Abstract
Thailand is one of the leading exporting countries of rambutan and rambutan peels are considered as a biological waste. In this study, rambutan (Nephelium lappaceum L. cv. Rong Rian) peel extracts (RPE) obtained by water extraction were analyzed for their phytochemical composition, antibacterial and antioxidant activities, and cytotoxicity. The bioactive compounds in RPE identified by GC-MS were mome inositol (35.99 mg/g), catechol (29.37 mg/g), 5-hydroxymethylfurfural (5.69 mg/g), 2-pentenal, (E)-(5.22 mg/g), acetic acid (3.69 mg/g), 1,2,3-propanetriol (3.67 mg/g), 2-furan-carboxaldehyde (2.66 mg/g), and other compounds. FT-IR analysis confirmed the presence of alcohol and phenol in the extract. Antibacterial activities of RPE against food pathogenic and spoilage bacteria showed that RPE could inhibited Bacillus subtilis, B. cereus, Staphylococcus aureus, Vibrio cholerae, V. parahaemolyticus, Pseudomonas aeruginosa, and P. fluorescens, with MIC values ranging between 1024 and 8192 µg/mL. The extract also showed antioxidant properties, as determined by DPPH and ABTS assays. The cytotoxicity analysis after 72 h of treatment showed the IC50 values at 194.97 ± 4.87, 205.92 ± 2.55, and 94.11 ± 1.33 µg/mL for L929, Vero, and MCF-7 cell lines, respectively. Therefore, this study provided a basis of knowledge of rambutan peels as an excellent source of natural bioactive compounds for various applications.
Keywords: antioxidant, antibacterial, bioactive compounds, GC-MS, FT-IR, rambutan
1. Introduction
Rambutan (Nephelium lappaceum L.) is an economically important tropical fruit widely cultivated in south-east Asia and the cultivar “Rong Rian” or Nasan School Rambutan has long been known as the most popular and delicious rambutan in Thailand. Thailand is one of the world’s leading producers and exporters of the rambutan fruit and a variety of rambutan products, especially canned rambutan in syrup. Thus, large amounts of rambutan peels were uselessly discarded and considered as biological wastes [1,2]. Previous studies have reported that peels of rambutan contain phytochemical compounds including flavonoids, saponin, tannins, geraniin, ellagic acid, and corilagin that act as a potential of antioxidant and antibacterial activities [3,4]. Moreover, the antidiabetic and antihypercholesterolemic activities of rambutan peel extract (RPE) has been described [5]. The amount and variety of phytochemical compounds may differ depending on several factors, such as the region or country of cultivation, geographical climate, and soil condition [6]. Therefore, investigations into the phytochemical compounds and biological activities of rambutan peels are still necessary for their potential applications.
Food safety is a major health concern. Unsafe food may be contaminated with microorganisms and/or chemical substances. Some strains of bacteria can cause food spoilages and food poisoning diseases in human, e.g., Bacillus spp., Clostridium perfringens, Staphylococcus aureus, Vibrio spp., Escherichia coli, Pseudomonas spp., and Salmonella sp. [7]. To reduce or eliminate food-associated pathogens, chemicals or antibiotics are used, and these may damage the health of workers, environments, and other organisms [8]. The discovery of bioactive compounds from plant, especially agricultural wastes, as the alternatives to chemicals and antibiotics is interesting because various parts of plants contain bioactive compounds known as phytochemicals that exhibit the antimicrobial activity, antioxidant activity, and other activities that benefit human [9]. Rambutan fruit peels are considered as wastes and contain such powerful anti-bacteria and antioxidants. In this regard, many studies reported about the antibacterial activity of rambutan peel extract against S. aureus, S. epidermidis, Listeria monocytogenes, E. coli, V. campbellii, V. cholerae, V. parahaemolyticus, V. anguillarum, P. aeruginosa, S. enteritidis, and Enterococcus faecalis [5,10]. Antioxidants refer to the compounds that act as the reducing agents of free radicals which are harmful to the human body. It has been reported that free radicals contributed to aging, heart diseases, cancer, and other diseases [11]. In rambutan peel, phenolic and flavonoid compounds (i.e., ellagitannins, phenolic acid, quercetin, and gallotannin) were related to its antioxidant activity. In addition, the presence of vitamin compounds in the peel of rambutan is also involved in the antioxidant activity [12,13]. Thus, the aims of this study are to extract and identify the bioactive compounds from peels of Nasan School Rambutan and investigate its antibacterial, antioxidant, and cytotoxic activities.
2. Materials and Methods
2.1. Bacterial Strains
Food pathogenic and spoilage bacteria used in this study were B. subtilis PSU4655, B. cereus PSU3874, S. aureus ATCC25923, V. cholerae PSU6072, V. parahaemolyticus ATCC17802, Salmonella sp. PSU411, E. coli ATCC25922, Pseudomonas aeruginosa ATCC27853, and P. fluorescens PSU6074. All isolates were recovered from the microbial culture collection, Division of Biological Science, Prince of Songkla University, and were preserved in 20% glycerol at −80 °C.
2.2. Chemicals
The reagents used for antioxidant activity assay were 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and potassium persulfate (K2S2O8), purchased from Sigma Aldrich (Darmstadt, Germany).
2.3. Preparation of Dried Rambutan Peels
Nasan School Rambutan (Ngo Rong Rean) used in this study were obtained directly from farm in Surat Thani province, southern Thailand, during fruiting season (July–September). Fresh rambutan peels were washed under running tap water and dried by hot air oven at 40 °C for 3 days or until dried. The dried rambutan peels were kept in a dry condition in the dark at room temperature until extraction.
2.4. Extraction of Rambutan Peel Extract (RPE) by Water Extraction
Dried rambutan peels were saturated in water using the method described by Hernandez et al. [7] with some modifications. Briefly, 100 g of dried rambutan peels was soaked in 500 mL of hot distilled water at 60 °C in ratio 1:5. The mixture was placed in a heating oven at 60 °C for 30 min. Before concentration of the extract, the peels were removed by filtrating through double layers of muslin. The solution was concentrated by freeze dryer (Martin Christ, Osterode am Harz, Germany). Briefly, the solution was frozen at −80 °C for 24 h. After freezing, frozen sample was placed in the freeze dryer at −45 °C for 5 days. The freeze-dried extract was stored at 4 °C in dark condition until further use. The extract yield of dried peels was expressed as mg of dried extract per g of dried peel matter.
2.5. GC-MS Analysis
Gas chromatography-electron ionization/mass spectrometry (GC-MS) analysis of phytochemical compounds present in RPE was carried out using Perkin Elmer Clarus 680 gas chromatography mass spectrophotometer (Agilent, Santa Clara, CA, USA). For sample preparation, RPE powder was dissolved in ethanol, centrifuged at 10,000 rpm for 10 min at 10 °C, and supernatant was used for GC-MS analysis. The GC-MS analysis was provided with an FID detector and Elite-5 capillary column ((5% biphenyl) 95% dimethylpolysiloxane), length 30 m × 0.25 mm ID and film thickness 250 µm df. Helium was used as carrier gas (flow rate, 1 mL/min). Injector and interface temperature was 260 °C. Column temperature was programmed from 60 to 300 °C at an increasing rate of 10 min, where it was held for 6 min. The spectrums of the compounds were compared with standard spectra available in Perkin Elmer GC-MS NIST library [14].
2.6. FT-IR Spectroscopy
The functional groups of compounds in RPE were identified by Fourier Transform Infrared Spectroscopy (FT-IR). A total of 10 mg of RPE powder was encapsulated in 100 mg of KBr pellet to prepare translucent sample disc. The powdered sample was then loaded in VERTEX 70 FT-IR spectrometer (Bruker Corporation, Leipzig, Germany), with a scan ranging from 500 to 3500 cm−1 and resolution of 4 cm−1 [14].
2.7. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays
The MIC of RPE was determined by broth microdilution method using 96-well microtiter plate recommended by Clinical and Laboratory Standard Institute (CLSI), 2015 [15]. The tested isolates were cultured in Mueller–Hinton broth (MHB) supplemented with (for Vibrio spp.) or without 1% NaCl for 4 h and adjusted to a concentration of 106 cfu/mL with fresh MHB supplemented with or without 1% NaCl. Then, 100 µL of bacterial suspension was inoculated into 100 µL of RPE solution at the final concentrations, ranging from 32 to 16,384 µg/mL, and incubated at 30 °C for 18–20 h. After incubation, the MIC values were evaluated by adding 20 µL of 0.1% resazurin solution to each well and incubating them for 4 h at 30 °C. The lowest concentration of RPE that inhibited the growth of isolates was recorded as the MIC value. Bacteria were removed from each well that showed no growth, cultured on Tryptic Soy agar (TSA) supplemented with or without 1% NaCl, and incubated at 30 °C for 18–24 h. The lowest concentration showing no visible growth was taken as MBC value.
2.8. Antioxidant Activity Assays
2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assays of RPE were examined in 96-well microtiter plate using the previous procedure described by Jitpakdee et al. [16].
2.8.1. DPPH Assay
For the DPPH assay, 20 µL of RPE was added into 280 µL of freshly prepared 107.14 µM DPPH solution. Trolox was used as a standard control. The mixture was incubated in dark at room temperature for 30 min and the absorbance was measured at 517 nm with spectrophotometer (LUMIstar, BMGLABTECH, Ortenberg, Germany).
2.8.2. ABTS Assay
The ABTS stock solution was prepared by adding 7 mM of ABTS with 8.75 mM of potassium persulfate and incubated in dark at room temperature for 16 h. Before use, the ABTS stock solution was diluted to 104.14 µM ABTS solution. Then, 20 µL of RPE or standard solution were mixed with 280 µL of 104.14 µM ABTS solution. After being mixed, the plate was incubated in dark at RT for 6 min and the absorbance was measured at 734 nm with spectrophotometer (LUMIstar, BMGLABTECH, Ortenberg, Germany).
2.8.3. Scavenging Activity of RPE
The scavenging activity of RPE was expressed as the inhibitory concentration (IC50) which presents the concentration of the RPE required to inhibit 50% of DPPH and ABTS radicals. The experiments were done in three independent replicates. The % DPPH and ABTS scavenging activities were calculated using this formula:
| [(control absorbance − sample absorbance)/control absorbance)] × 100 |
2.9. In Vitro Cytotoxicity of RPE
2.9.1. Cell Line Culture
L929 (mouse, fibroblast, normal cell line), Vero (monkey, kidney, normal cell line), and MCF-7 (human, breast, adenocarcinoma) cells were purchased from ATCC (Manassas, VA, USA). L929 and MCF-7 were grown in RPMI 1640 (Invitrogen, Inchinnan, UK) supplemented with 10% fetal bovine serum (Invitrogen, Inchinnan, UK), 50 units/mL of penicillin (Invitrogen, Inchinnan, UK), and 50 µg/mL of streptomycin (Invitrogen, Inchinnan, UK). Vero cells were grown in DMEM (Invitrogen, Inchinnan, UK) supplemented with 50 units/mL of penicillin (Invitrogen, Inchinnan, UK) and 100 µg/mL of streptomycin (Invitrogen, Inchinnan, UK). Cells were incubated at 37 °C with 5% CO2 in a humidified incubator.
2.9.2. Cytotoxicity Testing
The cytotoxicity testing of RPE on L929, MCF-7, and Vero cell lines took place using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. L929, MCF-7, and Vero cell lines, at a density of 2 × 104 cells, were incubated with RPE at concentration of 60–500 µg/mL in 96-well microtiter plate at 37 °C with 5% CO2 for 72 h. Then, cells were washed with 1× PBS and continued to incubate in 100 µL of 0.5 mg/mL MTT at 37 °C for 30 min. Avoiding light, the dark blue crystals of formazan (MTT metabolites) were dissolved with 100 µL of DMSO and incubated at 37 °C for 30 min. The absorbance at 570 and 650 nm were measured using microplate reader spectrophotometer [17]. The viable cells were presented as a percentage of survival and calculated using the following formula:
| Survival (%) = [(A570 of sample − A650 of sample) / (A570 of control − A650 of control)] × 100 |
2.10. Statistical Analysis
Results were presented as the means ± standard deviations (S.D.) of three replicates. One-way analysis of variance (ANOVA) was used for comparison to more than two means. Correlations between DPPH and ABTS assays of antioxidant activity were calculated using Bonferroni post-test. A difference was considered statistically significant when p ≤ 0.05.
3. Results
3.1. GC-MS Analysis
The extraction yield of dried rambutan peels was 134.1 mg/g of dried matter. A total of 28 compounds in RPE were identified by the GC-MS analysis, representing 81.65% of the total components (Table S1). Ten major compounds (93.94 mg/g or 70.05% of the extraction yield) identified were mome inositol, catechol, 5-hydroxymethylfurfural, 2-pentenal, (E)-, acetic acid, 1,2,3-propanetriol, 2-furan-carboxaldehyde, 2-cyclopenten-1-one, 2-hydroxy-, 1,2-diphenylethan-1-ol, and phenol (Table 1).
Table 1.
Ten major organic compounds identified in rambutan peel extract (RPE).
| No. | Name of Compound | Formula | Amount (mg/g of Dry Peel) |
Retention Time | Match Factor |
|---|---|---|---|---|---|
| 1 | Mome Inositol | C7H14O6 | 35.99 | 56.7432 | 87.8 |
| 2 | Catechol (1,2-benzenediol) | C6H6O2 | 29.37 | 45.6479 | 96.1 |
| 3 | 5-Hydroxymethylfurfural | C6H6O3 | 5.69 | 41.5651 | 94.9 |
| 4 | 2-Pentenal, (E)- | C5H8O | 5.22 | 20.9707 | 87.4 |
| 5 | Acetic acid | C2H4O2 | 3.69 | 15.5889 | 99.0 |
| 6 | 1,2,3-Propanetriol | C3H8O3 | 3.67 | 37.5876 | 96.2 |
| 7 | 2-furan-carboxaldehyde | C5H4O2 | 2.66 | 15.7927 | 96.1 |
| 8 | 2-Cyclopenten-1-one, 2-hydroxy- | C5H6O2 | 2.63 | 24.5133 | 96.0 |
| 9 | 1,2-Diphenylethan-1-ol | C14H14O | 2.63 | 40.2940 | 97.2 |
| 10 | Phenol | C6H6O | 2.40 | 30.6622 | 95.2 |
3.2. FT-IR Spectroscopy
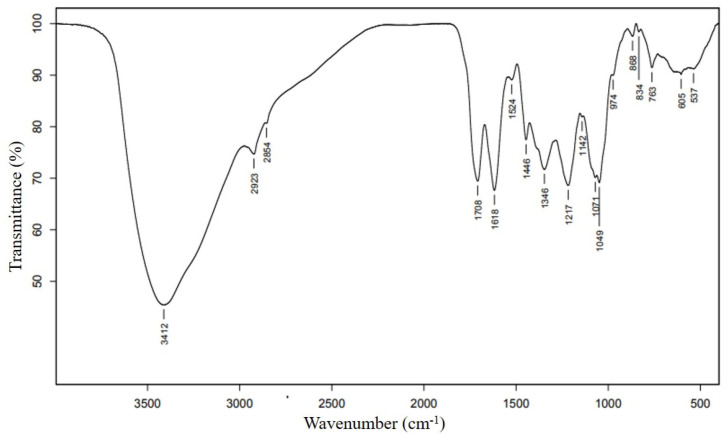
The FT-IR spectrum was analyzed to identify the functional groups of bioactive compounds. The result represented the peak range of 537 to 3412 cm−1 (Figure 1) and FT-IR band assignments are indicated in Table 2. Multiple peaks found in this study represented the complex nature of RPE. A broad band peak at 3412 cm−1 indicated the presence of the O–H functional group. This strong absorption is because major compounds in RPE contain an O–H hydroxyl group in their structures, such as mome-inositol, catechol, 5-hydroxymethylfurfural, 2,3-propanetriol, -cyclopenten-1-one, 2-hydroxy-, and phenol. The band of 1217 and 1071 cm−1 confirmed the existence of a hydroxyl compound. Narrow bands at 2923 and 2854 cm−1 are characteristic peaks corresponding to the aliphatic C–H stretching vibration. In addition, the C–H bending mode was found in the fingerprint region of 1346 and 763 cm−1. The C=O stretching band at 1708 cm−1 indicated the presence of carbonyl compounds such as carboxyl, aldehhyde, ketone, or esters. This is in agreement with the presence of the 5-hydroxymethylfurfural, 2-pentenal, (E)-, acetic acid, 2-furan-carboxaldehyde, and 1,2-Diphenylethan-1-ol. The bands at 1618, 1142, and 1049 cm−1 indicated the presence of C–O stretching. The adsorption bands around 1524 and 1446 cm−1 were attributed to the aromatic rings. The bands at 974, 868, 834 indicated the carbohydrate fingerprint regions. Meanwhile, the band at 763 cm−1 corresponded to the =CH bending. In addition, no peak was found in the triple bond region (2000–2500 cm−1).
Figure 1.
The FT-IR spectrum of rambutan peel extract (RPE).
Table 2.
Peak position (cm−1) and tentative assignments of FT-IR absorbance bands for RPE recorded in the spectral region from 500 to 3500 cm−1.
| Peak Position (cm−1) | Tentative Assignment | Reference |
|---|---|---|
| 3412 | O–H stretching (hydroxyl) | [18,19] |
| 2923, 2854 | C–H stretching (alkyl) | [18,19] |
| 1708 | C=O stretching (carboxyl, aldehyde, ketone, ester) | [18,19] |
| 1618 | C–O stretching | [20] |
| 1524 | C–C (aromatic ring) | [21] |
| 1446 | C–C (aromatic ring) | [18] |
| 1346 | C–H bending (alkyl) | [18,20,22] |
| 1217 | O–H bending (hydroxyl) | [18,19] |
| 1142 | C–O stretching | [21] |
| 1071 | O–H bending (hydroxyl) | [19] |
| 1049 | C–O stretching | [18] |
| 974 | Carbohydrate | [23] |
| 868 | Glycosidic linkage | [19] |
| 834 | Carbohydrate | [19,23] |
| 763 | =CH bending | [18] |
| 605 | Unknown | |
| 537 | Unknown |
3.3. Antibacterial Activity of RPE against Food Pathogenic and Spoilage Bacteria
The antibacterial activity of RPE against nine bacterial strains was evaluated using MIC and MBC assays. The result showed that RPE could inhibited B. subtilis, B. cereus, and S. aureus at MIC values of 8192, 1024, and 2048 µg/mL, respectively. RPE could also inhibit the growth of V cholerae and V. parahaemolyticus with an MIC value of 2048 µg/mL. The MIC values were higher against P. aeruginosa and P. fluorescens (8192 µg/mL). However, RPE at the tested concentration was unable to inhibit Salmonella sp. PSU411 and E. coli ATCC25922. In this study, the MBC values were found to be varied. Regarding MBC/MIC ratios, RPE had a bactericidal effect (MIC/MBC ≤ 4) on B. subtilis, B. cereus, V. cholerae, P. aeruginosa, and P. fluorescens (Table 3).
Table 3.
The MIC and MBC values of rambutan peel extract (RPE) against various food pathogenic and spoilage bacteria.
| Bacteria | MIC (µg/mL) | MBC (µg/mL) | MBC/MIC Ratio |
|---|---|---|---|
| Gram-positive bacteria | |||
| Bacillus subtilis PSU4655 | 8192 | 16,384 | 2 |
| Bacillus cereus PSU3874 | 1024 | 2048 | 2 |
| Staphylococcus aureus ATCC25923 | 2048 | >16,384 | ND |
| Gram-negative bacteria | |||
| Vibrio cholerae PSU6072 | 2048 | 8192 | 4 |
| Vibrio parahaemolyticus ATCC17802 | 2048 | >16,384 | ND |
| Salmonella sp. PSU411 | >16,384 | >16,384 | ND |
| Escherichia coli ATCC25922 | >16,384 | >16,384 | ND |
| Pseudomonas aeruginosa ATCC27853 | 8192 | 16,384 | 2 |
| Pseudomonas fluorescens PSU6074 | 8192 | 16,384 | 2 |
ND: not determined.
3.4. Antioxidant Activities of the Extract
The antioxidant activity of RPE was determined by the DPPH and ABTS methods. The scavenging effect of RPE at the highest concentration (2048 µg/mL) against ABTS was significantly higher than DPPH radicals, with an IC50 of 494.25 ± 6.35 and 561.53 ± 6.30 µg/mL, respectively (Table 4).
Table 4.
Antioxidant activity of rambutan peel extract (RPE).
| Samples | Antioxidant Activity | |
|---|---|---|
| DPPH IC50 a (µg/mL) | ABTS IC50 (µg/mL) | |
| RPE | 561.53 ± 6.30 * | 494.25 ± 6.35 * |
| Trolox | 30.38 ± 0.56 | 27.37 ± 0.15 |
a IC50 is the 50% inhibitory concentration. The value indicates the mean ± SD for three independent experiments performed in triplicates; * p < 0.05 compared between DPPH and ABTS.
3.5. Cytotoxicity of RPE
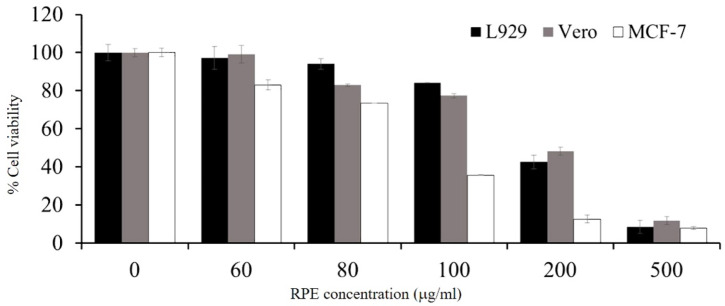
The cytotoxicity of RPE at different concentrations, ranging from 60 to 500 µg/mL, was tested against the proliferation of L929, Vero, and MCF-7 cell lines for 72 h. Cells were also evaluated by an MTT colorimetric assay. This study demonstrated that the growth inhibitory effect of RPE against each cell was dose-dependent. At the lowest concentration tested (60 µg/mL), RPE treatment resulted in cell viability of 97.17 ± 2.73% and 99.19 ± 0.46, for L929 and Vero normal cell lines, respectively. The cytotoxic effect of RPE on the MCF-7 cancer cell line (IC50; 94.11 ± 1.33 µg/mL) was stronger than that against L929 (IC50; 194.97 ± 4.87 µg/mL) and Vero (IC50; 205.92 ± 2.55 µg/mL) normal cell lines (Figure 2).
Figure 2.
Cytotoxic activity of RPE on two normal cell lines (L929 and Vero) and one cancer cell line (MCF-7). Cell survival was measured by MTT assay. Data are shown as the mean ± standard deviation.
4. Discussion
The cultivation of Nasan School Rambutan (N. lappaceum L. cv. Rong Rian) in Thailand began in 1926, in the Nasan School, Bannasan, Surat Thani Province, Thailand. Then, its cultivation spread to other areas of Thailand. It is considered as the most delicious and famous rambutan cultivar grown in Thailand. Its shape is oval and average fruit weight is 40–50 g. When compared with See-chompoo cultivars, another popular rambutan cultivar grown in Thailand, cv. Rongrien has bigger seeds and a thinner aril than cv. See-chompoo [24]. Recently, various methods were used for the extraction of the bioactive compounds from its plants and agricultural wastes. Water extraction is of interest due to its natural, non-toxic, and environmental-friendly properties. The result of this study showed that the extract yield was high (134.1 mg/g). A previous study reported an observation of approximately 2.06 g/100 g of lyophilized rambutan peel [10]. The variation the in phytochemical components in the peels may be due to the cultivars and cultivation conditions, such as the soil condition, climate, and other physical factors [6]. Thitilertdecha and Rakariyatham (2011) reported that cv. Rongrien had a significantly higher number of phenolic compounds than that of the cv. See-compoo [25]. Unfortunately, most of the studies did not indicate the cultivar of rambutan in their publications. Thus, it is difficult to compare the similarity or differences in phytochemical compounds and bioactivities among difference cultivars.
This study investigated the phytochemical compounds present in the RPE extracted using water by GC-MS and FT-IR analyses. The results confirmed the presence of bioactive compounds in RPE. From FT-IR and GC-MS spectral analysis, it is clear that alcoholic and phenolic compounds are present in RPE. A previous study showed that different parts of rambutan or solvents affect the presence of the extracted bioactive compounds. Aziz et al. reported that the extract from rambutan seeds in Malaysia contains the functional group of O–H, N–H, and C=O [26]. Rakariyatham et al. described how the water extraction of rambutan peel showed p-Coumaroyl glucose, vanillic acid, hexoside, carboxylic acid, ellagic acid, corilagin, and geraniin. Moreover, ethyl gallate and geraniin were obtained by ethanol extraction. The major compounds identified in the methanolic extract of rambutan peel were ellagic acid, corilagin, and geraniin [27]. Monrroy et al. (2020) reported 18 active compounds in RPE which had not previously been characterized [18]. However, some compounds, including catechol or 1,2-benzenediol (Table 1) and 5-methylfuran-2-carbaldehyde (Table S1), were also identified in this study.
In this study, nearly 50% of the major compounds were mome inositol and catechol which contain the -OH group. Mome inositol is a phytochemical compound found in several medicinal plants such as Zilla spinosa (Forssk.), Corbichonia decumbens (Forssk.), and Securigera securidaca (L.). It acts as an anti-alopecic, anti-cirrhotic, anti-neuropathic, cholesterolytic, lipotropic, and sweetening property [28]. Mome inositol was found to have anti-proliferation, anti-alopecic, anti-cirrhotic, and anti-neuropathic activities [13,29]. In addition, the catechol compound was responsible for antifungal, antibacterial, and antioxidant activities [30,31]. The content of catechol obtained in this study was 29.37 mg/g of dry rambutan peel. This was higher than a previous study with sweet orange, lemon, tangerine, and grapefruit extracted by water (range between 37.00 to 52.16 mg catechol/100g dry weight) [32].
The 5-hydroxymethylfurfural, acetic acid, and 2-furan-carboxaldehyde reported in this study were similar to the major organic compounds of Jatropha curcas L. kernel meal extract which exhibited antioxidant and antibacterial activities [33]. The antibacterial, antioxidant, and various biological activities of these compounds are widely reported in many fruit peels. For example, the 5-hydroxymethylfurfural from banana (Musa acuminata) peels possess antibiofilm and antivirulence against Pseudomonas aeruginosa [34]. The identification of 5-hydroxymethylfurfural was reported in the hydromethanolic extract of pomegranate (Punica granatum L. var. nana) peel and the extract exhibited antimicrobial activity against various plant pathogens [35]. 5-hydroxymethylfurfural and 2-furancarboxaldehyde were reported as two major compounds present in mango (Mangifera indica L.) peels and other plants, and their antioxidant and antiproliferative potential have been reported [36,37]. Acetic acid has been reported as an antimicrobial agent used for disinfection for more than 6000 years [38]. The presence of acetic acid, antioxidant properties, and antimicrobial activity has also been reported in banana peels [39]. The presence of phenol, unsaturated aldehydes, and ketones have been reported in many fruit peels, including melon, watermelon, orange, and mango [40,41]. The presence of these compounds contributes to fruit flavor and supports the antioxidant properties [42].
Previously, the bioactivities of RPE, such as anticancer, antiviral, antidiabetic, and anti-hypercholesterolemic activities, were reported [40]. In the present study, we investigated the antibacterial activity of RPE against three Gram-positive and six Gram-negative bacteria. The result showed that RPE could inhibit various pathogens, except Salmonella sp. and E. coli. Phuong et al. also examined the effect of RPE on the growth of bacterial pathogens. RPE at 1000 µg GAE/mL affected the growth of Vibrio spp. (including V. campbellii, V. parahaemolyticus, and V. anguillarum) and S. aureus. However, RPE had no effect on E. coli growth [5]. Tadtong et al. also reported that RPE at concentration of 2 mg/mL could inhibit S. aureus, but had no ability to inhibit E. coli by a disc diffusion assay. The results showed that flavonoids and tannins (members of phenolic compounds) were the major bioactive compounds [4]. In addition, Tsong et al. described that RPE could inhibit both Gram-positive and Gram-negative bacteria at a concentration of 0.5 mg/mL for S. typhi, B. cereus, and B. subtilis, and 1 mg/mL for E. coli [12]. In the study of Thitilertdecha et al., V. cholerae and S. aureus was inhibited by RPE at an MIC of 15.6 mg/mL and 31.2 mg/mL, respectively [10]. The antibacterial activity of RPE depends on the amount of bioactive and phenolics compounds present in the peel, such as geraniin, ellagic acid, rutin, quercetin, corilagin, flavonoid, tannin, and triterpenoid [5,12]. Moreover, bacterial cell structures may act as the important factors for bacteria which are resistant against antimicrobial agents [43]. This study demonstrated the potential of RPE as an antibacterial agent against important food pathogenic bacteria, especially B. cereus and V. cholerae.
In this study, the ability to scavenge the free radicals of RPE was determined by DPPH and ABTS assays. Both methods were widely used to examine the antioxidant activity of the extract, based on the ability of proton radical scavenging or hydrogen donating of the extract [44]. The DPPH and ABTS assays are convenient methods recommended for the primary screening of the antioxidant activity in plants and essential oils [45,46]. However, the disadvantage of these methods has been reported, especially the length of incubation time and the solution preparation [47]. In our study, the DPPH and ABTS radical scavenging activities of RPE were significantly different (p < 0.05). The difference may be due to the position of the aromatic ring of the hydroxyl group influencing the quenching of free radicals [48]. In a previous study, the free phenolic compound of RPE, obtained by microwave-assisted extraction, showed an IC50 of 3.55 ± 0.32 µg/mL by the DPPH assay [49]. Thitilertdecha et al. also reported the antioxidant activity of major phenolic compounds from the methanolic extract of rambutan peel, including of geraniin, corilagin, and ellagic acid, for which the IC50 were 0.79, 1.42, and 1.64 µM, respectively [50]. Previous studies reported that ellagitannin, phenolic acid, quercetin, and gallotannin were responsible for the antioxidant activity of RPE [10,44]. The differences in antioxidant activity may be due to the amount of antioxidant substances present in the extract, such as polyphenol, vitamin C and E, and carotenoids [51]. Thus, further study of the antioxidant activity of the purified active compounds is needed.
To evaluate the toxic effect of RPE, we also investigated the cytotoxic activity against various cell lines, including L929, Vero, and MCF-7 using an MTT assay. There are no previous data showing the toxicity levels against these cell lines. Our results showed that crude RPE showed a lower toxicity on L929 and Vero cell lines compared to MCF-7. Okonogi et al. evaluated the cytotoxicity of RPE on human colon adenoma (Caco-2) and normal peripheral blood mononuclear (PBMC) cell lines. The results showed that RPE exhibited non-toxic properties on a normal cell line with high IC50 values (>100 μg/mL) [52]. According to the standard National Cancer Institute (NCI) criteria and Geran protocol [53], the author also suggested that RPE is non-toxic to normal human cells and has the potential to develop as a cancer-preventive agent. Hernandez-Hernandez et al. revealed that the methanolic extract of yellow rambutan peel extract showed a cytotoxic effect on breast cancer cells (MDA-MB-231) and osteosarcoma cancer (MG-63) cell lines at a very low concentration (IC50 5.42 ± 1.67 and 6.97 ± 1.02 μg/mL, respectively), whereas no effect on the cervical cancer (HeLa) cell line was observed [54]. Our study demonstrated that RPE at 100 μg/mL could inhibit more than 50% of MCF-7, while 84.12 ± 3.59 and 77.41 ± 2.05% cell viability was observed in L929 and Vero cell lines, respectively.
Overall, the present study demonstrated that RPE obtained by the water extraction method contained phytochemical compounds with good bioactivities and a low toxicity. The characterization of the pure active compound is, therefore, attractive for further utilization and development of value-added products.
5. Conclusions
The results obtained in this study provided valuable information about the phytochemical composition and bioactivities of “Rong Rian” rambutan peel extract, including its antibacterial, antioxidant, and cytotoxic properties. This extract should, therefore, be used in many applications, such as in natural anti-microbial agents in the food industry or as a natural food preservative.
Acknowledgments
The authors would like to thank Jirayu Jitpakdee for technical support of the antioxidant assay. Our special thanks to Sutima Preeprem and Jiranan Pattano for substantial help on the statistical parts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11050956/s1, Table S1: Summary of phytochemical compounds in rambutan peel extract (RPE) by GC-MS.
Author Contributions
Conceptualization, P.M.-a.; Methodology, P.M.-a. and H.J.; Formal Analysis, P.M.-a. and H.J.; Investigation, H.J.; Resources, P.M.-a.; Writing—Original Draft Preparation, P.M.-a. and H.J.; Writing—Review and Editing, P.M.-a. and H.J.; Supervision, P.M.-a.; Project Administration, P.M.-a.; Funding Acquisition, P.M.-a. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was supported by a fund from Prince of Songkla University (SCI610391a). In addition, the first author (H.J.) would like to thank the Research Assistant (RA) Scholarship from the Faculty of Science Research Fund, Prince of Songkla University (contract no. 1-2563-02-005).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Office of Agricultural Economics (OAE) of Thailand. [(accessed on 20 February 2022)]. Available online: https://bit.ly/3K1IYfw.
- 2.Office of Agricultural Economics. Rambutan. [(accessed on 26 February 2022)]. Available online: https://bit.ly/3BToCSy.
- 3.Mahmood K., Kamilah H., Alias A.K., Ariffin F. Nutritional and therapeutic potentials of rambutan fruit (Nephelium lappaceum L.) and the by-products: A review. J. Food Meas. Charact. 2018;12:1556–1571. doi: 10.1007/s11694-018-9771-y. [DOI] [Google Scholar]
- 4.Tadtong S., Athikomkulchai S., Worachanon P., Chalongpol P., Chaichanachaichan P., Sareedenchai V. Antibacterial activities of rambutan peel extract. J. Health Res. 2011;25:35–37. [Google Scholar]
- 5.Phuong N.N.M., Le T.T., Van Camp J., Raes K. Evaluation of antimicrobial activity of rambutan (Nephelium lappaceum L.) peel extracts. Int. J. Food Microbiol. 2020;321:108539. doi: 10.1016/j.ijfoodmicro.2020.108539. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez C., Ascacio-Valdes J., De la Garza H., Wong-Paz J., Aguilar C.N., Martinez-Avila G.C., Castro-Lopez C., Aguilera-Carbo A. Polyphenolic content, in vitro antioxidant activity and chemical composition of extract from Nephelium lappaceum L. (Mexican rambutan) husk. Asian Pac. J. Trop. Med. 2017;10:1201–1205. doi: 10.1016/j.apjtm.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Gizaw Z. Public health risks related to food safety issues in the food market: A systematic literature review. Environ. Health Prev. Med. 2019;24:68. doi: 10.1186/s12199-019-0825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X., Hung Y.C. Development of a Chlorine Dosing Strategy for Fresh Produce Washing Process to Maintain Microbial Food Safety and Minimize Residual Chlorine. J. Food Sci. 2018;83:1701–1706. doi: 10.1111/1750-3841.14189. [DOI] [PubMed] [Google Scholar]
- 9.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thitilertdecha N., Teerawutgulrag A., Rakariyatham N. Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT-Food Sci. Technol. 2008;41:2029–2035. doi: 10.1016/j.lwt.2008.01.017. [DOI] [Google Scholar]
- 11.Bagchi K., Puri S. Free radicals and antioxidants in health and disease: A review. EMHJ-East. Mediterr. Health J. 1998;4:350–360. doi: 10.26719/1998.4.2.350. [DOI] [Google Scholar]
- 12.Tsong J.L., Goh L.P.W., Gansau J.A., How S.E. Review of Nephelium lappaceum and Nephelium ramboutan-ake: A High Potential Supplement. Molecules. 2021;26:7500. doi: 10.3390/molecules26227005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nik A.B., Vazifedoost M., Didar Z., Hajirostamloo B. The antioxidant and physicochemical properties of microencapsulated bioactive compounds in Securigera securidaca (L.) seed extract by co-crystallization. Food Qual. Saf. 2019;3:243–250. doi: 10.1093/fqsafe/fyz022. [DOI] [Google Scholar]
- 14.Arulmozhi P., Vijayakumar S., Kumar T. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected pathogenic microorganisms. Microb. Pathog. 2018;123:219–226. doi: 10.1016/j.micpath.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 15.CLSI . CLSI Guideline M45. 3rd ed. Clinical and Laboratory Standard Institute; Wayne, PA, USA: 2015. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. [Google Scholar]
- 16.Jitpakdee J., Kantachote D., Kanzaki H., Nitoda T. Selected probiotic lactic acid bacteria isolated from fermented foods for functional milk production: Lower cholesterol with more beneficial compounds. LWT. 2021;135:110061. doi: 10.1016/j.lwt.2020.110061. [DOI] [Google Scholar]
- 17.Sriwiriyajan S., Ninpesh T., Sukpondma Y., Nasomyon T., Graidist P. Cytotoxicity screening of plants of genus Piper in breast cancer cell lines. Trop. J. Pharm. Res. 2014;13:921–928. doi: 10.4314/tjpr.v13i6.14. [DOI] [Google Scholar]
- 18.Monrroy M., Araúz O., García J.R. Active compound identification in extracts of N. lappaceum peel and evaluation of antioxidant capacity. J. Chem. 2020;2020:4301891. doi: 10.1155/2020/4301891. [DOI] [Google Scholar]
- 19.Skenderidis P., Lampakis D., Giavasis I., Leontopoulos S., Petrotos K., Hadjichristodoulou C., Tsakalof A. Chemical properties, fatty-acid composition, and antioxidant activity of goji berry (Lycium barbarum L. and Lycium chinense Mill.) fruits. Antioxidants. 2019;8:60. doi: 10.3390/antiox8030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goudarzi M., Mir N., Mousavi-Kamazani M., Bagheri S., Salavati-Niasari M. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep32539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang J., Li M., Pu Y., Ragauskas A.J., Yoo C.G. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl. Sci. 2020;10:4345. doi: 10.3390/app10124345. [DOI] [Google Scholar]
- 22.Nandiyanto A.B.D., Oktiani R., Ragadhita R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019;4:97–118. doi: 10.17509/ijost.v4i1.15806. [DOI] [Google Scholar]
- 23.Chen R., Jin C., Tong Z., Lu J., Tan L., Tian L., Chang Q. Optimization extraction, characterization and antioxidant activities of pectic polysaccharide from tangerine peels. Carbohydr. Polym. 2016;136:187–197. doi: 10.1016/j.carbpol.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Chakraborty B., Mishra D., Hazarika B., Hazarika T., Ghosh S. Chapter 29 Rambutan (Nephelium lapspaceum) In: Ghosh S.N., editor. Breeding of Underutilized Fruit Crops. 1st ed. JAYA Publishing House; New Delhi, India: 2015. pp. 425–439. [Google Scholar]
- 25.Thitilertdecha N., Rakariyatham N. Phenolic content and free radical scavenging activities in rambutan during fruit maturation. Sci. Hortic. 2011;129:247–252. doi: 10.1016/j.scienta.2011.03.041. [DOI] [Google Scholar]
- 26.Aziz H.A., Rahmat N.S., Alazaiza M.Y.D. The Potential Use of Nephelium lappaceum Seed as Coagulant-Coagulant Aid in the Treatment of Semi-Aerobic Landfill Leachate. Int. J. Environ. Res. Public Health. 2022;19:420. doi: 10.3390/ijerph19010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakariyatham K., Zhou D., Rakariyatham N., Shahidi F. Sapindaceae (Dimocarpus longan and Nephelium lappaceum) seed and peel by-products: Potential sources for phenolic compounds and use as functional ingredients in food and health applications. J. Funct. Foods. 2020;67:103846. doi: 10.1016/j.jff.2020.103846. [DOI] [Google Scholar]
- 28.Al-Qahtani H., Alfarhan A.H., Al-Othman Z.M. Changes in chemical composition of Zilla spinosa Forssk. medicinal plants grown in Saudi Arabia in response to spatial and seasonal variations. Saudi J. Biol. Sci. 2020;27:2756–2769. doi: 10.1016/j.sjbs.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathi P., Nikhil K., Das S., Roy P., Bokka V.R., Botlagunta M. Evaluation of in vitro anticancer activity and GC-MS analysis from leaf Sophora interrupta Bedd. Int. J. Pharm. Pharm. Sci. 2015;7:303–308. [Google Scholar]
- 30.Kocaçalışkan I., Talan I., Terzi I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforsch. C. 2006;61:639–642. doi: 10.1515/znc-2006-9-1004. [DOI] [PubMed] [Google Scholar]
- 31.Justino G.C., Correia C.F., Mira L., Borges dos Santos R.M., Martinho Simões J.A., Silva A.M., Santos C., Gigante B. Antioxidant activity of a catechol derived from abietic acid. J. Agric. Food Chem. 2006;54:342–348. doi: 10.1021/jf052062k. [DOI] [PubMed] [Google Scholar]
- 32.Shehata M.G., Awad T.S., Asker D., El Sohaimy S.A., Abd El-Aziz N.M., Youssef M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021;4:326–335. doi: 10.1016/j.crfs.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oskoueian E., Abdullah N., Saad W.Z., Omar A.R., Ahmad S., Kuan W.B., Zolkifli N.A., Hendra R., Ho Y.W. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Jatropha curcas Linn. J. Med. Plant Res. 2011;5:49–57. [Google Scholar]
- 34.Vijayakumar K., Ramanathan T. Musa acuminata and its bioactive metabolite 5-Hydroxymethylfurfural mitigates quorum sensing (las and rhl) mediated biofilm and virulence production of nosocomial pathogen Pseudomonas aeruginosa in vitro. J. Ethnopharmacol. 2020;246:112242. doi: 10.1016/j.jep.2019.112242. [DOI] [PubMed] [Google Scholar]
- 35.Melgarejo-Sánchez P., Núñez-Gómez D., Martínez-Nicolás J.J., Hernández F., Legua P., Melgarejo P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021;8:1–29. doi: 10.1186/s40643-020-00351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M.-M., Wu L.-Y., Zhao T., Xiong L., Huang X., Liu Z.-H., Fan X.-L., Xiao C.-R., Gao Y., Ma Y.-B. The protective role of 5-HMF against hypoxic injury. Cell Stress Chaperones. 2011;16:267–273. doi: 10.1007/s12192-010-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali M.R., Yong M.J., Gyawali R., Mosaddik A., Ryu Y.C., Cho S.K. Mango (Mangifera indica L.) peel extracts inhibit proliferation of HeLa human cervical carcinoma cell via induction of apoptosis. J. Korean Soc. Appl. Biol. Chem. 2012;55:397–405. doi: 10.1007/s13765-012-1024-x. [DOI] [Google Scholar]
- 38.Ryssel H., Kloeters O., Germann G., Schäfer T., Wiedemann G., Oehlbauer M. The antimicrobial effect of acetic acid—An alternative to common local antiseptics? Burns. 2009;35:695–700. doi: 10.1016/j.burns.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Naksing T., Teeka J., Rattanavichai W., Pongthai P., Kaewpa D., Areesirisuk A. Determination of bioactive compounds, antimicrobial activity, and the phytochemistry of the organic banana peel in Thailand. J. Biosci. 2021;37:1981–3163. doi: 10.14393/BJ-v37n0a2021-56306. [DOI] [Google Scholar]
- 40.Asghar A., Tan Y.C., Zahoor M., Zainal Abidin S.A., Yow Y.-Y., Khan E., Lahiri C. A scaffolded approach to unearth potential antibacterial components from epicarp of Malaysian Nephelium lappaceum L. Sci. Rep. 2021;11:1–15. doi: 10.1038/s41598-021-92622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tahir T., Ashfaq M., Saleem M., Rafiq M., Shahzad M.I., Kotwica-Mojzych K., Mojzych M. Pyridine Scaffolds, Phenols and Derivatives of Azo Moiety: Current Therapeutic Perspectives. Molecules. 2021;26:4872. doi: 10.3390/molecules26164872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dembitsky V.M., Poovarodom S., Leontowicz H., Leontowicz M., Vearasilp S., Trakhtenberg S., Gorinstein S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Int. Food Res. J. 2011;44:1671–1701. doi: 10.1016/j.foodres.2011.03.003. [DOI] [Google Scholar]
- 43.Njeru S.N., Obonyo M.A., Nyambati S.O., Ngari S.M. Antimicrobial and cytotoxicity properties of the crude extracts and fractions of Premna resinosa (Hochst.) Schauer (Compositae): Kenyan traditional medicinal plant. BMC Complement. Altern. Med. 2015;15:295. doi: 10.1186/s12906-015-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W.-C., Wang S.-W., Li C.-W., Lin H.-R., Yang C.-S., Chu Y.-C., Lee T.-H., Chen J.-J. Comparison of various solvent extracts and major bioactive components from Portulaca oleracea for antioxidant, Anti-Tyrosinase, and Anti-α-Glucosidase Activities. Antioxidants. 2022;11:398. doi: 10.3390/antiox11020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koleva I.I., Van Beek T.A., Linssen J.P., de Groot A., Evstatieva L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 46.Proestos C., Lytoudi K., Mavromelanidou O.K., Zoumpoulakis P., Sinanoglou V.J. Antioxidant capacity of selected plant extracts and their essential oils. Antioxidants. 2013;2:11–22. doi: 10.3390/antiox2010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah P., Modi H. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015;3:636–641. [Google Scholar]
- 48.Bernini R., Barontini M., Cis V., Carastro I., Tofani D., Chiodo R.A., Lupattelli P., Incerpi S. Synthesis and Evaluation of the Antioxidant Activity of Lipophilic Phenethyl Trifluoroacetate Esters by In Vitro ABTS, DPPH and in Cell-Culture DCF Assays. Molecules. 2018;23:208. doi: 10.3390/molecules23010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L., Zhang H., Zhuang Y. Preparation of free, soluble conjugate, and insoluble-bound phenolic compounds from peels of rambutan (Nephelium lappaceum) and evaluation of antioxidant activities in vitro. J. Food Sci. 2012;77:C198–C204. doi: 10.1111/j.1750-3841.2011.02548.x. [DOI] [PubMed] [Google Scholar]
- 50.Thitilertdecha N., Teerawutgulrag A., Kilburn J.D., Rakariyatham N. Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules. 2010;15:1453–1465. doi: 10.3390/molecules15031453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dai J., Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okonogi S., Duangrat C., Anuchpreeda S., Tachakittirungrod S., Chowwanapoonpohn S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007;103:839–846. doi: 10.1016/j.foodchem.2006.09.034. [DOI] [Google Scholar]
- 53.Geran R., Greenberg N., Macdonald M., Schumacher A., Abbott B. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972;3:17–19. [Google Scholar]
- 54.Hernández-Hernández C., Aguilar C., Rodríguez-Herrera R., Flores-Gallegos A., Morlett-Chávez J., Govea-Salas M., Ascacio-Valdés J. Rambutan (Nephelium lappaceum L.): Nutritional and functional properties. Trends Food Sci. Technol. 2019;85:201–210. doi: 10.1016/j.tifs.2019.01.018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article.