Abstract
Objective
Despite massive efforts, there are no diagnostic blood biomarkers for knee osteoarthritis (KOA). This study investigated several candidate diagnostic biomarkers and the metabolic phenotype in end-stage KOA in the context of obesity.
Design
In this cross-sectional study, adult patients undergoing knee arthroplasty were enrolled and KOA severity was assessed using the Lequesne index. Blood biomarkers with an important role in obesity, the metabolic syndrome, or KOA (oxidized form of low-density lipoprotein [oxLDL], advanced glycation end product [AGE], soluble AGE receptor [sRAGE], fatty acid binding protein 4 [FABP4], phospholipase A2 group IIA [PLA2G2A], fibroblast growth factor 23 [FGF-23], ghrelin, leptin, and resistin) were measured using enzyme-linked immunosorbent assay (ELISA; n = 70) or Luminex technique (subgroup of n = 35). H1-NMR spectroscopy was used for the quantification of metabolite levels (subgroup of n = 31). The hip-knee-ankle angle was assessed. Multivariable and multivariate regression analysis was used to examine the relationship of biomarkers with body mass index (BMI) and KOA severity in complete case and multiple imputation analysis.
Results
While most of the investigated biomarkers were not associated with KOA severity, FABP4 and leptin were found to correlate with BMI and gender. Resistin was associated with Lequesne index in complete case analysis. Using a targeted metabolomics approach, BMI-dependent changes in the metabolome were hardly visible.
Conclusions
Our findings confirm studies on FABP4, leptin, and resistin with regard to obesity and the metabolic syndrome. There was no association of the investigated biomarkers with KOA severity, most likely due to the patient selection (end-stage KOA patients). Based on this absence of BMI-dependent changes in the metabolome, we might assume that BMI is not correlated with KOA severity in this specific patient group.
Keywords: FABP4, biomarkers, obesity, knee osteoarthritis
Introduction
Background
Knee osteoarthritis (KOA) is a major cause of disability and pain worldwide. 1 Despite massive efforts over the past decades, no blood serum marker has emerged as diagnostic marker so far. 2
Based on recent research results, inflammation, obesity, and the metabolic syndrome have been described as risk factors for KOA manifestation and progression of OA.
Obesity, defined as a body mass index (BMI) of greater than 30, is a silent epidemic associated with several chronic conditions and affecting more than 2.1 billion individuals worldwide.3,4 It is also a major contributing factor to the pathogenesis and progression of KOA due to adipocyte-derived systemic inflammation. Obesity-related osteoarthritis has been described as a complex, multifactorial condition that can cause significant impact on quality of life of affected patients. 5 There is a close correlation of BMI and clinical and functional consequences of KOA. 6 This observation is relevant when considering KOA treatment strategies based on obesity. Investigating candidate biomarkers to assess a possible correlation to BMI and/or KOA severity seems to be an important step in finding diagnostic blood biomarkers.
In addition, obesity represents a key finding for the metabolic syndrome (metS). The metS is defined as a combination of several disorders associated with cardiovascular risk. It causes local and systemic changes that induce a pro-inflammatory state leading to oxidative stress and eventually cartilage degradation. 7
Although adipocytokines and the metabolome are considered to be important pathogenic factors in obesity-related OA, there has been little research of these factors as diagnostic biomarkers.
Leptin, resistin, and grehlin play a major role in obesity and metS. These markers contribute to the imbalance of joint homeostasis and are involved in the pathogenesis of osteoarthritis.8,9
The oxidized form of low-density lipoprotein (oxLDL) is not only a marker of metabolic disease, but plays a major role in cartilage degeneration. 10
Fatty acid binding protein 4 (FABP4) is a adipokine that is closely associated with obesity and metabolic disease. In knock-out models and after pharmaceutical inhibition of FABP4, OA induced by high-fat diet in mice was alleviated. 11
The receptor/ligand system of advanced glycation end products (AGEs) and their cell receptor (RAGE) are similarly involved in the pathophysiology of metabolic diseases. The AGE-RAGE interaction results in increased generation of oxygen radicals and pro-inflammatory cytokines. Circulating soluble AGE receptors (sRAGE) interact with AGEs to counterbalance the negative effects of AGEs-RAGE interaction. 12
The finding that PLA2G2A is highly abundant in biological fluids of patients suffering from inflammatory diseases, such as arthritis, sepsis, and myocardial infarctions, emphasizes the important role of PLA2G2A in inflammation. Elevated levels of PLA2G2A have been described as biomarker for cardiovascular disease and the metS. 13
Fibroblast growth factor 23 (FGF-23) is a regulator of cartilage differentiation and might also play a role in the pathogenesis of osteoarthritis. 14
Previous metabolomics studies have identified changes in branched-amino acid (BCAA) and arginine levels in serum and synovial fluid of KOA patients. 15 Another study, using an untargeted metabolomics approach, found several differences in obese KOA patients compared with nonobese KOA patients. 16
To the best of our knowledge, these peptides and proteins with important roles in cartilage matrix turnover (PLA2G2A), glucose metabolism (AGE, soluble RAGE), and chondrocyte differentiation (FGF-23) have not been studied extensively in the context of obesity and end-stage KOA before.
In this article, we examined abovementioned biomarkers as markers of burden of disease in end-stage KOA, measured using the Lequesne index. This study was conducted to investigate whether any of these biomarkers are associated with obesity, the metS, and KOA and to test their ability in characterizing KOA severity.
Methods
This study was conducted as a cross-sectional study at the Department of Orthopedics and Trauma of the Medical University of Graz, Austria, from January 2019 to April 2020. The procedures described were in accordance with the ethical standards of the ethics committee of the Medical University of Vienna (IRB#2029/2016) and the Medical University of Graz (IRB#31-133ex18/19) and with the Helsinki Declaration.
Study Population
Adult patients undergoing knee arthroplasty were enrolled in the “Better Life in Osteoarthritis Registry” (BLOAR) after informed consent. The BLOAR is an Austrian multicenter registry for OA patients with the goal to further research in the prevention and treatment of osteoarthritis. A total of 70 patients were grouped based on BMI (group 1 = “underweight”: BMI < 20 [n = 19], group 2 = “normal weight”: 20 to 30 [n = 32], group 3 = “obese”: BMI > 30 [n = 19]) and chosen randomly (enrollment based on date of surgery and available resources on the day of enrollment) from the registry for analysis in this study.
Blood samples were taken preoperatively and centrifuged within 1 hour. Serum was stored at −20°C until further processing. To achieve a more distinct separation, and due to limited resources, only patients with the lowest and highest BMI in the underweight and obese group, respectively, were further analyzed with metabolic phenotyping and Luminex assays.
Patient Characteristics
Height, weight, and abdominal circumference and blood pressure measurements were taken. For the purpose of this study, obesity was defined as a BMI of greater than 30, normal weight as a BMI of 20 to 30, and underweight as a BMI less than 20. Previous nonoperative and operative treatment (including physiotherapy, physical therapy, weight-reduction program, intra-articular injections, topical and systemic analgesics) was recorded. In women, hormonal status was recorded (previous gynecologic surgeries, oral contraceptive pills, hormone-replacement treatment). Comorbidities and medication were recorded based on history taking and medication was grouped based on mechanism of action. Based on the National Cholesterol Education Program (NCEP) definition of the metabolic syndrome, patients were diagnosed with metabolic syndrome when 3 or more of the following conditions were met: (1) on antihypertensive medication, known arterial hypertension, or systolic office measurement greater than 130 mmHg; (2) known dyslipidemia or on lipid-lowering drugs; (3) known diabetes mellitus or on antidiabetic drugs; and (4) abdominal obesity (waist circumference > 102 cm for men or > 88 cm for women). 17 The Lequesne index, a patient-reported outcome index, is a questionnaire with 3 sections on pain and discomfort, walking distance, and activities of daily living. A higher score is associated with a greater disability. This index was used to assess KOA severity. 18 The ASA physical status classification system was used to assess the overall patient health status. The ASA physical status classification is a system that can be used to assess the health status and evaluate perioperative risks.
X-Ray Measurements
Preoperative full-length limb standing x-ray images were taken and analyzed for the hip-knee-ankle angle. 19 Measurements were performed using imageJ (version 1.52j) by P.S. and by M.F. in a subset of 16 patients. 20 Interrater agreement was assessed using intraclass correlation coefficient 3,A (ICC3,A). Interrater agreement was excellent (ICC3,A = 0.99, P = 0).
Enzyme-Linked Immunosorbent Assay (ELISA)
The following ready-to-use sandwich ELISA kits were used according to the manufacturer’s instruction: oxidized low-density lipoprotein cholesterol (oxLDL; Mercodia, Uppsala, Sweden), advanced glycation end products (AGE; Cell Biolabs, San Diego, CA), soluble receptor for advanced glycation end products (sRAGE; BioVendor, Brno, Czech Republic), fatty acid binding protein 4 (FABP4; BioVendor), and membrane-bound phospholipase A2 group IIA (PLA2G2A; RayBiotech Life, Peachtree Corners, GA). The AGE-sRAGE ratio was calculated based on these measurements. All measurements were performed in duplicates at 450 nm with a microplate reader (Infinite F50 from Tecan, Austria).
XMAP Human Bone Metabolism Magnetic Bead Panel
Using the Luminex xMAP platform in a magnetic bead format, we simultaneously analyzed the following targets from human serum samples in a subgroup of 35 patients who were selected based on BMI (see above; BMI group 1: n = 16, BMI group 3: n = 19): fibroblast growth factor 23 (FGF-23), ghrelin, leptin, and resistin. For detection, we used the commercially available ProcartaPlex (Thermo Fisher, Waltham, MA) on a Bio-Plex 200 system (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. There was no cross-reactivity between the antibodies for an analyte and any of the other analytes in this panel. Measurement of median fluorescence intensity (MFI) was performed using the Bio-Plex Manager software, version 4.1 (Bio-Rad Laboratories) and used for further analysis. 21
Metabolic Phenotyping
In another, partly overlapping subgroup of 31 patients (BMI group 1: n = 15, BMI group 3: n = 16), changes in metabolic phenotypes were explored using nuclear magnetic resonance (NMR) spectroscopy (Bruker BioSpin GmbH, Rheinstetten, Germany). Human serum samples from KOA patients were lyophilized and 500 µl of NMR buffer (5.56 g Na2HPO4, 0.4 g trimethylsilylpropanoic acid [TSP], 0.2 g NaN3, in 400 ml of D2O; pH 7.4) were added. All NMR experiments were performed at a temperature of 310 K on a Bruker AVANCE Neo Ultrashield 600 MHz spectrometer equipped with a triple resonance probe head and processed as described previously. 22 Bruker TopSpin version 3.1 (Bruker BioSpin GmbH) was used for NMR data acquisition and automatic processing (exponential line broadening of 0.3 Hz, phasing, and referencing to TSP at 0.0 ppm). Regions around water, TSP, and remaining MeOH signals were excluded. The spectra for all samples were further analyzed, deconvoluted, and quantified automatically, using the R package ASICS. 23 Probabilistic quotient normalization was performed to correct for sample metabolite dilution. Concentrations shown are normalized concentrations.
Statistical Analysis
All analyses were performed using R version 4.0.3 (2020-10-10) on Manjaro Linux 5.6.15-1. 24 The following R packages were used: mice, lmerTest, tidyverse, visreg, irr, ASICS, ClustOfVars, car.23,25 -31 For Luminex data analysis, a collection of R scripts provided by Breen et al. 32 was used.
In the ELISA data set, hierarchical cluster analysis, based on principal component analysis (PCA) for a mixture of continuous and categorical variables (PCAMIX), was performed on variables to gain a better understanding of the relationship among variables collected.30 For the purpose of this study, we cut the cluster dendogram based on aggregation levels and the Rand index into a total of 11 clusters.
Missing data and sensitivity analysis
There were missing data in the ELISA biomarkers and the Lequesne index (all less than 16%, see Suppl. Tables S1 and S2). It was assumed that data were missing completely at random (MCAR). Details on assumed mechanisms of missingness are outlined in the Supplement. Based on the MCAR assumption, missing values were imputed using multiple imputation chained equation (MICE) with the R package mice. 28 All analyses were performed on complete cases and the multiply imputed data sets. To explore violations of the MCAR assumption, sensitivity analysis was performed. The δ adjustment technique was used for this purpose:28,33 A δ of ±15% and ±30% of the complete case mean was added to the imputed values to understand how deviations from the MCAR assumptions influence analysis.
Inferential analysis
Normality was assessed using Shapiro-Wilk tests, Q-Q, and density plots. Means were compared using a Student t test or Wilcoxon rank sum test where appropriate. Correlations were assessed using Spearman rank correlation.
To answer our research questions, the following regression analyses were performed: (1) Multivariable linear regression was performed on each ELISA biomarker as dependent variable. All models were adjusted to the known risk factors such as age, gender, BMI, and hip-knee-ankle angle. To understand the relationship with the Lequesne index and the metabolic syndrome, these variables were also added to all models. To detect multicollinearity in fixed-effects models, the variance inflation factor (VIF) was calculated. All VIF values were smaller than 2. (2) Logarithmic transformation was applied to Luminex fluorescence values and these were used in linear mixed models as outcome variable.21,32 Patient identity was used as cluster variable in a model with random intercepts. Models were adjusted for age, gender, BMI, and HKAA. To understand the association with the Lequesne index and the metabolic syndrome, these variables were also added to the model. (3) For metabolic phenotyping, data were analyzed using PCA. The number of principal components was determined using the cumulative variance (exceeding 50% of cumulative variance). Partial least squares regression and discriminant Analysis (PLSR, PLS-DA) using the R package ASICS was used to test the relationship of metabolite concentrations and BMI group and test scores. PLS and PLS-DA models were assessed using R2 to estimate goodness of fit and Q2 to estimate predictive performance. The number of components added to the model was determined based on R2 and Q2 scores using 7-fold cross-validation. The predictive performance of the full model was assessed based on the cumulative Q2 score using permutation testing (1000 permutations). All models were assessed using diagnostic plots.
All P values were adjusted using the Benjamini-Hochberg correction as implemented in R and rounded to 3 digits, with a value of <.05 indicating significance.
Results
Study Population
A total of 70 patients were enrolled. Patient characteristics are shown in Table 1 . The mean age was 70.09 ± 8.92 and mean BMI was 30.42 ± 6.27. A total of 50 women (71.43%) were included. Supplementary Tables S3-A and S3-B show the subgroup patient characteristics.
Table 1.
Study Population by BMI Group.
| Underweight (n = 19) |
Normal (n = 32) |
Obese (n = 19) |
Total (N = 70) |
|
|---|---|---|---|---|
| Age | 75.16 (6.38) | 70.28 (9.41) | 64.68 (7.33) | 70.09 (8.92) |
| BMI | 22.88 (1.63) | 30.18 (2.62) | 38.35 (3.07) | 30.42 (6.27) |
| Female | 15 (78.9%) | 20 (62.5%) | 15 (78.9%) | 50 (71.4%) |
| HKAA | 7.40 (3.94) | 7.63 (4.36) | 8.52 (4.02) | 7.81 (4.12) |
| ASA | ||||
| 1 | 1 (5.3%) | 3 (9.4%) | 0 (0.0%) | 4 (5.7%) |
| 2 | 9 (47.4%) | 17 (53.1%) | 8 (42.1%) | 34 (48.6%) |
| 3 | 9 (47.4%) | 10 (31.2%) | 11 (57.9%) | 30 (42.9%) |
| 4 | 0 (0.0%) | 2 (6.2%) | 0 (0.0%) | 2 (2.9%) |
| Lequesne index | 14.30 (3.73) | 12.79 (3.82) | 14.83 (2.92) | 13.80 (3.61) |
| TUGT | 14.07 (3.84) | 12.89 (6.38) | 14.71 (5.92) | 13.71 (5.67) |
| Diabetes mellitus | 4 (21.1%) | 5 (15.6%) | 3 (15.8%) | 12 (17.1%) |
| Hyperlipidemia | 6 (37.5%) | 10 (38.5%) | 4 (21.1%) | 20 (32.8%) |
| Abdominal circumference | 89.08 (7.30) | 106.96 (11.22) | 122.76 (9.90) | 107.63 (15.95) |
| CAD | 4 (21.1%) | 5 (15.6%) | 3 (15.8%) | 12 (17.1%) |
| Hysterectomy | 0 (0.0%) | 5 (17.2%) | 7 (36.8%) | 12 (19.0%) |
BMI = body mass index; HKAA = hip-knee-ankle angle; ASA = ASA physical status classification system; CAD = coronary artery disease; TUGT = timed up and go test.
Hierarchical Cluster Analysis
Data on 66 variables were collected. Hierarchical cluster analysis groups highly correlated variables in clusters to give a better understanding of the meaning of each variable. Figure 1 shows the cluster dendogram. The clusters and variables are listed in Supplementary Table S4.
Figure 1.
Dendogram of hierarchical cluster analysis of variables. A synthetic variable is computed on the basis of principal component analysis for a mixture of continuous and categorical variables (PCAMIX). Variables are grouped based on the correlation with this variable. The cluster dendogram was cut (red line) into 11 clusters based on the agglomeration level and the Rand index. The dendogram height indicates the agglomeration level and thus lower height indicates more similarity. OCP = oral contraceptive pill; GI disorder = gastrointestinal disorder; EMF = dielectric heating; TENS = transcutaneous electrical nerve stimulation; CAD = coronary artery disease; ASA = ASA physical status classification system; HKAA = hip-knee-ankle angle; MS = metabolic syndrome; numbers indicate cluster number.
Hierarchical cluster analysis showed that FABP4 level was closely related to gender and height, whereas sRAGE and oxLDL were closely connected among each other and with hysterectomy. Furthermore, AGE and PLA2G2A revealed a close correlation with each other as well as with osteoporosis. The Lequesne index was in the same cluster as the HKAA. Conservative treatment options (physiotherapy and physical and occupational therapy) formed an additional cluster. These findings were as expected and showed the close connection of variables.
Association of ELISA/Luminex Measurements With Clinical Parameters
FABP4 level was associated with BMI and gender, but not Lequesne index, in KOA.
In univariable analysis, we found a significant positive correlation of BMI and FABP4 levels (ρ = 0.4851, P = 0). When adjusting for age, gender, HKAA, and metS in complete cases, FABP4 was still associated with BMI and gender (see Suppl. Table S5). The same result could be observed in the imputed data sets, where BMI and gender were associated with FABP4 after adjustment (see Table 2 , Fig. 2 ). Thus, higher FABP4 levels were found in women and obese patients. No association with the Lequesne index was observed.
Table 2.
Results of Linear Regression With FABP4 as the Dependent Variable in Multiple Imputation Models.
| Variable | Crude Est. | Adj. Est. | 2.5% | 97.5% | Adj. P Value |
|---|---|---|---|---|---|
| Age | −0.35 | 0.169 | –0.355 | 0.693 | .521 |
| BMI | 1.64 | 1.610 | 0.851 | 2.370 | .001*** |
| Gender (male) | –19.34 | –17.201 | –26.052 | –8.349 | .001*** |
| HKAA | 0.26 | –0.372 | –1.411 | 0.666 | .521 |
| Lequesne index | 1.25 | 0.858 | –0.416 | 2.131 | .425 |
| No metabolic syndrome | –8.49 | –3.871 | –12.659 | 4.916 | .521 |
The model was adjusted for the age, gender, BMI, HKAA, and metabolic syndrome. P values are adjusted. There is a significant positive association of FABP4 levels and BMI and female gender. Crude est. = crude estimate; Adj. est. = adjusted estimate; 2.5% and 97.5% = 2.5% and 97.5% confidence intervals; no metabolic syndrome = patient did not meet metS criteria as outlined in the text; FABP4 = fatty acid binding protein 4; BMI = body mass index; HKAA = hip-knee-ankle angle.
Figure 2.
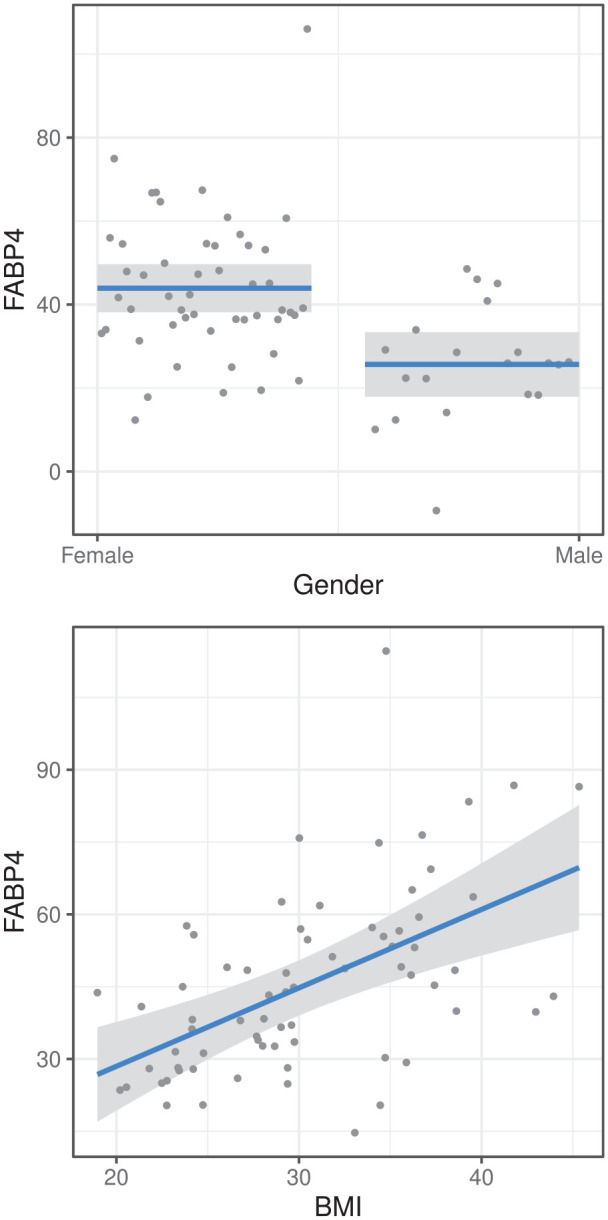
Top: Association of gender and FABP4 as predicted by linear regression. FABP4 is higher in women. Bottom: Correlation of BMI and FABP4 as predicted by regression. FABP4 increases with BMI. FABP4 = fatty acid binding protein 4; BMI = body mass index.
In the sensitivity analysis, results were stable (see Suppl. Tables S6-A and S6-B). Thus, even if the imputed values deviated by up to 30% from the mean of the observed values, we would still observe a similar effect.
All other biomarkers explored by using the ELISA technique (oxLDL, AGE, sRAGE, PLA2G2A) did not show a significant association with BMI, Lequesne index, or metS (Suppl. Tables S7-A to S7-E).
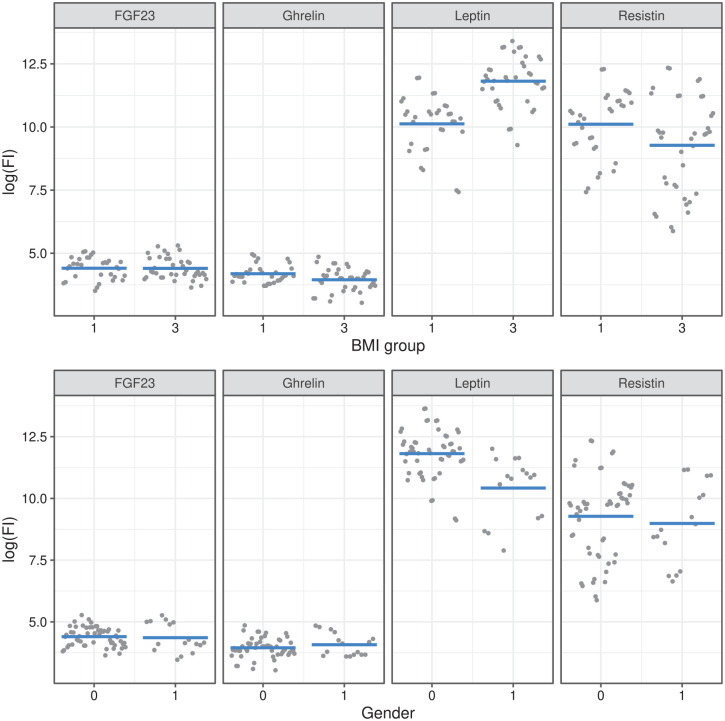
Leptin was associated with gender and BMI group
In complete cases, leptin was associated with BMI group and gender (Suppl. Table S8). The same results were observed after multiple imputation ( Table 3 ). This association was further examined using sensitivity analysis and remained stable (Suppl. Tables S9-A and S9-B). Leptin levels were higher in obesity compared with underweight and in women compared with men. Figure 3 shows the relationship of BMI and gender as predicted by the linear mixed model.
Table 3.
Results of Linear Mixed Models on Imputed Data Showing Interactions Only.
| Coefficient | Unadj. Est. | Adj. Est. | 2.5% | 97.5% | Adj. P Value |
|---|---|---|---|---|---|
| Ghrelin: Age | 0.002 | –0.004 | –0.058 | 0.049 | .972 |
| Ghrelin: BMI group 3 | –0.135 | –0.222 | –1.173 | 0.729 | .972 |
| Ghrelin: Gender (male) | 0.052 | 0.175 | –0.723 | 1.073 | .972 |
| Ghrelin: HKAA | –0.013 | –0.008 | –0.114 | 0.098 | .972 |
| Ghrelin: Lequesne Index | –0.012 | –0.019 | –0.141 | 0.102 | .972 |
| Ghrelin: No metabolic syndrome | –0.125 | –0.251 | –1.083 | 0.581 | .972 |
| Leptin: Age | –0.031 | 0.028 | –0.026 | 0.081 | .787 |
| Leptin: BMI group 3 | 1.014 | 1.642 | 0.688 | 2.596 | .011* |
| Leptin: Gender (male) | –1.039 | –1.418 | –2.323 | –0.513 | .021* |
| Leptin: HKAA | 0.033 | 0.040 | –0.069 | 0.149 | .972 |
| Leptin: Lequesne Index | 0.005 | –0.031 | –0.182 | 0.121 | .972 |
| Leptin: No metabolic syndrome | –0.565 | –0.535 | –1.383 | 0.313 | .669 |
| Resistin: Age | –0.003 | –0.028 | –0.082 | 0.026 | .787 |
| Resistin: BMI group 3 | –0.321 | –0.785 | –1.752 | 0.183 | .391 |
| Resistin: Gender (male) | –0.279 | –0.234 | –1.137 | 0.668 | .972 |
| Resistin: HKAA | 0.030 | 0.127 | 0.020 | 0.234 | .113 |
| Resistin: Lequesne Index | –0.097 | –0.166 | –0.301 | –0.030 | .113 |
| Resistin: No metabolic syndrome | 0.169 | –0.028 | –0.884 | 0.827 | .972 |
The model was adjusted for age, gender, BMI group, HKAA, and metabolic syndrome. There is a significant positive association of leptin levels and obesity (BMI group 3) and female gender. Unadj. est. = unadjusted estimate; Adj. est. = adjusted estimate; 2.5% and 97.5% = 2.5% and 97.5% confidence intervals; ‘:’ = interaction term; BMI = body mass index; HKAA = hip-knee-ankle angle.
P < .05.
Figure 3.
The relationship of BMI group (top) and gender (bottom) with fluorescence intensity per cytokine is shown as predicted by linear mixed modeling. Leptin levels are higher in women in obese patients. BMI = body mass index; BMI group 1 = underweight; BMI group 3 = obesity; 0 = female gender.
Resistin was associated with Lequesne index in complete case analysis
In complete cases, resistin showed an association with Lequesne index (Suppl. Table S8). After applying multiple imputation, this association was not observed, however ( Table 3 ). This was confirmed in the sensitivity analysis (Suppl. Tables S9-A and S9-B) demonstrating that only under the most were able to explain the data. extreme imputation scenario (+30%), resistin was associated with Lequesne index. Thus, this indicated that resistin levels were probably not correlated with Lequesne index.
FGF-23 and ghrelin were not associated with BMI or Lequesne index
Neither FGF-23 nor ghrelin was associated with BMI, metS, or Lequesne index in complete case analysis and multiple imputation (Suppl. Table S8, Table 3 , Suppl. Tables S9-A and S9-B).
Metabolic Phenotyping
Serum probes from the 2 BMI groups were analyzed for their metabolite composition and summarized in a heat map ( Fig. 4 ). While, on the heatmap, there is clearly a pattern, we were not able to detect an association with BMI or metS.
Figure 4.
Heat map of metabolite concentrations based on BMI group (dark green = underweight, light green = obese). While there is obviously a pattern in metabolite concentrations, we could not detect an association with BMI group or metabolic syndrome in our study. Numbers at the bottom = sample number; BMI = body mass index; IMG = inositol monophosphate; GMP = guanosine monophosphate; GTP = guanosine-5-triphosphate; NADP = nicotinamide adenine dinucleotide phosphate.
To understand the relationship of metabolites with clinical parameters, PCA, group comparison with t tests, and PLS or PLS-DA were performed.
PCA results did not indicate a possible difference in metabolites of BMI groups 1 (underweight) and 3 (obese; see Fig. 5 ). In univariate analysis, we did not detect any differences. Accordingly, in PLS-DA, we were not able to predict BMI group membership (R2Y = 0.96, Q2Y = 0.27, PR2Y = .27, PQ2 = .043).
Figure 5.

PCA score plot of the first 2 principal components. Samples were colored according to their BMI group. There is an overlap of BMI groups 1 and 3. “t1, t2” = first 2 principal components (explained variance) and deviation from the respective component (axis); s1-s31 = patient samples; PCA = principal component analysis; BMI = body mass index.
Using PLS and PLS-DA, we were also not able to predict scores of the Lequesne index or metabolic syndrome (Suppl. Tables S10-S12).
Discussion
In this study, we investigated several candidate biomarkers, as well as the metabolome, in the context of obesity, in end-stage KOA. A cross-sectional clinical/lab study was conducted in end-stage KOA patients using serum biomarkers as measured by ELISA/Luminex technique or NMR spectroscopy to conduct a multivariable and a multivariate analysis for possible associations with clinical parameters such as BMI and the Lequesne index. Our hypothesis was that biomarker levels change with respect to BMI and that biomarkers were associated with KOA severity.
We found that higher FABP4 and leptin were significantly associated with obesity and female gender. In agreement with previous studies, we did not detect an association of leptin and the Lequesne index, but BMI group and gender. Resistin did not show an association with Lequesne index in end-stage KOA patients. Finally, we were not able to detect BMI-dependent changes in the metabolome of KOA patients.
FABP4 is one of several members of a family of lipid chaperons. It is mainly expressed in adipocytes and is involved in intracellular fatty acid transportation. There is a strong correlation between FABP4 and obesity and FABP4 expression is higher in females than males. 34 In mice models of induced obesity, FABP4 knock-out and pharmaceutical inhibition alleviated cartilage degeneration. 11 In KOA patients, FABP4 concentrations are higher compared with non-KOA controls and are associated with obesity and KOA severity on the Kellgren-Lawrence scale. 35 In agreement with all these findings, our results confirm higher FABP4 levels in obese females in KOA, independent of age, HKAA, and metabolic syndrome. We did not detect an association with the Lequesne index, however. Only a homogeneous group of end-stage KOA patients on the day before knee arthroplasty was included in our study and this might explain why no such association could be observed.
PLA2G2A has been implicated in the pathogenesis of OA as a cartilage degrading enzyme. 36 To our knowledge, no study has previously assessed PLA2G2A as a blood biomarker for KOA. Our study did not demonstrate an association of PLA2G2A with BMI and/or KOA severity.
Hyperglycemia leads to an accumulation of advanced glycation end products (AGE), proteins modified by nonenzymatic glycosylation that cause local inflammation by binding to membrane-bound receptors (mRAGE). The interaction of AGEs with soluble receptors (sRAGE) is associated with anti-inflammatory responses by blocking interaction with mRAGE. 12 Our results did not indicate an association with BMI or KOA severity. A study on fasting in KOA also found no association of changes in pain and function scores and AGE/sRAGE. 37
oxLDL, the oxidized form of LDL-cholesterol, is a significant regulator of cartilage degeneration 10 : In KOA, oxLDL levels are higher compared with controls. Furthermore, in another study, oxLDL was found to be positively correlated with KOA severity. This study did not adjust for confounders, however. 38 Our study did not demonstrate such a relationship, neither with BMI nor with KOA severity, after adjusting for several important confounders. Furthermore, in contrast to the aforementioned study, only end-stage KOA patients were included for this investigation and differences in KOA severity might be minimal.
Leptin is mostly produced by adipocytes and leptin levels are positively associated with obesity and female gender.39,40 Leptin levels are increased in KOA compared with controls and there is an association with BMI and gender.41 -43 Our results confirm that obese female patients have higher serum leptin levels. In agreement with a previous study, we could not detect an association with KOA severity.42,43 This contrasts with another study including patients at different stages of KOA and might be explained by a more rigorous adjustment for confounding covariates in our study and the fact that we included end-stage KOA patients only. 41
In KOA patients with joint effusion, resistin is associated with severity even after adjusting for several confounders in serum and synovial fluid.43,44 Another case-control study in obese, female patients found higher resistin levels in KOA patients and resistin levels were positively correlated with WOMAC scores. 45 Another study in different KOA stages did not find an association of pain and resistin levels in serum, but synovial fluid only. 46 In contrast to aforementioned study in female patients with joint effusion, in our study resistin levels were weakly, inversely associated with the Lequesne index in complete case analysis. 44 This negative correlation was observed in complete case analysis and, in the most extreme scenario, in multiple imputation only. Our results are supported by a study showing that serum resistin levels are not associated with preoperative visual-analog scale (VAS) pain levels. Although not significant, in that study the association was also a negative one. 47 Similarly, a previous report found that serum resistin levels were not associated with cartilage damage in end-stage KOA. 42 It is unclear why there was a negative association in complete case analysis, if any, when compared with studies of KOA at different stages showing a positive association. It is possible that missing data introduced a bias in our data that was mitigated through multiple imputation. To summarize, our results show that resistin is not associated with Lequesne index.
In KOA, ghrelin was found to be associated with Western Ontario and McMaster Universities Osteoarthritic Index (WOMAC), even after adjusting for BMI, age, and gender. 48 We could not confirm this finding, possibly due to more rigorous adjustment applied in our study and due to the inclusion of end-stage KOA patients only.
FGF-23 plays an important role in chondrocyte differentiation. FGF-23 is upregulated in osteoarthritic cartilage compared with normal controls. 14 FGF-23 levels were found to be higher in FGF-23 KOA patients compared with normal patients in serum and there was a correlation of KOA severity and FGF-23 level. 49 In contrast, in our study we could not detect an association of serum FGF-23 level with KOA severity nor BMI. The aforementioned study did not control for confounding factors and this might explain the different findings.
Previous studies, using a targeted or untargeted metabolomics approaches, showed significant changes, particularly in branched-chain amino acids (BCAA) and arginine pathways, in KOA compared with control in agreement with epidemiologic studies indicating that changes in BCAA serum concentrations are associated with diabetes, cardiovascular disease, and metabolic syndrome.15,50 In another study, obese KOA patients showed higher oxidative stress levels compared with nonobese KOA patients. 16 In contrast, in our study we did not detect any BMI-dependent changes in end-stage KOA patients. Again, the reason might be that only end-stage KOA patients were included, there was no control group, and differences in metabolites may be minimal within this group. Furthermore, while the abovementioned study showed BMI-dependent changes in KOA patients, that study used an untargeted approach with mass spectroscopy and these differences in the methodology might explain the discrepancies compared with our findings. 16
Limitations
This study has the following limitations: Only a small sample size was enrolled in this study. Due to resource limitations, there were missing data. This could be mitigated by multiple imputation and sensitivity analysis, as demonstrated, however. Furthermore, blood samples were taken at random and patient fasting state was unknown. This might influence ELISA, Luminex, and NMR spectroscopy results. However, based on the agreement with previous studies, we are confident that our results are valid. Finally, our data are applicable to patients undergoing knee arthroplasty for end-stage KOA only.
Conclusion
This study investigated the relationship of several previously not extensively examined biomarkers with BMI and KOA severity. We could confirm the association of BMI and gender with FABP4 and leptin. None of the explored biomarkers was associated with KOA severity and no BMI-dependent changes in the metabolome of end-stage KOA patients were observed.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035211069251 for The Association of Blood Biomarkers and Body Mass Index in Knee Osteoarthritis: A Cross-Sectional Study by Paul Schadler, Birgit Lohberger, Bettina Thauerer, Martin Faschingbauer, Werner Kullich, Martin Helmut Stradner, Andreas Leithner, Valentin Ritschl, Maisa Omara and Bibiane Steinecker-Frohnwieser in CARTILAGE
Footnotes
Author Contributions: B.L. and B.S.F. contributed to conception and design. B.L., B.S.F., and P.S. contributed to analysis, interpretation, and drafting of the article. A.L., B.L., B.T., B.S.F., M.F., M.O., M.S., P.S., V.R., and W.K. contributed to collection and assembly of data. All authors critically revised and finally approved the article.
Acknowledgments and Funding: We would like to thank the many contributors to open-source R packages. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project and the “Better Life in Osteoarthritis Registry” were funded by the Ludwig Boltzmann Institute for Arthritis and Rehabilitation and the Medical University of Graz.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.L. reported institutional educational grants by Johnson & Johnson, Alphamed, Globus, and Implantec. M.S. reported financial support by Eli Lilly, Pfizer, Bristol Mayer Squibb, Takeda, AbbVie, Novartis, Roche, MSD, CSL Behring, and UCB. B.L., B.S.F.., B.T., M.F., M.O., P.S., V.R., and W.K. reported no conflict of interest.
Compliance With Ethical Standards: The procedures followed were in accordance with the ethical standards of the responsible committee (Ethics committee of the Medical University of Graz, Austria, IRB#31-133) on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
ORCID iDs: Paul Schadler  https://orcid.org/0000-0001-9633-1469
https://orcid.org/0000-0001-9633-1469
Bettina Thauerer  https://orcid.org/0000-0001-8357-4082
https://orcid.org/0000-0001-8357-4082
Valentin Ritschl  https://orcid.org/0000-0001-8763-8215
https://orcid.org/0000-0001-8763-8215
Data Availability Statement: All data and R code used in this study are available upon reasonable request.
Supplemental Material: Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2. Convill JG, Tawy GF, Freemont AJ, Biant LC. Clinically relevant molecular biomarkers for use in human knee osteoarthritis: a systematic review. Cartilage. 13;13:1511S-1531S. doi: 10.1177/1947603520941239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Obesity and overweight. World Health Organization. Accessed February 23, 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 4. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176-s185. [PubMed] [Google Scholar]
- 5. Chen L, Zheng JJY, Li G, Yuan J, Ebert JR, Li H, et al. Pathogenesis and clinical management of obesity-related knee osteoarthritis: impact of mechanical loading. J Orthop Translat. 2020;24:66-75. doi: 10.1016/j.jot.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raud B, Gay C, Guiguet-Auclair C, Bonnin A, Gerbaud L, Pereira B, et al. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci Rep. 2020;10(1):3601. doi: 10.1038/s41598-020-60587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8(12):729-37. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 8. Francisco V, Pérez T, Pino J, López V, Franco E, Alonso A, et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: when the levee breaks: adipokines in biomechanics, obesity, and osteoarthritis. J Orthop Res; 36(2):594-604. doi: 10.1002/jor.23788. [DOI] [PubMed] [Google Scholar]
- 9. Kumari R, Kumar S, Kant R. An update on metabolic syndrome: metabolic risk markers and adipokines in the development of metabolic syndrome. Diabetes Metab Syndr. 2019;13(4):2409-17. doi: 10.1016/j.dsx.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 10. Hashimoto K, Akagi M. The role of oxidation of low-density lipids in pathogenesis of osteoarthritis: a narrative review. J Int Med Res. 2020;48(6):0300060520931609. doi: 10.1177/0300060520931609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang C, Chiu KY, Chan BPM, Li T, Wen C, Xu A, et al. Knocking out or pharmaceutical inhibition of fatty acid binding protein 4 (FABP4) alleviates osteoarthritis induced by high-fat diet in mice. Osteoarthr Cartil. 2018;26(6):824-33. doi: 10.1016/j.joca.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 12. Asadipooya K, Uy EM. Advanced Glycation End Products (AGEs), Receptor for AGEs, diabetes, and bone: review of the literature. J Endocr Soc. 2019;3(10):1799-818. doi: 10.1210/js.2019-00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuefner MS, Pham K, Redd JR, Stephenson EJ, Harvey I, Deng X, et al. Secretory phospholipase A2 group IIA modulates insulin sensitivity and metabolism. J Lipid Res. 2017;58(9):1822-33. doi: 10.1194/jlr.M076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orfanidou T, Iliopoulos D, Malizos KN, Tsezou A. Involvement of SOX-9 and FGF-23 in RUNX-2 regulation in osteoarthritic chondrocytes. J Cell Mol Med. 2009;13(9B):3186-94. doi: 10.1111/j.1582-4934.2009.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhai G, Randell EW, Rahman P. Metabolomics of osteoarthritis: emerging novel markers and their potential clinical utility. Rheumatology. 2018;57(12):2087-95. doi: 10.1093/rheumatology/kex497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Senol O, Gundogdu G, Gundogdu K, Miloglu FD. Investigation of the relationships between knee osteoarthritis and obesity via untargeted metabolomics analysis. Clin Rheumatol. 2019;38(5):1351-60. doi: 10.1007/s10067-019-04428-1. [DOI] [PubMed] [Google Scholar]
- 17. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18. Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee. Validation–value in comparison with other assessment tests. Scand J Rheumatol Suppl. 1987;65:85-9. doi: 10.3109/03009748709102182. [DOI] [PubMed] [Google Scholar]
- 19. Durandet A, Ricci P-L, Saveh AH, Vanat Q, Wang B, Esat I, et al. Radiographic analysis of lower limb axial alignments, 2013. [Google Scholar]
- 20. Schneider CA, Rasband WS, Eliceiri KW. NIH image to imageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breen EJ, Polaskova V, Khan A. Bead-based multiplex immuno-assays for cytokines, chemokines, growth factors and other analytes: median fluorescence intensities versus their derived absolute concentration values for statistical analysis. Cytokine. 2015;71(2):188-98. doi: 10.1016/j.cyto.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 22. Alkan HF, Walter KE, Luengo A, Madreiter-Sokolowski CT, Stryeck S, Lau AN, et al. Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metab. 2018;28(5):706-20.e6. doi: 10.1016/j.cmet.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lefort G, Liaubet L, Canlet C, Tardivel P, Père M-C, Quesnel H, et al. ASICS: an R package for a whole analysis workflow of 1D 1H NMR spectra. Bioinformatics. 2019;35(21):4356-63. doi: 10.1093/bioinformatics/btz248. [DOI] [PubMed] [Google Scholar]
- 24. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/. [Google Scholar]
- 25. Breheny P, Burchett W. Visualization of regression models using Visreg. R J. 2017;9(2):56-71. [Google Scholar]
- 26. Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 27. Kuznetsova A, Brockhoff PB, Christensen RHB. LmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1-26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 28. Buuren S van, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. https://www.jstatsoft.org/v45/i03/. [Google Scholar]
- 29. Gamer M, Lemon J, Singh IFP. Irr: various coefficients of interrater reliability and agreement; 2019. https://CRAN.R-project.org/package=irr.
- 30. Chavent M, Kuentz-Simonet V, Liquet B, Saracco J. ClustOfVar: an r package for the clustering of variables. J of Stat Softw. 2012;50(13):1-16. doi: 10.18637/jss.v050.i13.25317082 [DOI] [Google Scholar]
- 31. Fox J, Weisberg S. An R companion to applied regression. 3rd ed. New York: SAGE; 2019. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. [Google Scholar]
- 32. Breen EJ. Protein multiplexed immunoassay analysis with R. In: Greening DW, Simpson RJ, editors Serum/Plasma Proteomics. Vol 1619. New York: Springer; 2017, p. 495-537. doi: 10.1007/978-1-4939-7057-5_35. [DOI] [PubMed] [Google Scholar]
- 33. Cro S, Morris TP, Kenward MG, Carpenter JR. Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: a practical guide. Stat Med. 2020;39(21):2815-42. doi: 10.1002/sim.8569. [DOI] [PubMed] [Google Scholar]
- 34. Furuhashi M. Fatty acid-binding protein 4 in cardiovascular and metabolic diseases. J Atheroscler Thromb. 2019;26(3):216-32. doi: 10.5551/jat.48710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, Li T, Chiu KY, Wen C, Xu A, Yan CH. FABP4 as a biomarker for knee osteoarthritis. Biomark Med. 2018;12(2):107-18. doi: 10.2217/bmm-2017-0207. [DOI] [PubMed] [Google Scholar]
- 36. Leistad L, Feuerherm A, Faxvaag A, Johansen B. Multiple phospholipase A2 enzymes participate in the inflammatory process in osteoarthritic cartilage. Scand J Rheumatol. 2011;40(4):308-16. doi: 10.3109/03009742.2010.547872. [DOI] [PubMed] [Google Scholar]
- 37. Drinda S, Franke S, Schmidt S, Stoy K, Lehmann T, Wolf G, et al. AGE-RAGE interaction does not explain the clinical improvements after therapeutic fasting in osteoarthritis. Complement Med Res. 2018;25(3):167-72. doi: 10.1159/000486237. [DOI] [PubMed] [Google Scholar]
- 38. Ertürk C, Altay MA, Bilge A, Çelik H. Is there a relationship between serum ox-LDL, oxidative stress, and PON1 in knee osteoarthritis? Clin Rheumatol. 2017;36(12):2775-80. doi: 10.1007/s10067-017-3732-4. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Chua S. Leptin function and regulation. In: Terjung R, editor. Comprehensive physiology. New York: John Wiley; 2017:351-69. doi: 10.1002/cphy.c160041. [DOI] [PubMed] [Google Scholar]
- 40. La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017;98:51-8. doi: 10.1016/j.cyto.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Staikos C, Ververidis A, Drosos G, Manolopoulos VG, Verettas D-A, Tavridou A. The association of adipokine levels in plasma and synovial fluid with the severity of knee osteoarthritis. Rheumatology. 2013;52(6):1077-83. doi: 10.1093/rheumatology/kes422. [DOI] [PubMed] [Google Scholar]
- 42. de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JWJ, Lafeber FPJG, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthr Cartil. 2012;20(8):846-53. doi: 10.1016/j.joca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 43. Calvet J, Orellana C, Giménez NA, Berenguer-Llergo A, Caixàs A, García-Manrique M, et al. Differential involvement of synovial adipokines in pain and physical function in female patients with knee osteoarthritis. A cross-sectional study. Osteoarthr Cartil. 2018;26(2):276-84. doi: 10.1016/j.joca.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 44. Calvet J, Orellana C, Gratacós J, Berenguer-Llergo A, Caixàs A, Chillarón JJ, et al. Synovial fluid adipokines are associated with clinical severity in knee osteoarthritis: a cross-sectional study in female patients with joint effusion. Arthritis Res Ther. 2016;18(1):207. doi: 10.1186/s13075-016-1103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alissa EM, Alzughaibi LS, Marzouki ZM. Relationship between serum resistin, body fat and inflammatory markers in females with clinical knee osteoarthritis. Knee. 2020;27(1):45-50. doi: 10.1016/j.knee.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 46. Song Y, Guan J, Wang H, Ma W, Li F, Xu F, et al. Possible involvement of serum and synovial fluid resistin in knee osteoarthritis: cartilage damage, clinical, and radiological links. J Clin Lab Anal. 2016;30(5):437-43. doi: 10.1002/jcla.21876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bas S, Finckh A, Puskas GJ, Suva D, Hoffmeyer P, Gabay C, et al. Adipokines correlate with pain in lower limb osteoarthritis: different associations in hip and knee. Int Orthop. 2014;38(12):2577-83. doi: 10.1007/s00264-014-2416-9. [DOI] [PubMed] [Google Scholar]
- 48. Wu J, Wang K, Xu J, Ruan G, Zhu Q, Cai J, et al. Associations between serum ghrelin and knee symptoms, joint structures and cartilage or bone biomarkers in patients with knee osteoarthritis. Osteoarthr Cartil. 2017;25(9):1428-35. doi: 10.1016/j.joca.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 49. Mohammed MA, Rady SAK, Mohammed RA, Fadda SMH. Relation of plasma fibroblast growth factor-23 (FGF-23) to radiographic severity in primary knee osteoarthritis patients. EgyptRheumatol. 2018;40(4):261-4. doi: 10.1016/j.ejr.2018.01.007. [DOI] [Google Scholar]
- 50. Zhai G. Alteration of metabolic pathways in osteoarthritis. Metabolites. 2019;9(1). doi: 10.3390/metabo9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035211069251 for The Association of Blood Biomarkers and Body Mass Index in Knee Osteoarthritis: A Cross-Sectional Study by Paul Schadler, Birgit Lohberger, Bettina Thauerer, Martin Faschingbauer, Werner Kullich, Martin Helmut Stradner, Andreas Leithner, Valentin Ritschl, Maisa Omara and Bibiane Steinecker-Frohnwieser in CARTILAGE