Abstract
Recently the use of plant-derived extracts for the green synthesis of nanoparticles has drawn considerable attention. In the present study silver and copper nanoparticles were synthesized using extracts of Andrographis paniculata which is found to possess various pharmacological properties. The synthesized nanoparticles were characterized using UV spectroscopy, SEM with EDS, XRD, TEM and DLS. Furthermore, an attempt is made to impregnate these nanoparticles onto cotton bandages. The structure and morphology of silver nanoparticles impregnated onto the cotton bandages were confirmed by SEM. The anti-bacterial activity of cotton bandages loaded with silver and copper nanoparticles was tested against Escherichia coli, Bacillus cereus, and Staphylococcus aureus using a modified disc diffusion assay. The results indicate that the cotton bandages biofabricated with nanoparticles exhibited anti-bacterial activity in terms of zone of inhibition of growth of tested bacteria suggesting their usage as medical textiles in various biomedical applications for the prevention of infections. Hence, the nanoparticles impregnated cotton fibers can be applied for the development of masks, aprons, etc. to protect against bacterial penetration and as well to counteract the present situation of the world.
Keywords: Metal nanoparticles, Andrographis paniculata, Green synthesis, Anti-bacterial activity, Medical textiles
Introduction
The development of anti-microbial and antibiotic-resistant pathogens makes the treatment of bacterial infections complicated leading to the exploration of novel approaches for circumventing this problem. Metal nanoparticles are acknowledged to possess strong inhibitory and anti-bacterial effects (Khodadadi et al. 2021; Urnukhsaikhan et al. 2021). These metal nanoparticles can be synthesized using physical, chemical and biological methods (Rónavári et al. 2021). However, physical and chemical methods involve high energy constraints and the use of toxic reagents for synthesis. This issue can be overcome by employing biological agents, such as bacteria, fungi, algae, actinomycetes, and plants for the synthesis of metal nanoparticles (John et al. 2021; Koul et al. 2021; Chugh et al. 2021; Rai et al. 2021; Dikshit et al. 2021). Recently, the use of plant extracts for nanoparticle synthesis has received much attention due to the rich diversity of phytochemicals, availability in bulk quantities, and simplicity (Balciunaitiene et al. 2021; Amjad et al. 2021; Gaddam et al. 2021; Aboyewa et al. 2021; Selvakesavan and Franklin 2021; Sawalha et al. 2021; Rajagopal et al. 2021).
Andrographis paniculata Nees (commonly known as the king of bitters) is a medicinal plant that belongs to the Acanthaceae family (Jayakumar et al. 2013; Niranjan et al. 2010). The plant is native to India and Srilanka and is distributed in northern parts of India, Java, Malaysia, Indonesia, West Indies, Hong Kong, Thailand, and Singapore (Jayakumar et al. 2013; Niranjan et al. 2010; Nugroho et al. 2013). The plant is used to treat common cold and fever and is found to exhibit pharmacological activities, such as immunostimulatory, anti-viral and anti-bacterial activities (Jayakumar et al. 2013). Major active phytoconstituents of the plant are andrographolide, neoandrographolide and dehydroandrographolide. Andrographolide exhibits a broad range of biological activities, such as anti-inflammatory, anti-bacterial, anti-tumor, anti-diabetic, anti-malarial, anti-snake venom, and hepatoprotective (Jayakumar et al. 2013; Niranjan et al. 2010). Anti-SARS-CoV-2 activity of A. paniculata was reported recently (Sa-Ngiamsuntorn et al. 2021).
Cotton fabrics such as the lab, surgical coats, and masks are more prone to microbial attack (Ravindra et al. 2010). Few reports have highlighted the use of nanoparticles in the production of anti-microbial fabrics. Vigneshwaran et al. (2007) reported a novel in situ synthesis protocol for the incorporation of silver nanoparticles onto cotton fabrics. El-Shishtawy et al. (2011) have employed silver nanoparticles coating on cotton fabrics. Khan et al. (2019) developed an anti-bacterial, UV-protected and self-cleaning surgical gown through the blending of zinc oxide nanoparticles with polyvinyl alcohol. Turakhia et al. (2019) utilized root extracts of Z. officinale for the synthesis of iron nanoparticles and further antimicrobial surgical cotton was developed using iron nanoparticles coating. Román et al. (2020) developed anti-bacterial cotton fabrics through functionalization with copper oxide nanoparticles. Tania and Ali (2021) applied zinc nanoparticles onto cotton fabrics using the mechanical thermo-fixation technique.
In the present study, an attempt was made to synthesize silver and copper nanoparticles using an aqueous extract of flowers and roots of A. paniculata. The biologically synthesized nanoparticles were characterized using various methods, such as UV spectral studies, SEM with EDS, XRD, TEM, and DLS. Furthermore, the work also attempted to impregnate the synthesized nanoparticles onto the cotton bandage and to evaluate its anti-bacterial potential. Hence this study will be helpful for the development of novel anti-bacterial medical textiles and wound dressing materials.
Materials and methods
Chemicals
Fresh flowers and roots of A. paniculata were collected from the Kalasalingam Academy of Research and Education Campus. All the chemicals used in the study were obtained from Himedia Laboratories, Mumbai.
Preparation of aqueous extracts of flowers and root of A. paniculata
Fresh flowers and roots were washed thoroughly in tap water, followed by distilled water. Dried flowers and roots were cut into minor pieces and 5 g of cut flowers and roots were boiled for 10 min in 100 mL distilled water and filtered through Whatman No. 1 filter paper (Padalia et al. 2015). The filtered extracts were used for the synthesis of silver and copper nanoparticles.
Synthesis of silver nanoparticles
1 mM aqueous solution of silver nitrate (AgNO3) was used for the synthesis of silver nanoparticles. 3 mL of flower and root extracts was added into 20 mL of AgNO3 solution, and incubated in dark at room temperature for 40 and 50 min, respectively. This causes the reduction of Ag+ ions into Ag nanoparticles (AgNPs). Purification of AgNPs was carried out by repeated centrifugation at 10,000 rpm for 10 min and air-dried. The purified AgNPs were then stored at 4 °C and used for the rest of the analysis (Padalia et al. 2015).
Synthesis of copper nanoparticles
To 20 mL of copper sulphate, 3 mL of flower and of root extracts were added separately in different flasks. The reaction mixture was incubated at room temperature for 60 and 40 min, respectively. This causes the reduction of Cu2+ ions into copper nanoparticles (CuNPs) Purification of nanoparticles was done by repeated centrifugation at 10,000 rpm for 10 min. The nanoparticle pellet was stored at 4 °C for further analysis (Padalia et al. 2015).
Characterization of synthesized silver and copper nanoparticles
The nanoparticles were dispersed in distilled water and UV–Vis spectra of the synthesized AgNPs and CuNPs were acquired using a spectrophotometer (Shimadzu) in the range of 400–700 nm. The FTIR spectra were recorded in the range of 400–4000 cm−1 using Fourier transform infrared spectroscopy (Shimadzu). The surface morphology of the nanoparticles was imaged using scanning electron microscopy (Carl Zeiss) at an accelerating voltage of 15 kV. Elemental analysis was performed using EDS (Bruker). XRD pattern was used to study the crystalline nature of the nanoparticles by a Bruker, ECO D8 Advance X-ray diffractometer operating at a voltage of 40 kV, and a current of 20 mA using CuKα radiation. Scherrer formula (D = 0.94 k/β cosθ) was used to calculate the crystallite domain size from the width of the XRD peaks, where D is the average crystallite domain size perpendicular to the reflecting planes, k is the X-ray wavelength (1.5406), β is the full width at half maximum (FWHM), and θ is the diffraction angle (Dinesh et al. 2012). The shape and size of the synthesized nanoparticles were studied through transmission electron microscopy (Jeol/JEM 2100) operating at 200 kV voltage. The size distribution of AgNPs and CuNPs was analyzed using a particle size analyzer (Horiba) through light scattering.
Impregnation of silver and copper nanoparticles onto cotton bandages
The surgical cotton bandage was cut into small pieces and pre-weighed pieces (2.5 g) of cotton bandage were immersed in 250 mL of 1 mM silver nitrate. To this mixture, 50 mL of extracts of A. paniculata was added and kept on an orbital shaker at 100 rpm at room temperature in dark for 24 h. Phytochemicals present in flower and root extracts result in the reduction of silver ions into silver, which nucleates into AgNPs on the cotton bandages. After 24 h, the pieces of cotton bandages were recovered from the mixture. The AgNP impregnated cotton bandage was washed with deionized water and oven-dried at 55 °C (Vivekanandhan et al. 2012). The same procedure was repeated for impregnating CuNPs on the cotton bandages.
Confirmation of nanoparticle impregnation using SEM
The surface morphology of AgNPs and CuNPs impregnated cotton bandages were analyzed using a scanning electron microscope (Carl Zeiss SEM) at an accelerating voltage of 15 kV. The normal cotton bandage was used as a control.
Anti-bacterial activity of nanoparticle impregnated cotton bandage
Dried pieces of AgNPs and CuNPs impregnated cotton bandages were evaluated for anti-bacterial activity against the test microorganisms, Escherichia coli, Bacillus cereus and Staphylococcus aureus. The overnight cultures prepared using nutrient broth were used for swabbing. The anti-bacterial susceptibility testing was studied similar to disc diffusion assay by placing the pieces of AgNPs and CuNPs impregnated cotton bandages in the form of discs in bacteria swabbed Mueller Hinton agar plates. The plates were inspected for the inhibition zones developed against the tested bacteria after incubation at 37 °C for 1 day. A cotton bandage disc without nanoparticles was used as control.
Results and discussion
Visual observation
With the addition of root and flower extract to the colorless silver nitrate solution, the color changed into reddish brown in the former and dark brown in the latter mixtures, due to the reduction of silver ions to silver nanoparticles as evidenced from Fig. 1a, b. The intensity of color was directly proportional to the formation of AgNPs (Padalia et al. 2015). Similarly, the addition of root and flower extract to copper sulphate results in the color change of the reaction mixture into light green and pale green, respectively, leading to the formation of CuNPs (Gopinath et al. 2014) as visualized from Fig. 1c, d.
Fig. 1.
Silver (a, b) and copper nanoparticles (c, d) synthesis using root extract and flower extract of Andrographis paniculata, respectively
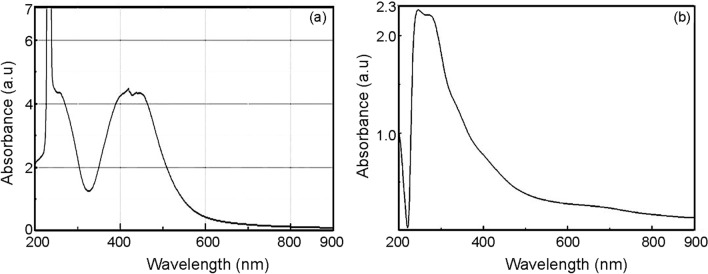
UV–visible spectroscopy
The UV spectrum (Fig. 2a) of the AgNPs synthesized using the root extract displayed a peak at 416 nm by a “surface plasmon resonance” (Sudhakar et al. 2014). Similarly, the AgNPs prepared from the flower extract exhibited an absorption peak at 429 nm. The UV spectrum of biosynthesized CuNPs from the root extract showed a peak at 251 nm (Fig. 2b) and CuNPs prepared from the flower extract revealed a peak at 308 nm by the surface plasmon resonance (Shobha et al. 2014).
Fig. 2.
UV–visible spectrum of silver and copper nanoparticles synthesized using root extract of Andrographis paniculata, respectively
FTIR spectral analysis
FTIR analysis was used to recognize the possible reducing biomolecules in the root and flower extracts. Figure 3a–d shows the FTIR spectra of aqueous AgNPs and CuNPs prepared from root and flower extracts of A. paniculata, respectively. The functional groups and their corresponding signal assignment are provided in Tables 1, 2, 3 and 4. The reducing agents promote the development of metallic nanoparticles from the respective ionic compounds. The reduction is mediated by plant biomolecules, such as sugars, proteins, organic compounds and numerous functional groups, such as C=C (alkenyl), C=N (amide), O=H (phenolic and alcohol), N–H (amine), C–H and COO– (carboxylic group) that are responsible for the synthesis of nanoparticles (Awwad et al. 2013). These metabolites are acknowledged as significant sources for controlling various acute diseases (Kuppusamy et al. 2016).
Fig. 3.
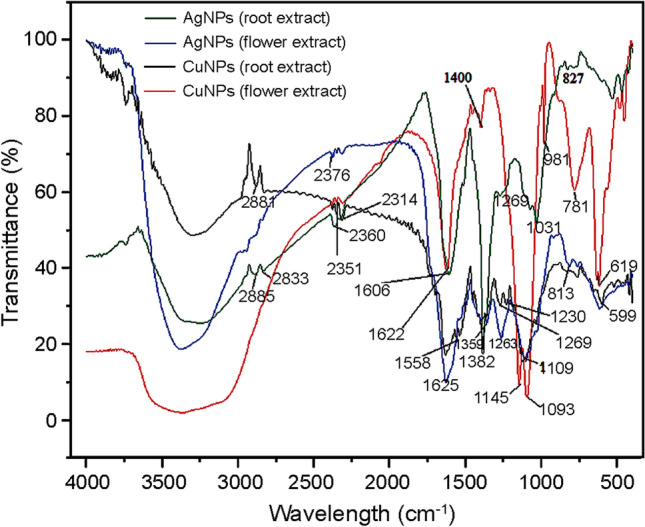
FTIR spectrum of silver (a, b) and copper nanoparticles (c, d) synthesized using root and flower extracts of A.paniculata, respectively
Table 1.
FTIR bands corresponding to the functional groups of silver nanoparticles synthesized from root extract A. paniculata
| Nanoparticles | Wavenumber (cm−1) | Functional group |
|---|---|---|
| Silver nanoparticles (root extract) | 1074.35, 1031.92 | Bending vibration of C–OH groups and anti-symmetric stretching bend of C–O–C groups of polysaccharide (Mollick et al. 2019) |
| 1382.96 | Asymmetrical stretching for nitro compounds (Rajeshkumar et al. 2014) | |
| 1606.60 | C=O stretching modes due to the free carboxylic group (Vijayan et al. 2014) | |
| 2885.51 | CH stretching modes of alkanes (Dinesh et al. 2012) |
Table 2.
FTIR bands corresponding to the functional groups of silver nanoparticles synthesized from flower extract of A. paniculata
Table 3.
FTIR bands corresponding to the functional groups of copper nanoparticles synthesized from flower extract of A. paniculata
| Nanoparticles | Wavenumber (cm−1) | Functional group |
|---|---|---|
| Copper nanoparticles (flower extract) | 619.15 | Vibration of alkyl halides (Rajeshkumar et al. 2014) |
| 1093.64 | Bending vibration of C–OH groups (Mollick et al. 2019) | |
| 1400.32 | C–N-stretching vibrations of aliphatic and aromatic amines, alcohols and phenols (Awwad et al. 2013) | |
| 1622.13 cm | Amide I group of proteins (Mollick et al. 2019) |
Table 4.
FTIR bands corresponding to the functional groups of copper nanoparticles synthesized from the root extract of A. paniculata
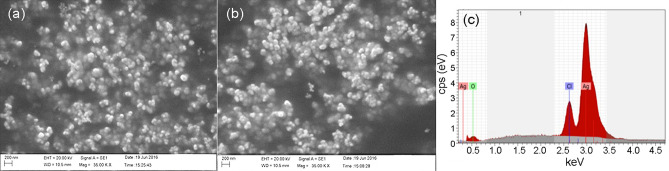
SEM and EDS analysis
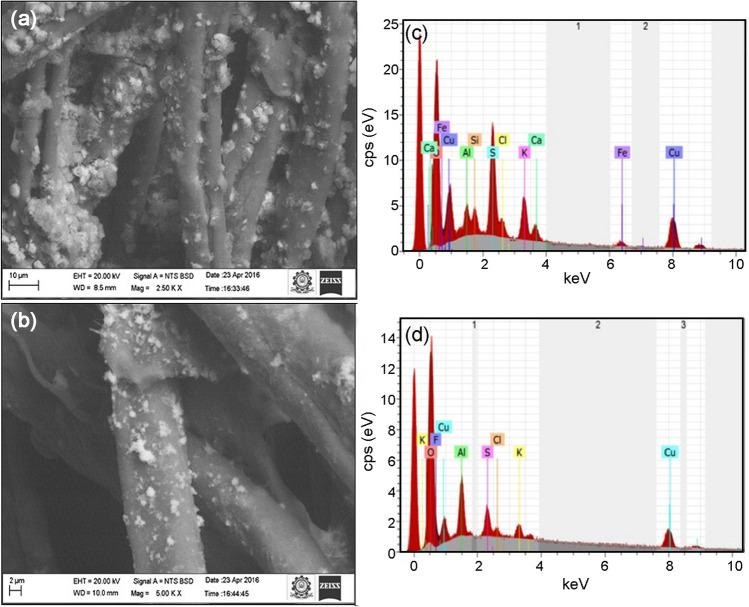
The SEM images of AgNPs synthesized with the root and flower extracts and CuNPs with the root and flower extracts of A. paniculata are shown in Figs. 4a, b and 5a, b, respectively. The EDS graphs of AgNPs and CuNPs synthesized using A. paniculata are depicted in Figs. 4c and 5c, respectively.
Fig. 4.
SEM image of silver (a, b) nanoparticles synthesized using root and flower extracts of A. paniculata; EDS graph of silver nanoparticles (c) synthesized using A. paniculata, respectively
Fig. 5.
SEM image of copper (a, b) nanoparticles synthesized using root and flower extracts of A. paniculata; EDS graph (c) copper nanoparticles synthesized using A. paniculata, respectively
Figure 4a, b shows that AgNPs are in spherical aggregated form and the corresponding EDS (Fig. 4c) confirms the presence of AgNPs with a broad peak at 3 keV (Oluwaniyi et al. 2016). Figure 5a shows that the shape of CuNPs synthesized using root extract is cuboidal, while the CuNPs synthesized using flower extract of A. paniculata is in spherical and aggregated (Fig. 5b). The corresponding EDS graph (Fig. 5c) shows a peak at 8 keV, which is characteristic of the absorption of metallic copper nanoparticles (Caroling et al. 2015).
XRD analysis
Figure 6a shows distinct peaks at 38° and 77° indexed as (111), (311), 27.5° and 32° (111), 38° (200), 46° (220), 55° (311) and 57.5° (222) lattice planes of face-centered cubic (fcc) structure of metallic silver of AgNPs synthesized from flower extract (Ponvel et al. 2015). Similarly, Fig. 6b shows distinct peaks at 38° and 77° which are indexed as (111), (311), 28° and 33° (111), 38° (200), 46.50° (220), 55° (311) and 57.5° (222) lattice planes of face-centered cubic (fcc) structure of metallic silver of AgNPs synthesized from root extract.
Fig. 6.
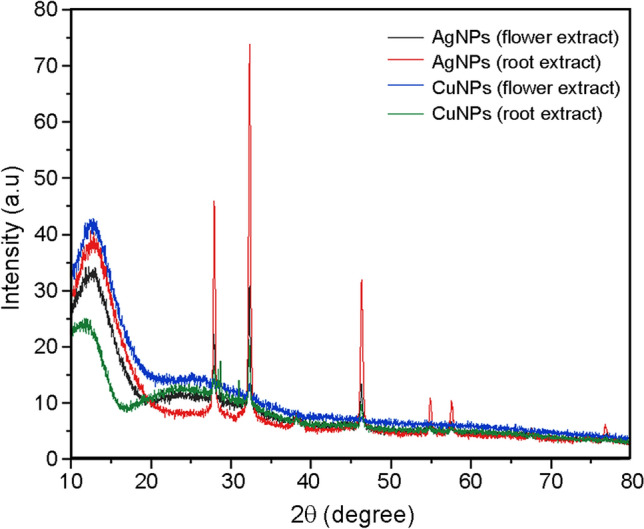
XRD pattern of the silver (a, b) and copper (c, d) nanoparticles synthesized using flower and root extracts of A. paniculata, respectively
Figure 6c, d shows the XRD pattern of the CuNPs synthesized from the flower and root extracts of A. paniculata. The orientation and crystalline nature of the nanoparticles were revealed through the XRD patterns. Peak position at 32° (Fig. 6c) is characteristic of face-centered cubic of copper lines indexed at (210), (111), (210) and (220) and peak position at 28°, 32°, 38°, 46° (Fig. 6d) are characteristic of face-centered cubic of copper lines indexed at (210), (111), (210) and (220), respectively (Awwad et al. 2013). The crystallite size of AgNPs and CuNPs synthesized using root and flower extracts of A. paniculata are calculated using the Scherrer formula and they are tabulated in Table 5. The crystallite size of nanoparticles ranges between 22 and 42 nm.
Table 5.
Crystallite size of the nanoparticles
| Nanoparticles | Crystallite size (nm) | |
|---|---|---|
| Root extract | Flower extract | |
| Silver nanoparticles | 24.73 | 22.098 |
| Copper nanoparticles | 25.96 | 33.65 |
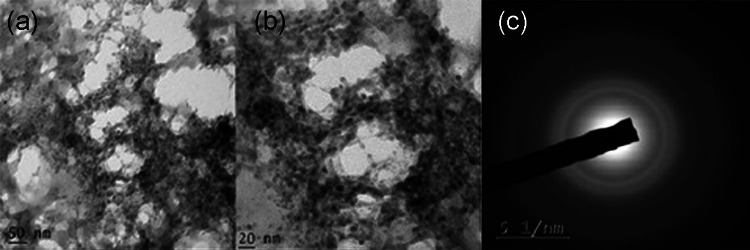
TEM analysis
The TEM images of silver and copper nanoparticles, synthesized from the root extract, are depicted in Figs. 7 and 8, respectively. The shape of the silver nanoparticles in Fig. 7a–c is triangular, spherical and ellipse with moderate variation in size that is polydisperse. The “selected-area electron diffraction” (SAED) patterns portrayed in Fig. 7d exhibit concentric rings with intermittent bright spots, representing that the nanoparticles are crystalline in nature (Padalia et al. 2015). Figure 8a, b shows that the copper nanoparticles are of irregular shape and in aggregate form. SAED pattern depicted in Fig. 8c exhibits diffuse rings indicating that the nanoparticles are amorphous (Caroling et al., 2015).
Fig. 7.
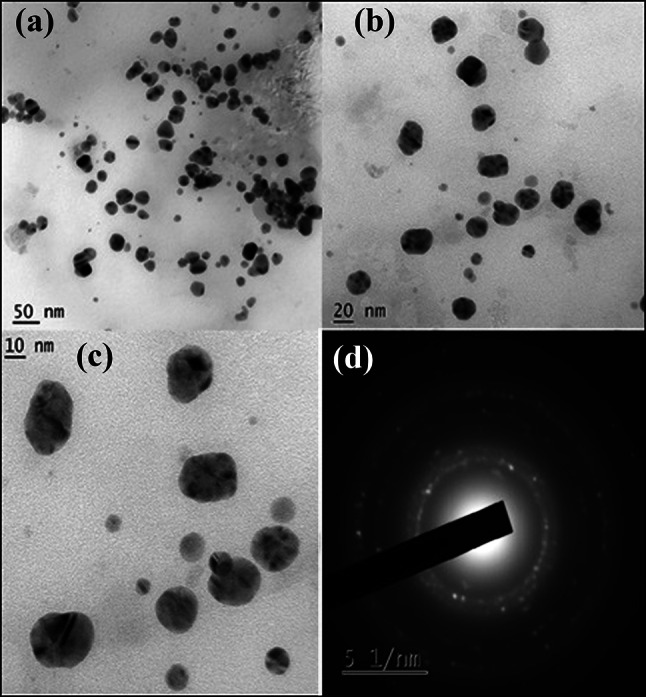
TEM images (a–c) of silver nanoparticles in low and high magnifications, (d) SAED pattern of silver nanoparticles synthesized using root extract of A.paniculata
Fig. 8.
TEM images (a, b) and (c) SAED pattern of copper nanoparticles synthesized using root extract of A.paniculata
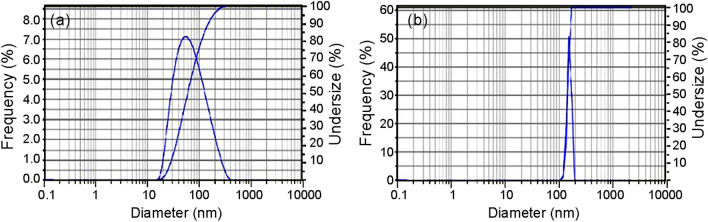
Particle size distribution analysis
The particle size distribution of AgNPs and CuNPs is shown in Fig. 9a, b, respectively. AgNPs have a mean size of 75.8 nm with a polydispersity index of 0.559 and the particle size distribution ranges from 19.03 to 279.04 nm. CuNPs have an average size of 144.8 nm with a polydispersity index of 1.505 and the particle size distribution ranges from 118.74 to 171.25 nm.
Fig. 9.
Particle size distribution of silver (a) and copper (b) nanoparticles prepared using root extract of A.paniculata
Confirmation of nanoparticle impregnation using SEM
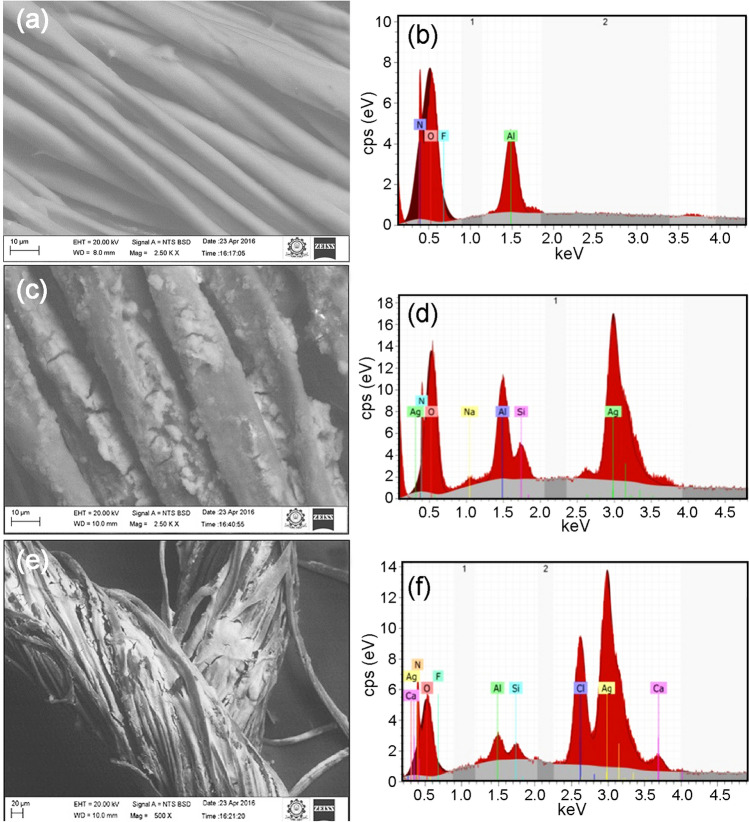
Figure 10a shows the SEM image and Fig. 10b displays the EDS pattern of the control bandage. Figure 10c, e shows the SEM images of AgNPs (flower extract) impregnated cotton bandage and AgNPs (root extract) impregnated cotton bandage, respectively. The corresponding EDS mapping images are given in Fig. 10d, f. Similarly, Fig. 11a, c shows their respective SEM images of CuNPs (flower extract) impregnated cotton bandage and CuNPs (root extract) impregnated cotton bandage. The EDS mapping images are given in Fig. 11b, d, respectively.
Fig. 10.
SEM image and EDS mapping pattern of control cotton bandage (a, b) and silver nanoparticle (flower extract—c, d and root extract—e, f) impregnated cotton bandage, respectively
Fig. 11.
SEM image and EDS mapping pattern of copper nanoparticles (flower extract—a, b, root extract—c, d, impregnated cotton bandage
From Fig. 10a, it is observed that the cotton bandage is smooth and no peak corresponding to silver and copper is displayed in the EDS pattern (Fig. 10b). Figure 10d, f shows that AgNPs (flower and root extract) have been impregnated in the cotton bandage and a peak at 3 keV confirms it as well. Figure 11b, d shows that CuNPs (flower and root extract) have been impregnated in the cotton bandage and is confirmed by a peak at 8 keV. The hydroxyl groups of polysaccharides present in root and flower extracts reduce the silver nitrate into AgNPs on the cotton bandage.
Cotton is a natural fiber that consists of cellulose with 1,4-D-glucose pyranose as its repeating units. These surface hydroxyl groups present in cotton bandage facilitates the adsorption of AgNPs proficiently onto cotton fibers (Ravindra et al. 2010).
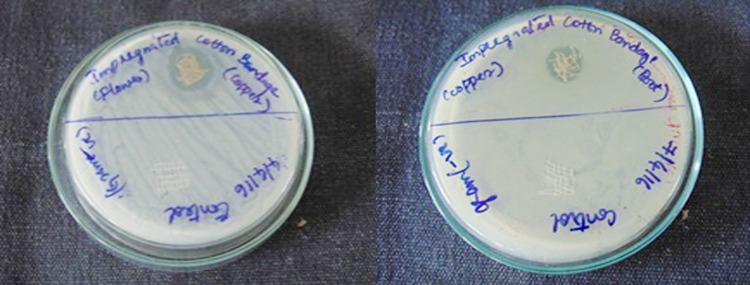
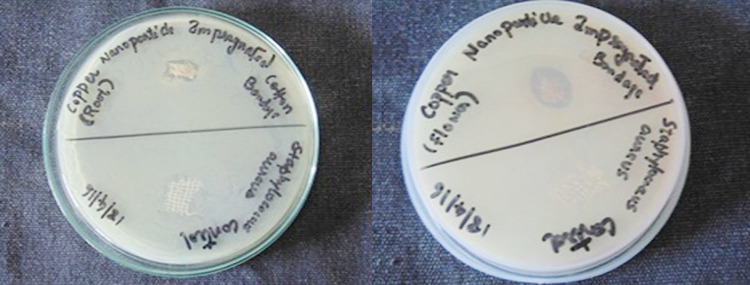
Anti-bacterial activity
Figures 12 and 13 show the anti-bacterial activity of CuNPs and AgNPs impregnated cotton bandage against B. cereus, respectively. Figures 14 and 15 show the anti-bacterial activity of copper and AgNPs impregnated cotton bandage against E. coli, respectively. Figures 16 and 17 show the anti-bacterial activity of CuNPs and AgNPs impregnated cotton bandage against S. aureus, respectively.
Fig. 12.
Anti-bacterial activity of copper nanoparticles (flower and root extract) impregnated cotton bandage against B. cereus, respectively
Fig. 13.
Anti-bacterial activity of silver nanoparticles (root and flower extract) impregnated cotton bandage against B. cereus, respectively
Fig. 14.
Anti-bacterial activity of copper nanoparticles (flower and root extract) impregnated cotton bandage against E. coli, respectively
Fig. 15.
Anti-bacterial activity of silver nanoparticles (root and flower extract) impregnated cotton bandage against E. coli, respectively
Fig. 16.
Anti-bacterial activity of copper nanoparticles (root and flower extract) impregnated cotton bandage against S. aureus, respectively
Fig. 17.
Anti-bacterial activity of silver nanoparticles (flower and root extract) impregnated cotton bandage against S. aureus, respectively
Table 6 shows the inhibition zones of exhibited by nanoparticles impregnated cotton bandage against E. coli, B. cereus, S. aureus, respectively. From the table, it can be inferred that the AgNPs impregnated cotton bandages exhibit better anti-bacterial activity against the tested bacteria.
Table 6.
Inhibition zone by nanoparticles impregnated cotton bandages
| Nanoparticle impregnated cotton bandage | Inhibition zone (cm) | ||
|---|---|---|---|
| Escherichia coli | Bacillus cereus | Staphylococcus aureus | |
| Silver (root) | 1.5 | 1.2 | 1.1 |
| Silver (flower) | 1.3 | 1.5 | 1.3 |
| Copper (root) | 1.2 | 1.1 | 1.2 |
| Copper (flower) | 1.4 | 1.1 | 1.2 |
Overall, the fabricated cotton bandages with AgNPs and CuNPs provided a similar inhibition pattern of all the tested bacteria. Though several studies regarding the green synthesis of metal nanoparticles using plant extracts were available, the present study was focused on the green synthesis of AgNPs and CuNPs using extracts of flower and root of A. paniculata which is not addressed in the literature before. To our knowledge, this is the first report highlighting the use of roots and flowers of A. paniculata for AgNPs and CuNPs synthesis. We have also reported the use of roots and flowers of A. paniculata for the synthesis of gallium nanoparticles (Monika et al. 2017).
Synthesis of silver nanoparticles using leaf extract of A. paniculata was reported by Sulochana et al. (2012). Synthesis of silver nanoparticles using A. paniculata leaf extract and its anti-bacterial activity was evaluated by Sinha and Paul (2015). Devasenan et al., attempted green synthesis of zinc nanoparticles using leaf extract of A. paniculata (Devasenan et al. 2016). Rajakumar et al. synthesized zinc oxide nanoparticles by exploiting the reducing and capping potential of A. paniculata leaf extract (Rajakumar et al. 2018). Anantharaman et al. used leaf extract of A. paniculata for the synthesis of AgNPs and evaluated its anti-microbial properties (Anantharaman et al. 2020). Biological synthesis of titanium dioxide nanoparticles was carried out with leaves extract of A. paniculata by Rajeshkumar et al. (2021).
Furthermore, very few studies have reported the development of medical textiles or cotton bandages fabricated with metal nanoparticles synthesized using physical or chemical methods. As the cotton bandages were fabricated with nanoparticles synthesized using herbal extract, it offers an additional advantage in view of the medical applications.
Conclusions
Metal nanoparticles synthesized through the green synthesis approach are gaining much attention in recent times. An extensive interest is growing particularly when phytochemicals are employed for the reduction of metal ions to nanoparticles. The present study focussed on a green synthesis strategy for the synthesis of silver and copper nanoparticles using extracts of A. paniculata, a medicinal plant used in the treatment of various diseases. The synthesized nanoparticles were characterized using UV spectroscopy, SEM with EDS, XRD, TEM, and DLS analyses. Furthermore, the nanoparticles were impregnated onto the cotton bandages and their anti-bacterial activity was assessed. This study showed that cotton fibres impregnated with nanoparticles through a green synthesis approach can be potentially applied for the development of medical textiles, such as bandages, masks, aprons, etc. for the prevention of infections.
Acknowledgements
The authors acknowledge the support provided by the International Research Centre, Kalasalingam Academy of Research and Education for material characterization facilities, such as FTIR, SEM–EDS, XRD, and Sophisticated Test and Instrumentation Centre (STIC), Cochin University for recording TEM images.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors give the consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aboyewa JA, Sibuyi N, Meyer M, Oguntibeju OO. Green synthesis of metallic nanoparticles using some selected medicinal plants from Southern Africa and their biological applications. Plants. 2021;10:1929. doi: 10.3390/plants10091929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjad R, Mubeen B, Ali SS, Imam SS, Alshehri S, Ghoneim MM, Alzarea SI, Rasool R, Ullah I, Nadeem MS, Kazmi I. Green synthesis and characterization of copper nanoparticles using Fortunella margarita leaves. Polymers. 2021;13:4364. doi: 10.3390/polym13244364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman S, Rego R, Muthakka M, Anties T, Krishna H. Andrographis paniculata-mediated synthesis of silver nanoparticles: antimicrobial properties and computational studies. SN Appl Sci. 2020;2:1–14. doi: 10.1007/s42452-020-03394-7. [DOI] [Google Scholar]
- Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its anti-bacterial activity. Int J Ind Chem. 2013;4:1–6. doi: 10.1186/2228-5547-4-29. [DOI] [Google Scholar]
- Balciunaitiene A, Viskelis P, Viskelis J, Streimikyte P, Liaudanskas M, Bartkiene E, Zavistanaviciute P, Zokaityte E, Starkute V, Ruzauskas M, Lele V. Green synthesis of silver nanoparticles using extract of Artemisia absinthium L., Humulus lupulus L. and Thymus vulgaris L., physico-chemical characterization, antimicrobial and antioxidant activity. Processes. 2021;9:1304. doi: 10.3390/pr9081304. [DOI] [Google Scholar]
- Caroling G, Priyadharshini MN, Vinodhini E, Ranjitham AM, Shanthi P. Biosynthesis of copper nanoparticles using aqueous guava extract-characterization and study of anti-bacterial effects. Int J Pharm Biol Sci. 2015;5:25–43. [Google Scholar]
- Chugh D, Viswamalya VS, Das B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J Genet Eng Biotechnol. 2021;19:1–21. doi: 10.1186/s43141-021-00228-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devasenan S, Beevi NH, Jayanth SS. Green synthesis and characterization of zinc zanoparticle using Andrographis paniculata leaf extract. Int J Pharm Sci Rev Res. 2016;39:243–247. [Google Scholar]
- Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, Gupta PK, Kim BS. Green synthesis of metallic nanoparticles: applications and limitations. Catalysts. 2021;11:902. doi: 10.3390/catal11080902. [DOI] [Google Scholar]
- Dinesh S, Karthikeyan S, Arumugam P. Biosynthesis of silver nanoparticles from Glycyrrhiza glabra root extract. Arch Appl Sci Res. 2012;4:178–187. [Google Scholar]
- El-Shishtawy RM, Asiri AM, Abdelwahed NAM, Al-Otaibi MM. In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose. 2011;18:75–82. doi: 10.1007/s10570-010-9455-1. [DOI] [Google Scholar]
- Gaddam SA, Kotakadi VS, Subramanyam GK, Penchalaneni J, Challagundla VN, DVRPasupuleti SGVR. Multifaceted phytogenic silver nanoparticles by an insectivorous plant Drosera spatulata Labill var. bakoensis and its potential therapeutic applications. Sci Rep. 2021;11:1–17. doi: 10.1038/s41598-021-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath M, Subbaiya R, Selvam MM, Suresh D. Synthesis of copper nanoparticles from Nerium oleander leaf aqueous extract and its anti-bacterial activity. Int J Curr Microbiol Appl Sci. 2014;3:814–818. [Google Scholar]
- Hegazy HS, Shabaan LD, Rabie GH, Raie DS. Biosynthesis of silver nanoparticles using cell free callus exudates of Medicago sativa L. Pak J Bot. 2015;47:1825–1829. [Google Scholar]
- Jayakumar T, Hsieh CY, Lee JJ, Sheu JR. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Based Complement Altern Med. 2013;2013:846740. doi: 10.1155/2013/846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John MS, Nagoth JA, Zannotti M, Giovannetti R, Mancini A, Ramasamy KP, Miceli C, Pucciarelli S. Biogenic synthesis of copper nanoparticles using bacterial strains isolated from an antarctic consortium associated to a psychrophilic marine ciliate: characterization and potential application as antimicrobial agents. Mar Drugs. 2021;19:263. doi: 10.3390/md19050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MQ, Kharaghani D, Nishat N, Shahzad A, Hussain T, Khatri Z, Zhu C, Kim IS. Preparation and characterizations of multifunctional PVA/ZnO nanofibers composite membranes for surgical gown application. J Mater Res Technol. 2019;8:1328–1334. doi: 10.1016/j.jmrt.2018.08.013. [DOI] [Google Scholar]
- Khodadadi S, Mahdinezhad N, Fazeli-Nasab B, Heidari MJ, Fakheri B, Miri A. Investigating the possibility of green synthesis of silver nanoparticles using Vaccinium arctostaphlyos extract and evaluating its antibacterial properties. Biomed Res Int. 2021;2021:1–13. doi: 10.1155/2021/5572252. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Koul B, Poonia AK, Yadav D, Jin JO. Microbe-mediated biosynthesis of nanoparticles: applications and future prospects. Biomolecules. 2021;11:886. doi: 10.3390/biom11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppusamy P, Yusoff MM, Maniam GP, Govindan N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications. Saudi Pharm J. 2016;24:473–484. doi: 10.1016/j.jsps.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollick MR, Rana D, Dash SK, Chattopadhyay S, Bhowmick B, Maity D, Mondal D, Pattanayak S, Roy S, Chakraborty M, Chattopadhyay D. Studies on green synthesized silver nanoparticles using Abelmoschus esculentus (L.) pulp extract having anti-cancer (in vitro) and anti-microbial applications. Arab J Chem. 2019;12:2572–2584. doi: 10.1016/j.arabjc.2015.04.033. [DOI] [Google Scholar]
- Monika S, Ponlakshmi SH, Sundar K, Vanavil B. Biological synthesis of gallium nanoparticles using extracts of Andrographis paniculata. Int J Eng Sci, Adv Comp Biotech. 2017;8:208–222. [Google Scholar]
- Niranjan A, Tewari SK, Lehri A. Biological activities of Kalmegh (Andrographis paniculata Nees) and its active principles. Ind J Nat Prod Resour. 2010;1:125–135. [Google Scholar]
- Nugroho AE, Lindawati NY, Herlyanti K, Widyasturi L, Paramona S. Anti-diabetic effect of a combination of andrographolide-enriched extract of Andrographis paniculata (Burm. f) Nees and asiaticoside-enriched extract of Centella asiatica L. in high fructose-fat fed rats. Indian J Exp Biol. 2013;51:1101–1108. [PubMed] [Google Scholar]
- Oluwaniyi OO, Adegoke HI, Adesuji ET, Alabi AB, Bodede SO, Labulo AH, Oseghale CO. Biosynthesis of silver nanoparticles using aqueous leaf extract of Thevetia peruviana Juss and its anti-microbial activities. Appl Nanosci. 2016;6:903–912. doi: 10.1007/s13204-015-0505-8. [DOI] [Google Scholar]
- Padalia H, Moteriya P, Chanda S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab J Chem. 2015;8:732–741. doi: 10.1016/j.arabjc.2014.11.015. [DOI] [Google Scholar]
- Ponvel KM, Narayanaraja T, Prabakaran J. Biosynthesis of silver nanoparticles using root extract of the medicinal plant Justicia adhatoda: characterization, electrochemical behavior and applications. Int J Nano Dimens. 2015;6:339–349. doi: 10.7508/IJND.2015.04.002. [DOI] [Google Scholar]
- Rai M, Bonde S, Golinska P, Trzcińska-Wencel J, Gade A, Abd-Elsalam KA, Shende S, Gaikwad S, Ingle AP. Fusarium as a novel fungus for the synthesis of nanoparticles: mechanism and applications. J Fungi. 2021;7:139. doi: 10.3390/jof7020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal G, Nivetha A, Sundar M, Panneerselvam T, Murugesan S, Parasuraman P, Kumar S, Kunjiappan IS, S, Mixed phytochemicals mediated synthesis of copper nanoparticles for anticancer and larvicidal applications. Heliyon. 2021;7:e07360. doi: 10.1016/j.heliyon.2021.e07360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumar G, Thiruvengadam M, Mydhili G, Gomathi T, Chung IM. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst Eng. 2018;41:21–30. doi: 10.1007/s00449-017-1840-9. [DOI] [PubMed] [Google Scholar]
- Rajeshkumar S, Malarkodi C, Kumar KP, Vanaja M, Gnanajobitha G, Annadurai G. Algae mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. Int J Met. 2014;2014:1–8. doi: 10.1155/2014/692643. [DOI] [Google Scholar]
- Rajeshkumar S, Santhoshkumar J, Jule LT, Ramaswamy K. Phytosynthesis of titanium dioxide nanoparticles using king of bitter Andrographis paniculata and its embryonic toxicology evaluation and biomedical potential. Bioinorg Chem Appl. 2021;2021:1–11. doi: 10.1155/2021/6267634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra S, Mohan YM, Reddy NN, Raju KM. Fabrication of antibacterial cotton fibers loaded with silver nanoparticles via green approach. Colloids Surf A Physicochem Eng Asp. 2010;367:31–40. doi: 10.1016/j.colsurfa.2010.06.013. [DOI] [Google Scholar]
- Román LE, Gomez ED, Solís JL, Gómez MM. Antibacterial cotton fabric functionalized with copper oxide nanoparticles. Molecules. 2020;25:5802. doi: 10.3390/molecules25245802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rónavári A, Igaz N, Adamecz DI, Szerencsés B, Molnar C, Kónya Z, Pfeiffer I, Kiricsi M. Green silver and gold nanoparticles: biological synthesis approaches and potentials for biomedical applications. Molecules. 2021;26:844. doi: 10.3390/molecules26040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa-Ngiamsuntorn K, Suksatu A, Pewkliang Y, Thongsri P, Kanjanasirirat P, Manopwisedjaroen S, Charoensutthivarakul S, Wongtrakoongate P, Pitiporn S, Chaopreecha J, Kongsomros S, Jearawuttanakul K, Wannalo W, Khemawoot P, Chutipongtanate S, Borwornpinyo S, Thitithanyanont A, Hongeng S. Anti-SARS-CoV-2 Activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J Nat Prod. 2021;84:1261–1270. doi: 10.1021/acs.jnatprod.0c01324. [DOI] [PubMed] [Google Scholar]
- Sawalha H, Abiri R, Sanusi R, Shaharuddin NA, Noor A, Ab Shukor NA, Abdul-Hamid H, Ahmad SA. Toward a better understanding of metal nanoparticles, a novel strategy from Eucalyptus plants. Plants. 2021;10:1–22. doi: 10.3390/plants10050929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakesavan RK, Franklin G. Prospective application of nanoparticles green synthesized using medicinal plant extracts as novel nanomedicines. Nanotechnol Sci Appl. 2021;14:179–195. doi: 10.2147/NSA.S333467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobha G, Vinutha M, Ananda S. Biological synthesis of copper nanoparticles and its impact—a review. Int J Pharm Sci Invent. 2014;3:6–28. [Google Scholar]
- Sinha SN, Paul D. Phytosynthesis of silver nanoparticles using Andrographis paniculata leaf extract and evaluation of their antibacterial activities. Spectrosc Lett. 2015;48:600–604. doi: 10.1080/00387010.2014.938756. [DOI] [Google Scholar]
- Sudhakar PS, Krishna KBM, Sundar S. Synthesis and characterization of silver nanoparticles using aqueous extract of Andrographis paniculata and their anti-microbial activities. Int J Sci Technol Manag. 2014;29:1962–1969. [Google Scholar]
- Sulochana S, Krishnamoorthy P, Sivaranjini K. Synthesis of silver nanoparticles using leaf extracts of Andrographis paniculata. J Pharmacol Toxicol. 2012;7:251–258. doi: 10.3923/jpt.2012.251.258. [DOI] [Google Scholar]
- Tania IS, Ali M. Coating of ZnO nanoparticle on cotton fabric to create a functional textile with enhanced mechanical properties. Polymers. 2021;13:2701. doi: 10.3390/polym13162701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turakhia B, Chikkala S, Shah S. Novelty of bioengineered iron nanoparticles in nanocoated surgical cotton: a green chemistry. Adv Pharmacol Sci. 2019;2019:1–10. doi: 10.1155/2019/9825969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnukhsaikhan E, Bold BE, Gunbileg A, Sukhbaatar N, Mishig-Ochir T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci Rep. 2021;11:1–12. doi: 10.1038/s41598-021-00520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramanya RH. Functional finishing of cotton fabrics using silver nanoparticles. J Nanosci Nanotechnol. 2007;7:1893–1897. doi: 10.1166/jnn.2007.737. [DOI] [PubMed] [Google Scholar]
- Vijayan SR, Santhiyagu P, Singamuthu M, Ahila NK, Jayaraman R, Ethiraj K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides and their anti-micro fouling activity. Sci World J. 2014;2014:1–14. doi: 10.1155/2014/938272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekanandhan S, Laura C, Manjusri M, Mohanty AK. Green process for impregnation of silver nanoparticles into microcrystalline cellulose and their anti-microbial bionanocomposite films. J Biomater Nanobiotech. 2012;3:371–376. doi: 10.4236/jbnb.2012.33035. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.