Abstract
Tebipenem-pivoxil hydrobromide, an orally bioavailable carbapenem, is currently in clinical development for the treatment of extended-spectrum β-lactamase- and AmpC-producing Enterobacterales. Previously, tebipenem was found to possess antimicrobial activity against the biothreat pathogens, Burkholderia pseudomallei and Burkholderia mallei. Thus, herein, tebipenem was evaluated against a panel of 150 curated strains of Burkholderia cepacia complex (Bcc) and Burkholderia gladioli, pathogens that infect people who are immunocompromised or have cystic fibrosis. Using the provisional susceptibility breakpoint of 0.12 mg/L for tebipenem, 100% of the Bcc and B. gladioli tested as being provisionally resistant to tebipenem. Bcc and B. gladioli possess two inducible chromosomal β-lactamases, PenA and AmpC. Using purified PenA1 and AmpC1, model β-lactamases expressed in Burkholderia multivorans ATCC 17616, PenA1 was found to slowly hydrolyze tebipenem, while AmpC1 was inhibited by tebipenem with a k2/K value of 1.9 ± 0.1 × 103 M−1s−1. In addition, tebipenem was found to be a weak inducer of blaPenA1 expression. The combination of the slow hydrolysis by PenA1 and weak induction of blaPenA1 likely compromises the potency of tebipenem against Bcc and B. gladioli.
Keywords: β-lactamase, Burkholderia, β-lactam, PenA, carbapenemase, tebipenem, carbapenem, AmpC
1. Introduction
The discovery of tebipenem-pivoxil, a novel orally bioavailable carbapenem, was first reported in the 1990s by Wyeth Lederle Japan, Co., Ltd. in Japan and was approved for pediatric clinical use in Japan in 2009 [1,2,3]. At that time, tebipenem was found to be highly active against Streptococcus pneumoniae and β-lactamase-nonproducing ampicillin-resistant Haemophilus influenzae, two pathogens that were increasingly prevalent in Japan [2,4,5].
Due to the scourge of multi-drug-resistant gram-negative bacterial infections in the United States, tebipenem-pivoxil hydrobromide, a slightly modified formulation that improves drug properties (e.g., stability), is currently in clinical development by Spero Therapeutics in the US for the treatment of multi-drug-resistant gram-negative pathogens [6]. In addition to tebipenem’s antimicrobial activity against gram-positive pathogens, tebipenem has demonstrated a potency against strains of Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes and Proteus mirabilis, producing extended-spectrum-β-lactamases (ESBLs) and plasmidic AmpC β-lactamases [7,8,9,10,11]. The inhibitory activity of tebipenem against Enterobacterales primarily lies in its ability to inactivate PBP2 [12]. Notably, the phase 3 clinical trial, ADAPT-PO, revealed that oral tebipenem was non-inferior to intravenous ertapenem for the treatment of complicated urinary tract infections and acute pyelonephritis [13].
In 2013, tebipenem was found to demonstrate some activity against the biothreat pathogen, Burkholderia pseudomallei [14]. Furthermore, in 2021 tebipenem was evaluated against a broader panel of biothreat pathogens, including B. pseudomallei and Burkholderia mallei, and minimum inhibitory concentrations (MICs) ranged between 1–4 mg/L and 0.25–1 mg/L, respectively [15]. The Burkholderia genus encompasses more than 30 species of mammalian pathogens. Major human and animal pathogens include B. mallei, B. pseudomallei complex, Burkholderia cepacia complex (Bcc), and Burkholderia gladioli. Populations particularly susceptible to acquiring Burkholderia spp. infections include those who are immunocompromised. For Bcc and B. gladioli, individuals with cystic fibrosis (CF) and chronic granulomatous disease are most vulnerable to infections [16,17,18,19,20]. Select β-lactam antibiotics (e.g., meropenem, ceftazidime) are often “first-line” agents and can be effective as a treatment option for infections due to Bcc and B. gladioli; unfortunately, their potency is declining [21,22,23,24]. Burkholderia spp. produce at least two chromosomal β-lactamases, a PenA-like family class A β-lactamase and an AmpC-like class C β-lactamase [25,26,27,28,29,30,31]. PenA1 and AmpC1 from Burkholderia multivorans, a member of the Bcc, were previously microbiologically and biochemically characterized, and PenA1 was found to be a carbapenemase with a very broad spectrum [32], while AmpC1 possessed a narrow spectrum that included some cephems [31]. In addition, some strains of Bcc also express an OXA class D β-lactamase [25,26,27,28,29,30,31]. In Bcc and B. gladioli, the expression of blaPenA and blaAmpC is regulated by PenRA, similar to the Enterobacterales’ blaAmpC/AmpR system [28,30,33]. Specifically, upon exposure to an inducing β-lactam, such as imipenem, the balance of the muropeptides within the bacteria is altered, which changes the repressor function of PenRA, and blaPenA and blaAmpC are expressed [30,31,33].
Previously, we have found that β-lactams and β-lactam-β-lactamase inhibitor combinations are some of the most potent antimicrobials against multi-drug- and extensively-drug-resistant Bcc and B. gladioli obtained from persons with CF in the US [23,34,35,36]. However, most of these agents are only available in intravenous formulations. Thus, a critical need exists to continue to explore novel treatment options, especially orally bioavailable agents, against Bcc and B. gladioli. Herein, the activity of tebipenem against Bcc and B. gladioli was explored, along with biochemical interactions of tebipenem with PenA1 and AmpC1, and the impact of tebipenem on the expression of blaPenA1 and blaAmpC1.
2. Results
2.1. Tebipenem Does Not Demonstrate Clinically Relevant Antimicrobial Activity against Bcc and B. gladioli
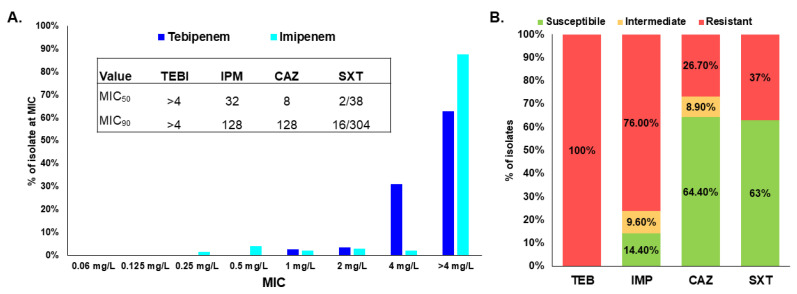
E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as controls for tebipenem integrity; the controls tested within the anticipated quality control ranges (Table 1). Thus, agar dilution testing was confirmed to provide equivalent results to the reference broth microdilution, as the agar dilution MICs for the quality control strains were within the proposed quality control ranges for tebipenem [37]. Antimicrobial susceptibility testing using the agar dilution methodology was further used to determine the MICs for a well-characterized panel of 150 clinical isolates of Bcc and B. gladioli [23,30,31,34,35,36,38]. One hundred percent of the isolates tested provisionally resistant to tebipenem, with all isolates possessing an MIC ≥ 1 mg/L (Figure 1A and Supplementary Table S1). The majority of the strains tested (63%) produced an MIC > 4 mg/L. A limitation of this study was that endpoint MICs were not determined; this decision was based on the provisional breakpoints for tebipenem. Different patterns in the susceptibility profiles to tebipenem based on Burkholderia species were not observed. The tebipenem MICs were compared to those of another carbapenem, imipenem; however, tebipenem MICs were overall lower when compared to imipenem (Figure 1A). However, using the percent provisional susceptibility to tebipenem and imipenem susceptibility breakpoint of Pseudomonas aeruginosa for Burkholderia species (as an imipenem breakpoint is not available), 76% of the isolates tested resistant to imipenem (Figure 1B) [23]. Comparatively, strains were 64.4% and 63% susceptible to the first-line agents ceftazidime and trimethoprim-sulfamethoxazole, respectively, used to treat infections due to Bcc and B. gladioli; in general, these are a highly resistant subset of bacteria [23]. Moreover, compared to tebipenem, MIC90 values for these former agents were in the resistant range (Figure 1, inset) [34,37].
Table 1.
MICs values in mg/L for selected control strains.
| Control Strain | Tebipenem |
|---|---|
| B. multivorans ATCC 17616 | >4 |
| E. coli ATCC 25922 | ≤0.06 |
| P. aeruginosa ATCC 27853 | >4 |
Quality control ranges: E. coli ATCC 25922 (0.008–0.03 mg/L) and P. aeruginosa ATCC 27853 (1–8 mg/L) [37].
Figure 1.
(A) Bar graph showing the % of isolates that had a specific MIC value. Inset: MIC50 and MIC90 values for antibiotics in next panel [34]. (B) Bar graph comparing the % susceptible, % intermediate, and % resistant of the isolates for tebipenem (TEB), imipenem (IMP), another carbapenem [23], and ceftazidime (CAZ) and trimethoprim-sulfamethoxazole (SXT), two first-line agents [23] used to treat infections caused by Burkholderia species.
2.2. Tebipenem Is Slowly Hydrolyzed by PenA1 but Inhibits AmpC1, but Eventually Forms a Stable Complex with Both β-lactamases
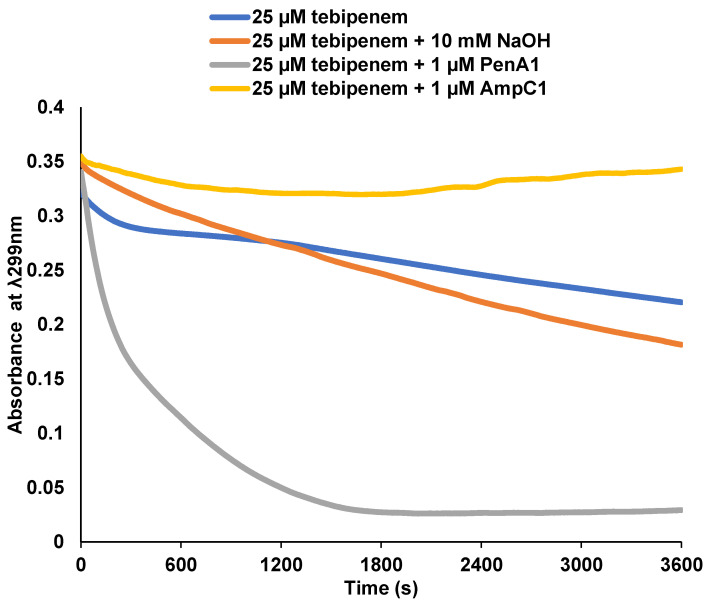
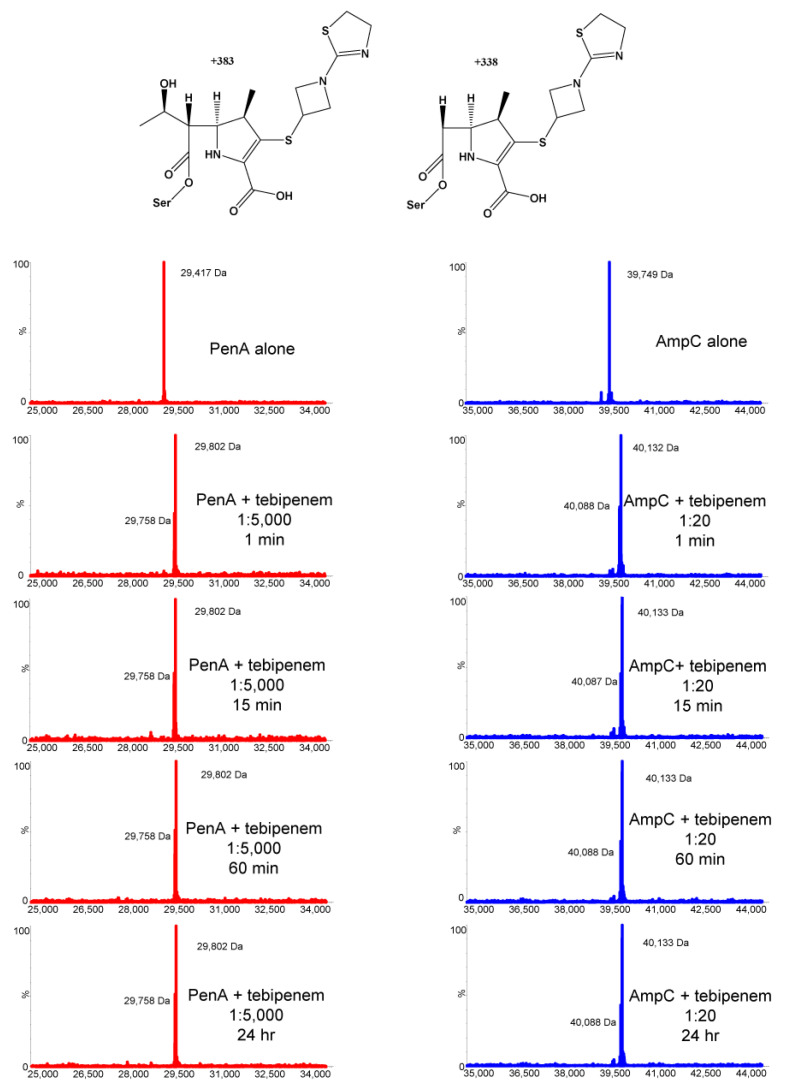
The kinetic characterization of purified PenA1 and AmpC1 from B. multivorans ATCC 17616 revealed that PenA1 slowly hydrolyzed tebipenem (Figure 2). One µM PenA1 hydrolyzed 25 µM tebipenem within 30 min. Conversely, AmpC1 did not hydrolyze tebipenem and lowered the background hydrolysis of tebipenem, likely due to AmpC1 being in an acyl-enzyme complex with tebipenem. The Ki app of PenA1 for tebipenem was 4.7 ± 0.5 µM; however, 4000 molecules of tebipenem were turned over in 15 min by PenA1 before it was inactivated, thus resulting in non-determinable k2/K and koff values (Table 2). As AmpC1 did not hydrolyze tebipenem, Ki app, k2/K, and koff values were determinable at 22 ± 2 µM, 1.9 ± 0.1 × 103 M−1s−1, and 3 ± 1 × 10−4 s−1, respectively (Table 2). In addition, the number of molecules of tebipenem hydrolyzed in 15 min (tn) before AmpC was inactivated was only 10. This is 400-fold less than that observed with PenA1. To assess the stability of the PenA1-tebipenem and AmpC1-tebipenem complexes over time and to determine if tebipenem is modified over time, ESI-MS was employed using enzyme-to-inhibitor (E:I) ratios greater than the tn at 15 min for each enzyme. At 1:5000 and 1:20 E:I ratios for PenA1 and AmpC1, respectively, tebipenem remained bound up to the last time point of 24 h (Figure 3). Thus, once the tebipenem acylated PenA1 and AmpC1, it remained in a stable complex. In addition, ESI-MS revealed that the hydroxyethyl side chain of tebipenem was lost upon acylating both PenA1 and AmpC1 with a loss of 45 Da. The loss of the hydroxyethyl side chain of carbapenems has previously been described by others [39,40,41,42,43].
Figure 2.
Progress curves showing the breakdown of 25 µM tebipenem over time alone (blue), and hydrolysis by the addition of 10 mM sodium hydroxide (NaOH) (orange), 1 µM PenA1 (gray) or 1 µM AmpC1 (yellow).
Table 2.
Steady-state inhibitory kinetics using tebipenem.
| Parameter | PenA1 | AmpC1 |
|---|---|---|
| Ki app (µM) | 4.7 ± 0.5 | 22 ± 2 |
| k2/K (M−1s−1) | N/D | 1.9 ± 0.1 × 103 |
| koff (s−1) | N/D | 3 ± 1 × 10−4 |
| tn at 15 min | 4000 | 10 |
Each data point was collected in triplicate, and each experiment was completed in duplicate. N/D, not determinable due to background hydrolysis of tebipenem, see Figure 2.
Figure 3.
ESI-MS of PenA1 (red) and AmpC1 (blue) with tebipenem at various time points (1 min, 15 min, 60 min, and 24 h) to reveal the intermediates formed upon incubation, i.e., acyl-enzyme (top left) and acyl-enzyme with loss of R1 hydroxyethyl side chain (top right). Both enzymes are also depicted without tebipenem to show the apo-enzyme’s molecular weight. The ratio of enzyme to tebipenem was chosen based on the tn at 15 min; see Table 2.
2.3. Tebipenem Is a Minor Inducer of blaPenA1 Expression
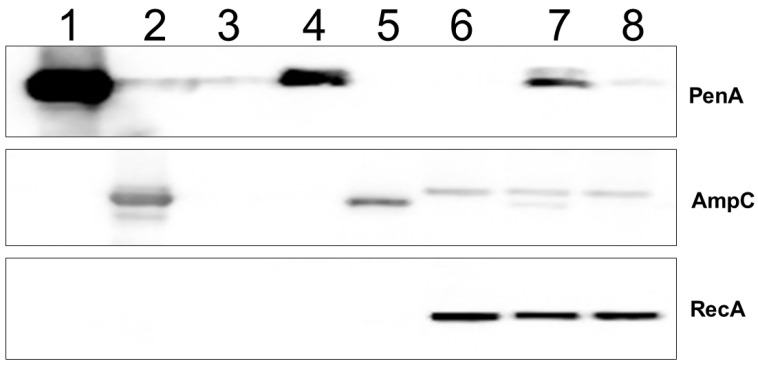
Using Western blotting against PenA1 and AmpC1, tebipenem was found to weakly induce the expression of blaPenA1, as evidenced by the faint band in the lane analyzing B. multivorans ATCC 17616 treated with 1 mg/L of tebipenem (Figure 4, lane 8). Conversely, a basal level of AmpC1 was detected in all lanes carrying crude extracts of B. multivorans ATCC 17616 (Figure 4, lane 7). Moreover, AmpC without its signal peptide was observed only in the plus imipenem lane, likely due to the samples being extracted prior to AmpC reaching the periplasm where the signal peptide is removed.
Figure 4.
Immunoblotting using anti-PenA1 peptide and anti-AmpC1 polyclonal antibodies as well as an anti-RecA antibody, as an internal standard for the uninduced and induced B. multivorans samples. B. multivorans ATCC 17616 was grown to log phase (OD600 nm ~0.6) in LB, and then cultures were either maintained in LB only (lane 6), or 1 mg/L of imipenem, a known inducer of blaPenA1 and blaAmpC1 expression [30], was added (lane 7), or 1 mg/L tebipenem was added (lane 8), and cultures were grown for an additional hour, pelleted, and crude extracts were prepared for immunoblotting. Purified PenA1 (lane 1), purified AmpC (lane 2), E. coli DH10B with pBC SK(+) (lane 3), E. coli DH10B with pBC SK(+) blaPenA1 (lane 4), and E. coli DH10B with pBC SK(+) blaAmpC1 (lane 5) were used as controls.
3. Discussion
Based on the provisional breakpoints provided by the sponsor of this study [15], tebipenem was found to lack antimicrobial activity against a strain panel of difficult-to-treat clinical isolates of Bcc and B. gladioli. The lack of activity is likely due to the slow hydrolysis of tebipenem by PenA1 as well as the weak induction of blaPenA1 expression. Thus, tebipenem is a slow substrate for PenA1; conversely, tebipenem inactivates AmpC1. These data are reminiscent of those previously observed with imipenem [23,32]. Imipenem is also slowly hydrolyzed by PenA, but not AmpC1 [32], and 76% of the isolates tested herein are resistant to imipenem using the Clinical Laboratory and Standards Institute’s (CLSI’s) P. aeruginosa susceptibility breakpoint of ≤2 mg/L [23,37]. The addition of a PenA1 inhibitor would likely improve the potency of tebipenem against Bcc and B. gladioli; however, as tebipenem-pivoxil hydrobromide is not being partnered with a β-lactamase inhibitor, this was not tested in this study. Indeed, adding relebactam to imipenem improved imipenem’s potency against this panel of isolates with 71.4% testing susceptible to the imipenem-relebactam combination [38]. Importantly, when tebipenem was tested against B. pseudomallei and B. mallei, MIC90 values of 2 mg/L and 1 mg/L, respectively, were observed [15]. Thus, tebipenem was more active versus that strain panel compared to the one tested herein; the MIC90 value was >4 mg/L (Figure 1, inset). The B. pseudomallei and B. mallei MICs ranged between 1–4 mg/L and 0.25–1 mg/L, respectively; based on those datasets and the provisional susceptibility breakpoint for tebipenem, these pathogens would also be provisionally resistant to tebipenem [15]. Given the provisional breakpoints for tebipenem, tebipenem lacks sufficient antimicrobial activity against Burkholderial pathogens.
4. Materials and Methods
Susceptibility Testing. An extensively characterized panel of 150 MDR clinical strains [44], including 140 Bcc (B. ambifaria, B. arboris, B. cenocepacia, B. cepacia, B. contaminans, B. diffusa, B. dolosa, B. multivorans, B. pseudomultivorans, B. pyrrocinia, B. seminalis, B. stabilis, B. ubonensis and B. vietnamiensis) and 10 B. gladioli obtained from the Burkholderia cepacia Research Laboratory and Repository (University of Michigan) were phenotypically characterized using agar dilution MICs (tebipenem range: 0.06–4 mg/L) by using a Steers replicator that deposited 104 colony forming units of bacteria per spot. All Burkholderia species were isolated and speciated as previously described [23]. In addition, E. coli ATCC 25922, P. aeruginosa ATCC 27853, and B. multivorans ATCC 17616 were used as control strains. Provisional MIC interpretations for tebipenem were provided by Spero Therapeutics: susceptible ≤ 0.12 mg/L, intermediate 0.25 mg/L, and resistant ≥0.5 mg/L [15]. Provisional breakpoints are used when official breakpoints have not been assigned by the Clinical Laboratory Standards Institute, European Committee on Antimicrobial Susceptibility Testing, or another leading agency that publishes antibiotic breakpoints for microorganisms.
Protein Purification and Steady-State Kinetic Analysis of Inhibition. The purification protocols for PenA1 and AmpC1 were previously described [31,32]. Using base hydrolysis (i.e., sodium hydroxide (NaOH)), the absorbance wavelength and extinction coefficient (–12,090.76 M−1cm−1 at 299 nm) for tebipenem was determined using the Beer–Lambert law. The stability of tebipenem for each β-lactamase was assessed by incubating 25 µM tebipenem with 1.0 µM of purified PenA1 or AmpC1—10 mM NaOH was used as a control base—and hydrolysis curves were monitored at ~25 °C for 1 h using an Agilent 8453 Diode Array spectrophotometer for measurements in 10 mM phosphate-buffered saline, pH 7.4 (PBS). In addition, Ki app, the k2/K value or acylation rate, koff or the off-rate and partition ratios (tn) at 15 min were attempted and determined if feasible, using previously described methods [45,46].
Timed-Electrospray Ionization-Mass Spectrometry (ESI-MS). ESI-MS was performed to assess the timing of the reaction course as well as discern the nature of the intermediates formed upon the reaction of tebipenem with the β-lactamases. The purified PenA1 and AmpC1 β-lactamases were incubated with tebipenem at 1:5000 and 1:20 enzyme-to-inhibitor (E:I) ratios for 1 min, 15 min, 60 min, and 24 h. A Waters SYNAPT G2-Si quadrupole-time-of-flight mass spectrometer equipped with a Waters Acquity H class Ultra Performance Liquid Chromatography (UPLC) was used according to previously described methods [47].
Induction Assays and Western Blotting. To measure β-lactamase induction in B. multivorans, Western blots were used to assess the level of PenA1 and AmpC1 induction; the methodology was previously described [30,31]. Briefly, polyclonal anti-PenA peptide, anti-AmpC, and anti-RecA antibodies were previously generated in rabbits for use against B. multivorans [30,31]. An anti-RecA antibody was used as a loading control for cellular samples. Strains were grown in lysogeny broth (LB) to an optical density at 600 nm (OD600 nm) of ~0.6, after which sub-MIC concentrations—that do not alter the growth of the bacteria—of imipenem (1 µg/mL), a known inducer [30], and tebipenem (1 µg/mL) were added. The cells were grown for an additional hour, and samples were prepared for Western blotting, as previously described [30,31]. Purified PenA1 and purified AmpC as well as E. coli DH10B with pBC SK(+), E. coli DH10B with pBC SK(+) blaPenA1, and E. coli DH10B with pBC SK(+) blaAmpC1 grown in LB only were used as controls.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11050674/s1, Table S1: Antimicrobial susceptibility testing results for 151 Bcc and B. gladioli conducted via agar dilution methodology.
Author Contributions
Conceptualization, K.M.P.-W.; methodology, S.A.B. and E.T.Z.; validation, K.M.P.-W., S.A.B. and E.T.Z.; formal analysis, K.M.P.-W.; resources, K.M.P.-W. and J.J.L.; writing—original draft preparation, K.M.P.-W.; writing—review and editing, K.M.P.-W., S.A.B., E.T.Z. and J.J.L.; supervision, K.M.P.-W.; project administration, K.M.P.-W. and S.A.B.; funding acquisition, K.M.P.-W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was funded through an investigator-initiated research grant from Spero Therapeutics. This research was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program BX002872 to K.M.P.-W. from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This research was also funded in part by the Cystic Fibrosis Foundation (K.M.P.-W. and J.J.L.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hikida M., Itahashi K., Igarashi A., Shiba T., Kitamura M. In vitro antibacterial activity of LJC 11,036, an active metabolite of L-084, a new oral carbapenem antibiotic with potent antipneumococcal activity. Antimicrob. Agents Chemother. 1999;43:2010–2016. doi: 10.1128/AAC.43.8.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi R., Konomi M., Hasegawa K., Morozumi M., Sunakawa K., Ubukata K. In vitro activity of tebipenem, a new oral carbapenem antibiotic, against penicillin-nonsusceptible Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2005;49:889–894. doi: 10.1128/AAC.49.3.889-894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A., Utley L., Parr T.R., Zabawa T., Pucci M.J. Tebipenem, the first oral carbapenem antibiotic. Expert Rev. Anti Infect. Ther. 2018;16:513–522. doi: 10.1080/14787210.2018.1496821. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa K., Chiba N., Kobayashi R., Murayama S.Y., Iwata S., Sunakawa K., Ubukata K. Rapidly increasing prevalence of β-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob. Agents Chemother. 2004;48:1509–1514. doi: 10.1128/AAC.48.5.1509-1514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuroki H., Tateno N., Ikeda H., Saito N. Investigation of pneumonia-causing pathogenic organisms in children and the usefulness of tebipenem pivoxil for their treatment. J. Infect. Chemother. 2010;16:280–287. doi: 10.1007/s10156-010-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEntee L., Johnson A., Farrington N., Unsworth J., Dane A., Jain A., Cotroneo N., Critchley I., Melnick D., Parr T., et al. Pharmacodynamics of tebipenem: New options for oral treatment of multidrug-resistant Gram-negative infections. Antimicrob. Agents Chemother. 2019;63:e00603-19. doi: 10.1128/AAC.00603-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thamlikitkul V., Lorchirachoonkul N., Tiengrim S. In vitro and in vivo activity of tebipenem against ESBL-producing E. coli. J. Med. Assoc. Thail. 2014;97:1259–1268. [PubMed] [Google Scholar]
- 8.Yao Q., Wang J., Cui T., Yang Z., Su M., Zhao P., Yan H., Zhan Y., Yang H. Antibacterial properties of tebipenem pivoxil tablet, a new rral carbapenem preparation against a variety of pathogenic bacteria in vitro and in vivo. Molecules. 2016;21:62. doi: 10.3390/molecules21010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio A., Pucci M.J., Jain A. Characterization of SPR994, an orally available carbapenem, with activity comparable to intravenously administered carbapenems. ACS Infect. Dis. 2018;4:1436–1438. doi: 10.1021/acsinfecdis.8b00188. [DOI] [PubMed] [Google Scholar]
- 10.Arends S.J.R., Rhomberg P.R., Cotroneo N., Rubio A., Flamm R.K., Mendes R.E. Antimicrobial activity evaluation of tebipenem (SPR859), an orally available carbapenem, against a global set of Enterobacteriaceae isolates, including a challenge set of organisms. Antimicrob. Agents Chemother. 2019;63:e02618-18. doi: 10.1128/AAC.02618-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotroneo N., Rubio A., Critchley I.A., Pillar C., Pucci M.J. In vitro and in vivo characterization of tebipenem, an oral carbapenem. Antimicrob. Agents Chemother. 2020;64:e02240-19. doi: 10.1128/AAC.02240-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacasse E., Brouillette E., Larose A., Parr T.R., Rubio A., Jr., Malouin F. In vitro activity of tebipenem (SPR859) against penicillin-binding proteins of Gram-negative and Gram-positive bacteria. Antimicrob. Agents Chemother. 2019;63:e02181-18. doi: 10.1128/AAC.02181-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sodhi V., Kronsberg K.A., Clark M., Cho J.C. Tebipenem pivoxil hydrobromide-No PICC, no problem! Pharmacotherapy. 2021;41:748–761. doi: 10.1002/phar.2614. [DOI] [PubMed] [Google Scholar]
- 14.Seenama C., Tiengrim S., Thamlikitkul V. In vitro activity of tebipenem against Burkholderia pseudomallei. Int. J. Antimicrob. Agents. 2013;42:375. doi: 10.1016/j.ijantimicag.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Clayton N.P., Jain A., Halasohoris S.A., Pysz L.M., Lembirik S., Zumbrun S.D., Kane C.D., Hackett M.J., Pfefferle D., Smiley M.A., et al. In vitro and in vivo characterization of tebipenem (TBP), an orally active carbapenem, against biothreat pathogens. Antimicrob. Agents Chemother. 2021;65:e02385-20. doi: 10.1128/AAC.02385-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marson F.A., Hortencio T.D., Aguiar K.C., Ribeiro J.D. Demographic, clinical, and laboratory parameters of cystic fibrosis during the last two decades: A comparative analysis. BMC Pulm. Med. 2015;15:3. doi: 10.1186/1471-2466-15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott I.J., Peleg A.Y. Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: Antimicrobial resistance and therapeutic strategies. Semin. Respir. Crit. Care Med. 2015;36:99–110. doi: 10.1055/s-0034-1396929. [DOI] [PubMed] [Google Scholar]
- 18.Hanulik V., Webber M.A., Chroma M., Uvizl R., Holy O., Whitehead R.N., Baugh S., Matouskova I., Kolar M. An outbreak of Burkholderia multivorans beyond cystic fibrosis patients. J. Hosp. Infect. 2013;84:248–251. doi: 10.1016/j.jhin.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Chiappini E., Taccetti G., de Martino M. Bacterial lung infections in cystic fibrosis patients: An update. Pediatr. Infect. Dis. J. 2014;33:653–654. doi: 10.1097/INF.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 20.Gautam V., Singhal L., Ray P. Burkholderia cepacia complex: Beyond Pseudomonas and Acinetobacter. Indian J. Med. Microbiol. 2011;29:4–12. doi: 10.4103/0255-0857.76516. [DOI] [PubMed] [Google Scholar]
- 21.Avgeri S.G., Matthaiou D.K., Dimopoulos G., Grammatikos A.P., Falagas M.E. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: A systematic review of the clinical evidence. Int. J. Antimicrob. Agents. 2009;33:394–404. doi: 10.1016/j.ijantimicag.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Wuthiekanun V., Peacock S.J. Management of melioidosis. Expert Rev. Anti Infect. Ther. 2006;4:445–455. doi: 10.1586/14787210.4.3.445. [DOI] [PubMed] [Google Scholar]
- 23.Papp-Wallace K.M., Becka S.A., Zeiser E.T., Ohuchi N., Mojica M.F., Gatta J.A., Falleni M., Tosi D., Borghi E., Winkler M.L., et al. Overcoming an extremely drug resistant (XDR) pathogen: Avibactam restores susceptibility to ceftazidime for Burkholderia cepacia complex isolates from Cystic Fibrosis patients. ACS Infect. Dis. 2017;3:502–511. doi: 10.1021/acsinfecdis.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dalem A., Herpol M., Echahidi F., Peeters C., Wybo I., De Wachter E., Vandamme P., Pierard D. In vitro susceptibility of Burkholderia cepacia complex isolated from Cystic Fibrosis patients to ceftazidime-avibactam and ceftolozane-tazobactam. Antimicrob. Agents Chemother. 2018;62:e00590-18. doi: 10.1128/AAC.00590-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung T.K., Ho P.L., Woo P.C., Yuen K.Y., Chau P.Y. Cloning and expression of class A β-lactamase gene blaA(BPS) in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 2002;46:1132–1135. doi: 10.1128/AAC.46.4.1132-1135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godfrey A.J., Wong S., Dance D.A., Chaowagul W., Bryan L.E. Pseudomonas pseudomallei resistance to β-lactam antibiotics due to alterations in the chromosomally encoded β-lactamase. Antimicrob. Agents Chemother. 1991;35:1635–1640. doi: 10.1128/AAC.35.8.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tribuddharat C., Moore R.A., Baker P., Woods D.E. Burkholderia pseudomallei class A β-lactamase mutations that confer selective resistance against ceftazidime or clavulanic acid inhibition. Antimicrob. Agents Chemother. 2003;47:2082–2087. doi: 10.1128/AAC.47.7.2082-2087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trepanier S., Prince A., Huletsky A. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrob. Agents Chemother. 1997;41:2399–2405. doi: 10.1128/AAC.41.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirel L., Rodriguez-Martinez J.M., Plesiat P., Nordmann P. Naturally occurring class A β-lactamases from the Burkholderia cepacia complex. Antimicrob. Agents Chemother. 2009;53:876–882. doi: 10.1128/AAC.00946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becka S.A., Zeiser E.T., Marshall S.H., Gatta J.A., Nguyen K., Singh I., Greco C., Sutton G.G., Fouts D.E., LiPuma J.J., et al. Sequence heterogeneity of the PenA carbapenemase in clinical isolates of Burkholderia multivorans. Diagn. Microbiol. Infect. Dis. 2018;92:253–258. doi: 10.1016/j.diagmicrobio.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becka S.A., Zeiser E.T., Barnes M.D., Taracila M.A., Nguyen K., Singh I., Sutton G.G., LiPuma J.J., Fouts D.E., Papp-Wallace K.M. Characterization of the AmpC β-lactamase from Burkholderia multivorans. Antimicrob. Agents Chemother. 2018;62:e01140-18. doi: 10.1128/AAC.01140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papp-Wallace K.M., Taracila M.A., Gatta J.A., Ohuchi N., Bonomo R.A., Nukaga M. Insights into β-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J. Biol. Chem. 2013;288:19090–19102. doi: 10.1074/jbc.M113.458315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhar S., Kumari H., Balasubramanian D., Mathee K. Cell-wall recycling and synthesis in Escherichia coli and Pseudomonas aeruginosa-their role in the development of resistance. J. Med. Microbiol. 2018;67:1–21. doi: 10.1099/jmm.0.000636. [DOI] [PubMed] [Google Scholar]
- 34.Papp-Wallace K.M., Shapiro A.B., Becka S.A., Zeiser E.T., LiPuma J.J., Lane D.J., Panchal R.G., Mueller J.P., O’Donnell J.P., Miller A.A. In vitro antibacterial activity and in vivo efficacy of sulbactam-durlobactam against pathogenic Burkholderia species. Antimicrob. Agents Chemother. 2021;65:e01930-20. doi: 10.1128/AAC.01930-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeiser E.T., Becka S.A., Barnes M.D., Taracila M.A., LiPuma J.J., Papp-Wallace K.M. Resurrecting old β-lactams: Potent inhibitory activity of temocillin against multidrug-resistant Burkholderia species isolates from the United States. Antimicrob. Agents Chemother. 2019;63:e02315-18. doi: 10.1128/AAC.02315-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeiser E.T., Becka S.A., Wilson B.M., Barnes M.D., LiPuma J.J., Papp-Wallace K.M. “Switching partners”: Piperacillin-avibactam is a highly potent xombination against multidrug-eesistant Burkholderia cepacia complex and Burkholderia gladioli Cystic Fibrosis isolates. J. Clin. Microbiol. 2019;57:e00181-19. doi: 10.1128/JCM.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptiblity Testing. CLSI Supplement M100 . 31st ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2021. [Google Scholar]
- 38.Becka S.A., Zeiser E.T., LiPuma J.J., Papp-Wallace K.M. Activity of imipenem-relebactam against multidrug- and extensively drug-resistant Burkholderia cepacia complex and Burkholderia gladioli. Antimicrob. Agents Chemother. 2021;65:e0133221. doi: 10.1128/AAC.01332-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremblay L.W., Fan F., Blanchard J.S. Biochemical and structural characterization of Mycobacterium tuberculosis beta-lactamase with the carbapenems ertapenem and doripenem. Biochemistry. 2010;49:3766–3773. doi: 10.1021/bi100232q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endimiani A., Doi Y., Bethel C.R., Taracila M., Adams-Haduch J.M., O’Keefe A., Hujer A.M., Paterson D.L., Skalweit M.J., Page M.G., et al. Enhancing resistance to cephalosporins in class C beta-lactamases: Impact of Gly214Glu in CMY-2. Biochemistry. 2010;49:1014–1023. doi: 10.1021/bi9015549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drawz S.M., Babic M., Bethel C.R., Taracila M., Distler A.M., Ori C., Caselli E., Prati F., Bonomo R.A. Inhibition of the class C beta-lactamase from Acinetobacter spp.: Insights into effective inhibitor design. Biochemistry. 2010;49:329–340. doi: 10.1021/bi9015988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hugonnet J.E., Tremblay L.W., Boshoff H.I., Barry C.E., Blanchard J.S., 3rd Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science. 2009;323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papp-Wallace K.M., Endimiani A., Taracila M.A., Bonomo R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011;55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coenye T., Spilker T., Martin A., LiPuma J.J. Comparative assessment of genotyping methods for epidemiologic study of Burkholderia cepacia genomovar III. J. Clin. Microbiol. 2002;40:3300–3307. doi: 10.1128/JCM.40.9.3300-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehmann D.E., Jahic H., Ross P.L., Gu R.F., Hu J., Durand-Reville T.F., Lahiri S., Thresher J., Livchak S., Gao N., et al. Kinetics of avibactam inhibition against Class A, C, and D β-lactamases. J. Biol. Chem. 2013;288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papp-Wallace K.M., Winkler M.L., Gatta J.A., Taracila M.A., Chilakala S., Xu Y., Johnson J.K., Bonomo R.A. Reclaiming the efficacy of β-lactam-β-lactamase inhibitor combinations: Avibactam restores the susceptibility of CMY-2-producing Escherichia coli to ceftazidime. Antimicrob. Agents Chemother. 2014;58:4290–4297. doi: 10.1128/AAC.02625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papp-Wallace K.M., Becka S.A., Taracila M.A., Winkler M.L., Gatta J.A., Rholl D.A., Schweizer H.P., Bonomo R.A. Exposing a β-lactamase “twist”: The mechanistic basis for the high level of ceftazidime resistance in the C69F variant of the Burkholderia pseudomallei PenI β-lactamase. Antimicrob. Agents Chemother. 2016;60:777–788. doi: 10.1128/AAC.02073-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.