Abstract
Objectives:
We hypothesized that prenatal cannabis exposure (PCE) would be associated with increased attention problems and altered neurocognition in young adolescents.
Methods:
Data were obtained from the Adolescent Brain Cognitive Development (ABCD study®), a cohort of approximately 12,000 children. Presence or absence of PCE after knowledge of pregnancy was measured by caregiver report. All participants with PCE (N=224) were included and compared to two control groups; those matched on tobacco and alcohol exposure and those without prenatal tobacco or alcohol exposures. Outcomes were measured with the ABCD baseline assessment when participants were 9-10 years old and included attention, internalizing, externalizing and total problems scales on the Child Behavior Checklist (CBCL). Teacher reports were used when available. Mixed effects modeling assessed the association between PCE and outcomes controlling for parental psychopathology, prematurity and socioeconomic status. For participants with available data, patterns of brain activity during three fMRI tasks (the Stop Signal Task measuring response inhibition, the Monetary Incentive Delay (MID) task measuring reward processing and the EN-Back task measuring working memory) were analyzed using Permutation Analyses of the Linear Model.
Results:
Compared to both control groups, participants with PCE had significantly higher attention problems, externalizing, and total problem scores. PCE did not impact cognitive performance or patterns of brain activation during fMRI tasks.
Conclusions:
There are long-term associations between PCE and early adolescent attention and behavioral problems. These are not reflected in cognitive performance or task fMRI measures, a finding that is consistent with reports that fewer than half of children with ADHD have any specific cognitive deficit (Nigg et al., 2005; Willcutt et al., 2005). The young age of the sample may also relate to this finding and future investigation of neurodevelopmental trajectories of youth with PCE is warranted.
Keywords: Child Behavior, Attention, Brain Development, Prenatal cannabis, fMRI
1. Introduction
Social acceptance of cannabis is increasing. Currently, 14 US states have legalized adult recreational cannabis use and 33 have medical marijuana programs. Moreover, an increasing number of women of childbearing age use marijuana(Ko et al., 2015; Volkow et al., 2019). For instance, the National Survey on Drug Use and Health estimates cannabis use in the past month among non-pregnant 18-44 years old women has increased from 6% to 11% between 2002 and 2017. Similarly, use among pregnant women has increased from 3% to 7% in the same time period (Volkow et al., 2019). Although use decreases throughout pregnancy, recent estimates indicate 2.5% of pregnant women continue to use in the second and third trimester. This is significantly increased from less than 1% in the early 2000s (Volkow et al., 2019). Importantly, not only has use in pregnancy increased but potency of cannabis has steadily increased since its use gained popularity in the mid 20th century. In the last 30 years Δ9-tetrahydrocannabinol (THC) concentrations have increased from 4% to 14% in recreational cannabis (ElSohly et al., 2021; ElSohly et al., 2016). This increase in use and potency raises concern for more widespread fetal exposure to THC.
Preclinical models of prenatal cannabis exposure (PCE) demonstrate that THC, a highly lipophilic molecule, crosses the placenta (Grant et al., 2018). Although the precise pharmacokinetics of fetal exposure in humans are not known, Falcon et al. showed that PCE can be associated with accumulation of THC in human fetal and placental tissue (Falcon et al., 2012). Molecular mechanisms for THC associated alterations in neuroregulation have been described in animal studies of the endocannabinoid system (ECS) (Jutras-Aswad et al., 2009). The ECS plays a critical role in early brain development. THC acts a partial agonist on cannabinoid receptors resulting in the down-regulation of endogenous endocannabinoid synthesis. In animal models, offspring with prenatal THC exposure have shown increased hyperactivity and locomotor habituation (Pinky et al., 2019; Schneider, 2009). Emotional alterations reported include increased separation-induced vocalization, inhibited social interactions and increased anxiety-related behaviors (Schneider, 2009). Lastly, long term memory impairment and learning deficits have also been demonstrated in rat pups prenatally exposed to THC (Antonelli et al., 2005; Campolongo et al., 2007).
While neurodevelopmental effects of prenatal cannabis exposure have been explored in animal models, data in children showing long-lasting cognitive or behavioral associations are less robust (El Marroun et al., 2018). To date, there have been six longitudinal studies that investigate the developmental correlates of PCE; The Ottawa Prenatal Prospective Study (OPPS), Maternal Health Practices and Child Development Study (MHPCD), Generation R study (GenR), Adolescent Brain Cognitive Development Study (ABCD), Lifestyle and Early Achievement in Families study (LEAF) and the Norwegian Mother and Child Cohort Study (MoBa). The advantage of the longitudinal nature of these studies is the ability to examine changes across developmental stages. OPPS and MHPCD have demonstrated associations between PCE and negative neurocognitive outcomes in children from infancy to late adolescence. Acknowledging these studies used different measurements of cognitive and behavioral outcomes, deficits in short term memory, impulse control and attention have been consistently associated with PCE. Sex-specific increases in aggression and attention problems have been reported in 18-month-old girls in the Gen R study (El Marroun et al., 2011). The LEAF study described similarly increased aggression in 3.5 year old participants with PCE (Murnan et al., 2021). Evidence suggests impairment in the ability to attend continues in early childhood. At 6 years the OPPS study found PCE was associated with decreased sustained attention and impulse control, as well as increased hyperactivity (Fried et al., 1992). The MHPCD cohort also demonstrated deficits in short term memory, decreased attention and increased impulsivity in 6-year-olds with PCE (Goldschmidt et al., 2008; Leech et al., 1999). Moreover, differences persist into early adolescence with both OPPS and MHPCD showing increased impulsivity in 9-12-year-olds (Fried et al., 1998; Goldschmidt et al., 2000), and the OPPS cohort has demonstrated decreased sustained attention as late as 16 years of age (Fried et al., 2003). The more recent ABCD cohort enrolled participants when they were between 9 and 10 years old. This cohort has shown findings similar to OPPS and MHPCD and reported increased attention problems associated with PCE. (Paul et al., 2020). In addition to attention problems, MHPCD, GenR and ABCD cohorts have demonstrated PCE was associated with increased externalizing problems reported on the CBCL in early adolescence. (El Marroun et al., 2019; Goldschmidt et al., 2000; Paul et al., 2020).
The aforementioned studies of PCE have largely accounted for alcohol and tobacco exposure with statistical corrections. However, substance use in pregnancy has been associated with multiple demographic differences, obstetric complications, infant-caregiver relationship differences, and parental mental health differences, all of which may have an impact on child behavior and development (Eiden et al., 2018; Gabrhelík et al., 2021; Shmulewitz & Hasin, 2019; Stroud et al., 2018; Walker et al., 2011; Young-Wolff et al., 2020). Although there may be substance specific correlates, there are several know associations common among women who use any substance during pregnancy. We postulate that there may also be correlates of prenatal substance use that have not yet been identified that are important to child development. Therefore, comparing populations with PCE to those with other prenatal substance exposure may be a more appropriate “control” to account for common risk factors which might also influence child developmental outcomes. Therefore, we sought to harness the robust sample size of the ABCD study, of nearly 12,000 adolescents, to examine attention problems postnatally while accounting for prenatal exposures prior to statistical analysis. To do so we compare those with PCE to a non-exposed control group, and to a group matched on alcohol and tobacco prenatal exposure (with no PCE). We believe this case-control study design controls for more of the unknown differences between pregnant women who use and do not use substances after they know they are pregnant. Based on extant literature we hypothesize the PCE group will have greater attention problems than both control groups.
Furthermore, the biological mechanisms of behavioral changes associated with PCE have yet to be established. Functional magnetic resonance imaging (fMRI) is a methodology that has begun to improve understanding of the neural networks involved in executive functioning in adolescents. fMRI uses neuronal activity related oxygen demand to identify brain regions involved in cognitive tasks. Increased neuron firing requires increased blood oxygen levels. This increase in blood oxygen level causes a measurable difference in the magnetic signaling termed the BOLD (blood oxygen level dependent) signal. Therefore, identifying fMRI differences among those with PCE could advance understanding of the neurological underpinnings of PCE associated behavioral changes.
Thus far evaluation of fMRI has been reported in small numbers of older adolescents and young adults who were exposed to cannabis in utero. Smith et al have evaluated participants (n=16 with PCE) in the OPPS cohort between 18-22 years of age and demonstrated differences in the several areas of the brain during specific tasks related to aspects of executive functioning. Briefly, Smith found increased activity in the left postcentral and precentral gyrus as well as the left superior frontal gyrus while assessing response inhibition with a Go/No-Go task (Smith et al., 2016). Poor response inhibition is correlated with impulsivity, often experienced by children with attention difficulties (Bari & Robbins, 2013). Working memory was assessed in the same population during a visuospatial 2-back and letter 2-back tasks. Although there was not a difference in performance on the tasks, those with PCE demonstrated a different pattern of brain activation. Those with PCE had increased activation of the left posterior cingulate, left middle occipital gyrus and left cerebellum (Smith et al., 2016). Changes in these areas are consistent with theoretical hypotheses of underlying changes caused by exposure to cannabis since cannabinoid receptors are particularly dense in the prefrontal cortex, hippocampus and cerebellum (Bara et al., 2021). The prefrontal and parietal cortex, hippocampus and cerebellum have also been shown to be involved in working memory in children and young adults (Alsameen et al., 2021; Arthursson et al., 2017; Klingberg et al., 2002). Importantly, it is well known that adolescence is a time of significant development of the prefrontal cortex. Additionally, brain regions involved in executive functioning tasks on fMRI have been demonstrated to evolve from early adolescence through adulthood (Zhang et al., 2021). Thus far, brain activations patterns in early adolescents with PCE have not been reported. Therefore, investigation of fMRI differences in young adolescents with PCE is warranted.
2. Methods
2.1. Sample
Data were obtained from the ABCD second public release 2.0 (See acknowledgments). ABCD is a national population-based cohort study of young adolescents enrolled between 9-10 years from 21 sites across the US. Participants are planned to be followed longitudinally for 10 years. Release 2.0 includes baseline data on 11,875 participants, of which 11,489 had data regarding PCE. All data for this analysis were gathered when adolescent participants were 9-10 years old. Informed consent and assent were provided by parents and children respectively; and the ABCD research protocol was IRB approved.
All participants with PCE after knowledge of pregnancy were included in this study (N=224). The tobacco/alcohol-exposed control group was first matched to the PCE group on presence or absence of tobacco and alcohol use after knowledge of pregnancy and then matched on age and sex. The non-exposed control group was without prenatal alcohol, tobacco, or cannabis exposures and was matched to the PCE group on age and sex. This resulted in a final sample of 672 adolescents, with 224 in each group.
2.2. Measures
2.2.1. Prenatal exposure
PCE was reported by parents and caregivers. Parents and caregivers were defined as the person with guardianship and/or custody of the participant at time of enrollment. 82% of parents in the study sample were biological mothers, similar to the full ABCD cohort in which 86% of respondents are biological mothers. Caregivers were asked, “Once you (or the biological mother) knew you were (she was) pregnant were you (was she) using any of the following?” The list that followed included tobacco, alcohol, marijuana, other illicit drugs as well as prescription drugs. Possible answers included, “yes”, “no”, and “don’t know.” Duration and trimester of use was not collected. For those who reported use, daily frequency was collected by parent or caregiver retrospective report.
2.2.2. Behavioral measures
Youth behavior was assessed using the parent-reported Child Behavior Checklist (CBCL)(Achenbach & Rescorla, 2000). Data used for this analysis are all from the baseline assessment. Thus, caregivers who reported the prenatal history also answered the CBCL questionnaire. The CBCL is a 113 item questionnaire in which caregivers rate behaviors in the past six months on scale from 0 (not true) to 2 (very true or often true). The CBCL measures dimensional psychopathology on eight syndrome scales, two composite scores and a total problems summative score. The Attention Problem syndrome score (range 0-20) was the primary dependent variable. Mean attention problem scores from a normative sample are 3.8 for boys and 3.2 for girls ages 6-11 (Achenbach & Rescorla, 2000). Secondary outcomes include the Thought problem (range 0-30) and Social problems (range 0-22) syndrome scores as they have been associated with PCE in the prior ABCD investigation (Paul et al., 2020). In addition, the summative CBCL scores: Internalizing (range 0-64), Externalizing (range 0-70), Total Problems (range 0-240) were investigated.
The Brief Problem Monitor – Teacher (BPM-T) was used when available as an additional informant measure of internalizing, externalizing and attention problems. The BPM-T is an 18 item questionnaire in which teachers report child behaviors. The BPM-T measures three dimensions of psychopathology: attention (0-12), externalizing (0-12) and internalizing behaviors (0-12)(Achenbach et al., 2017). The BPM-T was available on a subset of participants. Attention scores were available on 83 (37%), 67 (30%) and 60 (27%) of the PCE, tobacco/alcohol exposed and non-exposed groups respectively. Internalizing and Externalizing scores were available in 41% of the PCE group, 37% of the tobacco/alcohol exposed group and 36% of the non-exposed groups.
2.2.3. Neurocognitive measures
Seven tasks from the NIH Toolbox are used in ABCD (Luciana et al., 2018) We investigated five tasks: the flanker task, dimensional card sort, pattern recognition, picture completion, and list sort as these reflect the attentional processes we hypothesize to be related to PCE.
The flanker task assesses the degree to which outside stimuli affect participant response. It measures cognitive control and attention. Five arrows are presented to participants, with the 2 left-most and 2 right-most arrows facing in the same direction. The participant must push a button indicating the direction of the middle arrow. Scores are based on speed and accuracy (Luciana et al., 2018).
The dimensional card sort measures cognitive flexibility. Participants sort objects by color or shape to objects presented at the bottom of the screen. 3 sets of sorting occur, the first by either shape or color. The second run presents the dimension (shape or color) that was not tested on run 1. And the last trial consists of alternating between sorting by shape and color. Scores are based on both accuracy and reaction time (Luciana et al., 2018).
The pattern recognition task measures rapid visual processing. Impaired attention could prolong the time needed to process information. Participants are given two pictures and asked if they are the same. Their score is comprised of number correct in a given time period. (Luciana et al., 2018)
The picture completion task measures memory and visuospatial sequencing. Participants are shown 15 pictures in sequence and asked to reproduce the sequence. The task is scored by adding the number of correct adjacent pairs reproduced over 3 trials. Scores range from 0-42 (Luciana et al., 2018).
Finally, the list sort task measures working memory and participants are shown pictures of food or animals of different sizes and told the name associated with the picture. Then, they are asked to repeat back the pictures from smallest to largest. Participants start with 2 pictures of the same category and if successful, increase by increments of one picture to a maximum of 7. The second category is tested in a similar manner. All participants then move on to the combined trials where pictures from both categories are used and participants are instructed to repeat back pictures from one category at a time in ascending order of size (Luciana et al., 2018).
2.2.4. Functional brain imaging
Imaging data were available on a subset of participants. We examined differences in BOLD (blood oxygen level dependent) signal between groups during three fMRI tasks using all available data (Rosenberg et al., 2020).
Stop Signal Task (SST) data were available in 98 (44%), 112 (50%) and 109 (49%) of participants in the PCE, tobacco/alcohol exposed and non-exposed groups respectively. The SST is designed to study inhibitory control (Logan, 1994) and is the most correlated with ADHD in the literature. Participants are presented first with a fixation or warning stimulus followed by leftward or rightward facing arrows and instructed to indicate which way the arrow faces “as quickly and accurately as possible.” 17% of trials are followed with an upward facing arrow which is the “stop signal.” Stop signals indicate participants should try to stop their response to that particular trial. The timing of the stop signal is determined algorithmically to ensure participants only successfully stop on 50% of trials. This is done by increasing or decreasing the delay stop signal time by 50ms when participants are successful or unsuccessful respectively (Casey et al., 2018).
Performance on this task was measured with the stop signal reaction time. Activations maps were computed on the voxel and vertex level for the following contrasts: stop versus correct-go trials, correct go-trials versus fixation and correct-stop versus incorrect stop.
Working memory was assessed with the EN-Back task (Cohen et al., 2016). EN-Back data were available in 87 (39%), 117 (52%) and 104 (46%) participants in the PCE, tobacco/alcohol exposed and non-exposed groups respectively. This task consists of two runs of eight blocks. Each block consists of 10 trials and 4 fixation signals and begins with an instructional message of “2-back” or “target=” to indicate the target for 0-back blocks. In the 2-back blocks participants are asked if the current stimulus matches the one shown 2 trials prior. During the 0-back blocks participants attempt to match the current stimulus to the target picture. The colored fixation signal appears before each block to alert the participant to the changing of task (Casey et al., 2018).
Discrimination, measured by D’ was used to assess performance (Rosenberg et al., 2020). Contrasts of 0-back minus fixation; 2-back minus fixation, 2-back minus 0-back and face minus place were examined.
Monetary Incentive Delay task (MID) data were available in 115 (51%), 124 (55%), and 118 (53%) of participants in the PCE, tobacco/alcohol exposed and non-exposed groups respectively. The MID was used to measure reward processing (Knutson et al., 2000; Yau et al., 2012). There are five possible incentives for the MID task (Win $5.00, Win $0.20, Lose $5.00, Lose $0.20 and no money at stake - $0). Participants are informed of the incentive at the beginning of each trial. A variable target appears on the screen and the participant responds to either win or avoid losing money. The participant then sees the result of the trial. The task consists of 10 trials of each incentive (Casey et al., 2018).
Activation maps were computed for anticipation of large reward and large loss versus neutral anticipation, as well as reward positive and loss positive versus negative feedback.
2.2.5. Covariates
Factors shown to be related to childhood ADHD were used as covariates including parental psychopathology (Faraone et al., 2005; Waldman & Gizer, 2006). Parental psychopathology was measured using the total problems score on the Adult Self Report ASEBA instrument (ASR) (Achenbach & Rescorla, 2000). The ASR is a 120 item questionnaire that compliments the CBCL. Similarly, it has 8 syndrome scales, and a total problems summative score. Parental psychopathologies correlated with attention problems in children include attention problems, antisocial behavior, anxiety and depression (Galera et al., 2011). Therefore, we chose to use the total problems score as a covariate. Younger maternal age at time of delivery is recognized as a risk factor for ADHD and research suggests longer duration of breastfeeding, while multifactorial, is protective (Chang et al., 2014; Stadler et al., 2016). Therefore, months breastfed, and maternal age were included as covariates. Additional known neurodevelopmental factors of prematurity (less than 40 weeks gestation) and birthweight were also included. Lastly, race and household education level were included as sociodemographic covariates as these were not equivalent between groups (Table 1).
Table 1:
Frequencies and Means of Predictor Variables and Outcomes by Exposure Group
| PCE group N=224 |
No PCE, matched on tobacco, alcohol and demographics N=224 |
No PCE, tobacco or alcohol, matched on demographics N=224 |
P value | |
|---|---|---|---|---|
| Group matching characteristics | ||||
| Mean child participant age in months (SD) | 118 (7.3) | 117 (7.4) | 118 (7.3) | 0.975 |
| Child participant Sex | 0.548 | |||
| M | 97 (43%) | 107 (48%) | 97 (43%) | |
| F | 127 (57%) | 117 (52%) | 127 (57%) | |
| Maternal alcohol use after knowledge of pregnancy | 0.139 | |||
| No | 168 (75%) | 181 (81%) | 224 (100%) | |
| Yes | 56 (25%) | 43 (19%) | 0 (0%) | |
| Mean drinks per week among those using alcohol (SD) | 5.3 (10.9) | 6.3 (13.6) | 0 | 0.789* |
| Maternal tobacco use after knowledge of pregnancy | 1.0 | |||
| No | 115 (51%) | 115 (51%) | 224 (100%) | |
| Yes | 109 (49%) | 109 (49%) | 0 (0%) | |
| Mean cigarettes per day among those using tobacco (SD) | 8.7 (6.2) | 7.0 (6.4) | 0 | 0.107* |
| Other demographics | ||||
| Maternal Education | <0.001 | |||
| Less than high school | 13 (6%) | 4 (2%) | 1 (<0.5%) | |
| High school diploma or GED | 10 (5%) | 18 (8%) | 10 (4%) | |
| Some College | 45 (20%) | 28 (13%) | 20 (9%) | |
| Bachelor’s degree | 102 (46%) | 81 (36%) | 70 (31%) | |
| Graduate degree | 34 (15%) | 43 (19%) | 53 (24%) | |
| Not available | 20 (9%) | 50 (22%) | 70 (31%) | |
| Race | <0.001 | |||
| White | 85 (38%) | 124 (55%) | 130 (58%) | |
| Black | 81 (36%) | 35 (15%) | 30 (13%) | |
| Hispanic | 27 (12%) | 35 (15%) | 45 (20%) | |
| Asian | 1 (<0.5%) | 3 (1%) | 3 (1%) | |
| Other | 30 (13%) | 27 (12%) | 16 (7%) | |
| Covariates | ||||
| Premature | 0.097 | |||
| No | 184 (82%) | 184 (82%) | 181 (81%) | |
| Yes | 35 (16%) | 39 (17%) | 43 (19%) | |
| Don’t know | 5 (2%) | 1 (<0.5%) | 0 (0%) | |
| Breastfeeding duration Months (SD) | 4.2 (6.6) | 5.6 (8.1) | 8.1(9.7) | <0.001 |
| Mean maternal age in years (SD) | 25 (6.0) | 29 (6.6) | 29 (6.2) | <0.001 |
| Parental ASEBA total problem scores raw (SD) | 36 (24) | 25 (19) | 21 (19) | <0.001 |
| Gestational age at first knowledge of pregnancy weeks (SD) | 8 (7) | 7 (7) | 6 (5) | <0.001 |
| Behavioral Outcomes Measured on the CBCL | ||||
| Mean attention score (SD) | 5.4 (4.3) | 3.3 (3.6) | 3.1 (3.9) | <0.001 |
| Mean thoughts score (SD) | 3.2 (3.5) | 2.1 (2.5) | 1.7 (2.4) | <0.001 |
| Mean social score (SD) | 3.2 (3.1) | 2.2 (2.8) | 1.9 (2.8) | <0.001 |
| Mean externalizing score (SD) | 9.6 (9.1) | 5.1 (5.9) | 5.0 (6.6) | <0.001 |
| Mean internalizing score (SD) | 7.7 (7.4) | 5.3 (5.6) | 4.8 (5.8) | <0.001 |
| Mean total problems score (SD) | 33.3 (25.5) | 20.7 (18.5) | 18.9 (20.2) | <0.001 |
| Teacher report forms Measured on the BPM-T | ||||
| Mean attention score (SD) | 4.6 (3.8) | 2.6 (3.0) | 2.3 (3.1) | 0.001 |
| Mean externalizing score (SD) | 2.9 (3.6) | 1.6 (2.4) | 1.1 (2.4) | 0.001 |
| Mean internalizing score (SD) | 2.5 (2.7) | 1.7 (2.4) | 1.7 (2.2) | 0.056 |
| Neurocognitive outcomes | ||||
| Mean flanker task score (SD) | 19.7 (1.1) | 19.8 (0.7) | 19.8 (1.0) | 0.543 |
| Mean card sort task score (SD) | 27.4 (2.9) | 27.7 (2.4) | 27.8 (2.4) | 0.234 |
| Mean pattern task score (SD) | 36.0 (7.5) | 37.7 (7.1) | 37.8 (7.1) | 0.014 |
| Mean picture task score (SD) | 10.9 (5.5) | 12.5 (5.6) | 12.8 (6.2) | 0.001 |
| Mean list task score (SD) | 15.0 (3.2) | 15.3 (3.0) | 16.0 (3.3) | 0.003 |
| fMRI task performance | ||||
| Mean stop signal reaction time (SD) | 286.2 (120.4) | 301.4 (88.1) | 300.0 (79.4) | 0.289 |
| Mean D’ 0 back (SD) | 2.3 (0.9) | 2.4 (0.8) | 2.4 (0.9) | 0.168 |
| Mean D’ 2 back (SD) | 1.8 (0.8) | 2.0 (0.8) | 1.9 (0.8) | 0.276 |
Prematurity is defined as less than 40 weeks gestation. Missing data points are not included in this table. Chi square was used to assess differences in categorical variables, ANOVA was used for continuous variables.
Difference measured between groups with prenatal exposures only.
2.3. Statistical Analysis
Statistical analyses were performed using Stata 16.0 (StataCorps LLC, College Station, Texas). CBCL scores and BMT-T scores fit a Poisson distribution thus, Poisson mixed effects models were used to assess the association between PCE and attention symptoms. First, attention problems were predicted from PCE alone (base model), next a model including covariates as fixed effects, and families nested within site as random effects was used to isolate effect of PCE.
Other measures (CBCL scores, BPM-T scores, and neurocognitive performance) were analyzed using mixed effects models. We used the Benjamini, Krieger, Yekutieli method to account for multiple analyses(Benjamini et al., 2006). CBCL and neurocognition analyses included the same covariates as the Attention Problems analysis. Neurocognitive scores were normalized using a rank based inverse normalizing transformation. Teacher report models included race and highest household education as fixed effects.
Task fMRI data were pre-processed as described in Hagler et al (Hagler et al., 2019). Performance measures from the fMRI tasks were normally distributed, therefore did not require transformation. The Permutation Analysis of Linear Models (PALM)’s general linear model was used to generate subcortical and cortical functional activation maps for each of the three fMRI tasks by contrasting participants with PCE with each control group. Scanner ID was added to the covariates used in the primary analysis.
3. Results
3.1. Descriptive Statistics:
Of the 11,489 participants, 2% (N=224) were prenatally exposed to cannabis after the knowledge of pregnancy. Frequency of cannabis used on a daily basis after knowledge of pregnancy was available on 148 (66%); average use was twice daily (SD = 1.3). Of these cannabis exposed participants, 57% were female, 49% had concurrent prenatal tobacco exposure and 25% were exposed to alcohol while in utero. The THC exposed group was more diverse with white individuals making up 38% of the group, while in the two control groups, more than half of the participants were white. Maternal age at delivery was slightly younger in the PCE group compared to controls and both exposed groups reported breastfeeding for a shorter duration, with means less than 6 months. Although the groups differed on gestational age at first knowledge of pregnancy the means were between 6-8 weeks gestation, all in the middle of the first trimester. Importantly those who reported PCE had significantly higher scores of parental self-reported psychopathology, measured on the ASR. ASR mean total problem score was 36 for the PCE group vs 25 and 21 for the matched tobacco/alcohol exposed controls and the non-exposed controls respectively.
Outcome variables demonstrated several differences between participants with PCE and those without. The PCE group had the highest scores for all CBCL behavioral measures, and teacher reported behavioral measures. There was no difference between groups for the flanker or card sort cognitive tasks, however the PCE group had slightly lower scores on the pattern, picture and list tasks. Lastly, the performance on tasks during the fMRI were not different between groups.
3.2. Attention symptoms:
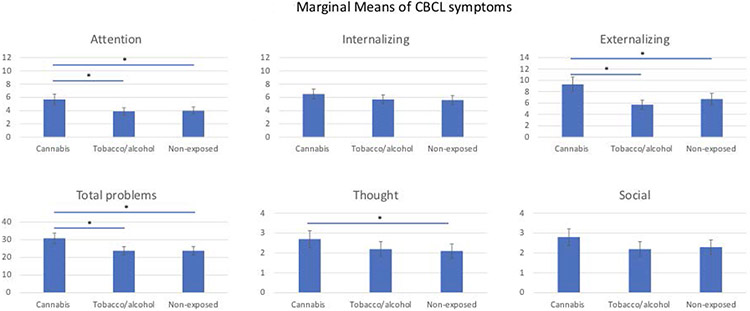
The PCE group had statistically significantly increased attention problem scores in both models (with and without covariates) compared to both control groups (Table 2). In fact, attention scores for both control groups were approximately 30% lower than the PCE group. These differences remained significant after accounting for multiple analyses. For interpretation, differences in marginal means are presented in Figure 1. The only significant fixed effect variable was parental psychopathology, with increasing ASR total problem scores being associated with higher attention scores. (RR 1.02; 95%CI 1.016-1.025).
Table 2:
Associations of CBCL symptoms with prenatal cannabis exposure compared to controls after including confounding variables
| CBCL measurement |
Prenatal tobacco and/or alcohol exposure | No prenatal exposure | ||||
|---|---|---|---|---|---|---|
| Rate ratio (95% CI) | P | FDR* p | Rate ratio (95% CI) | P | FDR* p | |
| Attention | 0.68 (0.54 to 0.85) | 0.001 | 0.007 | 0.69 (0.55 to 0.88) | 0.003 | 0.008 |
| Internalizing | 0.87 (0.72 to 1.06) | 0.175 | 0.079 | 0.86 (0.70 to 1.05) | 0.129 | 0.063 |
| Externalizing | 0.62 (0.49 to 0.78) | <0.001 | 0.007 | 0.72 (0.57 to 0.92) | 0.009 | 0.011 |
| Total | 0.77 (0.65 to 0.92) | 0.003 | 0.008 | 0.77 (0.65 to 0.92) | 0.004 | 0.009 |
| Thought | 0.84 (0.67 to 1.05) | 0.126 | 0.063 | 0.77 (0.61 to 0.98) | 0.034 | 0.031 |
| Social | 0.80 (0.64 to 1.01) | 0.065 | 0.052 | 0.81 (0.64 to 1.04) | 0.092 | 0.062 |
Fixed effects covariates include Parental total problems, maternal age, months breastfed, prematurity, race and highest household education.
False discovery rate corrected p values
Figure 1:
Marginal means and standard errors are plotted for interpretability. Significance determined by mixed effect modeling described. * Denotes significant false discovery rate (FDR) corrected p values <0.05 when compared to the cannabis exposed group.
3.3. Other CBCL symptoms:
After accounting for covariates, externalizing symptoms and total problems were significantly higher in participants with PCE compared to both control groups (Table 2). Internalizing symptoms and thought problems were increased compared only to the non-exposed group. Social problems were not significantly different between any group. Parental psychopathology was associated with higher scores on all CBCL domains. All associations persisted after correcting for multiple analyses.
3.4. Teacher report forms (BPM-T)
BPM-T scores were available for a subset of participants. 210 (31%) had teacher reported attention scores, 257 (38%) had externalizing and internalizing scores. After adjusting for covariates, PCE was associated with increased attention problem scores compared to the tobacco/alcohol exposed (p=0.009) and non-exposed group (p=0.007). When compared to the non-exposed group, those with PCE had increased teacher reported externalizing problems (p=0.005), however this increase was not seen when comparing to the tobacco/alcohol exposed group. There were no significant group differences on teacher reported internalizing symptoms.
3.5. Neurocognitive measures:
There were no significant group differences on any neurocognitive task after accounting for covariates (Table 3).
Table 3:
Mixed effect model coefficients (b) for associations between neurocognitive tasks and prenatal cannabis exposure
| Variable | NIH toolbox task | ||||
|---|---|---|---|---|---|
| Flanker | Card sort | Pattern | Picture | List | |
| Comparison group | |||||
| Tobacco and/or alcohol exposure | −0.08 | 0.01 | 0.08 | 0.11 | −0.09 |
| No prenatal exposure | −0.05 | −0.01 | 0.03 | 0.01 | −0.02 |
| Fixed effects | |||||
| Parental total problems score | 0.0001 | −0.0001 | −0.003 | −0.003 | −0.001 |
| Maternal age | 0.002 | 0.0003 | −0.008 | 0.006 | 0.010 |
| Months breastfed | −0.001 | −0.0003 | 0.008 | 0.001 | 0.02* |
| Prematurity | −0.05 | 0.10 | 0.05 | −0.05 | 0.03 |
| Race (compared to Caucasians) | |||||
| Black | −0.08 | −0.27* | −0.36* | −0.39* | −0.39* |
| Hispanic | 0.11 | −0.14 | −0.03 | 0.03 | −0.12 |
| Asian | 0.17 | −0.19 | 0.02 | 0.01 | 0.10 |
| Other | −0.11 | −0.40* | −0.29* | −0.24 | −0.33* |
| Highest household education | |||||
| 1 | −0.43* | −0.24 | 0.22 | −0.12 | −0.63* |
| 2 | −0.12 | −0.09 | 0.28 | −0.01 | −0.59* |
| 3 | −0.23 | −0.12 | 0.23 | −0.03 | −0.21 |
| 4 | −0.17 | −0.11 | 0.42 | 0.19 | −0.20 |
| 5 | −0.16 | 0.01 | 0.44 | 0.27 | −0.07 |
p<0.05
3.6. fMRI task performance:
Functional performance measures were available on 430 (64%) of participants. There were no significant performance differences between the 3 groups on either stop signal reaction time from the Stop task or D’ (0-back or 2-back conditions) of the EN-Back task.
3.7. Functional imaging:
Complete imaging and covariate data were available on 308, (46%), 357 (53%) and 319 (47%) participants for the EN-back task, MID task and SST, respectively. There were no significant differences in BOLD signal activation patterns between groups on any of the contrasts across tasks.
4. Discussion
Our findings demonstrate PCE is associated with increased attention, externalizing and total problem scores when compared to those without PCE. These results hold when the comparison is those with no prenatal exposure and those with matched presence of prenatal tobacco and alcohol exposure, supporting the hypothesis that PCE in and of itself is related to later attention/externalizing problems. Additionally, we report a lack of difference in cognitive functioning and functional brain imaging associated with PCE. These findings come from a cohort powered to find subtle differences in developmental trajectories therefore a null finding is unlikely to be due to small sample size.
While other studies have reported associations of PCE with behavioral and cognitive outcomes (including within the ABCD cohort), the current study has novel features and adds additional information (Paul et al., 2020). First, rather than controlling for tobacco and alcohol exposures in the entire ABCD sample, we created two sets of matched controls to compare to those who had similar tobacco and alcohol exposures as well as those who had no prenatal tobacco, alcohol or cannabis exposures. We chose this methodology in an attempt to account for innumerable potential confounding differences in those with and without substance use during pregnancy. It is possible that our more focused inclusion criteria help to constrain statistically significant relationships, which could potentially increase the validity of our findings. Moreover, we opted to use self-reported current mental health symptoms from the Adult Self Report, instead of reported family history, because current parental psychopathology can increase reports of problematic child behavior through cognitive errors or underlying psychological constructs (De Los Reyes & Kazdin, 2005; Haack et al., 2017). We found measurement of parental psychopathology to be significantly associated with each CBCL symptom score as well as youth cognitive task performance. Therefore, we believe its inclusion in analyses of the CBCL domains and cognitive performance is imperative. Finally, the use of multi-informant data (teacher reports) strengthens the results even though these data were only available in a sub-group of participants.
Our data support most of the findings of the extant literature and add to them in important ways. Goldschmidt et al report PCE associated increased externalizing symptoms without changes in attention scores in the MHPCD cohort (Goldschmidt et al., 2000). The fact that we find an increase in the CBCL attention scores in a sample, including those from high and low income households, suggests PCE plays a role in attention problems, but it may be more apparent in those of higher SES who have fewer competing baseline risk factors. Similarly, our study reproduces patterns reported using data from the GenR study that demonstrate increased externalizing behaviors in those with PCE, without an increase in internalizing symptoms (El Marroun et al., 2019). Recognizing there is variation between study designs, the consistency across results suggest PCE is associated with behavioral differences in children, in particular an increase in problems with attention and externalizing symptoms.
The finding that PCE is associated with behavioral symptoms and not cognitive deficits or altered patterns of brain activity has not been reported previously in this cohort. Previous cohorts identified only minor differences in aspects of executive functioning in early adolescents (9-12 years) with PCE (Fried et al., 1998). Behavior changes without cognitive changes have been described in previous studies of adolescents with ADHD. In fact, Jonsdottir et al question the role of executive function deficits in ADHD at all (Jonsdottir et al., 2006). Additionally, Barkley et al have found little correlation between executive functioning measurement tasks, such as those in the NIH toolbox, and executive functional deficits in daily life experienced by individuals with ADHD (Barkley & Murphy, 2011). That being said, cognitive differences among those with PCE have been identified in mid-adolescence (13-16 years) (Fried & Watkinson, 2001). It may be that PCE has stronger associations with differences in cognition and brain activity later in adolescence – an empirical proposition that can be tested in the ongoing ABCD study.
While the lack of cognitive difference may have been anticipated, the lack of brain changes was unexpected. It is likely that there are multiple small brain alterations reflecting the heterogeneity of the diagnosis of ADHD (Nigg et al., 2005; O'Halloran et al., 2018; Postema et al., 2021; Potter et al., 2006). Previous studies using fMRI have suggested sustained attention involves multiple neural networks working in tandem (O'Halloran et al., 2018). Therefore, if individuals with attention problems exhibit a diverse pattern of alterations in these neural networks, a unifying pattern of change may not be viable on BOLD signaling. Additionally, our exposure data are captured as any use after knowledge of pregnancy and variables such as dose of cannabinoids are not available. If specific brain changes differ depending on timing and dose of prenatal exposure to cannabinoids this diversity would result in the same inability to identify large differences in the entire exposed population.
Lastly, it is possible that our null result is valid. Additional studies evaluating functional changes in offspring exposed to intrauterine cannabis are necessary to replicate or refute this negative finding.
Our study has several important limitations to consider. The first and most important is the lag time between the exposure of interest (PCE) and data collection. Participants were asked to remember 10 years in the past and report cannabis use during pregnancy. This measure is prone to recall bias and may impact the validity of results. That said, a study comparing antenatal reporting of substance use with report 14 years later suggested retrospective report of prenatal drug exposures are at least as accurate as antenatal report (Hannigan et al., 2010). We opted to use cannabis use after knowledge of pregnancy as it more likely represents ongoing use throughout multiple stages of gestation. Even so, specific timing of use is not available therefore our data cannot be used to draw conclusions regarding trimester specific exposures. Additionally, we lack a precise measure of the amount and potency of cannabis used therefore we are unable to make any dose-based conclusions. Still, we feel our analysis is meaningful given the sample size, multiple matched controls and ability to account for potentially confounding variables.
Second, measures of attention and behavior typically rely on parental report. Using teacher reports in our study strengthens the findings as multi-informant data is the clinical gold standard and reduces bias in reporting (Association, 2013). Bias is of particular concern regarding parental psychopathology. Thus, we used both teacher reports and accounted for a dimensional measure of parental psychopathology in all analyses.
In sum, we found that prenatal cannabis exposure after the knowledge of pregnancy was associated with increased attention problems and externalizing symptoms in children 9-11 years of age. There were no differences in cognition, or functional imaging in this age group. Our results contribute to and reinforce the findings of prior cohort studies illuminating the long-term risks of PCE on behavioral outcomes and indicate the need for continued study on associations with cognitive and functional imaging outcomes.
Acknowledgements:
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9-10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Funding/Support:
U01DA051039; UG1OD024955
Abbreviations:
- ABCD
Adolescent Brain Cognitive Development study
- ADHD
Attention Deficit Hyperactivity Disorder
- CBCL
Child Behavior Checklist
- BPM-T
Teacher Report Forms
- SST
Stop Signal Task
- MID
Monetary Incentive Delay
- PALM
Permutation Analysis of Linear Models
- Gen R
Generation R study
- MHPCD
Maternal Health Practices and Child Development study
- OPPS
Ottawa Prenatal Prospective Study
- SES
Socioeconomic status
- THC
Tetrahydrocannabinol
- PCE
Prenatal Cannabis Exposure
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest relevant to this article to disclose.
References
- Achenbach T, McConaughy S, Ivanova M, & Rescorla L (2017). Manual for the ASEBA Brief Problem Monitor for Ages 6-18 (BPM/6-18). Burlington: University of Vermont Research Center for Children, Youth, and Families. [Google Scholar]
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms and profiles (Vol. 30). Burlington, VT: University of Vermont, Research center for children, youth & families. [Google Scholar]
- Alsameen M, DiFrancesco MW, Drummond SPA, Franzen PL, & Beebe DW (2021, Oct). Neuronal activation and performance changes in working memory induced by chronic sleep restriction in adolescents. J Sleep Res, 30(5), e13304. 10.1111/jsr.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli T, Tomasini MC, Tattoli M, Cassano T, Tanganelli S, Finetti S, Mazzoni E, Trabace L, Steardo L, Cuomo V, & Ferraro L (2005, Dec). Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb Cortex, 15(12), 2013–2020. 10.1093/cercor/bhi076 [DOI] [PubMed] [Google Scholar]
- Arthursson PSH, Thompson DK, Spencer-Smith M, Chen J, Silk T, Doyle LW, & Anderson PJ (2017, Dec). Atypical neuronal activation during a spatial working memory task in 13-year-old very preterm children. Hum Brain Mapp, 38(12), 6172–6184. 10.1002/hbm.23820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A. P. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Bara A, Ferland JN, Rompala G, Szutorisz H, & Hurd YL (2021, Jul). Cannabis and synaptic reprogramming of the developing brain. Nat Rev Neurosci, 22(7), 423–438. 10.1038/s41583-021-00465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, & Robbins TW (2013, Sep). Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol, 108, 44–79. 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Barkley RA, & Murphy KR (2011, 2011/June/01). The Nature of Executive Function (EF) Deficits in Daily Life Activities in Adults with ADHD and Their Relationship to Performance on EF Tests. Journal of Psychopathology and Behavioral Assessment, 33(2), 137–158. 10.1007/s10862-011-9217-x [DOI] [Google Scholar]
- Benjamini Y, Krieger AM, & Yekutieli D (2006). Adaptive linear step-up procedures that control the false discovery rate. Biometrika, 93(3), 491–507. [Google Scholar]
- Campolongo P, Trezza V, Cassano T, Gaetani S, Morgese MG, Ubaldi M, Soverchia L, Antonelli T, Ferraro L, Massi M, Ciccocioppo R, & Cuomo V (2007, Sep). Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol, 12(3-4), 485–495. 10.1111/j.1369-1600.2007.00074.x [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, Chaarani B, Mejia MH, Hagler DJ Jr., Daniela Cornejo M, Sicat CS, Harms MP, Dosenbach NUF, Rosenberg M, Earl E, Bartsch H, Watts R, Polimeni JR, Kuperman JM, Fair DA, Dale AM, & Workgroup, A. I. A. (2018, Aug). The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci, 32, 43–54. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, D'Onofrio BM, Almqvist C, Kuja-Halkola R, Sjölander A, & Larsson H (2014, Dec). Maternal age at childbirth and risk for ADHD in offspring: a population-based cohort study. Int J Epidemiol, 43(6), 1815–1824. 10.1093/ije/dyu204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson KA, Rudolph MD, Chein J, Richeson JA, Heller AS, Silverman MR, Dellarco DV, Fair DA, Galvan A, & Casey BJ (2016, Apr). When Is an Adolescent an Adult? Assessing Cognitive Control in Emotional and Nonemotional Contexts. Psychol Sci, 27(4), 549–562. 10.1177/0956797615627625 [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, & Kazdin AE (2005, Jul). Informant discrepancies in the assessment of childhood psychopathology: a critical review, theoretical framework, and recommendations for further study. Psychol Bull, 131(4), 483–509. 10.1037/0033-2909.131.4.483 [DOI] [PubMed] [Google Scholar]
- Eiden RD, Schuetze P, Shisler S, & Huestis MA (2018, Jul-Aug). Prenatal exposure to tobacco and cannabis: Effects on autonomic and emotion regulation. Neurotoxicol Teratol, 68, 47–56. 10.1016/j.ntt.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Bolhuis K, Franken IHA, Jaddoe VWV, Hillegers MH, Lahey BB, & Tiemeier H (2019, 02). Preconception and prenatal cannabis use and the risk of behavioural and emotional problems in the offspring; a multi-informant prospective longitudinal study. Int J Epidemiol, 48(1), 287–296. 10.1093/ije/dyy186 [DOI] [PubMed] [Google Scholar]
- El Marroun H, Brown QL, Lund IO, Coleman-Cowger VH, Loree AM, Chawla D, & Washio Y (2018, Nov). An epidemiological, developmental and clinical overview of cannabis use during pregnancy. Prev Med, 116, 1–5. 10.1016/j.ypmed.2018.08.036 [DOI] [PubMed] [Google Scholar]
- El Marroun H, Hudziak JJ, Tiemeier H, Creemers H, Steegers EA, Jaddoe VW, Hofman A, Verhulst FC, van den Brink W, & Huizink AC (2011, Nov 1). Intrauterine cannabis exposure leads to more aggressive behavior and attention problems in 18-month-old girls. Drug Alcohol Depend, 118(2-3), 470–474. 10.1016/j.drugalcdep.2011.03.004 [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Chandra S, Radwan M, Majumdar CG, & Church JC (2021, Jun). A Comprehensive Review of Cannabis Potency in the United States in the Last Decade. Biol Psychiatry Cogn Neurosci Neuroimaging, 6(6), 603–606. 10.1016/j.bpsc.2020.12.016 [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, & Church JC (2016, Apr 1). Changes in Cannabis Potency Over the Last 2 Decades (1995-2014): Analysis of Current Data in the United States. Biol Psychiatry, 79(7), 613–619. 10.1016/j.biopsych.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon M, Pichini S, Joya J, Pujadas M, Sanchez A, Vall O, Garcia Algar O, Luna A, de la Torre R, Rotolo MC, & Pellegrini M (2012, May 10). Maternal hair testing for the assessment of fetal exposure to drug of abuse during early pregnancy: Comparison with testing in placental and fetal remains. Forensic Sci Int, 218(1-3), 92–96. 10.1016/j.forsciint.2011.10.022 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, & Sklar P (2005, Jun). Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry, 57(11), 1313–1323. 10.1016/j.biopsych.2004.11.024 [DOI] [PubMed] [Google Scholar]
- Fried PA, & Watkinson B (2001, Sep-Oct). Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol, 23(5), 421–430. 10.1016/s0892-0362(01)00160-x [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, & Gray R (1992, Sep-Oct). A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol, 14(5), 299–311. 10.1016/0892-0362(92)90036-a [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, & Gray R (1998, May-Jun). Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol, 20(3), 293–306. 10.1016/s0892-0362(97)00091-3 [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, & Gray R (2003, Jul-Aug). Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol, 25(4), 427–436. 10.1016/s0892-0362(03)00029-1 [DOI] [PubMed] [Google Scholar]
- Gabrhelík R, Mahic M, Lund IO, Bramness J, Selmer R, Skovlund E, Handal M, & Skurtveit S (2021). Cannabis Use during Pregnancy and Risk of Adverse Birth Outcomes: A Longitudinal Cohort Study. Eur Addict Res, 27(2), 131–141. 10.1159/000510821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galera C, Cote SM, Bouvard MP, Pingault JB, Melchior M, Michel G, Boivin M, & Tremblay RE (2011, Dec). Early risk factors for hyperactivity-impulsivity and inattention trajectories from age 17 months to 8 years. Arch Gen Psychiatry, 68(12), 1267–1275. 10.1001/archgenpsychiatry.2011.138 [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Day NL, & Richardson GA (2000, May-Jun). Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol, 22(3), 325–336. 10.1016/s0892-0362(00)00066-0 [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford J, & Day NL (2008, Mar). Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry, 47(3), 254–263. 10.1097/CHI.0b013e318160b3f0 [DOI] [PubMed] [Google Scholar]
- Grant KS, Petroff R, Isoherranen N, Stella N, & Burbacher TM (2018, Feb). Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol Ther, 182, 133–151. 10.1016/j.pharmthera.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack LM, Jiang Y, Delucchi K, Kaiser N, McBurnett K, Hinshaw S, & Pfiffner L (2017, Sep). Parental Cognitive Errors Mediate Parental Psychopathology and Ratings of Child Inattention. Fam Process, 56(3), 716–733. 10.1111/famp.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr., Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey BJ, Barch DM, Harms MP, Watts R, Bjork JM, Garavan HP, Hilmer L, Pung CJ, Sicat CS, Kuperman J, Bartsch H, Xue F, Heitzeg MM, Laird AR, Trinh TT, Gonzalez R, Tapert SF, Riedel MC, Squeglia LM, Hyde LW, Rosenberg MD, Earl EA, Howlett KD, Baker FC, Soules M, Diaz J, de Leon OR, Thompson WK, Neale MC, Herting M, Sowell ER, Alvarez RP, Hawes SW, Sanchez M, Bodurka J, Breslin FJ, Morris AS, Paulus MP, Simmons WK, Polimeni JR, van der Kouwe A, Nencka AS, Gray KM, Pierpaoli C, Matochik JA, Noronha A, Aklin WM, Conway K, Glantz M, Hoffman E, Little R, Lopez M, Pariyadath V, Weiss SR, Wolff-Hughes DL, DelCarmen-Wiggins R, Feldstein Ewing SW, Miranda-Dominguez O, Nagel BJ, Perrone AJ, Sturgeon DT, Goldstone A, Pfefferbaum A, Pohl KM, Prouty D, Uban K, Bookheimer SY, Dapretto M, Galvan A, Bagot K, Giedd J, Infante MA, Jacobus J, Patrick K, Shilling PD, Desikan R, Li Y, Sugrue L, Banich MT, Friedman N, Hewitt JK, Hopfer C, Sakai J, Tanabe J, Cottler LB, Nixon SJ, Chang L, Cloak C, Ernst T, Reeves G, Kennedy DN, Heeringa S, Peltier S, Schulenberg J, Sripada C, Zucker RA, Iacono WG, Luciana M, Calabro FJ, Clark DB, Lewis DA, Luna B, Schirda C, Brima T, Foxe JJ, Freedman EG, Mruzek DW, Mason MJ, Huber R, McGlade E, Prescot A, Renshaw PF, Yurgelun-Todd DA, Allgaier NA, Dumas JA, Ivanova M, Potter A, Florsheim P, Larson C, Lisdahl K, Charness ME, Fuemmeler B, Hettema JM, Maes HH, Steinberg J, Anokhin AP, Glaser P, Heath AC, Madden PA, Baskin-Sommers A, Constable RT, Grant SJ, Dowling GJ, Brown SA, Jernigan TL, & Dale AM (2019, Nov 15). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage, 202, 116091. 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Chiodo LM, Sokol RJ, Janisse J, Ager JW, Greenwald MK, & Delaney-Black V (2010, Nov-Dec). A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol, 44(7-8), 583–594. 10.1016/j.alcohol.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir S, Bouma A, Sergeant JA, & Scherder EJ (2006, Aug). Relationships between neuropsychological measures of executive function and behavioral measures of ADHD symptoms and comorbid behavior. Arch Clin Neuropsychol, 21(5), 383–394. 10.1016/j.acn.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Jutras-Aswad D, DiNieri JA, Harkany T, & Hurd YL (2009, Oct). Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci, 259(7), 395–412. 10.1007/s00406-009-0027-z [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, & Westerberg H (2002, Jan 1). Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci, 14(1), 1–10. 10.1162/089892902317205276 [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, & Hommer D (2000, Jul). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage, 12(1), 20–27. 10.1006/nimg.2000.0593 [DOI] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Tong VT, Creanga AA, & Callaghan WM (2015, Aug). Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol, 213(2), 201.e201–201.e210. 10.1016/j.ajog.2015.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech SL, Richardson GA, Goldschmidt L, & Day NL (1999, Mar-Apr). Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol, 21(2), 109–118. 10.1016/s0892-0362(98)00042-7 [DOI] [PubMed] [Google Scholar]
- Logan GD (1994, Oct). Spatial attention and the apprehension of spatial relations. J Exp Psychol Hum Percept Perform, 20(5), 1015–1036. 10.1037//0096-1523.20.5.1015 [DOI] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, & Banich MT (2018, 08). Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci, 32, 67–79. 10.1016/j.dcn.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnan AW, Keim SA, Yeates KO, Boone KM, Sheppard KW, & Klebanoff MA (2021, Nov-Dec). Behavioral and Cognitive Differences in Early Childhood related to Prenatal Marijuana Exposure. J Appl Dev Psychol, 77. 10.1016/j.appdev.2021.101348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, & Sonuga-Barke EJ (2005, Jun 1). Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry, 57(11), 1224–1230. 10.1016/j.biopsych.2004.08.025 [DOI] [PubMed] [Google Scholar]
- O'Halloran L, Cao Z, Ruddy K, Jollans L, Albaugh MD, Aleni A, Potter AS, Vahey N, Banaschewski T, Hohmann S, Bokde ALW, Bromberg U, Buchel C, Quinlan EB, Desrivieres S, Flor H, Frouin V, Gowland P, Heinz A, Ittermann B, Nees F, Orfanos DP, Paus T, Smolka MN, Walter H, Schumann G, Garavan H, Kelly C, & Whelan R (2018, Apr 1). Neural circuitry underlying sustained attention in healthy adolescents and in ADHD symptomatology. Neuroimage, 169, 395–406. 10.1016/j.neuroimage.2017.12.030 [DOI] [PubMed] [Google Scholar]
- Paul SE, Hatoum AS, Fine JD, Johnson EC, Hansen I, Karcher NR, Moreau AL, Bondy E, Qu Y, Carter EB, Rogers CE, Agrawal A, Barch DM, & Bogdan R (2020, Sep 23). Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results From the ABCD Study. JAMA Psychiatry. 10.1001/jamapsychiatry.2020.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinky PD, Bloemer J, Smith WD, Moore T, Hong H, Suppiramaniam V, & Reed MN (2019, 05). Prenatal cannabinoid exposure and altered neurotransmission. Neuropharmacology, 149, 181–194. 10.1016/j.neuropharm.2019.02.018 [DOI] [PubMed] [Google Scholar]
- Postema MC, Hoogman M, Ambrosino S, Asherson P, Banaschewski T, Bandeira CE, Baranov A, Bau CHD, Baumeister S, Baur-Streubel R, Bellgrove MA, Biederman J, Bralten J, Brandeis D, Brem S, Buitelaar JK, Busatto GF, Castellanos FX, Cercignani M, Chaim-Avancini TM, Chantiluke KC, Christakou A, Coghill D, Conzelmann A, Cubillo AI, Cupertino RB, de Zeeuw P, Doyle AE, Durston S, Earl EA, Epstein JN, Ethofer T, Fair DA, Fallgatter AJ, Faraone SV, Frodl T, Gabel MC, Gogberashvili T, Grevet EH, Haavik J, Harrison NA, Hartman CA, Heslenfeld DJ, Hoekstra PJ, Hohmann S, Hovik MF, Jernigan TL, Kardatzki B, Karkashadze G, Kelly C, Kohls G, Konrad K, Kuntsi J, Lazaro L, Lera-Miguel S, Lesch KP, Louza MR, Lundervold AJ, Malpas CB, Mattos P, McCarthy H, Namazova-Baranova L, Nicolau R, Nigg JT, Novotny SE, Oberwelland Weiss E, O'Gorman Tuura RL, Oosterlaan J, Oranje B, Paloyelis Y, Pauli P, Picon FA, Plessen KJ, Ramos-Quiroga JA, Reif A, Reneman L, Rosa PGP, Rubia K, Schrantee A, Schweren LJS, Seitz J, Shaw P, Silk TJ, Skokauskas N, Soliva Vila JC, Stevens MC, Sudre G, Tamm L, Tovar-Moll F, van Erp TGM, Vance A, Vilarroya O, Vives-Gilabert Y, von Polier GG, Walitza S, Yoncheva YN, Zanetti MV, Ziegler GC, Glahn DC, Jahanshad N, Medland SE, Group, E. A. W., Thompson PM, Fisher SE, Franke B, & Francks C (2021, Mar 22). Analysis of structural brain asymmetries in attention-deficit/hyperactivity disorder in 39 datasets. J Child Psychol Psychiatry. 10.1111/jcpp.13396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA, & Bucci DJ (2006, Dec 15). Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav Brain Res, 175(2), 201–211. 10.1016/j.bbr.2006.09.015 [DOI] [PubMed] [Google Scholar]
- Rosenberg MD, Martinez SA, Rapuano KM, Conley MI, Cohen AO, Cornejo MD, Hagler DJ Jr., Meredith WJ, Anderson KM, Wager TD, Feczko E, Earl E, Fair DA, Barch DM, Watts R, & Casey BJ (2020, Jun 24). Behavioral and Neural Signatures of Working Memory in Childhood. J Neurosci, 40(26), 5090–5104. 10.1523/jneurosci.2841-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M (2009, Oct). Cannabis use in pregnancy and early life and its consequences: animal models. Eur Arch Psychiatry Clin Neurosci, 259(7), 383–393. 10.1007/s00406-009-0026-0 [DOI] [PubMed] [Google Scholar]
- Shmulewitz D, & Hasin DS (2019, Jul). Risk factors for alcohol use among pregnant women, ages 15-44, in the United States, 2002 to 2017. Prev Med, 124, 75–83. 10.1016/j.ypmed.2019.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Mioduszewski O, Hatchard T, Byron-Alhassan A, Fall C, & Fried PA (2016, Nov - Dec). Prenatal marijuana exposure impacts executive functioning into young adulthood: An fMRI study. Neurotoxicol Teratol, 58, 53–59. 10.1016/j.ntt.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Stadler DD, Musser ED, Holton KF, Shannon J, & Nigg JT (2016, Feb). Recalled Initiation and Duration of Maternal Breastfeeding Among Children with and Without ADHD in a Well Characterized Case-Control Sample. J Abnorm Child Psychol, 44(2), 347–355. 10.1007/s10802-015-9987-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, McCallum M, Kehoe T, Salisbury AL, & Huestis MA (2018, Nov-Dec). Prenatal tobacco and marijuana co-use: Impact on newborn neurobehavior. Neurotoxicol Teratol, 70, 28–39. 10.1016/j.ntt.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Han B, Compton WM, & McCance-Katz EF (2019, Jul 9). Self-reported Medical and Nonmedical Cannabis Use Among Pregnant Women in the United States. JAMA, 322(2), 167–169. 10.1001/jama.2019.7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID, & Gizer IR (2006, Aug). The genetics of attention deficit hyperactivity disorder. Clin Psychol Rev, 26(4), 396–432. 10.1016/j.cpr.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Walker MJ, Al-Sahab B, Islam F, & Tamim H (2011, Jul 12). The epidemiology of alcohol utilization during pregnancy: an analysis of the Canadian Maternity Experiences Survey (MES). BMC Pregnancy Childbirth, 11, 52. 10.1186/1471-2393-11-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005, Jun 1). Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry, 57(11), 1336–1346. 10.1016/j.biopsych.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, & Heitzeg MM (2012, Feb 15). Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J Neurosci, 32(7), 2544–2551. 10.1523/JNEUROSCI.1390-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Sarovar V, Alexeeff SE, Adams SR, Tucker LY, Conway A, Ansley D, Goler N, Armstrong MA, & Weisner C (2020, Sep 1). Trends and correlates of self-reported alcohol and nicotine use among women before and during pregnancy, 2009-2017. Drug Alcohol Depend, 214, 108168. 10.1016/j.drugalcdep.2020.108168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Peng P, Eickhoff SB, Lin X, Zhang D, & Wang Y (2021, Nov). Neural substrates of the executive function construct, age-related changes, and task materials in adolescents and adults: ALE meta-analyses of 408 fMRI studies. Dev Sci, 24(6), e13111. 10.1111/desc.13111 [DOI] [PubMed] [Google Scholar]