Abstract
Skin cancer is the most common type of cancer and its incidence is increasing. The objective of this study was to describe the trends in reimbursed drug and hospital costs of benign and (pre)malignant skin tumours, and to present future projections. Therefore, nationwide hospital and drug reimbursement data (for the period 2007–17) were used. In 2017, malignant skin tumours were the 4th most costly cancer in the Netherlands (after breast, colorectal, and lung cancer). The total costs for skin tumours increased from €278 million for 384,390 patients (in 2007) to €465 million for 578,355 patients (in 2017). Drug costs increased from €0.7 million to €121 million (over the period 2007–17), resulting in a 26% share of overall costs in 2017. Future costs are projected to reach €1.35 billion in 2030. In conclusion, the increasing costs of skin cancer are strongly affected by the increasing incidence and introduction of expensive drugs, and future projections are for an alarming increase.
Key words: skin cancer, skin neoplasm, healthcare costs, health expenditure
Skin cancer is the most common type of cancer in many Caucasian populations. It results in both a high burden and high financial impact (1–3). It is estimated that 1 in 5 persons will develop skin cancer in the Netherlands (4). With increasing incidence, the costs of management are also expected to rise (3, 5–7).
Knowledge of the healthcare costs related to its main components and trends is essential to enable health policy decision-makers to make well-informed decisions on potential interventions, and to be able to evaluate the future effect of these decisions. Several studies have reported skin cancer-related healthcare costs based on estimates for different countries. However, these are based on extrapolations of regional data, or are population- (e.g. Medicare), or diagnosis-specific (8–11). Furthermore, a comprehensive and up-to-date economic evaluation after the introduction of expensive drugs for the treatment of skin cancer (i.e. targeted therapy and immunotherapy) is lacking.
SIGNIFICANCE
Skin cancer is the most common type of cancer. Nationwide claims data in the Netherlands were obtained for the period 2007–2017 in order to assess the associated healthcare costs. Malignant skin tumours were the 4th most costly cancer in the Netherlands in 2017, after breast, colorectal, and lung cancer. The total costs for skin tumours increased from €278 million for 384,390 patients (in 2007) to €465 million for 578,355 patients (in 2017). Drug costs increased from €0.7 million to €121 million (over the period 2007–17), resulting in a 26% share of overall costs in 2017. Future total costs for skin cancer are projected to reach €1.35 billion in 2030. In conclusion, the increasing costs of skin cancer are strongly affected by the increasing incidence and introduction of expensive drugs, and future projections show an alarming increase.
The primary objective of this study was to describe national trends of reimbursed drug and hospital costs of benign, premalignant and malignant skin tumours over the past 11 years, and to compare the costs for malignant skin tumours with other malignancies in the Netherlands. Secondary objectives were to evaluate the proportion attributable to benign and malignant tumours, the distribution of the costs across different medical specialties, and the effect of the introduction of expensive drugs on the overall healthcare costs related to cutaneous malignancies. Furthermore, taking into account the expected increasing financial burden of skin cancer management, this study projected the current costs to 2030, based on published trends in incidence rates.
MATERIALS AND METHODS
Data source
The economic burden of skin tumour management was studied from a healthcare perspective using a top-down approach. Only reimbursed direct medical hospital care costs were investigated. Indirect and primary care costs were not included.
Nationwide data concerning reimbursed hospital and drug costs were obtained from Vektis, a central database collecting data on all reimbursed healthcare claims in the Netherlands; previous validation showed an overall accuracy of over 95% (12). Since 2006, the Dutch healthcare system has been based on a single compulsory insurance scheme (i.e. Health Insurance Act) (13). Reimbursement is based on a Diagnosis Related Groups (DRGs) system, in which all activities related to diagnosing or treating patients are included, resulting in a reimbursement claim.
Data extraction
All drug and DRG claims for all medical specialties contributing to the treatment of benign, premalignant and malignant skin tumours in the period 2007–17 were included (Table SI1). These DRG claims comprise all possible benign, premalignant and malignant skin tumours. Hospital claims data includes both inpatient as well as outpatient care. Data were aggregated such that the number of claims, unique number of patients (i.e. the number of patients with at least one relevant claim), year, costs, and mean costs were available. Also, information was obtained on Anatomical Therapeutic Chemical (ATC) classification codes of drugs related to skin tumour treatments, including the number of claims, number of patients, year, total and mean costs. In the Dutch healthcare system, drugs are divided into regular pharmaceuticals (relative low-cost) and add-on pharmaceuticals (relative high-cost) (Table SII1). Add-on pharmaceuticals are provided in an inpatient setting, although they were charged separately from DRGs. Both low-cost and high-cost drugs were included in the analyses.
Analysis of the cost-drivers for skin cancer management focused merely on skin cancer management costs in dermatology, as DRGs are less specific for skin cancer in other medical specialties. The healthcare products with their accompanying costs were aggregated into the following groups: diagnostic evaluations, outpatient visits, inpatient care, operative treatment, non-operative treatment, and other. This data was available for the period 2012–17.
In order to obtain a perspective on the scope of the economic burden of skin cancer management compared with all cancer types, claims for direct medical costs of all malignancies and medical specialties were extracted (Table SIII1).
Statistical analyses
No hypothesis testing and sample size calculations were performed. The costs were adjusted to 2018 Euros by using consumer price index inflation rates from StatBureau (www.statbureau.org). The descriptive analyses were performed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA).
Future projections for the reimbursed costs for skin tumours to 2030 were based on published estimates of incidence growth rates for basal cell carcinomas (BCCs), squamous cell carcinoma (SCCs) and melanomas (2, 14–16). The unique number of patients in 2017 was used to extrapolate future incidence based on an annual increasing incidence rate of 5% (scenario A). The annual incidence rates were multiplied by the mean costs per patient of 2017, including future annual inflation rates of 2% from the Organisation for Economic Co-operation and Development (OECD) inflation forecast (17). Furthermore, future costs (up to 2 years) for the market entry of new innovative drugs and broadening indications of existing drugs, as publicly published by Horizonscan, were also taken into account (18).
Sensitivity analyses
Compared with dermatology, for some medical specialties such as (plastic) surgery, ear, nose and throat (ENT) and radiotherapy, claims data are less specific for skin cancer management. For example, a plastic surgery DRG claim may concern both skin tumours and skin infections. Therefore, we performed a sensitivity analysis, in which 50% of the costs for non-specific claims (Table SI1) were included compared with 100% in the main analyses. This limit was chosen with the assumption that at least half of the included claims concerned skin cancer. Drug reimbursement costs were excluded from the sensitivity analysis, as these are not specific for a specialty. Given the unknown future trends in incidence rates 2 sensitivity analyses were performed for predicting the future costs: scenario B, an annual increasing incidence rate of 10%; and scenario C, stabilization with flattening of the incidence of skin cancer.
RESULTS
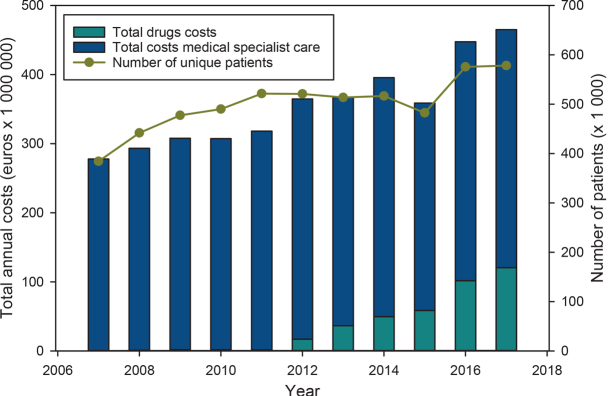
The total annual costs for benign, premalignant and malignant skin tumour management (including drug and hospital care) increased from €278 million for 384,390 patients in 2007 to €465 million for 578,355 patients in 2017 (Fig. 1). The mean costs per patient for benign, premalignant and malignant skin tumour management increased from €723 to €804 during the period 2007–2017.
Fig. 1.
Total annual costs of skin tumours (i.e. benign, in situ and malignant) for drugs and medical specialist care, vs the number of unique patients, 2007–2017. The relative trough level in 2015 was caused by shortening of the Diagnosis Related Group duration in 2015, causing artificially lower costs.
Malignant tumours show a higher mean annual cost per patient for hospital care compared with premalignant and benign tumours and naevi. For malignant skin tumours the mean annual costs per patient show an upward trend between 2007 and 2012 (i.e. 35% increase), while a downward trend is seen during the last 5 years (i.e. 20% decrease). In contrast to the decreasing mean annual costs per patient, the total annual costs for malignant skin tumours continue to increase, resulting from increasing incidence. In 2017, the annual costs of malignant skin tumours comprise 60% of the total skin tumour expenditure (€112.7/€188 in millions), representing 44% of all patients with skin tumours in dermatology (221,307/505,555).
Costs for skin tumour management per treating medical specialty
In 2017, the costs for dermatological skin tumour management were €188 million (comprising 85% of all patients with skin tumours and 56% of the total costs), whereas the total costs for other specialties remained below €50 million. Internal medicine is the most costly per patient, treating 0.5% of all patients with annual mean costs per patient increasing from €3,759 in 2007 to €7,085 in 2017. The total costs in 2017 for internal medicine comprised 6% of the total skin tumour-related hospital care costs.
Cost-drivers in skin cancer management
With the introduction of new agents, in particular for the treatment of advanced and metastatic melanoma, drug costs comprise an increasing part of the healthcare costs. The total drug costs increased from €0.7 million (2007) to €121 million (2017). The main contribution to the increase in the total drug costs concerned add-on pharmaceuticals, being €118 million in 2017 (Fig. 1) (for 8,351 users). The total costs for regular drugs increased from €0.7 million (2007; for 10,305 users) to €2.5 million (2017; for 45,662 users).
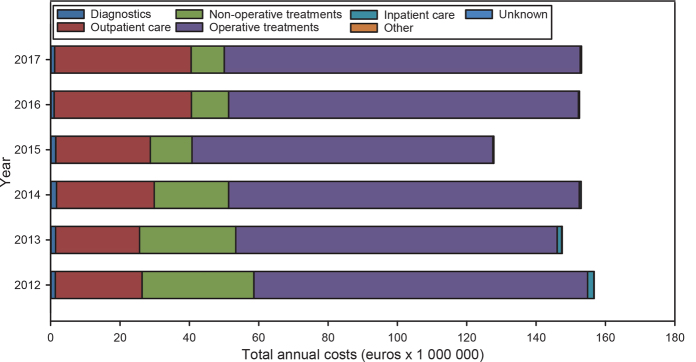
Excluding drug costs, the largest cost-drivers for the total costs of dermatological skin cancer management regarding premalignant and malignant tumours (€153/€188) were operative treatments, comprising 67% of the total costs in 2017 (Fig. 2). The next main cost-drivers were outpatient visits (26%) and non-operative treatments (6%).
Fig. 2.
Cost-drivers of skin cancer management (premalignant and malignant tumours) in dermatology medical specialist care, 2012–2017.
Costs of skin cancer compared with other cancers
The direct hospital costs of malignant skin tumour management comprised €244 million in 2017, making it the 4th most costly cancer in the Netherlands, with breast cancer, colorectal cancer and lung cancer being more costly (Table I).
Table I.
The top 10 most costly cancers, both invasive and in situ, in the Netherlands (2017) based on direct costs of hospital care
| Type of cancer | Total costs (in million €) |
|---|---|
| Breast cancer | 473 |
| Colorectal cancer | 368 |
| Lung cancer | 299 |
| Skin cancer | 244 |
| Leukaemias | 204 |
| Renal and kidney cancer | 148 |
| Cancer of the brain and nervous system | 137 |
| Prostate cancer | 128 |
| Hodgkin/non-Hodgkin lymphomas | 118 |
| Cancer of the bladder | 114 |
Projections to 2030
The reimbursed costs for benign, premalignant and malignant skin tumour management are projected to be €681 million in 2020, €959 million in 2025 and €1.35 billion in 2030, for the scenario based on an annual increase in incidence rate of 5% (Fig. 3).
Fig. 3.
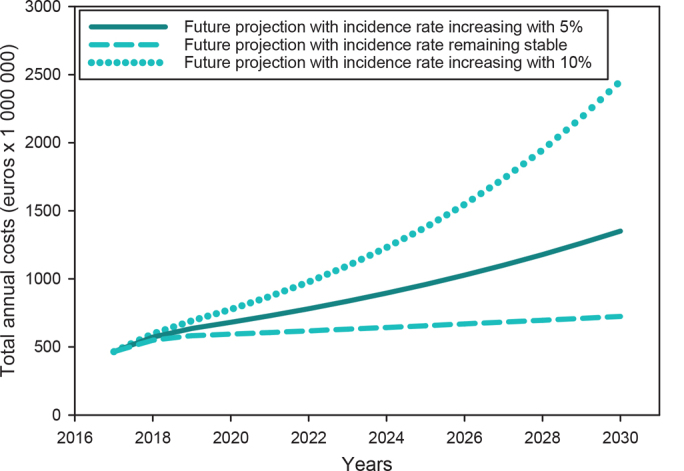
Future projects of the reimbursed costs for benign, premalignant and malignant skin tumour management out to 2030, based on 3 scenarios. The analyses with an annual 5% increasing incidence rate (solid line) is the most likely scenario.
Sensitivity analyses
The observed difference between the main and sensitivity analyses for total direct hospital costs was €27.7 million (8%) in 2017, with total costs for medical specialist care being €317 million in 2017 compared with €345 million in the main analyses. The sensitivity analyses for the future costs projections showed that, with a stable incidence rate, the overall costs would increase up to €602 million by 2030 due to inflation, compared with €465 million in 2017 (Fig. 3). The overall costs could be as high as €2.1 billion by 2030, with incidence rates increasing by 10% annually.
DISCUSSION
This study provides up-to-date quantified data on reimbursed drug and hospital care costs of skin tumour management and presents trends over the past decade in the Netherlands. Skin cancer is the 4th most costly cancer in the Netherlands, and is strongly affected by its increasing incidence and the introduction of expensive drugs. As expected, reimbursement costs for malignant skin tumours are both in total and per patient higher than for benign skin tumours. Large differences were observed in hospital care costs for skin tumour management among different medical specialties. Whereas internal medicine was the most costly per patient, although covering a small volume, dermatology was one of the least costly per patient, although with the highest volume. The high costs for internal medicine are the result of the introduction of expensive (add-on) drugs, including immunotherapy and targeted therapy, for treatment of advanced and metastatic skin cancer, in particular melanoma.
Taking costly (add-on) drug costs into account, the total annual skin tumour costs increased by 67%, with a 50% increase in total patient volume over the last decade. Drug costs represented 26% (€121/€465 million) of the total costs in 2017, and 0.3% (€0.7/€278 million) in 2007. This reflects the increasingly important role of pharmaceutical treatment in the management of skin cancer, especially with the introduction of new drugs. Another example contributing to the increasing role of drugs in skin cancer management involves the restricted reimbursement for photodynamic therapy since 2013 in the Netherlands. This resulted from studies demonstrating that inexpensive treatments, such as topical 5-fluoruracil and imiquimod, are non-inferior or even superior to photodynamic therapy for superficial BCC (19, 20).
When comparing different specialties, the mean annual reimbursed costs per patient for internal medicine is striking; an evident increase was observed after an initial decrease in 2011–12 (data not shown). This downfall may be largely attributable to Dutch participation in several clinical trials on BRAF-inhibition and checkpoint inhibition for advanced and metastatic melanoma (21–27). As these trials are sponsored by pharmaceutical companies, the costs are not included in the insurance registry data. After the approval and reimbursement of ipilimumab in 2012, additional expensive agents were approved, leading to increasing mean annual costs for internal medicine. In addition to the drug costs, treatment with these new agents is associated with other costs including frequent hospital visits, extensive diagnostics (i.e. laboratory tests and imaging), and management of drug-induced toxicities (10, 28). As a result of the potential high efficacy and durable systemic treatment for melanoma, it is expected that these costs will further increase with the expanding use of expensive drugs (for example, adjuvant therapy) for stage III melanoma (18, 29).
Several previous studies have estimated the costs associated with skin cancer management (8, 11, 30–32). However, in contrast to our study, these studies extrapolated the costs according to national incidence or prevalence data, or are subtype specific (i.e. melanoma). Furthermore, with the lack of formal registries of SCCs and BCCs in many countries, this creates further uncertainty and making direct comparisons difficult (2).
The rationale for restructuring the healthcare system in 2006 was to implement a system in which healthcare expenditures would become more controllable (33). Regardless of this restructuring, the healthcare expenditures continue to increase (33–35). This also applies to skin cancer: although the mean costs per patient in dermato-oncological care decreased, the overall expenditures increased. This has 2 main reasons: the increasing number of patients (1.5-fold increase) and therefore increasing use of healthcare services, and the increase in supply of healthcare services with the development of new (expensive) drugs. Our future projection, which is based solely on a 5% increasing incidence rate and inflation, shows the costs increasing to €1.35 billion by 2030. Although this excludes a further price-effect by new drugs introduced in the future, and an additional volume-effect by broadening indications of pharmaceutical treatment, it already shows an alarming increase in overall costs.
Considering the increasing costs of skin cancer management, and the fact that the majority of skin cancers are preventable, there is considerable potential regarding efficient prevention. Several skin cancer prevention programmes (e.g. melanoma screening, sensitization campaign, ban on sunbed use) may be cost-effective and also cost saving for governments (8, 30, 36), but until now prevention strategies initiated by the Dutch government have been restricted. In recent years, the Dutch Cancer Society (KWF) initiated more active campaigning on the prevention of skin cancer, although it focusses mainly on sun-tanning behaviour (37). We therefore believe that governments should play a larger role in initiating and supporting major public health campaigns for skin cancer prevention, more similar to that of tobacco control. With our study showing drug reimbursement costs contributing substantially to the total cost of skin tumour management, this is a key element for health policymakers.
Although the database used comprises a high sensitivity due to the obligatory aspect of registering DRGs, the described reimbursed healthcare may, however, still be an underestimation for 3 main reasons. Drug reimbursement costs were not provided by Vektis when the number of users was fewer than 10, as this may distort competition in the price negotiations between insurers and care providers. Secondly, drugs provided in a trial setting are not registered by insurers, and thus not included in our study. Finally, patients receiving a skin examination as a secondary healthcare request during other health-related visits may have not been recorded, although this amount is probably small due to a financial incentive for complete registration. Benign tumours were included in the evaluation of the total reimbursed costs for skin tumour management, as they may initially appear malignant, for which referral to secondary care is warranted. This may have resulted in a small overestimation of the economic burden.
Inherent to the design of our study, the primary care costs and indirect costs of skin tumour management were not included, since this information is not recorded by health insurance companies. Previous studies have shown indirect costs in skin tumour management to be lower compared with other types of cancer, due to a relatively low amount of production loss (38–40).
Since the claims system was not developed for research purposes, our study has some limitations. Medical specialties other than dermatology have a less specific coding system regarding skin tumour management, due to which we were unable to differentiate the costs between, for example, costs attributable to melanoma and non-melanoma skin cancer. This may also create some uncertainty regarding the specificity of the costs associated with skin cancer. Our sensitivity analysis showed this uncertainty to be 8% (€27.7 million) for 2017.
In conclusion, the results of this study shows that the hospital care costs for skin tumour management are increasing steeply, with skin cancer being the 4th most costly cancer in the Netherlands. As the affordability of healthcare is pivotal, hospital costs are under pressure. The increasing costs seem to be affected mainly by the introduction of expensive drugs, emphasizing the need for controlling drug costs by, for example, effective price negotiations. In addition, increasing skin cancer incidence contributed to the increasing costs, providing a potential target for intervention. The government has an important role in supporting and initiating effective prevention campaigns.
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Singleterry J. The costs of cancer. American Cancer Society Cancer Action Network 2017. [cited March 25 2019] Available from: http://www.acscan.org. [Google Scholar]
- 2.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–1080. [DOI] [PubMed] [Google Scholar]
- 3.Hollestein LM, de Vries E, Aarts MJ, Schroten C, Nijsten TE. Burden of disease caused by keratinocyte cancer has increased in the Netherlands since 1989. J Am Acad Dermatol 2014; 71: 896–903. [DOI] [PubMed] [Google Scholar]
- 4.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol 2010; 146: 279–282. [DOI] [PubMed] [Google Scholar]
- 5.Slaper HvD A, den Outer P, van Kranen H, Slobbe L. [UV radiation and health: Problem area and knowledge base at RIVM. https://www.rivm.nl/publicaties/uv-straling-en-gezondheid-probleemveld-en-kennisbasis-bij-rivm 2017. (in Dutch). [Google Scholar]
- 6.Hollestein L, Weinstock M, Le Roux E, Olsen C. More than many: how to manage the most frequent cancer? J Invest Dermatol 2017; 137: 1823–1826. [DOI] [PubMed] [Google Scholar]
- 7.Lim HW, Collins SAB, Resneck JS, Jr., Bolognia JL, Hodge JA, Rohrer TA, et al. The burden of skin disease in the United States. J Am Acad Dermatol 2017; 76: 958–972e2. [DOI] [PubMed] [Google Scholar]
- 8.Gordon LG, Rowell D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review. Eur J Cancer Prev 2015; 24: 141–149. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz ES, Morgan FC, Zigler CM, Besaw RJ, Schmults CD. National skin cancer expenditure analysis in the United States Medicare population, 2013. J Am Acad Dermatol 2019; 80: 275–278. [DOI] [PubMed] [Google Scholar]
- 10.Franken MG, Leeneman B, Jochems A, Schouwenburg MG, Aarts MJB, van Akkooi ACJ, et al. Real-world healthcare costs of ipilimumab in patients with advanced cutaneous melanoma in The Netherlands. Anti-Cancer Drugs 2018; 29: 579–588. [DOI] [PubMed] [Google Scholar]
- 11.Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, Acostamadiedo JM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol 2003; 48: 425–429. [DOI] [PubMed] [Google Scholar]
- 12.Eindhoven DC, van Staveren LN, van Erkelens JA, Ikkersheim DE, Cannegieter SC, Umans V, et al. Nationwide claims data validated for quality assessments in acute myocardial infarction in the Netherlands. Neth Heart J 2018; 26: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Ven WP, Schut FT. Universal mandatory health insurance in the Netherlands: a model for the United States? Health Aff (Millwood) 2008; 27: 771–781. [DOI] [PubMed] [Google Scholar]
- 14.Hollestein LM, van den Akker SA, Nijsten T, Karim-Kos HE, Coebergh JW, de Vries E. Trends of cutaneous melanoma in The Netherlands: increasing incidence rates among all Breslow thickness categories and rising mortality rates since 1989. Ann Oncol 2012; 23: 524–530. [DOI] [PubMed] [Google Scholar]
- 15.Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989–2008. Eur J Cancer 2012; 48: 2046–2053. [DOI] [PubMed] [Google Scholar]
- 16.Flohil SC, de Vries E, Neumann HA, Coebergh JW, Nijsten T. Incidence, prevalence and future trends of primary basal cell carcinoma in the Netherlands. Acta Derm Venereol 2011; 91: 24–30. [DOI] [PubMed] [Google Scholar]
- 17.OECD . Inflation forecast (indicator). 2019. [cited 2019 Mar 7]. doi: 10.1787/598f4aa4-en. [DOI] [Google Scholar]
- 18.Horizonscan . 2019. Horizonscan Geneesmiddelen Oncologie en hematologie [cited 2019 Apr 4]. Available from: https://www.horizonscangeneesmiddelen.nl/geneesmiddelen?hoofdniveau=domein&domein=oncologie-en-hematologie. [Google Scholar]
- 19.Arits AH, Mosterd K, Essers BA, Spoorenberg E, Sommer A, De Rooij MJ, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial. Lancet Oncol 2013; 14: 647–654. [DOI] [PubMed] [Google Scholar]
- 20.Nijsten T. Commentary on ‘Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial’. Br J Dermatol 2015; 172: 12. [DOI] [PubMed] [Google Scholar]
- 21.Ascierto PA, McArthur GA, Dreno B, Atkinson V, Liszkay G, Di Giacomo AM, et al. Cobimetinib combined with vemura-fenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016; 17: 1248–1260. [DOI] [PubMed] [Google Scholar]
- 22.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol 2017; 28: 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 26.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017; 390: 1853–1862. [DOI] [PubMed] [Google Scholar]
- 27.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377: 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehler E, Zhao Z, Pinar Bilir S, Munakata J, Barber B. Economic burden of toxicities associated with treating metastatic melanoma in eight countries. Eur J Health Econ 2017; 18: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggermont AMM, Dummer R. The 2017 complete overhaul of adjuvant therapies for high-risk melanoma and its consequences for staging and management of melanoma patients. Eur J Cancer 2017; 86: 101–105. [DOI] [PubMed] [Google Scholar]
- 30.Pil L, Hoorens I, Vossaert K, Kruse V, Tromme I, Speybroeck N, et al. Burden of skin cancer in Belgium and cost-effectiveness of primary prevention by reducing ultraviolet exposure. Prev Med 2016; 93: 177–182. [DOI] [PubMed] [Google Scholar]
- 31.Guy GP, Jr., Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med 2015; 48: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kandel M, Allayous C, Dalle S, Mortier L, Dalac S, Dutriaux C, et al. Update of survival and cost of metastatic melanoma with new drugs: estimations from the MelBase cohort. Eur J Cancer 2018; 105: 33–40. [DOI] [PubMed] [Google Scholar]
- 33.Kroneman M, Boerma W, van den Berg M, Groenewegen P, de Jong J, van Ginneken E. Netherlands: health system review. Health Syst Transit 2016; 18: 1–240. [PubMed] [Google Scholar]
- 34.Papanicolas I, Woskie LR, Jha AK. Health care spending in the united states and other high-income countries. JAMA 2018; 319: 1024–1039. [DOI] [PubMed] [Google Scholar]
- 35.Marino A, Morgan D, Lorenzoni L and James C (2017). Future trends in health care expenditure: A modelling framework for cross-country forecasts. OECD Health Working Papers, No. 95, OECD Publishing, Paris. 10.1787/247995bb-en. [DOI] [Google Scholar]
- 36.Krueger H. The economic burden of skin cancer in Canada: current and projected. 2010. [cited March 25 2019]. Available from: http://krueger.ca/wp-content/uploads/2015/08/skincancer.pdf. [Google Scholar]
- 37.de Roos KP, de Haas E. [Vision document on the prevention of skin cancer.] Nederlands Tijdschrift voor Dermatologie en Venereologie 2017; 27: 4 (in Dutch). [Google Scholar]
- 38.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 2013; 14: 1165–1174. [DOI] [PubMed] [Google Scholar]
- 39.Tinghog G, Carlsson P, Synnerstad I, Rosdahl I. Societal cost of skin cancer in Sweden in 2005. Acta Derm Venereol 2008; 88: 467–473. [DOI] [PubMed] [Google Scholar]
- 40.Eriksson T, Tinghog G. Societal cost of skin cancer in Sweden in 2011. Acta Derm Venereol 2015; 95: 347–348. [DOI] [PubMed] [Google Scholar]