Abstract
Opioid‐induced respiratory depression (OIRD) is a potentially life‐threatening complication of opioid consumption. Apart from naloxone, an opioid antagonist that has various disadvantages, a possible reversal strategy is treatment of OIRD with the hypothalamic hormone and neuromodulator thyrotropin‐releasing hormone (TRH). In this review, we performed a search in electronic databases and retrieved 52 papers on the effect of TRH and TRH‐analogs on respiration and their efficacy in the reversal of OIRD in awake and anesthetized mammals, including humans. Animal studies show that TRH and its analog taltirelin stimulate breathing via an effect at the preBötzinger complex, an important respiratory rhythm generator within the brainstem respiratory network. An additional respiratory excitatory effect may be related to TRH’s analeptic effect. In awake and anesthetized rodents, TRH and taltirelin improved morphine‐ and sufentanil‐induced respiratory depression, by causing rapid shallow breathing. This pattern of breathing increases the work of breathing, dead space ventilation, atelectasis, and hypoxia. In awake and anesthetized humans, a continuous infusion of intravenous TRH with doses up to 8 mg, did not reverse sufentanil‐ or remifentanil‐induced respiratory depression. This is related to poor penetration of TRH into the brain compartment but also other causes are discussed. No human data on taltirelin are available. In conclusion, data from animals and human indicate that TRH is not a viable reversal agent of OIRD in awake or anesthetized humans. Further human studies on the efficacy and safety of TRH’s more potent and longer lasting analog taltirelin are needed as this agent seems to be a more promising reversal drug.
Keywords: opioid, opioid adverse effects, opioid death, opioid‐induced respiratrory depression, respiratory stimulants, reversal, TRH
Opioid‐induced respiratory depression (OIRD) is a potentially life‐threatening complication of opioid consumption. A possible reversal strategy is treatment of OIRD with the hypothalamic hormone and neuromodulator thyrotropin‐releasing hormone, TRH. In this review, we report that TRH and taltirelin improve OIRD, by causing rapid shallow breathing. In a humans, a continuous infusion of intravenous TRH with doses up to 8 mg, did not reverse OIRD. No human data on taltirelin are available. In conclusion, data from animals and human indicate that TRH is not a viable reversal agent of opioid‐induced respiratory depression in humans.

Abbreviations
- EMG
electromyogram
- NMDAR
N‐methy‐D‐aspartate receptor
- OIRD
opioid‐induced respiratory depression
- TSH
thyroid‐stimulating hormone
- TRH
thyrotropin‐releasing hormone
- TRHR
thyrotropin‐releasing hormone receptor
1. INTRODUCTION
Opioid‐induced respiratory depression (OIRD) is a serious complication of opioid therapy and opioid abuse. 1 , 2 The cause of OIRD is exogenous opioid‐induced activation of μ ‐opioid receptors. These receptors are expressed on respiratory neurons in brainstem respiratory networks and, when activated, cause slowing and eventually the silencing of these respiratory neurons and consequently the cessation of breathing (apnea). 3 , 4 Recent studies indicate that particularly the pre‐Bötzinger complex, which is involved in respiratory rhythm generation, and the parabrachial/Kölliker‐Fuse complex, which provides excitatory input to the preBötzinger complex, are crucial areas in the brainstem for development of OIRD. 2 , 3 , 4 , 5
When serious OIRD occurs, various strategies are available to prevent unintentional death, such as endotracheal intubation followed by artificial ventilation, as occurs prior to surgery or in high care units, or when spontaneous breathing is required, administration of an opioid receptor antagonist such as naloxone. Endotracheal intubation and artificial ventilation demand not only well‐trained individuals but also specific well‐equipped locations that enable artificial ventilation and patient monitoring. In most other settings and circumstances, naloxone is the first choice in case of a life‐threatening OIRD. 1 , 2 , 6 Naloxone is a non‐competitive opioid antagonist that rapidly crosses the blood‐brain‐barrier. While in many cases the timely and adequately dosed administration of naloxone will restore breathing, there are situations where administration of this antagonist is undesired or inadequate. These include 2 : an overdose with high‐dose potent opioids (such as fentanyl) or with opioids with a high affinity for the opioid receptors (such as buprenorphine, carfentanil, or sufentanil). In these cases, naloxone is ineffective or short‐acting 6 ; the (ab)use of an opioid in combination with non‐opioid centrally‐acting respiratory depressants such as alcohol, benzodiazepines, or antidepressants. Such combinations have been proven to be potentially lethal due to additive or synergistic respiratory depression, which cannot be antagonized by naloxone 7 ; in case of an opioid‐use disorder when naloxone will cause immediate withdrawal symptoms, agitation, and possibly even aggressive behavior toward medical personnel 8 ; when naloxone will cause loss of analgesia, which may cause sympathoexcitation, stress and consequently various complications including pulmonary edema, cardiac arrhythmias, or epileptic seizures 8 ; and finally in case of mass poisoning with opioids released in the atmosphere, where supplies of naloxone may be insufficient or ineffective. 9 Finally, one needs to realize that naloxone has another important drawback and that is its short duration of action due to rapid metabolism and clearance from the brain. 2 , 8 Consequently, development of respiratory stimulants without naloxone’s shortcomings is greatly advantageous and currently an unmet need in the treatment of an opioid overdose. We recently reviewed current advances in the development of opioid‐reversal strategies. 2 A variety of respiratory stimulants have been tested to prevent or reverse OIRD but none are currently sufficiently scrutinized with respect to efficacy and safety. One of the older reversal strategies is the treatment of OIRD with thyrotropin‐releasing hormone (TRH). The first study on the stimulant effect of TRH on the efficacy of antagonism of morphine‐induced respiratory depression appeared more than 45 years ago. 10 In light of the importance of the topic, we here appraise the efficacy of TRH and its analogue taltirelin as reversal agents of OIRD.
In this short scoping review, we will first discuss the ability of TRH and analogues to stimulate breathing and next whether TRH is able to reverse OIRD in experimental animal and human models of OIRD. To get the full picture of the effects of TRH and its analog taltirelin on breathing, we performed a search in PubMed, Embase, Web of Science, and Cochrane Library on March 17, 2022. The search strategy was developed in collaboration with an information specialist of the Walaeus Library of Leiden University Medical Centre (Jan Schoones) and is available upon request from the authors (a.dahan@lumc.nl). No publication date limits were applied; only papers written in English were considered. We do agree that this is a major restriction as we are missing out on a number of papers that appeared in the Russian literature. Still, we managed to review the translation into English of some of these papers, some of which are included in the current review. The search resulted in 297 unique papers of which 52 were included in this review. We will first discuss TRH itself and its roles in the mammalian system, followed by the ventilatory effects of TRH and its analog taltirelin, TRH’s analeptic effects and finally the ability of TRH and taltirelin to reverse OIRD.
2. THYROTROPIN‐RELEASING HORMONE: NEUROMODULATOR WITH STATE‐DEPENDENT EFFECTS
TRH is a tripeptide, L‐pyro‐Glu‐His‐Pro‐NH2 (thyroliberin, in pharmaceutical form named protirelin), which is produced by the hypothalamus but also by other organs, such as the brain, heart, pancreas, and intestines. 11 , 12 While TRH is typically considered a hormone that plays a central role in regulating the pituitary‐thyroid axis by stimulating thyrotropin cells in the anterior pituitary to release thyroid‐stimulating hormone (TSH), Gary et al. 13 and Kamath et al. 14 proposed the TRH hypothesis of homeostatic system regulation. Within this concept TRH plays various roles: (i) the well‐known role for TRH within the hypothalamic‐hypohysiotropic neuroendocrine axis; (ii) activity of TRH within the brainstem, midbrain, and spinal cord; (iii) TRH activity within the limbic/cortical system and finally (iv) a role of TRH within the chronobiological system. In fact, the homeostatic principle is such that TRH enables return to a homeostatic baseline following a perturbation of the system. For example, in case of sedation TRH will have analeptic properties, while in case of a convulsion TRH acts as an anticonvulsant. 13 , 14 Important for breathing and OIRD is that TRH and its receptors are present in various brain areas involved in ventilatory control, including the medulla oblongata and striatum (role #ii), supporting a potential role for TRH as an excitatory neuromodulator or neurotransmitter within the respiratory network. 11 , 15 TRH mediates its effects by binding to the G‐protein‐coupled TRH receptor (TRHR). Humans have one TRH receptor, TRHR1, while rodents additionally possess a second receptor, TRHR2. 16 , 17
Although the different targets of TRH support its use in a variety of diseases, such as central nervous system diseases, including brain/spinal injury, schizophrenia, Alzheimer’s disease, epilepsy, spinocerebellar ataxia, amyotrophic lateral sclerosis, Parkinson’s disease, and depression, its use is still restricted because of various limitations. 18 , 19 , 20 , 21 As discussed earlier by Komath et al. 19 these restrictions are related to poor reproducibility of efficacy in clinical trials, short duration of action (half‐life 10 min; >90% is cleared from circulation within the first 20 min after intravenous administration), 22 low intestinal and central permeability, rapid degradation, and various (cardiac and endocrinological) side effects. Still, continuous TRH infusions or administration of long‐acting TRH analogs may circumvent these restrictions and may be useful in the reversal of OIRD, either by a direct effect within the respiratory network or via indirect analeptic effects.
3. EFFECT OF THYROTROPIN‐RELEASING HORMONE ON BREATHING
3.1. Animal studies
Hedner et al. 23 studied the effect of TRH and TRH analogues administered intracerebroventricularly (icv) in spontaneously breathing halothane‐anesthetized rats. TRH and TRH analogs produced respiratory stimulation causing hypocapnia and alkalosis, an effect that was potentiated by pretreatment with naloxone; direct injection of TRH into the nucleus tractus solitarius increased respiratory frequency. These TRH effects were unrelated to an interaction with opioid receptors. 23 Further studies in rats, cats, and fetal sheep all showed the respiratory stimulation following peripheral or central administration of TRH in α‐chloralose, urethane, or volatile anesthesia, 24 , 25 , 26 , 27 , 28 , 29 , 30 and awake condition. 31 Apart from areas within the brainstem respiratory network, TRH and its receptors were identified in the phrenic motor nucleus and hypoglossal motoneurons. 22 , 32 Later studies demonstrate that TRH exerts its effects through actions within the preBötzinger complex at TRH‐receptors, 15 , 33 , 34 , 35 , 36 , 37 although similar excitatory effects were observed from TRH injections at other nuclei involved in ventilatory control such as the dorsal respiratory group, area postrema, nucleus ambiguous, nucleus tractus solitarius, and retrotrapezoid nucleus, 38 , 39 , 40 , 41 , 42 , 43 Still, also respiratory depression was observed after injection of TRH into the Bötzinger complex of the anesthetized rabbit. 43 Finally, TRH analogue taltirelin restored the central ventilatory chemoreflex in rats with an inherent ventilatory insensitivity to hypercapnia. 44
In summary, these data indicate that in, mostly anesthetized, animals, TRH when administered at central sites, is a respiratory stimulant, it enhances rhythmogenic respiratory activity.
3.2. Human studies
The number of studies that specifically examined the effect of TRH on respiration in humans (as primary endpoint) is limited. Nink et al. 45 tested the effect of 0.2 and 0.4 mg intravenous TRH on resting and CO2‐stimulated ventilation in 45 healthy volunteers of either gender. They observed a rapid, short‐lived (3–4 min) increase in ventilation from 8 to 10.5 L/min, primarily elicited through an increase of tidal volume, after 0.4 mg but not after 0.2 mg TRH. Concomitant with the increase in ventilation, heart rate rose by 15 beats per min. The authors concluded that TRH elicits a dose‐dependent effect on ventilation and adverse events (dizziness, restlessness/agitation). Shortcomings of the study include lack of blinding, lack of randomization, lack of power calculation, and use of medical staff as study subjects. This may be the reason for the observation of modest but significant respiratory and hemodynamic effects after these relatively low TRH doses.
In a rather small study, Schulz et al. 46 tested the effect of 0.4 mg TRH versus placebo on the rebreathing response to CO2 in six healthy subjects in a single blinded fashion. They observed a small reduction of the apneic threshold by 1.7 mmHg, without any effect on the slope of the hypercapnic ventilatory response in just three of the six subjects. Since this study is underpowered it remains difficult to draw any meaningful conclusions from this study.
Finally, Peek et al. 47 studied the effect of TRH on fetal breathing‐movements in 75 pregnant women between week 26 and 34 of pregnancy in whom pharmacologically fetal lung maturation was indicated. After a 0.4 mg bolus TRH infusion, breathing movements increased by 35 breaths per hour from a baseline of 60 breaths/h. These data indicate the ability of TRH to interact with ventilatory control is active in the third trimester of pregnancy. Still, it was anticipated that just 2% of injected TRH would reach the fetus and also some subjectivity of operator‐dependent scoring may have occurred.
In summary, the rather limited number of human studies using relatively low TRH doses all point toward rather small and short‐lived respiratory effects. Various methodological issues are apparent that confounded the results of these studies. The best conclusions that we can draw from these studies is that at single low intravenous doses, insufficient TRH reaches the TRH receptors within the brainstem to cause a significant and long‐lasting respiratory effect.
4. ANALEPTIC EFFECTS BY THYROTROPIN‐RELEASING HORMONE DURING ANESTHESIA
As already indicated and previously discussed, TRH acts as a state‐dependent modulator of the central nervous system arousal state. 13 , 14 This is exemplified by the observation that TRH given to the hibernating ground squirrel results in behavioral arousal, while in the eurthermic awake squirrel, TRH produces inhibitory effects. 13 , 48 Increase in the level of arousal is important in OIRD as it may counteract part of the respiratory depression. 49 A series of experiments, performed in the 1970s and 1980s, explored the analeptic effects of TRH and its ability to cause arousal and reduce anesthesia time. Focus was on barbiturate and ethanol anesthesia but there are also studies on reversal of chloralose hydrate or diazepam anesthesia, 10 , 50 , 51 , 52 , 53 ketamine or inhalational anesthesia. 24 , 54 , 55 Experiments were performed in a series of species, including mice, rats, cats, dogs, rabbits, and non‐human primates. 10 , 51 , 56 , 57 , 58 Findings from the different studies agreed, showing that during anesthesia, systemic and centrally administered TRH enhances locomotor activity, increases muscle tone, body temperature, and respiratory rate and reduces sleep time. 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 Serbenyuk et al. 58 showed that hyperventilation‐induced apnea is reversed or prevented by intravenous TRH in the pentobarbital anesthetized cat, suggestive of a role of TRH in the “wakefulness drive” to breathe. 59 In one study, blood gas values showed the development of lactic acidosis in control (awake) and rats anesthetized with volatile anesthetics following TRH administration, although pH remained unaffected, possibly related to respiratory compensation. 54 Interestingly, in pentobarbital‐anesthetized, spontaneously‐breathing and intubated dogs, 1 mg TRH caused tachypnoea combined with a reduction in tidal volume. 56 This may cause an increase in dead‐space ventilation with worsening of the ventilation/perfusion (V/Q) mismatch and consequently acidosis and hypoxia. A crucial role for endogenous TRH in arousal mechanisms during anesthesia was demonstrated by Lighton et al., 60 who showed that passive immunization against TRH, by icv administration of anti‐TRH serum, prolongs barbiturate anesthesia.
The analeptic effects described above are best explained by an effect of TRH on the reticular activating system with possibly a crucial role for the cholinergic system. 10 , 61 These data suggest that apart from a direct respiratory stimulatory effect within the brainstem respiratory network, TRH may excite respiration through an analeptic effect, causing arousal and associated tachypnea.
5. ABILITY OF TRH TO REVERSE OPIOID‐INDUCED RESPIRATORY DEPRESSION
5.1. Animal studies
The first study on the ability of TRH to reverse OIRD, tested the effect of 0.05 and 0.1 mg TRH given to rabbits via the icv route following administration of 4 mg/kg intravenous morphine. 10 TRH had no effect on morphine‐induced sedation and caused no evident behavioral arousal but did increase respiratory rate and body temperature. In fact, after TRH the animals developed hyperthermia, which may have further enhanced respiratory rate.
Kharkevich et al. 62 measured the effect of systemic and icv administered TRH and analogue RGH 2202 on the diaphragm electrical activity in urethane‐anesthetized, vagotomized, artificially ventilated rats. The depression of diaphragmatic activity by 10 mg/kg intravenous morphine was fully restored by intravenous TRH (5 mg/kg). Similar results were observed after local application of the dorsal pontomedullary surface with morphine (20 mM) and TRH and RHG 2202 (both 3 mM) for 7 min. Interestingly, the TRH excitatory effects were attenuated by prior administration of N‐methyl‐D‐aspartate (NMDA) antagonists, ketamine and MK801, suggestive of a mechanistic role for NMDA receptor activation in the TRH‐reversal of OIRD.
Takita et al. 63 studied the effect of TRH on morphine‐induced respiratory depression in an artificial cerebrospinal fluid (aCSF) superfused ex vivo preparation of the brainstem‐spinal cord from 1 to 4‐day‐old rats with respiratory activity measured using suction electrodes at the C4 and C5 ventral roots. Morphine (10 μM) applied via the aCSF caused a reduction in respiratory frequency to 65% of control. Adding TRH (100 nM) caused the partial return of respiratory frequency to 88% of control. Additionally, TRH increased tonic motor activity at C4. The authors concluded that TRH partly reversed the respiratory depression induced by morphine in the medulla oblongata through activation of TRH receptors expressed on respiratory neurons.
In two articles, Cotten and colleagues report on the ability of TRH and its analogue taltirelin to reverse OIRD in the rat. 31 , 64 Taltirelin is a TRH analog that compared to TRH demonstrates an improved therapeutic selectivity, is 10–100 times more potent, is more rapid in crossing the blood‐brain barrier and has an 8‐times longer duration of action. It is registered in Japan for treatment of spinal cerebral degeneration. 19 , 31 Earlier studies showed that taltirelin increases respiratory rate in the rat when administered centrally. 65 TRH and taltirelin, given intravenously (1 mg/kg followed by 5 mg/kg per h) or intratracheally (5 mg/kg dissolved in 100 µl saline), reversed respiratory depression from 5 mg/kg intravenous morphine in the isoflurane‐anesthetized rat. 31 The TRH and taltirelin effects were primarily due to increased breathing rates. In contrast with TRH, which was unable to fully restore pH, PCO2 and PO2, taltirelin restored blood gas values but caused lactic acidosis. Next, Cotton’s group studied the effect of intravenous taltirelin on OIRD in awake rats. 64 Two opioids were studied, the natural opioid morphine and the highly potent synthetic opioid sufentanil. In agreement with their earlier study, 31 taltirelin (1 mg/kg given intravenously) reversed OIRD from both opioids by increased breathing rates but failed to fully correct hypoxia and lactic acidosis. The return of minute ventilation toward baseline levels was slow and takes about 45 min following taltirelin administration. Respiratory stimulation is due to an effect of taltirelin on breathing frequency (increase up to 150% of baseline).
Cotton’s group further studied the effect of 1 mg/kg intravenous taltirelin, 1 ml/kg intramuscular saline and 30 μg/kg intravenous dexmedetomidine on gastrocnemius EMG activity in awake, restrained rats following 10 μg/kg intravenous sufentanil administration. 66 Sufentanil increased EMG activity that was subsequently reduced by the α2‐adrenergic receptor agonist dexmedetomidine but not by taltirelin. In fact, taltirelin seems to increase EMG activity. Enhanced EMG activity from opioids is a known observation in animals and humans (i.e., the rigid cage syndrome), and may be reversed by naloxone. Muscle rigidity from opioids may be a cause of further impairment of ventilation next to the effect of the opioid at the brainstem. Non‐opioid interventions that are able to reduce muscle tone will enhance tidal volume and consequently improve ventilation and gas exchange. Apart from dexmedetomidine, volatile anesthetics and the α 1 ‐adrenergic receptor agonist prazosine reduce opioid‐enhanced muscle tone and consequently improve the ability of taltirelin (and other non‐opioid reversal agents) to reverse OIRD. 64
In summary, the animal data show that TRH and taltirelin have an excitatory effect on the ventilatory control system when ventilation is depressed by an opioid. Still, the observed respiratory phenotype of rapid shallow breathing seems disadvantageous and associated with persistent hypoxia and lactic acidosis, possibly from an increase in muscle rigidity, increase in work of breathing and atelectasis with a further mismatch of the V/Q ratio.
5.2. Human studies
Lenz and colleagues studied the effect of 0.4 mg TRH on respiration in 15 neurosurgical patients under neurolept‐anesthesia with fentanyl and flunitrazepam. 67 TRH had no effect on arousal or respiratory parameters (arterial PO2 and PCO2). These results are disappointing and contradict the results of animal studies. Still, it may be argued that the TRH dose was too low with insufficient TRH crossing the blood ‐brain‐barrier.
To investigate the effect of higher doses, we performed an exploratory study in six healthy male and female volunteers, 66 the volunteers received a continuous infusion of remifentanil (target plasma concentration 1.2 ng/mL) such that isohypercapnic ventilation was reduced by 45%–50%. When ventilation had reached a steady state, dose escalating intravenous TRH infusions were given. The first subject started was given 0.8 mg over 60 min (an initial bolus dose of 0.2 mg followed by an infusion of 0.2 mg over 30 min, which was repeated once). Since no respiratory effect was observed the dose was increased to a total dose of 1.6 mg (bolus 0.4 mg and infusion of 0.4 mg over 30 min, repeated once), 3.2 mg (0.8 mg/0.8 mg, repeated once), 4.8 mg (1.2 mg/1.2 mg, repeated once), 8 mg (2 mg/2 mg, repeated once), and 4 mg (2 mg/2 mg, not repeated, because of futility). None of the subjects showed any sign of consistent reversal of OIRD from remifentanil (Figure 1), which is in strong contrast with, for example, ketamine that showed a dose‐dependent return toward baseline ventilation in the same human model of OIRD. 68 One can argue that the dose (max. dose 8 mg) was too low to cause an effect. Still, it was a factor of 20 higher than the dose used in the study by Nink et al. 45 who did show a significant, albeit modest, effects from just 0.4 mg intravenous TRH. Furthermore, the dose of 8 mg TRH did reach central sites as all of the participating subjects experienced adverse effects (headache, nausea, warm/cold feelings, restlessness/agitation). We refrained from higher TRH doses to prevent worsening of side effects or the development of endocrinological effects. Additionally, pricing of the TRH was such (US$ 1000.‐ for an 8 mg intravenous solution) that we deemed higher doses an overly expensive and thus uneconomic therapy of OIRD.
FIGURE 1.
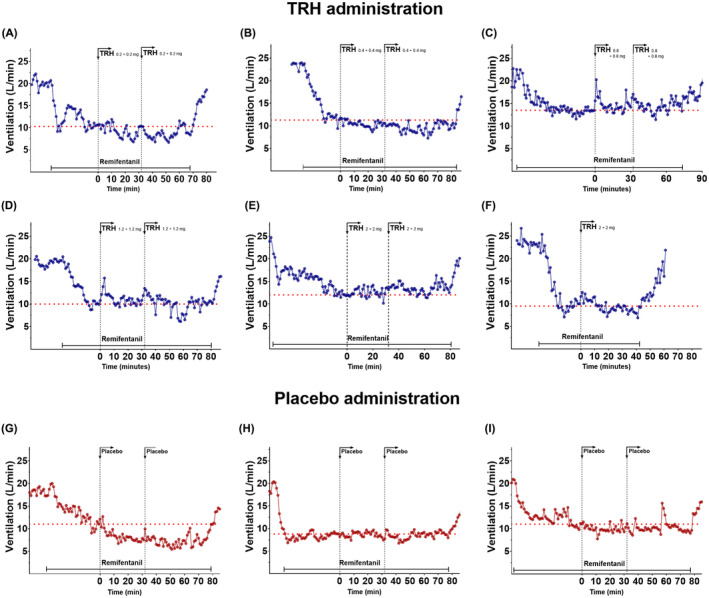
Influence of TRH or placebo (normal saline) on remifentanil‐induced respiratory depression at iso‐hypercapnia in human volunteers. Initially, the end‐tidal PCO2 was elevated so that minute ventilation increased to 20 ± 5 L/min. After reaching steady‐state ventilation, remifentanil was titrated to cause a depression of ventilation of 50%. Subsequently TRH (panels A‐F) or placebo (panels G‐I) were administered as a bolus dose followed by a continuous infusion over 30 min. In all but one subject (Panel F), this dosing sequence was repeated once. The data are obtained from seven healthy volunteers of either gender. Data from panels A and G and B and H are from the same subjects. Each symbol represents the minute ventilation averaged over 1‐min. The red dotted line gives the average ventilation prior to TRH or placebo administration.
6. DISCUSSION
The picture that emerges from this critical appraisal is that in animals, TRH interacts with the ventilatory control system by acting at the preBötzinger complex, the primary rhythm‐generator of the brainstem, via activation of local TRH receptors. The efficacy of TRH or its analog taltirelin to overcome OIRD in rodents was limited with rapid shallow breathing associated with lactic acidosis, irrespective of the presence of anesthesia or not. In humans, just low doses of TRH were tested (<0.4 mg), showing small excitatory effects on ventilation, while continuous infusions of doses up to 8 mg seemed ineffective in reversing OIRD in awake and anesthetized states. In this respect animal and human data agree, and consequently, the animal and human data currently preclude the use of TRH (bolus or continuous infusions) as respiratory stimulant to reverse or prevent OIRD from potent opioids such as the synthetic opioids fentanyl, carfentanil, sufentanil, or remifentanil, both within the clinical setting or outside the clinical setting when illicit opioids (fentanyl or other opioids are often laced with fentanyl) are abused or overdosed. As earlier discussed, 19 TRH has various shortcomings that make its use in humans as single bolus dose ineffective, such as its short duration of action, difficulty in passing the blood‐brain‐barrier, and endocrinological side effects. Engel et al. 69 studied the effect of TRH on motor function in patients with amyotrophic lateral sclerosis and observed tachypnoea (mentioned as an adverse effect of treatment) after administration of 500 mg TRH in some of their patients. This indicates that much higher doses of TRH may be needed, which is currently overly uneconomic. Given these limitations, we can be confident in stating that there is definitely no place for TRH as respiratory stimulant counteracting the respiratory effects of opioids or other respiratory depressants. Subsequently we have to give a negative answer the question “Is TRH a viable reversal agent of OIRD.” With respect to the TRH analog taltirelin, the animal data were not overly positive and further human studies are necessary. While taltirelin is more rapid in crossing the blood‐brain barrier and is acting at about a 100‐fold lower dose than TRH, 16 return of minute ventilation was slow and coincided with development of lactic acidosis. Human studies are possible since taltirelin has been approved for human use in the treatment of neurodegenerative diseases in Japan, but is not registered in the EU or US, presently.
Another analog of interest is rovatirelin. 70 Rovatirelin is a relatively new analog of TRH and has been tested in clinical studies for treatment of spinocerebellar ataxia. 71 It has an unnatural 3‐(thiazol‐4‐yl)‐L‐alanine amino acid moiety in the middle part, instead of L‐histidine. 72 Compared to taltirelin, oral rovatirelin shows greater absorption and brain penetration in rats due to its high lipophilicity, and shows stable brain concentrations lasting for hours. 70 These data indicate that brain uptake is best for rovatirelin compared to its parent and to taltirelin (brain uptake TRH <taltirelin <rovatirelin). The rapid uptake and prolonged central presence of rovatirelin may be of advantage when considering this drug as alternative to naloxone for reversal of OIRD. However, pharmacokinetic and pharmacodynamic studies in humans are needed to get definite answers to our question whether taltirelin or any other TRH analog is able to effectively reverse OIRD.
NOMENCLATURE OF TARGETS AND LIGANDS
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), 73 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019). 74
DISCLOSURE
None.
ETHICS STATEMENT
This review paper was exempt from formal ethics approval per decision of the departmental review board.
AUTHOR CONTRIBUTIONS
Marieke Hyke Algera, Joseph F. Cotten, Monique van Velzen, Marieke Niesters, Martijn Boon, Daniel S. Shoham, Kaye E. Dandrea, Rutger van der Schrier, Albert Dahan: writing of the editorial; commenting on the manuscript; approval of the final version. Marieke Hyke Algera, Daniel S. Shoham, Kaye E. Dandrea: performed experiments, Joseph F. Cotten, analyzed data.
ACKNOWLEDGMENTS
None.
Algera MH, Cotten JF, van Velzen M, et al. Are thyrotropin‐releasing hormone (TRH) and analog taltirelin viable reversal agents of opioid‐induced respiratory depression? Pharmacol Res Perspect. 2022;10:e00974. doi: 10.1002/prp2.974
Funding information
Support was provided from institutional and/or departmental sources. This work is part of the research project Tackling and Preventing the Opioid Epidemic (TAPTOE; Dutch Research Council in the framework of the NWA‐ORC Call [Grant NWA.1160.18.300]). Dr. Dahan received grants/awards from ZonMW (The Hague, the Netherlands) and US Food and Drug Administration (Silver Spring, MD, USA). Dr. Cotten is funded by Massachusetts General Hospital Department of Anesthesia, Critical Care & Pain Medicine and by Countermeasures Against Chemical Threats (CounterACT) Program supplemental awards to National Institutes of Health National Institute on Drug Abuse [Grant R21‐DA045231].
DATA AVAILABILITY STATEMENT
The search strategy is available upon request from the authors (a.dahan@lumc.nl).
REFERENCES
- 1. Dahan A, Aarts L, Smith TW. Incidence, reversal and prevention of opioid‐induced respiratory depression. Anesthesiology. 2010;112:226‐238. [DOI] [PubMed] [Google Scholar]
- 2. van der Schrier R, Dahan JDC, Boon M, et al. Advances in reversal strategies of opioid‐induced respiratory depression. Anesthesiology. 2022;136:618‐632. [DOI] [PubMed] [Google Scholar]
- 3. Baertsch NA, Bush NE, Burgraff NJ, Ramirez JM. Dual mechanisms of opioid‐induced respiratory depression in the inspiratory rhythm‐generating network. eLife. 2021;10:e67523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palkovic B, Callison JJ, Marchenko V, et al. Dose‐dependent respiratory depression by remifentanil in the rabbit parabrachial nucleus/Kölliker‐Fuse complex and preBötzinger complex. Anesthesiology. 2021;135:649‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varga AG, Reid BT, Kieffer BL, Levitt ES. Differential input of two critical respiratory centers in opioid‐induced respiratory depression in awake mice. J Physiol. 2002;598:189‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahan A, van der Schrier R, Smith T, et al. Averting opioid‐induced respiratory depression without affecting analgesia. Anesthesiology. 2018;128:1027‐1037. [DOI] [PubMed] [Google Scholar]
- 7. van der Schrier R, Roozekrans M, Olofsen E, et al. Influence of ethanol on oxycodone‐induced respiratory depression: a dose‐escalating study in young and elderly individuals. Anesthesiology. 2017;126:534‐542. [DOI] [PubMed] [Google Scholar]
- 8. van Dorp ELA, Yassen A, Dahan A. Naloxone treatment in opioid addiction: the risks and benefits. Exp Opin Drug Saf. 2007;6:125‐132. [DOI] [PubMed] [Google Scholar]
- 9. Yeung DT, Bough KJ, Harper JR, Platoff GE. National Institute of Health (NIH) executive meeting summary: developing medical countermeasures to rescue opioid‐induced respiratory depression (a trans‐agency meeting)‐August 6/7, 2019. J Med Toxicol. 2020;16:87‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horita A, Carino MA, Chesnut RM. Influence of thyrotropin releasing hormone (TRH) on drug‐induced narcosis and hypothermia in rabbits. Psychopharmacol. 1976;49:57‐62. [DOI] [PubMed] [Google Scholar]
- 11. Wong‐Riley MTT, Liu Q. Neurochemical development of brain stem nuceli involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83‐98. [DOI] [PubMed] [Google Scholar]
- 12. Strabák V. Pancreatic thyrotropin releasing hormone and mechanism of insulin secretion. Cell Physiol Biochem. 2018;50:378‐384. [DOI] [PubMed] [Google Scholar]
- 13. Gary KA, Sevarino KA, Yarbrough GG, Prange AJ, Winokur A. The thyrotropin‐releasing hormone (TRH) hypothesis of homeostatic regulation implications for TRH‐based therapeutics. J Pharmacol Ther. 2003;305:410‐416. [DOI] [PubMed] [Google Scholar]
- 14. Kamath J, Yarbrough GG, Prange AJ, Winokur A. The thryrotropin‐releasing hormone (TRH)‐immune system homeostatic hypothesis. Pharmacol Ther. 2009;121:20‐28. [DOI] [PubMed] [Google Scholar]
- 15. Ballanyi K, Ruangkittisakul A. Structure‐function analysis of rhythmogenic inspiratory pre‐Bötzinger complex networks in “calibrated” newborn rat brainstem slices. Respir Physiol Neurobiol. 2009;168:158‐178. [DOI] [PubMed] [Google Scholar]
- 16. Monga V, Meena CL, Kaur N, Jain R. Chemistry and biology of thyrotropin‐releasing hormone (TRH) and its analogs. Curr Med Chem. 2008;15:2718‐2733. [DOI] [PubMed] [Google Scholar]
- 17. Sun Y, Lu X, Gershengorn MC. Thyrotropin‐releasing hormone receptors ‐ similarities and differences. J Mol Endocrinol. 2003;30:87‐97. [DOI] [PubMed] [Google Scholar]
- 18. Congia S, Tronci S, Ledda M, Porcella A, Coppola G. Low doses of TRH in amyotrophic lateral sclerosis and in other neurological diseases. Int J Neurol Sci. 1991;12:193‐199. [DOI] [PubMed] [Google Scholar]
- 19. Khomane KS, Meena CL, Jain R, Bansal AK. Novel thyrotropin‐releasing hormone analogs: a patent review. Exp Opin Ther Patents. 2011;21:1673‐1691. [DOI] [PubMed] [Google Scholar]
- 20. Marangell LB, George MS, Callahan AM, et al. Effects of intrathecal thyrotropin‐releasing hormone (protirelin) in refractory depressed patients. Archives Gen Psychiatry. 1997;54:14‐222. [DOI] [PubMed] [Google Scholar]
- 21. Takeuchi Y, Takano T, Abe J, Takikita S, Ohno M. Thyrotropin‐releasing hormone: role in the treatment of West syndrome and related epileptic encephalopathies. Brain Dev. 2001;23:662‐667. [DOI] [PubMed] [Google Scholar]
- 22. Bassiri RM. Utiger RD (1973) Metabolism and excretion of exogenous thyrotropin‐releasing hormone in humans. J Clin Invest. 1973;52:1616‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hedner J, Hedner T, Wessberg P, Lundberg D, Jonason J. Effects of TRH and TRH analogues on the central regulation of breathing in the rat. Acta Physiol Scand. 1983;117:427‐437. [DOI] [PubMed] [Google Scholar]
- 24. Bhargava HN, Yousif DJ, Matwyshyn GA. Mathwshyn GA (1983) Interactions of thyrotropin releasing hormone, its metabolites and analogues with endogenous and exogenous opiates. Gen Pharmacol. 1983;14:565‐570. [DOI] [PubMed] [Google Scholar]
- 25. Bennet L, Gluckman PD, Johnston BM. The central effects of thyrotropin‐releasing hormone on the breathing movements and electrocortical activity of the fetal sheep. Ped Res. 1988;23:72‐75. [DOI] [PubMed] [Google Scholar]
- 26. Hedner J, McCrown TJ, Mueller RA, et al. Respiratory stimulant effects of TRH into the mesencephalic region in the rat. Acta Physiol Scand. 1987;130:69‐75. [DOI] [PubMed] [Google Scholar]
- 27. Holtman JR, Buller AL, Hamosh P, Gillis RA. Hamosh P and Gillis RA (1986) Central respiratory stimulation produced by thyrotropin‐releasing hormone in the cat. Peptides. 1986;7:207‐212. [DOI] [PubMed] [Google Scholar]
- 28. Koivusalo F, Paakkari I, Leppäluoto J, Karppanen H. The effect of centrally administered TRH on blood pressure, heart rate and ventilation in rat. Acta Physiol Scand. 1979;196:83‐86. [DOI] [PubMed] [Google Scholar]
- 29. Mulkey DK, Rosin DL, West G, et al. Serotonergic neurons activate chemosensitive retrotrapezoid nucelus neurons by a pH‐independent mechanism. J Neurosci. 2007;27:14128‐14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paakkari I, Nurminen ML, Sirén AL. Cardiovascular effects of TRH in anaesthetized rats: role of the brain stem. Eur J Pharmacol. 1986;122:131‐134. [DOI] [PubMed] [Google Scholar]
- 31. Boghosian JD, Luethy A, Cotten JF. Intravenous and intratracheal thyrotropin releasing hormone and its analog taltirelin reverse opioid‐induced respiratory depression in isoflurane anesthetized rats. J Pharmacol Exp Ther. 2018;366:105‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holtman JR, Norman WP, Skirboll L, et al. Evidence of 5‐hydroxytryptamine, substance P, and thyrotropin‐releasing hormone in neurons innervating the phrenic motor nucleus. J Neurosci. 1984;4:1064‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vonhof S, Sirén AL, Feuerstein GZ. Central ventilatory effects of thyrotropin‐releasing hormone in the conscious rat. Neuropeptides. 1991;18:93‐98. [DOI] [PubMed] [Google Scholar]
- 34. Bayliss DA, Viana F, Berger AJ. Mechanisms underlying effects of thyrotropin‐releasing hormone on rat hypoglossal motorneurons in vitro. J Neurophysiol. 1973;68:1733‐1745. [DOI] [PubMed] [Google Scholar]
- 35. Greer JJ, Al‐Zubaidy Z, Carter JO. Thyrotropin‐releasing hormone stimulates perinatal rat respiration in vitro. Am J Physiol. 1996;271:R1160‐R1164. [DOI] [PubMed] [Google Scholar]
- 36. Inyushkin AN, Merkulova NA, Chepurnov SA. The pre‐Bötzinger complex participates in generating the respiratory effects of thyroliberin. Neurosci Behav Physiol. 1999;29:285‐292. [DOI] [PubMed] [Google Scholar]
- 37. Pagliardini S, Ren J, Gray PA, et al. Central respiratory rhythmogenesis is abnormal in Lbx1‐deficient mice. J Neurosci. 2008;28:11030‐11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cream C, Nattie E, Li A. TRH microdialysis into the RTN of the conscious rat increases breathing, metabolism, and temperature. J Appl Physiol. 1999;87:673‐682. [DOI] [PubMed] [Google Scholar]
- 39. Dekin MS, Richerson GB, Getting PA. Thyrotropin‐releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67‐69. [DOI] [PubMed] [Google Scholar]
- 40. Hou L, Zhou X, Chen Y, et al. Thyrotropin‐releasing hormone causes a tonic excitatory postsynaptic current and inhibits the phasic inspiratory inhibitory inputs in inspiratory‐inhibited aiway vagal preganglionic neurons. Neurosci. 2012;202:184‐191. [DOI] [PubMed] [Google Scholar]
- 41. McCowan TJ, Hedner JA, Towle AC, Breese GR, Mueller ERA. Brainstem localization of a thyrotropin‐releasing hormone‐induced change in respiratory function. Brain Res. 1986;373:189‐196. [DOI] [PubMed] [Google Scholar]
- 42. Prange AJ, Breese GR, Cott JM, et al. Thyrotropin releasing hormone: antagonism of pentobarbital in rodents. Life Sci. 1974;14:447‐455. [DOI] [PubMed] [Google Scholar]
- 43. Mutolo D, Bongianni F, Carfì M, Pantaleo T. Respiratory responses to thyrotropin‐releasing hormone microinjected into the rabbit medulla oblongata. Am J Physiol. 1999;277:R1331‐R1338. [DOI] [PubMed] [Google Scholar]
- 44. Puissant MM, Echert AE, Yang C, et al. RNASeq‐derived transcriptome comparisons reveal neuromodulatory deficiency in CO2 insensitive brown Norway rat. J Physiol. 2015;593:415‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nink M, Krause U, Lehnert H, et al. Thyrotropin‐releasing hormone has stimulatory effects on ventilation in humans. Act Physiol Scand. 1991;141:309‐318. [DOI] [PubMed] [Google Scholar]
- 46. Schulz R, Nink M, Werner GS, et al. Human corticotropin‐releasing hormone and thyrotropin‐releasing hormone modulate the hypercapnic ventilatory response in humans. Eur J Clin Invest. 1996;26:989‐995. [DOI] [PubMed] [Google Scholar]
- 47. Peek MJ, Bajoria R, Talbert D, Fisk NM. Effects of thyrotropin‐releasing hormone on fetal heart rate and breathing movements. Fetal Diagn Ther. 1998;13:100‐105. [DOI] [PubMed] [Google Scholar]
- 48. Winokur A. The relevance of thyrotropin‐releasing hormone to psychiatric disorders. In: Nemeroff CB, ed. Neuropeptides and psychiatric disorders (Progress in Psychiatry). American Psychiatry Association Press; 1991:15‐18. [Google Scholar]
- 49. Boon M, van Dorp E, Broens S, Overdyk F. Combining opioids and benzodiazepines: effects on mortality and severe respiratory events. Ann Palliat Med. 2020;9:542‐557. [DOI] [PubMed] [Google Scholar]
- 50. Breese GR, Cott JM, Cooper BR, Prange AJ, Lipton MA. Antagonism of ethanol narcosis by thyrotropin releasing hormone. Life Sci. 1974;14:1053‐1063. [DOI] [PubMed] [Google Scholar]
- 51. Breese GR, Cott JM, Cooper BR, et al. Effects of thyrotropin‐releasing hormone (TRH) on the actions of pentobarbital and other centrally acting drugs. J Pharmacol Exp Ther. 1975;193:11‐22. [PMC free article] [PubMed] [Google Scholar]
- 52. Cott JM, Breese GR, Cooper BR, Barlow TS, Prange AJ. Investigations into the mechanism of reduction of ethanol sleep by thyrotropin‐releasing hormone (TRH). J Pharmacol Exp Ther. 1976;196:594‐604. [PMC free article] [PubMed] [Google Scholar]
- 53. Sharp T, Tulloch IF, Bennett GW, et al. Analeptic effects of centrally injected TRH and analogues in the pentobarbitone‐anaesthetized rat. Neuropharmacol. 1984;23:339‐348. [DOI] [PubMed] [Google Scholar]
- 54. Bhargava HN. Antagonism of ketamine‐induced anesthesia and hypothermia by thyrotropin‐releasing hormone and cyclo(His‐Pro). Neuropharmacol. 1981;20:699‐702. [DOI] [PubMed] [Google Scholar]
- 55. Schaefer CF, Brackett DJ, Biber B, et al. Respiratory and cardiovascular effects of thyrotropin‐releasing hormone as modified by isoflurane, enflurane, pentobarbital and ketamine. Reg Peptides. 1989;24:269‐282. [DOI] [PubMed] [Google Scholar]
- 56. Hernandez DE, Meyer RE, Irving PE, Crane SW. Reversal of pentobarbital‐induced narcosis by thyrotropin‐releasing hormone (TRH) in dogs. Pharmacol Res Comm. 1987;19:567‐577. [DOI] [PubMed] [Google Scholar]
- 57. Kraemer GW, Mueller R, Breese GR, et al. Thyrotropin releasing hormone: antagonism of pentobarbital narcosis in the monkey. Pharmacol Biochem Behav. 1976;4:709‐712. [DOI] [PubMed] [Google Scholar]
- 58. Serbenyuk TV, Gurskaya IE, Slyuta AD, Roze GY, Romanovskii PY. Restoration of disturbed respiration in cats by thyrotropin releasing hormone. Byull Eksper Biol Med. 1988;106:17‐19. [Google Scholar]
- 59. Meah MS, Gardner WN. Post‐hyperventilation apnoea in conscious humans. J Phsyiol. 1994;477:527‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lighton C, Bennett GW, Mardsen CA. Chronic immunization of endogenous thyrotropin‐releasing hormone (TRH) in brain alters the behavioral response to pentobarbital and a TRH analogue. Brain Res. 1986;378:385‐389. [DOI] [PubMed] [Google Scholar]
- 61. Nguyen V, Zharikova A, Prokai‐Tatrai K, Prokai L. [Glu2]TRH dose‐dependently attenuates TRH‐evoked analeptic effect in mice. Brain Res Bull. 2010;82:83‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kharkevich DA, Chizh BA, Kasparov SA. Stimulant effect of thyrotropin‐releasing hormone and its analog, RGH 2202, on the diaphragm respiratory activity, and their antagonism with morphine: possible involvement of the N‐methyl‐D‐aspartate receptors. Brain Res. 1991;51:110‐115. [DOI] [PubMed] [Google Scholar]
- 63. Takita K, Herlenius E, Yamamoto Y, Lindahl SGE. Effects of neuroactive substances on the morphine‐induced respiratory depression; an in vitro study. Brain Res. 2000;884:201‐205. [DOI] [PubMed] [Google Scholar]
- 64. Dandrea KE, Cotten JF. A comparison of breathing stimulants for reversal of synthetic opioid‐induced respiratory depression in conscious rats. J Pharmacol Exp Ther. 2021;378:146‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu WY, Liu H, Aggarwal J, Huang ZL, Horner RL. Differential activating effects of thyrotropin‐releasing hormone and its analog taltirelin on motor output to the tongue musculature in‐vivo. Sleep. 2020;43(9):zsaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Algera MH, Cotten JF, van Velzen M, et al. Respiratory effects of thyrotropin‐releasing hormone (TRH) and its analogue during opioid‐induced respiratory depression. Br J Anaesth. 2022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lenz G, Hempel V, Glöser A, Dangelmaier R. Failure of thyrotropin releasing hormone to reverse fentanyl‐flunitrazepam anesthesia in man. Acta Anaesthesiol Belg. 1986;37:219‐223. [PubMed] [Google Scholar]
- 68. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid‐induced respiratory depression. Br J Anaesth. 2018;120:1117‐1127. [DOI] [PubMed] [Google Scholar]
- 69. Engel WK, Siddique T, Nicoloff JT. Effect on weakness and spasticity in amyotrophic lateral sclerosis of thyrotropin‐releasing hormone. Lancet. 1983;8341:73‐75. [DOI] [PubMed] [Google Scholar]
- 70. Kobayashi K, Abe Y, Harada H, Oota E, Endo T, Takeda H. Non‐clinicsl pharmacokinetic profiles of rovatirelin, an orally available thryrotropin‐releasing hormone analogue. Xenobiotica. 2019;49:106‐119. [DOI] [PubMed] [Google Scholar]
- 71. Nishizawa M, Onodera O, Hirakawa A, Shimizu Y, Yamada M. On behalf of the rovatirelin study group. Effect of rovatirelin in patients with cerebellar ataxia: two randomized double‐blind placebo‐controlled phase 3 trials. J Neurol Neurosurg Psychiatry. 2020;91:254‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kobayashi N, Sato N, Fujimura Y, et al. Discovery of the orally effective Thyrotropin‐Releasing Hormone mimetic: 1‐{N‐[(4S,5S)‐(5‐Methyl‐2‐oxooxazolidine‐4‐yl)carbonyl]‐3‐ (thiazol‐4‐yl)‐L‐alanyl}‐(2R)‐2‐methylpyrrolidine trihydrate (Rovatirelin Hydrate). ACS Omega. 2018;3:13647‐13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2019: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46:D1091‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alexander SPH, Kelly E, Mathie A, et al. The concise guide to pharmacology 2019/2020. Br J Pharmacol. 2019;176:S1‐S20. https://bpspubs.onlinelibrary.wiley.com/toc/14765381/2021/178/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The search strategy is available upon request from the authors (a.dahan@lumc.nl).


