Abstract
Objective
Nasal septum cartilage is a hyaline cartilage that provides structural support to the nasal cavity and midface. Currently, information on its cellular and mechanical properties is widely dispersed and has often been inferred from studies conducted on other cartilage types such as the knee. A detailed understanding of nasal cartilage properties is important for several biological, clinical, and engineering disciplines. The objectives of this scoping review are to (1) consolidate actual existing knowledge on nasal cartilage properties and (2) identify gaps of knowledge and research questions requiring future investigations.
Design
This scoping review incorporated articles identified using PROSPERO, Cochrane Library (CDSR and Central), WOS BIOSIS, WOS Core Collection, and ProQuest Dissertations and Theses Global databases. Following the screening process, 86 articles were considered. Articles were categorized into three groups: growth, extracellular matrix, and mechanical properties.
Results
Most articles investigated growth properties followed by extracellular matrix and mechanical properties. NSC cartilage is not uniform. Nasal cartilage growth varies with age and location. Similarly, extracellular matrix composition and mechanical properties are location-specific within the NSC. Moreover, most articles included in the review investigate these properties in isolation and only very few articles demonstrate the interrelationship between multiple cartilage properties.
Conclusions
This scoping review presents a first comprehensive description of research on NSC properties with a focus on NSC growth, extracellular matrix and mechanical properties. It additionally identifies the needs (1) to understand how these various cartilage properties intersect and (2) for more granular, standardized assessment protocols to describe NSC.
Keywords: nasal septum, nasal cartilage composition, cartilage growth, extracellular matrix, mechanical properties
Introduction
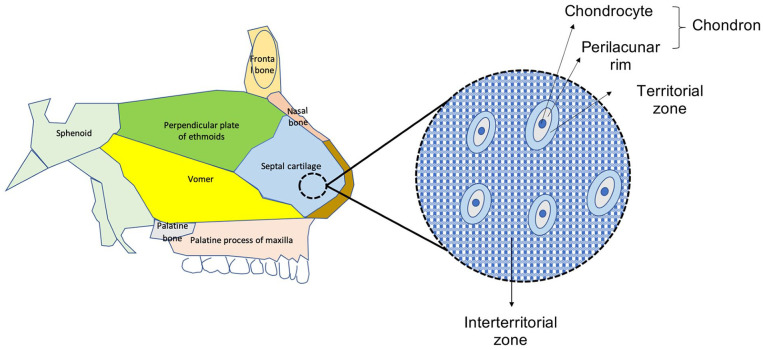
The nasal septum (NS) divides the nasal cavity into two, lending central support to the nasal dorsum. The main components of the NS are the nasal septum cartilage (NSC), the perpendicular plate of the ethmoid (PPE), and the vomer bone ( Fig. 1 ). 1 The thickest part of the NSC is the area connected to the vomer and PPE, which has clinical implications during reconstructive surgery and tissue engineering.1-6 NSC is avascular, devoid of neural and lymphatic supply, and is surrounded by perichondrium and respiratory epithelium. According to its location, it articulates with the frontal bone, nasal bone, cribriform plate, sphenoid bone, maxillary crest, vomer, and palatine bone. Implied from NSC pathologies such as nasal septum deviation, the NSC is thought to play an important role during breathing and mastication, as well as in the cosmetic appearance and growth of the face.7,8 Chondrocytes are the exclusive residents of the NSC. They are organized as units called chondrons and their immediate environment is referred to as the territorial area, while the area in between those areas is named interterritorial area. 9 In addition to the chondrocytes, the extracellular matrix (ECM) of the NSC is composed mainly of Collagen II, Aggrecan and Hyaluronic acid, among other proteins. 10 Interestingly, the origin of the NSC and PPE are the same, a cartilage that at the most posterior aspect undergoes endochondral ossification becoming the PPE, while the most anterior remains cartilaginous and is known as the NSC. 1 The complex composition and organization provide NSC with specific properties, some of them common to other hyaline cartilages, but others exclusive of it, that have been studied over the last decades.
Figure 1.
Anatomy of the nasal septum cartilage.
There is interest from several disciplines in understanding NSC ( Fig. 2 ). Clinically, NS deviation is one of the most frequent deformations resulting in cosmetic and respiratory alterations. Severe cases require surgical intervention to alleviate breathing obstructions and may require implantation of new cartilage. Further NSC is one of the primary and commonly used donor sites for autologous cartilage graft in nasal reconstructive and cosmetic surgery. 11 It is also used for autologous grafting to other locations such as the knee, as it is relatively easy to harvest hyaline cartilage and shows superior tissue stability compared to auricular or costal cartilages. 11 However, its small size and limited tissue availability pose significant limitations, in particular when previous surgical intervention/trauma to the nose has occurred. 11 Several studies have demonstrated that nasal cartilage can serve as a source to replace lost hyaline cartilage in joints.2-6 There is an unmet clinical need to be able to engineer hyaline cartilage that is functionally stable and able to respond to local requirements, such as mechanical compression, lubrication, ability to withstand inflammatory signals. A comprehensive literature review addressing those topics in relation to tissue engineering of the nasal cartilage was recently published 11 and thus they will not be further considered here. In addition to regenerative medicine, several branches of biology are interested in understanding NSC development and its contribution to facial growth as well as its structural properties ( Fig. 2 ). Thus, from various angles, there is interest for an in-depth understanding of NSC properties.
Figure 2.
Understanding nasal cartilage properties will benefit an interdisciplinary team of individuals.
NSC has been a focus of investigations since the 19th century. A breadth of knowledge has accumulated over time, conducted in a variety of species using diverse methodological approaches. Most studies focus primarily on a single property. Studies are often invested in identifying novel capabilities of nasal chondrocytes for cartilage regeneration, but very rarely consider in-depth, the underlying biological properties that contribute to these capabilities. There appears to be an overall lack of understanding of how these properties collectively contribute to form the mature NSC. While for other cartilages, in particular knee cartilage, research has significantly contributed to understanding cartilage biology, it is less clear what has been demonstrated for NSC or has been inferred from studies involving other cartilages. Since most of the effort invested has been sporadic, some attempt to streamline the research objectives is required. Hence, we decided on the format of a scoping review to unify specific knowledge on NSC, potentially resolving controversies and challenges associated with its development and function. This review synthesizes published studies on NSC properties to (1) consolidate the current state of knowledge on extracellular, mechanical, and growth properties, and (2) identify the areas that need further investigation.
Methods
Study Design
A scoping review rather than a systematic or narrative review was used since it is designed to allow unbiased exploration and reporting on a broader research topic. Arksey and O’Malley’s 12 five-stage methodological framework was used to conduct this scoping review. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-R) checklist was used to provide the reader with a review outline ( Table 1 ) as well as demonstrate methodological transparency and reproducibility.
Table 1.
Preferred Reporting Items for Systematic Review and Meta Analyses Statement (PRISMA-S) Checklist.
| Section/Topic | # | Checklist Item | Location(s) Reported |
|---|---|---|---|
| INFORMATION SOURCES AND METHODS | |||
| Database name | 1 | Name each individual database searched, stating the platform for each. | Methods |
| Multi-database searching | 2 | If databases were searched simultaneously on a single platform, state the name of the platform, listing all of the databases searched. (Cochrane Library (Reviews and Trials) | Methods |
| Study registries | 3 | List any study registries searched. | Methods |
| Online resources and browsing | 4 | Describe any online or print source purposefully searched or browsed (e.g., tables of contents, print conference proceedings, web sites), and how this was done. | N/A |
| Citation searching | 5 | Indicate whether cited references or citing references were examined, and describe any methods used for locating cited/citing references (e.g., browsing reference lists, using a citation index, setting up email alerts for references citing included studies). | N/A |
| Contacts | 6 | Indicate whether additional studies or data were sought by contacting authors, experts, manufacturers, or others. | N/A |
| Other methods | 7 | Describe any additional information sources or search methods used. | N/A |
| SEARCH STRATEGIES | |||
| Full search strategies | 8 | Include the search strategies for each database and information source, copied and pasted exactly as run. | Supplemental Appendix 1 |
| Limits and restrictions | 9 | Specify that no limits were used, or describe any limits or restrictions applied to a search (e.g., date or time period, language, study design) and provide justification for their use. | Methods |
| Search filters | 10 | Indicate whether published search filters were used (as originally designed or modified), and if so, cite the filter(s) used. | N/A |
| Prior work | 11 | Indicate when search strategies from other literature reviews were adapted or reused for a substantive part or all of the search, citing the previous review(s). | N/A |
| Updates | 12 | Report the methods used to update the search(es) (e.g., rerunning searches, email alerts). | Methods |
| Dates of searches | 13 | For each search strategy, provide the date when the last search occurred. | Supplemental Appendix 1 |
| PEER REVIEW | |||
| Peer review | 14 | Describe any search peer review process. | N/A |
| MANAGING RECORDS | |||
| Total Records | 15 | Document the total number of records identified from each database and other information sources. | Supplemental Appendix 1 |
| Deduplication | 16 | Describe the processes and any software used to deduplicate records from multiple database searches and other information sources. | Methods |
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, PRISMA-S Group. Last updated February 27, 2020.
Stage 1: Identifying the Research Questions
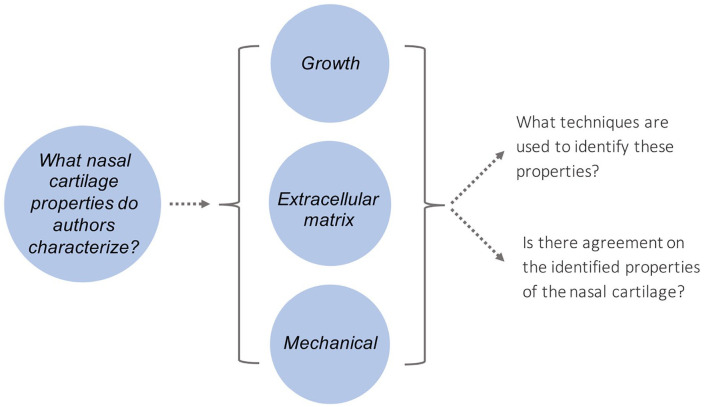
This study reviewed published literature from various databases as outlined in Supplemental Appendix 1 to demonstrate the existing knowledge on NSC. We focussed on what type of properties of the nasal cartilage do authors characterize.
Based on this, we developed the search strategy, organization, and analysis of the scoping review findings. The NSC properties identified from the extracted data were categorized into three groups to assess the state of research conducted on each property ( Fig. 3 ).
Figure 3.
Conceptual framework of the scoping review.
Stage 2: Identifying Relevant Studies—Search Strategy
A search was executed by an expert searcher/health librarian (SC) on the following databases: PROSPERO, Cochrane Library (CDSR and Central), WOS BIOSIS, WOS Core Collection, and ProQuest Dissertations and Theses Global using controlled vocabulary and text words representing the concepts “nasal cartilage” and “biological or physical properties.” No limits or search filters were applied. Databases were searched from inception to April 2021 and were updated July 19, 2021. Detailed search strategies are available in Supplemental Appendix 1.
Stage 3: Study Selection
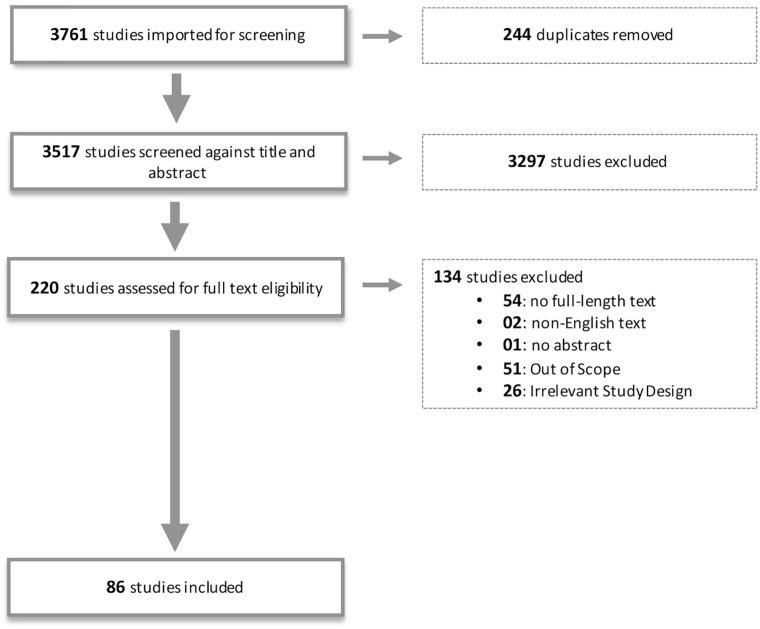
An extended PRISMA-R approach was used to report on the findings ( Fig. 4 ). Articles from the above-mentioned databases and search terms identified 3761 articles that were exported to COVIDENCE review management software, where 244 duplicates were removed. A moderate interrater agreement was established using Cohen’s kappa. The inclusion and exclusion criteria outlined in Figure 4 and Table 2 yielded 86 articles which were thus assessed in this review.
Figure 4.
Preferred Reporting Items for Systematic Review and Meta Analyses (PRISMA) flow chart outlining the scoping review process.
Table 2.
Inclusion and Exclusion Criteria Used in the Review.
| Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Language | English or English translated articles | Non-English articles with no English translation |
| Year of Publication | All articles published | None |
| Peer-reviewed | Yes | None |
| Study Design | Original Research, In vivo and In vitro research, Literature reviews, Human, rodent, rabbit and pig studies |
Tissue engineering using nasal chondrocytes Surgical case studies Nasal cartilage pathologies |
Stage 4: Extracting and Charting Results
The identified articles were reviewed and charted by two evaluators using duplicate screening verification (PB and FB). A consensus-based discussion was performed to resolve inconsistencies and disagreements. The articles were organized by author, title, year, country, type of article and central theme of the article. The 86 articles selected for this review were published between 1963 and 2021. The geographic breakdown revealed that 41.86% (36/86) originated from the United States, 6.98% (6/86) from Canada, 5.81% (5/86) from Germany, 4.65% (4/86) from the United Kingdom, 4.65% (4/86) from Turkey, 5.81% (5/86) from South Korea, 4.65% (4/86) from the Netherlands, 3.49% (3/86) from Japan, 3.49% (3/86) from France, 3.49% (3/86) from Switzerland, 2.32 % (2/86) from Greece, 2.32% (2/86) from Iran, 2.32 % (2/86) from Denmark, and 1.16% (1/86) article each from Australia, Austria, Hong Kong, Hungary, Norway, Portugal, and Sweden.
Stage 5: Reporting Results
The findings from the 86 articles were classified based on 3 groups identified in Figure 3 . The three groups included:
Growth
ECM and collagen composition
Mechanical properties
Each of the common themes was surveyed based on the research question identified in stage 1.
Availability of Data
The data used in the review are available upon request from the corresponding author.
Results
Overview
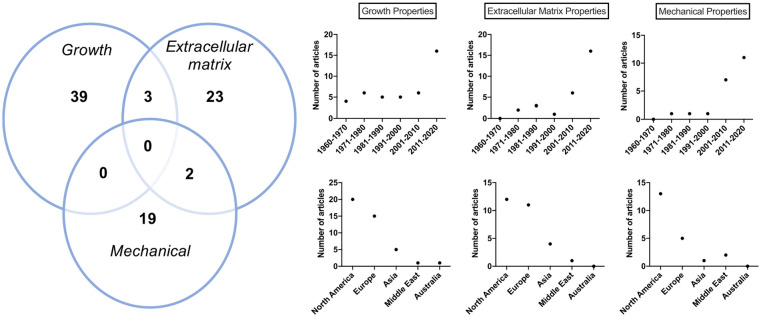
The 86 articles in the review were categorized based on the 3 groups of NSC properties. Most research articles discussed the growth properties of NSC (45.34%, 39/86)8,13-50 followed by articles on ECM (26.74%, 23/86)9,10,51-71 and mechanical properties (22.09%, 19/86)7,9,72-82,83(p20),84-89 ( Fig. 5 ). Only 3.49% of articles (3/86) addressed both cartilage growth and ECM properties,90-92 while 2.32% (2/86) discussed ECM in connection with mechanical properties of the NSC.93,94 No articles investigating growth in conjunction with mechanical properties were identified. None of the articles incorporated all three aspects (growth, ECM, and mechanical) of the NSC. Table 3 lists the articles characterized as studies investigating NSC growth properties, whereas Tables 4 and 5 list ECM, and mechanical properties, respectively. Table 6 lists articles that discussed more than one property.
Figure 5.
Descriptive information of articles categorized into three nasal cartilage categories. (Left panel) 39 articles discussed growth properties of the nasal septum cartilage while 23 commented on extracellular matrix properties and 19 articles assessed mechanical properties. (Right panel) Articles categorized based on the 3e cartilage properties and graphed as a function of time and geographical location. Note articles published in 2021 were added to 2011-2020.
Table 3.
Articles That Investigated Growth Properties of the Nasal Cartilage.
| Author | Year | Title | Country |
|---|---|---|---|
| Al Dayeh and Herring | 2014 | Cellular proliferation in the nasal septal cartilage of juvenile minipigs | USA |
| Al Dayeh et al. | 2013 | Real-time monitoring of the growth of the nasal septal cartilage and the nasofrontal suture | USA |
| Amano et al. | 2020 | Indian hedgehog in craniofacial neural crest cells links to skeletal malocclusion by regulating associated cartilage formation and gene expression | Japan |
| Ashique et al. | 2002 | Signaling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo | Canada |
| Babula et al. | 1970 | Role of cartilaginous nasal septum in midfacial growth | USA |
| Bruintjes et al. | 1996 | Review of the functional anatomy of the cartilages and muscles of the nose | Netherlands |
| Burdi | 1965 | Sagittal growth of the nasomaxillary complex during the second trimester of human prenatal development | USA |
| Carnevale and Tatum | 1996 | Chondrocyte volume fraction distribution in porcine septal cartilage: An initial stereoscopic evaluation | USA |
| Catala and Johnston | 1980 | Interstitial growth of septal cartilage in the young albino-rat | USA |
| Copray | 1986 | Growth of the nasal septal cartilage of the rat in vitro | Netherlands |
| Daultrey et al. | 2018 | The Caucasian Nasal Septum: An In Vivo Computed Tomography Study | UK |
| Delaire and Precious | 1987 | Interaction of the Development of the Nasal Septum the Nasal Pyramid and the Face | France |
| Diewert | 1980 | Differential Changes in Cartilage Cell Proliferation and Cell Density in the Rat Cranio Facial Complex during Secondary Palate Development | Canada |
| Elsaesser et al. | 2016 | Characterization of a migrative subpopulation of adult human nasoseptal chondrocytes with progenitor cell features and their potential for in vivo cartilage regeneration strategies | Germany |
| Adegani et al. | 2013 | A comparison of pluripotency and differentiation status of four mesenchymal adult stem cells | Iran |
| Foster and Holton | 2016 | Variation in the Developmental and Morphological Interaction Between the Nasal Septum and Facial Skeleton | USA |
| Goergen et al. | 2017 | Morphological interaction between the nasal septum and nasofacial skeleton during human ontogeny | USA |
| Granstrom and Magnusson | 1992 | Histochemical analysis of enzymes involved in the formation and metabolism of the nasal septal cartilage | Sweden |
| Grymer and Bosch | 1997 | The nasal septum and the development of the midface. A longitudinal study of a pair of monozygotic twins | Denmark |
| Hall and Precious | 2013 | Cleft lip, nose, and palate: the nasal septum as the pacemaker for midfacial growth | Canada |
| Holton et al. | 2011 | Nasal Septal and Premaxillary Developmental Integration: Implications for Facial Reduction in Homo | USA |
| Holton et al. | 2012 | Nasal septal and craniofacial form in European- and African-derived populations | USA |
| Howe et al. | 2004 | The growth of the nasal septum in the 6-9 week period of fetal development—Warfarin embryopathy offers a new insight into prenatal facial development | Australia |
| Hwang et al. | 2010 | Mapping Thickness of Nasal Septal Cartilage | Korea |
| Kaucka et al. | 2018 | Signals from the brain and olfactory epithelium control shaping of the mammalian nasal capsule cartilage | Austria |
| Kim et al. | 2008 | Analysis of the Development of the Nasal Septum according to Age and Gender Using MRI | Korea |
| Kim et al. | 2010 | Anatomical Variation of the Nasal Septum: Correlation Among Septal Components | Korea |
| Kvinnsland | 1974 | Partial resection of cartilaginous nasal-septum in rats—its influence on growth | Norway |
| Long et al. | 1968 | Regional Variations in Chondrocyte Proliferation in the Cartilaginous Nasal Septum of the Growing Rabbit | USA |
| Marulanda et al. | 2017 | Matrix Gla protein deficiency impairs nasal growth, causing midface hypoplasia | Canada |
| Melsen | 1976 | Histological Analysis of the Post Natal Development of the Nasal Septum | Denmark |
| Pavlov et al. | 2003 | Chondrogenic differentiation during midfacial development in the mouse: In vivo and in vitro studies. | France |
| Searles | 1977 | A Radioautographic Study of Chondrocytic Proliferation in Nasal Septal Cartilage of the New Born Rat | USA |
| Seltzer | 1963 | The problem of the nasal septum | USA |
| Van Loosen et al. | 1988 | The Nasal Septal Cartilage in the Newborn | Netherlands |
| Van Loosen et al. | 1997 | Nasal cartilage maturation assessed by automated computer-assisted image analysis | UK |
| Vetter et al. | 1983 | Growth-activity in human septal cartilage—age dependent incorporation of labeled sulfate in different anatomic locations | Germany |
| Vetter et al. | 1984 | Postnatal-growth of the human septal cartilage—preliminary report | Germany |
| Vidic et al. | 1972 | The Structure and Pre Natal Morphogenesis of the Nasal Septum in the Rat | USA |
Table 4.
Articles That Investigated Extracellular Matrix Properties of the Nasal Cartilage.
| Author | Year | Title | Country |
|---|---|---|---|
| Aksoy et al. | 2011 | Structural characteristics of septal cartilage and mucoperichondrium | Turkey |
| Ayad and Weiss | 1984 | A New Look at Vitreous Humor Collagen | UK |
| Boukla | 1990 | Purification and Properties of Bovine Nasal Hyaline Cartilage Collagenase | Greece |
| Evans et al. | 1983 | Localization of Collagen Types and Fibronectin in Cartilage by Immuno Fluorescence | UK |
| Glant et al. | 1977 | The Localization of Proteo Glycans and Glyco Proteins in the Hyaline Cartilage | Hungary |
| Holden et al. | 2008 | Human Nasal Cartilage Ultrastructure: Characteristics and Comparison Using Scanning Electron Microscopy | USA |
| Kim et al. | 2018 | Characteristics of Nasal Septal Cartilage-Derived Progenitor Cells during Prolonged Cultivation | Korea |
| Lee et al. | 2013 | Age-Related Histologic Changes in Human Nasal Cartilage | USA |
| Neuman et al. | 2013 | A compositional analysis of cadaveric human nasal septal cartilage | USA |
| Pelttari et al. | 2017 | Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine | Switzerland |
| Perin et al. | 1978 | Comparative Studies on Human and Bovine Nasal Cartilage Proteo Glycan Complex Components | France |
| Riedler et al. | 2017 | Age-Related Histologic and Biochemical Changes in Auricular and Septal Cartilage | USA |
| Rotter et al. | 2001 | Age dependence of cellular properties of human septal cartilage—Implications for tissue engineering | USA |
| Sasano et al. | 1992 | Distribution of type I collagen, type II collagen and PNA binding glycoconjugates during chondrogenesis of three distinct embryonic cartilages | USA |
| Sasano et al. | 2001 | Gene and protein expressions of type I collagen are regulated tissue-specifically in rat hyaline cartilages in vivo | Japan |
| Shafiee et al. | 2011 | Nasal Septum-Derived Multipotent Progenitors: A Potent Source for Stem Cell-Based Regenerative Medicine | Iran |
| Takahashi et al. | 2012 | Histological study of the nasal septal cartilage in BALB/c-bm/bm mouse which spontaneously induces malocclusion | Japan |
| Theocharis et al. | 2002 | Isolation and characterization of matrix proteoglycans from human nasal cartilage—Compositional and structural comparison between normal and scoliotic tissues | Greece |
| Ustunel et al. | 2003 | Immunohistochemical distribution patterns of collagen type II, chondroitin 4-sulfate, laminin and fibronectin in human nasal septal cartilage | Turkey |
| Wan et al. | 2018 | A six-gene expression toolbox for the glands, epithelium and chondrocytes in the mouse nasal cavity | USA |
| Wiggenhauser et al. | 2018 | The distribution patterns of COMP and matrilin-3 in septal, alar and triangular cartilages of the human nose | Germany |
| Yu et al. | 2017 | Expression of Noggin and Gremlin1 and its implications in fine-tuning BMP activities in mouse cartilage tissues | USA |
| Zhang et al. | 2012 | Expression of dentine sialophosphoprotein in mouse nasal cartilage | USA |
Table 5.
Articles That Investigated Mechanical Properties of the Nasal Cartilage.
| Author | Year | Title | Country |
|---|---|---|---|
| Al Dayeh et al. | 2009 | Deformation of Nasal Septal Cartilage During Mastication | USA |
| Alkan et al. | 2011 | Tensile Characteristics of Costal and Septal Cartilages Used as Graft Materials | Turkey |
| Al Dayeh and Herring | 2014 | Compressive and tensile mechanical properties of the porcine nasal septum | USA |
| Bos et al. | 2018 | Structural and Mechanical Comparison of Human Ear, Alar, and Septal Cartilage | Netherlands |
| Caffrey et al. | 2013 | Flexural Properties of Native and Tissue-Engineered Human Septal Cartilage | USA |
| Colombo et al. | 2013 | Mechanical behavior of bovine nasal cartilage under static and dynamic loading | Switzerland |
| Correro-Shahgaldian et al. | 2016 | Properties and Mechanobiological Behavior of Bovine Nasal Septum Cartilage | Switzerland |
| Flam | 1974 | The Tensile and Flexural Properties of Bovine Nasal Cartilage | USA |
| Grellmann et al. | 2006 | Determination of strength and deformation behavior of human cartilage for the definition of significant parameters | Germany |
| Iwanaga et al. | 2018 | Distribution of the internal nasal branch of the infraorbital nerve to the nasal septum: Application to rhinoplasty | USA |
| Reuther et al. | 2012 | In vivo oxygen tension in human septal cartilage increases with age | USA |
| Richmon et al. | 2006 | Compressive biomechanical properties of human nasal septal cartilage | USA |
| Richmon et al. | 2005 | Tensile biomechanical properties of human nasal septal cartilage | USA |
| Rotter et al. | 2002 | Age-related changes in the composition and mechanical properties of human nasal cartilage | USA |
| Tekke et al. | 2014 | Importance of nasal septal cartilage perichondrium for septum strength mechanics: a cadaveric study | Turkey |
| Tsao and Chuah | 1988 | Development of Bone-Like Substance in Cartilaginous Rat Nasal Septum Under Experimental Conditions | Hong Kong |
| Westreich et al. | 2007 | Defining nasal cartilage elasticity—Biomechanical testing of the tripod theory based on a cantilevered model | USA |
| Xia et al. | 2012 | Anisotropic properties of bovine nasal cartilage | USA |
| Youn et al. | 2000 | Optical and thermal properties of nasal septal cartilage | USA |
Table 6.
Articles That Investigated More Than One Nasal Cartilage Property.
| Author | Year | Title | Country | Cartilage Property |
|---|---|---|---|---|
| Baddam et al. | 2021 | Nasal Septum Deviation as the Consequence of BMP-Controlled Changes to Cartilage Properties | Canada | Growth and Extracellular Matrix Properties |
| Bong-Soo et al. | 2021 | Septal chondrocyte hypertrophy contributes to midface deformity in a mouse model of Apert syndrome | South Korea | Growth and Extracellular Matrix Properties |
| Baddam et al. | 2021 | Histological and molecular characterization of the growing nasal septum in mice | Canada | Growth and Extracellular Matrix Properties |
| Fertuzinhos et al. | 2020 | Thermo-Mechanical Behavior of Human Nasal Cartilage | Portugal | Extracellular Matrix and Mechanical Properties |
| Naumann et al. | 2002 | Immunochemical and mechanical characterization of cartilage subtypes in rabbit | USA | Extracellular Matrix and Mechanical Properties |
Nasal Septum Cartilage Growth
Growth properties were most frequently investigated over the past six decades (42/86), with an exponential increase in studies in the last decade. Most studies were conducted in North America (20) and Europe (15), followed by Asia (5), Middle East (1), and Australia (1) ( Fig. 5 ). 21 articles studied human samples, whereas the rest used various animal models such as rats (7), mice (8), pigs (4), rabbit (1), and chicken (1) ( Fig. 6 ).
Figure 6.
Species used to investigate nasal cartilage properties.
Human studies commented either on NSC development, NSC growth, or the role of NSC in midfacial growth. Most of the studies investigated NSC growth and its role in midfacial growth. Only a few studies addressed the development of the NSC itself. They established that the NSC originates from the ectomesenchyme (neural crest) and first develops during the third month of embryonic development. 41 Its overall shape is influenced both by the vertical growth and the time the ossifying PPE meets the vomer. 23 Cartilage thickness and appearance vary throughout development. The anterior base of the NS and the postero-superior area were described as being the thickest parts while the antero-inferior part above the base is thinnest.32,54,83 The cartilaginous area of the NS decreases with age while the total area remains constant over time.29,34 This reduction was attributed to the ossification of the PPE. Sex differences were observed during NSC development where males have a larger NSC area than females.43,44 An inverse relationship between NSC and age was observed for both sexes.29,45,48 One study investigated size differences between different ethnic populations finding that those of European descent have a larger NS than individuals of African descent. 30
NSC growth was assessed using proliferation and cell density measurements. The NS develops both by appositional and interstitial growth with cell densities decreasing and cell size increasing from birth to adulthood. A rapid increase in cell size was noted during the first two years of life. Much more limited proliferation was noted up to the age of 35 years in the anterior central part of the NS.43,46 However, it is not clear whether this compensates for cell apoptosis. Studies on minipigs also described a high proliferative capacity of the anterior central cartilage, as well as decreasing cellularity with age.14,15 Several studies correlated changes to cellularity with episodic growth of the NSC in humans.8,13,29,43,46 NSC growth is rapid during the first 2 years of life and plateaus by early adulthood (20 years). Data for children and adolescents are very limited. A similar episodic growth pattern was also observed in mice, pigs, and rats, although the timing of the bursts and pauses in growth was variable. Because of this growth potential early in development, investigators considered the NSC as a primary growth center for midfacial growth. Currently unresolved, two contradictory lines of thought persist: where one from Scott et al. proposes that the NSC is a growth center that pushes the midfacial bones downward and forward during the prenatal period of development. In contrast, Moss et al. proposed the functional matrix theory where the NS is a strut, and its growth is passive and merely compensatory to the expansion of the nasal cavity and midfacial bones. Due to the inability to directly test these theories in humans, tests in various animal models have been done/undertaken.30,31,35 Many of those studies were done on rats. For instance, NS was extirpated and the effect on septum height was assessed. Extirpated snouts31,35 showed reduced nasal height without the overall height of the face being affected. Altering the timing of extirpation led to different growth outcomes. None of the experiments resolved the controversy if NS directly drives or only accompanies midfacial growth.
A variety of techniques were used to assess NSC development. Investigations involving humans commonly used computed tomography (CT), magnetic resonance imaging (MRI), and cephalometric analysis. Studies of human cadavers allowed for a detailed description of histological appearance and staging of cartilage maturation adding to the understanding of NSC growth and development. Tissue dissection and histology were predominantly used in studies involving animals. Access to CT imaging was historically limited, however, in the past decade, CT analysis has been more widely used. This disparity in techniques used between human and animal studies has for a considerable time prevented direct data comparison and knowledge translation.
Even though NSC growth is one of the most investigated aspects, gaps, and controversies related to its development remain. Single time-point analysis and investigations at various ages resulted in variable, not directly comparable results. The lack of standardized measures for nasal growth is a direct consequence of the use of diverse methodology. Snout resection studies in animals presented contradictory results and furthered the controversy in determining whether NSC is a critical structure for midfacial growth as these studies used animals of varying sizes and ages. Variation in location of resection added to the divide in findings. To overcome poor understanding of developmental timelines and the controversy regarding the role of nasal growth for midfacial growth, investigations need to utilize multiple methodologies to query cartilage thickness, proliferation, and growth as the NSC develops. This would provide a strong foundation for developing hypotheses related to the role of NSC in midfacial growth. To illustrate this point, the two opposing theories outlining the role of nasal septum in midfacial growth were in part the result of limited methodology and understanding of NS growth at the time these experiments were performed. Thus, there is a need to conduct age-matched standardized experiments to resolve this controversy.
ECM and Collagen Composition of Nasal Septum Cartilage
Of the 86 included studies, 28 focused on ECM components of the NSC (Table 4). Again, most were carried out in North America (12) and Europe (11), with a few coming from Asia (4) and the Middle East (1) (Fig. 5). Almost half of the papers (10) came from the USA alone. More than half of the studies were published within the last ten years (16) with 21.43% published between 2001 and 2010 (6), 10.71% in the period from 1981 to 1990, and 7.14% in the 1970s (Fig. 5). Half of the studies used human samples, while the other half used different various animal models, specifically mice (8), bovine (5), rats (1), and rabbits (1) (Fig. 6).
A variety of methods were employed for studying the ECM, in particular basic histological techniques as well as immunohistochemistry/immunofluorescence. The overarching aim was to describe ECM components and their relative location throughout the NSC. In the last couple of decades, in-situ hybridization and various biochemical assays were employed to support traditional histology.42,44 More recently, quantitative data from micro-CT, RT-qPCR, chromatography, and biochemical assays for proteoglycan and glycosaminoglycan content were more frequently used.
Most studies were descriptive and identified the ECM as an irregularly organized network of fine filaments and granules formed of collagen and non-collagen components.10,54 In particular, collagen fibers fill the interstitial space of the network.54,60,70 Its composition depends on the site of origin within the cartilage likely reflecting local, functional, and physiological demands on the cartilage. 90 The predominant collagen is Collagen II (85%–90%) which provides tensile strength and, in a lesser proportion, other types of collagens (Collagen I, VI, IX, X, and XI).9,52,68 There is controversy on the presence of Collagen V.9,65,66,94 Collagen II is found throughout the entire cartilage while Collagens I and III are restricted around chondrocytes (territorial zone).9,94 Given the variety of collagens, their different localization within the matrix with some of them difficult to recognize due to masking by proteoglycans (PG), hyaluronic acid digest is commonly used to expose the minor protein components located in the center of the cartilage. 10 Their specific distribution has been described from embryonic development onwards. At early stages, Collagen I is found predominantly within the precursor blastema of the NSC. 62 Following a transition phase with the expression of both Collagen I and II, Collagen II becomes dominant throughout the cartilage while Collagen I is predominantly found on the periphery. 62
The non-collagenous protein fraction consists mainly of proteins such as Laminin, glycoproteins (GP) such as Fibronectin, and proteoglycans (PG) such as aggrecan.51,65,66 There is a high content of glycosaminoglycans (GAG; Hyaluronic acid, chondroitin sulfate, heparan sulfate, and keratan sulfate), essential for maintaining an adequate level of water in the structure. 66 PG seems to be arranged in two levels: a PG-aggregate and a PG-collagen. PG-aggregate connects the core protein and link proteins (glycoproteins), while the PG-collagen binds the core proteins to the surrounding collagen. In the same way, there is an interaction between link proteins and collagen fibers.10,55 We identified only one study that compared PG content from human and bovine cartilage. 59 Although the study demonstrated some differences such as the chondroitin sulfate/keratan sulfate content, PG properties, and link protein distribution, it did not discuss their functional relevance to the development/function of the NSC. The most abundant PG is Aggrecan, which is entrapped within Collagen II providing resistance against compressive forces. 65 A decrease in the amount of PGs with age was reported. It is thought that this does not compromise adult NSC as a source of cells for tissue engineering. 55
Fibronectin, an essential link protein, is distributed through the interterritorial matrix (in between territorial areas that surround chondrocytes), but it was also found within the territorial area as well as connecting units formed by a single chondrocyte (chondron) and its immediate surroundings (Fig. 1). These macromolecules are thought to play an important role in both cell-matrix adhesion and matrix-matrix cohesion in the territorial area. 55
Regarding GAG content, chondroitin sulfate is predominant, followed by hyaluronic acid, keratan sulfate, and in minor proportion dermatan sulfate. A special role has been attributed to hyaluronic acid, which binds Aggrecan and link proteins. Interestingly, the GAG composition of healthy human NSC differs from scoliotic NSC, lending support to the idea that NSC composition and organization are connected to the ability to withstand functional stresses.65,66
Several reports refer to a differential and specific distribution of collagen and non-collagen components from the center of the cartilage to the periphery, as well as in the anteroposterior direction.51,62 This specific pattern could hold the clue to the particular mechanical properties that differentiate the NSC from other hyaline cartilage such as the knee articular cartilage.
The detailed description of ECM characteristics is technically challenging. Results, as in any other field, are technique sensitive and this might be the origin of some of the discrepancies noted. For example, separating collagens from the non-collagen portion is difficult and this likely affects the visualization of the cartilage composition. Few studies compare cartilages from different species, making it difficult to translate findings from non-human studies to humans, in particular since the shape and size of the NSC determine functional abilities. Although there is a high agreement between studies concerning cartilage composition, most studies are qualitative and merely descriptive. Samples are from different locations and ages, making direct comparison difficult. More detailed and comprehensive studies are required to fully understand the functional importance of the ECM organization and disposition in the NSC.
Mechanical Properties of the Nasal Cartilage
Nineteen of the 86 studies included in the review investigated mechanical properties of the NSC (Table 5). Most of the investigations were conducted in North America (13) followed by Europe (5), Middle East (2) and Asia (1) (Fig. 5). Most of the studies (85.71%) were published in the two last decades. An equal number of human (9/21) and animal (9/21) studies were conducted (Fig. 6). Equilibrium modulus, elastic modulus, compression, hydraulic permeability, stress, strain, and tensile strength were measured in the NSC.
Studies measuring the equilibrium modulus82,88,95 observed a decrease in modulus with increasing donor age. The most prominent decrease in equilibrium occurred around 25-35 years of age.82,88 This decrease in equilibrium was accompanied by a decrease in glycosaminoglycan content. 82 No differences between the sexes were observed. Hydraulic permeability measurements assessing flow resistance and collagen content based on hydroxyproline levels demonstrated a decrease in flow resistance and collagen content with age. 82 Studies comparing nasal cartilage to various articular (knee) and elastic (ear) cartilages revealed that NSC is more permeable than those other cartilages. 75 Similarly, a decrease in elastic modulus was noted with age, which was attributed to NSC remodeling becoming more fibrous with age.77,78,82,85 One study calculated the contribution of the perichondrium that lines the NSC to elastic modulus and deflection strength. 83 NSC with undisturbed perichondrium is 18% more effective in preserving shape. However, these results might be treated with caution, as they were based on a small sample size, no statistical analysis was performed, and ECM quality might have been compromised due to the use of human cadaver material. Proteoglycans are expected to be important for those measurements have a short half-life after isolation.
Between several studies, stress, strain, compression, and tensile measurements on the NSC displayed contradictory results. One study reported that mechanical properties of the NSC were comparable to other load-bearing cartilages. 78 Compression studies revealed that the septo-ethmoidal junction rather than the septum itself was under anteroposterior compression caused by occlusal loading. 7 The NS was found to absorb energy and forces transmitted through the skull. One study commented that the NS is overall stiff, capable to move only at the vomer. 7 Variable stiffness based on orientation was described. A higher stiffness was observed in the caudal-cephalic and vertical orientation than in the horizontal plane. Stress relaxation post compression measurements aligned with the heterogeneity of stiffness observed along the NS. In general, when studying different locations along the NSC height, the central part demonstrated greater tissue relaxation potentially due to reduced stiffness in that region.77,84 Studies on tensile strength of the NSC were limited, with one study comparing nasal and articular cartilages, concluding that NSC has weaker tensile strength than articular cartilage. 88 Another study compared compression and tensile properties and concluded that the NSC was more under the influence of compression rather than tension. 74
The methodologies used to investigate mechanical properties of the NS are diverse, making it challenging to compare different studies involving assessments of the same properties directly. There is a need to develop more universally applicable measurement systems that ideally can be used irrespective of species tested. The use of small sample sizes, performing measurements on isolated tissues rather than on intact tissues, and age differences could all contribute to the incongruence in findings. This makes translation of findings across species challenging. In particular, it remains unclear to what degree mechanical properties of the NSC are heterogeneous and depend on location within the NS.
Articles Addressing Multiple Properties of the NSC
Only 5/86 papers reported on more than one property of the NSC (Table 6). Two of these papers reported on ECM and mechanical properties while the three papers reported on growth and ECM properties. The studies on ECM and mechanical properties demonstrated that the expression of collagen V and collagen X in NSC makes it stiffer than epiglottal and articular cartilage. 94 In contrast, collagen VI was only found in elastic and articular cartilage and not the NSC. Thus, expression of various collagens appears to be associated with diverse cartilage properties. The distribution of collagens might be also important because, for instance, expression of Collagen X in the NSC is pericellular, whereas in articular cartilage it is distributed throughout the matrix. 94 Of the three studies integrating growth and ECM properties, one reiterates that NS is a heterogeneous structure with a dynamic growth rate that involves bursts and pauses of growth during postnatal development of NSC. 90 This investigation although in mice, confirms that during normal NSC development, cell density decreases, and cell size increases over time as previously described in other rodent and human samples. Moreover, this study also demonstrates that the NS matures through chondrocyte hypertrophy in an anterior-posterior and dorsal-ventral direction over time. However, two studies investigated alterations to NSC growth and ECM properties during nasal septum deviation.91,92 In both studies septum deviation was associated with reduced midfacial growth and an increase in proteins commonly associated with hypertrophic chondrocytes such as Collagen X was observed, suggesting that alterations to chondrocyte properties might be directly associated with nasal septum deviation.
In conclusion, although attempts have been made to integrate multiple cartilage properties and delineate the functional relationship between them, they ultimately still describe the properties in isolation. They fail to demonstrate how differences in these properties functionally act together and contribute to the development of the NSC.
Discussion
Investigations to define NSC properties have been performed for many decades. Here, we present the first scoping review summarizing accumulated knowledge on these properties. The most prominent aspects investigated are growth, ECM, and mechanical properties; hence, this review is structured to reflect this.
Studies assessing growth properties revealed that the timeline of growth is variable. Existing data suggest trajectories of growth bursts and pauses during development in several species. Variability in the timeline of growth stems from diverse ages of human and animal samples used. Investigations across different species also demonstrated that cell density decreases and cell size increases with age in the NSC. If and to what degree, the NSC directly contributes to midfacial growth remains controversial. Follow-up studies to validate or diffuse Scott et al.’s functional matrix theory 31 using rats 35 remained inconclusive. The influence of genetics on NSC growth and differentiation has not been widely explored. We identified only one study on twins that assessed how nasal trauma affects septum growth. 28 Extrapolating from these results it appears that NSC growth is governed by significant genetic predisposition.
Understanding how ECM properties contribute or define NSC properties has been a long-standing interest of the research field. ECM properties have consistently been characterized using classic histological methods and more recently from advanced quantitative methodologies such as proteomics. Comparing expression patterns of ECM markers across species is relatively easier. Studies have demonstrated that the NSC shows a dynamic expression of collagens and proteoglycans during development. Collagens I and II are most extensively studied showing that both Collagens I and II are present at early stages of NSC development with Collagen I expression subsiding as the NSC matures. Conversely, Collagen II expression increases as the NSC differentiates. Differential staining of proteoglycans like Aggrecans was also observed during the development of NSC. Despite the detailed qualitative description, functional significance of differences in these ECM markers remains unclear. Furthermore, description of expression patterns of ECM components is often limited to one specific location and point in time or comparison to other cartilage types. This makes it challenging to appreciate how changes in ECM components contribute to NSC development and maturation. There are age- and region-specific changes to chondrocyte density and size. It is fair to assume that similar differences also exist in relation to ECM components. Surprisingly, only 2/86 studies have investigated age-specific changes to ECM while only 1/86 studies explored both age and region-specific changes to expression of ECM markers. Understanding age and region-specific expression patterns of ECM components become important when comparing native nasal cartilage against tissue-engineered cartilage.
Mechanical properties of the NSC were only relatively recently investigated. To date, in-depth studies considering the age and location of the NSC are lacking. Most studies were conducted at a single time point and location. Given the heterogeneous structure of NSC, it is fair to assume that location-dependent mechanical properties may exist. Moving forward, investigations considering age and location when assessing mechanical properties should clarify if, and how, local variations in properties contribute to the physiological function of the NSC.
Gaps of Knowledge
NSC properties are not only defined by growth/maturation, ECM, and mechanical properties. Little is known about the roles nerves, vasculature, and perichondrium play in the formation and maintenance of healthy NSC. If, and how, the cartilage remodels throughout life is also unknown. While compiling articles for this review it became obvious that significant literature exists for each of the three selected aspects of NSC. However, information on how the three properties intersect remains comparatively scarce. Most studies addressed a single property in isolation, contributing to the data heterogeneity.
Although there is sufficient evidence showing that NSC grows rapidly during early stages of development, the time point when this growth stabilizes remains poorly defined. The mechanisms underlying growth stabilization similarly remain unexplored. Several articles have demonstrated that cell density and cell size are inversely related during NSC development. What regulates the switch between a decrease in cell density and an increase in cell size and how this contributes to NSC growth remains unclear. A better understanding of the growth mechanism would help clinicians with treatment planning for growth-dependent pathologies such as cleft palate or midfacial hypoplasia.
The expression patterns of ECM components in the mature NSC are mostly well established. Detailed information on age and location-specific differences are, however, lacking. Little is known about the ability of the NSC to remodel and adapt. We found only one report investigating cartilage proteinases (Collagenase 1 and 2) in NSC. 53 Outlining expression of collagenases, proteinases and their inhibitors becomes important when correlating cartilage remodeling to NSC growth. A few articles commented on collagen fibril organization, but no interpretation of how collagen fibril organization would contribute to cartilage maturation and properties was provided. Several studies referred to a similar GAG content in NSC as found in various other hyaline cartilages, although they differ in their properties. 78 Why this may be is still unclear. Understanding these nuances is clearly important when trying to delineate how a particular cartilage meets its physiological demands.
To date, mechanical properties of the NSC have been least explored and various gaps of knowledge persist. Firstly, there is no standardized methodology to test different parameters such as tension, compression of NSC. The existing methodologies are rather specific to species, size, and location of the sample. While this is to some degree necessary, developing methodological approaches that have wider applicability is important. Second, some articles have tested equilibrium and elastic modulus of the NSC. It is currently unclear to what degree the elastic modulus varies within given groups (e.g., patient populations). Similarly, NSC from different species depending on biological constraints is expected to differ.
In addition to the above-identified specific gaps of knowledge, we identified two more general research needs:
There is frequently the implicit assumption that NSC properties between different species or different hyaline cartilage-bearing tissues are comparable. Given the structural and functional heterogeneity, it is reasonable to assume that this is not necessarily the case. Thus, there is a need to conduct species- and hyaline cartilage type-specific studies.
Knowledge is still limited regarding the extent and implication of changes to cartilage properties as part of NSC pathologies. Recent studies on nasal septum deviation in the mouse indicate substantial changes to ECM.91,92 Similar studies are yet to be conducted in humans.
Strengths and Limitations
This review to our knowledge provides the first comprehensive description of the state of research on NSC properties. It identifies the need to incorporate already existing knowledge to generate functionally stable cartilage. It underscores the need to establish more granular, standardized assessment protocols to describe NSC. Technological advances will continue to facilitate this.
A limitation of the review is that it was restricted to NSC. It was not the scope of the review to assess all cartilages. It is possible that a better understanding of cartilage properties exists for other cartilages, such as knee articular cartilage, however, it would be challenging to translate this directly to the NSC. Furthermore, the heterogeneity of the studies posed a challenge to coherently integrate existing knowledge of NSC properties. The need for standardization of experiments and methodologies became evident; however, it was not the scope of this review to critically evaluate the various approaches. Although a broad search strategy was applied, a possibility remains that some of the articles were not considered in this review.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035221087696 for Properties of the Nasal Cartilage, from Development to Adulthood: A Scoping Review by Pranidhi Baddam, Francy Bayona-Rodriguez, Sandra M. Campbell, Hamdy El-Hakim and Daniel Graf in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Author Contributions: PB: conceptualization, data curation, writing - original draft preparation, writing - review and editing, visualization, project administration.
FB: data curation, writing - original draft preparation, writing - review and editing, visualization, project administration.
SC: developed and executed search, methodology, data curation, reviewed draft.
HEH: writing - review and editing.
DG: conceptualization, writing - original draft preparation, writing - review and editing, visualization, project administration.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the ressearch, authorship, and/or publication of this article: This work was supported by the American Association of Orthodontics Foundation (AAOF) 2021 B.F. Dewel Memorial Biomedical Research Award and the Geoffrey & Robyn Sperber Craniofacial Biology Research Funds (DG). The authors would also like to acknowledge the support of trainees through NSERC Postgraduate Doctoral Scholarship (PB).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Daniel Graf  https://orcid.org/0000-0003-1163-8117
https://orcid.org/0000-0003-1163-8117
References
- 1. Hur MS, Won HS, Kwak DS, Chung IH, Kim IB. Morphological patterns and variations of the nasal septum components and their clinical implications. J Craniofac Surg. 2016;27(8):2164-7. doi: 10.1097/SCS.0000000000002974. [DOI] [PubMed] [Google Scholar]
- 2. Scotti C, Osmokrovic A, Wolf F, Miot S, Peretti GM, Barbero A, et al. Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1β and low oxygen. Tissue Eng Part A. 2012;18(3-4):362-72. doi: 10.1089/ten.TEA.2011.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Candrian C, Vonwil D, Barbero A, Bonacina E, Miot S, Farhadi J, et al. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum. 2008;58(1):197-208. doi: 10.1002/art.23155. [DOI] [PubMed] [Google Scholar]
- 4. Pušić M, Brezak M, Vukasović Barišić A, Vučković M, Kostešić P, Šećerović A, et al. Morphological and molecular evaluation of the tissue repair following nasal septum biopsy in a sheep model. Cartilage. 2021;13(2 Suppl):521S-9S. doi: 10.1177/19476035211046040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kafienah W, Jakob M, Démarteau O, Frazer A, Barker MD, Martin I, et al. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8(5):817-26. [DOI] [PubMed] [Google Scholar]
- 6. Asnaghi MA, Power L, Barbero A, Haug M, Köppl R, Wendt D, et al. Biomarker signatures of quality for engineering nasal chondrocyte-derived cartilage. Front Bioeng Biotechnol. 2020;8:283. doi: 10.3389/fbioe.2020.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al Dayeh AA, Rafferty KL, Egbert M, Herring SW. Deformation of nasal septal cartilage during mastication. J Morphol. 2009;270(10):1209-18. doi: 10.1002/jmor.10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goergen MJ, Holton NE, Grünheid T. Morphological interaction between the nasal septum and nasofacial skeleton during human ontogeny. J Anat. 2017;230(5):689-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans HB, Ayad S, Abedin MZ, Hopkins S, Morgan K, Walton KW, et al. Localisation of collagen types and fibronectin in cartilage by immunofluorescence. Ann Rheum Dis. 1983;42(5):575-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glant T, Levai G, Hadhazy C. The localization of proteoglycans and glycoproteins in the hyaline cartilage. Histochemistry. 1977;53(4):291-9. doi: 10.1007/BF00509246. [DOI] [PubMed] [Google Scholar]
- 11. Bagher Z, Asgari N, Bozorgmehr P, Kamrava S, Alizadeh R, Seifalian A. Will tissue-engineering strategies bring new hope for the reconstruction of nasal septal cartilage? Curr Stem Cell Res Ther. 2020;15(2):144-54. doi: 10.2174/1574888X14666191212160757. [DOI] [PubMed] [Google Scholar]
- 12. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19-32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 13. Kim J, Cho J, Kim S, Kim B, Lee D. Anatomical variation of the nasal septum: correlation among septal components. Clin Anat. 2010;23(8):945-9. doi: 10.1002/ca.21045. [DOI] [PubMed] [Google Scholar]
- 14. Al Dayeh AA, Herring SW. Cellular proliferation in the nasal septal cartilage of juvenile minipigs. J Anat. 2014;225(6):604-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al Dayeh AA, Rafferty KL, Egbert M, Herring SW. Real-time monitoring of the growth of the nasal septal cartilage and the nasofrontal suture. Am J Orthod Dentofacial Orthop. 2013;143(6):773-83. doi: 10.1016/j.ajodo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 16. Amano K, Okuzaki D, Aikawa T, Kogo M. Indian hedgehog in craniofacial neural crest cells links to skeletal malocclusion by regulating associated cartilage formation and gene expression. FASEB J. 2020;34(5):6791-807. [DOI] [PubMed] [Google Scholar]
- 17. Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int J Dev Biol. 2002;46(2):243-53. [PubMed] [Google Scholar]
- 18. Babula W, Smiley G, Dixon A. The role of the cartilaginous nasal septum in midfacial growth. Am J Orthod. 1970;58(3):250-63. doi: 10.1016/0002-9416(70)90088-6. [DOI] [PubMed] [Google Scholar]
- 19. Bruintjes TD, van Olphen AF, Hillen B. Review of the functional anatomy of the cartilages and muscles of the nose. Rhinology. 1996;34(2):66-74. [PubMed] [Google Scholar]
- 20. BURDI AR. SAGITTAL GROWTH OF THE NASOMAXILLARY COMPLEX DURING THE SECOND TRIMESTER OF HUMAN PRENATAL DEVELOPMENT. J Dent Res. 1965;44:112-25. [DOI] [PubMed] [Google Scholar]
- 21. Copray J. Growth of the nasal septal cartilage of the rat invitro. J Anat. 1986;144:99-111. [PMC free article] [PubMed] [Google Scholar]
- 22. Daultrey C, Hardman J, Anari S. The caucasian nasal septum: an in vivo computed tomography study. Aesthet Surg J. 2018;38(7):717-22. doi: 10.1093/asj/sjx249. [DOI] [PubMed] [Google Scholar]
- 23. Delaire J, Precious D. Interaction of the development of the nasal septum the nasal pyramid and the face. Int J Pediatr Otorhinolaryngol. 1987;12(3):311-26. [DOI] [PubMed] [Google Scholar]
- 24. Diewert VM. Differential changes in cartilage cell proliferation and cell density in the rat cranio facial complex during secondary palate development. Anatomical Record. 1980;198(2):219-28. [DOI] [PubMed] [Google Scholar]
- 25. Elsaesser AF, Schwarz S, Joos H, Koerber L, Brenner RE, Rotter N. Characterization of a migrative subpopulation of adult human nasoseptal chondrocytes with progenitor cell features and their potential for in vivo cartilage regeneration strategies. Cell Biosci. 2016;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adegani FJ, Langroudi L, Arefian E, Shafiee A, Dinarvand P, Soleimani M. A comparison of pluripotency and differentiation status of four mesenchymal adult stem cells. Mol Biol Rep. 2013;40(5):3693-703. [DOI] [PubMed] [Google Scholar]
- 27. Foster A, Holton N. Variation in the developmental and morphological interaction between the nasal septum and facial skeleton. Anat Rec. 2016;299(6):730-40. [DOI] [PubMed] [Google Scholar]
- 28. Grymer LF, Bosch C. The nasal septum and the development of the midface. A longitudinal study of a pair of monozygotic twins. Rhinology. 1997;35(1):6-10. [PubMed] [Google Scholar]
- 29. Hall BK, Precious DS. Cleft lip, nose, and palate: the nasal septum as the pacemaker for midfacial growth. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(4):442-7. [DOI] [PubMed] [Google Scholar]
- 30. Scott JH. The growth of the human face. Proc R Soc Med. 1954;47(2):91-100. doi: 10.1177/003591575404700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moss ML, Bromberg BE, Song IC, Eisenman G. The passive role of nasal septal cartilage in mid-facial growth. Plast Reconstr Surg. 1968;41(6):536-42. doi: 10.1097/00006534-196806000-00004. [DOI] [PubMed] [Google Scholar]
- 32. Hwang K, Huan F, Kim D. Mapping thickness of nasal septal cartilage. J Craniofac Surg. 2010;21(1):243-4. doi: 10.1097/SCS.0b013e3181c5a203. [DOI] [PubMed] [Google Scholar]
- 33. Kaucka M, Petersen J, Tesarova M, Szarowska B, Kastriti ME, Xie M, et al. Signals from the brain and olfactory epithelium control shaping of the mammalian nasal capsule cartilage. Elife. 2018;7:e34465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim I, Lee M, Lee K, Kim H, Chung Y. Analysis of the development of the nasal septum according to age and gender using MRI. Clin Exp Otorhinolaryngol. 2008;1(1):29-34. doi: 10.3342/ceo.2008.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kvinnsland S. Partial resection of cartilaginous nasal-septum in rats—its influence on growth. Angle Orthod. 1974;44(2):135-40. [DOI] [PubMed] [Google Scholar]
- 36. Long R, Greulich RC, Sarnat BG. Regional variations in chondrocyte proliferation in the cartilaginous nasal septum of the growing rabbit. J Dent Res. 1968;47(3):505. [DOI] [PubMed] [Google Scholar]
- 37. Marulanda J, Eimar H, Mckee MD, Berkvens M, Nelea V, Roman H, et al. Matrix Gla protein deficiency impairs nasal septum growth, causing midface hypoplasia. J Biol Chem. 2017;292(27):11400-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melsen B. Histological analysis of the postnatal development of the nasal septum. J Dent Res. 1976;55(Spec Issue B):B296. [DOI] [PubMed] [Google Scholar]
- 39. Pavlov MI, Sautier J, Oboeuf M, Asselin A, Berdal A. Chondrogenic differentiation during midfacial development in the mouse: in vivo and in vitro studies. Biol Cell. 2003;95(2):75-86. [DOI] [PubMed] [Google Scholar]
- 40. Searles JC. A radioautographic study of chondrocytic proliferation in nasal septal cartilage of the newborn rat. Am J Anat. 1977;150(4):659-63. doi: 10.1002/aja.1001500405. [DOI] [PubMed] [Google Scholar]
- 41. Seltzer AP. The problem of the nasal septum. J Albert Einstein Med Cent. 1963;11(4):283-99. [PubMed] [Google Scholar]
- 42. Van Loosen J. The nasal septal cartilage in the newborn. Rhinology. 1988;26(3):161-6. [PubMed] [Google Scholar]
- 43. Vetter U, Pirsig W, Heinze E. Growth-activity in human septal cartilage: age-dependent incorporation of labeled sulfate in different anatomic locations. Plast Reconstr Surg. 1983;71(2):167-71. doi: 10.1097/00006534-198302000-00001. [DOI] [PubMed] [Google Scholar]
- 44. Vetter U, Pirsig W, Heinze E. Postnatal-growth of the human septal cartilage—preliminary report. Acta Otolaryngol. 1984;97(1-2):131-6. doi: 10.3109/00016488409130973. [DOI] [PubMed] [Google Scholar]
- 45. Vidic B, Greditzer HG, Litchy WJ. The structure and pre natal morphogenesis of the nasal septum in the rat. J Morphol. 1972;137(2):131-48. doi: 10.1002/jmor.1051370202. [DOI] [PubMed] [Google Scholar]
- 46. van Loosen J, Yiang J, Howard CV, van Zanten BA, Verwoerd-Verhoef HL, Verwoerd CD, et al. Nasal cartilage maturation assessed by automated computer-assisted image analysis. Adv Otorhinolaryngol. 1997;51:51-60. [DOI] [PubMed] [Google Scholar]
- 47. Holton NE, Yokley TR, Figueroa A. Nasal septal and craniofacial form in European- and African-derived populations. J Anat. 2012;221(3):263-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carnevale G, Tatum S. Chondrocyte volume fraction distribution in porcine septal cartilage: an initial stereoscopic evaluation. Otolaryngol Head Neck Surg. 1996;115(4):365-9. doi: 10.1016/S0194-5998(96)70052-9. [DOI] [PubMed] [Google Scholar]
- 49. Catala A, Johnston LE., Jr. Interstitial growth of septal cartilage in the young albino rat. J Dent Res. 1980;59(8):1453-6. doi: 10.1177/00220345800590081601. [DOI] [PubMed] [Google Scholar]
- 50. Granström G, Magnusson BC. Histochemical analysis of enzymes involved in the formation and metabolism of the nasal septal cartilage. Acta Otolaryngol Suppl. 1992;492:15-21. [DOI] [PubMed] [Google Scholar]
- 51. Aksoy F, Yildirim YS, Demirhan H, Özturan O, Solakoglu S. Structural characteristics of septal cartilage and mucoperichondrium. J Laryngol Otol. 2012;126(1):38-42. [DOI] [PubMed] [Google Scholar]
- 52. Ayad S, Weiss JB. A new look at vitreous humor collagen. Biochem J. 1984;218(3):835-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boukla A. Purification and properties of bovine nasal hyaline cartilage collagenase. Int J Biochem. 1990;22(11):1273-82. [DOI] [PubMed] [Google Scholar]
- 54. Holden P, Liaw L, Wong B. Human nasal cartilage ultrastructure: characteristics and comparison using scanning electron microscopy. Laryngoscope. 2008;118(7):1153-6. doi: 10.1097/MLG.0b013e31816ed5ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim D, Lim J, Kim S, Lee W, Park SH, Kwon MY, et al. Characteristics of nasal septal cartilage-derived progenitor cells during prolonged cultivation. Otolaryngol Head Neck Surg. 2018;159(4):774-82. doi: 10.1177/0194599818777195. [DOI] [PubMed] [Google Scholar]
- 56. Lee J, McHugh J, Kim J, Baker S, Moyer J. Age-related histologic changes in human nasal cartilage. JAMA Facial Plast Surg. 2013;15(4):256-62. doi: 10.1001/jamafacial.2013.825. [DOI] [PubMed] [Google Scholar]
- 57. Neuman M, Briggs K, Masuda K, Sah R, Watson D. A compositional analysis of cadaveric human nasal septal cartilage. Laryngoscope. 2013;123(9):2120-4. doi: 10.1002/lary.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pelttari K, Mumme M, Barbero A, Martin I. Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine. Curr Opin Biotechnol. 2017;47:1-6. [DOI] [PubMed] [Google Scholar]
- 59. Perin JP, Bonnet F, Jolles P. Comparative studies on human and bovine nasal cartilage proteoglycan complex components. Mol Cell Biochem. 1978;21(2):71-82. [DOI] [PubMed] [Google Scholar]
- 60. Riedler K, Shokrani A, Markarian A, Fisher L, Pepper J. Age-related histologic and biochemical changes in auricular and septal cartilage. Laryngoscope. 2017;127(11):E399-407. doi: 10.1002/lary.26807. [DOI] [PubMed] [Google Scholar]
- 61. Rotter N, Bonassar L, Tobias G, Lebl M, Roy A, Vacanti C. Age dependence of cellular properties of human septal cartilage—implications for tissue engineering. Arch Otolaryngol Head Neck Surg. 2001;127(10):1248-52. doi: 10.1001/archotol.127.10.1248. [DOI] [PubMed] [Google Scholar]
- 62. Sasano Y, Mizoguchi I, Kagayama M, Shum L, Bringas P, Jr, Slavkin HC. Distribution of type I collagen, type II collagen and PNA binding glycoconjugates during chondrogenesis of three distinct embryonic cartilages. Anat Embryol. 1992;186(3):205-13. [DOI] [PubMed] [Google Scholar]
- 63. Shafiee A, Kabiri M, Ahmadbeigi N, Yazdani SO, Mojtahed M, Amanpour S, et al. Nasal septum-derived multipotent progenitors: a potent source for stem cell-based regenerative medicine. Stem Cells Dev. 2011;20(12):2077-91. doi: 10.1089/scd.2010.0420. [DOI] [PubMed] [Google Scholar]
- 64. Takahashi K, Kajii T, Tsukamoto Y, Saito F, Wada S, Sugawara-Kato Y, et al. Histological study of the nasal septal cartilage in BALB/c-bm/bm mouse which spontaneously induces malocclusion. Orthod Craniofac Res. 2012;15(2):84-91. doi: 10.1111/j.1601-6343.2011.01538.x. [DOI] [PubMed] [Google Scholar]
- 65. Theocharis A, Karamanos N, Papageorgakopoulou N, Tsiganos C, Theocharis D. Isolation and characterization of matrix proteoglycans from human nasal cartilage. Compositional and structural comparison between normal and scoliotic tissues. Biochim Biophys Acta. 2002;1569(1-3):117-26. doi: 10.1016/S0304-4165(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 66. Ustünel I, Cayli S, Güney K, Celik-Ozenci C, Tanriöver G, Sahin Z, et al. Immunohistochemical distribution patterns of collagen type II, chondroitin 4-sulfate, laminin and fibronectin in human nasal septal cartilage. Acta Histochem. 2003;105(2):109-14. [DOI] [PubMed] [Google Scholar]
- 67. Wan Y, Rogers MB, Szabo-Rogers HL. A six-gene expression toolbox for the glands, epithelium and chondrocytes in the mouse nasal cavity. Gene Expr Patterns. 2018;27:46-55. [DOI] [PubMed] [Google Scholar]
- 68. Wiggenhauser PS, Schwarz S, Rotter N. The distribution patterns of COMP and matrilin-3 in septal, alar and triangular cartilages of the human nose. Histochem Cell Biol. 2018;150(3):291-300. doi: 10.1007/s00418-018-1672-y. [DOI] [PubMed] [Google Scholar]
- 69. Yu X, Kawakami H, Tahara N, Olmer M, Hayashi S, Akiyama R, et al. Expression of Noggin and Gremlin1 and its implications in fine-tuning BMP activities in mouse cartilage tissues. J Orthop Res. 2017;35(8):1671-82. doi: 10.1002/jor.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang R, Chen FM, Zhao SL, Xiao MZ, Smith AJ, Feng JQ. Expression of dentine sialophosphoprotein in mouse nasal cartilage. Arch Oral Biol. 2012;57(6):607-13. doi: 10.1016/j.archoralbio.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 71. Sasano Y, Takahashi I, Zhu JX, Ohtani H, Mizoguchi I, Kagayama M. Gene and protein expressions of type I collagen are regulated tissue-specifically in rat hyaline cartilages in vivo. Eur J Morphol. 2001;39(3):149-54. [DOI] [PubMed] [Google Scholar]
- 72. Reuther M, Briggs K, Schumacher B, Masuda K, Sah R, Watson D. In vivo oxygen tension in human septal cartilage increases with age. Laryngoscope. 2012;122(11):2407-10. doi: 10.1002/lary.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alkan Z, Yigit O, Acioglu E, Bekem A, Azizli E, Kocak I, et al. Tensile characteristics of costal and septal cartilages used as graft materials. Arch Facial Plast Surg. 2011;13(5):322-6. [DOI] [PubMed] [Google Scholar]
- 74. Al Dayeh AA, Herring SW. Compressive and tensile mechanical properties of the porcine nasal septum. J Biomech. 2014;47(1):154-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bos E, Pluemeekers M, Helder M, Kuzmin N, van der Laan K, Groot ML, et al. Structural and mechanical comparison of human ear, alar, and septal cartilage. Plast Reconstr Surg Glob Open. 2018;6(1):e1610. doi: 10.1097/GOX.0000000000001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Caffrey J, Kushnaryov A, Reuther M, Wong VW, Briggs KK, Masuda K, et al. Flexural properties of native and tissue-engineered human septal cartilage. Otolaryngol Head Neck Surg. 2013;148(4):576-81. doi: 10.1177/0194599812474228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Colombo V, Cadova M, Gallo LM. Mechanical behavior of bovine nasal cartilage under static and dynamic loading. J Biomech. 2013;46(13):2137-44. [DOI] [PubMed] [Google Scholar]
- 78. Correro-Shahgaldian M, Introvigne J, Ghayor C, Weber FE, Gallo LM, Colombo V. Properties and mechanobiological behavior of bovine nasal septum cartilage. Ann Biomed Eng. 2016;44(5):1821-31. [DOI] [PubMed] [Google Scholar]
- 79. Flam E. The tensile and flexural properties of bovine nasal cartilage. J Biomed Mater Res. 1974;8(5):277-82. [DOI] [PubMed] [Google Scholar]
- 80. Grellmann W, Berghaus A, Haberland E, Jamali Y, Holweg K, Reincke K, et al. Determination of strength and deformation behavior of human cartilage for the definition of significant parameters. J Biomed Mater Res A. 2006;78(1):168-74. [DOI] [PubMed] [Google Scholar]
- 81. Iwanaga J, Watanabe K, Oskouian R, Tubbs R. Distribution of the internal nasal branch of the infraorbital nerve to the nasal septum: application to rhinoplasty. J Plast Reconstr Aesthet Surg. 2018;71(5):665-9. doi: 10.1016/j.bjps.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 82. Rotter N, Tobias G, Lebl M, Roy AK, Hansen MC, Vacanti CA, et al. Age-related changes in the composition and mechanical properties of human nasal cartilage. Arch Biochem Biophys. 2002;403(1):132-40. [DOI] [PubMed] [Google Scholar]
- 83. Tekke N, Alkan Z, Yigit O, Bekem A, Unal A, Sahin F, et al. Importance of nasal septal cartilage perichondrium for septum strength mechanics: a cadaveric study. Rhinology. 2014;52(2):167-71. doi: 10.4193/Rhino13.199. [DOI] [PubMed] [Google Scholar]
- 84. Tsao SW, Chuah MI. Development of bone-like substance in cartilaginous rat nasal septum under experimental conditions. Anat Rec. 1988;221(4):834-40. doi: 10.1002/ar.1092210407. [DOI] [PubMed] [Google Scholar]
- 85. Westreich R, Courtland H, Nasser P, Jepsen K, Lawson W. Defining nasal cartilage elasticity—biomechanical testing of the tripod theory based on a cantilevered model. Arch Facial Plast Surg. 2007;9(4):264-70. doi: 10.1001/archfaci.9.4.264. [DOI] [PubMed] [Google Scholar]
- 86. Xia Y, Zheng S, Szarko M, Lee J. Anisotropic properties of bovine nasal cartilage. Microsc Res Tech. 2012;75(3):300-6. doi: 10.1002/jemt.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Richmon JD, Sage A, Wong VW, Chen AC, Sah RL, Watson D. Compressive biomechanical properties of human nasal septal cartilage. Am J Rhinol. 2007;21(1):135. [DOI] [PubMed] [Google Scholar]
- 88. Richmon JD, Sage AB, Wong VW, Chen AC, Pan C, Sah RL, et al. Tensile biomechanical properties of human nasal septal cartilage. Am J Rhinol. 2005;19(6):617-22. [PubMed] [Google Scholar]
- 89. Youn J, Telenkov S, Kim E, Bhavaraju NC, Wong BJ, Valvano JW, et al. Optical and thermal properties of nasal septal cartilage. Lasers Surg Med. 2000;27(2):119-28. doi: 10.1002/1096-9101(2000)27:2<119::AID-LSM3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 90. Baddam P, Kung T, Adesida AB, Graf D. Histological and molecular characterization of the growing nasal septum in mice. J Anat. 2021;238(3):751-64. doi: 10.1111/joa.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baddam P, Young D, Dunsmore G, Nie C, Eaton F, Elahi S, et al. Nasal septum deviation as the consequence of BMP-controlled changes to cartilage properties. Front Cell Dev Biol. 2021;9:696545. doi: 10.3389/fcell.2021.696545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim BS, Shin HR, Kim HJ, Yoon H, Cho Y-D, Choi K-Y, et al. Septal chondrocyte hypertrophy contributes to midface deformity in a mouse model of Apert syndrome. Sci Rep. 2021;11(1):7979. doi: 10.1038/s41598-021-87260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fertuzinhos A, Teixeira M, Ferreira M, Fernandes R, Correia R, Malheiro AR, et al. Thermo-mechanical behaviour of human nasal cartilage. Polymers. 2020;12(1):177. doi: 10.3390/polym12010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Naumann A, Dennis JE, Awadallah A, Carrino DA, Mansour JM, Kastenbauer E, et al. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem. 2002;50(8):1049-58. [DOI] [PubMed] [Google Scholar]
- 95. Richmon J, Sage A, Wong V, Chen A, Sah R, Watson D. Compressive biomechanical properties of human nasal septal cartilage. Am J Rhinol. 2006;20(5):496-501. doi: 10.2500/ajr.2006.20.2932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035221087696 for Properties of the Nasal Cartilage, from Development to Adulthood: A Scoping Review by Pranidhi Baddam, Francy Bayona-Rodriguez, Sandra M. Campbell, Hamdy El-Hakim and Daniel Graf in CARTILAGE
Data Availability Statement
The data used in the review are available upon request from the corresponding author.