemic, the effect on the ASCEND trials was limited. Extensive surveying of sites within weeks of the onset of the pandemic and rapid analysis of survey data allowed study leadership to provide guidance to sites to adjust and adapt study procedures. This limited the effect of the pandemic on study centers, research personnel, and participants.
Survey data were also important in driving modifications of study procedures. Mitigation strategies included conducting study visits remotely in participants’ homes, collecting and processing blood samples in a local laboratory or at a participant’s home to inform randomized treatment dosing, and using couriers to deliver randomized treatment to participants’ homes. In some hard-hit areas, sites were temporarily closed because hospital or research facilities were instructed to lockdown and/or study personnel were restricted to working remotely or were deployed elsewhere. In addition, some participants were transferred temporarily to facilities where study personnel could not ensure they would receive treatment according to study protocols. In these cases, the risk of continuing randomized treatment outweighed the potential benefit, and a small number of participants were temporarily switched to standard of care anemia treatment or to no anemia treatment.
Nevertheless, most participants were able to continue to receive randomized treatment according to study protocols. More participants not receiving dialysis had their randomized treatment temporarily interrupted than participants who were on dialysis. Temporary site closures in nondialysis settings were more disruptive to providing randomized treatment because monitoring of Hb was not possible, compared with dialysis settings where routine Hb measurement occurs even outside of study activities.
The approach used by the ASCEND trials during the COVID-19 pandemic had several strengths and some limitations. An important strength was that survey data were disseminated to operational and scientific leaders in real time during the first and subsequent waves of the pandemic to enable mitigation strategies to be developed and used. Second, the high survey response rates of 95%–100% meant that the surveys were highly representative of what was happening globally. Potential limitations included a lack of information about what was happening in the minority of nonresponding sites, especially with respect to whether these sites were closed. Despite site closures in the early stages of the pandemic, study sites became more resilient as the pandemic evolved. Indeed, from May to September 2020, the number of temporarily closed sites decreased substantially. The reasons for fewer closures were likely a combination of lower rates of COVID-19 over time and better adaptation to COVID-19, even when rates were high.
A key lesson learned early during the COVID-19 pandemic for the ASCEND trials was that because COVID-19 occurred in waves that varied across regions and countries, adaptations to study conduct were required. Finding safe ways to continue the trial were creative and diverse and included conducting study visits by telephone or telehealth.
A second important lesson was that Hb assessment needed to be flexible. When the HemoCue Hb assessment could not be done at study sites, allowing Hb assessment at local laboratories to inform dosing of randomized treatment was key to success as evidenced by the small percentage of participants whose randomized treatments were discontinued.
Our lessons on mitigating study disruption during a pandemic may assist the wider clinical research community to consider modifying their approach in designing or conducting clinical trials. The overarching guiding principles were the importance of early and continued assessment of site capabilities and the necessity to act nimbly with site-specific responses, given different patterns and surges of disruptions in different countries. The ultimate goal was to provide optimal care for participants while protecting the integrity of study performance and data collection. Implementation of mitigating strategies that were flexible and targeted limited the extent of disruption to the conduct of the ASCEND trials due to COVID-19.
Disclosures
A. Acharya reports consultancy fees from GlaxoSmithKline. B. Cizman, A.R. Cobitz, and A.M. Meadowcroft are employees of and stockholders in GlaxoSmithKline. R. Correa-Rotter reports scientific consulting and financial support for participation in clinical trials: AstraZeneca DAPA-CKD trial steering committee, GlaxoSmithKline for ASCEND Investigator and National Leader, Novonordisk FLOW national leader and investigator; is an advisory board member for Amgen, AbbVie, Boehringer Ingelheim, and Medtronic; and is a speaker for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, and Sanofi. I. Dasgupta reports research grants from Medtronic and Sanofi-Genzyme and is an advisory board member for AstraZeneca and GlaxoSmithKline. K.L. Johansen reports consultancy fees from GlaxoSmithKline and Akebia. V. Kher reports local INDA consultancy agreements with AstraZeneca, Biocon Pharmaceuticals, GlaxoSmithKline, Intas Pharmaceuticals, Novartis, Panacea, Roche, RPG Life Sciences, Sanofi Aventis, and Torrent Pharmaceuticals; research funding from Astellas India, Novartis India, and Sanofi Aventis India; honoraria from Astellas India, Intas India, JB Pharmaceuticals India, Novartis India, Reddy’s India, Roche India, and Torrent India; is a scientific advisor for Biocon India, Medtronics, Novartis India, Reddy’s India, Roche India, Sanofi Aventis, Torrent, and Wockhardt India; participates in a speakers’ bureau for AstraZeneca India, Biocon India, Intas India, JB Pharmaceuticals, Johnson and Johnson, Novartis India, Panacea India, Pfizer, Roche India, and Sanofi Aventis India. R.D. Lopes reports grants and personal fees from Bristol-Myers Squibb and Pfizer, personal fees from Bayer AG and Boehringer Ingelheim, and research grants from Amgen, Inc., GlaxoSmithKline, Medtronic plc, and Sanofi Aventis. B. Rayner reports honoraria for CME talks from AstraZeneca, Boehringer Ingelheim, Merck, Novartis, Sandoz, and Servier, and has served on a Servier Advisory Board. A.L. Silver reports research funding from Akebia, Ardelyx, Boehringer Ingelheim, Bayer AG, DiaMedica, Fibrogen/AstraZeneca, GlaxoSmithKline, Goldfinch Bio, Novartis, ProKidney, Reata, and Retrophin; consultancy fees from Ardelyx, Boehringer Ingelheim, Novartis, ProKidney, and Reata; and participates in a speakers’ bureau for Amgen, AstraZeneca, and Aurinia. A.K. Singh reports consultancy fees from GlaxoSmithKline. All remaining authors have nothing to disclose.
Funding
The ASCEND clinical program is funded by GlaxoSmithKline (ASCEND-D: NCT02879305, study 200807; ASCEND-ND: NCT02876835; study 200808; ASCEND-ID: NCT03029208; study 201410).
Acknowledgments
The authors would like to acknowledge Debby Mattioli for her efforts in helping to create the site survey. Editorial support in the form of assembling figures, grammatical editing and referencing was provided by Jonathan Plumb, of Fishawack Indicia, part of Fishawack Health, and was funded by GlaxoSmithKline.
Author Contributions
B. Cizman, A.R. Cobitz, K.L. Johansen, R.D. Lopes, L. Matsumoto, A.M. Meadows, O. Merege, A.K. Singh, H. Thomas contributed to conception or design. R. Correa-Rotter, I. Dasgupta, V. Kher, B. Rayner, A.L. Silva contributed to acquisition of data. All authors contributed to the data analysis or interpretation, critically reviewed the manuscript and approved the final version for submission.
Data Sharing Statement
Partial restrictions to the data and/or materials apply. Anonymized individual patient data and study documents can be requested for further research from https://www.clinicalstudydatarequest.com/.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006212021/-/DCSupplemental.
Brief description of ASCEND studies.
Complete survey administered to sites. Download Supplemental Materials, PDF file, 317 KB (316.8KB, pdf)
References
- 1.Psotka MA, Abraham WT, Fiuzat M, Filippatos G, Lindenfeld J, Ahmad T, Bhatt AS, Carson PE, Cleland JGF, Felker GM, Januzzi JL Jr, Kitzman DW, Leifer ES, Lewis EF, McMurray JJV, Mentz RJ, Solomon SD, Stockbridge N, Teerlink JR, Vaduganathan M, Vardeny O, Whellan DJ, Wittes J, Anker SD, O’Connor CM: Conduct of clinical trials in the era of COVID-19: JACC scientific expert panel. J Am Coll Cardiol 76: 2368–2378, 2020. 10.1016/j.jacc.2020.09.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siontis GC, Sweda R, Windecker S: Cardiovascular clinical trials in the era of a pandemic. J Am Heart Assoc 9: e018288, 2020. 10.1161/JAHA.120.018288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuttle KR: Impact of the COVID-19 pandemic on clinical research. Nat Rev Nephrol 16: 562–564, 2020. 10.1038/s41581-020-00336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Brief description of ASCEND studies.
Complete survey administered to sites. Download Supplemental Materials, PDF file, 317 KB (316.8KB, pdf)