Abstract
Osteosarcoma (OS) is the most common primary bone tumor in children and adolescents. The etiology of OS is largely unknown but may be informed by comparisons of incidence and trends between geographic regions. Using the Cancer Incidence in Five Continents (CI5) data from 1988–2012, we present OS age-standardized incidence rates (ASRs; cases/million) and average annual percent change (AAPC) and 95% confidence intervals (CI) by geographic region among the age groups 0–9, 10–19, 20–29, 30–59, 60–79, 0–79. Among the 10–19 age group, we also used the most recent data (2008–2012) to present the ASRs for each country. We observed little variation in OS incidence between geographic regions in 2008–2012 across all age-groups. Overall, the ASR for 0–79 ranged from 2 cases per million in Southern Asia to 4.2 in Sub-Saharan Africa. A bimodal distribution in incidence was observed with peaks in the 10–19 and 60–79 age-groups across all regions over time. Overall, OS incidence was relatively stable across 1988–2012 with the only statistically significant increases in the 0–79 age group observed in Eastern Asia (AAPC: 1.8; 95%CI: 0.6, 1.9) and Sub-Saharan Africa (AAPC: 3.1; 95% CI: 0.5, 5.8). The small variation in incidence between regions and the stability in incidence over time suggests that OS carcinogenesis is not influenced by environmental or time-varying exposures.
Keywords: Osteosarcoma, Incidence, International, Bone Cancer, Epidemiology
Introduction
Primary malignant bone tumors account for 3 to 5 % of cancers in children and adolescents, but are rare in adults, corresponding to 1% of all cancers in adulthood.1–3 Osteosarcoma (OS) is the most commonly diagnosed bone tumor, accounting for 20–40% of all bone cancers.4 The average 5-year overall survival for individuals with localized OS is 80%.5 However, individuals with metastatic disease present lower short- and long-term survival rates.6,7 Osteosarcoma (OS) incidence has a bimodal age distribution, with peaks of incidence occurring in the 2nd-3rd and 7th-8th decades of life, respectively.4
Prior irradiation, chemotherapy with alkylating agents,8 heritable retinoblastoma,9 Li Fraumeni Syndrome, Rothmund Thomson Syndrome10–12 and Paget’s disease of bone (PDB)13 have all been associated with increased risk for OS, but account for a small proportion of cases. The etiology of most OS remains unknown and international comparisons can help in the generation of new hypotheses. Thus, here we evaluate the pattern of incidence of OS in the Cancer Incidence in Five Continents (CI5) using the most recent dataset available. We also present an analysis of trends in incidence by evaluating the average annual percent change (AAPC) over the 25-year period from 1988 to 2012, for the first time, by geographic regions and stratified by age-groups.
Materials and Methods
We extracted data from the last five available volumes of the CI5 series to evaluate incidence rates and trends of osteosarcoma. Registries within the CI5 series adhere to strict quality criteria for inclusion and, since 1988, report histological classifications, facilitating an analysis of OS specifically rather than combined bone tumors.14,15 The data are available in five-year periods: 1988–1992, 1993–1997, 1998–2002, 2003–2007, 2008–2012.16–20 We pooled registries within countries and further classified the registries into regional categories as defined by the United Nations Statistics Division (UNSD).21 Small sample sizes precluded us from categorizing regions in Africa or Oceania by the smaller subdivisions included in the UNSD scheme. We also excluded Central Asia as the CI5 data only had one country at one time period in the region. Therefore, the regions were categorized as North Africa, Sub-Saharan Africa, Caribbean, Central America, South America, North America, Eastern Asia, South-eastern Asia, Southern Asia, Western Asia, Eastern Europe, Northern Europe, Southern Europe, Western Europe, and Oceania.
The age-standardized incidence rates (ASRs) are expressed per million population and were calculated using the World Health Organization (WHO) 2000–2025 world standard population.22 In order to evaluate peaks in incidence during the latest time-period (2008–2012), cases were aggregated into five age-groups (0–9, 10–19, 20–29, 30–59, 60–79) and stratified by sex and geographic region. The 80–84 and 85+ age-groups were excluded from the analysis due to insufficient population sizes, specifically some regions had no data for this age group limiting any comparability. ASRs with less than 5 cases were not presented in tables. We calculated 95% confidence intervals (CI) using Poisson approximation.
We assessed trends in incidence of OS by calculating the average annual percent change (AAPC) and 95% CIs using Joinpoint software. This technique applies regression lines on a logarithmic scale which best fit over the ASRs according to underlying joinpoints.23 The slope coefficients obtained represent the annual percent change (APC) of each joinpoint segment and AAPC is calculated as the weighted average of the coefficients.23 As there was no joinpoint observed in the five time-periods, the AAPC is equal to the APC. We calculated AAPC in all ages (0–79), in five age-groups from 0–9 to 60–79, and sex-stratified in combined and specific age-groups. AAPCs with less than 5 cases for more than 2 time periods were excluded from the analysis.
All analyses were performed using Stata 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.) and Joinpoint Regression Program Version 4.7.0.0 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute). Figures 1, 3 and 4 were created in GraphPad Prism v8.0.0 (GraphPad Software, La Jolla, CA). Figure 2 was created in ArcGIS Desktop 10.5.1 (Environmental Systems Research Institute, Redlands, CA). Statistical significance was determined using two-sided hypothesis tests (alpha of 0.05).
Figure 1:

Osteosarcoma (OS) age standardized incidence rates (ASR) and 95% confidence intervals (CI) by age group, sex, and geographic region using the Cancer Incidence in Five Continents (CI5) data (2008–2012).
Figure 3:
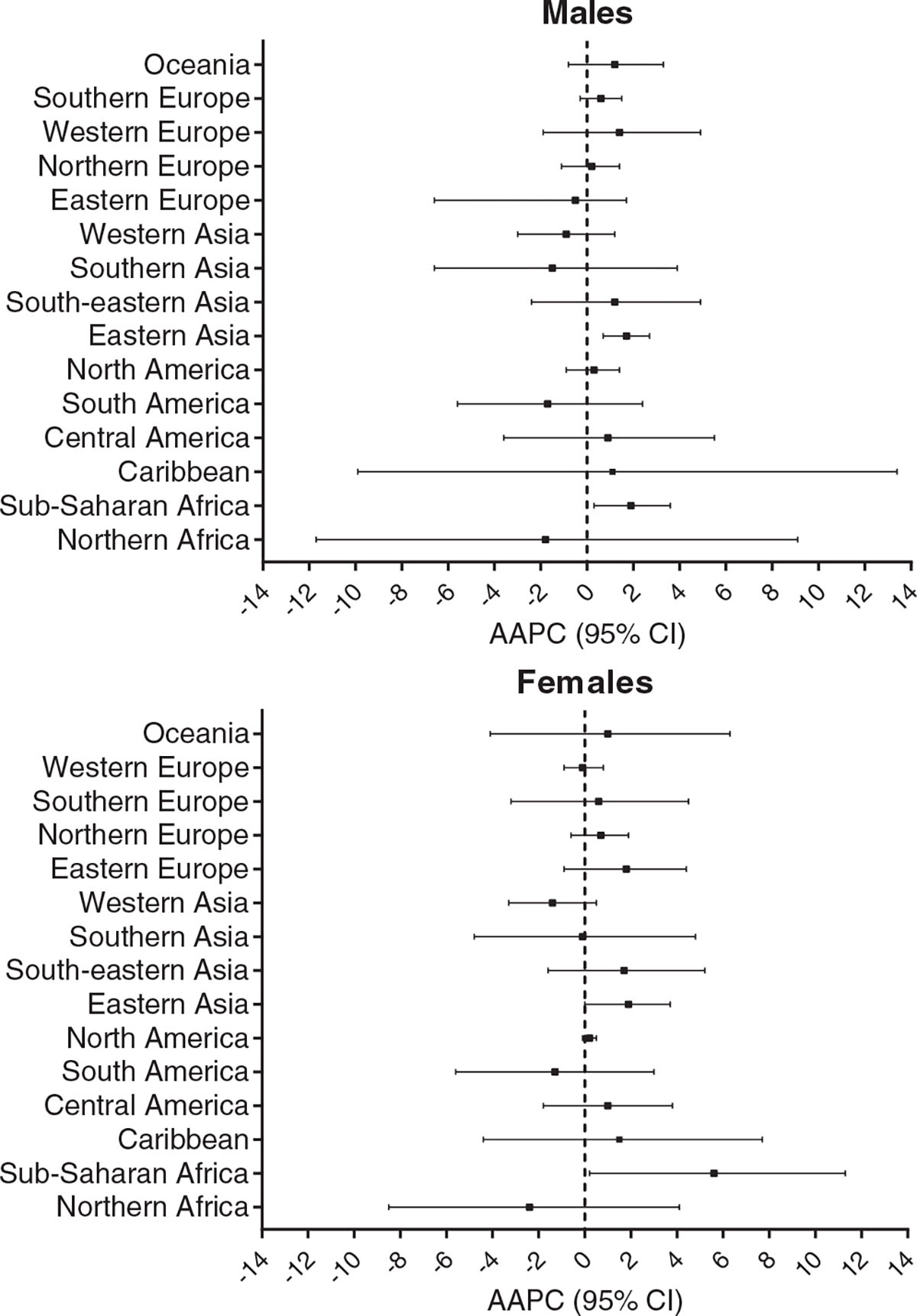
Overall average annual percent change (AAPC) and 95% confidence interval (CI) in each geographic region for males and females using the Cancer Incidence in Five Continents (CI5) data from 1988 to 2012.
Figure 4:
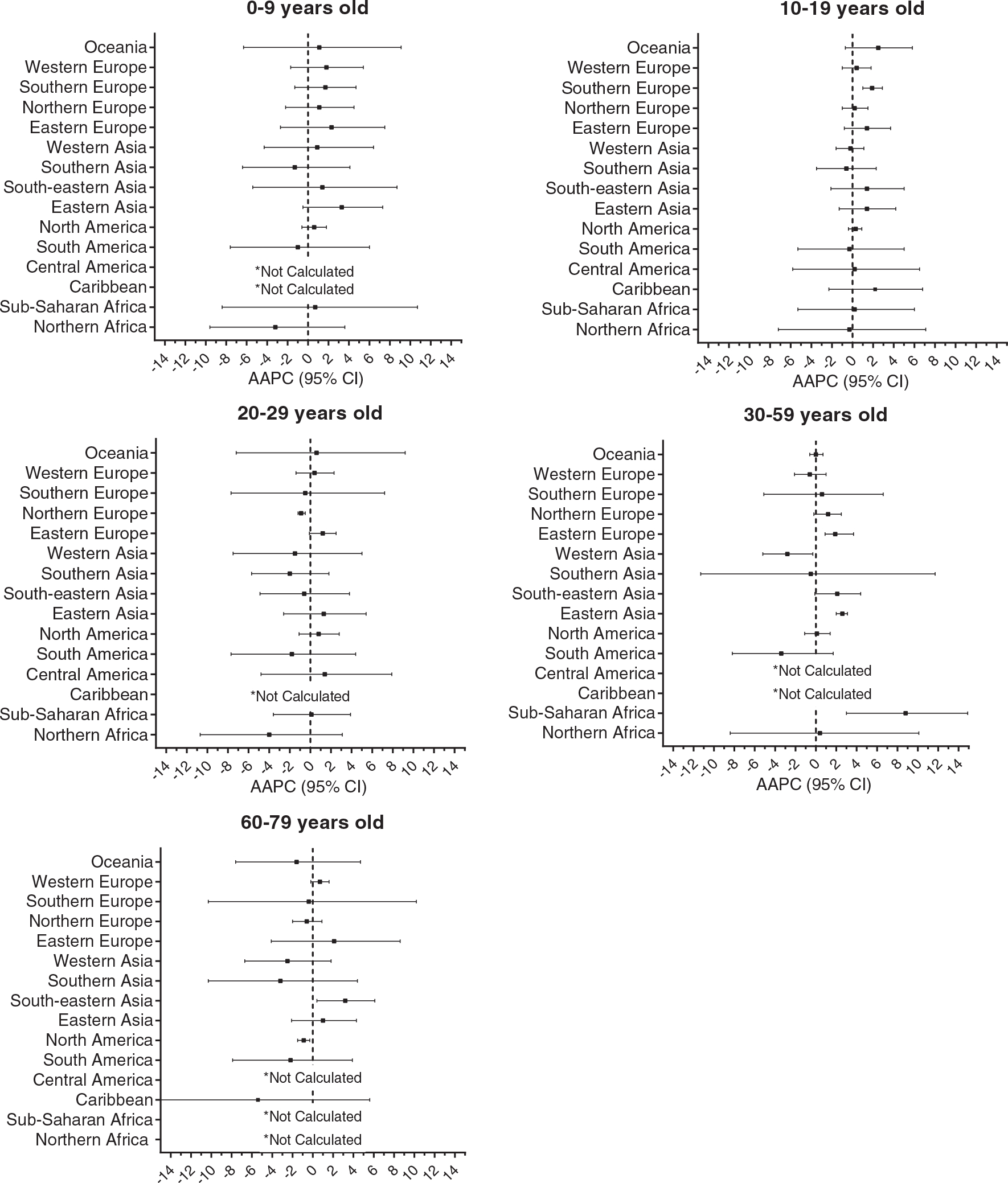
Average annual percent change (AAPC) and 95% confidence interval (CI) in by geographic region in each age group using the Cancer Incidence in Five Continents (CI5) data from 1988 to 2012. *AAPCs were not calculated when there were less than 5 cases in three or more time-periods.
Figure 2:
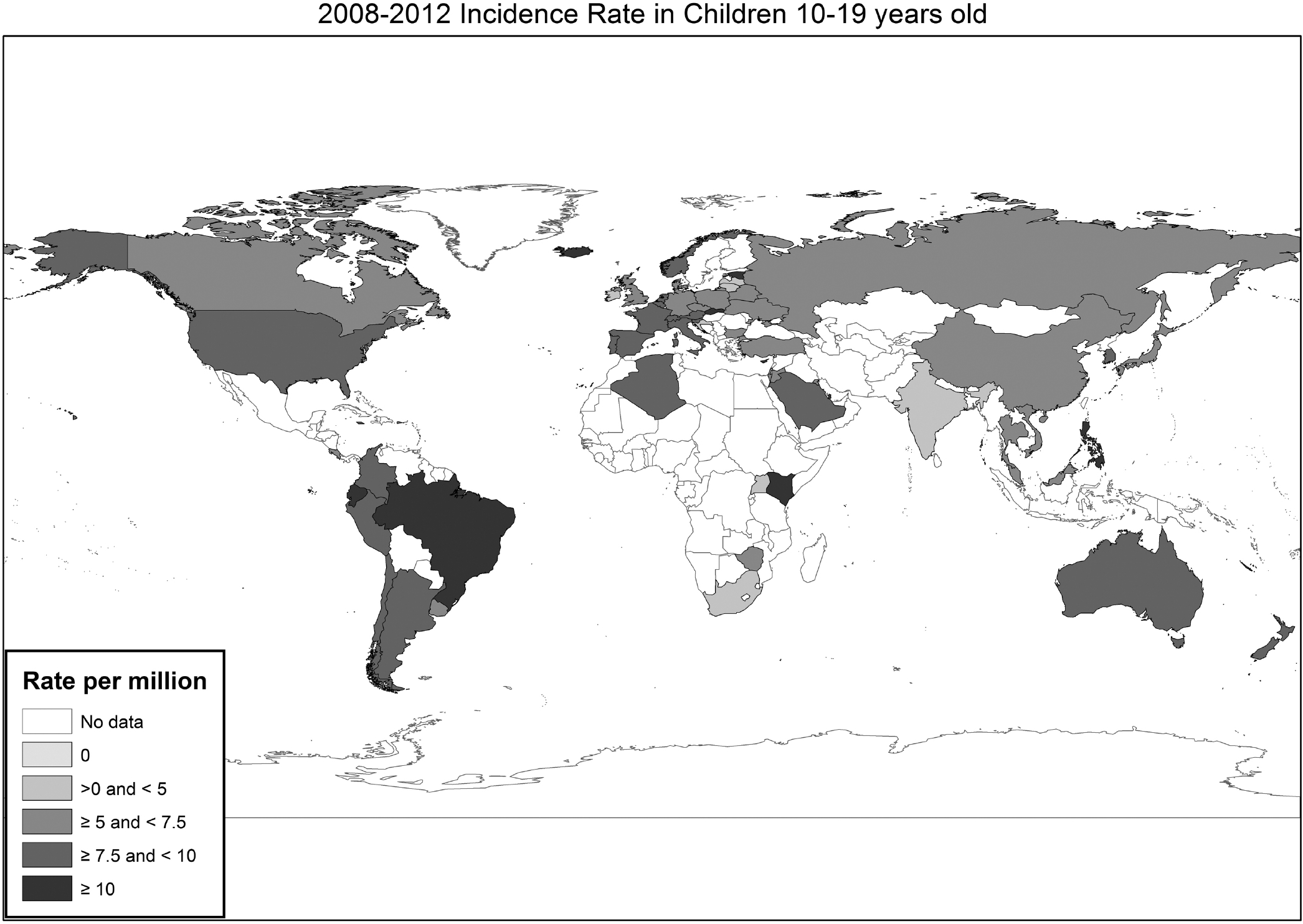
Osteosarcoma (OS) age standardized incidence rates (ASR) per country in the 10–19-year-old age category in the Cancer Incidence in Five Continents (CI5) in 2008–2012.
Results
Standardized Incidence Rates - CI5 Volume XI (2008–2012)
Overall incidence of OS displayed little variation across geographic regions. Age-standardized rates (ASR) for 0–79 years ranged from 2 cases per million in Southern Asia, to 4.2 in Sub-Saharan Africa (Table 1). After stratifying by sex, rates in males ranged from 2.2 cases per million in Southern Asia, to 4.8 in North Africa (Figure 1). Rates in females ranged from 1.8 cases per million in Southern Asia to 4 in Sub-Saharan Africa.
Table 1.
Osteosarcoma (OS) age standardized incidence rates (ASR) per million and number of cases (N) by time period and age category and the average annual percent change (AAPC) in incidence over 1988–2012 from the Cancer Incidence in Five Continents (CI5) data
| Age Standardized Incidence Rates1 (N) | |||||||
|---|---|---|---|---|---|---|---|
| Age group | 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | 2008–2012 | AAPC (95% CI)2,3 | |
|
| |||||||
| Northern Africa | 0–9 yrs | -(2) | 2.7(6) | 1.8(10) | 1.3(12) | -(3) | −3.2 (−9.6, 3.6) |
| 10–19 yrs | -(4) | 11.9(29) | 7.2(40) | 8.2(81) | 9.4(24) | −0.3 (−7.2, 7.1) | |
| 20–29 yrs | -(3) | 7.7(18) | 5.0(23) | 3.5(31) | 4.1(11) | −4.0 (−10.7, 3.1) | |
| 30–59 yrs | -(2) | 3.2(10) | 1.9(13) | 1.9(29) | 3.4(13) | 0.4 (−8.4, 10.1) | |
| 60–79 yrs | -(0) | 10.4(8) | -(3) | 0.9(4) | -(2) | - | |
| 0–79 yrs | 2.5(11) | 5.9(71) | 3.3(89) | 3.1(157) | 4.1(53) | −2.1 (−9.8, 6.3) | |
|
| |||||||
| Sub-Saharan Africa | 0–9 yrs | -(3) | -(1) | 1.3(5) | 1.1(7) | 0.9(8) | 0.7 (−8.4, 10.7) |
| 10–19 yrs | 3.8(11) | 7.8(33) | 8.7(35) | 6.2(40) | 6.8(53) | 0.2 (−5.3, 6.0) | |
| 20–29 yrs | 4.0(12) | 2.8(11) | 2.4(10) | 3.2(19) | 3.5(33) | 0.1 (−3.6, 3.9) | |
| 30–59 yrs | -(4) | -(4) | 2.3(7) | 2.8(16) | 4.7(37) | 8.8 (3.0, 14.9) | |
| 60–79 yrs | -(3) | -(1) | -(3) | 5.9(5) | 4.9(6) | - | |
| 0–79 yrs | 2.5(33) | 2.3(50) | 3.7(60) | 3.5(87) | 4.2(137) | 3.1 (0.5, 5.8) | |
|
| |||||||
| Caribbean | 0–9 yrs | -(0) | -(4) | -(1) | 1.7(7) | 1.6(5) | - |
| 10–19 yrs | -(0) | 5.0(10) | 7.2(6) | 6.1(27) | 7.3(26) | 2.2 (−2.3, 6.8) | |
| 20–29 yrs | -(0) | -(4) | -(2) | 2.9(12) | 2.1(7) | - | |
| 30–59 yrs | -(0) | -(2) | -(2) | 1.6(18) | 1.4(13) | - | |
| 60–79 yrs | -(0) | 4.4(6) | -(0) | 2.6(10) | 1.7(6) | −5.4 (−16.9, 7.6) | |
| 0–79 yrs | -(0) | 2.2(26) | 2.3(11) | 2.7(74) | 2.6(57) | 1.1 (−2.2, 4.6) | |
|
| |||||||
| Central America | 0–9 yrs | -(4) | -(3) | 1.2(5) | -(2) | -(3) | - |
| 10–19 yrs | 5.2(16) | 9.7(14) | 8.5(35) | 5.6(24) | 7.3(25) | 0.2 (−5.8, 6.5) | |
| 20–29 yrs | 2.1(6) | -(1) | 2.9(10) | 3.1(12) | 2.4(8) | 1.4 (−4.8, 7.9) | |
| 30–59 yrs | -(4) | -(1) | -(4) | 0.8(6) | 1.6(11) | - | |
| 60–79 yrs | -(0) | -(0) | -(1) | -(1) | -(2) | - | |
| 0–79 yrs | 1.8(30) | 2.4(19) | 2.5(55) | 1.9(45) | 2.6(49) | 1.1 (−2.3, 4.6) | |
|
| |||||||
| South America | 0–9 yrs | 1.4(12) | 1.6(9) | 2.5(39) | 1.6(39) | 1.5(37) | −1.0 (−7.6, 6.0) |
| 10–19 yrs | 8.9(80) | 8.6(51) | 13.5(228) | 11.7(307) | 9.2(243) | −0.3 (−5.3, 5.0) | |
| 20–29 yrs | 4.3(37) | 3.4(20) | 5.9(101) | 4.3(112) | 3.2(83) | −1.8 (−7.7, 4.4) | |
| 30–59 yrs | 3.1(40) | 1.7(18) | 3.0(96) | 1.8(94) | 1.6(90) | −3.4 (−8.2, 1.7) | |
| 60–79 yrs | 5.2(16) | 5.6(14) | 2.1(17) | 3.3(42) | 3.3(48) | −2.2 (−7.9, 3.9) | |
| 0–79 yrs | 4.2(185) | 3.6(112) | 5.1(481) | 4.1(594) | 3.3(501) | 0.3 (−0.5, 1.0) | |
|
| |||||||
| North America | 0–9 yrs | 1.5(53) | 1.7(63) | 1.5(107) | 1.6(125) | 1.7(125) | 0.6 (−0.6, 1.8) |
| 10–19 yrs | 7.4(251) | 7.9(296) | 8.2(575) | 7.9(637) | 8.1(626) | 0.3 (−0.4, 0.9) | |
| 20–29 yrs | 2.3(92) | 3.0(116) | 3.1(211) | 3.0(241) | 3.0(240) | 0.8 (−1.1, 2.8) | |
| 30–59 yrs | 1.5(149) | 1.8(203) | 1.8(362) | 1.7(426) | 1.7(399) | 0.1 (−1.1, 1.4) | |
| 60–79 yrs | 3.6(120) | 3.6(133) | 3.2(194) | 3.1(235) | 3.0(243) | −0.9 (−1.5, −0.3) | |
| 0–79 yrs | 2.9(665) | 3.2(811) | 3.2(1449) | 3.1(1664) | 3.2(1633) | 0.3 (−0.5, 1.0) | |
|
| |||||||
| Eastern Asia | 0–9 yrs | 0.7(16) | 1.0(34) | 1.6(71) | 1.5(79) | 1.7(105) | 3.3 (−0.5, 7.3) |
| 10–19 yrs | 5.8(128) | 5.7(240) | 7.5(372) | 8.3(518) | 7.3(579) | 1.4 (−1.3, 4.2) | |
| 20–29 yrs | 1.3(33) | 2.1(102) | 2.5(147) | 2.0(150) | 2.4(247) | 1.3 (−2.6, 5.4) | |
| 30–59 yrs | 1.1(83) | 1.3(171) | 1.6(250) | 1.7(395) | 2.0(650) | 2.6 (2.0, 3.1) | |
| 60–79 yrs | 3.2(70) | 3.2(120) | 2.4(114) | 2.9(208) | 3.6(415) | 1.0 (−2.1, 4.3) | |
| 0–79 yrs | 2.1(330) | 2.4(667) | 2.8(954) | 3.0(1350) | 3.1(1996) | 1.8 (0.6, 2.9) | |
|
| |||||||
| South-eastern Asia | 0–9 yrs | -(4) | 1.3(33) | 1.6(23) | 1.5(38) | 1.3(36) | 1.4 (−5.4, 8.7) |
| 10–19 yrs | 6.6(70) | 8.0(208) | 11.0(145) | 10.6(267) | 9.2(267) | 1.4 (−2.1, 5.0) | |
| 20–29 yrs | 2.3(26) | 3.4(97) | 4.0(54) | 3.4(93) | 2.6(93) | −0.6 (−4.9, 3.8) | |
| 30–59 yrs | 1.3(22) | 1.7(82) | 2.2(60) | 2.3(131) | 2.2(158) | 2.1 (−0.1, 4.4) | |
| 60–79 yrs | 2.5(8) | 2.8(26) | 3.3(19) | 4.7(50) | 4.4(61) | 3.2 (0.4, 6.1) | |
| 0–79 yrs | 2.4(130) | 3.1(446) | 4.0(301) | 4.0(579) | 3.6(615) | 1.4 (−1.9, 4.8) | |
|
| |||||||
| Southern Asia | 0–9 yrs | 0.7(15) | 0.6(23) | 0.9(31) | 0.7(28) | 0.5(19) | −1.3 (−6.4, 4.1) |
| 10–19 yrs | 6.1(117) | 6.1(219) | 6.2(222) | 6.7(324) | 4.9(220) | −0.6 (−3.5, 2.3) | |
| 20–29 yrs | 3.0(60) | 3.5(222) | 2.6(96) | 3.0(149) | 2.0(101) | −2.0 (−5.7, 1.8) | |
| 30–59 yrs | 1.0(30) | 1.3(324) | 2.9(168) | 1.5(115) | 1.4(128) | −0.5 (−11.3, 11.7) | |
| 60–79 yrs | 2.5(12) | 3.2(220) | 6.7(72) | 2.5(40) | 1.8(36) | −3.2 (−10.3, 4.4) | |
| 0–79 yrs | 2.3(234) | 2.6(1102) | 3.5(589) | 2.6(656) | 2.0(504) | −1.1 (−2.9, 0.7) | |
|
| |||||||
| Western Asia | 0–9 yrs | 0.9(6) | 1.1(12) | 0.8(14) | 1.4(27) | 1.1(34) | 0.9 (−4.3, 6.4) |
| 10–19 yrs | 7.0(40) | 6.9(66) | 7.1(116) | 7.5(136) | 6.6(200) | −0.2 (−1.6, 1.1) | |
| 20–29 yrs | 4.2(21) | 3.6(29) | 2.0(31) | 3.8(70) | 2.6(81) | −1.5 (−7.5, 5.0) | |
| 30–59 yrs | 1.8(18) | 2.1(32) | 1.8(55) | 1.6(58) | 1.2(81) | −2.8 (−5.2, −0.3) | |
| 60–79 yrs | 2.9(10) | 2.2(8) | 3.1(24) | 2.0(19) | 1.8(27) | −2.5 (−6.7, 1.8) | |
| 0–79 yrs | 3.1(95) | 3.0(147) | 2.7(240) | 3.0(310) | 2.4(423) | −1.1 (−2.9, 0.7) | |
|
| |||||||
| Eastern Europe | 0–9 yrs | 0.7(18) | 0.5(12) | 1.0(24) | 0.8(32) | 1.1(48) | 2.3 (−2.7, 7.5) |
| 10–19 yrs | 4.8(120) | 4.1(114) | 5.9(177) | 5.4(334) | 6.1(322) | 1.4 (−0.8, 3.7) | |
| 20–29 yrs | 1.8(39) | 1.6(40) | 1.8(60) | 2.0(144) | 2.1(158) | 1.2 (−0.1, 2.5) | |
| 30–59 yrs | 1.1(70) | 0.9(68) | 1.2(106) | 1.4(274) | 1.5(320) | 1.9 (0.9, 3.7) | |
| 60–79 yrs | 2.9(66) | 1.5(42) | 2.6(96) | 4.0(322) | 3.4(285) | 2.1 (−4.1, 8.6) | |
| 0–79 yrs | 2.0(313) | 1.6(276) | 2.3(463) | 2.3(1106) | 2.5(1133) | 1.6 (−0.5, 3.8) | |
|
| |||||||
| Northern Europe | 0–9 yrs | 1.0(40) | 1.3(81) | 1.3(70) | 1.1(61) | 1.5(74) | 1.1 (−2.2, 4.5) |
| 10–19 yrs | 6.2(262) | 7.0(400) | 7.2(426) | 6.5(406) | 7.2(368) | 0.2 (−1.0, 1.5) | |
| 20–29 yrs | 2.7(132) | 2.6(166) | 2.6(148) | 2.4(146) | 2.3(129) | −0.9 (−1.2, −0.5) | |
| 30–59 yrs | 1.1(142) | 1.2(221) | 1.4(271) | 1.3(268) | 1.5(259) | 1.2 (−0.2, 2.5) | |
| 60–79 yrs | 3.1(148) | 2.3(171) | 2.2(166) | 2.3(189) | 2.3(168) | −0.6 (−2.0, 0.9) | |
| 0–79 yrs | 2.7(724) | 2.6(1039) | 2.7(1081) | 2.5(1070) | 2.7(998) | 0.4 (−0.7, 1.4) | |
|
| |||||||
| Southern Europe | 0–9 yrs | 1.4(17) | 1.2(17) | 1.3(26) | 1.3(28) | 1.8(37) | 1.7 (−1.3, 4.7) |
| 10–19 yrs | 5.2(81) | 6.2(103) | 7.0(165) | 7.3(164) | 7.9(162) | 1.9 (1.0, 2.9) | |
| 20–29 yrs | 2.2(36) | 2.1(42) | 3.4(102) | 2.6(73) | 1.8(42) | −0.5 (−7.7, 7.2) | |
| 30–59 yrs | 1.2(55) | 1.3(75) | 2.1(85) | 1.8(172) | 1.5(135) | 0.6 (−5.1, 6.6) | |
| 60–79 yrs | 2.3(43) | 2.2(56) | 4.8(197) | 3.1(133) | 2.3(95) | −0.4 (−10.3, 10.2) | |
| 0–79 yrs | 2.2(232) | 2.4(293) | 3.3(675) | 2.9(570) | 2.8(471) | 1.0 (−2.5, 4.7) | |
|
| |||||||
| Western Europe | 0–9 yrs | 0.8(14) | 1.3(25) | 1.2(37) | 1.1(40) | 1.4(75) | 1.8 (−1.7, 5.4) |
| 10–19 yrs | 7.3(139) | 8.5(154) | 8.0(280) | 8.9(345) | 8.1(476) | 0.4 (−1.0, 1.8) | |
| 20–29 yrs | 2.4(58) | 3.1(69) | 2.6(93) | 2.9(120) | 2.8(180) | 0.4 (−1.4, 2.3) | |
| 30–59 yrs | 2.0(116) | 1.7(107) | 1.7(210) | 1.5(222) | 1.7(383) | −0.6 (−2.1, 1.0) | |
| 60–79 yrs | 2.3(52) | 2.5(58) | 2.7(135) | 2.8(175) | 2.7(275) | 0.7 (−0.2, 1.6) | |
| 0–79 yrs | 2.8(379) | 3.1(413) | 2.9(755) | 3.1(902) | 3.1(1392) | 0.3 (−0.4, 1.0) | |
|
| |||||||
| Oceania | 0–9 yrs | 0.6(8) | 0.9(14) | 1.4(23) | 1.2(19) | 0.8(14) | 1.1 (−6.3, 9.1) |
| 10–19 yrs | 4.4(60) | 6.8(105) | 7.7(126) | 8.8(148) | 8.2(143) | 2.5 (−0.7, 5.8) | |
| 20–29 yrs | 1.2(17) | 2.9(49) | 3.5(58) | 2.1(35) | 2.6(49) | 0.6 (−7.2, 9.2) | |
| 30–59 yrs | 1.5(50) | 1.7(71) | 1.5(74) | 1.6(79) | 1.6(85) | 0.0 (−0.6, 0.7) | |
| 60–79 yrs | 2.5(30) | 4.7(74) | 3.4(52) | 2.5(43) | 2.9(58) | −1.6 (−7.6, 4.7) | |
| 0–79 yrs | 1.9(165) | 2.9(313) | 3.1(333) | 2.9(324) | 2.9(349) | 1.2 (−2.1, 4.5) | |
Rates were not calculated for strata with less than 5 cases are denoted by a dash (-).
Estimates in bold are statistically significant.
AAPCs were not calculated for categories with < 5 cases for 3 or more time periods, as denoted by a dash (-).
Primary peak incidence comparisons (10–19 age group)
The 10–19 age-group presented the first peak in incidence of OS as well as largest difference in male and female rates (Figure 1). Among regional groups, incidence ranged from 4.9 to 9.4 cases per million (Table 1). The highest rates were observed in Northern Africa, Southeastern Asia and South America at 9.4, 9.2 and 9.2 cases per million. The lowest incidence rates were seen in Southern Asia, Eastern Europe, and Western Asia at 4.9, 6.1, and 6.6 cases per million (Table 1). The highest rate among males was observed in South America (10.8), while the highest rate among females was seen in South-eastern Asia (7.7) (Figure 1). The lowest male and female rates were observed in Southern Asia (ASR: 5.3) and Southern Europe (ASR: 5.1), respectively. Southern Europe presented the highest male to female ratio of 2:1 in the 10–19 age-group.
When we evaluated rates by country, the highest rates were seen in Iceland (ASR:13.5), Slovakia (ASR:13) and the Philippines (ASR: 12.5) (Figure 2). Rates varied among countries within each region. The lowest rates were seen in South Africa (ASR: 2.8), Malta (ASR: 3.7) and Lithuania (ASR: 3.9).
Secondary peak incidence comparisons (60–79 age group)
The second peak was seen in the 60–79 age-group, where incidence ranged from 1.7 cases per million in the Caribbean to 4.9 cases per million in Sub-Saharan Africa (Table 1). Generally, males had higher incidence than females in most regions and age-groups. Rates among men ranged from 1.3 in Southern Asia to 4.6 in Eastern Europe, whilst among women, ranged from 1 in Caribbean, to 5.6 in Sub-Saharan Africa. In this age-group, Sub-Saharan Africa presented a male to female ratio of 0.72 :1 (Figure 1).
Trends in Incidence of Osteosarcoma (1988–2012)
In most regions, we did not observe a significant change in incidence of OS from 1988 – 2012 overall (Figure 3). Age-stratified results also supported that OS incidence was relatively stable over the time-period.
Eastern Asia and Sub-Saharan Africa were the only regions with statistically significant increasing trends in incidence overall (Table 1). Represented by China, Japan and Korea, Eastern Asia showed a slight increasing trend (AAPC: 1.8; 95% CI: 0.6, 2.9), which was similar for men (AAPC: 1.7; 95% CI: 0.7 ,2.7) and women (AAPC: 1.9; 95% CI: 0.0, 3.7). While Sub Saharan Africa showed a comparable trend among males (AAPC: 1.9; 95% CI: 0.3, 3.6), incidence in women rose much faster with an average annual increase of 5.6 % (95% CI: 0.2, 11.3) (Figure 3).
Age group 0 to 9 years
AAPCs ranged from −1% (95% CI: −7.6, 6.0) observed in South America, to 3.3% (95% CI: −0.5,7.3) in Eastern Asia (Table 1, Figure 4). There were few cases in many regions within this age group.
Age group 10 to 19 years
Southern Europe experienced a small, but relatively precise increase in incidence (AAPC:1.9; 95% CI: 1.0, 2.9). There were no other statistically significant trends observed in this age group. The other regions presented AAPCs ranging from −0.6%, Southern Asia, to 2.5%, in Oceania.
Age group 20 to 29 years
AAPCs ranged from −1.8, South America (95% CI: −7.7, 4.4) to 1.3 (95% CI: −2.6, 5.4) observed in Eastern Asia. A slight, but statistically significant decrease was observed in Northern Europe (AAPC: −0.9; 95% CI: −1.2, −0.5). There were no other estimates displaying potential change over time in this age group. Rates remained particularly stable in Sub-Saharan Africa (AAPC: 0.1; 95% CI: −3.6, 3.9), Southern Europe (AAPC: −0.5; 95% CI: −7.7, 7.2) , Western Europe (AAPC: 0.4; 95% CI: −1.4, 2.3) and Oceania ( AAPC: 0.6; 95% CI: −7.2, 9,2).
Age group 30 to 59 years
An increasing trend was observed in Eastern Asia (AAPC: 2.6; 95% CI: 2.0, 3.1) and Eastern Europe (AAPC: 1.9; 95% CI: 0.9, 3.7). Western Asia had an average annual decrease of 2.8 % (95% CI: −5.2, −0.3). The other regions did not show significant AAPCs for this age group.
Age group 60 to 79 years
There were decreasing AAPC estimates in South America (AAPC:−2.2; 95% CI: −7.9, 3.9), Western Asia ( AAPC:−2.5; 95% CI: −6.7, 1.8), Oceania ( AAPC: −1.6; 95% CI: −7.6, 4.7) and North America ( AAPC: −0.9; 95% CI: −0.3 , −1.5), but most estimates lacked precision. Rates remained stable in Northern Europe (AAPC: −0.6; 95% CI: −2.0, 0.9), Southern Europe (AAPC: −0.5; 95% CI: −10.2, 10.3) and Western Europe (AAPC: 0.7; 95% CI: −0.2, 1.6). Increasing trends were observed in Eastern Europe (AAPC: 2.1; 95% CI: −4.0, 8.6), Eastern Asia (AAPC: 1; 95% CI: −2.1, 4.3) and South-eastern Asia (AAPC: 3.2; 95% CI: 0.4, 6.1).
Discussion
This study provides a comprehensive comparison of osteosarcoma incidence rates over geographic regions, across multiple age groups and over time. In most regions, we observed OS incidence rates to follow a bimodal incidence peak, with the first peak occurring in the 10–19-year age group and the secondary peak occurring after 60 years of age. We also observed a slight male excess, particularly in the 10–19-year age group. Across geographic regions, the age-standardized incidence rates showed little variability overall. Additionally, we observed incidence rates to remain relatively stable over the 25-year timespan of this study, with slight overall increases observed in Sub-Saharan Africa and Eastern Asia. Age-stratified trend analyses generally lacked enough precision or consistency across age groups to draw conclusions.
The primary incidence peak in the 10–19 age-group has long been associated with height attainment during the pubertal growth spurt.24,25 While incidence rates in 2008–2012 among the 10–19 age group were variable by country, we observed some of the highest OS incidence rates among the tallest populations, including those in The Netherlands, Iceland, Slovakia, and Czech Republic.26,27 A large meta-analysis has suggested that individuals who are taller are at greater risk of OS.28 Given the multifactorial nature of height and its complex genetic architecture, some observational studies may be limited for investigating association between height attainment and OS risk. However, more than 400 loci have been identified in relation to adult height29 and studies using these loci to construct a polygenic height score (PHS) have found heritable height associated with increases in OS risk.30 Further studies using mendelian randomization have estimated a 75% increase in OS risk per 10-cm increase in adult height.30,31 Height is related to the number of bone stem cell divisions and this may be the underlying mechanism relating height to bone cancer risk.32
Overall, we observed few significant changes in the incidence of OS over the time period. An exception was noted in Southern Europe in the 10–19-year-old age group and Eastern Asia overall, where OS incidence rates increased over time. Interestingly, populations in both regions (South Korea, Japan, Hong Kong, Spain, Italy, Croatia, and Portugal) have reported notable increases in height over the last century.27 We also observed slight increasing rates in Sub-Saharan Africa, however, the heterogeneity in countries available for this region in the CI5 dataset could explain this increase. Few observed increases in other regions were notable, consistent or displayed precision in their estimates, a finding consistent with previous investigations.33–36 As noted previously, bone stem cells divisions have previously been highly correlated to lifetime risk of OS, indicating that DNA replication errors would substantially account for etiology of OS, rather than environmental exposures or hereditary factors.32 Thus, in our interpretation these findings support the possibility that random cellular disaster plays a greater role than exogenous risk factors in the etiology of osteosarcoma. In fact, more than 75% of osteosarcomas were recently found to have the signature of chromothripsis37, in which complex chromosomal rearrangements are generated in a single catastrophic event. Where we saw significant trends we cannot, however, rule out secular changes in environmental factors such as diet and endocrine disruptors.
We also observed osteosarcoma to occur more often in males compared to females. Other analyses have also reported that there is a slight male excess in osteosarcoma, particularly during the onset of puberty.5 Male excess is observed in many common and rare cancers, but within OS, the male excess was only consistent in ages 10–19. These differences in bone cancer risk at puberty have been known to parallel sex differences in skeletal growth with the peak occurring earlier and being lower in females compared to males.38 In some regions females had slightly higher incidence rates in older age groups, but these differences mainly occurred in regions with very small case counts in higher age groups.
The secondary incidence peak in individuals above 60 years old has been associated with Paget’s disease of bone (PDB).39,40 There is a scarcity of data regarding the incidence of PDB, but it has been suggested by a meta-analysis that prevalence, incidence, and severity of PDB is decreasing over the last decades.41 Bone cancers in this age range may also be associated with previous cancer treatment as prior irradiation and chemotherapy are known risk factors for OS.8 Cohort studies have reported incidence of OS as a second primary cancer in adults.42–45 Bone tumors as second primary cancers are increasing7, however, bone tumors are still rare second primary neoplasms.45–49
Analyses of trends over time can be influence by diagnostic substitution. Due to this limitation, we also assessed whether unspecified bone tumors changed in incidence over time. We observed many decreases in the incidence of unspecified tumors over time (Supplementary Table S1). Specifically, we observed that there were often large, consistent decreases in Eastern Europe and Oceania. It is unclear what proportion of unspecified tumors may be OS. These changes were also often based on small rates that would limit the influence of our estimates. However, if these decreasing trends are mostly comprised of OS, then many of our positive AAPCs may be even closer to the null.
Among the limitations of this study, the CI5 dataset consists not only of national, but smaller registries which may not be representative of the whole country. In addition, the regions of Southern Asia, Western Asia, Northern Africa and Central America encompass a small number of countries limiting the representativeness of findings in those geographic regions. Sub-Saharan Africa also has different countries represented in each time-period, which may not adequately represent the region and could account for variation in rates over time-periods. Another limitation of this study is the instability of some rates over time with small case counts.50 It is also possible that metastasis to the bones was misdiagnosed or miscoded as primary bone cancer, particularly in the elderly in whom bone metastasis is more common. Lastly, 95% CIs should be interpreted cautiously as our study did not adjust for multiple statistical comparisons.
The literature presents the incidence of OS through a restricted number of population-based studies which analyze different age-groups and time frames. Our study provides international comparisons using the most recent 25 years of CI5 in which morphological classification of cancers were added, therefore enhancing data validity. Moreover, monitoring trends in incidence stratified by age-groups may be relevant as variation may represent differences in risk factors over time or by region. Overall, our findings are consistent with previous literature36 indicating little variation between geographic regions and little change over time in OS incidence. Our findings support the possibility that OS is driven by random cellular disaster rather than locally or time-varying exogenous risk factors.
Supplementary Material
Novelty and Impact:
Osteosarcoma (OS) is the most diagnosed bone tumor, but few etiologic factors have been identified. Geographic comparisons and time-trend analyses may elucidate potential biological mechanisms. Our analysis showed little variation in OS incidence between geographic regions and small changes over time in OS incidence. Our results support the hypothesis that OS is driven by random cellular disaster rather than environment or other time-varying risk factors.
Funding
This work was supported by the National Institutes of Health (T32 CA099936 to AKH).
Abbreviations:
- AAPC
average annual percent change
- ASR
age-standardized incidence rates
- CI
confidence interval
- CI5
Cancer Incidence in Five Continents
- OS
osteosarcoma
- PDB
Paget’s disease of bone
Footnotes
Conflict of Interest
The authors have no conflict of interest relevant to this article to disclose.
Ethics Statement
This study used publicly-available, group-level data which did not require ethical approval.
Data Availability Statement
The Cancer Incidence in Five Continents data is publicly available at: https://ci5.iarc.fr/Default.aspx. Processed datasets used in the analysis are available from the corresponding author upon reasonable request.
References
- 1.Bleyer A O’Leary M, Barr R, Ries LAG: Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age. Natl Cancer Inst NIH Pub. No. 06–5767:218. [Google Scholar]
- 2.Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18(6):719–731. doi: 10.1016/S1470-2045(17)30186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ries LAG, Smith MA, Gurney JG, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. NIH Pub No 99–4649. Published online 1999:179 pp. [Google Scholar]
- 4.Valery PC, Laversanne M, Bray F. Bone cancer incidence by morphological subtype: a global assessment. Cancer Causes Control. 2015;26(8):1127–1139. doi: 10.1007/s10552-015-0607-3 [DOI] [PubMed] [Google Scholar]
- 5.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kager L, Zoubek A, Pötschger U, et al. Primary Metastatic Osteosarcoma: Presentation and Outcome of Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J Clin Oncol. 2003;21(10):2011–2018. doi: 10.1200/JCO.2003.08.132 [DOI] [PubMed] [Google Scholar]
- 7.Gorlick R, Janeway K, Lessnick S, Randall RL, Marina N, on behalf of the COG Bone Tumor Committee. Children’s Oncology Group’s 2013 blueprint for research: Bone tumors. Pediatr Blood Cancer. 2013;60(6):1009–1015. doi: 10.1002/pbc.24429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkins MM, Wilson LMK, Burton HS, et al. Radiotherapy, Alkylating Agents, and Risk of Bone Cancer After Childhood Cancer. JNCI J Natl Cancer Inst. 1996;88(5):270–278. doi: 10.1093/jnci/88.5.270 [DOI] [PubMed] [Google Scholar]
- 9.Yu C-L, Tucker MA, Abramson DH, et al. Cause-Specific Mortality in Long-Term Survivors of Retinoblastoma. JNCI J Natl Cancer Inst. 2009;101(8):581–591. doi: 10.1093/jnci/djp046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mai PL, Best AF, Peters JA, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort: Cancer Risk in TP53 Mutation Carriers. Cancer. 2016;122(23):3673–3681. doi: 10.1002/cncr.30248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mai PL, Khincha PP, Loud JT, et al. Prevalence of Cancer at Baseline Screening in the National Cancer Institute Li-Fraumeni Syndrome Cohort. JAMA Oncol. 2017;3(12):1640. doi: 10.1001/jamaoncol.2017.1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hameed M, Mandelker D. Tumor Syndromes Predisposing to Osteosarcoma: Adv Anat Pathol. 2018;25(4):217–222. doi: 10.1097/PAP.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s Disease of Bone. J Bone Miner Res. 2006;21(S2):P58–P63. doi: 10.1359/jbmr.06s211 [DOI] [PubMed] [Google Scholar]
- 14.Bray F, Parkin DM. Evaluation of data quality in the cancer registry: Principles and methods. Part I: Comparability, validity and timeliness. Eur J Cancer. 2009;45(5):747–755. doi: 10.1016/J.EJCA.2008.11.032 [DOI] [PubMed] [Google Scholar]
- 15.Parkin DM, Ferlay J, Shanmugaratnam K, Sobin L, Teppo L, Whelan SL. Chapter 4. Histological groups. In: Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J, editors. Cancer Incidence in Five Continents, Vol VIII. IARC Scientific Publications, 1997; No. 143:34–44. Lyon, IARC. [Google Scholar]
- 16.Parkin DM, Whelan SL, Ferlay J Raymond L, Young J editors. Cancer Incidence in Five Continents, Vol. VII. IARC Scientific Publications, 1997; No. 143, Lyon, IARC. [Google Scholar]
- 17.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors, Cancer Incidence in Five Continents, Vol VIII. IARC Scientific Publications, 2002; No. 155, Lyon, IARC. [Google Scholar]
- 18.Curado. MP, Edwards. B, Shin. HR, Storm. H, J. Ferlay., Heanue. M and Boyle. P, eds (2007). Cancer Incidence in Five Continents, Vol. IX. IARC Scientific Publications, 2007; No. 160, Lyon, IARC. [Google Scholar]
- 19.Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R and Ferlay J editors. Cancer Incidence in Five Continents, Vol. X. IARC Scientific Publication, 2014; No. 164, Lyon, IARC. [DOI] [PubMed] [Google Scholar]
- 20.Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R and Ferlay J editors. Cancer Incidence in Five Continents, Vol. XI. IARC Scientific Publications, 2017; No. 166, Lyon, IARC. [Google Scholar]
- 21.United Nations Statistics Division. UNSD — Methodology. Series M, No. 49. Accessed February 25, 2018. https://unstats.un.org/unsd/methodology/m49/ [Google Scholar]
- 22.Ahmad OB, Boschi-Pinto C, Lopez Christopher AD, Murray JL, Lozano R, Inoue M. AGE STANDARDIZATION OF RATES: A NEW WHO STANDARD; 2001, No. 31. [Google Scholar]
- 23.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682.\ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotterill SJ, Wright CM, Pearce MS, Craft AW. Stature of young people with malignant bone tumors. Pediatr Blood Cancer. 2004;42(1):59–63. [DOI] [PubMed] [Google Scholar]
- 25.Longhi A, Pasini A, Cicognani A, et al. Height as a Risk Factor for Osteosarcoma: J Pediatr Hematol Oncol. 2005;27(6):314–318. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Martinez A, Zhou B, Sophiea MK, et al. Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. The Lancet. 2020;396(10261):1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NCD Risk Factor Collaboration (NCD-RisC). A century of trends in adult human height. eLife. 2016;5:e13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora RS, Kontopantelis E, Alston RD, Eden TO, Geraci M, Birch JM. Relationship between height at diagnosis and bone tumours in young people: a meta-analysis. Cancer Causes Control. 2011;22(5):681–688. [DOI] [PubMed] [Google Scholar]
- 29.The Electronic Medical Records and Genomics (eMERGE) Consortium, The MIGen Consortium, The PAGE Consortium, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46(11):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Morimoto LM, de Smith AJ, et al. Genetic determinants of childhood and adult height associated with osteosarcoma risk: Height Genetics and Osteosarcoma. Cancer. 2018;124(18):3742–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khankari NK, Shu X-O, Wen W, et al. Association between Adult Height and Risk of Colorectal, Lung, and Prostate Cancer: Results from Meta-analyses of Prospective Studies and Mendelian Randomization Analyses. PLOS Med. 2016;13(9):e1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomasetti C, Vogelstein B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2124–2135. doi: 10.1016/j.ejca.2006.05.015 [DOI] [PubMed] [Google Scholar]
- 34.Kollár A, Rothermundt C, Klenke F, et al. Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 2019;63:101596. doi: 10.1016/j.canep.2019.101596 [DOI] [PubMed] [Google Scholar]
- 35.Eyre R, Feltbower RG, Mubwandarikwa E, et al. Incidence and survival of childhood bone cancer in northern England and the West Midlands, 1981–2002. Br J Cancer. 2009;100(1):188–193. doi: 10.1038/sj.bjc.6604837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125(1):229–234. doi: 10.1002/ijc.24320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortes-Ciriano I, Lee JJ, Xi R, Jain D, Jung YL, Yang L, Gordenin D, Klimczak LJ, Zhang C, Pellman DS, PCAWG Structural Variation Working Group, Park PJ, PCAWG Consortium. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nature Genetics. 2020; 52(3): 331–341. doi: 10.1038/s41588-019-0576-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva I dos S, Swerdlow AJ. Sex Differences in the Risks of Hormone-dependent Cancers. Am J Epidemiol. 1993;138(1):10–28. doi: 10.1093/oxfordjournals.aje.a116773 [DOI] [PubMed] [Google Scholar]
- 39.Longhi A, Errani C, Gonzales-Arabio D, Ferrari C, Mercuri M. Osteosarcoma in Patients Older Than 65 Years. J Clin Oncol. 2008;26(33):5368–5373. doi: 10.1200/JCO.2007.14.9104 [DOI] [PubMed] [Google Scholar]
- 40.Wick MR, Siegal GP, Unni KK, McLeod RA, Greditzer HG. Sarcomas of bone complicating osteitis deformans (Paget’s disease): fifty years’ experience. Am J Surg Pathol. 1981;5(1):47–59. doi: 10.1097/00000478-198101000-00008 [DOI] [PubMed] [Google Scholar]
- 41.Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, Ralston S. Epidemiology of Paget’s disease of bone: A systematic review and meta-analysis of secular changes. Bone. 2013;55(2):347–352. doi: 10.1016/j.bone.2013.04.024 [DOI] [PubMed] [Google Scholar]
- 42.Boivin J-F, Hutchison GB, Zauber AG, et al. Incidence of Second Cancers in Patients Treated for Hodgkin’s Disease. JNCI J Natl Cancer Inst. 1995;87(10):732–741. doi: 10.1093/jnci/87.10.732 [DOI] [PubMed] [Google Scholar]
- 43.Swerdlow AJ, Douglas AJ, Hudson GV, Hudson BV, Bennett MH, MacLennan KA. Risk Of Second Primary Cancers After Hodgkin’s Disease By Type Of Treatment: Analysis Of 2846 Patients In The British National Lymphoma Investigation. BMJ. 1992;304(6835):1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dores GM, Metayer C, Curtis RE, et al. Second Malignant Neoplasms Among Long-Term Survivors of Hodgkin’s Disease: A Population-Based Evaluation Over 25 Years. J Clin Oncol. 2002;20(16):3484–3494. doi: 10.1200/JCO.2002.09.038 [DOI] [PubMed] [Google Scholar]
- 45.Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. 10.1056/NEJMoa1505949. doi: 10.1056/NEJMoa1505949 [DOI] [PubMed]
- 46.Berrington de Gonzalez A, Curtis RE, Gilbert E, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010;102(1):220–226. doi: 10.1038/sj.bjc.6605435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chattopadhyay S, Sud A, Zheng G, et al. Second primary cancers in non-Hodgkin lymphoma: Bidirectional analyses suggesting role for immune dysfunction. Int J Cancer. 2018;143(10):2449–2457. doi: 10.1002/ijc.31801 [DOI] [PubMed] [Google Scholar]
- 48.Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89(9):1638–1644. doi: 10.1038/sj.bjc.6601319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaturvedi AK, Engels EA, Gilbert ES, et al. Second Cancers Among 104760 Survivors of Cervical Cancer: Evaluation of Long-Term Risk. JNCI J Natl Cancer Inst. 2007;99(21):1634–1643. doi: 10.1093/jnci/djm201 [DOI] [PubMed] [Google Scholar]
- 50.Robison LL, Shu X-O. Assessing temporal trends in pediatric cancer incidence. Pediatr Blood Cancer. 2010;54(7):n/a–n/a. doi: 10.1002/pbc.22474 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Cancer Incidence in Five Continents data is publicly available at: https://ci5.iarc.fr/Default.aspx. Processed datasets used in the analysis are available from the corresponding author upon reasonable request.


