Background
Diabetes is the leading cause of ESKD worldwide. It is estimated that 30% of individuals with type 1 diabetes mellitus and 40% of individuals with type 2 diabetes mellitus (T2DM) develop kidney disease (1). Deaths related to diabetic kidney disease (DKD) are higher compared with any other type of CKD (2). This excess mortality is mostly attributable to cardiovascular (CV) disease, and most patients with DKD will die without progression to ESKD (1). Thus, the unmet need for therapies that decrease the risks of DKD is enormous.
Mechanisms of Action of Mineralocorticoid Antagonism in the Diabetic Kidney
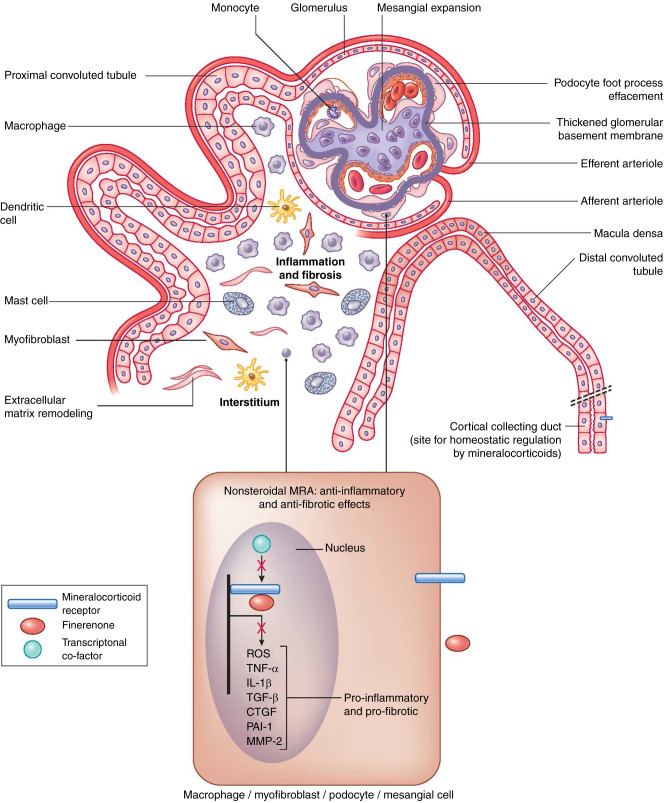
The mechanisms by which diabetes causes kidney disease are myriad. Hemodynamic changes, metabolic disturbances, proinflammatory, and profibrotic processes culminate in structural changes within the kidney. The hallmarks of DKD include glomerular hypertrophy, glomerular basement membrane thickening and mesangial expansion, glomerulosclerosis, tubulointerstitial fibrosis and inflammation, and arteriosclerosis (1). In recent years, our armamentarium to treat DKD has increased with the addition of sodium-glucose cotransporter-2 (SGLT-2) inhibitors and, most recently, with nonsteroidal mineralocorticoid receptor antagonist (MRA), finerenone. MRs are physiologic binding sites for aldosterone, cortisol, and, to a lesser extent, progesterone. They are expressed in various cells of the kidney, including epithelial cells of the collecting duct, podocytes, mesangial cells, tubulointerstitial fibroblasts, and macrophages (Figure 1). In the collecting duct, MR activation promotes sodium reuptake, potassium secretion, and fluid retention. In nonepithelial cells, the MR controls expression of many proinflammatory and profibrotic genes associated with DKD progression. The MR can be inappropriately overactivated in DKD, causing increased NADPH oxidase activity and upregulation of proinflammatory cytokines (e.g., TNF-α, IL-1β) and profibrotic proteins (e.g., connective tissue growth factor, transforming growth factor β1, plasminogen activator inhibitor 1, matrix metalloproteinase 2). The resulting damage culminates in glomerulosclerosis and progressive tubulointerstitial injury (3).
Figure 1.
Schematic of a diseased nephron with structural changes associated with diabetes. Binding of finerenone to the mineralocorticoid receptor disrupts transcription of proinflammatory and profibrotic factors. ROS, reactive oxygen species; CTGF, connective tissue growth factor; PAI-1, plasminogen activator inhibitor 1; MMP-2, matrix metalloproteinase 2.
Finerenone’s binding to the MR changes its configuration such that ligands (e.g., aldosterone) are unable to bind, which prevents downstream transcription of proinflammatory and profibrotic factors (Figure 1) (3). Finerenone has differential pharmacokinetics and physiologic effects compared with steroidal mineralocorticoid antagonists that influence therapeutic actions for DKD and serum potassium. Unlike the steroidal MRAs (spironolactone and eplerenone), the nonsteroidal MRA finerenone has higher selectivity for the MR and acts as an inverse agonist, whereas steroidal MRAs act as partial agonists. Thus, finerenone blocks activation of the receptor in the absence of a ligand with more complete disruption of recruitment of transcriptional cofactors (4). At comparable natriuretic doses, finerenone provides more potent inhibition of proinflammatory and profibrotic genes in the kidney than spironolactone or eplerenone. It also has less hyperkalemia risk. Effects that favor less hyperkalemia with finerenone include its shorter half-life, absence of active metabolites, a balanced distribution of MR selectivity between kidney and heart, and less epithelial sodium channel upregulation (5). Furthermore, unlike steroidal MRAs, finerenone also binds to mutant MR S180L, a receptor that is paradoxically activated by progesterone, which is usually an MR antagonist, leading to either gestational hypertension or worsening hypertension during pregnancy. This unique property suggests its potential use in this condition (5).
Clinical Evidence
To date, there have been two notable trials for nonsteroidal MRAs. The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial evaluated finerenone’s role in slowing CKD progression and reducing cardiovascular mortality in patients with T2DM and CKD (n=5674) on the background of treatment with an angiotensin converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). The participants had a mean (SD) eGFR of 44.3 (12.6) ml/min per 1.73 m2 and median (interquartile range) urine albumin-creatine ratio of 852 (446–1634) mg/g. There was an 18% reduction in the primary kidney disease outcome (≥40% decrease in eGFR, kidney failure, or death from kidney causes). Additionally, there was a 14% reduction in the secondary CV outcome (death from CV causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) (6).
The Efficacy and Safety of Finerenone in Subjects with Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD) trial (n=7352) evaluated the same CV outcome as in FIDELIO-DKD as the primary outcome and the kidney disease outcome as a main secondary outcome in patients with T2DM and less severe CKD with mean (SD) eGFR 67.8 (21.7) ml/min per 1.73 m2 and median (IQR) urine albumin-creatine ratio 308 (108–740) mg/g. There was a 13% reduction in the primary CV outcome in the finerenone group compared with placebo. Although there was a reduction in the secondary kidney disease outcome in the finerenone group, it did not reach statistical significance (7).
To examine the safety and efficacy of finerenone in individuals with T2DM across a wide range of CKD, a prespecified analysis of combined FIDELIO-FIGARO trials called FIDELITY was conducted. For this pooled analysis population (n=13,026) with median follow-up of 3 years, there was a 14% decrease in the CV outcome and a 23% decrease in a composite kidney disease outcome (time to kidney failure, ≥57% sustained decline in eGFR, or kidney-related death) (8). As a result of these clinical trials, finerenone received US Food and Drug Administration approval for reducing CKD progression, CV events, and heart failure hospitalizations in those with CKD due to T2DM.
The standard-of-care for DKD is an ACE inhibitor or an ARB and an SGLT2 inhibitor. On the basis of the available evidence, finerenone can be considered an excellent choice for patients with DKD who cannot take an SGLT2 inhibitor, or for those with persistently increased albuminuria despite treatment with the standard of care. The dose and titration of finerenone depend on both eGFR and serum potassium. The full dose of finerenone is 20-mg daily. Those with an eGFR 25–60 ml/min per 1.73 m2 or serum potassium 4.8–5 mEq/L, are recommended to take the lower dose of 10 mg daily, per the US Food and Drug Administration label (Table 1).
Table 1.
Dose considerations for finerenone in patients with type 2 diabetes and CKD
| Finerenone | eGFR >60 ml/min per 1.73 m2 | eGFR ≥25 to 60 ml/min per 1.73 m2 | eGFR <25 ml/min per 1.73 m2 | Serum Potassium ≤4.8 mEq/L | Serum Potassium >4.8 to <5 mEq/L | Serum Potassium >5.5 mEq/L |
|---|---|---|---|---|---|---|
| Initial daily dose | 20 mg | 10 mg | Not recommended | 10 or 20 mg | 10 mg | Withhold |
| Dose adjustments Monitor K and eGFR at baseline and 4 weeks after initiation or dose adjustment FDA label (labeling.bayerhealthcare. com/html/products/pi/ Kerendia_PI.pdf) | Maintain if eGFR does not decline >30% Reduce to 10 mg or withhold if eGFR declines ≥30% | Maintain if eGFR does not decline >30% Withhold if eGFR declines ≥30% | Not applicable | Maintain if serum K ≤5.5 mEq/L Withhold if serum K >5.5 mEq/L May start 10 mg if serum K declines to ≤5.0 mEq/L | Increase to 20 mg if serum K ≥4.8 mEq/L Maintain if serum K >4.8–5.5 mEq/L Withhold if serum K >5.5 mEq/L May start 10 mg if serum K declines to ≤5.0 mEq/L | May start 10 mg if serum K declines to ≤5.0 mEq/L |
K, potassium; FDA, Food and Drug Administration.
Hyperkalemia Risk
A major risk of MRAs is hyperkalemia, and increased risk of hyperkalemia was observed in the finerenone group in FIDELIO-FIGARO. Overall, the risk of hyperkalemia seems to be lower with a nonsteroidal MRA compared with steroidal MRAs. In a rat model of CKD, the risk of hyperkalemia was significantly lower with the nonsteroidal MRA PF-03882845 versus eplerenone (5). The Mineralocorticoid Receptor Antagonist Tolerability Study (ARTS) was a phase 2 trial of assessing safety of finerenone (BAY 94–8862), compared with spironolactone in patients with heart failure with reduced ejection fraction, and mild or moderate CKD. Finerenone was associated with lower mean increase in serum potassium level compared with spironolactone (9). Although the maximum daily dose of finerenone in ARTS was 10 mg compared with 20 mg in FIDELIO-FIGARO, the preclinical model of CKD (5) and data from ARTS are supportive of a lower risk of hyperkalemia with finerenone compared with steroidal MRAs. Moreover, the risk of hyperkalemia from MRA may be mitigated by SGLT-2 inhibition. By increasing urine flow in the cortical connecting tubule, SGLT-2 inhibitors could promote potassium excretion (10). Interestingly, big potassium channels that secrete potassium are activated via the mechanosensing of urine flow by intercalated cells, without directly requiring exchange for sodium, although long-term increases in urine flow may activate the epithelial sodium channel and promote sodium reuptake in neighboring principal cells, and thereby, help to maintain electrochemical balance in the filtrate (11). Indeed, a recent post hoc analysis from CREDENCE found that canaglifozin compared with placebo reduced the risk for hyperkalemia by approximately 20% in study participants with DKD (12). Another post hoc analysis from FIDELIO-DKD showed that risk of hyperkalemia was reduced by more than half among SGLT-2 inhibitor users (13). However, careful monitoring of serum potassium, with dose adjustment or treatment interruption as needed, is required in patients with CKD treated with finerenone (Table 1).
Effects on BP
It is important to note the anti-inflammatory and antifibrotic effects of nonsteroidal MRA are largely independent of BP lowering. In FIDELIO-FIGARO, the effects of finerenone on BP were nominal (6,7). In ARTS, spironolactone reduced systolic BP, whereas BP did not vary between the placebo and finerenone (9). Lesser BP lowering with finerenone has been attributed to its shorter half-life and reduced effect on natriuresis (7). Therefore, the steroidal mineralocorticoid antagonists have a more potent BP-lowering effects that make these agents preferred for treatment of hypertension.
Future Directions
With SGLT-2 inhibitors and finerenone gaining regulatory approval for treatment of DKD, we now have new therapies to actualize kidney health. ACE inhibitors and ARBs, SGLT-2 inhibitors, and finerenone work by complementary mechanisms to protect the kidneys and heart. Future research will elucidate how these therapies can be used in combination and personalized to individual patients. We foresee a future of kidney care shifting from treating advanced kidney disease and kidney failure to promoting kidney health and prevention of disease. Now we have a growing armamentarium of safe and effective therapies, and it is imperative to focus on their dissemination and implementation for millions of people worldwide with DKD who can benefit from them.
Disclosures
K. Tuttle reports current employment with Providence Health Care and the University of Washington; reports having consultancy agreements with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, and Novo Nordisk; reports receiving research funding from Bayer and Goldfinch Bio; reports receiving honoraria from Bayer, Gilead, and Goldfinch Bio; and reports being a scientific advisor or member of CJASN, Kidney Health Initiative, Lancet Diabetes Endocrinology, National Institute of Diabetes and Digestive and Kidney Diseases, and Nature Reviews Nephrology. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
J.K. Ghuman wrote the original draft; J.K. Ghuman and K.R. Tuttle conceptualized the study, and reviewed and edited the manuscript; and K.R. Tuttle provided supervision.
References
- 1.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017. 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas B: The global burden of diabetic kidney disease: Time trends and gender gaps. Curr Diab Rep 19: 18, 2019. 10.1007/s11892-019-1133-6 [DOI] [PubMed] [Google Scholar]
- 3.Bauersachs J, Jaisser F, Toto R: Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension 65: 257–263, 2015. 10.1161/HYPERTENSIONAHA.114.04488 [DOI] [PubMed] [Google Scholar]
- 4.Grune J, Beyhoff N, Smeir E, Chudek R, Blumrich A, Ban Z, Brix S, Betz IR, Schupp M, Foryst-Ludwig A, Klopfleisch R, Stawowy P, Houtman R, Kolkhof P, Kintscher U: Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone’s antifibrotic activity. Hypertension 71: 599–608, 2018. 10.1161/HYPERTENSIONAHA.117.10360 [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, Zannad F: Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 42: 152–161, 2021. 10.1093/eurheartj/ehaa736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators : Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383: 2219–2229, 2020. 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD Investigators : Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 385: 2252–2263, 2021. 10.1056/NEJMoa2110956 [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, Bakris GL; FIDELIO-DKD and FIGARO-DKD investigators : Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis [published online ahead of print]. Eur Heart J 2021. 10.1093/eurheartj/ehab777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F: Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur Heart J 34: 2453–2463, 2013. 10.1093/eurheartj/eht187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khuri RN, Strieder WN, Giebisch G: Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975. 10.1152/ajplegacy.1975.228.4.1249 [DOI] [PubMed] [Google Scholar]
- 11.Verschuren EHJ, Castenmiller C, Peters DJM, Arjona FJ, Bindels RJM, Hoenderop JGJ: Sensing of tubular flow and renal electrolyte transport. Nat Rev Nephrol 16: 337–351, 2020. 10.1038/s41581-020-0259-8 [DOI] [PubMed] [Google Scholar]
- 12.Neuen BL, Oshima M, Perkovic V, Agarwal R, Arnott C, Bakris G, Cannon CP, Charytan DM, Edwards R, Górriz JL, Jardine MJ, Levin A, Neal B, De Nicola L, Pollock C, Rosenthal N, Wheeler DC, Mahaffey KW, Heerspink HJL: Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: The CREDENCE trial. Eur Heart J 42: 4891–4901, 2021. 10.1093/eurheartj/ehab497 [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Joseph A, Anker S, Filippatos G, Rossing P, Ruilope LM, Pitt B, Kolkhof P, Scott C, Lawatscheck R, Wilson DJ, Bakris GL; FIDELIO-DKD Investigators : Hyperkalemia risk with finerenone: Results from the FIDELIO-DKD trial. J Am Soc Nephrol 33: 225–237, 2021. 10.1681/ASN.2021070942 [DOI] [PMC free article] [PubMed] [Google Scholar]