Abstract
Introduction
During acute feeding trials, consuming a large dose of whey protein (WP) before meals improves postprandial glucose regulation in people with type 2 diabetes. It is unclear if the reported benefits of premeal WP supplementation are translatable to everyday care or are associated with clinically meaningful, real-world glycemic outcomes. This study examined the application of a novel, premeal shot containing a low dose of WP on parameters of free-living glycemic control in people with type 2 diabetes.
Research design and methods
In a randomized, placebo-controlled, single-blind crossover design, 18 insulin naive individuals with type 2 diabetes ((mean±SD) age, 50±6 years; HbA1c (glycated hemoglobin), 7.4%±0.8%; duration of diabetes, 6±5 years) consumed a ready-to-drink WP shot (15 g of protein) or a nutrient-depleted placebo beverage 10 min before breakfast, lunch, and dinner over a 7-day free-living period. Free-living glucose control was measured by blinded continuous glucose monitoring and determined by the percentage of time spent above range (>10 mmol/L), in euglycemic range (3.9–10.0 mmol/L), below range (<3.9 mmol/L) and mean glucose concentrations.
Results
Mealtime WP supplementation reduced the prevalence of daily hyperglycemia by 8%±19% (30%±25% vs 38%±28%, p<0.05), thereby enabling a 9%±19% (~2 hours/day) increase in the time spent in euglycemia (p<0.05). Mean 24-hour blood glucose concentrations were 0.6±1.2 mmol/L lower during WP compared with placebo (p<0.05). Similar improvements in glycemic control were observed during the waken period with premeal WP supplementation (p<0.05), whereas nocturnal glycemic control was unaffected (p>0.05). Supplemental compliance/acceptance was high (>98%), and no adverse events were reported.
Conclusions
Consuming a novel premeal WP shot containing 15 g of protein before each main meal reduces the prevalence of daily hyperglycemia, thereby enabling a greater amount of time spent in euglycemic range per day over 7 days of free-living in people with type 2 diabetes.
Trial registration number
ISRCTN17563146; www.isrctn.com/ISRCTN17563146
Keywords: Diabetes Mellitus, Type 2
WHAT IS ALREADY KNOWN ON THIS TOPIC
Postprandial glycemic excursions are predominant contributors to overall glycemic control. During laboratory-based feeding trials, the consumption of whey protein prior to a meal reduces postprandial glycemia in individuals with type 2 diabetes.
It is unclear if the reported benefits of premeal whey protein supplementation are translatable to everyday care or are associated with clinically meaningful glycemic outcomes.
WHAT THIS STUDY ADDS
This is the first study to investigate the application of a bespoke-made, premeal shot containing a low dose of whey protein hydrolyzate on parameters of day-to-day glycemic control in free-living people with type 2 diabetes.
Our study shows that consuming a small amount of whey protein before each main meal reduced the daily time spent in hyperglycemia in adults with type 2 diabetes, compared with the ingestion of placebo treatment.
The reduction in hyperglycemia enabled a 2-hour increase in time spent within euglycemia per day and reduced mean daily glucose concentrations without compromising the risk of hypoglycemia.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
The prevalence of hyperglycemia remains an underappreciated problem for people with controlled type 2 diabetes treated with available therapies.
Given the financial implications associated with antihyperglycemic pharmacotherapies, the provision of premeal whey protein supplementation may offer an effective non-pharmaceutical approach to regulate glycemia.
Introduction
For people with controlled type 2 diabetes (T2D), the regulation of postprandial glycemia (PPG) is essential to achieving optimal glycemic control,1 2 which may reduce the risk of complications associated with hyperglycemia.3 4 Indeed, PPG excursions are predominant contributors to overall glycemic control, as measured by glycated hemoglobin (HbA1c).1 2 However, there is growing recognition that PPG are not only determinants of HbA1c but are also an independent cardiovascular risk factor,5 6 thus supporting the development of strategies that limit PPG excursions. Given the reluctance from patients to initiate new antihyperglycemic medications or to intensify existing treatment regimens,7 the use of non-pharmaceutical approaches to regulate PPG is desirable.
Nutrition plays an integral role in the management of T2D8 and represents an opportunity to optimize glycemic control in a cost-effective manner.9 We10 and others11–13 have demonstrated that consuming whey protein (WP) at a fixed interval before a main meal (preload) effectively reduces PPG excursions in individuals with T2D. The consumption of a WP preload stimulates the early and sustained release of insulin and several gut peptides, including glucagon-like peptide 1 (GLP-1), and delays gastric emptying, thereby reducing the glycemic response to a meal. However, despite promising acute evidence, the application of mealtime WP on key clinical outcomes is unclear. For instance, although one study reported a statistically significant, but clinically modest, reduction in HbA1c following the chronic application of premeal WP,14 HbA1c is unable to provide insight into day-to-day glycemic variability or the frequency of hyperglycemic events.15 There is also a wide range of possible mean glucose values and the time spent within desirable glycemic ranges with a given HbA1c that limits the precision by which it can be used to detect changes in glycemic control at the individual level.15 16 Accordingly, the utility of mealtime WP supplementation on day-to-day glucose control remains to be established.
It must be recognized that achieving meaningful and sustainable improvements in glycemic control requires approaches that are applicable and deliverable within the real world.17 To date, WP preloads have been presented as unpalatable, powdered supplements that require dilution and mixing with flavoring immediately prior to their consumption.10–13 This often produces solutions that are large in volume and cumbersome in their delivery.18 While such approaches have proven effective under tightly controlled clinical trials, the degree to which these results can be extrapolated into everyday care is uncertain.19 Indeed, there is a general unwillingness to consume powdered protein supplements publicly20 with patient embarrassment and challenging social conditions highlighted as key deterrents to adherence to diabetes treatments.21 To maximize the real-world application of mealtime WP supplementation, premeal interventions need to be translatable into treatments that are compatible with contemporary living.
In the present study, we examined the application of a novel, ready-to-drink WP preload that was created specifically for free-living glucose management over 7 days of free-living in individuals with T2D. Given PPG excursions are predominant contributors to overall glycemic control,1 2 we hypothesized that thrice daily mealtime WP supplementation would reduce daily hyperglycemic exposure, thereby increasing the time spent in euglycemia, as measured by continuous glucose monitoring (CGM).
Research design and methods
Participants
Patients, recruited by study advertisements, were aged 30–60 years with a duration of diabetes of ≥1 year, treated with lifestyle and/or oral antihyperglycemic medications, which were stable for ≥3 months preceding study enrollment. All participants were required to have an HbA1c of <80 mmol/mol (9.5%) and be of stable body mass and with a body mass index of 40 kg/m2. Individuals treated with injectable therapies (exogenous insulin and GLP-1 receptor agonists) and those with a history of gastrointestinal disease or a requirement for medications known to affect gastrointestinal function or appetite were excluded. Respondents who met our inclusion criteria were invited to attend our Newcastle National Institute for Health Research Clinical Research Facility (Newcastle upon Tyne Hospitals, Newcastle upon Tyne, UK) for a screening visit. All participants provided their written informed consent prior to enrollment into the study in accordance with Good Clinical Practice. Participant recruitment and testing were conducted between March 2019 and September 2021.
Study design
Patients entered into a single-blind, randomized, placebo-controlled, crossover design assessing the influence of premeal WP consumption on free-living glycemic control. In a counterbalanced order, patients randomly consumed a WP-rich, ready-to-drink shot (WP containing 15.6 g of dietary protein) or a protein-depleted placebo (PLA) shot 10 min before each of their main meals over a 7-day free-living period. Treatment sequences were determined by an online randomizer (www.randomization.com) that randomly assigned trial order in a balanced permutation. Free-living glycemic control was measured by a blinded CGM (Dexcom G6; Dexcom, San Diego, California, USA) that was implanted into subcutaneous tissue of the anterior-medial aspect of the lower abdomen. This device has a reported mean absolute relative difference of ~10% compared with a reference instrument and demonstrates consistent accuracy throughout its 10-day lifespan.22 Dexcom CGMs were fitted ~48 hours prior to the start of the trial and were removed in-clinic on completion of each free-living week. To account for compression artifacts, the anatomical location of CGM placement was chosen on the participants’ non-dominant sleeping side; this anatomical site was kept consistent throughout. Physical activity patterns were quantified by a wrist-worn activity monitor (GeneActiv, ActivInsights, UK). An ~14-day washout period separated each free-living phase. All medications were kept stable and unaltered throughout the study.
Throughout the duration of the intervention, participants were instructed to make no changes to their habitual dietary or physical activity patterns. Although the consumption of alcohol was permitted during this study, participants were asked to refrain from consuming excessive amounts of alcoholic beverages, which was defined as consuming alcohol (>3 alcoholic beverages per day) >3 days per week. The duration of the study was scheduled to coincide with periods representing participant’s normal daily life (ie, there were no planned activities, vacations, or unusual bouts of strenuous activity).
Free-living dietary intake was assessed by completion of an online, multipass 24-hour dietary recall system (https://intake24.co.uk/). Dietary recalls were completed daily with participants submitting a log of all foods and drinks consumed from the previous 24 hours. All foods within the Intake24 system are linked to the UK National Diet and Nutrition Survey database. Participants were given detailed instructions on how to use this application prior to study enrollment. Paper supplement logs were also completed to document the timings of both supplement consumption and the commencement of the main meals. This paper log was used to identify self-reported postprandial events and to cross-reference with the timing of meals submitted by the Intake24 application; the latter was used to measure supplement adherence. Participants did not include the WP or PLA shots into their dietary recalls.
Intervention
Patients were instructed to consume a WP or PLA preload shot 10 min prior to each of their main meals (breakfast, lunch, and dinner) over a 7-day free-living period. The premeal WP and PLA shots were produced by Arla Foods Ingredients Group P/S (Viby J, Denmark) specifically for free-living glucose management. Both treatments were stable at both temperate and chilled environmental conditions and had a shelf-life of 6 months. The premeal drinks were presented in a contemporary, ready-to-drink format as a 100 mL beverage ‘shot’ and were of similar viscosity and texture. To account for any subtle differences in mouthfeel and to maintain supplement blinding, the premeal supplements were presented as two different flavors: WP, cocoa-cappuccino; PLA, strawberry. The premeal WP treatment used a hydrolyzed WP ingredient (Lacprodan DI-6820; Arla Food Ingredients Group P/S) to produce a palatable, ready-to-drink beverage. Each WP shot (418 kJ) contained 15.6 g of dietary protein, and a small amount of dietary carbohydrates (3 g) and fat (2.3 g) from 100 mL of low-viscosity liquid. Further detailed information regarding the product development of the novel WP shot has been published elsewhere.23 The PLA shot (<142 kJ) contained small amounts of dietary carbohydrate (3.9 g) and fat (2.2 g), with negligible protein content (<0.1 g).
Outcomes
Interstitial glucose concentrations were measured every fifth minute (288 values per day) over a period of 7 days. CGM data were accepted if >90% of the available daily data and >70% of the available weekly data were collected.24 Data from the Dexcom CGMs were stored using the Dexcom Clarity Professional web software (Dexcom, USA). Physical activity data were converted into 15 s epoch files using the GENEActiv PC software V.3.2, which were subsequently analyzed using Microsoft Excel Macro files provided by the manufacturer. Energy and macronutrient intake collected by the Intake24 dietary recall were exported as a Microsoft Excel spreadsheet.
The primary outcome of this study was the time spent in hyperglycemia (>10 mmol/L) during a 7-day free-living period. In addition to the primary outcome, secondary outcomes included the time spent in euglycemic range (TIR), time below range, time above range, PPG events (incremental area under the curve (iAUC) and the peak incremental change in glucose (PPG)), measures of intraday (coefficent of variance (%CV), mean amplitude of glycemic excursions (MAGE)) and interday (mean of daily differences (MODD)) glycemic variability, and indices of hypoglycemic and hyperglycemic risk (low blood glucose index and high blood glucose index, respectively). Glucose management indicator (GMI), which gives an approximation of HbA1c based on the average glucose levels collected by CGM, was also calculated.25
Glycemic ranges were defined as <3.0 mmol/L (time below range level 1), 3.0–3.8 mmol/L (time below range level 2), 3.9–7.8 mmol/L (time in tight euglycemic range), 3.9–10.0 mmol/L (TIR), 10.0–13.9 mmol/L (time above range level 1), and >13.9 mmol/L (time above range level 2).24 26 %CV was calculated as the SD divided by mean glucose multiplied by 100. GMI (mmol/mol) was calculated using the formula proposed by Bergenstal et al.25 Risk indices for high and low blood glucose, MAGE, and MODD were computed using an automated software package (EasyGV V.9.0R2; University of Oxford, UK). Daytime and nocturnal glycemia were defined as 06:00–23:55 hours and 24:00–05:55 hours, respectively.26 The iAUC for cumulative PPG events was calculated using the trapezoidal rule, depicting the area above baseline concentrations, which was accepted as the reported timing of consumption of the preload. PPG excursions were accepted if there were no self-reported eating occassions within 120 min following the commencement of the meal. Where the time of meal commencement could not be established, the data were excluded from analysis. PPG events were identified and averaged per individual: that is, if 10 iAUC events were identified, the iAUC was calculated per event and then averaged per 10 to give a single iAUC event.
Sample size
A sample size estimate was made using interstitial glucose collected during a 6-hour laboratory visit from preliminary data.10 To detect a difference of at least 10% in time spent above 10 mmol/L in the postprandial period, 18 participants were required to fully complete two trials (WP vs PLA), to test the null hypothesis that the population means are equal across trials with a probability of 0.8 and a type 1 error of 0.05. Sample size calculation was completed using Stata, with PLA mean time above 10 mmol/L at 63.0% vs 51.5% following WP (SD of the mean differences of 16%).
Statistical analysis
All data were assessed for normal distribution by a Shapiro-Wilks test. Non-parametric data were logarithmically transformed and reassessed for distribution. Where transformation failed, data were assessed non-parametrically. Paired sampled t tests or Wilcoxon signed-rank test were used to explore treatment differences on variables that displayed normal or non-normal distribution, respectively. Inferential statistics were conducted using the software package IBM SPSS Statistics (V.27; IBM, USA). Significance was set at alpha p<0.05. Treatment differences in the time spent in glycemic ranges are expressed as the absolute percentage point change. Data are presented as means±SD unless stated otherwise.
Results
Participants
The Consolidated Standards of Reporting Trials flow diagram is shown in online supplemental figure 1. A total of 26 participants were recruited for this study. From this cohort, eight participants were withdrawn for the following reasons: one participant withdrew their consent prior to randomization; three participants were withdrawn due abnormal laboratory findings (laboratory measured HbA1c >80 mmol/mol (9.5%)); two participants were withdrawn due to the COVID-19 pandemic and the cessation of research activities during March–September 2020; one due to a change in glucose-lowering medication during the trial; and one due to non-adherence. Therefore, data are analyzed and presented on n=18. Patient characteristics are presented in table 1. In brief, participants had a mean HbA1c of 57.4±9.2 mmol/mol (7.4%±0.8%) and a self-reported diabetes duration of 6.2±4.9 years. All participants were of white Europid descent. The most common antihyperglycemic treatments were either metformin monotherapy (n=5, 28%) or the combination of metformin and a sulfonylurea (n=6, 33%). Hypertensive and statin therapies were prescribed for 78% (n=14) and 56% (n=10) of participants, respectively. Five of the 18 participants were women (28%).
Table 1.
Patient characteristics
| Characteristics (n=18) | |
| Sex (male/female) | 13/5 |
| Age (years) | 50±6 (40–60) |
| Stature (cm) | 172±10 |
| Body mass (kg) | 98.7±19.5 (66.8–130.5) |
| BMI (kg/m2) | 33.3±5.7 (21.3–43.6) |
| HbA1c (mmol/mol) | 57.4±9.2 (42–73) |
| HbA1c (%) | 7.4±0.8 (6.0–8.8) |
| Duration of diabetes (years) | 6.2±4.9 (2–20) |
| Family history of diabetes (n) | 8 |
| Diabetes treatment | |
| Metformin | 5 |
| SU | 1 |
| Metformin+SU | 6 |
| Metformin+SGLT2 i | 2 |
| Metformin, SU+TZD | 1 |
| Diet and lifestyle | 3 |
Data are presented as mean±SD unless stated otherwise. Data in parenthesis are ranges.
BMI, body mass index; HbA1c, glycated hemoglobin; SGLT2i, sodium–glucose cotransporter 2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
bmjdrc-2022-002820supp001.pdf (553.6KB, pdf)
Glycemic control
Mean 24-hour glucose control
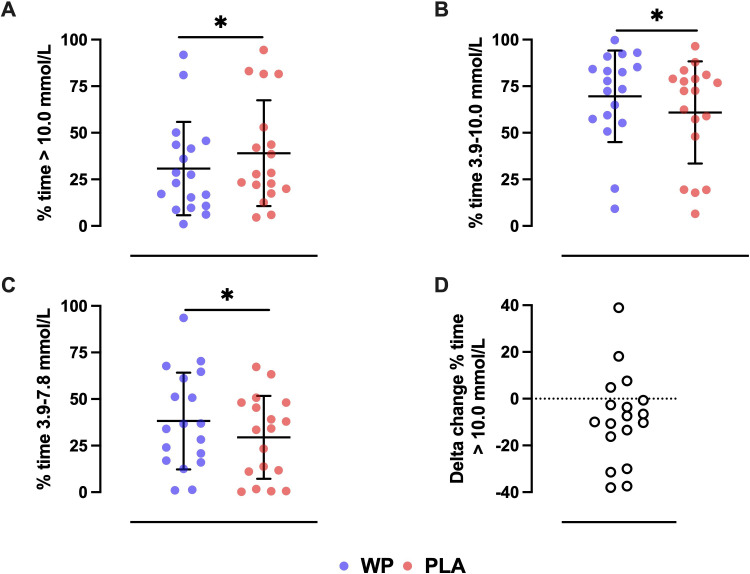
Key glycemic parameters during 7 days of free-living are presented in figure 1 and table 2. During the PLA free-living week, patients spent 38.1%±28.4% of the 24- hour period at blood glucose >10.0 mmol/L. The prevalence of daily hyperglycemia (>10.0 mmol/L) was reduced by −8.3%±19.3% following treatment with mealtime WP supplementation (figure 1A), resulting in −117±276 min less per 24 hours spent in hyperglycemia (p=0.024). The subsequent reduction in hyperglycemic exposure enabled patients to achieve an increase in TIR of +8.7%± 19.0%, compared with PLA (p=0.035; figure 1B). Within this euglycemic range, premeal WP supplementation increased the time spent between glucose concentrations of 3.9–7.8 mmol/L by +8.8%±14.7% (p=0.022), corresponding to an average increase of +127±210 min per day (figure 1C). The time spent within hyperglycemic levels 1 and 2 were numerically, but not statistically (p=0.089 and p=0.352, respectively), lower following the WP preload (table 2).
Figure 1.
The mean±SD percentage of time spent per day in (A) hyperglycemia (>10.0 mmol/L), (B) euglycemia (3.9–10.0 mmol/L), and (C) normoglycemia (3.9–7.8 mmol/L) during 7 days of free-living with premeal supplementation of a whey protein (WP) (blue circles) or placebo (PLA) (red circles) preload. Panel (D) depicts the per patient delta change in the percentage of time spent >10.0 mmol/L with premeal WP supplementation relative to PLA. *Denotes a statistical treatment effect as measured by a paired samples t test or Wilcoxon signed rank test (p<0.05).
Table 2.
Free-living glycemic control parameters
| WP | PLA | P value | |
| Data collected (hour:min) | 2946:09 | 2950:28 | – |
| Glucose parameters | |||
| Mean 24-hour glucose (mmol/L) | 8.9±1.8 | 9.5±2.0# | 0.045 |
| GMI (mmol/mol)* | 54.6±8.6 | 57.4±9.5# | 0.045 |
| iAUC 0–120 min (mmol/L/min) | 1.6±0.6 | 2.1±0.9# | 0.003 |
| PPG (mmol/L) | 3.0±1.0 | 3.7±1.3# | 0.007 |
| Time in ranges | |||
| <3.0 mmol/L (%)* Time (hour:min) |
0.1±0.3 0:00±0:06 |
0.1±0.3 0:00±0:06 |
0.317 |
| 3.0–3.8 mmol/L (%)* Time (hour:min) |
0.5±1.4 0:06±0:18 |
1.0±3.4 0:12±0:48 |
0.953 |
| 3.9–7.8 mmol/L (%) Time (hour:min) |
38.3±26.0 9:02±6:02 |
29.5±22.2# 7:05±5:18 |
0.021 |
| 3.9–10.0 mmol/L (%)* Time (hour:min) |
69.6±24.6 16:42±5:54 |
60.9±27.4# 14:37±6:36 |
0.035 |
| 10.0–13.9 mmol/L (%) Time (hour:min) |
24.7±18.6 5:55±4:30 |
30.0±17.9 7:11±4:48 |
0.089 |
| > 13.9 mmol/L (%)* Time (hour:min) |
5.5±7.9 1:19±1:54 |
8.5±12.9 2:02±3:06 |
0.352 |
| > 10 mmol/L (%)† Time (hour:min) |
29.8±25.1 7:09±6:01 |
38.1±28.4# 9:08±6:48 |
0.024 |
| Glycemic variability | |||
| %CV | 23.8±5.8 | 23.1±5.0 | 0.609 |
| MAGE (mmol/L) | 5.0±1.1 | 5.1±1.0 | 0.653 |
| High blood glucose index† | 7.0±4.6 | 8.5±5.6 | 0.052 |
| Low blood glucose index* | 0.8±1.0 | 0.9±1.4 | 0.689 |
| MODD (mmol/L) | 1.9±0.5 | 2.0±0.5 | 0.349 |
Boldface and # indicates a statistical difference between treatments determined by a paired sampled t-test or Wilcoxon signed-rank test (p<0.05). All data are presented as means±SDs unless stated otherwise.
*Data were analyzed non-parametrically by a Wilcoxon signed-rank test.
†Data were logarithmically transformed prior to analysis.
%CV, coefficient of variance; GMI, glucose management indicator; iAUC, incremental area under the curve; MAGE, mean amplitude of glycemic excursions; MODD, mean of daily differences; PLA, placebo; ∆PPG, peak incremental change in glucose; WP, whey protein.
Patient’s GMI, which is an estimate of overall glycemic control, was reduced by −2.9±5.6 mmol/mol during the WP treatment arm compared with PLA (p=0.045), indicative of improved glycemic control and a reduction in hyperglycemia (table 2). Indeed, relative to PLA, mean 24-hour glucose concentrations were −0.6±1.2 mmol/L lower following the premeal WP treatment (p=0.045). Despite a reduction in mean 24-hour glucose concentrations and time spent >10.0 mmol/L, this was not accompanied by an increase in time below range or low blood glucose index (all p>0.317). Markers of glycemic variability were similar between treatments (table 2).
Diurnal and nocturnal glucose control
Mean glucose concentrations during the waken hours (06:00–23:55 hours) were −0.7±1.2 mmol/L lower during the WP week compared with PLA (p=0.023). Similarly, TIR was +8.7%±20.5% greater after treatment with premeal WP supplementation compared with PLA (p=0.048), and correspondingly, less time was spent in hyperglycemia (−8.9%±20.9%; p=0.048). On the other hand, parameters of glycemic control during the nocturnal period (24:00 –05:55 hours) were comparable between treatments (online supplemental table 1).
Postprandial glucose control
A total of n=652 postprandial events were identified and analyzed. The number of postprandial events were similar between treatments (WP, n=321; PLA, n=331). Relative to PLA, PPG iAUC0–120min were −24%±29% lower during the WP free-living week (p=0.003). On average, PPG was reduced by −0.7±0.9 mmol/L (p=0.007). The within-subject timing of meals were similar between free-living weeks (mean difference, 27±24 min), as shown in online supplemental figure 2.
Energy intake
There was a modest reduction in self-reported energy intake during the WP week compared with PLA (−631±1314 kJ per day), although this was statistically insignificant (p=0.057). When accounting for the energy content associated with the preloads, cumulative energy intake was similar between free-living weeks (p=0.635).
Physical activity
There were no differences in the time spent at differing physical activity levels between the WP and PLA weeks (p>0.605).
Compliance
All participants tolerated the PLA and WP treatments well and reported no gastrointestinal side effects. Supplemental compliance during the intervention weeks was exemplary (WP, 97.5%±2.4%; PLA, 99.3%±2.4%).
Discussion
This study examined the application of premeal WP supplementation on daily glycemic control over 1 week in people with T2D. For the first time, we demonstrate that daily hyperglycemia can be significantly reduced by the provision of a low dose of WP (15 g) ingested prior to each main meal over 7 days of free-living. This enabled patients to achieve 2 hours more per day spent within euglycemia, without increasing the risk of hypoglycemia. These results occurred without a change in patient medication, dietary intake, or physical activity levels, thereby demonstrating the utility of premeal WP supplementation for the management of hyperglycemia.
The present study extends from previous acute laboratory work highlighting the PPG-lowering efficacy of premeal WP for people with T2D.10–13 However, for the first time, we report the translatability and reproducibility of these findings under real-world and free-living conditions. It was found that premeal ingestion of our novel WP shot effectively mitigated free-living PPG excursions, thereby reducing the prevalence of hyperglycemia by ~2 hours per day, compared with PLA. This subsequently enabled an absolute daily increase in TIR of 8.7%, the magnitude of which is substantial. Indeed, an increase in TIR of 5% is considered clinically significant27 and may be associated with a reduced risk of developing vascular complications.28 29 For instance, for every 10% reduction in TIR (roughly equivalent to the increase in TIR in the present study with mealtime WP supplementation), the risk of developing retinopathy or microalbuminuria was increased by 64% and 40%, respectively, in the Diabetes Control and Complications Trial cohort.29 A similar 10% reduction in TIR has also been associated with a 13% and 25% increased risk of developing cardiovascular autonomic neuropathy and peripheral neuropathy, respectively, in people with long-standing T2D.30 31 Promisingly, the preload treatments were well accepted among participants, as demonstrated by their exemplary compliance, and no adverse side effects were reported supporting the application of this novel mealtime therapy.
The findings presented herein are of interest given our data demonstrate that the intervention of available oral antihyperglycemic medications are insufficient in the protection against hyperglycemia. Current recommendations state that people with T2D should strive for <6 hours per day at glucose concentrations >10.0 mmol/L.24 However, despite the continued use of patient’s antihyperglycemic agents, our cohort spent up to 9 hours (38%) of the day at glucose concentrations >10.0 mmol/L during the PLA free-living week. This observation is similar to what has been reported by others32 and underscores the need for further strategies designed to reduce the prevalence of hyperglycemia in people with T2D.
Our primary finding that premeal WP supplementation reduces the prevalence of hyperglycemia corroborates with data demonstrating a reduction in overall hyperglycemia following the application of PPG-lowering therapies.33 Indeed, our observed reduction in time spent >10.0 mmol/L derived from the waken/feeding period, as shown by the reduction in diurnal but not nocturnal hyperglycemia. Although the free-living nature of our study makes it difficuilt to discern mechanisms associated with our findings, prior literature has demonstrated that a WP preload of similar amounts (15–20 g) elevates GLP-1 above preprandial concentrations for ~180 min following ingestion of a meal.12 13 23 The ingestion of WP also modestly stimulates the secretion of glucose-dependent insulinotropic polypeptide (GIP)12; though the relevance of this to the observed improvement in glycemic control is likely minimal since endogenous GIP has little to no effect on PPG in individuals with T2D.34 Herein, it is possible that diurnal increases in GLP-1 secretion from thrice daily WP supplementation may have enhanced β-cell glucose sensitivity and delayed the rate of gastric emptying, thereby slowing the systemic appearance of meal-derived glucose and augmenting an efficient islet response.35 36 These effects are consistent with what was previously observed following adminstration of a protein preload given prior to an oral glucose load,37 supporting this assertion. Nonetheless, literature examining the glucoregulatory effects of premeal WP have been conducted solely following an overnight fast. Considering the regulation of PPG displays a clear circadian pattern,38 whether a low dose of mealtime WP supplementation is sufficient to augmenting a PPG-lowering milieu to meals consumed later in the day is unclear and requires future study.
The present analyses demonstrates that the addition of premeal WP to patient care has the potential to reduce daily hyperglycemia and increase TIR without increasing the risk of hypoglycemia, as shown by the low blood glucose index and time spent below range. An increase in TIR is also suggestive that the frequency of erratic glycemic swings were reduced with mealtime WP.39 Fluctuations in glycemia are posited to be implicated in the pathogenesis of diabetes-related vascular complications, supporting the development of strategies designed to reduce glycemic variability.40 Although there were no changes in amplitude markers of glycemic variability with WP supplementation (ie, %CV), this likely reflects the relative glycemic stability of the cohort studied since all patients had a %CV <36%.24 Nevertheless, the risk of microvascular complications has recently been shown to be inversely related to TIR, independent of %CV.28 Therefore, although there were no changes in amplitude markers of glycemic variability with the WP treatment, this is unlikely to affect the clinical value of our results.
To the best of the authors’ knowledge, this is the first study designed and powered to examine the use of premeal WP on free-living glycemic management captured by masked CGM. Our study is rendered timely as its design benefits from incorporating current recommendations that endorse the use of CGM when assessing glycemic control at the individual level.26 Offering ecological validity to our findings, patient’s GMI during PLA was identical to their laboratory-measured HbA1c. This suggests that our reported data reflects a true change from participant’s habitual glycemic control.25 In this regard, an increase in TIR of ~9% with premeal WP supplementation is projected to confer a ~5–7 mmol/mol (0.6%) reduction in HbA1c.16 27 This assertion is supported by our findings of a reduction in mean daily glucose concentrations (−0.6 mmol/L). In the context of available treatments for T2D, the magnitude of this projected reduction in HbA1c is akin to what would be expected from the adminstration of thiazolidinediones, sodium–glucose cotransporter 2 inhibitors and dipeptidyl peptidase-IV inhibitors.41 Considering adherence to pharmacological agents can be poor,7 the data presented herein may hold important implications for the management of hyperglycemia.
When accounting for the energy associated with the preloads, daily energy intake was similar between treatments. This was despite patients consuming an additional ~836 kJ/day when adherent to the WP shot, compared with PLA. Therefore, patients may modestly adjust their energy intake to account for the caloric load associated with a small WP preload. This is in line with previous observations that reported no change in body mass following the long-term ingestion of mealtime WP supplementation (~753 kJ/day) in people with T2D.14 These collective findings are appealing and suggest that the adherence to a low dose of premeal WP is unlikely to compromise weight management in obese and dysglycemic populations.
There are several strengths associated with our study including our randomized, placebo-controlled, crossover design, and the counterbalanced administration of treatments to minimize treatment order effects. Furthermore, and unique to this study, patients were provided with premeal WP and PLA shots created specifically for the real-world application of mealtime WP supplementation. Nonetheless, our study is not without its limitations. First, our analyses were conducted on people of white, Europid descent; thus, the applicability of these results to other ethnic groups and races is unclear. Second, although our study was powered to test the primary outcome, we acknowledge that our analyses are conducted on a small sample of people with T2D (n=18). Since our patients were of relatively controlled diabetes and treated with oral therapies, our findings cannot be extrapolated to the wider T2D population. Finally, although our findings indicate an improvement in glycemic control with mealtime WP supplementation, the long-term evidence supporting our data is lacking with only one study to date reporting a modest improvement in HbA1c with premeal WP supplementation.14 Whether the results presented herein are sustainable longer term or are associated with improvements in clinically relevant end points cannot be inferred and require further investigation. Importantly, however, the glucose-lowering mechanisms by which premeal WP supplementation regulates PPG remain functionable after its chronic application14 supporting these findings.
In summary, we show that thrice daily consumption of a novel preload shot containing a low dose of WP reduces daily hyperglycemia, increasing the time spent in euglycamia by ~2 hours per day during 7 days of free-living. This is of importance given our analysis clearly demonstrates that the prevelance of hyperglycemia is an underappreciated problem for people with controlled T2D treated with available oral medications. The provision of a contemporary WP preload shot may represent an effective sole or adjunctive therapy for the treatment of hyperglycemia, which could also have important financial implications at a time where public health budgets are constrained.
Acknowledgments
The authors thank the study participants for their time, effort, and commitment, as well as the research teams at the Newcastle National Institute for Health Research Clinical Research Facility, Newcastle upon Tyne, for their assistance with data collection.
Footnotes
Contributors: KS, EJS, and DJW designed the research. KS conducted the research, analyzed the data, and wrote the manuscript. DJW analyzed the data and wrote the manuscript. GST, MW, KABD, LHB and EJS reviewed and edited the manuscript. DJW is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of data analysis.
Funding: This work was supported by a grant awarded to DJW and EJS (grant no: BH172513) from Arla Foods Ingredients Group P/S (Viby J, Denmark). Arla Foods Ingredients Group P/S produced whey protein and placebo treatments. Arla Foods Ingredients Group P/S had no role in the collection, analysis, or interpretation of data. CGM equipment were provided by an equipment award to DJW from Dexcom (San Diego, California, USA).
Competing interests: DJW and EJS have received research funding, travel expenses, and consultancy fees from Arla Foods Ingredients Group P/S. EJS has received research funding from The Dairy Council. LHB is an employee of Arla Foods Ingredients Group P/S.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This randomized controlled, crossover trial was conducted in line with Good Clinical Practice and the revised 1983 Declaration of Helsinki. This study involves human participants. Ethical approval was obtained from the North East–Newcastle & North Tyneside 1 Research Ethics Committee (reference: 18/NE/0372). Ethics granted: January 16, 2019. Participants gave informed consent to participate in the study before taking part.
References
- 1.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881–5. 10.2337/diacare.26.3.881 [DOI] [PubMed] [Google Scholar]
- 2.Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007;77:280–5. 10.1016/j.diabres.2006.11.011 [DOI] [PubMed] [Google Scholar]
- 3.Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med Overseas Ed 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 4.Esposito K, Ciotola M, Carleo D, et al. Post-meal glucose peaks at home associate with carotid intima-media thickness in type 2 diabetes. J Clin Endocrinol Metab 2008;93:1345–50. 10.1210/jc.2007-2000 [DOI] [PubMed] [Google Scholar]
- 5.Cavalot F, Pagliarino A, Valle M, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga diabetes study. Diabetes Care 2011;34:2237–43. 10.2337/dc10-2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meigs JB, Nathan DM, D'Agostino RB, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham offspring study. Diabetes Care 2002;25:1845–50. 10.2337/diacare.25.10.1845 [DOI] [PubMed] [Google Scholar]
- 7.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence 2016;10:1299–307. 10.2147/PPA.S106821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–54. 10.2337/dci19-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin Y, Davies A, Briggs A, et al. Type 2 diabetes remission: 2 year within-trial and lifetime-horizon cost-effectiveness of the Diabetes Remission Clinical Trial (DiRECT)/Counterweight-Plus weight management programme. Diabetologia 2020;63:2112–22. 10.1007/s00125-020-05224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King DG, Walker M, Campbell MD, et al. A small dose of whey protein co-ingested with mixed-macronutrient breakfast and lunch meals improves postprandial glycemia and suppresses appetite in men with type 2 diabetes: a randomized controlled trial. Am J Clin Nutr 2018;107:550–7. 10.1093/ajcn/nqy019 [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Stevens JE, Cukier K, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009;32:1600–2. 10.2337/dc09-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu T, Little TJ, Bound MJ, et al. A protein preload enhances the glucose-lowering efficacy of vildagliptin in type 2 diabetes. Diabetes Care 2016;39:511–7. 10.2337/dc15-2298 [DOI] [PubMed] [Google Scholar]
- 13.Watson LE, Phillips LK, Wu T, et al. Differentiating the effects of whey protein and guar gum preloads on postprandial glycemia in type 2 diabetes. Clin Nutr 2019;38:2827–32. 10.1016/j.clnu.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 14.Watson LE, Phillips LK, Wu T, et al. A whey/guar “preload” improves postprandial glycaemia and glycated haemoglobin levels in type 2 diabetes: A 12‐week, single‐blind, randomized, placebo‐controlled trial. Diabetes, Obesity and Metabolism 2019;21:930–8. 10.1111/dom.13604 [DOI] [PubMed] [Google Scholar]
- 15.Beck RW, Connor CG, Mullen DM, et al. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care 2017;40:994–9. 10.2337/dc17-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019;13:614–26. 10.1177/1932296818822496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care 2017;40:1425–32. 10.2337/dc16-1974 [DOI] [PubMed] [Google Scholar]
- 18.Smith K, Bowden Davies KA, Stevenson EJ, et al. The clinical application of mealtime whey protein for the treatment of postprandial hyperglycaemia for people with type 2 diabetes: a long whey to go. Front Nutr 2020;7:587843. 10.3389/fnut.2020.587843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carls GS, Tuttle E, Tan R-D, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care 2017;40:1469–78. 10.2337/dc16-2725 [DOI] [PubMed] [Google Scholar]
- 20.MacDonald A, Lilburn M, Davies P, et al. 'Ready to drink' protein substitute is easier is for people with phenylketonuria. J Inherit Metab Dis 2006;29:526–31. 10.1007/s10545-006-0234-y [DOI] [PubMed] [Google Scholar]
- 21.Davies MJ, Gagliardino JJ, Gray LJ, et al. Real-world factors affecting adherence to insulin therapy in patients with type 1 or type 2 diabetes mellitus: a systematic review. Diabet Med 2013;30:512–24. 10.1111/dme.12128 [DOI] [PubMed] [Google Scholar]
- 22.Welsh JB, Zhang X, Puhr SA, et al. Performance of a Factory-Calibrated, real-time continuous glucose monitoring system in pediatric participants with type 1 diabetes. J Diabetes Sci Technol 2019;13:254–8. 10.1177/1932296818798816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith K, Taylor GS, Allerton DM, et al. The postprandial glycaemic and hormonal responses following the ingestion of a novel, Ready-to-Drink shot containing a low dose of whey protein in centrally obese and lean adult males: a randomised controlled trial. Front Endocrinol 2021;12:696977. 10.3389/fendo.2021.696977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International consensus on time in range. Diabetes Care 2019;42:1593–603. 10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1c from continuous glucose monitoring. Diabetes Care 2018;41:2275–80. 10.2337/dc18-1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–40. 10.2337/dc17-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vigersky RA, McMahon C. The relationship of hemoglobin A1c to Time-in-Range in patients with diabetes. Diabetes Technol Ther 2019;21:81–5. 10.1089/dia.2018.0310 [DOI] [PubMed] [Google Scholar]
- 28.Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–6. 10.2337/dc18-1131 [DOI] [PubMed] [Google Scholar]
- 29.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–5. 10.2337/dc18-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MY, Kim G, Park JY, et al. The association between continuous glucose Monitoring-Derived metrics and cardiovascular autonomic neuropathy in outpatients with type 2 diabetes. Diabetes Technol Ther 2021;23:434–42. 10.1089/dia.2020.0599 [DOI] [PubMed] [Google Scholar]
- 31.Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020;8:e000991. 10.1136/bmjdrc-2019-000991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dijk J-W, Manders RJF, Hartgens F, et al. Postprandial hyperglycemia is highly prevalent throughout the day in type 2 diabetes patients. Diabetes Res Clin Pract 2011;93:31–7. 10.1016/j.diabres.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 33.Rayner CK, Watson LE, Phillips LK, et al. Effects of sustained treatment with Lixisenatide on gastric emptying and postprandial glucose metabolism in type 2 diabetes: a randomized controlled trial. Diabetes Care 2020;43:1813–21. 10.2337/dc20-0190 [DOI] [PubMed] [Google Scholar]
- 34.Stensen S, Gasbjerg LS, Krogh LL, et al. Effects of endogenous GIP in patients with type 2 diabetes. Eur J Endocrinol 2021;185:33–45. 10.1530/EJE-21-0135 [DOI] [PubMed] [Google Scholar]
- 35.Kjems LL, Holst JJ, Vølund A, et al. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003;52:380–6. 10.2337/diabetes.52.2.380 [DOI] [PubMed] [Google Scholar]
- 36.Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab 2010;95:215–21. 10.1210/jc.2009-1503 [DOI] [PubMed] [Google Scholar]
- 37.Tricò D, Baldi S, Tulipani A, et al. Mechanisms through which a small protein and lipid preload improves glucose tolerance. Diabetologia 2015;58:2503–12. 10.1007/s00125-015-3710-9 [DOI] [PubMed] [Google Scholar]
- 38.Lindgren O, Mari A, Deacon CF, et al. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J Clin Endocrinol Metab 2009;94:2887–92. 10.1210/jc.2009-0366 [DOI] [PubMed] [Google Scholar]
- 39.Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care 2016;39:502–10. 10.2337/dc15-2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol 2019;7:221–30. 10.1016/S2213-8587(18)30136-0 [DOI] [PubMed] [Google Scholar]
- 41.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care 2009;32:193–203. 10.2337/dc08-9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2022-002820supp001.pdf (553.6KB, pdf)
Data Availability Statement
Data are available on reasonable request.