Abstract
An enzymatic route for the depolymerization of a heteropolysaccharide (xanthan) in Bacillus sp. strain GL1, which was closely related to Brevibacillus thermoruber, was determined by analyzing the structures of xanthan depolymerization products. The bacterium produces extracellular xanthan lyase catalyzing the cleavage of the glycosidic bond between pyruvylated mannosyl and glucuronyl residues in xanthan side chains (W. Hashimoto et al., Appl. Environ. Microbiol. 64:3765–3768, 1998). The modified xanthan after the lyase reaction was then depolymerized by extracellular β-d-glucanase to a tetrasaccharide, without the terminal mannosyl residue of the side chain in a pentasaccharide, a repeating unit of xanthan. The tetrasaccharide was taken into cells and converted to a trisaccharide (unsaturated glucuronyl-acetylated mannosyl-glucose) by β-d-glucosidase. The trisaccharide was then converted to the unsaturated glucuronic acid and a disaccharide (mannosyl-glucose) by unsaturated glucuronyl hydrolase. Finally, the disaccharide was hydrolyzed to mannose and glucose by α-d-mannosidase. This is the first complete report on xanthan depolymerization by bacteria. Novel β-d-glucanase, one of the five enzymes involved in the depolymerization route, was purified from the culture fluid. This enzyme was a homodimer with a subunit molecular mass of 173 kDa and was most active at pH 6.0 and 45°C. The enzyme specifically acted on xanthan after treatment with xanthan lyase and released the tetrasaccharide.
Xanthan is an extracellular heteropolysaccharide produced by a plant-pathogenic bacterium, Xanthomonas campestris, and is composed of cellulosic backbone with linear trisaccharide side chains consisting of a mannosyl-glucuronyl-mannose sequence attached at the C-3 position on alternate glucosyl residues (16, 27). The internal and terminal mannosyl residues of the side chain are frequently acetylated and pyruvylated, respectively, depending on both the growth conditions and the bacterial strains (30). Xanthan has been widely used as a gelling and stabilizing agent in the food, pharmaceutical, and oil industries (29) because the polysaccharide shows superior rheological properties, such as pseudoplasticity, high viscosity at low concentration, and tolerance toward a wide range of pHs and temperatures (15, 19).
However, the polymer is considered to play a key role in the virulence of Xanthomonas bacterial cells against plants (5). Therefore, a biosynthetic pathway of xanthan in X. campestris has been well characterized, especially with respect to the relationship between pathogenicity and the polysaccharide (5, 18). A cluster of 12 genes has been suggested to be involved in the biosynthesis of xanthan (18). However, a depolymerization pathway of xanthan by organisms has not been elucidated. Although some microorganisms and their enzymes have been reported to participate in the depolymerization of the polysaccharide (2, 4, 22, 33, 34), enzymes responsible for the complete depolymerization of xanthan have not been identified.
In the course of a study on the assimilation of heteropolysaccharides by microbes, we have isolated a bacterium, Bacillus sp. strain GL1, that is able to depolymerize a bacterial heteropolysaccharide (gellan) (9, 12). Gellan is composed of polymerized tetrasaccharide repeating units [→3)-β-D-Glcp-(1→4)-β-d-GlcAp-(1→4)-β-d-Glcp-(1→4)-α-L-Rhap-(1→] (17, 26). Recently, Bacillus sp. strain GL1 cells have been also found to utilize xanthan for their growth, and xanthan lyase acting on the side chain of xanthan has been characterized (13). Subsequent to this study, we attempted to clarify the xanthan depolymerization pathway of the bacterium and to characterize one of the xanthan depolymerization enzymes, β-d-glucanase, which hydrolyzes the main chain of xanthan.
MATERIALS AND METHODS
Materials.
Pyruvylated xanthan (average molecular mass, 2 × 106; pyruvylation of terminal mannosyl residue in the side chain, 50%) was a kind gift from The Kohjin Co., Tokyo, Japan. Modified xanthan was prepared as follows. Xanthan (0.5 g) was treated at 30°C for 90 h with 0.5 U of purified xanthan lyase, precipitated with ethanol to remove pyruvylated mannose, and dissolved in distilled water. Gellan (molecular mass, 5 × 105; deacetylated) and pectin were purchased from Wako Pure Chemicals Co., Osaka, Japan. Silica gel 60/Kieselguhr F254 thin-layer chromatography (TLC) plates were obtained from E. Merck, Darmstadt, Germany. Butyl- and DEAE-Toyopearl 650M were purchased from Tosoh Co., Tokyo, Japan, and DEAE-Sepharose CL-6B and Sephacryl S-200HR were from Pharmacia Biotech Co., Uppsala, Sweden. Bio-Gel P2 was from Bio-Rad Laboratories, Hercules, Calif. p-Nitrophenyl (pNP)–sugars, lichenan from Cetraria islandica, and β-glucan from barley were from Sigma Chemical Co., St. Louis, Mo. Xylan from oat spelt was from Nacalai Tesque Co., Kyoto, Japan.
Microorganism and culture conditions.
For the purification of β-d-glucanase, Bacillus sp. strain GL1 cells were aerobically cultured at 30°C and at 100 rpm for 48 h in a liquid xanthan medium consisting of 0.1% (NH4)2SO4, 0.1% KH2PO4, 0.1% Na2HPO4, 0.01% MgSO4 · 7H2O, 0.01% yeast extract, and 0.5% xanthan (pH 7.2). To investigate the activities of xanthan depolymerization enzymes, xanthan in the medium was replaced with gellan or pectin (0.5%).
DNA isolation and 16S rRNA analysis.
A genomic DNA of Bacillus sp. strain GL1 was isolated as described previously (28). A 16S rRNA gene of the bacterium was amplified by PCR by using the genomic DNA as a template and 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGCTACCTTGTTACGACTT-3′) as primers (21). The nucleotide sequence of the amplified 16S rDNA was determined by the dideoxy-chain termination method by using a model 377 automated DNA sequencer (Applied Biosystems Division of Perkin-Elmer, Foster City, Calif.) (31). Phylogenetic analysis was performed by using the FASTA and CLUSTALW programs on the DDBJ server (Shizuoka, Japan).
Assay for enzymes.
β-d-Glucanase was incubated at 30°C for 30 min in 0.5 ml of a reaction mixture containing 0.1% xanthan after treatment with xanthan lyase and 50 mM potassium phosphate buffer (KPB), pH 7.0, and the activity was determined by measuring the release of a reducing sugar according to the method of Lever (23). One unit of the enzyme activity was defined as the amount of enzyme required to release 1 μmol of the reducing sugar from the substrate per min. α-d-Mannosidase and β-d-glucosidase were assayed by using 0.4 mM pNP-sugars as substrates. One unit of the enzyme activity was defined as the amount of enzyme required to release 1 μmol of p-nitrophenol from the substrate per min. Unsaturated glucuronyl hydrolase was incubated at 30°C in 1 ml of a reaction mixture containing 0.005% tetrasaccharide from gellan by gellan lyase and 50 mM KPB, pH 7.0, and the activity was determined by monitoring the decrease of the A235 arising from the double bond in the tetrasaccharide. One unit of the enzyme activity was defined as the amount of enzyme required to produce the decrease of 1.0 in the A235 per min (10). The protein content was determined by the method of Lowry et al. (24), with bovine serum albumin as a standard, or by measuring the A280, assuming that an E280 of 1.0 corresponds to 1 mg/ml.
Preparation of extra- and intracellular fractions.
Cells grown at 30°C for 48 h were harvested by centrifugation at 13,000 × g at 4°C for 10 min, and the resulting culture fluid was used as an extracellular enzyme source. Collected cells were washed in 20 mM KPB, pH 7.0, and then resuspended in the same buffer. The cells were ultrasonically disrupted (Insonator, Kubota model 201M; Kubota, Tokyo, Japan) at 0°C and 9 kHz for 5 min, and the clear solution obtained after centrifugation at 13,000 × g at 4°C for 20 min was dialyzed against 20 mM KPB (pH 7.0) overnight. The dialysate was used as an intracellular enzyme source.
TLC.
Xanthan depolymerization products by extra- and intracellular enzymes were analyzed by TLC with a solvent system of 1-butanol–acetic acid–water (2:1:1 [vol/vol]). The products were visualized by heating the TLC plate at 110°C for 5 min after spraying it with 10% (vol/vol) sulfuric acid in ethanol.
Purification of xanthan depolymerization products.
Xanthan depolymerization products were applied to a gel filtration of Bio-Gel P2 column (0.9 by 122 cm) previously equilibrated with distilled water. The products were eluted at room temperature with distilled water, and 0.8-ml fractions were collected every 7 min. The eluted products were detected by TLC.
Mass spectrometry.
Each purified xanthan depolymerization product was analyzed by electrospray ionization-mass spectrometry (ESI-MS) by using an API III triple quadrupole mass spectrometer (Perkin-Elmer Sciex; Perkin-Elmer, Thornhill, Ontario, Canada) equipped with an atmospheric pressure ionization ion source. The mass spectrometer was operated in the positive or negative modes. The mass spectrum was scanned from 150 to 1,500 atomic mass units (amu) in 0.1-amu steps. Molecular weights were calculated on the basis of deconvolution mass spectra.
Purification of β-d-glucanase.
Unless otherwise specified, all operations were done at 0 to 4°C. Bacillus sp. strain GL1 cells were cultured at 30°C and 100 rpm for 48 h in 10 liters of xanthan medium (1 liter/flask). After cultivation, the cells were removed by centrifugation at 13,000 × g at 4°C for 10 min. The fluid (crude enzyme solution, 8.4 liters) was applied to a DEAE-Sepharose CL-6B column (4 by 22 cm) previously equilibrated with 20 mM KPB (pH 7.0). The enzyme was eluted with a linear gradient of NaCl (0 to 1 M) in 20 mM KPB (pH 7.0; 2 liters), and 18-ml fractions were collected every 9 min. The active fractions, which were eluted with 0.5 M NaCl, were combined, dialyzed against 20 mM KPB (pH 7.0), and applied to a DEAE-Toyopearl 650M column (4 by 8 cm) previously equilibrated with 20 mM KPB (pH 7.0). The enzyme was eluted with a linear gradient of NaCl (0 to 0.5 M) in 20 mM KPB (pH 7.0; 0.5 liters), and 4.2-ml fractions were collected every 3 min. The active fractions, which were eluted with 0.3 M NaCl, were combined and saturated with ammonium sulfate (30%), and then the enzyme solution was applied to a Butyl-Toyopearl 650M column (2.7 by 3.5 cm) previously equilibrated with 20 mM KPB (pH 7.0) and saturated with ammonium sulfate (30%). The enzyme was eluted with a linear gradient of ammonium sulfate (30 to 0%) in 20 mM KPB (pH 7.0; 100 ml), and 0.83-ml fractions were collected every minute. The active fractions, which were eluted with 20 mM KPB (pH 7.0) and saturated with ammonium sulfate (10%), were combined, concentrated to about 3 ml by ultrafiltration with an Amicon model 8200 apparatus (Amicon Co., Beverly, Mass.) by using a membrane with a molecular-weight cutoff of >10,000, and applied to a Sephacryl S-200HR column (2.7 by 64 cm) previously equilibrated with 20 mM KPB (pH 7.0) and containing 0.15 M NaCl. The enzyme was eluted with the same buffer, and 3-ml fractions were collected every 6 min. The enzyme was eluted between fractions 51 and 58. These fractions were combined, dialyzed against 20 mM KPB (pH 7.0), and then used as the purified enzyme.
Electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described previously (20).
Nucleotide sequence accession number.
The nucleotide sequence for 16S rDNA reported here has been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB024598.
RESULTS AND DISCUSSION
Phylogenetic analysis of Bacillus sp. strain GL1.
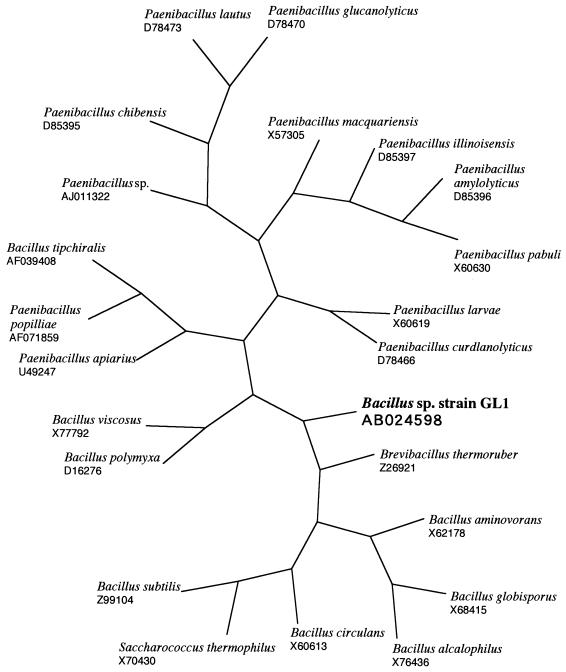
Bacillus sp. strain GL1 isolated from a soil can utilize gellan and xanthan as a carbon source for its growth. The taxonomic properties of the bacterium have been described previously (12). To determine the phylogenetic position of the bacterium, the nucleotide sequence of 16S rRNA gene (1,514 bp) was determined. From the detailed taxonomical properties of Bacillus sp. strain GL1, the bacterium has been found to resemble Bacillus circulans (12). The gene of the 16S rRNA exhibited the highest identity score (91%) with that of Paenibacillus sp. (GenBank accession number AJ011322) and high similarity with those of Bacillus circulans (82%) (X60613) and Bacillus subtilis (82%) (Z99104). The phylogenetic gene tree which resulted from multiple-sequence analysis is shown in Fig. 1. The bacterium was most closely related to Brevibacillus thermoruber (Z26921) and was closer to Bacillus circulans (X60613) than to Paenibacillus sp. (AJ011322).
FIG. 1.
Phylogenetic tree based on the 16S rRNA sequences of Bacillus sp. strain GL1 and bacteria close to it. The accession numbers of bacterial 16S rRNA sequences are indicated.
Activities of xanthan depolymerization enzymes in Bacillus sp. strain GL1.
Sufficient growth of Bacillus sp. strain GL1 was observed when cultured in the medium containing xanthan, gellan, or pectin as a sole carbon source, and the activities of possible xanthan depolymerization enzymes such as xanthan lyase, β-d-glucanase, and exoglycosidases were determined (Table 1). Extracellular xanthan lyase (13) and β-d-glucanase were produced only in the xanthan medium. The following three kinds of exoglycosidases are thought to be localized in cytosol because no signal sequence has been found in the deduced amino acid sequences from their nucleotide ones (10, 11, 25). α-d-Mannosidase was specifically induced in the xanthan medium. β-d-Glucosidase activity was found in all of the media tested. Unsaturated glucuronyl hydrolase, which catalyzes the hydrolysis of unsaturated oligosaccharides produced by polysaccharide lyases, was induced in the presence of gellan or xanthan (10).
TABLE 1.
Activities of xanthan depolymerization enzymes in Bacillus sp. strain GL1
| Enzymes | Sp act (mU/mg of protein)a
|
|||||
|---|---|---|---|---|---|---|
| Gellan
|
Pectin
|
Xanthan
|
||||
| Intra. | Extra. | Intra. | Extra. | Intra. | Extra. | |
| Xanthan lyase | ND | ND | ND | ND | ND | 320 |
| β-d-Glucanase | ND | ND | ND | ND | ND | 12.4 |
| β-d-Glucosidase | 29.0 | ND | 35.9 | ND | 6.17 | ND |
| Unsaturated glucuronyl hydrolaseb | 20.7 | ND | ND | ND | 16.4 | ND |
| α-d-Mannosidase | 0.906 | ND | 0.744 | ND | 20.3 | ND |
Bacillus sp. strain GL1 cells were cultured in a medium containing gellan, pectin, or xanthan as a sole carbon source as indicated. The activities of enzymes were determined as described in Materials and Methods. The data are averages of two or four independent measurements. Values agreed within ±20%. Intra., intracellular fraction; Extra., extracellular fraction; ND, not detected.
Data cited from Hashimoto et al. (10).
Enzymatic route for xanthan depolymerization in Bacillus sp. strain GL1.
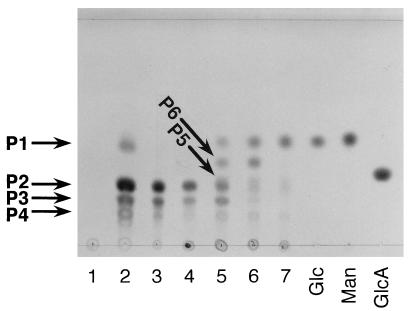
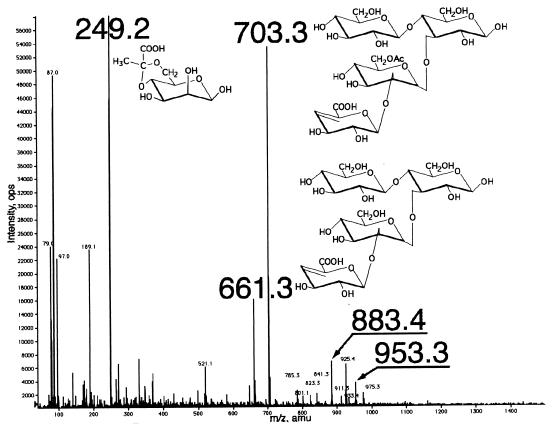
When xanthan was incubated with the extracellular enzyme source of Bacillus sp. strain GL1 cells grown in the xanthan medium, three major products with Rf values from a maximum of 0.44 (P1) to 0.26 (P2) and 0.19 (P3), along with a minor product (0.14 [P4]), were observed as the final products on the TLC plate (Fig. 2). Xanthan depolymerization products produced by enzymes in the extracellular fraction were analyzed by ESI-MS (Fig. 3). The molecular weights of three major products (P1 to P3) were determined to be 250 (P1), 662 (P2), and 704 (P3), which were calculated from m/z 249, 661, and 703 ions corresponding to the deprotonated ions [M-H]− in the negative mode of ESI-MS (Fig. 3), respectively, judging from the determination of the molecular weight of each purified product by ESI-MS. The product P1 was confirmed to be a pyruvylated mannose first liberated from xanthan by xanthan lyase (13). Based on the ESI-MS and information on the known structure of xanthan, the product P2 was identified as a tetrasaccharide consisting of an unsaturated glucuronic acid, an acetylated mannose, and two glucose residues. The product P3 is thought to be a deacetylated form of P2. Therefore, the products P2 and P3 were considered to be released by β-d-glucanase acting on the main chain of xanthan. The product P4 was found to be a pentasaccharide, a repeating unit of xanthan, for the following two reasons. (i) The purified product (P4) was degraded to tetrasaccharides (P2 and P3) and pyruvylated mannose (P1) by purified xanthan lyase (data not shown). (ii) The minor peaks around m/z 900 in the mass spectrum (Fig. 3) are thought to be derived from pentasaccharides with or without acetylation and/or pyruvation. For example, molecular weights of 954 and 884 from m/z 953 and 883 ions corresponding to the deprotonated ions coincided with those of pentasaccharides with or without pyruvation. These results indicate that in Bacillus sp. strain GL1, extracellular xanthan lyase attacks xanthan side chains, and then extracellular β-d-glucanase hydrolyzes the linkages of the main chain to give the tetrasaccharide. However, the formation of pentasaccharide was observed after incubation of xanthan with extracellular enzyme fraction, thus indicating that β-d-glucanase can partly act on xanthan prior to xanthan lyase.
FIG. 2.
Degradation of xanthan by extra- and intracellular enzymes of Bacillus sp. strain GL1. Lane 1, xanthan; lane 2, xanthan depolymerization products by extracellular enzymes. The degradation of the mixture of P2 and P3 (tetrasaccharides) by intracellular enzymes of Bacillus sp. strain GL1 is also shown. The tetrasaccharides were incubated at 30°C with intracellular enzymes. Samples of 10 μl were periodically removed from the reaction mixture and analyzed by TLC. Lane 3, tetrasaccharide (mixture of P2 and P3). The reaction times were as follows: lane 4, 0 min; lane 5, 40 min; lane 6, 2 h; and lane 7, 12 h. Lanes Glc, Man, and GlcA represent authentic d-glucose, d-mannose, and d-glucuronic acid, respectively.
FIG. 3.
ESI-mass spectrum of xanthan depolymerization products by extracellular enzyme source of Bacillus sp. strain GL1.
The tetrasaccharides were degraded by enzymes in an intracellular fraction to monosaccharides by way of two intermediates (P5 and P6) (Fig. 2). The molecular weights of the purified products (P5 and P6) were determined to be 542 and 342, calculating from m/z 543 and 365 ions corresponding to the protonated [M+H]+ and the sodium adduct [M+Na]+ ions in the positive mode of ESI-MS, respectively. The molecular weight of P5 coincided with that of a trisaccharide composed of an unsaturated glucuronic acid, an acetylated mannose, and a glucose residue. The product (P6) was revealed to be a disaccharide of mannosyl-glucose, since the disaccharide was hydrolyzed to mannose and glucose by purified α-d-mannosidase from Bacillus sp. strain GL1 cells (25). Therefore, the product (P5) was identified as unsaturated glucuronyl-acetylated mannosyl-glucose produced from P2 through the release of glucose by β-d-glucosidase, and the product (P6) was generated from P5 by the action of unsaturated glucuronyl hydrolase (10) and deacetylase.
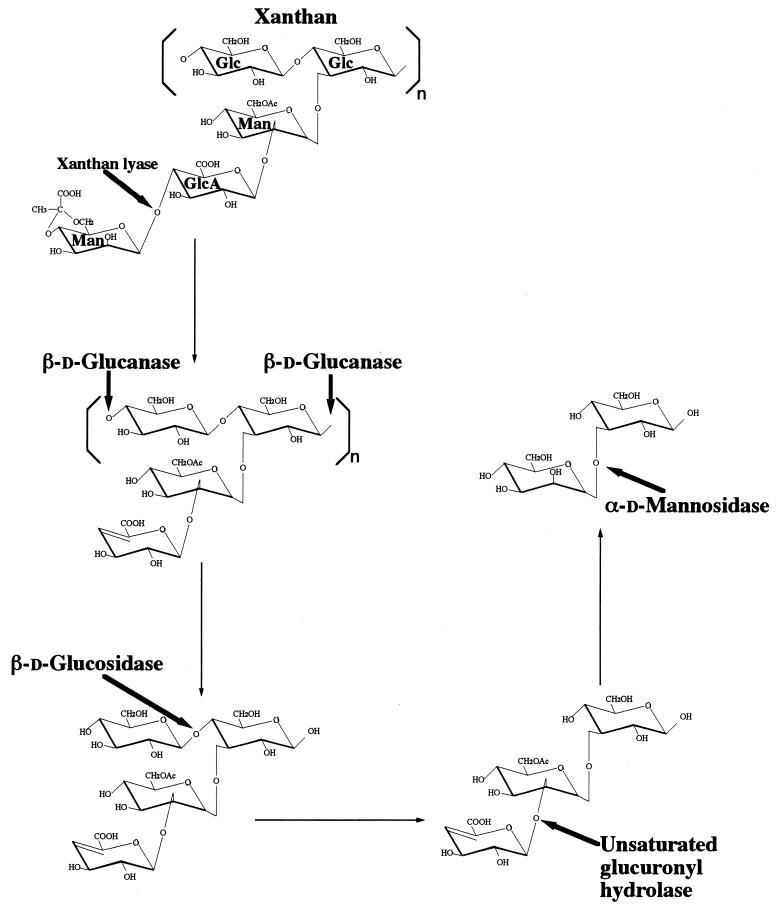
The overall xanthan depolymerization pathway in Bacillus sp. strain GL1 is summarized in Fig. 4. Xanthan is first depolymerized to pyruvylated mannose and tetrasaccharide by extracellular xanthan lyase and β-d-glucanase, and the tetrasaccharide is then degraded to the trisaccharide (unsaturated glucuronyl-acetylated mannosyl-glucose) by intracellular β-d-glucosidase. The unsaturated glucuronic acid is removed from the trisaccharide by intracellular unsaturated glucuronyl hydrolase. The resultant disaccharide (mannosyl-glucose) is finally hydrolyzed by α-d-mannosidase to mannose and glucose. Thus, five enzymes (xanthan lyase [13], β-d-glucanase, β-d-glucosidase [11], unsaturated glucuronyl hydrolase [10], and α-d-mannosidase [25]) were found to be responsible for the complete depolymerization of xanthan. Since four enzymes other than β-d-glucanase have been already analyzed in detail, purification and characterization of β-d-glucanase were performed.
FIG. 4.
Xanthan depolymerization pathway in Bacillus sp. strain GL1. The cleavage sites of xanthan depolymerization enzymes are indicated by thick arrows. Thin arrows show the pathway of xanthan depolymerization. The deacetylase possibly releases the acetyl group from acetylated mannose during the transformation of tri- to disaccharide.
Purification and properties of β-d-glucanase.
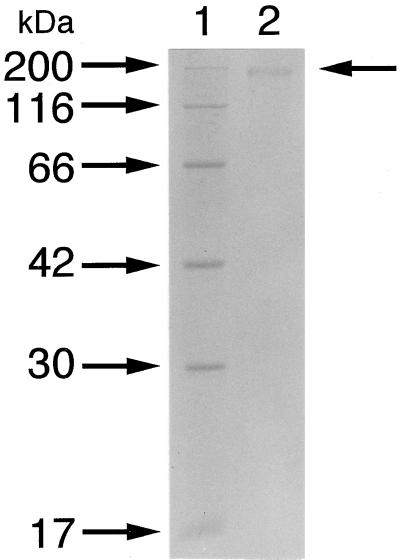
β-d-Glucanase involved in the hydrolysis of the main chain of xanthan was purified 332-fold with a recovery of 9.6% from the culture fluid (Table 2). The purified enzyme was homogeneous on an SDS-PAGE gel (Fig. 5).
TABLE 2.
Purification of β-d-glucanase
| Step | Total protein (mg) | Total activity (U) | Sp act (U/mg) | Yield (%) | Purifi-cation (fold) |
|---|---|---|---|---|---|
| Crude enzyme | 1,800 | 16.6 | 0.0092 | 100 | 1.0 |
| DEAE-Sepharose CL-6B | 85.9 | 16.8 | 0.196 | 101 | 21.2 |
| DEAE-Toyopearl 650M | 32.5 | 6.00 | 0.185 | 36.1 | 20.1 |
| Butyl-Toyopearl 650M | 3.14 | 2.68 | 0.854 | 16.1 | 92.6 |
| Sephacryl S-200HR | 0.538 | 1.64 | 3.06 | 9.88 | 331 |
FIG. 5.
Electrophoretic profile of β-d-glucanase. The purified β-d-glucanase was subjected to SDS-PAGE. Lane 1, molecular size standards (from top): myosin (200 kDa), β-galactosidase (116 kDa), bovine serum albumin (66 kDa), aldolase (42 kDa), carbonic anhydrase (30 kDa), and myoglobin (17 kDa); lane 2, purified β-d-glucanase. The arrow to the right indicates the position of β-d-glucanase.
(i) Molecular mass.
The molecular mass of the enzyme was determined to be 355 kDa by gel permeation chromatography (Sephacryl S-200HR) (data not shown) and 173 kDa when determined by SDS-PAGE with a gel at a low concentration of 6%, indicating that the enzyme was a homodimer.
(ii) pH and temperature.
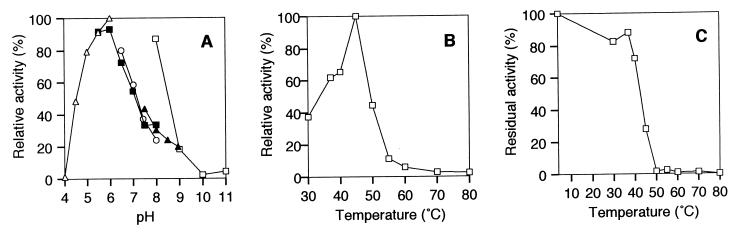
The enzyme was most active at pH 6.0 in sodium acetate buffer and at 45°C (Fig. 6A and B). The enzyme was stable below 40°C (Fig. 6C). About 30% of activity was lost after incubation at 40°C and pH 6.0 for 10 min (Fig. 6C).
FIG. 6.
Effect of pH and temperature on the activity and stability of β-d-glucanase. Experiments were carried out at 30°C with 0.05% xanthan after treatment with xanthan lyase and purified β-d-glucanase. (A) Effect of pH. Reactions were performed at 30°C for 30 min in the following 50 mM buffers: sodium acetate (▵), potassium phosphate (■), sodium-HEPES (○), Tris-HCl (▴), and glycine-NaOH (□). Activity at pH 6.0 in sodium acetate buffer was relatively taken as 100%. (B) Optimal temperature. Reactions were performed for 30 min at various temperatures in 50 mM sodium acetate buffer (pH 6.0). The activity at 45°C was relatively taken as 100%. (C) Thermal stability. After preincubation of the enzyme for 10 min at various temperatures, the residual activity was measured at 30°C for 30 min in 50 mM sodium acetate buffer (pH 6.0). The activity of the enzyme preincubated at 4°C was taken as 100%.
(iii) Metal ions and others.
The reaction was performed in the presence or absence of various compounds, and the residual activity was measured. Cu2+ inhibited the reaction by ca. 80% at 1 mM. Other divalent metal ions (Ca2+, Co2+, Fe2+, Hg2+, Mg2+, Mn2+, and Zn2+) had little effect on the enzyme activity at 1 mM. Thiol reagents (dithiothreitol, glutathione [reduced form], 2-mercaptoethanol, iodoacetamide, and N-ethylmaleimide) and a chelator (EDTA) (1 mM each) revealed no significant effect on the enzyme activity.
(iv) Substrate specificity.
To examine the substrate specificity of β-d-glucanase, the enzyme was incubated at 30°C for 30 min in a mixture containing 50 mM sodium acetate buffer (pH 6.0) and various substrates (0.1%). The enzyme was specific to xanthan after treatment with xanthan lyase and liberated tetrasaccharide when analyzed by TLC. Native xanthan, carboxymethyl cellulose, and β-glucan were slightly degraded (Table 3). After prolonged reaction of the enzyme in the presence of native xanthan, the enzyme was confirmed to produce a small amount of the pentasaccharide (P4) but not tetrasaccharides (P2 and P3) by TLC analysis (data not shown). This result supports the appearance of a small amount of pentasaccharide in xanthan depolymerization products produced by extracellular enzymes.
TABLE 3.
Substrate specificity of β-d-glucanase
| Substrate | Activitya (%) |
|---|---|
| Modified xanthanb | 100 |
| Xanthan | 8.2 |
| Carboxymethyl cellulose | 1.6 |
| Lichenan [β (1,3-1,4)-glucan] from Cetraria islandica | NDc |
| β-Glucan [β (1,3-1,4)-glucan] from barley | 1.0 |
| Xylan [β (1,4)-Xylose]n from oat spelt | ND |
| pNP-β-d-Glc | ND |
| pNP-β-d-Cellobiose | ND |
| pNP-β-d-Cellotriose | ND |
| pNP-β-d-Cellotetraose | ND |
Reactions were carried out for 30 min at 30°C and pH 6.0 (sodium acetate buffer). Activity toward modified xanthan was taken as 100%.
Modified xanthan was prepared from xanthan by treatment with xanthan lyase.
ND, not detected.
Thus, for the first time, we have clarified the enzymatic depolymerization route of xanthan in Bacillus sp. strain GL1. Xanthan was depolymerized to constituent monosaccharides by two extracellular and three intracellular enzymes, including a novel unsaturated glucuronyl hydrolase (10). As far as we know, β-d-glucanase of Bacillus sp. strain GL1 was the first purified enzyme hydrolyzing the main chain of xanthan from a specific producer, although the purification of the enzyme from mixed culture (1, 14) and the characterization of xanthanase, a mixture of xanthan-depolymerizing enzymes (3), have been reported. Hou et al. (14) reported that the purified β-d-glucanase from the mixed culture has a molecular weight of 60,000 and liberates saccharides consisting of 15 to 58 monosaccharides. The xanthan depolymerase (β-d-glucanase) purified from the mixed culture by Ahlgren is a monomeric enzyme with a molecular mass of 170 kDa, although the action of the enzyme toward modified xanthans has not been clarified (1). However, the β-d-glucanase presented here has a huge molecular mass of 355 kDa and produces tetrasaccharide from xanthan after treatment with xanthan lyase. Therefore, the enzyme of Bacillus sp. strain GL1 is quite different from those of the mixed culture (1, 14).
The Xanthomonas bacterium producing xanthan is a pathogen of cruciferous plants, including food vegetables such as cabbage and broccoli (6), and xanthan has been reported to be involved in the pathogenicity (7). Xanthan is widely used in various industries due to its excellent physicochemical properties. Low-molecular-weight and low-viscosity xanthans will develop new application areas of xanthan in biopolymer-based industries. Therefore, Bacillus sp. strain GL1 cells and their xanthan-depolymerizing enzymes are thought to be useful materials for the treatment of Xanthomonas infectious disease and for the preparation of modified xanthans with novel physicochemical properties. Since oligosaccharides have been reported to possess potent physiological functions such as bifidus factor (8) and plant elicitor (32), similar functions may be expected for the oligosaccharides produced from xanthan by xanthan-depolymerizing enzymes of Bacillus sp. strain GL1.
REFERENCES
- 1.Ahlgren J A. Purification and properties of a xanthan depolymerase from a heat-stable salt-tolerant bacterial consortium. J Ind Microbiol. 1993;12:87–92. [Google Scholar]
- 2.Cadmus M C, Jackson L K, Burton K A, Plattner R D, Slodki M E. Biodegradation of xanthan gum by Bacillus sp. Appl Environ Microbiol. 1982;44:5–11. doi: 10.1128/aem.44.1.5-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadmus M C, Slodki M E, Nicholson J J. High-temperature, salt-tolerant xanthanase. J Ind Microbiol. 1989;4:127–133. [Google Scholar]
- 4.Cheetham N W H, Mashimba E N M. Characterisation of some enzymic hydrolysis products of xanthan. Carbohydr Polym. 1991;15:195–206. [Google Scholar]
- 5.Chou F-L, Chou H-C, Lin Y-S, Yang B-Y, Lin N-T, Weng S-F, Tseng Y-H. The Xanthomonas campestris gumD gene required for synthesis of xanthan gum is involved in normal pigmentation and virulence in causing black rot. Biochem Biophys Res Commun. 1997;233:265–269. doi: 10.1006/bbrc.1997.6365. [DOI] [PubMed] [Google Scholar]
- 6.Daniels M J, Collinge D B, Dow J M, Osbourn A E, Roberts I N. Molecular biology of the interaction of Xanthomonas campestris with plants. Plant Physiol Biochem. 1987;25:353–359. [Google Scholar]
- 7.Denny T P. Involvement of bacterial polysaccharides in plant pathogenesis. Annu Rev Phytopathol. 1995;33:173–197. doi: 10.1146/annurev.py.33.090195.001133. [DOI] [PubMed] [Google Scholar]
- 8.Gibson G R, Wang X. Enrichment of bifidobacteria from human gut contents by oligofructose using continuous culture. FEMS Microbiol Lett. 1994;118:121–127. doi: 10.1111/j.1574-6968.1994.tb06813.x. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto W, Inose T, Nakajima H, Sato N, Kimura S, Murata K. Purification and characterization of microbial gellan lyase. Appl Environ Microbiol. 1996;62:1475–1477. doi: 10.1128/aem.62.4.1475-1477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto, W., E. Kobayashi, H. Nankai, N. Sato, T. Miya, S. Kawai, and K. Murata. Unsaturated-glucuronyl hydrolase of Bacillus sp. GL1: novel enzyme prerequisite for metabolism of unsaturated oligosaccharides produced by polysaccharide lyases. Submitted for publication. [DOI] [PubMed]
- 11.Hashimoto W, Miki H, Nankai H, Sato N, Kawai S, Murata K. Molecular cloning of two genes for β-d-glucosidase in Bacillus sp. GL1 and identification of one as a gellan-degrading enzyme. Arch Biochem Biophys. 1998;360:1–9. doi: 10.1006/abbi.1998.0929. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto W, Maesaka K, Sato N, Kimura S, Yamamoto K, Kumagai H, Murata K. Microbial system for polysaccharide depolymerization: enzymatic route for gellan depolymerization by Bacillus sp. GL1. Arch Biochem Biophys. 1997;339:17–23. doi: 10.1006/abbi.1996.9851. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto W, Miki H, Tsuchiya N, Nankai H, Murata K. Xanthan lyase of Bacillus sp. strain GL1 that liberates pyruvylated mannose from xanthan side chains. Appl Environ Microbiol. 1998;64:3765–3768. doi: 10.1128/aem.64.10.3765-3768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou C T, Barnabe N, Greaney K. Purification and properties of a novel xanthan depolymerase from a salt-tolerant bacterial culture, HD1. Appl Environ Microbiol. 1986;52:37–44. doi: 10.1128/aem.52.1.37-44.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janes A, Pittsley J E, Senti F R. Polysaccharide B-1459: a new hydrocolloid polyelectrolyte produced from glucose by bacterial fermentation. J Appl Polym Sci. 1961;5:519–526. [Google Scholar]
- 16.Jansson P-E, Kenne L, Lindberg B. Structure of the extracellular polysaccharide from Xanthomonas campestris. Carbohydr Res. 1975;45:275–282. doi: 10.1016/s0008-6215(00)85885-1. [DOI] [PubMed] [Google Scholar]
- 17.Jansson P-E, Lindberg B, Sandford P A. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohydr Res. 1983;124:135–139. [Google Scholar]
- 18.Katzen F, Ferreiro D U, Oddo C G, Ielmini M V, Becker A, Pühler A, Ielpi L. Xanthomonas campestris pv. campestris gum mutants: effects on xanthan biosynthesis and plant virulence. J Bacteriol. 1998;180:1607–1617. doi: 10.1128/jb.180.7.1607-1617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy J F, Bradshaw I J. Production, properties and applications of xanthan. Prog Ind Microbiol. 1984;19:319–371. [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 115–175. [Google Scholar]
- 22.Lesley S M. Degradation of the polysaccharide of Xanthomonas phaseoli by an extracellular bacterial enzyme. Can J Microbiol. 1961;7:815–825. doi: 10.1139/m61-097. [DOI] [PubMed] [Google Scholar]
- 23.Lever M. Carbohydrate determination with 4-hydroxybenzoic acid hydrazide (PAHBAH): effect of bismuth on the reaction. Anal Biochem. 1977;81:21–27. doi: 10.1016/0003-2697(77)90594-2. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Nankai, H., W. Hashimoto, S. Kawai, and K. Murata. Unpublished data.
- 26.Pollock T J. Gellan-related polysaccharides and the genus Sphingomonas. J Gen Microbiol. 1993;139:1939–1945. [Google Scholar]
- 27.Rogovin S P, Anderson R F, Cadmus M C. Production of polysaccharide with Xanthomonas campestris. J Biochem Microbiol Technol Eng. 1961;3:51–63. [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Sandford P A, Baird J. Industrial utilization of polysaccharides. In: Aspinall G O, editor. The polysaccharides. Vol. 2. New York, N.Y: Academic Press, Inc.; 1983. pp. 411–490. [Google Scholar]
- 30.Sandford P A, Pittsley J E, Knutson C A, Watson P R, Cadmus M C, Janes A. Variation in Xanthomonas campestris NRRL B-1459: characterization of xanthan products of differing pyruvic acid content. In: Sandford P A, Laskin A, editors. Extracellular microbial polysaccharides. American Chemical Society Symposium Series, no. 45. Washington, D.C: American Chemical Society; 1977. pp. 192–210. [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharp J K, McNeil M, Albersheim P. The primary structures of one elicitor-active and seven elicitor-inactive hexa(β-d-glucopyranosyl)-d-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinea. J Biol Chem. 1984;259:11321–11336. [PubMed] [Google Scholar]
- 33.Sutherland I W. An enzyme system hydrolyzing the polysaccharides of Xanthomonas species. J Appl Microbiol. 1982;53:385–393. [Google Scholar]
- 34.Sutherland I W. Hydrolysis of unordered xanthan in solution by fungal cellulases. Carbohydr Res. 1984;131:93–104. [Google Scholar]