Abstract
Malodour from the axilla is commonly caused by specific microbes, and may be inhibited by zinc oxide. The aim of this study was to determine the effects of zinc oxide on the axillary microbiota, odour and pH in a randomized, double-blind, placebo-controlled trial in 30 healthy volunteers. In each participant one axilla was treated with zinc oxide and the other with a placebo for 13 days. The microbiota and pH were ana-lysed before and during treatment. At the final visit, the participants judged their own axillary odour for comparison. With zinc oxide treatment total bacterial growth and, specifically, that of odour-producing Corynebacterium spp. and Staphylococcus hominis, decreased (p<0.05), despite an increase (p<0.0005) in skin-surface pH. Compared with the placebo, zinc oxide treatment reduced (p = 0.005) self-perceived malodour. In vitro, Corynebacterium spp. (19 isolated strains) survival was reduced (p<0.0005) at pH 5.0 compared with pH 6.0; growth inhibition by zinc oxide occurred at ≤400 mg/l, and cell death occurred at ≤10,000 mg/l for 12 (63%) of the strains. In conclusion, application of zinc oxide reduced malodour and the counts of causative bacteria, but increased the pH of the axilla.
Key words: microbiota, malodour, topical antiseptics, susceptibility tests, skin pH
Bothersome odour from the axilla is commonly caused by decomposition of apocrine gland secretions by corynebacteria and staphylococci (1–5). There is a need for effective anti-odour treatments (6). Zinc oxide (ZnO) is a white, odour-free powder and a common ingredient in dermatological topical preparations due to its protective, antiphlogistic and antibacterial activities (7–11). The first commercial deodorant (“Mum”) contained ZnO. Interestingly, topical ZnO reduces the occurrence of Corynebacterium spp. and malodour in open wounds (12). The release of odorants from Corynebacterium spp. mediated by zinc-dependent bacterial proteinases may counteract this anti-odour effect (13).
SIGNIFICANCE
Corynebacterium spp. thrive in the intertriginous axilla, and can cause malodour. Zinc oxide is a mild antiseptic compound effective against Gram-positive organisms. In healthy volunteers, this study found that, compared with a placebo, zinc oxide formulated in an oil-in-water emulsion reduced the counts of aerobic microbiota, including corynebacteria, and decreased self-evaluated axillary malodour. The isolated Corynebacterium spp. showed reduced survival at pH 5.0 and susceptibility to zinc oxide in vitro. This study provides novel data that may prove useful for basic microbiome research and the development of efficient deodorant products for the axilla.
The generation of zinc ions (Zn2+) is the major mechanism for the antibacterial activity of ZnO (9). The solubility of ZnO in pure water is low, but increases many-fold in the presence of zinc-binding ligands, such as proteins (9, 14). pH is another important factor for the ionization of ZnO (15, 16), and the solubility of ZnO in an aqueous solution is ˜0.25 g/l at pH 7 and ˜25 g/l at pH 6 (14). Thus, pH is a determinant of the antibacterial efficacy of ZnO. Furthermore, the effect of ZnO treatment on skin-surface pH is unknown.
The primary aim of this randomized, double-blind, placebo-controlled trial was to investigate whether applications of ZnO reduces colonization by Corynebacterium spp. in the axillae compared with that of an oil-in-water emulsion placebo in healthy volunteers.
MATERIALS AND METHODS
The trial was approved by the Committees of Health Research Ethics in the Capital Region of Denmark (H-16045754) and registered at ClinicalTrials.gov (NCT03221699). The trial was conducted at the Digestive Disease Center, Bispebjerg Hospital, University of Copenhagen, Copenhagen, Denmark.
Participants
Healthy non-smoking volunteers between 18 and 65 years of age were recruited via www.forsoegsperson.dk and were included after providing written informed consent. Individuals with skin disorders; those who were pregnant, breastfeeding or receiving immunosuppressive treatment; and/or those who were hypersensitive to zinc and/or other ingredients in the formulations were excluded.
Study design, bacterial sampling, pH measurements, randomization, application of formulations and odour evaluation
Participants discontinued their habitual application of deodorants, antiperspirants or other cosmetic products to the axillae within 8 h before their first visit to hospital (day 0) and throughout the entire trial period. At baseline, day 0, the participants’ axilla vault was swabbed by rotating (1 s/rotation) the tip of a dry nylon-flocked swab (eSwab, Copan, Brescia, Italy) 3 times clockwise and then 3 times counter-clockwise. This process was repeated using the side of the swab. The recovery of corynebacteria was not increased by moistening the swab with sterile saline (0.9% (w/v) NaCl (5). The pH of the non-hairy axillary fossa was measured with an InLab® surface electrode connected to a pH meter 1140 (Mettler-Toledo, Greifensee, Switzerland). Before each measurement, the calibrated electrode was rinsed in distilled water, dabbed dry and moistened with a non-woven swab saturated in distilled water. The electrode was positioned vertically on the skin surface and the pH was recorded when the signal had been stable for 7 s (17). The ZnO and placebo formulations were compared in each participant and were randomly assigned to the right or left axilla (with a different formulation in the contralateral axilla). The allocation (1:1 ratio) was computer-generated in blocks of 10 and concealed. Starting on day 0, participants applied 0.5 ml of the indistinguishable oil-in-water emulsion containing 1.1% (w/w) ZnO and an oil-in-water emulsion placebo from masked 1-ml syringes labelled with the participant’s consecutive number and right or left axilla, from the vault to 5 cm beneath the hairline, using a powder-free, disposable latex finger cot. Participants entered the time of application as morning (06:00–11:00 h), noon (11:00–14:00 h) or evening (15:00–22:00 h) in their log chart. Washing of the treatment areas within 8 h of application was not allowed. Participants were requested to reapply the assigned formulations after showers. On days 8 and 13, bacterial swabs were again obtained, and the pH was measured at the hospital; on day 13, the participants were asked: (i) whether they had observed a difference in the odour from the left and right axillae and, if so, (ii) which axilla they considered to be less odorous. Pain was reported by the participants using the short version of the validated McGill questionnaire (18).
Microbiology
Culture of swabs, semi-quantification and bacteria identification. The swab was inserted into a tube containing 1 ml Amies transport medium and kept at 4°C until processing, which was performed within 24 h. Samples (10 µl) were applied to blue agar plates with modified Conradi-Drigalski diagnostic substrate selective for Gram-negative bacteria and to 5% horse blood agar (19). The blue agar plates were incubated in ambient air for 24 h at 35°C and the red blood agar plates were incubated in 5% CO2/95% ambient air for 48 h at 35°C.
Bacterial growth was estimated semiquantitatively as 0 (no growth), 1×102, 1× 103, 1×104 and ≥ 1×105 CFU/ml.
Isolated bacteria were identified by light microscopy and Microflex matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Billerica, MA, USA) with FlexAnalysis™ software. Scores ≥2.0 were required for identification at the species level and ≥ 1.7 at the genus level (20–22). The individual isolated strains were cryopreserved in 10% glycerol at –80°C.
In vitro investigations on corynebacteria. The strains of Corynebacterium spp. isolated from the axillae at baseline were used for these investigations.
The survival of each bacterial strain was investigated in 1 ml saline (0.5 McFarland standard) buffered at pH 5.0, pH 6.0 and pH 7.0 with 0.20 M 4-morpholineethanesulfonic acid (16). The turbidity was measured at λ=580 nm by spectrophotometry (DensiCHEK Plus; BioMérieux, Durham, NC, USA). The bacterial suspensions were incubated at 35°C, and aliquots (100 µl) were taken after 0 (only saline), 4 and 24 h of incubation. Serial 10-fold dilutions were made in saline, and 10 µl was seeded on 5% horse blood agar plates, which were incubated in 5% CO2/95% ambient air for 48 h at 35°C. Colonies were counted, and the concentrations (CFU/ml) were calculated (23).
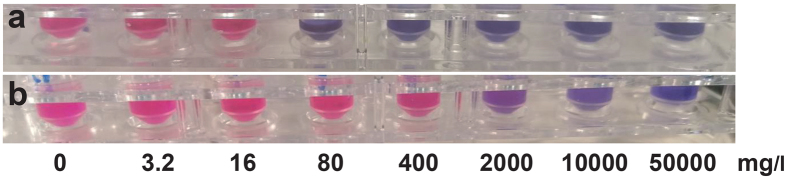
The antibacterial effect of ZnO was investigated using a broth dilution method. Initial tests showed that Müller-Hinton broth did not support the growth of the fastidious corynebacteria; therefore, another broth (0.5% Lab-Lemco beef extract powder, 1.0% special peptone, 0.3% NaCl, 0.2% Na2HPO4 · 2H2O, pH 7.4) that was supplemented with 5% (v/v) horse serum was used for serial 5-fold dilutions of 5.5% ZnO with vigorous vortex mixing in sterile 2-ml screw-capped microcentrifuge polypropylene tubes (Sarstedt, Nümbrecht, Germany). The primary particle size of ZnO (EMSURE®, Ph Eur; Merck KGaA, Darmstadt, Germany) was ˜5 µm. The 200-µl ZnO suspensions and control (200 µl broth with 5% horse serum alone) were inoculated with 20 µl of the respective strain in sterile saline (1.0 McFarland standard corresponding to ˜1 × 105 CFU/ml) resulting in final ZnO concentrations of 50,000, 10,000, 2,000, 400, 80, 16 and 3.2 mg/l. Protein precipitation occurred at Zn2+ concentration > 400 mg/l. The same tests were performed in parallel on a reference strain (S. aureus ATCC® 25923). The tubes were incubated with continuous shaking (500 rpm) in humidified ambient air for 24 h at 35°C. Aliquots (10 µl) were seeded on 5% horse blood agar plates, which were incubated in ambient air for 48 h at 35°C. The minimum bactericidal concentration (MBC) was determined by the lowest ZnO concentration causing no growth. Sterile resazurin (R7017; Sigma-Aldrich) solution at 0.015% in phosphate-buffered saline (pH 7.4) was then added (60 µl) to the tubes, which were incubated in ambient air for another 24 h at 35°C (24). The minimum inhibitory concentration (MIC) was determined from the shift of pink to violet/blue colour (Fig. 1).
Fig. 1.
Determinationofminimum inhibitory concentration (MIC)for zincoxide (ZnO)using the viability dye resazurin. Two experimental setups with the ZnO dilution series (from left to right: 0, 3.2, 16, 80, 400, 2,000, 10,000 and 50,000 mg/l) are shown for (a) Corynebacterium ureicelerivorans and (b) S. aureus ATCC® 25923. Breakpoints were taken as the shift from pink, representing viable bacterial cells, to violet/blue colour. Accordingly, the MIC values were determined to 80 mg/l for (a) C. ureicelerivorans and (b) 2,000 mg/l for S. aureus ATCC® 25923.
pH and zinc ion measurements of the formulations. One millilitre of the ZnO formulation or 1 ml placebo formulation was centrifuged at 17,800 rpm at 4°C for 3 h in 1.5-ml Eppendorf tubes. The pH of the almost clear top layer (200–300 µl) was determined using Hamilton® MiniTrode pH electrode (Merck) connected to a PHM 95 pH/Ion meter (Radiometer, Copenhagen, Denmark). For zinc determination, the top layer after the first centrifugation (200–300 µl) was transferred to a 0.22-µm centrifuge filter unit (Ultra free-MC, Millipore) that was centrifuged for 1.5 h at 17,800 rpm at 4°C. The clear filtrate (˜15 µl/ml formulation) was diluted with 2% ultrapure grade HNO3 (69%, Sigma-Aldrich), and the zinc content was determined by inductively coupled plasma-optical emission spectrometry (Avio 200 ICP-OES; PerkinElmer, Waltham, MA, USA) equipped with a Meinhard concentric glass nebulizer (type K1 LDV). Calibration was performed on samples containing 0.000, 0.050, 0.100, 0.500, 1.000 and 5.000 ppm free Zn2+ in 2% HNO3. The zinc emission lines used in this study were 206.200 nm, 213.857 nm and 202.548 nm. Milli-Q® water was used for sample dilution. Two syringes of each formulation were analysed.
Blinding
All investigators and participants were blinded. The identities of the formulations were decoded after the analyses had been completed, and conclusions were drawn.
Sample size calculation and statistical analyses
It was expected that Corynebacterium spp. would dominate in the axillae of 40% of the participants, and that ZnO treatment would antiperspirants. reduce this value to 8% (5, 12). Based on these assumptions along One woman withdrew from the study after the baseline with a 1-tailed McNemar’s test (α =5%, β =20%), we calculated visit. The ZnO treatment was allocated to the right axilla that a sample size of n =27 was necessary. Thirty participants were included to account for dropouts.
The log-transformed microbiota data and pH data were analysed applied once daily for 13 days in 16 participants and once by analysis of variance (ANOVA). Self-evaluated odour rating was analysed by the Pearson χ2 test. The effect of different pH values on Corynebacterium spp. survival was analysed by the t-test. p-values < 0.05 were considered significant.
RESULTS
pH and zinc ion concentrations of the formulations
The pH of the aqueous phase of the ZnO formulation was 7.77, and the pH of the placebo formulation was 5.70. The Zn2+ concentration in the aqueous phase of the ZnO formulation, obtained after centrifugation and elimination of solid ZnO particles, was 1,450 mg/l. The placebo formulation contained 0.6 mg/l Zn2+.
Participants, product application/acceptance and timing of bacterial swabs/pH measurements
Thirty non-smoking Caucasians (age 21–39 years, mean ±standard deviation [SD] 25.6 ±4.7 years), 15 females and 15 males, were included from 2 February 2017 to 9 March 2017. All female and no male participants had shaved axillae. Eighteen participants used deodorants alone, 10 used antiperspirants alone, one woman used both deodorants and antiperspirants, and one man used neither deodorants nor antiperspirants.
One woman withdrew from the study after the baseline visit. The ZnO treatment was allocated to the right axilla in 13 volunteers. The ZnO and placebo formulations were applied once daily for 13 days in 16 participants and once for 9–12 days and twice for 1–4 days in 13 participants, distributed into 153 applications in the morning, 105 at noon and 150 in the evening. None of the participants experienced discomfort with any formulation, except for one female who reported itching from the 5th application onwards of the placebo formulation.
Bacteria were sampled, and pH was measured in the morning in 75 of the 88 measurement sessions at the hospital.
Microbiology
At least 3 different species of the genus Corynebacterium were isolated and identified at baseline from 19 participants (63%), namely, 9 women and 10 men. The typical morphology of an isolated Corynebacterium strain is shown in Fig. 2. Staphylococcus hominis inhabited the naive axillae of 4 women and 4 men. The most prevalent bacterium in the axilla was Staphylococcus epidermidis, isolated from 28 (93%) of the participants. Gram-negative bacteria occurred only sporadically. The baseline composition of the microbiota was similar for the ZnO and placebo groups (Table I).
Fig. 2.
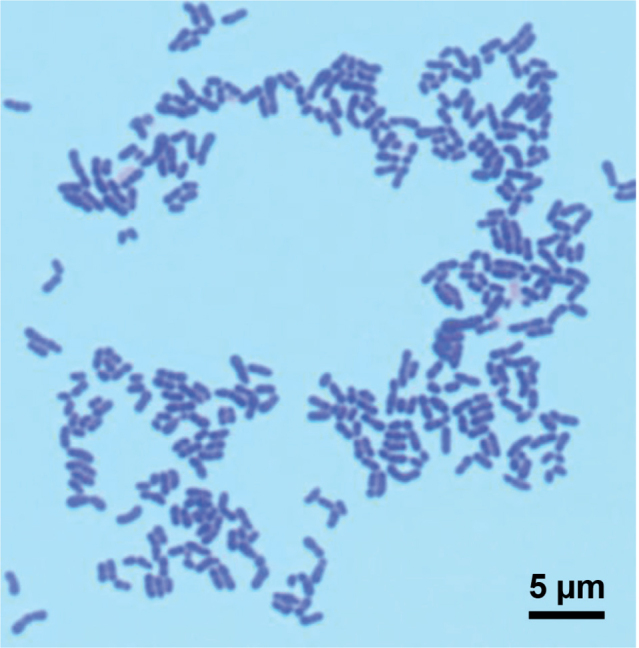
Corynebacterium tuberculostearicum. The bacteria were flame-fixed, stained, cover-slipped with Pertex® mounting medium and examined with oil immersion 100× objective. Bar: 5 µm. Gram stain.
Table I.
Prevalence of bacteria isolated from the axillae of 30 healthy volunteers before the initiation of treatment (baseline) and after treatment with zinc oxide (ZnO) or a placebo for 8 and 13 daysa
| Bacteria | Baseline | Day 8 | Day 13 | |||
|---|---|---|---|---|---|---|
| ZnO (n = 30) | Placebo (n = 30) | ZnO (n = 29) | Placebo (n = 29) | ZnO (n = 29) | Placebo (n = 29) | |
| C. jeikeium | 0 | 0 | 1 | 0 | 0 | 0 |
| C. kroppenstedtiib | 2 | 2 | 0 | 0 | 0 | 0 |
| C. tuberculostearicumb | 4 | 6 | 0 | 7 | 2 | 7 |
| C. ureicelerivorans | 8 | 6 | 1 | 1 | 0 | 0 |
| C. spp. | 3 | 3 | 0 | 2 | 1 | 1 |
| Enterobacter aerogenes | 1 | 1 | 1 | 1 | 1 | 0 |
| Enterobacter cloacae | 0 | 0 | 0 | 0 | 1 | 0 |
| Micrococcus luteus | 4 | 1 | 6 | 3 | 7 | 5 |
| Moraxella atlantae | 0 | 1 | 0 | 1 | 0 | 1 |
| Moraxella spp. | 0 | 0 | 1 | 2 | 0 | 0 |
| Propionibacterium acnes | 2 | 3 | 1 | 1 | 4 | 2 |
| Pseudomonas spp. | 0 | 0 | 1 | 0 | 0 | 0 |
| Sphingomonas paucimobilis | 0 | 0 | 0 | 0 | 1 | 0 |
| S. aureus | 0 | 1 | 0 | 0 | 0 | 0 |
| S. capitis | 1 | 1 | 3 | 0 | 5 | 2 |
| S. caprae | 1 | 1 | 0 | 0 | 0 | 0 |
| S. epidermidis | 27 | 27 | 21 | 23 | 22 | 23 |
| S. haemolyticus | 1 | 0 | 1 | 1 | 1 | 0 |
| S. hominis | 5 | 6 | 5 | 17 | 8 | 16 |
| S. lugdunensis | 3 | 4 | 4 | 0 | 4 | 3 |
| S. pasteuri | 0 | 0 | 1 | 0 | 0 | 0 |
| S. warneri | 1 | 2 | 0 | 0 | 1 | 0 |
| S. spp. | 1 | 1 | 0 | 2 | 0 | 2 |
The numbers of axillae with the indicated bacteria are presented.
C. kroppenstedtii and C. tuberculostearicum were isolated from both axillae of one female participant at baseline.
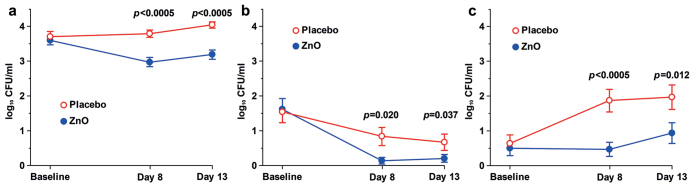
ZnO treatment significantly reduced the overall bacterial growth (Fig. 3a) and, specifically, the growth of Corynebacterium spp. (Fig. 3b) and S. hominis (Fig. 3c).
Fig. 3.
Effect of zinc oxide (ZnO) on the growth of: (a) all retrievable bacteria; (b) Corynebacterium spp.; (c) S. hominis. Mean±standard error of the mean (SEM).
Odour evaluation
Thirteen participants reported differences in odour between the 2 treated axillae, 11 (5 women and 6 men) of whom (10 with Corynebacterium spp.) rated the axilla treated with ZnO as having less odour than the other axilla, which was treated with placebo (p =0.005).
Skin-surface pH
The mean ±SD baseline axillary pH of women was 5.44 ±0.47, and that of men was 5.38 ±0.41 (p =0.60). Compared with the placebo, the ZnO treatment increased (p <0.0005) the skin surface pH by ˜0.5 on days 8 and 13 (Fig. 4).
Fig. 4.
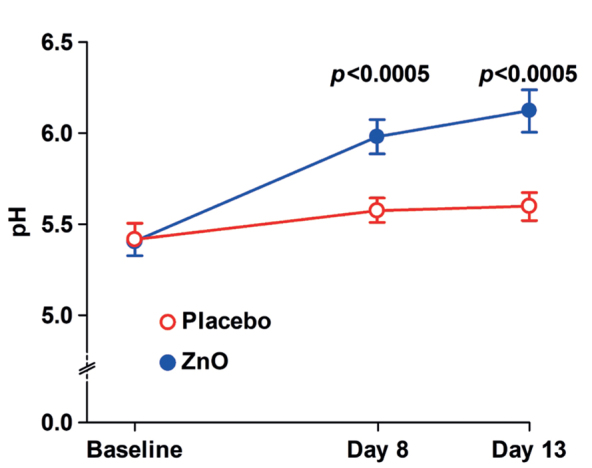
Effect of zinc oxide (ZnO) on the axillary skin surface pH. Mean±standard error of the mean (SEM).
In vitro investigations
These investigations were carried out on the 19 isolated strains (C. kroppenstedtii, n =2; C. tuberculostearicum, n =7; C. ureicelerivorans, n =8; C. spp., n =2).
The survival of the corynebacteria decreased (p<0.0005) from 4 h onwards at pH 5.0 compared with pH 6.0 and pH 7.0, while survival did not differ between pH 6.0 and pH 7.0 at 4 h (p = 0.69) or 24 h (p =0.39) (Fig. 5).
Fig. 5.
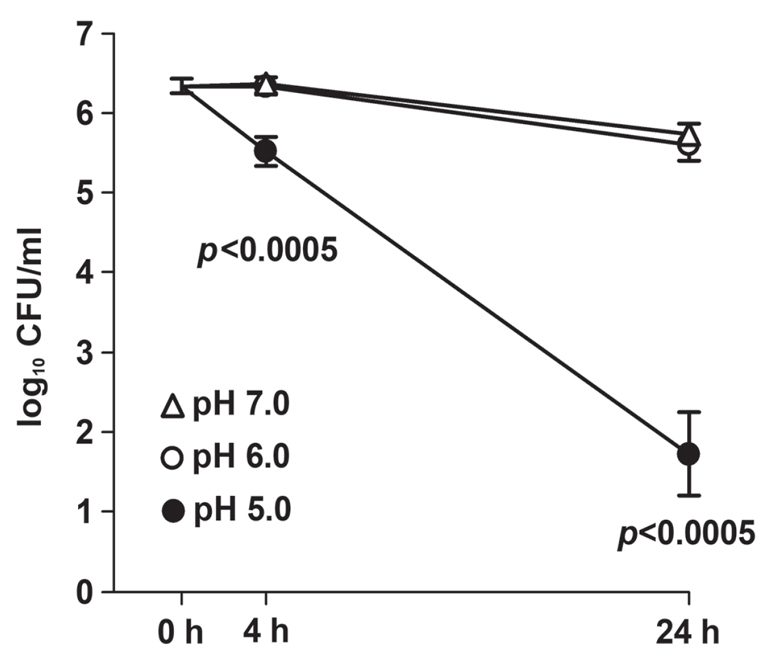
Effect of pH on the survival of the 19 isolates of Corynebacterium spp. after incubation at 35°C for 0, 4 and 24 at pH 5.0, 6.0 and 7.0. Mean±standard error of the mean (SEM).
In vitro, ZnO inhibited the growth of Corynebacterium spp. isolates with MIC values ranging from 3.2 to 400 mg/l and MBC of ≤10,000 mg/l for 12 (63%) of the 19 tested strains (Table II). The MIC for ZnO against S. aureus ATCC® 25923 was determined as 2,000 mg/l.
Table II.
Susceptibility of Corynebacterium spp. isolates (19 strains) to zinc oxide (ZnO) in vitro
| Strains growth-inhibited (MIC) or dead (MBC) at each ZnO concentration, n | |||||||
|---|---|---|---|---|---|---|---|
| ≢3.2 mg/l | 16 mg/l | 80 mg/l | 400 mg/l | 2,000 mg/l | 10,000 mg/l | >50,000 mg/l | |
| Minimum inhibitory concentration (MIC) | 2a | 5b | 10c | 2d | |||
| Minimum bactericidal concentration (MBC) | 1e | 3f | 8g | 7h | |||
1 C. kroppenstedtii and 1 C. ureicelerivorans.
1 C. kroppenstedtii and 4 C. tuberculostearicum.
2 C. tuberculostearicum, 7 C. ureicelerivorans and 1 C. spp.
1 C. tuberculostearicum and 1 C. spp.
1 C. kroppenstedtii.
2 C. tuberculostearicum and 1 C. ureicelerivorans.
1 C. kroppenstedtii, 4 C. tuberculostearicum, 2 C. ureicelerivorans and 1 C. spp.
1 C. tuberculostearicum, 5 C. ureicelerivorans and 1 C. spp.
DISCUSSION
In the present randomized double-blind trial, the topical ZnO treatment decreased the levels of Corynebacterium spp. in the axillae of healthy volunteers. The levels of Corynebacterium spp. correlate strongly with axillary malodour (1–4) and may explain the reduced self-perceived axilla malodour with ZnO compared with the placebo formulation. S. hominis was recently identified as a malodour producer (4, 25) and its growth in the axillae was also reduced by ZnO exposure.
The anti-odour effect of ZnO was obvious for 11 of 13 participants. Exclusion of subjects with low axillary odour and right-left asymmetry would probably have increased the power of the current trial (26). Furthermore, a panel of judges trained in malodour assessment may have detected more subtle differences than the participants could, as recommended by the cosmetic industry (26). Finally, we cannot exclude the possibility that the placebo formulation possessed anti-odour activity.
Corynebacterium spp. were isolated from 63% of the participants’ axillae. Earlier studies reported a comparatively higher (85–93%) prevalence of Corynebacterium spp. in the axillae (1, 3). The discrepancy may be explained by their use of the more vigorous sampling scrub cup technique with buffer solutions, which presumably also recover bacteria inhabiting hair follicles (27). Environmental factors, such as climate and season, also influence the microbiota (5).
Culture-independent molecular methods may yield different results (3–5), although the MALDI-TOF technique correctly identifies most members of the genus Corynebacterium (20). On the other hand, the culture method allowed us to semi-quantify the growth of all bacteria.
All women had shaved their axillae, but the presence of hair in the axilla does not appear to appreciably alter the microbiota (1).
Skin pH is important for skin integrity and microbial colonization (28). It is thought that lowering the pH in the axilla mitigates malodour via inhibition of the growth of corynebacteria (29). To our knowledge, the effect of pH on growth of Corynebacterium spp. has not been investigated previously. The current study found that the survival of the Corynebacterium spp. drastically decreased at pH 5.0 compared with pH 6.0. Notably, the pH in the hairy axillary vault is approximately 6.0, which is 0.5 units higher than that in the non-hairy axillary fossa (30). Interestingly, the malodour from cultured corynebacteria is maximal at pH 6.0 (2).
The increased pH of the formulation and of the skin surface with the addition of ZnO was attributed to the generation of alkaline hydroxide ions together with zinc ions (ZnO(s) + H2O⇄Zn2+(aq) + 2OH–(aq)) (14). The current study found that approximately 16% of the solid ZnO was solubilized in the deodorant formulation. The pH-modulating effect of ZnO may counteract its antibacterial activity due to increased survival of Corynebacterium spp. organisms with increasing pH. Nonetheless, low skin pH promotes the dissolution of ZnO via acid-catalysed hydrolysis (ZnO(s) + 2H+(aq)⇄Zn2+(aq) + H2O), thereby increasing the zinc ion (Zn2+) concentration and the antibacterial activity of ZnO (9, 31). In vitro tests in the current study indicated that ZnO is bacteriostatic at ≤400 mg/l and bactericidal at ≤10,000 mg/l for 63% of the clinical isolates of corynebacteria. Furthermore, the corynebacteria appeared to be more sensitive to ZnO than did S. aureus (9, 10).
Apart from its antibacterial activity (7–10), ZnO quenches volatile malodorous short-chain fatty acids (32). This mechanism may contribute to less malodorous axillae.
In conclusion, ZnO treatment effectively reduced self-perceived malodour originating from the axillae of healthy individuals, and this effect was most likely mediated by the antibacterial action of ZnO, primarily against Corynebacterium spp.
ACKNOWLEDGEMENTS
The authors thank Dr Chris Callewaert for commenting on the manuscript. The underarm formulations and some funding were provided by Colgate-Palmolive Company, Piscataway, NJ, USA.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Leyden JJ, McGinley KJ, Holzle E, Labows JN, Kligman AM. The microbiology of the human axilla and its relationship to axillary odor. J Invest Dermatol 1981; 77: 413–416. [DOI] [PubMed] [Google Scholar]
- 2.Rennie PJ, Gower DB, Holland KT, Mallet AI, Watkins WJ. The skin microflora and the formation of human axillary odour. Int J Cosmet Sci 1990; 12: 197–207. [DOI] [PubMed] [Google Scholar]
- 3.Taylor D, Daulby A, Grimshaw S, James G, Mercer J, Vaziri S. Characterization of the microflora of the human axilla. Int J Cosmet Sci 2003; 25: 137–145. [DOI] [PubMed] [Google Scholar]
- 4.Troccaz M, Gaia N, Beccucci S, Schrenzel J, Cayeux I, Starkenmann C, et al. Mapping axillary microbiota responsible for body odours using a culture-independent approach. Microbiome 2015; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callewaert C, Kerckhof FM, Granitsiotis MS, Van Gele M, Van de Wiele T, Boon N. Characterization of Staphylococcus and Corynebacterium clusters in the human axillary region. PLoS One 2013; 8: e70538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callewaert C, Lambert J, Van de Wiele T. Towards a bacterial treatment for armpit malodour. Exp Dermatol 2017; 26: 388–391. [DOI] [PubMed] [Google Scholar]
- 7.Bandyopadhyay P, Joseph MT. Quantification of in vitro malodor generation by anionic surfactant-induced fluorescent sensor property of tryptophan. Anal Biochem 2010; 397: 89–95. [DOI] [PubMed] [Google Scholar]
- 8.Abendrot M, Kalinowska-Lis U. Zinc-containing compounds for personal care applications. Int J Cosmet Sci 2018; 40: 319–327. [DOI] [PubMed] [Google Scholar]
- 9.Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D, Bolzinger MA. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2014; 457: 263–274. [Google Scholar]
- 10.Söderberg TA, Sunzel B, Holm S, Elmros T, Hallmans G, Sjöberg S. Antibacterial effect of zinc oxide in vitro. Scand J Plast Reconstr Surg Hand Surg 1990; 24: 193–197. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin S, Odio MR, Haines SL, O’Connor RJ, Englehart JS, Lane AT. Skin benefits from continuous topical administration of a zinc oxide/petrolatum formulation by a novel disposable diaper. J Eur Acad Dermatol Venereol 2001; 15 Suppl 1: 5–11. [DOI] [PubMed] [Google Scholar]
- 12.Ågren MS, Ostenfeld U, Kallehave F, Gong Y, Raffn K, Crawford ME, et al. A randomized, double-blind, placebo-controlled multicenter trial evaluating topical zinc oxide for acute open wounds following pilonidal disease excision. Wound Repair Regen 2006; 14: 526–535. [DOI] [PubMed] [Google Scholar]
- 13.Natsch A, Gfeller H, Gygax P, Schmid J, Acuna G. A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. J Biol Chem 2003; 278: 5718–5727. [DOI] [PubMed] [Google Scholar]
- 14.Dirkse TP. The solubility product constant of ZnO. J Electrochem Soc 1986; 133: 1656–1657. [Google Scholar]
- 15.Han J, Qiu W, Gao W. Potential dissolution and photo-dissolution of ZnO thin films. J Hazard Mater 2010; 178: 115–122. [DOI] [PubMed] [Google Scholar]
- 16.Ågren MS. Percutaneous absorption of zinc from zinc oxide applied topically to intact skin in man. Dermatologica 1990; 180: 36–39. [PubMed] [Google Scholar]
- 17.Ehlers C, Ivens UI, Moller ML, Senderovitz T, Serup J. Comparison of two pH meters used for skin surface pH measurement: the pH meter ‘pH900’ from Courage & Khazaka versus the pH meter ‘1140’ from Mettler Toledo. Skin Res Technol 2001; 7: 84–89. [DOI] [PubMed] [Google Scholar]
- 18.Melzack R. The short-form McGill Pain Questionnaire. Pain 1987; 30: 191–197. [DOI] [PubMed] [Google Scholar]
- 19.Larsen HF, Ahlström MG, Gjerdrum LMR, Mogensen M, Ghathian K, Calum H, et al. Noninvasive measurement of reepithelialization and microvascularity of suction-blister wounds with benchmarking to histology. Wound Repair Regen 2017; 25: 984–993. [DOI] [PubMed] [Google Scholar]
- 20.Alatoom AA, Cazanave CJ, Cunningham SA, Ihde SM, Patel R. Identification of non-diphtheriae corynebacterium by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2011; 50: 160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konrad R, Berger A, Huber I, Boschert V, Hörmansdorfer S, Busch U, et al. Matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry as a tool for rapid diagnosis of potentially toxigenic Corynebacterium species in the laboratory management of diphtheria-associated bacteria. Euro Surveill 2010; 15; pii 19699. [DOI] [PubMed] [Google Scholar]
- 22.Dubois D, Leyssene D, Chacornac JP, Kostrzewa M, Schmit PO, Talon R, et al. Identification of a variety of Staphylococcus species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2010; 48: 941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szweda P, Gorczyca G, Tylingo R. Comparison of antimicrobial activity of selected, commercially available wound dressing materials. J Wound Care 2018; 27: 320–326. [DOI] [PubMed] [Google Scholar]
- 24.Elshikh M, Ahmed S, Funston S, Dunlop P, McGaw M, Mar-chant R, et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett 2016; 38: 1015–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bawdon D, Cox DS, Ashford D, James AG, Thomas GH. Identification of axillary Staphylococcus sp. involved in the production of the malodorous thioalcohol 3-methyl-3-sufanylhexan-1-ol. FEMS Microbiol Lett 2015; 362. [DOI] [PubMed] [Google Scholar]
- 26.ASTM Subcommittee E18.07 . Standard guide for sensory evaluation of axillary deodorancy. ASTM E1207 – 14. West Conshohocken, PA, USA: ASTM International; 2014. [Google Scholar]
- 27.Ring HC, Bay L, Kallenbach K, Miller IM, Prens E, Saunte DM, et al. Normal skin microbiota is altered in pre-clinical hidradenitis suppurativa. Acta Derm Venereol 2017; 97: 208–213. [DOI] [PubMed] [Google Scholar]
- 28.Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol 2013; 93: 261–267. [DOI] [PubMed] [Google Scholar]
- 29.Stenzaly-Achtert S, Scholermann A, Schreiber J, Diec KH, Rippke F, Bielfeldt S. Axillary pH and influence of deodorants. Skin Res Technol 2000; 6: 87–91. [DOI] [PubMed] [Google Scholar]
- 30.Burry JS, Coulson HF, Esser I, Marti V, Melling SJ, Rawlings AV, et al. Erroneous gender differences in axillary skin surface/ sweat pH. Int J Cosmet Sci 2001; 23: 99–107. [DOI] [PubMed] [Google Scholar]
- 31.Holmes AM, Song Z, Moghimi HR, Roberts MS. Relative penetration of zinc oxide and zinc ions into human skin after application of different zinc oxide formulations. ACS Nano 2016; 10: 1810–1819. [DOI] [PubMed] [Google Scholar]
- 32.Kanda F, Yagi E, Fukuda M, Matsuoka M. Quenching short chain fatty acids (SCFA) responsible for human body odors. Cosmetics & Toiletries 1993; 108: 67–72. [Google Scholar]