Abstract
Para-selective C–H functionalization of free phenols by metal carbenoids is rather challenging due to the generally more favorable competing O–H insertion. Herein, with the use of the combination of Rh(II) and a Xantphos ligand as the catalyst, a novel multicomponent reaction of free phenols, diazoesters, and allylic carbonates was successfully developed, affording a wide variety of phenol derivatives, bearing an all-carbon quaternary center and a synthetically useful allylic unit. This reaction is likely to occur through a tandem process of carbene-induced para-selective C–H functionalization, followed by Rh(II)/Xantphos-catalyzed allylation. The distinctive reactivity of para-selective C–H rather than O–H insertion for the carbenoid intermediate, combined with features of excellent functional group compatibility, high atom and step economy, and ease in further diversification of the products, might render this protocol highly attractive in facile functionalization of unprotected phenols.
Short abstract
Overcoming O−H insertion to para C−H functionalization of phenols with readily accessible diazo compounds and allylic compounds is successfully achieved through a novel Rh(II)/Xantphos catalysis.
I. Introduction
Due to the importance of phenol motifs in natural products, pharmaceuticals, and functional materials, transformations of inexpensive and abundant phenols into their structurally more complex homologues have attracted much attention for a long time.1,2 In particular, phenol derivatives that contain a diaryl all-carbon quaternary center α to the para-position have shown unique biological activities (Scheme 1a).3−6 Therefore, the development of synthetic routes for para-selective C(sp2)–H functionalization of phenols is highly attractive.7−12 Although great advances have been made in the area of metal carbenoid induced C–H functionalization of (hetero)arenes,13−26 the direct C(sp2)–H functionalization of free phenols in a chemo- and regioselective manner with diazo compounds is rather challenging and surprisingly rare, probably because the competitive O–H bond insertion is often found more favorable than C(sp2)–H functionalization for carbenoids generated from a range of typical transition metals (e.g., Rh, Cu, Pd, Ag; Scheme 1b-i).27−41 Very recently, remarkable advances have been achieved in highly para-selective C(sp2)–H functionalization of free phenols with diazoesters, as reported independently by Zhang42−44 and Shi,45 using the specific carbophilicity of gold catalysts, wherein the chemoselectivity is largely dependent on the nature of the supporting ligands (Scheme 1b-ii).
Scheme 1. Catalytic Transformations of Free Phenols with Metal Carbene Derived from Diazo Compounds.
On the other hand, dirhodium(II)-catalyzed multicomponent reactions (MCRs) involving metal carbenes offer an elegant and powerful tool to rapidly generate structural complexity and diversity by modulations of each component in an atom-economical and convergent fashion.46,47 Remarkably, Hu and co-workers have developed dirhodium(II)-catalyzed MCRs of diazoesters, electron-rich arenes, and imines, wherein the metal carbene induced zwitterionic intermediates were trapped by electrophilic imines to enable direct C(sp2)–H functionalization of electron-rich aromatic rings such as indole and N,N-disubstituted anilines.48−51 Nevertheless, implementing such a methodology with free phenols might be rather difficult, as the competitive O–H bond insertion is often the preferential process in reactions of a metal carbenoid with a phenol.27−41,52 In addition, the introduction of allylic substrate as the electrophile in Rh(II)-catalyzed MCRs is rather challenging since activation of the allylic reactant via oxidative addition would be unfavorable for Rh(II).53−62
As a continuation of our interest in multicomponent reactions under a unique dirhodium(II)/diphosphine catalysis,63−70 we envisioned a novel multicomponent reaction of unprotected phenols, diazo compounds, and allylic compounds, which may proceed via a sequence of para-selective C(sp2)–H functionalization followed by an allylic alkylation (Scheme 1c). While such a strategy can provide a straightforward route to phenol derivatives bearing diaryl-substituted all-carbon quaternary centers, some uncertainties might severely impede the implementation of this strategy; e.g., (1) the catalytic reactivity of the dirhodium(II)/ligand for the proposed individual steps of the tandem process is unclear; (2) C(sp2)–H functionalization reaction of free phenols with a Rh(II) carbenoid still remains an unknown challenge; (3) the conceivable competitive reactions of O–H insertion, cyclopropanation71 of a metal carbene and C=C bond, and direct C/O-allylation of phenol with allylic compounds72,73 can cause considerable difficulties in chemoselectivity or site-selectivity control.
Herein, we disclose a dirhodium(II)/Xantphos catalyzed multicomponent reaction of free phenols, diazoesters, and allylic compounds, affording various phenol derivatives bearing an allylic moiety and an all-carbon quaternary center (Scheme 1c). Mechanistic studies suggested that the reaction proceeds via alkali-promoted para-selective C(sp2)–H functionalization of phenols to Rh(II) carbenoid, followed by allylic alkylation of the resulting intermediate. Notably, the allyl aryl ether products can undergo facile and selective O-deallylation under mild conditions, furnishing the corresponding free phenol derivatives bearing all-carbon quaternary centers, together with an allylic unit as a valuable handle for further synthetic manipulation to diverse complex molecular structures.
II. Results and Discussion
1. Reaction Development
Our studies began with the reaction of phenol (1a), methyl phenyldiazoacetate (2a), and allyl ethyl carbonate (3) using Rh2(Oct)4 as the catalyst and Cs2CO3 as a base in CH3CN at 60 °C. All the results are summarized in Table 1. No multicomponent coupling product 6aa was detected in the absence of a ligand, and only the direct phenol O-allylation product 4aa and the para C–H functionalization product 5aa were found in 74 and 31% yields, respectively (entry 1). On the other hand, use of BINAP as the ligand led to the formation of the target product 6aa, albeit only in a rather low yield (3%), along with substantial amounts of 4aa and 5aa (entry 2). Encouraged by this result, several phosphines or NHC ligands were screened in the reaction (entries 3–6). Pleasingly, Xantphos showed better performance, furnishing the product 6aa in a promising albeit still low yield of 18% (entry 3), thus attesting for the feasibility of the proposed protocol. However, further attempts to use a catalytic amount of dppb, PPh3, or iPrNHC as the ligand failed to improve the yield of 6aa (entries 4–6). Gratifyingly, Rh2(OPiv)4 was then identified as a more effective rhodium precursor for this reaction, delivering the target product 6aa in 85% yield (entry 8). Other metal salts, such as CuCl and [Pd(PhCN)2Cl2], which are commonly used in carbene transfer involving phenols and diazo compounds, were also tested in combination with Xantphos as the catalyst in the reaction, resulting the formation of 4aa as the detectable products in these cases (entries 9 and 10). Notably, with the change from the Rh(II) carboxylate to the Rh(I) salt [Rh(COD)2]BF4 under otherwise identical conditions, the reaction gave exclusively 4aa in 99% yield (entry 11). In addition, no 6aa was observed in the absence of a dirhodium catalyst (entry 12), demonstrating the essential role of a Rh(II)2 salt in promoting the reaction. All these results clearly indicated that this dirhodium/Xantphos catalysis displayed a unique catalytic reactivity and selectivity that is distinct from other metal catalysts in this reaction.
Table 1. Optimization of the Reaction Conditionsa.
| yield (%) |
|||||
|---|---|---|---|---|---|
| entry | [M] cat. | ligand (mol %) | 4aab | 5aab | 6aab |
| 1 | Rh2(Oct)4 | – | 74 | 31 | 0 |
| 2 | Rh2(Oct)4 | BINAP (1.5) | 75 | 26 | 3 |
| 3 | Rh2(Oct)4 | Xantphos (1.5) | 76 | 7 | 18 |
| 4 | Rh2(Oct)4 | dppb (1.5) | 86 | 0 | 5 |
| 5 | Rh2(Oct)4 | PPh3 (3.0) | 86 | 8 | 0 |
| 6 | Rh2(Oct)4 | iPr-NHCc (3.0) | 53 | 4 | 15 |
| 7 | Rh2(TPA)4 | Xantphos (1.5) | 91 | 0 | 12 |
| 8 | Rh2(OPiv)4 | Xantphos(1.5) | 42 | 0 | 85 |
| 9 | CuCl | Xantphos (1.5) | 27 | 0 | 0 |
| 10 | [Pd(PhCN)2Cl2] | Xantphos (1.5) | 99 | 0 | 0 |
| 11 | Rh(COD)2BF4 | Xantphos (1.5) | 99 | 0 | 0 |
| 12 | – | Xantphos (1.5) | 0 | 0 | 0 |
1a (0.375 mmol, 1.5 equiv), 2a (0.25 mmol, 1.0 equiv), 3 (0.75 mmol, 3.0 equiv), MeCN (2.0 mL), [M] catalyst (1.0 mol %), ligand (1.5 mol %), Cs2CO3 (3.5 equiv), 60 °C, 6.0 h.
GC yields. Yields for 4aa were calculated based on 1a as the limiting substrate. Yields for 5aa and 6aa were calculated based on 2a as the limiting substrate.
iPr-NHC = 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride.
2. Scope and Synthetic Applications
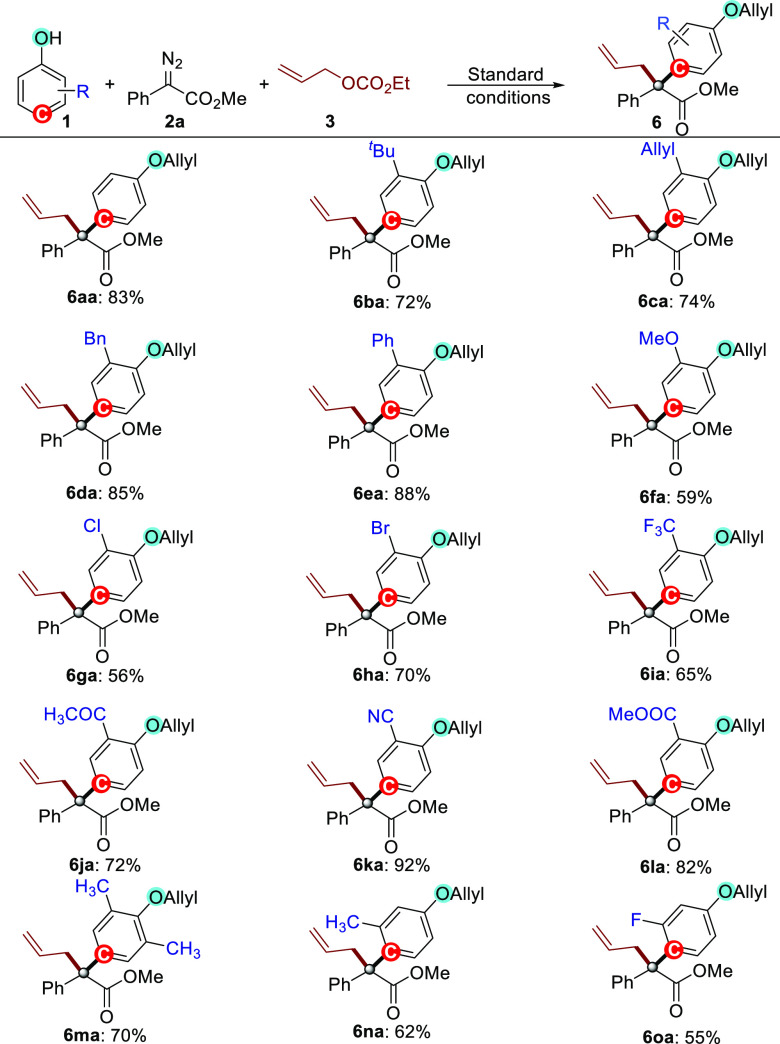
With the optimal conditions in hand, the phenol coupling partners 1 for this protocol were evaluated first in substrate scope studies. As depicted in Scheme 2, various commercially available free phenols smoothly participated in these MCRs with 2a and 3, furnishing site-specific and chemo-specific para C–H functionalization/allylation products 6aa–6oa in moderate to high yields (55–92%). Phenols with either electron-donating (1b–1f) or electron-withdrawing substituent(s) (1g–1l) on the ortho-position of the phenyl ring were effective coupling partners, affording the corresponding products 6ba–6la in 56–92% yields. It is noteworthy that the reaction of the phenol (1c) bearing an ortho allylic group still gave the corresponding product 6ca in 74% yield without formation of any cyclopropanation product, indicating that the para C–H bond functionalization is more favorable than the potential cyclopropanation of a C=C bond in this dirhodium catalysis. Importantly, halogen substituents such as chloride (1g) and bromide (1h) on the phenol substrates are well tolerated in the reactions, delivering the corresponding products that can be used as good platform molecules for downstream transformations by cross coupling. In addition, other functional groups, including ketone (1j), cyano (1k), and ester (1l), could be readily introduced in the reaction, offering a useful handle for further potential synthetic manipulations. Interestingly, 2,6-dimethyl substituted phenol 1m was also found as a competent substrate in the reaction, providing the multisubstituted product 6ma in 70% yield. Notably, the reactions of m-Me substituted phenol 1n or m-F substituted 1o containing sterically hindered para C–H bonds still gave the corresponding para C–H functionalization products 6na and 6oa, respectively, in 62 and 55% yields, suggesting that the para C–H functionalization is a more preferential process compared with O–H insertion for this catalyst system. With the use of p-methylphenol 1p as the substrate under standard conditions, the reaction failed to give the desired ortho C–H functionalization product 6pa (for details, see the Supporting Information).
Scheme 2. Scope of Phenols 1.
1 (0.375 mmol, 1.5 equiv), 2a (0.25 mmol, 1.0 equiv), 3 (0.75 mmol, 3.0 equiv), MeCN (2.0 mL), Rh2(OPiv)4 (1.0 mol %), Xantphos (1.5 mol %), Cs2CO3 (3.5 equiv), 60 °C, 6.0 h. Isolated yields.
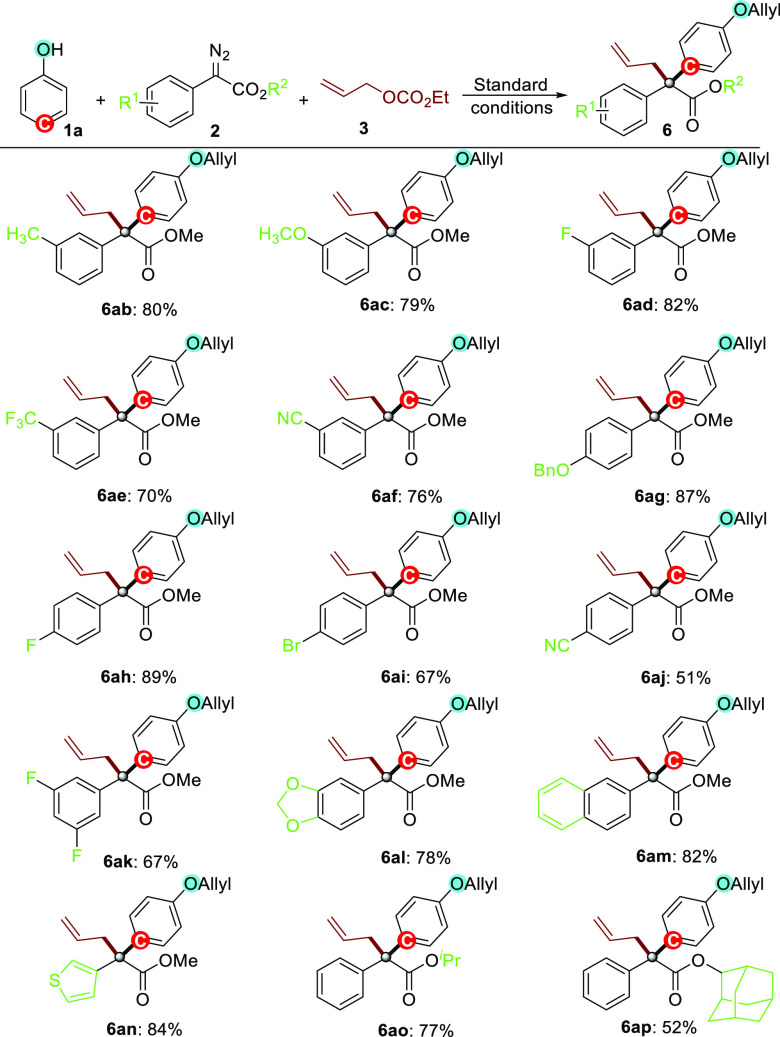
Subsequently, the scope of diazo esters 2 was investigated in reactions with 1a and 3 under otherwise identical conditions. As shown in Scheme 3, various diazo esters with different groups on the meta-position of the benzene ring, including −Me, −OMe, −F, −CF3, and −CN, all performed well in the reactions, delivering the corresponding phenol derivatives 6ab–6af in moderate to good yields (70–82%). When diazo esters possessing either electron-donating (−OBn) or electron-withdrawing (−F, −Br, −CN) substituents on the para-position of the phenyl moiety were employed, the corresponding products 6ag–6aj were isolated in 51–89% yields. Additionally, 3,5-difluorine substituted phenyl and [1,3]dioxonyl diazo substrates 2k and 2l also reacted smoothly as competent coupling partners in this reaction, giving the corresponding multisubstituted products 6ak and 6al in good yields (67 and 78%, respectively). Interestingly, the diazo substrates containing heteroaryl rings, such as 2-naphthyl (2m) and 3-thienyl motif (2n), were also suitable substrates for this reaction, affording the corresponding products (6am and 6an) in good yields (82 and 84%). Moreover, with changing the methyl ester of the diazo reactant to isopropyl (2o) or adamantan-2-yl ester (2p), the reactions also worked smoothly, leading to the corresponding products (6ao and 6ap) in 77 and 52% yields, respectively. It is worth mentioning that all these reactions afforded the corresponding phenol derivatives 6 bearing all-carbon quaternary centers without detection of any byproducts via O–H insertion or cyclopropanation. Subsequently, we turned our attention to the development of the asymmetric version of this transformation. However, preliminary attempts showed that no appreciable stereoselectivity was achieved currently either by employing chiral disphosphine ligands with Rh2(OPiv)4 or by using chiral Rh(II) precursors with Xantphos (for details, see the Supporting Information).
Scheme 3. Scope of Diazo Esters 2.
1a (0.375 mmol, 1.5 equiv), 2 (0.25 mmol, 1.0 equiv), 3 (0.75 mmol, 3.0 equiv), MeCN (2.0 mL), Rh2(OPiv)4 (1.0 mol %), Xantphos (1.5 mol %), Cs2CO3 (3.5 equiv), 60 °C, 6.0 h. Isolated yields.
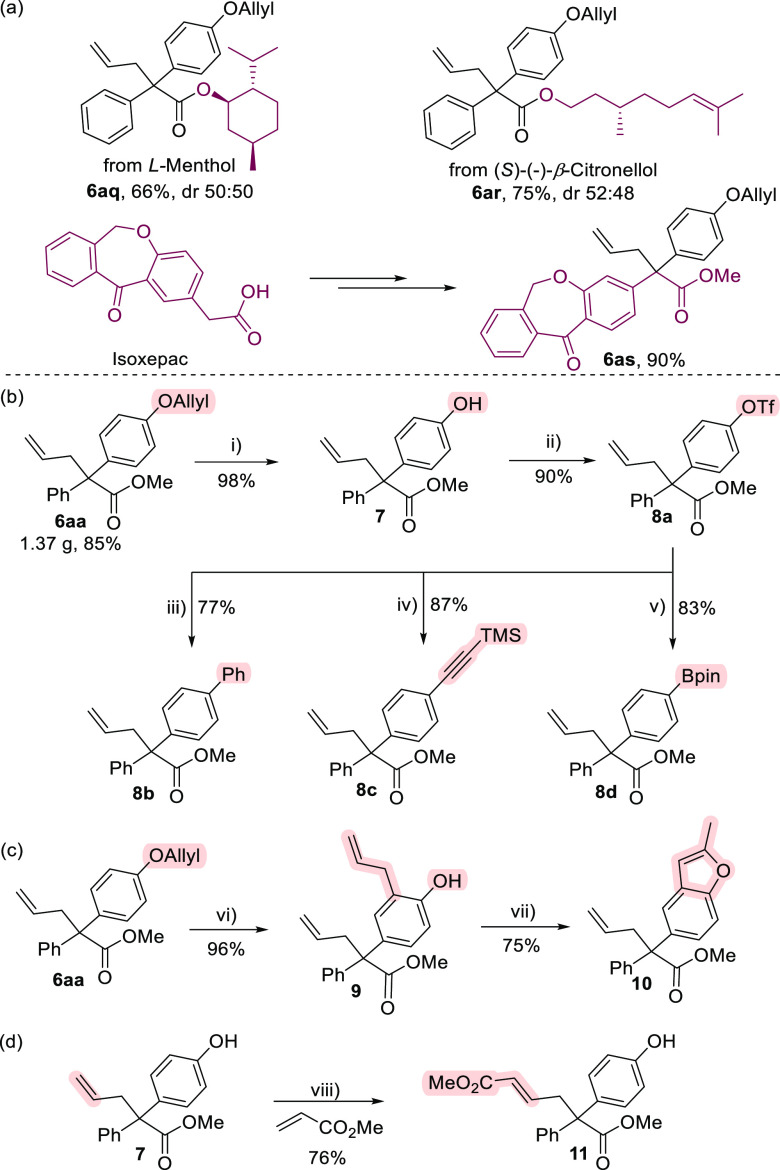
To show the synthetic utility of the methodology, the transformations using drug molecules, natural products, and their derivatives as the reaction partners were conducted. As depicted in Scheme 4a, the α-diazo esters that were easily derived from l-menthol and (S)-(−)-β-citronellol worked well in the reactions with 1a and 3, giving 6aq and 6ar in 66 and 75% yields, respectively. Additionally, α-diazo ester prepared from isoxepac was also found to react smoothly in MCRs with 1a and 3, successfully incorporating synthetically useful allylic group and phenol unit into the α-site of the acid derivative (6as, 90%). Moreover, a gram-scale (5.0 mmol) reaction of 1a, 2a, and 3 proceeded smoothly under standard conditions, affording the product 6aa in 85% yield (1.37 g), highlighting the practicality of the approach (Scheme 4b). Notably, Pd-catalyzed selective O-deallylation of 6aa can be readily achieved under mild conditions, providing the corresponding product 7 bearing a free hydroxyl group in very high yield. Importantly, the hydroxyl group can be used as a versatile synthetic handle for further transformation. For example, triflate 8a was easily prepared in 90% yield, which can then serve as versatile synthons in Pd-catalyzed coupling reactions, giving products bearing diphenyl (8b, 77%), synthetically important alkynyl (8c, 87%), or boron groups (8d, 83%). Furthermore, treatment of compound 6aa with Et2AlCl offered product 9 efficiently in 96% yield through ortho Claisen rearrangement, which underwent a Pd(II)-catalyzed intramolecular oxidative cyclization of alkene with hydroxyl group to furnish the product 10 with a 2-substituted benzofuran unit (75% yield, Scheme 4c), which is a core structure of some bioactive molecules.74 Finally, the cross-metathesis reaction of compound 7 with methyl acrylate was realized in the presence of second-generation Grubbs catalyst, giving the corresponding product 11 in 76% yield (Scheme 4d).
Scheme 4. Late-Stage Functionalization of Complex Architectures and Synthetic Transformation.
(i) Pd(PPh3)4, K2CO3, MeOH, rt. (ii) Tf2O, DMAP, Et3N, DCM, 0 °C to rt. (iii) PhB(OH)2, Pd(PPh3)4, K3PO4, 1,4-dioxane, 110 °C. (iv) Ethynyltrimethylsilane, Pd(PPh3)2Cl2, CuI, Et3N, DMF, 90 °C. (v) B2Pin2, Pd(dppf)Cl2, AcOK, 1,4-dioxane, 120 °C. (vi) Et2AlCl, hexane, 80 °C. (vii) PdCl2, Cu(OAc)2, DMF, 100 °C. (viii) Second-generation Grubbs catalyst (5 mol %), DCM (0.2 M), 40 °C.
3. Mechanistic Studies
To gain insight into the mechanism for the MCR, several experiments were conducted. First, the kinetic profiles for the reaction of 1a, 2a, and 3 under standard conditions were obtained through GC analysis of aliquots taken at specified periods. As depicted in Scheme 5a, the yield of compound 4aa showed a rapid increase in the first 5 min, and after that time remained almost constant during the whole reaction course. On the other hand, in the initial period (ca. 5 min) a rapid accumulation of 5aa was also observed, followed by a gradual decay in the rest of the reaction. This was accompanied by a slower but steady growth in the amount of the multicomponent coupling product 6aa, suggesting a tandem process, wherein 5aa might act as a nucleophile in the further reaction with 3. To confirm this hypothesis, the reaction of isolated compound 5aa with allylic partner 3 was performed under standard conditions. Indeed, the target product 6aa was isolated in 99% yield in this case (Scheme 5b). In contrast, almost no 6aa was detected in the absence of Rh2(OPiv)4, Xantphos, or Cs2CO3 (99% versus 0–7%). Next, when the reaction of 1a with 2a and 3 was conducted in a stepwise addition sequence of the reagents, 6aa was generated in 88% yield (Scheme 5c), further suggesting that this MCR reaction is a tandem process. Additionally, no formation of 6aa and no conversion of 4aa were observed in the reaction of 4aa and 2a with 3 under standard conditions (Scheme 5d). These results suggested that compound 5aa rather than 4aa should be involved as a plausible intermediate. It worth mentioning that neither the C–H functionalization nor cyclopropanation reaction took place in this multicomponent system (Scheme 5d), and the same was true for the two-component reaction of 4aa and 2a (for details, see the Supporting Information). Moreover, when compound 5aa′ prepared from phenol 1a with 2a was subjected to react with allylic compound 3, the target product 6aa was afforded in 90% yield (Scheme 5e). This result, together with the reactions in Scheme 5d, imply that C–H functionalization proceeds prior to O-allylation in the reaction steps for the formation of 5aa. On the basis of previous reports63−70 and these experimental results, we propose that the reaction is most likely to proceed through a tandem process of Rh(II) carbenoid induced C–H functionalization and [Rh2]/Xantphos-catalyzed allylic alkylation, which is distinctive from the well-known [Rh(II)2]-catalyzed MCRs, wherein an active ylide/zwitterionic intermediate generated in situ was directly trapped by an electrophile.46,47 The necessity of the Xantphos ligand in this catalysis may be owing to the coordination modification of the active center of dirhodium, leading to a novel catalytic activity for allylic alkylation.63−70 Nonetheless, the possibility of the formation of monorhodium species cannot be completely ruled out at the present stage. To gain some further insights into the allylic substitution process, compound 5aa was treated with deuterated allyl methyl carbonate under standard conditions, and two products, 6a-D and 6a-D′, were obtained in 50:50 ratio (Scheme 5f). The result showed that O-allyl remained unchanged and C-allyl of 6aa-D (6aa-D′) was completely from deuterated allyl substrate. In other words, the migration of the O-allyl to C-allyl might be unlikely involved in this reaction (for details, see the Supporting Information). Moreover, when allyl phenyl ether 4aa was introduced to the mixture of 2-benzylphenol 1d with 2a under standard conditions, no multicomponent coupling product 6da was detected by GC–MS (Scheme 5g), further indicating that the allyl phenyl ether could not serve as an allyl source in this multicomponent transformation.
Scheme 5. Reaction Profiles of the MCR (a) and Controlled Experiments (b–i).

To understand the para C–H functionalization selectivity of phenol with diazo compound in the current dirhodium catalysis, several two component reactions of 1a with 2a were conducted. As depicted in Scheme 5h, compound 5aa′ generated by the para C–H functionalization was delivered as the main product (76 and 78% isolated yields, respectively), and no O–H insertion product 12 was detectable under standard conditions and conditions without Xantphos ligand. In contrast, without Cs2CO3, only a trace amount of 5aa′ was generated under standard conditions, indicating the essential role of Cs2CO3. Accordingly, it was speculated that a facile formation of phenate intermediate from the free hydroxyl group and Cs2CO3 might promote the para-selective C–H functionalization in this dirhodium catalysis. Published data show that, due to the electropositive character of the Cs+ and the electron delocalization from the negatively charged oxygen to the aromatic ring, the electron density of the C4-carbon atom of phenolate is obviously increased as compared with 1a bearing the neutral OH group, thus enhancing the nucleophilic ability of the aromatic ring.75−79 To further evaluate this hypothesis, several commercially available alkali metal carbonates were tested as additives in the reaction of 1a and 2a (Scheme 5i). It was found that product 5aa′ was formed in 5–94% yields in the presence of Cs2CO3, K2CO3, or Na2CO3. No 5aa′ was detected when Li2CO3 was used or in the absence of any base. It is worth mentioning that the yields of 5aa′ by using different alkali metal carbonates in the reactions are consistent with their electron density ranking from 13C NMR of the phenolic models in the literature,75,76 which can be attributed to the different Coulombic interactions between the alkali metal cation and phenolate anion. These results demonstrated that the base additive plays a critical role in improving the reactivity and selectivity for para C–H functionalization of phenol with diazo compound in this dirhodium catalysis.
Based on these results, a possible reaction pathway is proposed in Scheme 6. In the presence of the base Cs2CO3, phenolate salt I with different resonance forms is generated first, which then undergoes [Rh]2-catalyzed para-selective C–H functionalization with 2 to afford the intermediate II due to the higher electron density of the C4-carbon atom of the phenolate. Subsequently, O-allylation of II with allylic substrate 3 takes place under [Rh]2 or [Rh]2/Xantphos catalysis, delivering the intermediate 5. Finally, product 6 is produced by [Rh]2/Xantphos catalyzed allylic alkylation of 5 with allylic substrate 3.
Scheme 6. Possible Reaction Pathway.
III. Summary and Conclusions
In conclusion, an unprecedented multicomponent reaction of free phenols, diazoesters, and allylic compounds has been developed, providing versatile phenol derivatives bearing acyclic all-carbon quaternary centers with synthetic useful allyl units. Mechanistic studies suggest that the reaction is likely to proceed via a tandem process of carbene-induced C–H functionalization and sequential Rh(II)/Xantphos-catalyzed allylation. Moreover, it is found that the base additives play an essential role in the para-selective C–H functionalization of free phenol with diazo compound in this dirhodium catalysis, which would broaden the application of dirhodium complex in carbene transfer reactions. The salient features of this protocol, including easily available starting materials, mild reaction conditions, good substrate scope, and versatile synthetic transformations of the products, would render the protocol highly appealing for late-stage modification of pharmaceuticals.
Acknowledgments
This work was generously supported by the National Natural Science Foundation of China (21821002).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.2c00004.
Experimental procedures, complete characterization data, and copies of 1H, 13C, and 19F NMR spectra (PDF)
Author Contributions
∥ Y.Y. and B.L. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Rappoport Z.The Chemistry of Phenols; Wiley-VCH: Weinheim, 2003. [Google Scholar]
- Quideau S.; Deffieux D.; Douat-Casassus C.; Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- Snyder S. A.; Thomas S. B.; Mayer A. C.; Breazzano S. P. Total syntheses of hopeanol and hopeahainol A empowered by a chiral Bronsted acid induced pinacol rearrangement. Angew. Chem. Int. Ed. 2012, 51, 4080–4084. 10.1002/anie.201107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunden H.; Ma J. N.; Hansen L. K.; Gustavsson A. L.; Burstein E. S.; Olsson R. Design of a highly selective and potent class of non-planar estrogen receptor β agonists. ChemMedChem. 2013, 8, 1283–1294. 10.1002/cmdc.201300175. [DOI] [PubMed] [Google Scholar]
- Wu H.; Wang Q.; Zhu J. Catalytic Enantioselective Pinacol and Meinwald Rearrangements for the Construction of Quaternary Stereocenters. J. Am. Chem. Soc. 2019, 141, 11372–11377. 10.1021/jacs.9b04551. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang W. Y.; Xie J. H.; Yu Z. L.; Tan J. H.; Zheng C.; Hou X. L.; You S. L. Enantioselective Desymmetrization of Bisphenol Derivatives via Ir-Catalyzed Allylic Dearomatization. J. Am. Chem. Soc. 2020, 142, 19354–19359. 10.1021/jacs.0c09638. [DOI] [PubMed] [Google Scholar]
- Xu X.; Luo J. Transition Metal-Catalyzed Directing-Group-Assisted C-H Activation of Phenols. ChemSusChem 2019, 12, 4601–4616. 10.1002/cssc.201901951. [DOI] [PubMed] [Google Scholar]
- Al Mamari H. H.; Štefane B.; Žugelj H. B. Metal-catalyzed C-H bond functionalization of phenol derivatives. Tetrahedron 2020, 76, 130925. 10.1016/j.tet.2020.130925. [DOI] [Google Scholar]
- Huang Z.; Lumb J.-P. Phenol-Directed C–H Functionalization. ACS Catal. 2019, 9, 521–555. 10.1021/acscatal.8b04098. [DOI] [Google Scholar]
- For template-assisted para C–H functionalization of phenols, see:Patra T.; Bag S.; Kancherla R.; Mondal A.; Dey A.; Pimparkar S.; Agasti S.; Modak A.; Maiti D. Palladium-Catalyzed Directed para C-H Functionalization of Phenols. Angew. Chem. Int. Ed. 2016, 55, 7751–7755. 10.1002/anie.201601999. [DOI] [PubMed] [Google Scholar]
- Ciana C. L.; Phipps R. J.; Brandt J. R.; Meyer F. M.; Gaunt M. J. A highly para-selective copper(II)-catalyzed direct arylation of aniline and phenol derivatives. Angew. Chem. Int. Ed. 2011, 50, 458–462. 10.1002/anie.201004703. [DOI] [PubMed] [Google Scholar]
- Sagadevan A.; Charpe V. P.; Ragupathi A.; Hwang K. C. Visible Light Copper Photoredox-Catalyzed Aerobic Oxidative Coupling of Phenols and Terminal Alkynes: Regioselective Synthesis of Functionalized Ketones via C≡C Triple Bond Cleavage. J. Am. Chem. Soc. 2017, 139, 2896–2899. 10.1021/jacs.6b13113. [DOI] [PubMed] [Google Scholar]
- Ye T.; McKervey M. A. Organic Synthesis with α-Diazo Carbonyl Compounds. Chem. Rev. 1994, 94, 1091–1160. 10.1021/cr00028a010. [DOI] [Google Scholar]
- Padwa A. Domino reactions of rhodium(II) carbenoids for alkaloid synthesis. Chem. Soc. Rev. 2009, 38, 3072–3081. 10.1039/b816701j. [DOI] [PubMed] [Google Scholar]
- Doyle M. P.; Duffy R.; Ratnikov M.; Zhou L. Catalytic Carbene Insertion into C-H Bonds. Chem. Rev. 2010, 110, 704–724. 10.1021/cr900239n. [DOI] [PubMed] [Google Scholar]
- Davies H. M. L.; Morton D. Guiding principles for site selective and stereoselective intermolecular C-H functionalization by donor/acceptor rhodium carbenes. Chem. Soc. Rev. 2011, 40, 1857–1869. 10.1039/c0cs00217h. [DOI] [PubMed] [Google Scholar]
- Zhu S.-F.; Zhou Q.-L. Transition-Metal-Catalyzed Enantioselective Heteroatom–Hydrogen Bond Insertion Reactions. Acc. Chem. Res. 2012, 45, 1365–1377. 10.1021/ar300051u. [DOI] [PubMed] [Google Scholar]
- Gillingham D.; Fei N. Catalytic X–H insertion reactions based on carbenoids. Chem. Soc. Rev. 2013, 42, 4918–4931. 10.1039/c3cs35496b. [DOI] [PubMed] [Google Scholar]
- Ford A.; Miel H.; Ring A.; Slattery C. N.; Maguire A. R.; McKervey M. A. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. 10.1021/acs.chemrev.5b00121. [DOI] [PubMed] [Google Scholar]
- Hu F.; Xia Y.; Ma C.; Zhang Y.; Wang J. C-H bond functionalization based on metal carbene migratory insertion. Chem. Commun. 2015, 51, 7986–7995. 10.1039/C5CC00497G. [DOI] [PubMed] [Google Scholar]
- Li Y.-P.; Li Z.-Q.; Zhu S.-F. Recent advances in transition-metal-catalyzed asymmetric reactions of diazo compounds with electron-rich (hetero-) arenes. Tetrahedron Lett. 2018, 59, 2307–2316. 10.1016/j.tetlet.2018.04.055. [DOI] [Google Scholar]
- Zhao X.; Wu G.; Zhang Y.; Wang J. Copper-catalyzed direct benzylation or allylation of 1,3-azoles with N-tosylhydrazones. J. Am. Chem. Soc. 2011, 133, 3296–3299. 10.1021/ja111249p. [DOI] [PubMed] [Google Scholar]
- Xu B.; Li M. L.; Zuo X. D.; Zhu S. F.; Zhou Q. L. Catalytic Asymmetric Arylation of α-Aryl-α-diazoacetates with Aniline Derivatives. J. Am. Chem. Soc. 2015, 137, 8700–8703. 10.1021/jacs.5b05086. [DOI] [PubMed] [Google Scholar]
- Li Z.; Chen Y.; Wang C.; Xu G.; Shao Y.; Zhang X.; Tang S.; Sun J. Construction of C-C Axial Chirality via Asymmetric Carbene Insertion into Arene C-H Bonds. Angew. Chem. Int. Ed. 2021, 60, 25714–25718. 10.1002/anie.202110430. [DOI] [PubMed] [Google Scholar]
- Zhu D. X.; Liu J. G.; Xu M. H. Stereodivergent Synthesis of Enantioenriched 2,3-Disubstituted Dihydrobenzofurans via a One-Pot C-H Functionalization/Oxa-Michael Addition Cascade. J. Am. Chem. Soc. 2021, 143, 8583–8589. 10.1021/jacs.1c03498. [DOI] [PubMed] [Google Scholar]
- Zhu D. X.; Xia H.; Liu J. G.; Chung L. W.; Xu M. H. Regiospecific and Enantioselective Arylvinylcarbene Insertion of a C-H Bond of Aniline Derivatives Enabled by a Rh(I)-Diene Catalyst. J. Am. Chem. Soc. 2021, 143, 2608–2619. 10.1021/jacs.0c13191. [DOI] [PubMed] [Google Scholar]
- Song X. G.; Zhu S. F.; Xie X. L.; Zhou Q. L. Enantioselective copper-catalyzed intramolecular phenolic O-H bond insertion: synthesis of chiral 2-carboxy dihydrobenzofurans, dihydrobenzopyrans, and tetrahydrobenzooxepines. Angew. Chem. Int. Ed. 2013, 52, 2555–2558. 10.1002/anie.201209455. [DOI] [PubMed] [Google Scholar]
- Maier T. C.; Fu G. C. Catalytic enantioselective O-H insertion reactions. J. Am. Chem. Soc. 2006, 128, 4594–4595. 10.1021/ja0607739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Zhu S. F.; Liu B.; Wang L. X.; Zhou Q. L. Highly enantioselective insertion of carbenoids into O-H bonds of phenols: An efficient approach to chiral α-aryloxycarboxylic esters. J. Am. Chem. Soc. 2007, 129, 12616–12617. 10.1021/ja074729k. [DOI] [PubMed] [Google Scholar]
- Liang X. S.; Li R. D.; Wang X. C. Copper-Catalyzed Asymmetric Annulation Reactions of Carbenes with 2-Iminyl- or 2-Acyl-Substituted Phenols: Convenient Access to Enantioenriched 2,3-Dihydrobenzofurans. Angew. Chem. Int. Ed. 2019, 58, 13885–13889. 10.1002/anie.201907943. [DOI] [PubMed] [Google Scholar]
- Harada S.; Tanikawa K.; Homma H.; Sakai C.; Ito T.; Nemoto T. Silver-Catalyzed Asymmetric Insertion into Phenolic O-H Bonds using Aryl Diazoacetates and Theoretical Mechanistic Studies. Chem. Eur. J. 2019, 25, 12058–12062. 10.1002/chem.201902126. [DOI] [PubMed] [Google Scholar]
- Xie X. L.; Zhu S. F.; Guo J. X.; Cai Y.; Zhou Q. L. Enantioselective palladium-catalyzed insertion of α-aryl-α-diazoacetates into the O-H bonds of phenols. Angew. Chem. Int. Ed. 2014, 53, 2978–2981. 10.1002/anie.201309820. [DOI] [PubMed] [Google Scholar]
- Shen H.-Q.; Xie H.-P.; Sun L.; Zhou Y.-G. Enantioselective Carbene Insertion into O–H of Phenols with Chiral Palladium/2,2′-Biimidazole Complexes. Organometallics 2019, 38, 3902–3905. 10.1021/acs.organomet.9b00219. [DOI] [Google Scholar]
- Zhang Y.; Yao Y.; He L.; Liu Y.; Shi L. Rhodium(II)/Chiral Phosphoric Acid-Cocatalyzed Enantioselective O-H Bond Insertion of α-Diazo Esters. Adv. Synth. Catal. 2017, 359, 2754–2761. 10.1002/adsc.201700572. [DOI] [Google Scholar]
- Nakayama H.; Harada S.; Kono M.; Nemoto T. Chemoselective Asymmetric Intramolecular Dearomatization of Phenols with α-Diazoacetamides Catalyzed by Silver Phosphate. J. Am. Chem. Soc. 2017, 139, 10188–10191. 10.1021/jacs.7b04813. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Li Y.; Shi J.; Ma B.; Liu L.; Zhang J. (C6F5)3B Catalyzed Chemoselective and ortho-Selective Substitution of Phenols with α-Aryl α-Diazoesters. Angew. Chem. Int. Ed. 2016, 55, 14807–14811. 10.1002/anie.201608937. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Zhang X. F.; Li M.; Li C.; Liu J. Q.; Jiang Y. Y.; Ji X.; Liu L.; Wu Y. C. Mechanistic Insights into the Chemo- and Regio-Selective B(C6F5)3 Catalyzed C-H Functionalization of Phenols with Diazoesters. J. Org. Chem. 2019, 84, 14508–14519. 10.1021/acs.joc.9b02035. [DOI] [PubMed] [Google Scholar]
- Ma B.; Tang Z.; Zhang J.; Liu L. Copper-catalysed ortho-selective C-H bond functionalization of phenols and naphthols with α-aryl-α-diazoesters. Chem. Commun. 2020, 56, 9485–9488. 10.1039/D0CC04495D. [DOI] [PubMed] [Google Scholar]
- Hu S.; Lu Z.; Liu M.; Xu H.; Wu J.; Chen F. TfOH-Catalyzed Cascade C-H Activation/Lactonization of Phenols with α-Aryl-α-diazoesters: Rapid Access to α-Aryl Benzofuranones. J. Org. Chem. 2020, 85, 14916–14925. 10.1021/acs.joc.0c01583. [DOI] [PubMed] [Google Scholar]
- Guo R. T.; Zhang Y. L.; Tian J. J.; Zhu K. Y.; Wang X. C. Rhodium-Catalyzed ortho-Selective Carbene C-H Insertion of Unprotected Phenols Directed by a Transient Oxonium Ylide Intermediate. Org. Lett. 2020, 22, 908–913. 10.1021/acs.orglett.9b04452. [DOI] [PubMed] [Google Scholar]
- For a In(OTf)3-catalyzed C–H allylation reaction of free phenols with vinyldiazoacetates, see:Zhao D.; Luo J.; Liu L.; Liu Y. Regiospecific and site-selective C–H allylation of phenols with vinyldiazo compounds catalyzed by In(III). Org. Chem. Front. 2021, 8, 6252–6258. 10.1039/D1QO01184G. [DOI] [Google Scholar]
- Yu Z.; Ma B.; Chen M.; Wu H. H.; Liu L.; Zhang J. Highly site-selective direct C-H bond functionalization of phenols with α-aryl-α-diazoacetates and diazooxindoles via gold catalysis. J. Am. Chem. Soc. 2014, 136, 6904–6907. 10.1021/ja503163k. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Yu Z.; Zhang J. Z.; Liu L.; Xia F.; Zhang J. Origins of unique gold-catalysed chemo- and site-selective C-H functionalization of phenols with diazo compounds. Chem. Sci. 2016, 7, 1988–1995. 10.1039/C5SC04319K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Li Y.; Zhang P.; Liu L.; Zhang J. Ligand and counteranion enabled regiodivergent C-H bond functionalization of naphthols with α-aryl-α-diazoesters. Chem. Sci. 2019, 10, 6553–6559. 10.1039/C9SC01657K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y.; Su Y.; Yu Z.; Dong B.; McClain E. J.; Lan Y.; Shi X. Chemoselective carbophilic addition of α-diazoesters through ligand-controlled gold catalysis. Angew. Chem. Int. Ed. 2014, 53, 9817–9821. 10.1002/anie.201404946. [DOI] [PubMed] [Google Scholar]
- Guo X.; Hu W. Novel multicomponent reactions via trapping of protic onium ylides with electrophiles. Acc. Chem. Res. 2013, 46, 2427–2440. 10.1021/ar300340k. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Hu W. Asymmetric Multicomponent Reactions Based on Trapping of Active Intermediates. Chem. Rec 2017, 17, 739–753. 10.1002/tcr.201600124. [DOI] [PubMed] [Google Scholar]
- Qiu H.; Li M.; Jiang L. Q.; Lv F. P.; Zan L.; Zhai C. W.; Doyle M. P.; Hu W. H. Highly enantioselective trapping of zwitterionic intermediates by imines. Nat. Chem. 2012, 4, 733–738. 10.1038/nchem.1406. [DOI] [PubMed] [Google Scholar]
- Jia S.; Xing D.; Zhang D.; Hu W. Catalytic asymmetric functionalization of aromatic C-H bonds by electrophilic trapping of metal-carbene-induced zwitterionic intermediates. Angew. Chem. Int. Ed. 2014, 53, 13098–13101. 10.1002/anie.201406492. [DOI] [PubMed] [Google Scholar]
- Kang Z.; Zhang D.; Xu X.; Hu W. Privilege-Structure-Oriented Three-Component Asymmetric Aminomethylation: Assembly of Chiral 3-Aminomethyl Indolones. Org. Lett. 2019, 21, 9878–9883. 10.1021/acs.orglett.9b03787. [DOI] [PubMed] [Google Scholar]
- Yu S.; Hua R.; Fu X.; Liu G.; Zhang D.; Jia S.; Qiu H.; Hu W. Asymmetric Multicomponent Reactions for Efficient Construction of Homopropargyl Amine Carboxylic Esters. Org. Lett. 2019, 21, 5737–5741. 10.1021/acs.orglett.9b02139. [DOI] [PubMed] [Google Scholar]
- For an elegant Au-catalyzed three-component reaction of phenols and diazoesters with allenamides through O–H insertion, see:Yu S.; Chen J.; Liu G.; Lei J.; Hu W.; Qiu H. A gold(I)-catalysed chemoselective three-component reaction between phenols, α-diazocarbonyl compounds and allenamides. Chem. Commun. 2020, 56, 1649–1652. 10.1039/C9CC09470A. [DOI] [PubMed] [Google Scholar]
- Tsuji J.; Minami I.; Shimizu I. Allyation of carbonucleophiles with allylic carbonates under neutral conditions catalyzed by rhodium complexes. Tetrahedron Lett. 1984, 25, 5157–5160. 10.1016/S0040-4039(01)81551-3. [DOI] [Google Scholar]
- Turnbull B. W. H.; Evans P. A. Asymmetric Rhodium-Catalyzed Allylic Substitution Reactions: Discovery, Development and Applications to Target-Directed Synthesis. J. Org. Chem. 2018, 83, 11463–11479. 10.1021/acs.joc.8b00583. [DOI] [PubMed] [Google Scholar]
- Thoke M. B.; Kang Q. Rhodium-Catalyzed Allylation Reactions. Synthesis 2019, 51, 2585–2631. 10.1055/s-0037-1611784. [DOI] [Google Scholar]
- Chen Z.-S.; Huang X.-Y.; Gao J.-M.; Ji K. Relay Rh(II)/Pd(0) Dual Catalysis: Selective Construction of Cyclic All-Quaternary Carbon Centers. Org. Lett. 2016, 18, 5876–5879. 10.1021/acs.orglett.6b02958. [DOI] [PubMed] [Google Scholar]
- Chen Z.-S.; Huang X.-Y.; Chen L.-H.; Gao J.-M.; Ji K. Rh(II)/Pd(0) Dual Catalysis: Regiodivergent Transformations of Alkylic Oxonium Ylides. ACS Catal. 2017, 7, 7902–7907. 10.1021/acscatal.7b02909. [DOI] [Google Scholar]
- Huang L.-Z.; Xuan Z.; Jeon H. J.; Du Z.-T.; Kim J. H.; Lee S.-g. Asymmetric Rh(II)/Pd(0) Relay Catalysis: Synthesis of α-Quaternary Chiral β-Lactams through Enantioselective C–H Insertion/Diastereoselective Allylation of Diazoamides. ACS Catal. 2018, 8, 7340–7345. 10.1021/acscatal.8b01687. [DOI] [Google Scholar]
- Chen L.-H.; Ma Y.-T.; Yang F.; Huang X.-Y.; Chen S.-W.; Ji K.; Chen Z.-S. Chemo-selective Rh(II)/Pd(0) Dual Catalysis: Synthesis of All-Carbon C3-Quaternary Allylic Oxindoles from N-Aryl-α-Diazo-β-Keto-Amides with Functionalized Allyl Carbonates. Adv. Synth. Catal. 2019, 361, 1307–1312. 10.1002/adsc.201801346. [DOI] [Google Scholar]
- Wang X. X.; Huang X. Y.; Lei S. H.; Yang F.; Gao J. M.; Ji K.; Chen Z. S. Relay Rh(II)/Pd(0) dual catalysis: synthesis of α-quaternary β-keto-esters via a [1,2]-sigmatropic rearrangement/allylic alkylation cascade of α-diazo tertiary alcohols. Chem. Commun. 2020, 56, 782–785. 10.1039/C9CC08559A. [DOI] [PubMed] [Google Scholar]
- Kang Z.; Chang W.; Tian X.; Fu X.; Zhao W.; Xu X.; Liang Y.; Hu W. Ternary Catalysis Enabled Three-Component Asymmetric Allylic Alkylation as a Concise Track to Chiral α,α-Disubstituted Ketones. J. Am. Chem. Soc. 2021, 143, 20818–20827. 10.1021/jacs.1c09148. [DOI] [PubMed] [Google Scholar]
- For an elegant example of Ru(II)/Pd(0) dual catalyzed two-component reactions of diazo compounds with allylic compounds, see:Yamamoto K.; Qureshi Z.; Tsoung J.; Pisella G.; Lautens M. Combining Ru-Catalyzed C-H Functionalization with Pd-Catalyzed Asymmetric Allylic Alkylation: Synthesis of 3-Allyl-3-aryl Oxindole Derivatives from Aryl α-Diazoamides. Org. Lett. 2016, 18, 4954–4957. 10.1021/acs.orglett.6b02423. [DOI] [PubMed] [Google Scholar]
- Lu B.; Liang X.; Zhang J.; Wang Z.; Peng Q.; Wang X. Dirhodium(II)/Xantphos-Catalyzed Relay Carbene Insertion and Allylic Alkylation Process: Reaction Development and Mechanistic Insights. J. Am. Chem. Soc. 2021, 143, 11799–11810. 10.1021/jacs.1c05701. [DOI] [PubMed] [Google Scholar]
- Trindade A. F.; Coelho J. A. S.; Afonso C. A. M.; Veiros L. F.; Gois P. M. P. Fine Tuning of Dirhodium(II) Complexes: Exploring the Axial Modification. ACS Catal. 2012, 2, 370–383. 10.1021/cs200597a. [DOI] [Google Scholar]
- Hong B.; Shi L.; Li L.; Zhan S.; Gu Z. Paddlewheel dirhodium(II) complexes with N-heterocyclic carbene or phosphine ligand: New reactivity and selectivity. Green Synth. Catal. 2022, 10.1016/j.gresc.2022.03.001. [DOI] [Google Scholar]
- Wang D.; Zhao Y.; Yuan C.; Wen J.; Zhao Y.; Shi Z. Rhodium(II)-Catalyzed Dehydrogenative Silylation of Biaryl-Type Monophosphines with Hydrosilanes. Angew. Chem. Int. Ed. 2019, 58, 12529–12533. 10.1002/anie.201906975. [DOI] [PubMed] [Google Scholar]
- Fu L.; Li S.; Cai Z.; Ding Y.; Guo X.-Q.; Zhou L.-P.; Yuan D.; Sun Q.-F.; Li G. Ligand-Enabled Site-Selectivity in a Versatile Rhodium(II)-Catalysed Aryl C–H Carboxylation with CO2. Nat. Catal. 2018, 1, 469–478. 10.1038/s41929-018-0080-y. [DOI] [Google Scholar]
- Vora H. U.; Silvestri A. P.; Engelin C. J.; Yu J. Q. Rhodium(II)-Catalyzed Nondirected Oxidative Alkenylation of Arenes: Arene Loading at One Equivalent. Angew, Chem. Int. Ed. 2014, 53, 2683–2686. 10.1002/anie.201310539. [DOI] [PubMed] [Google Scholar]
- Kwak J.; Kim M.; Chang S. Rh(NHC)-Catalyzed Direct and Selective Arylation of Quinolines at the 8-Position. J. Am. Chem. Soc. 2011, 133, 3780–3783. 10.1021/ja111670s. [DOI] [PubMed] [Google Scholar]
- Gois P. M. P.; Trindade A. F.; Veiros L. F.; André V.; Duarte M. T.; Afonso C. A. M.; Caddick S.; Cloke F. G. N. Tuning the Reactivity of Dirhodium(II) Complexes with Axial N-heterocyclic Carbene Ligands: the Arylation of Aldehydes. Angew. Chem. Int. Ed. 2007, 46, 5750–5753. 10.1002/anie.200700924. [DOI] [PubMed] [Google Scholar]
- Lebel H.; Marcoux J. F.; Molinaro C.; Charette A. B. Stereoselective cyclopropanation reactions. Chem. Rev. 2003, 103, 977–1050. 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Toste F. D. Asymmetric O- and C-alkylation of phenols. J. Am. Chem. Soc. 1998, 120, 815–816. 10.1021/ja972453i. [DOI] [Google Scholar]
- Evans P. A.; Leahy D. K. Regioselective and enantiospecific rhodium-catalyzed intermolecular allylic etherification with ortho-substituted phenols. J. Am. Chem. Soc. 2000, 122, 5012–5013. 10.1021/ja0003831. [DOI] [Google Scholar]
- Choi M.; Jo H.; Park H. J.; Sateesh Kumar A.; Lee J.; Yun J.; Kim Y.; Han S. B.; Jung J. K.; Cho J.; Lee K.; Kwak J. H.; Lee H. Design, synthesis, and biological evaluation of benzofuran- and 2,3-dihydrobenzofuran-2-carboxylic acid N-(substituted)phenylamide derivatives as anticancer agents and inhibitors of NF-κB. Bioorg. Med. Chem. Lett. 2015, 25, 2545–2549. 10.1016/j.bmcl.2015.04.050. [DOI] [PubMed] [Google Scholar]
- Haupt R. A.; Renneckar S. Chemical shifts of phenolic monomers in solution and implications for addition and self-condensation. Magn. Reson. Chem. 2013, 51, 95–101. 10.1002/mrc.3914. [DOI] [PubMed] [Google Scholar]
- Kremer T.; Schleyer P. V. R. Charge-localizing effect in alkali-metal enolates and phenolates. Structure and aromaticity of the phenolate anion. Organometallics 1997, 16, 737–746. 10.1021/om960763o. [DOI] [Google Scholar]
- Davies A. G. Metals as surrogates for hydrogen in organic chemistry: anything hydrogen can do, a metal can do better. J. Chem. Soc., Perkin Trans. 2000, 1, 1997–2010. 10.1039/a909835f. [DOI] [Google Scholar]
- Conner A. H.; Lorenz L. F.; Hirth K. C. Accelerated cure of phenol-formaldehyde resins: Studies with model compounds. J. Appl. Polym. Sci. 2002, 86, 3256–3263. 10.1002/app.11106. [DOI] [Google Scholar]
- Kinart W. J.; Kinart C. M. Studies on the catalysis of the reaction of organotin phenoxides with diethyl azodicarboxylate by lithium perchlorate. J. Organomet. Chem. 2003, 665, 233–236. 10.1016/S0022-328X(02)02129-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.