Abstract
Background
The indiscriminate use and the similarity of prescribed antibiotics especially beta-lactams in human and small animal medicine, along with the close communication between pets and humans, increases the risk of the transfer of antibiotic-resistant bacteria and/or resistance elements especially integrons, between them. Therefore, we aimed to compare the frequencies of extended spectrum beta-lactamase (ESBL)-producing strains, major ESBL genes, classes 1 and 2 integrons, and antibiotic resistance patterns of fecal Escherichia coli (E. coli) isolates from dogs and their owners.
Methods
The present study was conducted on 144 commensal E. coli isolates from the feces of 28 healthy dog-owner pairs and 16 healthy humans who did not own pets. Phenotypic confirmatory test was used to identify the frequencies of ESBL-producing E. coli. Frequencies of blaCTX-M, blaSHV, and blaTEM genes, and also classes 1 and 2 integrons were determined by polymerase chain reaction. Resistance against 16 conventional antibiotics was determined by disk diffusion technique.
Results
ESBL-production status was similar between the E. coli isolates of 71.4% of dog-owner pairs. The E. coli isolates of 75, 60.7, and 85.7% of dog-owner pairs were similar in terms of the presence or absence of blaCTX-M, blaTEM, and blaSHV genes, respectively. The presence or absence of class 1 and class 2 integrons was the same in E. coli isolates of 57.1% of dog-owner pairs. Prevalence of resistance to chloramphenicol and tetracycline was significantly higher in E. coli isolates of dogs than owners, but for other 10 (83.3%) tested antibiotics, no statistically significant difference was found in prevalence of antibiotic resistance between dogs and owners isolates. Furthermore, the antibiotic-resistance profile was the same in the E. coli isolates of 14.3% of dog-owner pairs.
Conclusions
The results of current research highlight the seriousness of the drug-resistance problem and the need to prevent further increases and spread of antibiotic-resistance to reduce treatment failure. Moreover, relatively similar characteristics of the E. coli isolates of dogs and their owners can show the risk of sharing resistant bacteria and/or resistance elements between them.
Keywords: Escherichia coli, Extended spectrum beta-lactamase, Integrons, Antibiotic-resistance, Dog, Dog-owner
Background
In the last decades, the indiscriminate use of antibiotics in human and veterinary medicine imposed selection pressure on bacteria, particularly the microbiota. Moreover, the similarity of prescribed antibiotics in human and small animal medicine, along with the close communication between companion animals and humans, increased the risk of the transfer of antibiotic-resistant bacteria between pets and humans [1, 2].
Integrons are one of the genetic elements that have a role in the horizontal transmission of antibiotic resistance genes [3]. Gut microbiota consists of various bacterial species which accumulate in close vicinity. Therefore, multi-resistance integrons in the microbiota can have a major role in preserving and spreading antibiotic resistance, as well as the development of multi-drug resistance (MDR) phenotypes among pathogenic and commensal bacteria [4].
Beta-lactam antibiotics are broad-spectrum drugs widely prescribed for treating infections in humans and animals. Resistance to these medications is mainly mediated by the production of beta-lactamase enzymes by bacteria [5]. The rising global rates of extended-spectrum beta-lactamase (ESBL) producing bacteria, particularly Escherichia coli (E. coli) and also methicillin-resistant staphylococci (MRS), such as methicillin-resistant Staphylococcus aureus (MRSA) among humans and pets may cause the inefficacy of treatment with such important medicines [6]. In addition, the concurrency of ESBL production and resistance to other antibiotic classes can further limit the therapeutic options for bacterial infections [7].
The spread of antibiotic resistance genes and resistant bacteria among humans and companion animals is of public health importance, and microbiota has a significant role in this process. Therefore, we aimed to determine and compare the frequencies of ESBL-producing strains, major ESBL genes including blaCTX-M, blaSHV, and blaTEM, classes 1 and 2 integrons, and antibiotic resistance patterns among commensal E. coli isolates in the feces of healthy dogs and their owners in Shiraz, Iran. Furthermore, the associations between ESBL production, the presence of major ESBL genes, the presence of class 1 and class 2 integrons, and resistance to some conventional antibiotics were investigated in this study.
Results
Phenotypic identification of ESBL-producing E. coli
The prevalence of ESBL-producing E. coli among all 144 E. coli isolates and in each studied group is separately reported in (Table 1). The results of statistical analysis showed no significant difference in the prevalence of ESBL-producing E. coli between the isolates of dogs and owners (P = 0.188), between dog and control human isolates (P = 0.705), and also between owner and control human isolates (P = 0.122).
Table 1.
Prevalence of ESBL-producing, blaCTX-M, blaSHV, and blaTEM-positive, class 1 and class 2 integrons-positive, MDR, and antibiotic-resistant E. coli among the studied isolatesa
| Characteristics | In all isolates (n = 144) | In dog’s isolates (n = 56) | In owner’s isolates (n = 56) | In control’s isolates (n = 32) |
|---|---|---|---|---|
| ESBL-producing | 19 (13.2) | 6 (10.7) | 11 (19.6) | 2 (6.2) |
| blaCTX-M-positive | 21 (14.6) | 7 (12.5) | 12 (21.4) | 2 (6.2) |
| blaSHV-positive | 7 (4.9) | 4 (7.1) | 1 (1.8) | 2 (6.2) |
| blaTEM-positive | 61 (42.4) | 24 (42.9) | 25 (44.6) | 12 (37.5) |
| Class 1 integron-positive | 52 (36.1) | 23 (41.1) | 22 (39.3) | 7 (21.9) |
| Class 2 integron-positive | 16 (11.1) | 4 (7.1) | 7 (12.5) | 5 (15.6) |
| MDR | 42 (29.2) | 23 (41.1) | 13 (23.2) | 6 (18.8) |
| Cephalexin-resistant | 19 (13.2) | 5 (8.9) | 12 (21.4) | 2 (6.2) |
| Cefoxitin-resistant | 1 (0.7) | 0 (0.0) | 1 (1.8) | 0 (0.0) |
| Ceftazidime-resistant | 29 (20.1) | 12 (21.4) | 15 (26.8) | 2 (6.2) |
| Cefotaxime-resistant | 46 (31.9) | 20 (35.7) | 22 (39.3) | 4 (12.5) |
| Cefepime-resistant | 19 (13.2) | 8 (14.3) | 9 (16.1) | 2 (6.2) |
| Aztreonam-resistant | 9 (6.2) | 4 (7.1) | 5 (8.9) | 0 (0.0) |
| Amikacin-resistant | 7 (4.9) | 3 (5.4) | 4 (7.1) | 0 (0.0) |
| Streptomycin-resistant | 60 (41.7) | 27 (48.2) | 21 (37.5) | 12 (37.5) |
| Norfloxacin-resistant | 5 (3.5) | 5 (8.9) | 0 (0.0) | 0 (0.0) |
| Nalidixic acid-resistant | 37 (25.7) | 8 (14.3) | 14 (25.0) | 15 (46.9) |
| Chloramphenicol-resistant | 20 (13.9) | 13 (23.2) | 3 (5.4) | 4 (12.5) |
| Tetracycline-resistant | 56 (38.9) | 30 (53.6) | 17 (30.4) | 9 (28.1) |
aValues are shown as number (%)
Among 28 dog-owner pairs, E. coli isolates of 20 (71.4%) pairs were similar in terms of ESBL-production as E. coli isolates of 2 (7.1%) and 18 (64.3%) dog-owner pairs were ESBL-producer and not ESBL-producer, respectively.
Detection of major ESBL genes in E. coli isolates
The prevalence of major ESBL genes-harboring E. coli among all 144 E. coli isolates, and in each studied group is separately reported in (Table 1). Among the three studied ESBL genes, the blaTEM gene had the highest prevalence followed by blaCTX-M and blaSHV in all E. coli isolates and the E. coli isolates of dogs and owners separately. The prevalence of blaTEM, blaCTX-M, and blaSHV genes in these isolates was significantly different (P < 0.001). However, blaCTX-M and blaSHV genes had a similar prevalence (P = 1) in control human E. coli isolates, significantly lower than the prevalence of the blaTEM gene (P < 0.001) (Table 1). The results of statistical analysis showed no significant difference in the prevalence of ESBL genes between dogs and owners isolates, between dogs and control human isolates, and also between owners and control human isolates (P > 0.05).
Overall, none of the isolates had the three ESBL genes simultaneously. Seventy (48.6%) isolates comprising 28 (50.0%) dog isolates, 24 (42.8%) owner isolates, and 18 (56.2%) control human isolates were negative for all the three tested ESBL genes. The statistical analysis revealed no significant association between the simultaneous presences of ESBL genes (P > 0.05).
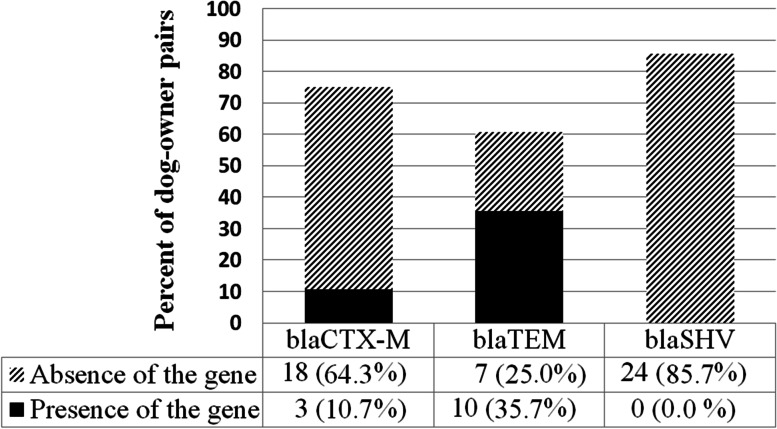
Out of 28 dog–owner pairs, E. coli isolates of 8 (28.6%) pairs had the same patterns of the presence of ESBL genes, and E. coli isolates of 5 (17.9%) pairs had none of the tested ESBL genes. E. coli isolates of 24 (85.7%), 21 (75.0%), and 17 (60.7%) dog-owner pairs had the similar status of presence or absence of blaSHV, blaCTX-M, and blaTEM genes, respectively. The proportion of dog-owner pairs who had E. coli isolates with a similar status of presence or absence of ESBL genes are demonstrated in (Fig. 1).
Fig. 1.
Proportion of dog-owner pairs who had E. coli isolates with a similar status of presence or absence of ESBL genes
Detection of class 1 and class 2 integrons in E. coli isolates
The prevalence of class 1 integron- and class 2 integron-positive E. coli among all 144 E. coli isolates, and in each of the studied groups are separately reported in (Table 1). We found that the prevalence of class 1 integrons was significantly higher than class 2 integrons in all 144 E. coli isolates and each of the three studied groups (P < 0.001).
Among all 144 E. coli isolates, 15 (10.4%) had both class 1 and class 2 integrons, 37 (25.7%) only had class 1 integrons, 1 (0.7%) only had class 2 integrons, and 91 (63.2%) were negative for both class 1 and class 2 integrons.
The statistical analysis indicated a significant association between the presence of class 1 integron and class 2 integron (P < 0.001). As a result, 93.8% of E. coli isolates which had class 2 integron also had class 1 integron simultaneously.
Out of 28 dog-owner pairs, E. coli isolates of 8 (28.6%) pairs had similar integron classes, and in 8 (28.6%) pairs, the isolates of dogs and their owners were negative for both classes 1 and 2 integrons.
Overall, the results of statistical analysis showed no significant difference in the prevalence of class 1 and class 2 integrons between dogs and owners isolates, between dogs and control human isolates, and between owners and control human isolates (P > 0.05).
Antibiotic resistance patterns of E. coli isolates
The prevalence of antibiotic-resistant E. coli in all 144 E. coli isolates and each of the studied groups is separately presented in (Table 1). Noteworthy, all the control human isolates were susceptible to cefoxitin, amikacin, aztreonam, and norfloxacin. The frequency of resistance to cephalexin, ceftazidime, and cefepime was below 10.0% in these isolates. In dogs, all isolates were susceptible to cefoxitin and the frequency of resistance to amikacin, aztreonam, cephalexin, and norfloxacin was below 10.0%. In owners, all isolates were susceptible to norfloxacin and the frequency of resistance to cefoxitin, chloramphenicol, amikacin, and aztreonam was below 10.0% (Table 1).
We observed that the prevalence of resistance to chloramphenicol (P = 0.007) and tetracycline (P = 0.013) was significantly higher in E. coli isolates of dogs than in owners. However, no significant difference was found in the prevalence of antibiotic resistance between dogs and owners isolates for the other tested antibiotics (P > 0.05). Furthermore, the prevalence of resistance to cefotaxime (P = 0.019) and tetracycline (P = 0.021) in dogs isolates was significantly higher than in control human isolates. In contrast, the prevalence of resistance to nalidixic acid was significantly higher in E. coli isolates of control humans than dogs (P = 0.001). No significant difference was found in the prevalence of antibiotic resistance between dogs and control human isolates for the other tested antibiotics (P > 0.05).
Comparison of the results of antibiotic resistance in E. coli isolates of owners and control humans showed that resistance to ceftazidime (P = 0.019) and cefotaxime (P = 0.008) was significantly higher in isolates of owners than in control humans. On the other hand, resistance to nalidixic acid was significantly higher in E. coli isolates of control humans than in owners (P = 0.036). Regarding other tested antibiotics, no significant difference was found in the prevalence of antibiotic resistance between owners and control humans isolates (P > 0.05).
Resistance or susceptibility to cefoxitin, amikacin, norfloxacin, cephalexin, cefepime, aztreonam, chloramphenicol, nalidixic acid, tetracycline, ceftazidime, cefotaxime, and streptomycin was similar in 96.2, 85.7, 82.1, 78.6, 78.6, 78.6, 67.9, 60.7, 60.7, 57.1, 50.0, and 42.9% of the dog-owner pairs, respectively. In four (14.3%) dog-owner pairs, E. coli isolates of dogs and their owners had the same antibiotic resistance profiles.
Among 144 E. coli isolates, 42 (29.2%) were resistant to at least one antimicrobial agent in three or more classes of antimicrobial drugs, being considered MDR isolates [8]. The prevalence of MDR E. coli isolates in dogs was significantly higher than in owners (P = 0.043) and control humans (P = 0.032). Although the prevalence of MDR E. coli isolates in owners (23.2%) was higher than in control humans (18.8%), this difference was not statistically significant (P = 0.624).
Associations of ESBL production, presence of major ESBL genes, presence of class 1 and class 2 integrons, and resistance to antibiotics
Overall, the results of statistical analysis showed a significant association between ESBL production and resistance to cephalexin, ceftazidime, cefotaxime, cefepime, and aztreonam (P < 0.001). Moreover, resistance to the above antibiotics was significantly associated with the blaCTX-M gene (P < 0.001). The presence of the blaTEM gene was significantly associated with resistance to streptomycin (P = 0.024), chloramphenicol (P = 0.007), and tetracycline (P = 0.004). However, no significant association was found between the presence of the blaSHV gene and resistance to any of the tested antibiotics (P > 0.05).
Among 19 ESBL -producing E. coli isolates, 18 (94.7%) were positive for at least one of the tested ESBL encoding genes. In addition, blaCTX-M, blaTEM, and blaSHV genes were present in 78.9, 57.9, and 0.0% of the ESBL producer isolates, respectively. However, only the presence of the blaCTX-M gene was significantly associated with the ESBL production ability of E. coli isolates (P < 0.001).
Investigation of the association between integrons and resistance to antibiotics revealed that among the tested antibiotics, only resistance to chloramphenicol (P = 0.004) and tetracycline (P = 0.040) was significantly associated with class 1 integron.
The results of statistical analysis showed no significant association between the presence of class 1 and class 2 integrons and ESBL production and also the presence of the tested ESBL genes in E. coli isolates (P > 0.05).
Discussion
In the present study, ESBL production status was similar between the E. coli isolates of 71.4% of dog-owner pairs. Moreover, the E. coli isolates of 75, 60.7, and 85.7% of dog-owner pairs were similar in terms of the presence or absence of blaCTX-M, blaTEM, and blaSHV genes, respectively. The presence or absence of class 1 and class 2 integrons was the same in E. coli isolates of 57.1% of dog-owner pairs. Furthermore, we observed a high similarity in resistance or susceptibility to 12 tested antibiotics in the E. coli isolates of dog-owner pairs, along with the absence of a significant difference in the prevalence of resistance to 83.3% of tested antibiotics between dogs and owners isolates. The antibiotic-resistance profile was the same in the E. coli isolates of 14.3% of dog-owner pairs. All the mentioned findings may indicate the possibility of transmitting resistant bacteria and/or resistance elements between dogs and their owners.
The possibility of the circulation of antibiotic-resistant E. coli clones or the direct/ indirect transmission of resistance elements such as plasmids or integrons between humans and pets in the same environment has been reported in previous studies [9–11]. For instance, in the study by Harada et al. (2012) relatively similar characteristics, including phylogenetic groups, antibiotic resistance, and virulence profiles, were observed in fecal E. coli isolated from dogs and their owners [11]. Likewise, in an investigation by Stenske et al. [10], no significant difference was found in the prevalence of resistance to 17 antibiotics between dogs and owners E. coli isolates. Carvalho et al. [9] also revealed similar resistance patterns, ESBL genes, and ESBL production ability in E. coli isolates from dogs and their owners in the same households. Ljungquist et al. [12] reported that dogs with owners carrying extended-spectrum cephalosporin-resistant Enterobacteriaceae (ESCRE) strains had identical ESCRE strains. On the other hand, when owners did not carry ESCRE strains, these strains were not found in dogs. According to these results, they reported the transfer of ESCRE strains between humans and dogs. These finding are in agreement with ours.
In the present study, the prevalence of MDR E. coli isolates in dogs was significantly higher than in owners and control humans. Likewise, several studies indicated the importance of MDR E. coli strains in pets and their effect on the treatment of in contact human infections [9, 13]. Moreover, Carvalho et al. [9] suggested that dogs can be a source of MDR E. coli in a household. Similarly, in a study by Abbas et al. [13], the prevalence of ESBL-producing E. coli (81.8% in dogs vs. 59% in owners) and multidrug-resistant ESBL-producing E. coli (44.4% in dogs vs. 30.77% in owners) was higher in dogs than owners. In line with the previous investigations, in our study, the frequency of resistance to cefotaxime and tetracycline was higher in dogs isolates, and resistance to ceftazidime and cefotaxime was higher in owners isolates in comparison with control humans isolates. Exclusively resistance to nalidixic acid was higher in control humans isolates than in dogs and owners isolates. These findings can indicate the importance of dogs and close contact with them in spreading antibiotic-resistant E. coli strains.
The prevalence of antibiotic-resistant and MDR E. coli isolates was variable in dogs and owners in the previous studies [9–11, 13]. Overall, the different prevalence of antibiotic resistance and MDR between the E. coli isolates of our study and previous reports could be due to variations in the origins of the isolates, type and amount of antibiotics used in humans and pets of different countries and therefore differences in the selective pressures in the E. coli strains of diverse geographical areas, and variation in the type and extent of communication between dogs and their owners in distinct cultures. In Iran, as an Islamic country, the extent of communication between dogs and their owners may be somewhat less than in other countries. For example some behaviors might be less frequent, such as allowing dogs to lick the owners’ faces. Moreover, Iranian dog owners usually wash their hands after petting dogs and before eating food. In addition, the higher level of antibiotic resistance in the E. coli isolates of the present study could be attributed to the free access and arbitrary use of antibiotics in Iran, which is a major problem for both medicine and veterinary medicine.
In the current research, associations were found between ESBL production, the presence of the bla gene, and resistance to beta-lactam antibiotics. In addition, the presence of the blaTEM gene was significantly associated with resistance to streptomycin, chloramphenicol, and tetracycline. These findings agree with those reported by Demirel et al. [7], who declared therapeutic complications caused by the simultaneous presence of ESBL genes and other classes of antibiotic-resistance genes including aminoglycosides, chloramphenicol, tetracyclines, fluoroquinolones and trimethoprim-sulfamethoxazole resistance genes in the identical plasmids.
Sáenz et al. [14] found various resistance genes in commensal E. coli strains from humans and animals. They stated that normal flora could play a major role in transmitting antibiotic-resistance elements, especially integrons. In the present study, no significant difference was found in the prevalence of class 1 and class 2 integrons between dogs and owners isolates, between dogs and control human isolates, and between owners and control human isolates. These results are consistent with those observed by Skurnik et al. [15]. The latter researcher reported that the distribution of gene cassettes in the integrons of pet E. coli isolates was very similar to those of human commensal E. coli isolates.
In line with our study, the prevalence of class 1 integrons was significantly higher than class 2 integrons in the previous studies [16–18]. Furthermore, the prevalence of class 1 and class 2 integrons was almost similar in these studies and our research. However, in a study by Akya et al. [19], the prevalence (92.3%) of class 1 integrons was much more than in our study. This inconsistency can be due to variations in the origin and type of studied strains, as their study was on ESBL-positive E. coli strains which were isolated from humans with urinary tract infections.
Investigation of the association between class 1 and class 2 integrons and resistance to antibiotics revealed a significant association between the presence of class 1 integrons and resistance to chloramphenicol and tetracycline. As well, Akya et al. [19] found a significant association between the presence of class 1 integrons and resistance to ceftazidime, streptomycin, and trimethoprim-sulfamethoxazole. Moreover, Kheiri & Akhtari [18] reported a significant association between dfr (dihydrofolate reductase) and aad (aminoglycoside adenyltransferase) gene cassettes in the integrons and resistance to trimethoprim-sulfamethoxazole and streptomycin, respectively. These findings can demonstrate the role of integrons in the preservation and transmission of antibiotic resistance genes.
Overall, relatively similar characteristics such as antimicrobial-resistance profiles and the presence or absence of ESBL genes and integrons in the E. coli isolates of dogs and their owners can indicate the possibility of the transmission of resistant bacteria and/or resistance elements between dogs and their owners. However, further studies such as pulsed-field gel electrophoresis (PFGE) or core genome multi-locus sequence typing (MLST), are needed to confirm that the strains are identical. In our previous study, the genetic relatedness of E. coli isolates from dogs and their owners was investigated by PFGE, enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR), and randomly amplified polymorphic DNA (RAPD) analyses [20].
Conclusion
The results of the current research highlight the seriousness of the drug-resistance problem and the need to prevent further increases and spread of antibiotic-resistance to reduce treatment failure. Moreover, relatively similar characteristics of the E. coli isolates of dogs and their owners can show the risk of sharing resistant bacteria and/or resistance elements between them.
Methods
Bacterial isolates
The present study was conducted on a total of 144 E. coli isolates from the feces of 28 healthy dog-owner pairs and 16 healthy humans who did not own the pet animals, as a control group. Two microbiologically confirmed E. coli isolates from each of the stool samples previously collected in a study by Naziri et al. [21] were included in this study. Briefly, the stool samples were previously streaked on Eosin Methylene Blue agar (Merck, Germany) and were incubated at 44 °C for 24 hours. Two random colonies with metallic green sheen morphology were selected from each sample. Gram-staining, oxidase, catalase, and IMViC (indole, motility, Voges-Proskauer, and citrate) tests were applied to these selected colonies to confirm the E. coli isolates.
All human participants were over 18 years old and filled out the informed consent. Dogs and dog owners were selected from the clients of several veterinary clinics in Shiraz, Iran and also the clients of the small animal clinics of the School of Veterinary Medicine, Shiraz University.
Phenotypic identification of ESBL-producing E. coli
The clinical and laboratory standards institute (CLSI) phenotypic confirmatory test was used to identify ESBL-producing E. coli [22]. At least five millimeters rise in the zone of inhibition around the cefotaxime-clavulanic acid or ceftazidime-clavulanic acid discs versus cefotaxime or ceftazidime discs alone was considered as a sign of ESBL-production by E. coli isolates. E. coli ATCC® 25,922 strain was also included in this test for quality control [22].
Detection of major ESBL genes in E. coli isolates
To detect major ESBL genes in E. coli isolates, the DNA of E. coli isolates was extracted by boiling methods. Detection of blaCTX-M, blaSHV, and blaTEM genes was performed using PCR and agar gel electrophoresis techniques, as previously described [23].
Detection of class 1 and class 2 integrons in E. coli isolates
A duplex PCR was conducted to amplify the 483-bp fragment of class 1 integron-integrase (intI1) gene and the 789-bp fragment of class 2 integron-integrase (intI2) gene, according to a protocol by Shaheen et al. [24] with minor modifications in the annealing temperature, which was adjusted at 64 °C. Electrophoresis of the PCR products was completed on 1% agarose (Parstous, Iran) gel containing a safe stain (YTA, Iran). Next, the amplicons were visualized under an ultraviolet-transilluminator (UVitec, UK).
Determination of antibiotic resistance patterns of E. coli isolates
The Kirby-Bauer disk diffusion test was applied for determination of antibiotic resistance patterns of E. coli isolates against cephalexin (30 μg), cefoxitin (30 μg), ceftazidime (30 μg), cefotaxime (30 μg), cefepime (30 μg), aztreonam (30 μg), amikacin (30 μg), streptomycin (10 μg), norfloxacin (10 μg), nalidixic acid (30 μg), chloramphenicol (30 μg), and tetracycline (30 μg) (Padtan Teb, Iran). Interpretation of the results was conducted according to the guidelines of Clinical and Laboratory Standards Institute (CLSI) [21]. The strain of E. coli ATCC® 25,922 was included in this study as a quality control [21].
Statistical analysis
Analysis of data was performed with SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA). The Chi-square test was used to compare the results in different studied groups. A value of P ≤ 0.05 was considered significant.
Acknowledgements
The cooperation of dog-owners and control humans who participated in this study was greatly appreciated. We are grateful to the small animal veterinary clinics of Shiraz, Iran, especially Shiraz Mehr clinic and Dr. A. Shojaee Tabrizi for their contribution in the sample collection. The authors would like to thank Mr. MS. Golvajoee for his technical assistance and kind support. We are indebted to Professor A. Derakhshandeh for providing the primers of the beta-lactamase genes.
Abbreviations
- MDR
Multi-drug resistance
- ESBL
Extended spectrum beta-lactamase
- bla
Beta-lactamase
- E. coli
Escherichia coli
- ESCRE
Extended-spectrum cephalosporin-resistant Enterobacteriaceae
- dfr
Dihydrofolate reductase
- aad
Aminoglycoside adenyltransferase
- PFGE
Pulsed-field gel electrophoresis
- MLST
Multi-locus sequence typing
- ERIC
Enterobacterial repetitive intergenic consensus
- RAPD
Randomly amplified polymorphic DNA
- PCR
Polymerase chain reaction
- CLSI
Clinical and laboratory standards institute
- ATCC®
American type culture collection
- intI
Integron-integrase
- SPSS
Statistical product and service solutions
Authors’ contributions
Design and supervision of the study, fecal culture, isolation and identification of bacteria, and also drafting of the manuscript were carried out by ZN. The experiments were performed by MP and AGO. Data analysis was carried out by ZN, MP and AGO. All authors (ZN, MP and AGO) read and approved the final version of manuscript.
Funding
This study was supported by a Grant (Numbers: 97GCU1M344385; 98GCU1M344385) from the Shiraz University, Iran.
Availability of data and materials
The data are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The present study was conducted according to the Declaration of Helsinki principles, and was approved by the Ethics Committee of the School of Veterinary Medicine, Shiraz University (Register numbers: PHD891925; MSC9631251). Participation in this study was voluntarily and all human participants filled and signed the informed consent form for their contributions as well as about dog-owners for their contributions and their dogs’ participations. In this study, we observed the information confidentiality of human subjects.
Consent for publication
Not applicable.
Competing interests
There is nothing to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zahra Naziri, Email: z.naziri@shirazu.ac.ir, Email: naziri_65@yahoo.com.
Meisam Poormaleknia, Email: maysamvpn69@gmail.com.
Azar Ghaedi Oliyaei, Email: azarghaedi@ymail.com.
References
- 1.Costa D, Poeta P, Sáenz Y, Coelho AC, Matos M, Vinué L, Rodrigues J, Torres C. Prevalence of antimicrobial resistance and resistance genes in faecal Escherichia coli isolates recovered from healthy pets. Vet Microbiol. 2008;127(1–2):97–105. doi: 10.1016/j.vetmic.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Guardabassi L, Schwarz S, Lloyd DH. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemother. 2004;54(2):321–332. doi: 10.1093/jac/dkh332. [DOI] [PubMed] [Google Scholar]
- 3.Odetoyin BW, Labar AS, Lamikanra A, Aboderin AO, Okeke IN. Classes 1 and 2 integrons in faecal Escherichia coli strains isolated from mother-child pairs in Nigeria. PLoS One. 2017;12(8):e0183383. doi: 10.1371/journal.pone.0183383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravi A, Avershina E, Ludvigsen J, L'Abée-Lund TM, Rudi K. Integrons in the intestinal microbiota as reservoirs for transmission of antibiotic resistance genes. Pathogens. 2014;3(2):238–248. doi: 10.3390/pathogens3020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgopapadakou NH. Penicillin-binding proteins and bacterial resistance to beta-lactams. J Antimicrob Chemother. 1993;37(10):2045–2053. doi: 10.1128/AAC.37.10.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hordijk J, Schoormans A, Kwakernaak M, Duim B, Broens E, Dierikx C, Mevius D, Wagenaar JA. High prevalence of fecal carriage of extended spectrum β-lactamase/AmpC-producing Enterobacteriaceae in cats and dogs. Front Microbiol. 2013;4:242. doi: 10.3389/fmicb.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demirel I, Kinnunen A, Önnberg A, Söderquist B, Persson K. Comparison of host response mechanisms evoked by extended spectrum beta lactamase (ESBL)-and non-ESBL-producing uropathogenic E. coli. BMC Microbiol. 2013;13(1):1–9. doi: 10.1186/1471-2180-13-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant,extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho AC, Barbosa AV, Arais LR, Ribeiro PF, Carneiro VC, Cerqueira AM. Resistance patterns, ESBL genes, and genetic relatedness of Escherichia coli from dogs and owners. Braz J Microbiol. 2016;47:150–158. doi: 10.1016/j.bjm.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenske KA, Bemis DA, Gillespie BE, D'Souza DH, Oliver SP, Draughon FA, Matteson KJ, Bartges JW. Comparison of clonal relatedness and antimicrobial susceptibility of fecal Escherichia coli from healthy dogs and their owners. Am J Vet Res. 2009;70(9):1108–1116. doi: 10.2460/ajvr.70.9.1108. [DOI] [PubMed] [Google Scholar]
- 11.Harada K, Okada E, Shimizu T, Kataoka Y, Sawada T, Takahashi T. Antimicrobial resistance, virulence profiles, and phylogenetic groups of fecal Escherichia coli isolates: a comparative analysis between dogs and their owners in Japan. Comp Immunol Microbiol Infect Dis. 2012;35(2):139–144. doi: 10.1016/j.cimid.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Ljungquist O, Ljungquist D, Myrenås M, Rydén C, Finn M, Bengtsson B. Evidence of household transfer of ESBL−/pAmpC-producing Enterobacteriaceae between humans and dogs–a pilot study. Infect Ecol Epidemiol. 2016;6(1):31514. doi: 10.3402/iee.v6.31514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas G, Khan I, Mohsin M. High rates of CTX-M group-1 extended-spectrum β-lactamases producing Escherichia coli from pets and their owners in Faisalabad, Pakistan. Infect Drug Resist. 2019;12:571. doi: 10.2147/IDR.S189884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sáenz Y, Briñas L, Domínguez E, Ruiz J, Zarazaga M, Vila J, Torres C. Mechanisms of resistance in multiple-antibiotic-resistant Escherichia coli strains of human, animal, and food origins. Antimicrob Agents Chemother. 2004;48(10):3996–4001. doi: 10.1128/AAC.48.10.3996-4001.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skurnik D, Ruimy R, Andremont A, Amorin C, Rouquet P, Picard B, Denamur E. Effect of human vicinity on antimicrobial resistance and integrons in animal faecal Escherichia coli. J Antimicrob Chemother. 2006;57(6):1215–1219. doi: 10.1093/jac/dkl122. [DOI] [PubMed] [Google Scholar]
- 16.Cocchi S, Grasselli E, Gutacker M, Benagli C, Convert M, Piffaretti JC. Distribution and characterization of integrons in Escherichia coli strains of animal and human origin. FEMS Immunol Med Microbiol. 2007;50(1):126–132. doi: 10.1111/j.1574-695X.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 17.Azam H, Ghezeljeh SM, Mahmoud S. Prevalence of class 1 and 2 integrons among the multidrug resistant uropathogenic strains of Escherichia coli. Asian Biomed. 2015;9(1):49–54. doi: 10.5372/1905-7415.0901.367. [DOI] [Google Scholar]
- 18.Kheiri R, Akhtari L. Antimicrobial resistance and integron gene cassette arrays in commensal Escherichia coli from human and animal sources in IRI. Gut Pathog. 2016;8(1):1–10. doi: 10.1186/s13099-016-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akya A, Lorestani RC, Rostamian M, Elahi A, Baakhshii S, Aliabadi M, et al. The relationship of class I integron gene cassettes and the multidrug-resistance in extended-spectrum β-lactamase producing isolates of Escherichia coli. Arch Pediatr Infect Dis. 2019;7(3):e87961.
- 20.Naziri Z, Derakhshandeh A, Firouzi R, Motamedifar M, Shojaee TA. DNA fingerprinting approaches to trace Escherichia coli sharing between dogs and owners. J Appl Microbiol. 2016;120(2):460–468. doi: 10.1111/jam.13003. [DOI] [PubMed] [Google Scholar]
- 21.Naziri Z, Firouzi R, Derakhshandeh A, Tabrizi AS. Comparative analysis of phylogenetic group and antimicrobial resistance pattern of fecal Escherichia coli isolates between healthy dogs and their owners. Comp Clin Pathol. 2015;24(5):1211–1220. doi: 10.1007/s00580-015-2062-7. [DOI] [Google Scholar]
- 22.CLSI . Performance standards for antimicrobial susceptibility testing CLSI supplement M100. 28. Wayne: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 23.Naziri Z, Derakhshandeh A, Borchaloee AS, Poormaleknia M, Azimzadeh N. Treatment failure in urinary tract infections: a warning witness for virulent multi-drug resistant ESBL-producing Escherichia coli. Infect Drug Resist. 2020;13:1839–1850. doi: 10.2147/IDR.S256131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaheen BW, Oyarzabal OA, Boothe DM. The role of class 1 and 2 integrons in mediating antimicrobial resistance among canine and feline clinical E. coli isolates from the US. Vet Microbiol. 2010;144(3–4):363–370. doi: 10.1016/j.vetmic.2010.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on reasonable request.