Abstract
AFLR, a DNA-binding protein of 444 amino acids, transactivates the expression of aflatoxin biosynthesis genes in Aspergillus parasiticus and Aspergillus flavus, as well as the sterigmatocystin synthesis genes in Aspergillus nidulans. We show here by fusion of various aflR coding regions to the GAL4 DNA-binding coding region that the AFLR carboxyl terminus contained a region that activated GAL1::lacZ gene expression in Saccharomyces cerevisiae and that the AFLR internal region was required for the activation activity. Compared to the AFLR carboxy-terminal fusion protein (AFLRC), a mutant AFLRC retained approximately 75% of the activation activity after deletion of three acidic amino acids, Asp365, Glu366, and Glu367, in a previously identified acidic stretch. Removal of the carboxy-terminal amino acid, Glu444, did not affect the activation activity. Substitutions of acidic Glu423, Asp439, or Asp436/Asp439 with basic amino acids, Lys and His, resulted in 10- to 15-fold-lower activation activities. Strikingly, the Asp436His mutation abolished the activation activity. Substitutions of basic His428 and His442 with acidic Asp resulted in 20 and 40% decreases in the activation activities, respectively. Simultaneous substitutions of Arg427, Arg429, and Arg431 with Leu also significantly decreased the activation activity; the decrease was approximately 50-fold. Results suggest that the AFLR carboxy-terminal region is involved in transcription activation and that total acidity in this region is not a major determinant of AFLR’s activation ability in S. cerevisiae.
Aflatoxins are a family of toxic secondary metabolites produced by some Aspergillus flavus group fungi, mainly A. flavus, A. parasiticus, and A. nomius (27). These toxins commonly contaminate agricultural commodities, such as corn, cottonseed, peanuts, and tree nuts (3). Aflatoxin B1 is by far the most potent hepatocarcinogen among the known mycotoxins.
Aflatoxin biosynthesis, with acetate as the building block, requires a polyketide synthase and two fatty acid synthases to form the initial anthraquinone and the C6 side chain, respectively (32). Subsequent steps involve numerous enzymatic conversions leading to the formation of several stable intermediates (4, 27).
Research efforts in the cloning of aflatoxin pathway genes have established the clustering of aflatoxin biosynthesis genes in A. parasiticus and A. flavus (35), as well as in Aspergillus nidulans, which produces sterigmatocystin in a pathway similar to that of aflatoxin (5). Studies have shown that one of these clustered genes, aflR, is involved in the regulation of aflatoxin and sterigmatocystin biosynthesis (8, 16, 33, 34). The aflR gene product, AFLR (8, 33, 34), belongs to the family of binuclear zinc-finger DNA-binding proteins, which are pathway-specific regulatory proteins found in filamentous fungi and yeast cells (10, 11, 29). To date, this family consists of more than 80 known proteins. The majority are commonly the regulatory proteins of various metabolic pathways (11, 29), but at least one study has shown that the “fluffy” gene product of Neurospora crassa, FL, is involved in conidiophore morphogenesis (2).
Several lines of evidence indicate that AFLR is involved in autoregulation as well as in the regulation of transcription of other aflatoxin pathway genes. Transformation of A. flavus containing a mutated aflR locus with a wild-type copy of aflR restored transcription of the structural genes (16). Wild-type and blocked A. parasiticus strains overproduced aflatoxin and/or its precursors upon transformation with an additional copy of intact aflR (8). Disruption of A. nidulans aflR prevented the synthesis of transcripts for genes involved in sterigmatocystin production (34). The A. flavus AFLR protein activates transcription of the genes of sterigmatocystin in A. nidulans, which suggests a conservation in AFLR function (34). Subsequently, electrophoretic mobility shift assays and footprinting studies have demonstrated that AFLR binds to specific sites in the promoters of other aflatoxin biosynthetic genes, as well as to a specific site in the aflR promoter (8, 13, 15).
AFLR contains a distinct highly acidic region, TEERVLHHPSMVGEDCVDEEDQPRVADS (8). Moreover, in the carboxy-terminal region of AFLR, from the predicted amino acid residues 360 to 444, approximately one-sixth of the amino acids are acidic. In Saccharomyces cerevisiae, acidic regions are necessary for the DNA-binding proteins, such as GAL4 and GCN4 (21, 25), to activate transcription of other pathway related genes. Most recently, Koh et al. (23) have shown that the acidic GAL4 transcription activation domain binds to the SRB4 (SRB stands for suppressors of RNA polymerase B) subunit of the RNA polymerase II holoenzyme.
In this study, we used an S. cerevisiae expression (one-hybrid) system to identify the region(s) of AFLR which was associated with transcription activation activity. A region in the AFLR carboxyl terminus was found to activate GAL1::lacZ gene expression. Amino acid substitutions which increased or decreased the total acidity in the carboxy-terminal 23-amino-acid region decreased the activation activity to different extents. The change of Asp436 in AFLR to His abolished such an activation activity in S. cerevisiae.
MATERIALS AND METHODS
Fusion of A. parasiticus aflR with S. cerevisiae GAL4 DNA-binding domain coding region.
The 1.5-kb SmaI-BamHI fragment of aflR (8), encoding the complete AFLR protein except for the first seven amino acid residues, was ligated to SmaI-BamHI-digested pAS2-1 (Clontech, Palo Alto, Calif.). The resulting construct was digested with NdeI, end filled, and self-ligated to create the in-frame fusion construct, pASaflR, which contained the S. cerevisiae GAL4 DNA-binding, the aflR DNA-binding, and the carboxy-terminal half coding region. The plasmid pASaflR was digested with SalI and self-ligated to generate pASaflRN, where the carboxy-terminal half coding region was deleted. In addition, the 0.9-kb EcoRI-BamHI fragment of aflR which encoded the carboxy-terminal half without the DNA-binding domain was fused to the S. cerevisiae GAL4 DNA-binding domain coding region to generate pASaflRC.
Unidirectional deletion of aflR carboxy-terminal coding region.
The plasmid pASaflRC was digested with BamHI and PstI to create 5′ and 3′ overhangs, respectively. Unidirectional deletion of the aflR 3′ portion was carried out with an Exo Mung Bean Deletion Kit (Stratagene, La Jolla, Calif.) with a scaled-down protocol (2 μg of BamHI- and PstI-digested pASaflRC in a final volume of 50 μl). Exonuclease III (ExoIII) digestion of double-stranded DNA to single-stranded DNA was performed at room temperature. Five-microliter aliquots of the reaction mixture were removed at 10 1-min intervals and placed into a tube containing 35 μl of diluted mung bean nuclease buffer to stop the digestion. Samples were then heated at 68°C for 15 min to inactivate the ExoIII and placed on ice. To each tube, 3 U of mung bean nuclease was added, and the tubes were incubated at 30°C for 30 min to remove the single-stranded DNA. The extent of deletion at each time point was determined by agarose (0.8%) gel electrophoresis. Samples obtained at 3 and 5 min which showed the desired extents of deletion were purified with a QIAquick Nucleotide Removal Kit (Qiagen, Valencia, Calif.), end filled with Klenow enzyme, self-ligated, and transformed into Escherichia coli DH5α (Life Technologies, Grand Island, N.Y.).
Site-directed mutagenesis.
PCR-based site-directed mutagenesis (20) was used to introduce stop codons or amino acid substitutions into the aflR gene, which rendered various pretermination or changes of amino acid residues in the translated AFLR protein. This method consists of two rounds of PCR with three pairs of oligonucleotide primers. The 5′ primer was located upstream of an EcoRI site, and the 3′ primer was located downstream of a BamHI site (8). F primer contained the designated change of nucleotide, and R primer was the reverse complementary sequence of F primer. First-round PCR with two pairs of primers (5′ primer and R primer amplified the region from the 5′ end to the mutation site, and F primer and 3′ primer amplified the region from the mutation site to the 3′ end) gave two slightly overlapping PCR products. The two PCR products were separated from the aflR template by agarose gel electrophoresis and purified. Second-round PCR with 5′ primer and 3′ primer with the two PCR products as the template gave the desired DNA fragment containing the designated mutation and the restriction sites, EcoRI and BamHI, for cloning into the yeast vector pAS2-1.
(i) The F primers for the generation of preterminations were as follows (with the stop codons underlined): ADT1, 5′-CTGTCTGACGTAAGAGCGCG-3′; ADT2, 5′-ATGGTGGGCTAGGATTGTGT-3′; ADT3, 5′-TTCTGAGCTAACTGCACTGA-3′; ADT4, 5′-AGCGCCTGCAATAAGGTGGA-3′; ADT5, 5′-AGTGGCCTCTAAGCAAATCT-3′; and ADT6, 5′-CCTGCATCGATAATGAAGAA-3′.
(ii) The F primers for the substitutions of acidic amino acids with basic amino acids in the carboxy-terminus of AFLR were as follows: E/K, 5′-AGTGGCCTCAAAGCAAATCT-3′ (for Glu423 to Lys); D1/H, 5′-GGTCCTCCCACATTATCGAT-3′ (for Asp436 to His); D2/H, 5′-GACATTATCCATTACCTGCA-3′ (for Asp439 to His); and 2D/2H, 5′-GTCCTCCCACATTATCCATTACCTGC-3′ (for Asp436 and Asp439 to His).
(iii) The F primers for the substitutions of basic amino acids with acidic or neutral amino acids in the carboxyl terminus of AFLR were as follows: H1/D, 5′-AAATCTCCGCGACCGCTTGC-3′ (for His428 to Asp); H2/D, TTACCTGGATCGAGAATGAA (for His442 to Asp); and 3R/3L, 5′-CAAATCTCCTCCACCTCTTGCTCGCCGTGT-3′ (for Arg427, Arg429, and Arg431 to Leu).
(iv) The 5′ primer was F2423 (5′-CCTTGGAGGAGATCTGGCTGGTCA-3′) and the 3′ primer was R450 (5′-TCCATGACAAAGACGGATCC-3′).
(v) For the pMut3 fusion construct, the 0.9-kb EcoRI-BamHI fragment of pAFmut3 where amino acids Asp365, Glu366, and Glu367 were deleted (12) was subcloned into EcoRI-BamHI-digested pAS2-1.
Internal deletion of aflR.
In addition to deletions generated from the 3′ end of aflR, coding regions in between the 3′ and 5′ ends of aflR were deleted by PCR. The forward primers were AD818 (5′-TTTGAATTCATCTCGGGGAACAAGAAGGCT-3′) and AD985 (5′-AAAGAATTCAACAGTGGCAGCTGTAGCAAC-3′); the reverse primer was R450. The PCR products were digested with EcoRI and BamHI and ligated to EcoRI-BamHI-digested pAS2-1.
Yeast transformation and selection.
pAS2-1-based aflR deletion constructs were transformed into S. cerevisiae Y190 (MATa ura3-52 his3-200 lys2-801 ade2-101 trp1-901 leu2-3,112 gal4Δ gal80Δ cyhr2 LYS2::GAL1UAS-HIS3TATA-HIS URA3::GAL1UAS-GAL1TATA-LacZ) by a PEG-LiAc protocol (22). Transformants were selected on Minimal Synthetic Drop Agar (Clontech) plates supplemented with required amino acids minus tryptophan (SD/−trp).
Assays for β-galactosidase activities of aflR deletion yeast transformants.
Qualitative determination of the β-galactosidase activities (blue-white screening) of the yeast transformants was carried out by colony-lift filter paper assay. Ten streaked yeast colonies grown on SD/−trp agar plates were lifted by placing a filter paper on each plate. The yeast cells were permeabilized by quick freezing in liquid nitrogen and thawed at room temperature. Each filter, with the colony side up, was then placed on top of a Whatman number 5 filter paper presoaked with Z buffer (Na2HPO4 · 7H2O, 16.1 g; NaH2PO4, 5.5 g; KCl, 0.75 g; MgSO4 · 7H2O, 0.246 g [per liter]; pH 7.0) containing β-mercaptoethanol and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid; 0.5 mg/ml). The filters were incubated at 30°C and checked periodically for the development of blue color.
For quantitative assay of the β-galactosidase activity by using o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate, yeast liquid cultures were prepared. Ten yeast transformants examined by colony-lift assays were pooled as the inoculum to reduce clone variability. The yeast cells were inoculated into 15 ml of SD/−trp medium and incubated at 30°C for 12 to 15 h in a gyroshaker with shaking at 200 rpm. The resulting yeast cultures were adjusted to an optical density at 600 nm of approximately 0.6 with SD/−trp medium to reduce the discrepancy in the amounts of yeast cells used in the assays. Determinations in triplicate were carried out as described in the protocol of Matchmaker Two-Hybrid System 2 (Clontech). One unit of β-galactosidase was defined as the amount of enzyme which hydrolyzed 1 μmol of ONPG to o-nitrophenol and d-galactose per min (26).
Nucleotide sequence accession number.
The GenBank accession number for the updated A. parasiticus aflR cDNA sequence is L26222.
RESULTS
Examination of regions of AFLR associated with transcription activation in S. cerevisiae.
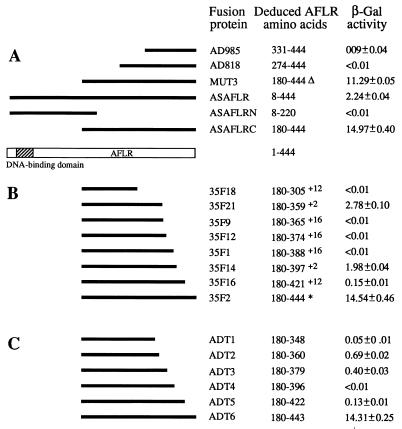
Figure 1A shows the activation activities of various regions of AFLR when fused to the GAL4 DNA-binding domain and examined in S. cerevisiae. The full-length AFLR fusion protein, ASAFLR, which contained both the GAL4 and AFLR DNA-binding domains and the AFLR carboxy-terminal half had much less activity than the fusion protein which contained the carboxy-terminal half (ASAFLRC) only. The GAL4 DNA-binding domain, as control, and ASAFLRN which contained two DNA-binding domains lacked activation activity. These results suggested that the carboxy-terminal portion of AFLR contained a putative domain which was associated with transcription activation in S. cerevisiae. Previously, we identified a highly acidic stretch of amino acids, one similar to the GAL4 and GCN4 acidic transcription activation domains (21, 25), in AFLR from amino acid residues 349 to 368 (8). To investigate the role of this acid stretch in AFLR, we deleted three amino acids, Asp365, Glu366, and Glu367, and examined the activation activity of the resulting fusion protein, MUT3, on GAL1::lacZ gene expression. In spite of a decrease in acidity in MUT3, it retained approximately 75% of the activation activity compared to the ASAFLRC. This result suggested that these acidic amino acids might not play a significant role in activating GAL1::lacZ gene expression in S. cerevisiae.
FIG. 1.
Examination of regions of AFLR for transcription activation activity with an S. cerevisiae GAL4 one-hybrid system. (A) Internal deletion. For MUT3, “▵” indicates that three acidic amino acids, Asp365, Glu366, and Glu367, in AFLR are deleted. (B) Deletion of carboxy-terminal regions by ExoIII-mung bean nuclease digestion. The superscript values indicate additional amino acids at the carboxyl terminus which are derived from the vector, pAS2-1: +16, AKLIRGEFLMIYDFYY; +2, PS; +12, SQANSGRISYDL. For 35F2, the asterisk indicates that the deletion is in the 3′ noncoding region of aflR. (C) Truncation of carboxy-terminal regions by incorporating a stop codon into the aflR coding region, i.e., GAA or GAG that encodes Glu was changed to TAA or TAG. The β-galactosidase (β-Gal) activity was as follows: 1 U of β-galactosidase activity is defined as the amount of enzyme which hydrolyzes 1 μmol of ONPG to o-nitrophenol and d-galactose per min at 30°C.
Unlike GAL4, where as much as 80% of the internal portion can be deleted without significantly affecting its activation activity (25), fusion proteins containing a deletion of the internal region of AFLR resulted in significant or complete loss of the activation activity (AD818 and AD985; Fig. 1A).
Carboxy-terminal deletions.
Figure 1B shows that AFLR fusion proteins containing the 23-amino-acid deletion of the carboxyl terminus significantly decreased or completely lost the activation activity in S. cerevisiae. A deletion at the aflR 3′ noncoding region, 35F2, did not affect the activation activity. One concern with regard to these GAL4-AFLR fusion proteins generated by ExoIII-mung bean nuclease deletion is that additional amino acids encoded by the pAS2-1 vector sequence might interfere with the activation function of these AFLR fusion proteins. For example, 35F14 and 35F21, which contained an additional two amino acids, appeared to retain partial activation activity. In contrast, others which contained more additional amino acids, such as 35F9, 35F12, 35F16, and 35F18, had a significant decrease or loss in the activation activity. Consequently, the carboxy-terminal deletion was refined by introducing a stop codon into the aflR coding region.
The results obtained from the pretermination experiments (Fig. 1C) were consistent with those of the ExoIII-mung bean nuclease deletion studies. The AFLRC mutants, ADT1 to ADT5, which had been truncated by 22 or more amino acids in the carboxyl terminus showed a significant decrease or loss of the activation activity. However, removal of Glu444 (ADT6) did not affect the observed activation function. Taken together, both results suggested that the AFLR carboxy-terminal portion containing Leu422 to Arg443 was an important region which was associated with transcription activation of the GAL1::lacZ gene in S. cerevisiae.
Amino acid substitutions in the AFLR carboxy-terminal 23-amino-acid region.
Deletion analysis indicates that the internal region of AFLR is also important for the activation activity in S. cerevisiae. However, due to technical complexity in the analysis of large segments of deletion, we focused on the dissection of the carboxy-terminal region of AFLR. For the AFLRC mutants, the increase in total acidity in the carboxy-terminal region by substitution of basic amino acid(s) with acidic or neutral amino acid(s) did not increase but decreased the activation activity to various extents (Table 1 and Fig. 2A). The decrease was approximately 20% for His428Asp and 40% for His442Asp compared to the activation activity of the wild-type ASAFLRC. Simultaneous substitutions of Arg427Leu, Arg429Leu, and Arg431Leu decreased the activation activity very significantly; the decrease was approximately 50-fold.
TABLE 1.
Amino acid substitutions in the carboxy-terminal 23 amino acids of AFLR
FIG. 2.
Site-directed mutagenesis of the A. parasiticus AFLR carboxy-terminal region. (A) Substitutions of basic or acidic amino acids in the carboxy-terminal 23 amino acids of A. parasiticus AFLR (Ap). Amino acid substitutions are shown in boldface in the second sequence. The bottom sequence is the corresponding 23 amino acids of A. nidulans AFLR (An). The number indicates the position of the amino acid. (B) Changes of the secondary structure resulting from an amino acid substitution(s) in A. parasiticus AFLR. The secondary structures of the corresponding regions of A. parasiticus AFLR and A. nidulans AFLR are also shown. Abbreviations: X, α-helix; –, β-sheet as predicted by the Garnier method (17).
Substitutions which decreased acidity of AFLRC also resulted in significant decreases in the activation activity (Table 1). The decrease (ca. 10- to 15-fold) was more pronounced than the decrease which resulted from the substitutions of basic amino acids with acidic amino acids. The mutant with Asp436His plus Asp439His substitutions retained a low level of activation activity similar to that found with Asp439His (1.70 versus 1.73; Table 1). Strikingly, the AFLRC mutant with a single amino acid substitution, Asp436His, completely lost its activation activity when fused to the GAL4 DNA-binding domain.
Changes in secondary structure and amino acid substitutions.
Studies have suggested that some activation domains form amphipathic α-helices (18, 30), whereas Van Hoy et al. (31) have suggested that the activation domains of GAL4 (activating region II) and GCN4 form β-sheets. The AFLR carboxy-terminal 23 amino acids formed extensive α-helical and β-sheet structures based on Garnier’s analysis (reference 17 and Fig. 2B). Moreover, substitutions which affected the activation function less significantly, such as His428Asp and His442Asp, did not change the secondary structure in this region (Table 1; Fig. 2). Asp436 was situated in the middle of a β-sheet. A change to His at this position extended the α-helical structure and shortened the β-sheet (Fig. 2B). Similar changes which extended the α-helical structure (D1/H, D2/H, and 2D/2H) and changes which shortened the α-helical structure (E1/K) or disrupted the basic secondary structure (3R/3L) in this region also significantly affected the activation activity.
DISCUSSION
Available information suggests that zinc binuclear cluster regulatory proteins are fungal and yeast specific. This type of protein is not present in Caenorhabditis elegans (9), and none has been reported from bacteria, Drosophila spp., or mammals. Thus, interactions between binuclear cluster regulatory proteins and the transcription machinery in lower eukaryotes most likely are conserved to some extent, as suggested by the GAL4-AFLR fusion in this study. Whereas the precise function of the carboxy-terminal half of AFLR in activating the GAL1::lacZ gene expression remains to be determined, recent studies of yeast cells have suggested that the targets for transcription activators appear to be a subset of proteins called SRB (23). Also, the GAL4 activation domain has been shown to bind to two segments of the SRB4 subunit of the RNA polymerase II holoenzyme (23). However, the paucity of direct evidence in the literature with regard to the mechanism(s) of transcription activation makes it impractical to draw a generalization. Nonetheless, activation of the GAL1::lacZ gene expression by AFLRC suggests that AFLRC is engaged in protein-protein interaction(s). The transcription initiation apparatus consists of more than 50 polypeptides (23); AFLRC probably makes contact with either the basal transcription factor(s), the scaffolding protein(s), or the RNA polymerase II in S. cerevisiae. This explanation, however, does not exclude the possibility that in A. parasiticus AFLR might interact with another aflatoxin pathway-specific factor(s) that is present under aflatoxin-conducive growth conditions.
Inclusion of the AFLR DNA-binding domain as well as deletion of internal regions of AFLR altered the activation activities of the resulting fusion proteins (Fig. 1A). The decrease most probably resulted from a change in conformation (1) which is important for making contact with the transcription machinery. Alternatively, the internal region of AFLR might contain a domain necessary for transcription activation in S. cerevisiae, although the GAL4-AFLR result (Fig. 1A, ASAFLR) seems to argue against such a possibility. The internal region of GAL4 is dispensable in transactivating the GAL1 gene expression (25). However, the internal region of NIT4, the nitrogen pathway-specific binuclear cluster regulatory protein of Neurospora crassa, is essential for function (14). The areA-300 mutation, which results in an in-frame tandem duplication of the entire DNA-binding domain of the positive-acting regulatory protein of nitrogen metabolism, AREA, also has been shown to alter the specificity of gene activation (6). Deletion of three acidic amino acids, Asp365, Glu366, and Glu367, in the identified acidic stretch of AFLR does not drastically affect the activation activity of MUT3 in S. cerevisiae. Ehrlich et al. (12) have shown that A. parasiticus transformed with aflR containing the same mutation (deletion of the coding sequence for Asp365, Glu366, and Glu367) produced an elevated level of aflatoxin intermediates, indicating that these three acidic amino acids do not play a significant role in transcription activation even in A. parasiticus.
Biological precedents have implied the involvement of acidic domains in transcription activation (14, 21, 24, 28, 30). These acidic domains are located either in the amino-terminal region or in the carboxy-terminal region depending on the type of activator. The acidic transactivation domains in bZIP activators, such as GCN (21), Luman (24), and VBP (28), are commonly located in the amino-terminal region. In contrast, the acidic transactivation domains in the zinc binuclear cluster activators, e.g., GAL4 (25), FacB (30), and NIT4 (14), are usually located in the carboxy-terminal region. Approximately one-sixth of the amino acids in the AFLR carboxy-terminal region, from residues 360 to 444, are acidic. On the basis of AFLR structure similarities (DNA-binding motif and acidity) to other binuclear cluster regulatory proteins, the carboxy-terminal portion of AFLR is probably a transcription activation domain. Two lines of evidence support such a notion. First, Watson et al. (36) recently reported that A. sojae and A. oryzae, which are closely related to A. parasiticus and do not produce aflatoxins, contain an aflR homolog with a specific point mutation. This amber mutation at Arg383 of AFLR results in truncation of the last 62 amino acids in the carboxyl terminus. No transcripts of aflR and other aflatoxin biosynthesis genes, such as nor1, ver1, and omtA, were detected in these fungi. Second, Cary et al. (7) disrupted the functional copy of aflR of A. parasiticus SU-1 which contains two copies of aflR. The predicted second AFLR of SU-1, coincidently, contained a specific amino acid change, Asp439 to a neutral Asn (7). Northern analysis showed that the SU-1 aflR disruptant lacks transcripts of aflR, nor1, ver1, and omtA.
The carboxy-terminal 23-amino-acid region of AFLR appears to be important for the activation of GAL1::lacZ gene expression in S. cerevisiae. GAL4-AFLRC fusion proteins containing a single amino acid substitution but maintaining the basic secondary structure (Fig. 2B, H1/D and H2/D) retain most of the activation activity. Substitutions of Asp436 and Asp439 with His resulted in partial activation activity, but it is not clear why the Asp436His substitution resulted in a loss of activation activity. It is hard to draw a correlation between protein-enzyme function and the amino acid sequence (primary structure). The result might imply the importance of tertiary structure (conformation) or quaternary structure (dimer formation) of the AFLR portion under study. In the corresponding region of A. nidulans AFLR, this position is the only place where a conservative substitution was found, i.e., Asp to Glu (34). Probably a negative charge in this position is necessary, even when the secondary structure of this region of A. nidulans is different from that of A. parasiticus (Fig. 2B).
Mutational analysis of the GAL4 transcription activator has revealed a strong correlation between activation activity and acidity (19). Although the role of the 23 amino acids of AFLRC remains to be tested in A. parasiticus in vivo, the results from this study show that substitutions that increase the acidity of the carboxyl terminus decrease the activation activities of the resulting fusion proteins. This result suggests that total acidity in the 23-amino-acid region is not a major determinant of AFLR’s activation ability in S. cerevisiae.
ACKNOWLEDGMENT
We are grateful to Karen Gillespie for her excellent technical assistance.
REFERENCES
- 1.Ascone I, Lenouvel F, Sequeval D, Dexpert H, Felenbok B. First experimental evidence of a zinc binuclear cluster in AlcR protein, mutational and X-ray absorption studies. Biochim Biophys Acta. 1997;1343:211–220. doi: 10.1016/s0167-4838(97)00112-x. [DOI] [PubMed] [Google Scholar]
- 2.Bailey L A, Ebbole D L. The fluffy gene of Neurospora crassa encodes a Gal4p-type C6 zinc cluster protein required for conidial development. Genetics. 1998;148:1813–1820. doi: 10.1093/genetics/148.4.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatnagar D, Cleveland T E, Cotty P J. Mycological aspects of aflatoxin formation. In: Eaton D L, Groopman J D, editors. The toxicology of aflatoxins: human health, veterinary, and agricultural significance. San Diego, Calif: Academic Press, Inc.; 1994. pp. 327–346. [Google Scholar]
- 4.Bhatnagar D, Ehrlich K C, Cleveland T E. Oxidation-reduction reactions in biosynthesis of secondary metabolites. In: Bhatnagar D, Lillehoj E B, Arora D K, editors. Handbook of applied mycology. Vol. 5. New York, N.Y: Marcel Dekker; 1992. pp. 255–285. [Google Scholar]
- 5.Brown D W, Yu J H, Kelkar H S, Fernandes M, Nesbitt T C, Keller N P, Adams T H, Leonard T J. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc Natl Acad Sci USA. 1996;93:1418–1422. doi: 10.1073/pnas.93.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caddick M X, Arst H N., Jr Nitrogen regulation in Aspergillus: are two fingers better than one? Gene. 1990;95:123–127. doi: 10.1016/0378-1119(90)90422-n. [DOI] [PubMed] [Google Scholar]
- 7.Cary, J. W., K. C. Ehrlich, M. S. Maureen, and D. Bhatnagar. A second copy of the aflatoxin pathway regulatory gene, aflR, in Aspergillus parasiticus is non-functional. Submitted for publication.
- 8.Chang P-K, Ehrlich K C, Yu J, Bhatnagar D, Cleveland T E. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl Environ Microbiol. 1995;61:2372–2377. doi: 10.1128/aem.61.6.2372-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chervitz S A, Aravind L, Sherlock G, Ball C A, Koonin E V, Dwight S S, Hariss M A, Dolinski K, Mohr S, Smith T, Weng S, Cherry J M, Botstein D. Comparison of the complete protein sets of worm and yeast: orthology and divergence. Science. 1998;282:2022–2028. doi: 10.1126/science.282.5396.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corton J C, Moreno E, Johnston S A. Alterations in the GAL4 DNA-binding domain can affect transcriptional activation independent of DNA binding. J Biol Chem. 1998;273:13776–13780. doi: 10.1074/jbc.273.22.13776. [DOI] [PubMed] [Google Scholar]
- 11.Dhawale S S, Lane C A. Compilation of sequence-specific DNA-binding proteins implicated in transcriptional control in fungi. Nucleic Acids Res. 1993;24:5537–5546. doi: 10.1093/nar/21.24.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich K C, Montalbano B G, Bhatnagar D, Cleveland T E. Alteration of different domains in AFLR affects aflatoxin pathway metabolism in Aspergillus parasiticus transformants. Fungal Genet Biol. 1998;23:279–287. doi: 10.1006/fgbi.1998.1045. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich, K. C., B. G. Montalbano, and J. W. Cary. Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticus. Gene, in press. [DOI] [PubMed]
- 14.Feng B, Marzluf G A. The regulatory protein NIT4 that mediates nitrate induction in Neurospora crassa contains a complex tripartite activation domain with a novel leucine-rich motif. Curr Genet. 1996;29:537–548. doi: 10.1007/BF02426958. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes M, Keller N P, Adams T H. Sequence-specific binding by Aspergillus nidulans AFLR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol Microbiol. 1998;28:1355–1365. doi: 10.1046/j.1365-2958.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty J E, Payne G A. Overexpression of aflR leads to upregulation of pathway gene transcription and increased aflatoxin production in Aspergillus flavus. Appl Environ Microbiol. 1997;63:3995–4000. doi: 10.1128/aem.63.10.3995-4000.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garnier J, Osguthorpe D J, Bobson B. Analysis of the accuracy and implication of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978;120:97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- 18.Giniger E, Ptashne M. Transcription in yeast activated by a putative amphipathic α-helix linked to a DNA binding unit. Nature. 1987;330:670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- 19.Gill G, Ptashne M. Mutants of GAL4 protein altered in an activation function. Cell. 1987;51:121–126. doi: 10.1016/0092-8674(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 20.Ho S N, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 21.Hope I A, Mahadevan S, Struhl K. Structural and functional characterization of the short acidic transcriptional activation region of yeast GCN4 protein. Nature. 1988;333:635–640. doi: 10.1038/333635a0. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Fukada Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh S S, Ansari A Z, Ptashne M, Young R A. An activator target in the RNA polymerase II holoenzyme. Mol Cell. 1998;1:895–904. doi: 10.1016/s1097-2765(00)80088-x. [DOI] [PubMed] [Google Scholar]
- 24.Lu R, Yang P, O’Hare P, Misra V. Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol. 1997;17:5117–26. doi: 10.1128/mcb.17.9.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987;48:847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- 26.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 27.Payne G A, Brown M P. Genetics and physiology of aflatoxin biosynthesis. Annu Rev Phytopathol. 1998;36:329–362. doi: 10.1146/annurev.phyto.36.1.329. [DOI] [PubMed] [Google Scholar]
- 28.Smidt M P, Snippe L, van Keuken G, Ab G. The bZip transcription factor vitellogenin-binding protein is post transcriptional down regulated in chicken liver. Eur J Biochem. 1998;15:106–111. doi: 10.1046/j.1432-1327.1998.2560106.x. [DOI] [PubMed] [Google Scholar]
- 29.Todd R B, Andrianopoulos A. Evolution of a fungal regulatory gene family: the Zn(II)2Cys6 binuclear cluster DNA binding motif. Fungal Genet Biol. 1997;21:388–405. doi: 10.1006/fgbi.1997.0993. [DOI] [PubMed] [Google Scholar]
- 30.Todd R B, Kelly J M, Davis M A, Hynes M J. Molecular characterization of mutants of the acetate regulatory gene facB of Aspergillus nidulans. Fungal Genet Biol. 1997;22:92–102. doi: 10.1006/fgbi.1997.1007. [DOI] [PubMed] [Google Scholar]
- 31.Van Hoy M, Leuther K K, Kodadek T, Johnston S A. The acidic activation domains of the GCN4 and GAL4 proteins are not α helical but form β sheets. Cell. 1993;72:587–594. doi: 10.1016/0092-8674(93)90077-4. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe C M, Wilson D, Linz J E, Townsend C A. Demonstration of the catalytic roles and evidence for the physical association of type I fatty acid synthases and a polyketide synthase in the biosynthesis of aflatoxin B1. Chem Biol. 1996;3:463–469. doi: 10.1016/s1074-5521(96)90094-0. [DOI] [PubMed] [Google Scholar]
- 33.Woloshuk C P, Foutz K R, Brewer J F, Bhatnagar D, Cleveland T E, Payne G A. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl Environ Microbiol. 1994;60:2408–2414. doi: 10.1128/aem.60.7.2408-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J-H, Butchko R A E, Fernandes M, Keller N P, Leonard T J, Adams T H. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr Genet. 1996;29:549–555. doi: 10.1007/BF02426959. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Chang P-K, Cary J W, Wright M, Bhatnagar D, Cleveland T E, Payne G A, Linz J E. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus. Appl Environ Microbiol. 1995;61:2365–2371. doi: 10.1128/aem.61.6.2365-2371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson A J, Fuller L J, Jeenes D J, Archer D B. Homologs of aflatoxin biosynthesis genes and sequence of aflR in Aspergillus oryzae and Aspergillus sojae. Appl Environ Microbiol. 1999;65:307–310. doi: 10.1128/aem.65.1.307-310.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]