Abstract
Objective
Stem-cell therapy is a promising treatment for cartilage defects. The newly identified urine-derived stem cells (USCs), which have multipotency and sufficient proliferative ability, are promising candidates for several tissue engineering therapies. In this study, we investigated the role of USC extracellular vehicles (EVs) in promoting the proliferation and migration of chondrocytes.
Design
USCs were characterized by measuring induced multipotent differentiation and flow cytometry analysis of surface marker expression. The EVs were isolated from USCs under normoxic conditions (nor-EVs) and hypoxic conditions (hypo-EVs). Transmission electron microscopy and western blot analysis characterized the EVs. The chondrocytes were cultured in the USC-EVs. CCK-8 assay and EdU staining detected the proliferation of chondrocytes, and transwell assay detected their migration. miR-26a-5p expression in EVs was detected by quantitative real-time polymerase chain reaction (qRT-PCR). The target relationship of miR-26a-5p and phosphatase and tensin homolog (PTEN) was predicted and confirmed. The roles of EVs-miR-26a-5p and PTEN on the proliferation and migration of chondrocytes were also investigated.
Results
Hypo-EVs showed a superior effect in promoting the proliferation and migration of chondrocytes than nor-EVs. Mechanistically, USC-EVs delivered miR-26a-5p into chondrocytes to overexpress miR-26a-5p. PTEN was identified as an miR-26a-5p target in chondrocytes. The effects of EVs-miR-26a-5p on promoting the proliferation and migration of chondrocytes were mediated by its regulation of PTEN.
Conclusion
Our study suggested that hypoxic USC-EVs may represent a promising strategy for osteoarthritis by promoting the proliferation and migration of chondrocytes via miR-26a-5p transfer.
Keywords: osteoarthritis, urine-derived stem cells, extracellular vesicles, miR-26a-5p, hypoxia
Introduction
Osteoarthritis (OA), the most widespread joint disease, affects the entire joint and causes pain and deformity. 1 As reported, the incidence of OA is 40% to 80% in middle-aged people and is rising owing to the growing population of older adults. In addition, high incidence and disability rates also cause a huge burden on individuals and society.2,3 Cartilage damage is one of the influencing factors of osteoarthritis, ascribed to the loss of chondrocytes in the articular cartilage. However, the self-healing potential of articular cartilage is very limited.4,5 Therefore, it is necessary to study ways in which damaged articular cartilage can be repaired and regenerated. Currently, the treatment used focuses on relieving pain and controlling inflammation. However, these traditional therapies cannot cure OA thoroughly.6-8
Recently, several researchers have focused on mesenchymal stem cells (MSCs) to promote the healing of damaged articular cartilage. Among them, MSCs from bone marrow have been widely studied. Numerous studies have reported their potential to differentiate into muscle, bone, and cartilage. 9 This potential for multidirectional differentiation makes bone marrow mesenchymal stem cells (BMSCs) a promising treatment strategy for OA.10,11 However, obtaining BMSCs from the autologous bone marrow of patients requires surgical sampling, which has disadvantages of considerable risk of infection and prohibitive cost. As a newly found stem cell, the urine-derived stem cells (USCs) have gradually become a research hotspot owing to the advantages of obtaining them, which include convenient access, non-invasive, no cost, and no ethical problems.12-14 Furthermore, USCs show robust proliferation and multilineage differentiation potential. 15 These make USCs likely to replace MSCs in OA research. 16 Increasing evidence has revealed that MSCs could provide therapeutic benefits by releasing specialized extracellular vehicles (EVs). 17 These EVs are communication carriers that transfer nucleic acids, proteins, and bioactive lipids between cells.18,19 Transplantation of EVs has been shown to have biological effects similar to that of MSCs, including repairing tissue damage.20,21 Therefore, as effective cell-free therapeutic agents, stem-cell EVs may have potential applications. 22 Numerous studies have reported that MSC-EVs could ameliorate OA by accelerating chondrocyte proliferation and migration and alleviating cartilage damage and inflammation.23-25 Guo et al. 26 reported that USC-EVs could alleviate intervertebral disc degeneration by promoting the proliferation of nucleus pulposus cells and ECM synthesis. USC-EVs protect against renal ischemia/reperfusion injury by inhibiting the infiltration of inflammatory cells. 27 However, the effect of USC-EVs on the regulation of growth of chondrocytes has not been reported.
Besides, hypoxic preconditioning is considered to be an effective way to enhance the repairability of stem cells. 28 Furthermore, short-term hypoxia is reported to promote the survival of MSCs, 29 and BMSCs under hypoxia enhanced the chondrogenic potential of BMSCs. 30 Mechanically, EVs can release microRNAs and carry them into several human pathological processes. 31 For example, Ouyang et al. 32 identified 465 human miRNAs in USC-EVs. Among these, miR-26a-5p was reported to attenuate the OA progression.33,34 Recent studies showed that PTEN overexpression induced the apoptosis of chondrocytes and inhibited chondrocytes growth.35,36 This study attempted to investigate the mechanism of USC-EVs in OA. We also explored whether hypoxic preconditioning could enhance the beneficial effects of USC-EVs on chondrocytes. We hypothesized that USC-EVs carried miR-26a-5p into chondrocytes to upregulate miR-26a-5p and inhibit PTEN, thereby promoting the proliferation and migration of chondrocytes.
Methods
Cell Lines
USCs were isolated as described in previous studies.12,37 In brief, a sterile urine sample (at least 150 mL each) was collected from five healthy adult donors (25–28 years old) and then centrifuged at 400 g for 10 minutes. Afterward, the supernatants were carefully discarded, and the remaining cell pellets were suspended in phosphate-buffered saline (PBS) and centrifuged again at 200 g for 10 min. Then, the supernatants were removed and the cell pellets were resuspended in the primary culture medium (keratinocyte serum-free medium and embryonic fibroblast medium at a ratio of 1:1). Keratinocyte serum-free medium was supplemented with 5 ng/mL epidermal growth factor, 50 ng/mL bovine pituitary extract, 30 ng/mL cholera toxin, 100 U/mL penicillin, and 1 mg/mL streptomycin. Embryonic fibroblast medium was Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), 10 ng/mL human epidermal growth factor, 5 ng/mL insulin, 10 mg/mL transferrin, 20 ng/mL triiodothyronine, and 1% penicillin-streptomycin. Nonadherent cells were discarded after 7 days of culture, and the medium was renewed. The culture medium was refreshed every other 3 days. When the cells reached approximately 80% confluence, they were passaged using 0.25% trypsin containing 1 mM EDTA. Cells at passage 3 were used in the following experiments.
The chondrocyte CHON-001 was obtained from the American Type Culture Collection. Following the manufacture’s instruction, the cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, NY, USA) supplemented with 10% FBS (Gibco). The cell culture condition was 37°C, 95% humidity, and 5% CO2.
Characterization of USCs
After the isolation of USCs, we used the Alizarin red staining, Oil red O staining, and Alcian blue staining to verify their multilineage differentiation potential. For osteogenic differentiation, USCs were incubated with an osteogenic induction medium (Gibco). On day 21, after the induction, the cells were stained with Alizarin Red S (Sigma, USA) for observation. For adipogenic differentiation, USCs were incubated with an adipogenic medium (Gibco). On day 21, after the induction, USCs were stained with Oil Red O (Sigma) for observation. For the chondrogenic differentiation, USCs were incubated with a chondrogenic medium (Gibco). After 21 days of induction, allicin blue staining (Sigma) was performed for observation. We also performed the flow cytometry analysis to detect the stem-cell surface markers (CD44, CD73, CD90, CD105, CD31, CD34, and CD45). The USCs were incubated with these antibodies (BD, USA), washed with PBS, and analyzed with a flow cytometry analyzer (Beckman, USA).
Isolation and Identification of EVs
For normal conditions, there was 5% CO2 and 21% O2, and for the hypoxic condition, there was 1% O2. USCs were cultured in a serum-free culture medium for 24 h. Then, the culture medium was collected for gradient centrifugation (500 g for 10 min, 2,000 g for 20 min, 10,000 g for 30 min). Then, the supernatant was retained, centrifuged at 100,000 g for 2 h, and resuspended in PBS. The morphology of the EVs was observed under transmission electron microscopy (TEM). The number and size distribution of EVs were analyzed using the NanoSight tracking detector (Malvern, England). The EVs surface proteins, including CD63, TSG101, Syntenin, and Calnexin was detected by western blot.
EVs Uptake Detection
Dil labeling was used for the EVs uptake detection. In brief, Dil solution was added to PBS and incubated with the USCs. Then, excess Dil dye was removed by ultracentrifugation at 100,000 g for 1 h. After that, the Dil-labeled EVs (150 μg/mL) were cultured with chondrocytes for 24 h, rinsed with PBS, and fixed in 4% paraformaldehyde (PFA). A laser confocal microscope was used for the observation of EVs uptake. The fluorescence intensity of Dil was evaluated by Image J software (Ver. 1.53i).
Cell Transfection
miR-26a-5p mimics, miR-26a-5p inhibitor, short hairpin RNA targeting PTEN (sh-PTEN), and negative controls were obtained from GenePharma (Shanghai, China). Cell transfection was performed with Lipofectamine 2000 reagent (Invitrogen, CA, USA).
Western Blot
Total protein was collected and then lysed in radioimmune precipitation assay (RIPA) lysis buffer (Solarbio, Beijing, China). After protein concentration determination, the protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. Subsequently, the PVDF membrane was blocked with 5% skimmed milk and incubated with primary antibodies at 4°C overnight and the secondary antibody for 1 h. Protein bands were detected with the efficient chemiluminescence kit (Millipore, MA, USA). The antibodies information was listed in Supplementary Table S1.
QRT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) and then reverse-transcribed by the Primer Script RT reagent Kit (Takara, Japan). For miR-26a-5p detection, a mirVana qRT-PCR miRNA Detection kit (Thermo Fisher Scientific) was used. The qPCR was performed with the SYBR Green PCR kit (Applied Biosystems, CA, USA) on an ABI7900 system. 2−ΔΔCt method was used to calculate the relative expression, with GAPDH and U6 as internal references. The primers are shown in Supplementary Table S2.
CCK-8 Assay
For the CCK-8 assay, chondrocytes were seeded in a 96-well plate (2 × 103 cells/well) and co-cultured with USC-EVs (150 μg/mL). Then, 10 μL CCK-8 regents (Dojindo, Kumamoto, Japan) were added and incubated for 2 h at 37°C. After that, the optical density (OD) value at 450 nm was detected, and the cell viability was determined.
5-Ethynyl-2′-Deoxyuridine (EdU) Staining
In EdU staining, chondrocytes were seeded onto a 24-well plate (2 × 104 cells/well) and cultured for 24 h with EdU kit (RiboBio, Guangzhou, China). After cells were further cultured for 4 h, the supernatant was removed. Subsequently, 4% paraformaldehyde was added to the cells for a 15-min blockade. Then, cells were incubated with 0.2% glycine for 10 min and cleared with 0.5% TritonX-100 for 10 min. Subsequently, the cells were incubated with 1× Apollo staining solution for 30 min in the dark. Finally, the nucleus was stained with DAPI, and the EdU positive cells were visualized with fluorescence microscopy (Olympus) in five visual fields.
Transwell Migration Assay
In brief, 2 × 104 chondrocytes were seeded in the upper chamber of a 24-well transwell chamber (Corning, NY, USA), and 600 μL/well of different treatment media (containing 150 μg/mL USC-EVs) was introduced into the lower chamber. After being incubated for 24 h, the cells that migrated to the lower surface were fixed and stained with 0.5% crystal violet for 10 min. Then, the stained chondrocytes were counted.
Dual-Luciferase Reporter Assay
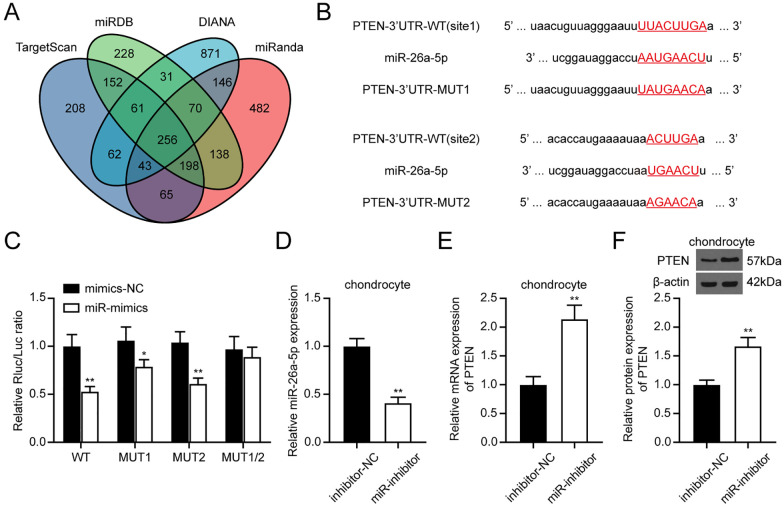
Four online data sets, including TargetScan, miRDB, DIANA, and miRanda, were applied to predict miR-26a-5p targets. Then, the PTEN wild-type (WT) or mutant (MUT) luciferase reporter vector was constructed (GeneScript, Nanjing, China) based on the predicted binding sites. The WT and MUT luciferase reporter vectors were co-transfected into chondrocytes with miR-26a-5p mimics or mimics NC. Forty-eight hours later, cells were lysed, and the luciferase signal was measured with the luciferase detection kit (Promega, Madison, WI, USA).
Statistical Analysis
Three independent tests were conducted in all experiments. The normally distributed data were statistically analyzed with student t test (between two groups) or one-way analysis of variance (among multiple groups) using GraphPad software (Ver. 9.1.1). Data were presented as mean ± standard deviation (SD). A significant difference was considered at P < 0.05.
Results
Characterization of the Isolated USCs
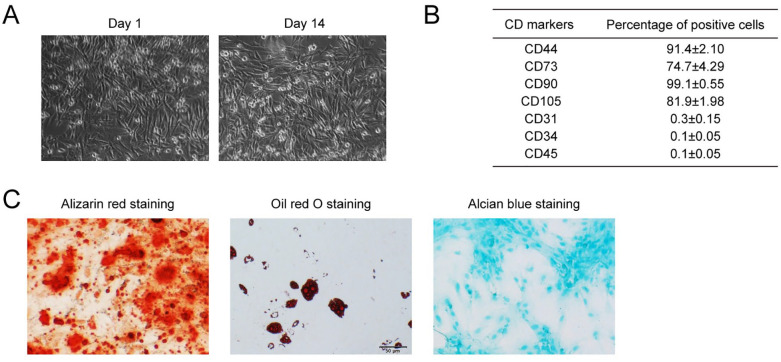
Urine-derived stem cells were isolated from fresh urine from five healthy adults. The USCs showed fibroblast-like morphology ( Fig. 1A ) in accordance with the previous study. 38 Cell surface markers were analyzed with the flow cytometry assay. The isolated USCs were strongly positively for CD44 and CD90; weakly positively for CD73 and CD105; and negatively for CD31, CD34, and CD45 ( Fig. 1B , Suppl. Fig. S1). When cultured in special culture medium, USCs underwent osteogenic, adipogenic, or chondrogenic differentiation, as indicated by positive Alizarin red staining, Oil red O staining, and Alcian blue staining, respectively ( Fig. 1C ). Therefore, the features of USCs were in line with the criteria for defining multipotent MSCs.
Figure 1.
Characterization of USCs. (A) The morphology of USCs under optical microscopy. (B) USC was characterized by flow cytometry analysis using the surface markers CD44, CD73, CD90, CD105, CD31, CD34, and CD45. (C) Verification of the multidirectional differentiation potential of USCs. Osteogenic differentiation ability was accessed by Alizarin red staining, adipogenic differentiation ability was accessed by Oil red O staining, Alcian blue staining accessed the chondrogenic differentiation ability. USCs = urine-derived stem cells.
USC-EVs Promote the Proliferation and Migration of Chondrocytes
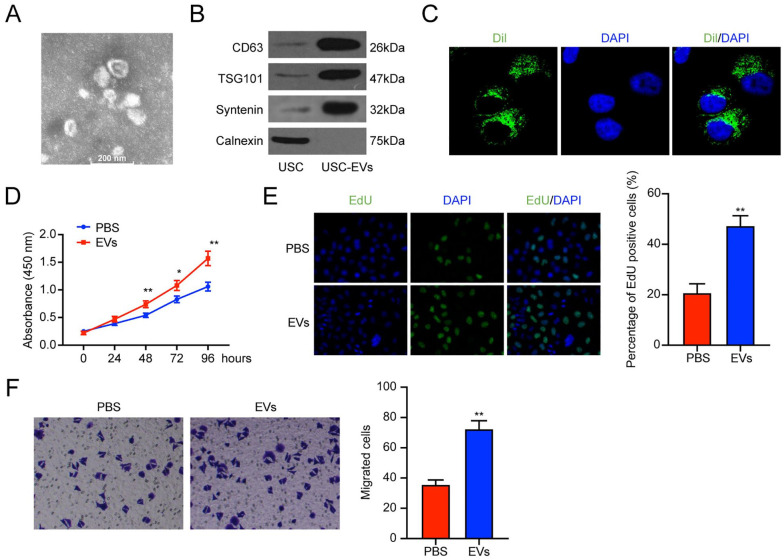
We explored the role of USC-EVs in regulating the chondrocytes’ cell function. The EVs were isolated and observed under the TEM. As presented in Figure 2A , the EVs were spherical vesicles with 30–100 nm diameters. Western blot analysis revealed that the EVs surface biosignatures CD63, TSG101, and Syntenin were expressed in USC-EVs, while the endoplasmic reticulum-specific protein Calnexin was not ( Fig. 2B ). The above results revealed that the isolated USC-EVs could be used in subsequent experiments.
Figure 2.
USC-EVs promoted the chondrocytes proliferation and migration. (A) TEM scanning of the USC-EVs. (B) EV surface biosignatures CD63, TSG101, Syntenin, and Calnexin, were detected by western blot. (C) The Chondrocytes uptake USC-EVs. (D) CCK-8 assay detection of the cell viability of chondrocytes. (E) EdU staining detection of the proliferation of chondrocytes. (F) Transwell assay was applied to detect the migration ability of chondrocytes. USCs = urine-derived stem cells; EVs = extracellular vehicles; TEM = transmission electron microscopy; CCK-8 = cell counting kit-8; EdU = 5-Ethynyl-2’-Deoxyuridine; DAPI = 4’,-6-diamidino-2-phenylindole. *P < 0.05, ** P < 0.01, compared with the PBS group.
Then, we co-cultured the Dil-labeled EVs with the chondrocytes for 24 h. The fluorescence imaging showed that EVs could be uptaken by chondrocytes in vitro ( Fig. 2C ). Subsequent CCK-8 assay and EdU staining revealed that EVs treatment improved cell proliferation of chondrocytes (Fig. 2D and E). We also used the Transwell assay to investigate the effect of EVs on chondrocyte migration. Similarly, compared with the PBS group, EV treatment enhanced the migration of chondrocytes ( Fig. 2F ). Altogether, USC-EVs treatment showed a promoting effect on the proliferation and migration of chondrocytes.
Hypoxia Treatment Enhances the EVs Release
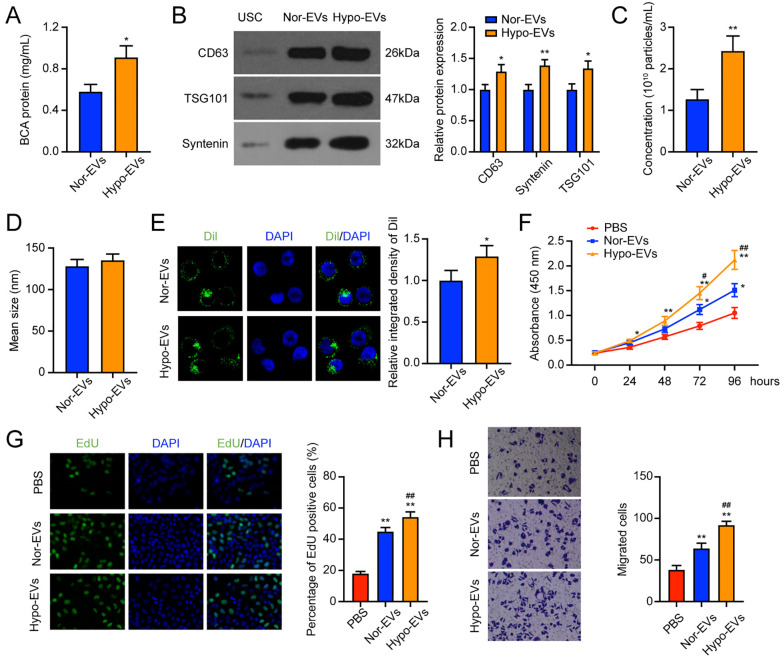
As has been reported, hypoxia can enhance the cell properties of MSCs. 39 In this study, we wanted to explore whether hypoxia affects the secretion of EVs by USCs. We first examined the cell viability and apoptosis of USCs under normoxic and hypoxic conditions. The cell viability was significantly higher in the hypoxia group than in the normoxia group (Suppl. Fig. S2A). However, the hypoxia group showed a lower apoptosis rate, but the difference was not significant (Suppl. Fig. S2B). Then, we detected the protein content in the two groups. It was found that compared with the normoxia group, the hypoxia group showed a significantly higher protein content ( Fig. 3A ). The expression of CD63, TSG101, and Syntenin was also found to increase after 1% O2 exposure ( Fig. 3B ). The two groups showed no significant differences in the mean size of EVs ( Fig. 3C ). While the number of EVs taken up by chondrocytes was higher in the hypoxia group than in the normoxia group ( Fig. 3D ). As shown in Figure 3E , the number of EVs taken up by chondrocytes in the hypoxia group was markedly higher. Thus, according to the above results, it was proved that hypoxia enhances the release of EVs from USCs, and EVs acquired from the hypoxic state were more easily absorbed by chondrocytes.
Figure 3.
Hypo-EVs enhanced the promoting effect of USC-EVs on chondrocytes. (A) BCA method to determine the protein concentration of the nor-EVs and hypo-EVs. (B) Western blot detection of EV surface biosignatures CD63, Syntenin, and TSG101. (C-D) The concentration (C) and mean size (D) of nor-EVs and hypo-EVs. (E) Dil-labeled EVs in chondrocytes. (F) CCK-8 assay detection of the cell viability of chondrocytes. (G) EdU staining of the cell proliferation of chondrocytes. (H) Transwell assay was applied to detect the migration ability of chondrocytes. EVs = extracellular vehicles; USCs = urine-derived stem cells; BCA = bicinchoninic acid; EdU = 5-Ethynyl-2’-Deoxyuridine; DAPI = 4’,-6-diamidino-2-phenylindole; PBS = phosphate-buffered saline; CCK-8 = cell counting kit-8. *P < 0.05, **P < 0.01, compared with the PBS group; #P < 0.05, ##P < 0.01, compared with the Nor-EVs group.
We also compared the effects of nor-EVs and hypo-EVs on the chondrocytes. CCK-8 assay results revealed that hypo-EVs treatment significantly promoted the chondrocytes viability compared with the nor-EVs group ( Fig. 3F ). Comparable results were observed in EdU staining, that hypo-EVs treatment showed a higher EdU positive rate ( Fig. 3G ). In the transwell assay, more migrated cells were observed in the hypo-EVs than in the nor-EVs group ( Fig. 3H ). To conclude, these results suggested that hypo-EVs could promote proliferation and migration to a greater extent than the nor-EVs.
USCs Transferred miR-26a-5p to Chondrocytes through EVs
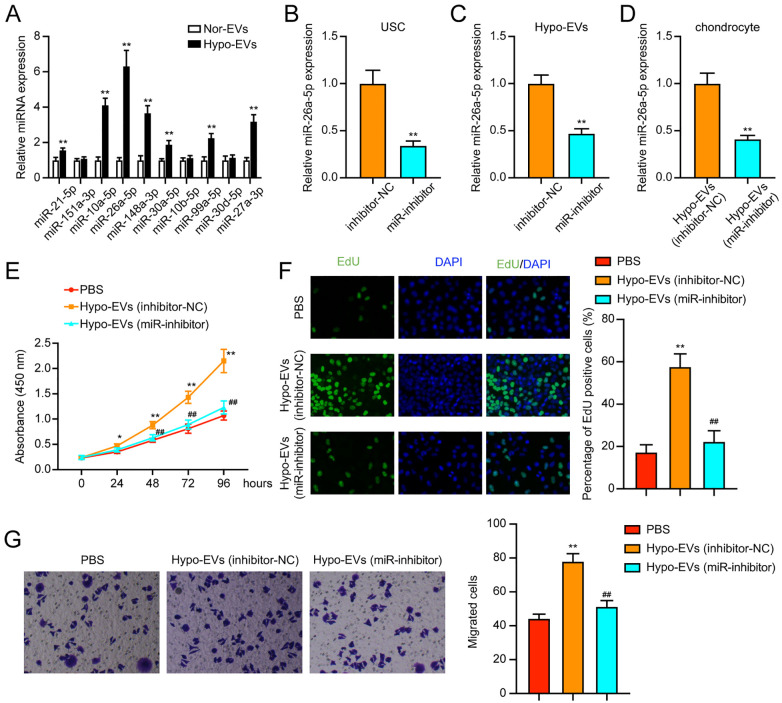
Studies have reported that miRNA in EVs play key roles in cell crosstalk and ultimately regulate biological functions.40-43 According to a previous study, 32 several miRNAs were found to be enriched in the USC-EVs, including miR-21-5p, miR-151a-3p, miR-10a-5p, miR-26a-5p, miR-148a-3p, miR-30a-5p, miR-10b-5p, miR-99a-5p, miR-30d-5p, and miR-27a-3p. In this study, we detected the expression changes of these miRNAs in nor-EVs and hypo-EVs. In Figure 4A , most miRNAs showed significantly higher expression in the hypo-EVs, and miR-26a-5p showed the greatest fold change. Thus, we selected miR-26a-5p in the following experiments.
Figure 4.
miR-26a-5p is transferred to chondrocytes through USC-EVs. (A) Expression of miRNAs in nor-EVs and hypo-EVs was detected by qRT-PCR. (B) The expression of miR-26a-5p after transfection was assessed by qRT-PCR in USCs. (C) The expression of miR-26a-5p in EVs. (D) The expression of miR-26a-5p in chondrocytes after treatment. (E-F) Detection of cell proliferation using CCK-8 and EdU staining assays. (G) Transwell assay detection of cell migration. USCs = urine-derived stem cells; EVs = extracellular vehicles; NC = negative control; qRT-PCR = quantitative real-time polymerase chain reaction; CCK-8 = cell counting kit-8; EdU = 5-Ethynyl-2’-Deoxyuridine; DAPI = 4’,-6-diamidino-2-phenylindole; PBS = phosphate-buffered saline. *P < 0.05, **P < 0.01, compared with the PBS group; ##P < 0.01, compared with the hypo-EVs (inhibitor-NC) group.
To explore whether hypo-EVs promoted the chondrocyte cell function through miR-26a-5p transfer, we inhibited the miR-26a-5p expression in USCs ( Fig. 4B ). Then, the EVs were collected, and the miR-26-5p expression in the hypo-EVs was found to be suppressed by miR-inhibitor ( Fig. 4C ). Also, compared with the treatment with EVs from the inhibitor-NC-hypo-USCs, the miR-26a-5p expression was decreased in chondrocytes treated with EVs from miR-inhibitor-hypo-USCs ( Fig. 4D ).
Furthermore, we aimed to investigate whether miR-26a-5p serves as a biological messenger between EVs and chondrocytes. We found that treatment with miR-inhibitor-hypo-EVs could remarkably suppress the chondrocytes viability and proliferation (Fig. 4E and F). Similarly, in the transwell migration assay, miR-inhibitor-hypo-EVs could also repress the cell migration of chondrocytes ( Fig. 4G ). In conclusion, miR-26a-5p suppression attenuated the promoting effect of hypo-EVs on the proliferation and migration of chondrocytes.
EVs miR-26a-5p Directly Targeted PTEN
Using four algorithms, we identified 256 targets of miR-26a-5p ( Fig. 5A ). By placing these 256 targets in the Enrichr webtool (https://maayanlab.cloud/Enrichr/), we performed the Gene Ontology (GO) enrichment analysis. The results are listed in Supplementary Table S3. It was found that these genes were enriched in the regulation of cell growth (e.g., negative regulation of the apoptotic process, negative regulation of developmental growth, and negative regulation of cell population proliferation). Then, we placed these 256 genes in the STRING webtool to analyze their interactions (Suppl. Fig. S3). In the protein-protein network, PTEN in the middle had more degrees, suggesting an important regulatory role among these proteins. Binding sites between miR-26a-5p and PTEN are illustrated in Figure 5B and verified with the dual-luciferase reporter assay. It was shown that transfection with miR-mimics significantly decreased the luciferase activity in the PTEN-WT vector, while the luciferase activity in the PTEN-MUT1/2 vector was not affected ( Fig. 5C ). In addition, knockdown of miR-26a-5p in chondrocytes remarkedly promoted the PTEN expression in chondrocytes ( Fig. 5D-F ). Thus, we confirmed that PTEN is negatively regulated by miR-26a-5p in chondrocytes.
Figure 5.
miR-26a-5p targeted PTEN in chondrocytes. (A) Prediction of miR-26-5p targets using TargetScan, miRDB, DIANA, and miRanda. (B) PTEN binding sites with miR-26-5p. (C) Dual-luciferase reporter assay confirmed the target relationship between miR-26a-5p and PTEN. (D) miR-26-5p expression in chondrocytes detected by qRT-PCR. (E-F) PTEN expression in chondrocytes after transfection. miRDB = MicroRNA Target Prediction Database (mirdb.org); DIANA = DIANA-microT-CDS (www.microrna.gr/microT-CDS), TargetScan = TargetScan Database (targetscan.org); PTEN = phosphatase and tensin homolog; WT = wild-type; MUT = mutant type; NC = negative control; qRT-PCR = quantitative real-time polymerase chain reaction. *P < 0.05, **P < 0.01.
EVs miR-26a-5p Promotes Chondrocyte Proliferation and Migration via Mediating PTEN
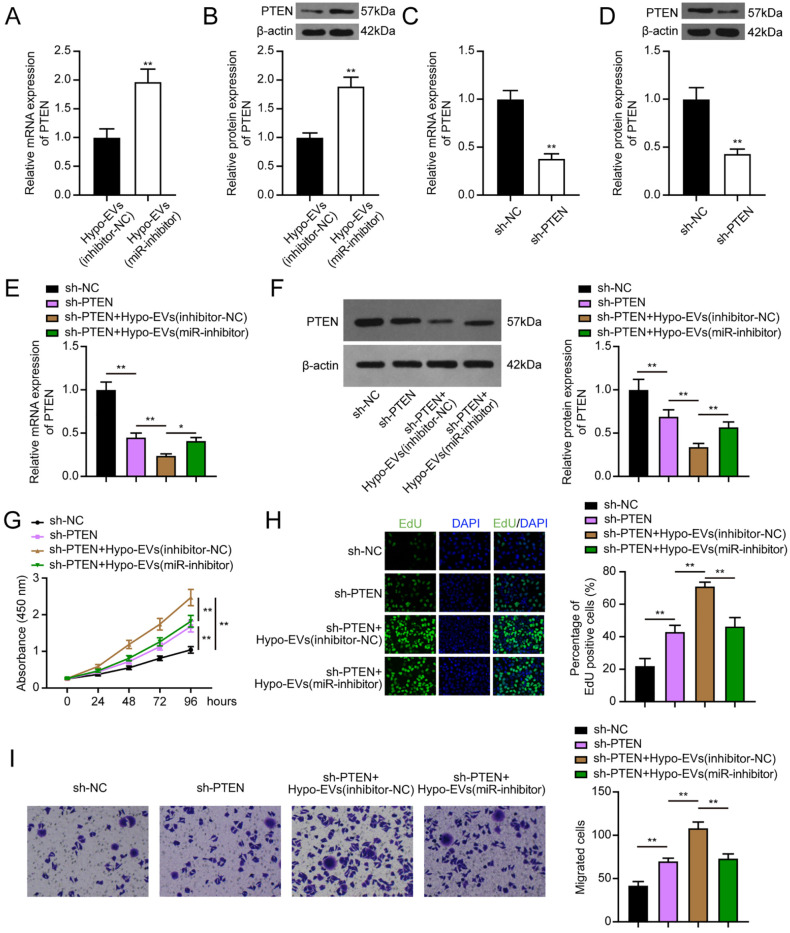
Rescue experiments were performed to investigate the association between EVs-miR-26a-5p and PTEN. Compared with the chondrocytes treated with inhibitor-NC-hypo-EVs, the PTEN expression was increased in chondrocytes treated with miR-inhibitor-hypo-EVs (Fig. 6A and B). The transfection with sh-PTEN also significantly suppressed the PTEN expression in chondrocytes (Fig. 6C and D). Moreover, co-treated with sh-PTEN and inhibitor-NC-hypo-EVs further reduced the PTEN expression in chondrocytes, and the PTEN expression was recovered in chondrocytes co-treated with sh-PTEN and miR-inhibitor-hypo-EVs (Fig. 6E and F). In the CCK-8 assay, EdU staining, and transwell assay, transfection of sh-PTEN promoted the cell proliferation and migration ability of chondrocytes, and this effect was rescued by the additional use of miR-inhibitor-hypo-EVs ( Fig. 6G-I ). Therefore, it is concluded that EVs-miR-26a-5p enhanced the proliferation and migration of chondrocytes through mediating PTEN.
Figure 6.
EV miR-26a-5p promoted chondrocyte proliferation and migration through mediating PTEN. (A-B) PTEN expression in chondrocytes after treatment with inhibitor-NC-Hypo-EVs or miR-inhibitor-Hypo-EVs. (C-D) PTEN expression in chondrocytes after sh-NC or sh-PTEN transfection. (E-F) After transfection with sh-NC or sh-PTEN and administration of inhibitor-NC-Hypo-EVs or miR-inhibitor-Hypo-EVs, the expression level of PTEN was measured in chondrocytes. (G-I) CCK-8, EdU, and transwell assays were applied to evaluate cell proliferation and migration. EVs = extracellular vehicles; PTEN = phosphatase and tensin homolog; NC = negative control; CCK-8 = cell counting kit-8; EdU = 5-Ethynyl-2’-Deoxyuridine; DAPI = 4’,-6-diamidino-2-phenylindole. *P < 0.05, **P < 0.01.
Discussion
OA is a ubiquitous degenerative joint disease, and the major symptom of OA is cartilage damage. 44 Research is focused on promoting the proliferation and migration of chondrocytes to intercept and control OA. 45 Several studies reported that proper implantation of stem cells into biomaterials could promote cartilage regeneration at the defective sites.46,47 In several clinical trials, it was observed that MSC-based treatments attenuate inflammation and pain of OA.48,49 USCs as novel stem cells can be conveniently obtained from urine through non-invasive methods, which have extensive self-renewal properties and are easy to culture and differentiate. 50 In this study, we isolated the USCs from human urine. Through flow cytometry analysis, we found that the isolated USCs expressed some traditional MSC-surface markers, such as CD44 and CD90. However, unlike the previous studies,26,51 the isolated USCs were weakly positively for CD73 and CD105. The difference in immunophenotype between different USCs may be attributed to the cell heterogeneity of USCs.
It is generally believed that paracrine function rather than differentiation is the main repair mechanism of stem-cell therapy.52,53 USC-EVs have been shown to be therapeutic in rett syndrome, 54 intervertebral disc degeneration, 26 and renal ischemia/reperfusion injury. 55 In this study, we investigated the role of USC-EVs in OA. We found that USC-EVs carried miR-26a-5p into chondrocytes to elevate miR-26a-5p and inhibit PTEN, thereby promoting the proliferation and migration of chondrocytes.
Initially, we found that USC-EVs promoted the proliferation and migration of chondrocytes. However, the common cell culture in vitro environment does not refer to a physiological stem-cell niche. The in vitro oxygen concentration is about 20% O2, whereas the oxygen concentration is 5% in cartilage. 56 Hypoxia preconditioning improves the therapeutic potential of stem cells. 57 Therefore, we treated the USCs under the hypoxia conditions to investigate whether USC-EVs play a greater therapeutic role under hypoxia conditions than under normoxic conditions. No significant morphological differences were observed between the nor-EVs and hypo-EVs. However, the production of EVs was facilitated under hypoxic conditions. Also, hypo-EVs can be more easily absorbed by chondrocytes than the nor-EVs. Subsequent cell experiments results revealed that the therapeutic effects of USC-EVs were enhanced by the hypoxic pretreatment, which was consistent with the previous research. 58
It was reported that miRNAs, a group of 20–24 nt small non-coding RNAs, are enriched in the EVs and can be transferred to target cells or tissues to induce mRNA degradation or translational inhibition.59-62 The miRNAs from MSC-EVs are shown to play an integrated role in reducing inflammatory response and cartilage reconstruction of OA. 63 Thus, we suspected that the higher benefic effect of hypo-EVs is related to the secretion of miRNAs. Ouyang et al. 32 previously identified 465 human miRNAs in USC-EVs. Based on this previous study, we detected 10 higher expressed miRNAs in nor-EVs and hypo-EVs. The results showed that miR-26-5p had the highest fold change in the hypo-EVs, suggesting that the miR-26a-5p expression difference between nor-EVs and hypo-EVs may lead to the biological differences. Jin et al. 64 reported that miR-26a-5p from the BMSC-derived exosomes alleviates damage of synovial fibroblasts in OA. Lu et al. 65 found that the synovial mesenchymal stem-cells EVs carried miR-26a-5p into chondrocytes to eliminate apoptosis and inflammation in OA. Similarly, we found that after treatment of hypo-EVs, miR-26a-5p was effectively transferred to the target chondrocytes. Also, the knockdown of miR-26a-5p in hypo-EVs revealed the effects of hypo-EVs on promoting the proliferation and migration of chondrocytes. Thus, we concluded that hypo-EVs from USCs could enhance chondrocyte proliferation and migration via delivering miR-26a-5p.
We also investigated the mechanism underlying the effect of EVs-miR-26a-5p on promoting the proliferation and migration of chondrocytes. Using the online prediction tools, we found 256 potential targets of miR-26a-5p. Through GO enrichment analysis, we found that these targets were mostly enriched in the cell growth regulation, suggesting that miR-26a-5p may regulate the growth of the chondrocytes in OA. Furthermore, the STRING network identified PTEN as a potential target of miR-26a-5p, confirmed by the dual-luciferase reporter assay. As an important tumor suppressor, PTEN is widely reported in various cancers.66,67 However, little is reported about its role in OA. Yuan et al. 35 found that PTEN is overexpressed in OA and positively regulates the apoptosis of chondrocytes induced by lipopolysaccharide (LPS). Another study reported that PTEN negatively regulated Col2a1 and Aggrecan in chondrocytes. 68 Lu et al. 65 reported that PTEN inhibited the chondrocyte proliferation and promoted apoptosis and inflammation. In accordance with the previous evidence, our results found that knockdown of PTEN promoted the proliferation and migration of chondrocytes. Furthermore, knockdown of PTEN reversed the adverse events caused by the suppression of the expression of miR-26a-5p in hypo-EVs, suggesting that the EVs miR-26a-5p promoted the proliferation and migration of chondrocytes via inhibiting PTEN.
However, there are some limitations in the current research. The use of primary chondrocytes or more than one cell line may make the conclusion more reliable. The progression of OA is also associated with cartilage inflammation. The effects of USC-EVs on regulating the OA chondrocytes inflammation could be investigated in vitro and in vivo in future studies. In addition, we did not exclude the possibility that other substances in USC-EVs may also promote the chondrocyte proliferation and migration. In the mechanism investigation, PTEN is not the only target of miR-26a-5p; some other targets may also regulate the cell function of chondrocytes, which needs to be further investigated.
In conclusion, USCs could release EVs to promote the proliferation and migration of chondrocytes, and hypoxia pretreatment of USCs could enhance this beneficial effect. Mechanistically, EVs transfer miR-26a-5p to chondrocytes and suppress PTEN expression in chondrocytes. Thus, hypoxia-pretreated USCs may be a promising approach for OA treatment.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035221077401 for Extracellular Vesicles from Hypoxic Pretreated Urine-Derived Stem Cells Enhance the Proliferation and Migration of Chondrocytes by Delivering miR-26a-5p by Sha Wan, Dingsu Bao, Jia Li, Kefu Lin, Qi Huang, Qiang Li and Lang Li in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Author Contributions: LL, SW, and KL have given substantial contributions to the conception and the design of the manuscript, JL, QH, and QL to acquisition, analysis, and interpretation of the data. All authors have participated to drafting the manuscript, and LL, DB, and SW revised it critically. All authors read and approved the final version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Acknowledgments and Funding: This work was supported by Local projects based on central guidance of China (No. XZ202001YD0026C) and Youth innovative Scientific Research Project of Sichuan Medical Association (No. Q19014).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The design of the present study adhered to the tenets of the Declaration of Helsinki and the protocol was reviewed and approved by the Ethics Committee of Hospital of Chengdu Office of People’s Government of Tibetan Autonomous Region.
ORCID iD: Lang Li  https://orcid.org/0000-0002-1247-2983
https://orcid.org/0000-0002-1247-2983
References
- 1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019. Apr 27;393(10182):1745-59. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2. Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015-2040. Arthritis Rheumatol. 2016. Jul;68(7):1582-7. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun X, Zhen X, Hu X, Li Y, Gu S, Gu Y, et al. Osteoarthritis in the middle-aged and elderly in China: prevalence and influencing factors. Int J Environ Res Public Health. 2019. Nov 26;16(23):4701. doi: 10.3390/ijerph16234701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toh WS, Brittberg M, Farr J, Foldager CB, Gomoll AH, Hui JH, et al. Cellular senescence in aging and osteoarthritis. Acta Orthop. 2016. Dec;87(Suppl 363):6-14. doi: 10.1080/17453674.2016.1235087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yasui Y, Ando W, Shimomura K, Koizumi K, Ryota C, Hamamoto S, et al. Scaffold-free, stem cell-based cartilage repair. J Clin Orthop Trauma. 2016. Jul-Sep;7(3):157-63. doi: 10.1016/j.jcot.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fibel KH, Hillstrom HJ, Halpern BC. State-of-the-art management of knee osteoarthritis. World J Clin Cases. 2015. Feb 16;3(2):89-101. doi: 10.12998/wjcc.v3.i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCormick F, Harris JD, Abrams GD, Frank R, Gupta A, Hussey K, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014. Feb;30(2):222-6. doi: 10.1016/j.arthro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 8. Seo SJ, Mahapatra C, Singh RK, Knowles JC, Kim HW. Strategies for osteochondral repair: focus on scaffolds. J Tissue Eng. 2014;5: 2041731414541850. doi: 10.1177/2041731414541850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. See EY, Toh SL, Goh JC. Multilineage potential of bone-marrow-derived mesenchymal stem cell cell sheets: implications for tissue engineering. Tissue Eng Part A. 2010. Apr;16(4):1421-31. doi: 10.1089/ten.TEA.2009.0501. [DOI] [PubMed] [Google Scholar]
- 10. He L, He T, Xing J, Zhou Q, Fan L, Liu C, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020. Jul 10;11(1):276. doi: 10.1186/s13287-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang R, Ma J, Han J, Zhang W, Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11(10):6275-89. [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008. Nov;180(5):2226-33. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 13. Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, et al. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells. 2013. Sep;31(9):1840-56. doi: 10.1002/stem.1424. [DOI] [PubMed] [Google Scholar]
- 14. Chun SY, Kim HT, Lee JS, Kim MJ, Kim BS, Kim BW, et al. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology. 2012. May;79(5):1186.e1-7. doi: 10.1016/j.urology.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 15. Guan JJ, Niu X, Gong FX, Hu B, Guo SC, Lou YL, et al. Biological characteristics of human-urine-derived stem cells: potential for cell-based therapy in neurology. Tissue Eng Part A. 2014. Jul;20(13-4):1794-806. doi: 10.1089/ten.TEA.2013.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen L, Li L, Xing F, Peng J, Peng K, Wang Y, et al. Human urine-derived stem cells: potential for cell-based therapy of cartilage defects. Stem Cells Int. 2018;2018:4686259. doi: 10.1155/2018/4686259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017. Jul;67:56-64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 18. Lai RC, Tan SS, Yeo RW, Choo AB, Reiner AT, Su Y, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. doi: 10.3402/jev.v5.29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007. Jun;9(6):654-9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 20. Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta. 2015. Jan;1852(1):1-11. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017. Nov 24;7(1):16214. doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017. Apr;35(4):851-8. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 23. Tao Y, Zhou J, Wang Z, Tao H, Bai J, Ge G, et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-kappaB signaling pathway. Bioorg Chem. 2021. Aug;113:104978. doi: 10.1016/j.bioorg.2021.104978. [DOI] [PubMed] [Google Scholar]
- 24. Wang K, Li F, Yuan Y, Shan L, Cui Y, Qu J, et al. Synovial mesenchymal stem cell-derived EV-packaged miR-31 downregulates histone demethylase KDM2A to prevent knee osteoarthritis. Mol Ther Nucleic Acids. 2020. Dec 4;22:1078-91. doi: 10.1016/j.omtn.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xia Q, Wang Q, Lin F, Wang J. MiR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021. Dec;12(2):11225-38. doi: 10.1080/21655979.2021.1995580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo Z, Su W, Zhou R, Zhang G, Yang S, Wu X, et al. Exosomal MATN3 of urine-derived stem cells ameliorates intervertebral disc degeneration by antisenescence effects and promotes NPC proliferation and ECM synthesis by activating TGF-beta. Oxid Med Cell Longev. 2021;2021:5542241. doi: 10.1155/2021/5542241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Liao J, Su X, Li W, Bi Z, Wang J, et al. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics. 2020;10(21):9561-78. doi: 10.7150/thno.42153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang XR, Huang YZ, Gao HW, Jiang YL, Hu JG, Pi JK, et al. Hypoxic preconditioning of human urine-derived stem cell-laden small intestinal submucosa enhances wound healing potential. Stem Cell Res Ther. 2020. Apr 6;11(1):150. doi: 10.1186/s13287-020-01662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsai CC, Yew TL, Yang DC, Huang WH, Hung SC. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am J Blood Res. 2012;2(3):148-59. [PMC free article] [PubMed] [Google Scholar]
- 30. Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012. Mar 2;3(2):9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019. Jan;21(1):9-17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 32. Ouyang B, Xie Y, Zhang C, Deng C, Lv L, Yao J, et al. Extracellular vesicles from human urine-derived stem cells ameliorate erectile dysfunction in a diabetic rat model by delivering proangiogenic MicroRNA. Sex Med. 2019. Jun;7(2):241-50. doi: 10.1016/j.esxm.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rasheed Z, Al-Shobaili HA, Rasheed N, Mahmood A, Khan MI. MicroRNA-26a-5p regulates the expression of inducible nitric oxide synthase via activation of NF-kappaB pathway in human osteoarthritis chondrocytes. Arch Biochem Biophys. 2016. Mar 15;594:61-7. doi: 10.1016/j.abb.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 34. Zhang Z, Yang B, Zhou S, Wu J. CircRNA circ_SEC24A upregulates DNMT3A expression by sponging miR-26b-5p to aggravate osteoarthritis progression. Int Immunopharmacol. 2021. Oct;99:107957. doi: 10.1016/j.intimp.2021.107957. [DOI] [PubMed] [Google Scholar]
- 35. Yuan X, Zhang Y, Cai C, Liu C, Xie J, Yi C. Circular RNA circZNF652 is overexpressed in osteoarthritis and positively regulates LPS-induced apoptosis of chondrocytes by upregulating PTEN. Autoimmunity. 2021. Nov;54(7):415-21. doi: 10.1080/08916934.2021.1951716. [DOI] [PubMed] [Google Scholar]
- 36. Zhou Z, Ma J, Lu J, Chen A, Zhu L. Circular RNA CircCDH13 contributes to the pathogenesis of osteoarthritis via CircCDH13/miR-296-3p/PTEN axis. J Cell Physiol. 2021. May;236(5):3521-35. doi: 10.1002/jcp.30091. [DOI] [PubMed] [Google Scholar]
- 37. Guan J, Zhang J, Guo S, Zhu H, Zhu Z, Li H, et al. Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/beta-catenin signaling pathway. Biomaterials. 2015. Jul;55:1-11. doi: 10.1016/j.biomaterials.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 38. Chen AJ, Pi JK, Hu JG, Huang YZ, Gao HW, Li SF, et al. Identification and characterization of two morphologically distinct stem cell subpopulations from human urine samples. Sci China Life Sci. 2020. May;63(5):712-23. doi: 10.1007/s11427-018-9543-1. [DOI] [PubMed] [Google Scholar]
- 39. Kwon SY, Chun SY, Ha YS, Kim DH, Kim J, Song PH, et al. Hypoxia enhances cell properties of human mesenchymal stem cells. Tissue Eng Regen Med. 2017. Oct;14(5):595-604. doi: 10.1007/s13770-017-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015. Jul 1;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang CC, Kang M, Lu Y, Shirazi S, Diaz JI, Cooper LF, et al. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020. Jun;109:182-94. doi: 10.1016/j.actbio.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, et al. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018. Nov 21;9(1):320. doi: 10.1186/s13287-018-1069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015. May;23(5):812-23. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012. Jun;64(6):1697-707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015. Oct 30;16(11):26035-54. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Filova E, Rampichova M, Litvinec A, Drzik M, Mickova A, Buzgo M, et al. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int J Pharm. 2013. Apr 15;447(1-2):139-49. doi: 10.1016/j.ijpharm.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 47. Grigolo B, Roseti L, Fiorini M, Fini M, Giavaresi G, Aldini NN, et al. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001. Sep;22(17):2417-24. doi: 10.1016/s0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 48. Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019. Mar;8(3):215-24. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016. Jul;5(7):847-56. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burdeyron P, Giraud S, Hauet T, Steichen C. Urine-derived stem/progenitor cells: a focus on their characterization and potential. World J Stem Cells. 2020. Oct 26;12(10):1080-96. doi: 10.4252/wjsc.v12.i10.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zidan AA, Al-Hawwas M, Perkins GB, Mourad GM, Stapledon CJM, Bobrovskaya L, et al. Characterization of urine stem cell-derived extracellular vesicles reveals B cell stimulating cargo. Int J Mol Sci. 2021. Jan 5;22(1):459. doi: 10.3390/ijms22010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011. Jul 8;9(1):11-5. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Windt TS, Vonk LA, Slaper-Cortenbach IC, van den Broek MP, Nizak R, van Rijen MH, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017. Jan;35(1):256-64. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 54. Pan W, Xu X, Zhang M, Song X. Human urine-derived stem cell-derived exosomal miR-21-5p promotes neurogenesis to attenuate Rett syndrome via the EPha4/TEK axis. Lab Invest. 2021. Jul;101(7):824-36. doi: 10.1038/s41374-021-00574-w. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Wang J, Yang B, Qiao R, Li A, Guo H, et al. Transfer of MicroRNA-216a-5p from exosomes secreted by human urine-derived stem cells reduces renal ischemia/reperfusion injury. Front Cell Dev Biol. 2020;8:610587. doi: 10.3389/fcell.2020.610587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005. Jul;204(1):184-91. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 57. Haider H, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res. 2010. Apr;3(2):89-102. doi: 10.1007/s12265-009-9161-2. [DOI] [PubMed] [Google Scholar]
- 58. Rong Y, Zhang J, Jiang D, Ji C, Liu W, Wang J, et al. Hypoxic pretreatment of small extracellular vesicles mediates cartilage repair in osteoarthritis by delivering miR-216a-5p. Acta Biomater. 2021. Mar 1;122:325-42. doi: 10.1016/j.actbio.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 59. Ching RC, Wiberg M, Kingham PJ. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther. 2018. Oct 11;9(1):266. doi: 10.1186/s13287-018-1017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010. Apr 6;107(14):6328-33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015. Feb;13(1):17-24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017. Aug 3;548(7665):52-7. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ragni E, Perucca Orfei C, De Luca P, Colombini A, Vigano M, de Girolamo L. Secreted factors and EV-miRNAs orchestrate the healing capacity of adipose mesenchymal stem cells for the treatment of knee osteoarthritis. Int J Mol Sci. 2020. Feb 26;21(5):1582. doi: 10.3390/ijms21051582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jin Z, Ren J, Qi S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int Immunopharmacol. 2020. Jan;78:105946. doi: 10.1016/j.intimp.2019.105946. [DOI] [PubMed] [Google Scholar]
- 65. Lu L, Wang J, Fan A, Wang P, Chen R, Lu L, et al. Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A-26a-5p ameliorate cartilage damage of osteoarthritis. J Gene Med. 2021. Jul 23;23:e3379. doi: 10.1002/jgm.3379. [DOI] [PubMed] [Google Scholar]
- 66. Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998. Aug;19(4):348-55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 67. Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018. Sep;19(9):547-62. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 68. Iwasa K, Hayashi S, Fujishiro T, Kanzaki N, Hashimoto S, Sakata S, et al. PTEN regulates matrix synthesis in adult human chondrocytes under oxidative stress. J Orthop Res. 2014. Feb;32(2):231-7. doi: 10.1002/jor.22506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035221077401 for Extracellular Vesicles from Hypoxic Pretreated Urine-Derived Stem Cells Enhance the Proliferation and Migration of Chondrocytes by Delivering miR-26a-5p by Sha Wan, Dingsu Bao, Jia Li, Kefu Lin, Qi Huang, Qiang Li and Lang Li in CARTILAGE