Abstract
An immunocompetent man in his 40s presented with 3 months of mid-thoracic back pain which progressed to include progressive paraesthesias and lower extremity weakness. Investigations revealed thoracic spine osteomyelitis with signs of cord compression. He underwent neurosurgical intervention, including laminectomy, spinal cord decompression and partial resection of an epidural mass. Initial intraoperative biopsy and surgical pathology results were concerning for an acid-fast bacillus as the causative pathogen, and the patient was given empiric therapy for presumed Mycobacterium tuberculosis. However, microbiology speciation revealed the presence of the non-tuberculous mycobacterium (NTM) Mycobacterium kansasii, which resulted in an alteration of his antimicrobial therapy. This case highlights the importance of considering NTM as a possible aetiology of spinal osteomyelitis, even among immunocompetent individuals or in low-prevalence regions.
Keywords: Bone and joint infections, TB and other respiratory infections
Background
Mycobacterium kansasii is a slow-growing, acid-fast non-tuberculous mycobacterium (NTM) found in abundance in soil, tap water and aquatic environments.1 It is a known human pathogen that causes both limited and invasive infectious syndromes. Typically, invasive infections are limited to patients with immunocompromised states, such as those with HIV or those on chronic immunosuppressive therapies.2 In immunocompetent hosts, M. kansasii most commonly causes pulmonary disease (in over 90% of cases), although infections of the lymph nodes, skin, soft tissue and bones have also been described.3 Vertebral osteomyelitis secondary to M. kansasii is exceedingly rare, with very few documented cases in immunocompetent individuals.2 4 Diagnosis is often difficult secondary to low clinical suspicion in these individuals, in addition to significant clinical overlap with Mycobacterium tuberculosis osteomyelitis.3 Consequently, delays in diagnosis and treatment can result in disease progression and further complications. This is an important consideration in vertebral osteomyelitis cases, given the proximity to the spinal cord.
Case presentation
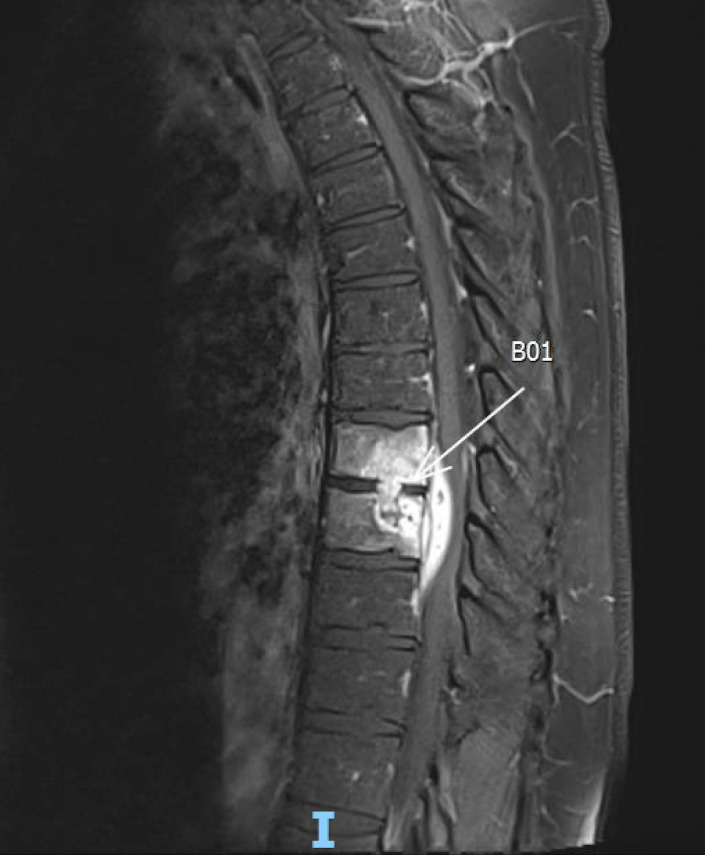
We present a man in his 40s, who presented to his primary care physician with progressively worsening, non-traumatic mid-back pain, which did not improve despite conservative therapies over a 3 month period. Spinal MRI was performed, which showed discitis and osteomyelitis at the T8 and T9 levels with relative preservation of the disk space (figure 1). While awaiting outpatient neurosurgical evaluation, the patient developed worsening pain, bilateral paraesthesias and difficulty walking, so he was admitted for urgent surgical intervention.
Figure 1.
Thoracic spine MRI showing T8-T9 osteomyelitis with relative preservation of the disc space and severe spinal canal stenosis.
The patient had no medical history. He worked as a mason and septic tank servicer. His social history was notable for encounters with domesticated felines, frequent trips to the Central America for leisure, consumption of unpasteurised cow and goat dairy products, and gardening. He spent much of his childhood living in close quarters with his grandmother and great maternal uncle, who were previously diagnosed and treated for M. tuberculosis. There was no history of intravenous drug use or high-risk sexual activity.
On examination, his blood pressure was 164/92 mm Hg, he was afebrile and his other vital signs were within normal limits. General examination showed a healthy-appearing man. There was no palpable lymphadenopathy globally, and evaluation of his back showed no visible abnormalities, although he had significant pain to palpation of his thoracic spine. There was no lower extremity weakness; however, he had bilateral paraesthesia distal to the calves while his lower extremity reflexes were grossly normal.
Investigations
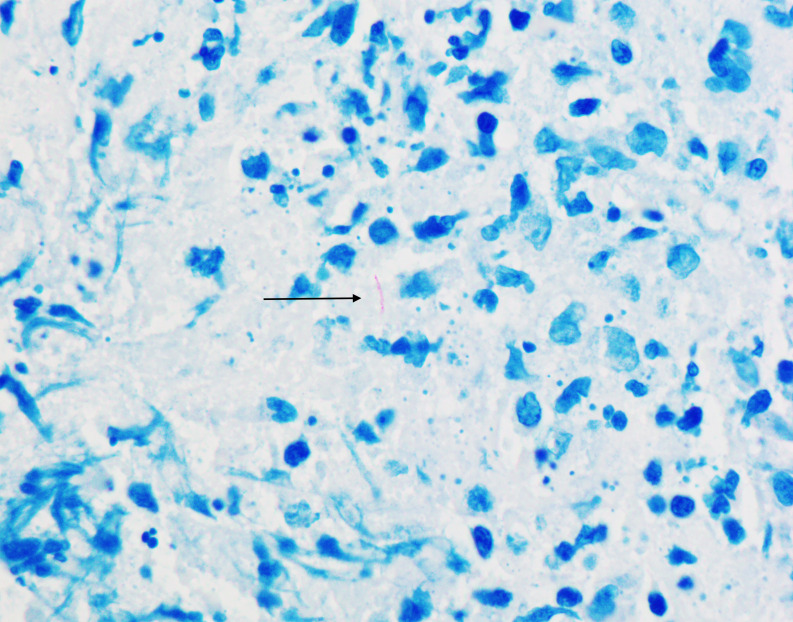
Repeat MRI of the thoracic spine confirmed a severe narrowing of the spinal cord with cord compression and increased signal noted on the cord at these levels. Given the progression of his symptoms and associated MRI findings, he underwent T8-T9 laminectomy with spinal cord decompression. Intraoperatively he was noted to have a T9 epidural mass described as soft, friable and greyish in colour, which was resected. Pathology from the epidural mass was consistent with granulomatous inflammation containing very few, slightly elongated, thick, coarsely beaded acid-fast structures (figure 2). These intraoperative findings raised concern for M. tuberculosis infection. Given his presentation, he was evaluated for HIV coinfection, and this testing was negative. Ultimately his NTM was identified as M. kansasii, not M. tuberculosis almost 40 days after cultures were obtained. CT scan of the chest was negative for a primary pulmonary lesion.
Figure 2.
Mixed inflammatory infiltrate with rare Mycobacterium kansasii (acid-fast stain, ×1000). Organism (indicated by the black arrow) exhibits somewhat beaded appearance and is typically longer and thicker than Mycobacterium tuberculosis.
Treatment
Given his clinical presentation, exposure histories, preliminary surgical pathology and culture results, he was initially treated with empiric rifampin, isoniazid, pyrazinamide and ethambutol (RIPE therapy) for presumed M. tuberculosis osteomyelitis. When the acid-fast bacillus was identified as M. kansasii, his treatment was changed to rifampin, azithromycin and ethambutol. Susceptibility testing confirmed that the M. kansasii was susceptible to macrolides, fluoroquinolones, aminoglycosides and rifamycins.
Outcome and follow-up
One month into therapy with rifampin, ethambutol and azithromycin, he developed moderate leucopenia, so his rifampin was changed to moxifloxacin with subsequent normalisation of his white blood cell count. At 3 month follow-up, his paraesthesias had significantly improved, but his thoracic spinal pain persisted. Repeat thoracic MRI showed stable T8-T9 vertebral body osteomyelitis with associated epidural enhancement and stable postsurgical changes. There was no recurrence of his epidural mass and no evidence of metastatic disease. The decision was made to continue antimicrobial therapy for at least 6 months.
Discussion
M. kansasii is one of the most frequently isolated NTM in the USA and Europe and the second most common NTM secondary to Mycobacterium avium complex.5 6 Although a majority of NTM are isolated from natural water sources or soil, M. kansasii is almost exclusively isolated from municipal water sources.5 The mechanism of transmission is thought to be aerosolisation, with human-to-human transmission being extremely unlikely.6 It shares similar microbiological characteristics with other slow-growing mycobacteria. Although NTM are considered to have relatively low virulence, M. kansasii is thought to be more virulent than the majority of other slow-growing NTM species.7
The risk factors most associated with M. kansasii infection include alcoholism, chronic lung disease, pneumoconiosis, prior mycobacterial disease, malignancy, HIV infection and other immunocompromising conditions.5 Most patients with M. kansasii infections develop pulmonary syndromes identical to M. tuberculosis infections. Studies suggest that extrapulmonary infections occur roughly 0.6%–9.0% of the time.8 9 Musculoskeletal infections are often limited to tendon-sheath infections or septic arthritis in immunocompetent individuals, typically following a history of skin injury, steroid injection or recent surgery.6 Significant immunocompromise in the absence of these events appears to be the greatest risk factor for invasive skeletal disease.10 Vertebral osteomyelitis is rare, with only a few case reports currently in literature, including our patient.11 Our patient was not immunocompromised, though he did encounter municipal water during his work servicing septic tanks.
Signs and symptoms of NTM osteomyelitis are often clinically indistinguishable from those of M. tuberculosis osteoarticular infections and include localised pain, joint stiffness and swelling, fevers, chills, anorexia, malaise and weight loss. The time from symptom onset to diagnosis remains protracted secondary to under-recognition and other disease modalities causing skeletal pain being more prominent.3
Diagnosis of M. kansasii poses a challenge. Clinicians cannot rely on clinical or radiographic tools given the overlapping features with M. tuberculosis and other NTM. As in our patient, initial histopathology often suggests mycobacterial disease, and patients are frequently placed on empiric M. tuberculosis therapy while awaiting culture growth and speciation/identification by phenotypic, molecular and biochemical testing.12 This process can take several days to weeks, resulting in suboptimal treatment regimens. Detailed histological evaluation often shows differences between the two species; M. kansasii appear slightly thicker, more beaded and longer compared with those of M. tuberculosis. Additionally, M. kansasii will produce a yellow pigment when exposed to light, consistent with classification as a photochromogen (Runyon I).13
Treatment regimens for M. kansasii osteomyelitis are not standardised but often include a macrolide, rifampin, ethambutol and isoniazid. In contrast to treatment for M. tuberculosis, pyrazinamide is not recommended given the innate resistance patterns of M. kansasii. Rifampin is a mainstay of therapy; however, it is important to note that some isolates show resistance. Prolonged use of rifampin can be limited by leucopenia, as seen in our patient, in which case substitution with moxifloxacin has been shown to be effective.5 Overall, the prognosis of vertebral osteomyelitis secondary to NTM, including M. kansasii, is poor.14 Consequently, antimicrobial treatment often spans at least 6–12 months. In addition, adjunctive surgery is often needed for debulking infected tissue.7
Learning points.
Mycobacterium kansasii is a slow growing (>7 days), non-tuberculous mycobacterium (NTM) that is a photochromogen (Runyon I) as it produces a yellow pigment when exposed to light. Under light microscopy, the gram-positive rods of M. kansasii appear slightly thicker, more beaded and longer compared with those of Mycobacterium tuberculosis.
M. kansasii causes infections of the lungs; however, it can also cause infection of the skin, musculoskeletal system and lymph nodes.
Infections with M. kansasii are often clinically indistinguishable from M. tuberculosis.
Invasive infections secondary to NTM are uncommon in immunocompetent individuals. The rarity and subacute presentation often lead to delayed diagnosis and suboptimal treatment regimens.
Footnotes
Contributors: MS and MG identified this patient as a valuable case report. TB performed the literature review and wrote initial drafts of the manuscript under the expert guidance of all other authors. MP performed the pathological evaluation of surgical specimens and provided microbiological expertise. All authors were involved in the drafting, editing, formatting and final approval of this case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Turk T, Almahmoud MF, Raslan K. Isolated Mycobacterium kansasii wound infection and Osteomyelitis in an immunocompetent patient. Oxf Med Case Reports 2016;2016:omw087. 10.1093/omcr/omw087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu H, Mizuno Y, Nakamura I, et al. Vertebral osteomyelitis caused by non-tuberculous mycobacteria: case reports and review. J Infect Chemother 2013;19:972–7. 10.1007/s10156-013-0550-8 [DOI] [PubMed] [Google Scholar]
- 3.Piersimoni C, Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis 2009;15:1351–8. 10.3201/eid1509.081259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddadzadeh M. A 14-year-old girl with thoracolumbar pain. Kardiochir Torakochirurgia Pol 2014;11:83–5. 10.5114/kitp.2014.41939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, David Griffith E E, Catanzaro A, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 6.Johnston JC, Chiang L, Elwood K. Mycobacterium kansasii. Microbiol Spectr 2017;5. 10.1128/microbiolspec.TNMI7-0011-2016 [DOI] [PubMed] [Google Scholar]
- 7.Sharma SK, Upadhyay V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J Med Res 2020;152:185–226. 10.4103/ijmr.IJMR_902_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore JE, Kruijshaar ME, Ormerod LP, et al. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995-2006. BMC Public Health 2010;10:612. 10.1186/1471-2458-10-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamden K, Watson JM, Knerer G, et al. Opportunist mycobacteria in England and Wales: 1982 to 1994. Commun Dis Rep CDR Rev 1996;6:R147–51. [PubMed] [Google Scholar]
- 10.Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection 2004;32:257–70. 10.1007/s15010-004-4001-4 [DOI] [PubMed] [Google Scholar]
- 11.Tsai C-W, Wang J-T, Tsai C-C, et al. Disseminated Mycobacterium kansasii infection in an HIV-negative patient presenting with mimicking multiple bone metastases. Diagn Microbiol Infect Dis 2006;54:211–6. 10.1016/j.diagmicrobio.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 12.Gundavda MK, Patil HG, Agashe VM, et al. Nontuberculous mycobacterial infection of the musculoskeletal system in immunocompetent hosts. Indian J Orthop 2017;51:205–12. 10.4103/0019-5413.201718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attorri S, Dunbar S, Clarridge JE. Assessment of morphology for rapid presumptive identification of Mycobacterium tuberculosis and Mycobacterium kansasii. J Clin Microbiol 2000;38:1426–9. 10.1128/JCM.38.4.1426-1429.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi S, Hu F-S, Yu H-Y, et al. Nontuberculous mycobacterial osteomyelitis. Infect Dis 2015;47:673–85. 10.3109/23744235.2015.1040445 [DOI] [PMC free article] [PubMed] [Google Scholar]