Abstract
The microaerophilic nature of Campylobacter species implies an inherent sensitivity towards oxygen and its reduction products, particularly the superoxide anion. The deleterious effects of exposure to superoxide radicals are counteracted by the activity of superoxide dismutase (SOD). We have shown previously that Campylobacter coli possesses an iron cofactored SOD. The sodB gene of C. coli UA585 was insertionally inactivated by the site-specific insertion of a tetO cassette. Organisms harboring the inactivated gene failed to produce a biologically functional form of the enzyme. While the ability of this mutant to grow in aerobic conditions was unchanged relative to the parental strain, its survival was severely compromised when nongrowing cells were exposed to air. Accordingly, the SOD-deficient mutant was unable to survive for prolonged periods in model foods. Furthermore, inactivation of the sodB gene decreased the colonization potential in an experimental infection of 1-day-old chicks. In contrast, strain CK100, which is deficient in catalase activity, showed the same survival and colonization characteristics as the parental strain. These results indicate that SOD, but not catalase, is an important determinant in the ability of C. coli to survive aerobically and for optimal colonization within the chicken gut.
Campylobacter jejuni and Campylobacter coli are now recognized as the most common causal agents of acute bacterial enteritis worldwide (19). Although to respire these pathogens require oxygen as a terminal electron acceptor, they were not considered to be able to grow in the concentration of oxygen present in air and are, therefore, classified as microaerophilic (11, 12). Recently, however, it has been shown that some campylobacters are able to adapt to an aerobic metabolism and thereby grow in the presence of air after the provision of a humid environment (13). Although the adaptation to aerobic metabolism was accompanied by a change in colonial morphology and outer membrane profile, the serotype and the ability of strains to colonize mice were unchanged by this process.
The oxidative stress imposed upon organisms surviving under aerobic conditions will be significantly higher than that encountered in a microaerobic environment. Cellular defenses against the damaging effects of oxidative stress will therefore play an important role in survival during exposure to air. In this respect a number of enzymes, including superoxide dismutases (SODs), catalases, peroxidase, glutathione synthetase, and glutathione reductase, are thought to provide the primary protection against oxygen toxicity in bacteria (6). SOD is a metallo-enzyme that has been isolated from a wide range of both prokaryotic and eukaryotic organisms (7). This enzyme is considered to provide the first line of defense against the toxic effects of reactive oxygen derivatives. SOD catalyzes the conversion of oxygen radicals (O2−) to hydrogen peroxide and dioxygen, thus providing a protective role against the effects of oxidative stress. Bacteria commonly contain two distinct types of SOD, either MnSOD (SodA) or FeSOD (SodB). Mn- and Fe-containing SODs share a high degree of amino acid homology and can be present in the same bacterial cells. If superoxide radicals are not efficiently scavenged, damage can occur to almost all known biological molecules, including DNA, membrane lipids, and other vital cellular components. Accordingly, bacterial mutants which are deficient in SOD show hypersensitivity towards oxygen and free-radical-generating agents (3, 15, 16).
At present our understanding of how campylobacters counter the effects of oxidative stress is limited. Both C. jejuni and C. coli possess a single catalase activity, encoded by the katA gene, which provides protection against oxidative stress by converting H2O2 to H2O and O2 (8). Accordingly, a C. coli mutant (CK100) deficient in this enzyme displays hypersensitivity to H2O2 (8). Previously, we and others have cloned and characterized an FeSOD from C. jejuni and C. coli (17, 18). Unlike Escherichia coli, which expresses three distinct types of SOD, there is no biochemical evidence to support the existence of other classes of SOD in either C. coli or C. jejuni. Furthermore, analysis of the recently completed C. jejuni genome sequence (18a) confirms the absence of genes encoding MnSODs or CuZnSODs from the chromosome of this species. Consequently, since it is probable that the FeSOD represents the only SOD activity in C. coli and C. jejuni, it is very likely that this enzyme plays a crucial role in the defense against oxidative stress in these organisms. In this context, there is preliminary evidence that an SOD-deficient mutant of C. jejuni is compromised in its ability to survive the oxidative stress imposed during its invasion of epithelial cells (17). In this study we have assessed the contribution of SOD towards the survival of C. coli UA585 after its exposure to air and during its persistence on foods. In addition, the role that this enzyme plays during the colonization of the gastrointestinal tract of the chick by C. coli was assessed by using an in vivo model of orally infected 1-day-old chicks.
MATERIALS AND METHODS
Bacterial strains, growth, and media.
C. coli UA585, originally isolated from a diarrheic pig, was a generous gift from D. E. Taylor (University of Alberta, Edmonton, Alberta, Canada). CK100, the catalase-deficient mutant derived from this parental strain, has been described previously (8). Routinely, C. coli was grown under microaerobic conditions on Mueller-Hinton agar (MHA) by using a gas-generating kit (Oxoid-Unipath, Basingstoke, United Kingdom). E. coli strains were grown at 37°C on Luria-Bertani agar containing ampicillin (100 μg ml−1) or tetracycline (10 μg ml−1) when necessary.
To assess the resistance of strains to oxidative-stress-generating agents, aliquots of overnight cultures of the C. coli parental strain and mutants were inoculated (1 in 50) into prewarmed Mueller-Hinton broth (MHB) containing various concentrations of methyl viologen (Sigma-Aldrich Chemical Co., Poole, United Kingdom), and cells were incubated aerobically in 500-ml flasks at 37°C with shaking (150 rpm). During the course of the experiment growth was monitored by assessing the optical density at 600 nm (OD600).
C. coli was cultured on solid agar under aerobic growth conditions essentially as described by Jones et al. (13). Briefly, the medium used was blood agar base no. 2 with 5% difibrinated horse blood (Oxoid). Inoculated agar plates were incubated either in unsealed plastic bags containing moistened tissues or in anaerobic gas jars (Oxoid) with open valves containing a beaker of water. In both cases the jars or bags were opened and ventilated every 8 h to ensure continuous aerobic conditions.
Allelic exchange, natural transformation, and complementation.
The structural sodB gene from C. coli UA585 present in the plasmid pSOD1 (18) was disrupted by the introduction of a 2.3-kb BglII tetracycline resistance cassette (5) into a unique internal BglII site. The resulting suicide plasmid, pMUT1, was introduced into C. coli UA585 by natural transformation (20). Transformants were recovered after 48 h, after microaerophilic incubation at 37°C on MHA-containing tetracycline (10 μg ml−1). The SOD-deficient mutant generated in this manner was designated CCSD1.
Complementation was achieved by first generating a plasmid able to replicate in C. coli and expressing SodB. A DNA fragment containing the C. coli UA585 sodB gene was generated by PCR. Primers SOD1 (5′-GGA TCC GAA ATT TAA AGA AAT AAT TAA AGA CTT TCT-3′) and SOD3 (5′-GGA TCC TGG ATG AGC TTA ATT CTC AAG GAC-3′) with homology to the 5′ end of the sodB promoter region and the DNA region downstream of sodB, respectively, were constructed. A commercial Taq DNA polymerase system (Perkin-Elmer Cetus, Norwalk, Conn.) was used to amplify chromosomal DNA from C. coli UA585 in the presence of 200 μmol of deoxynucleotides and 0.2 μmol of each primer. After an initial denaturation at 95°C for 3 min, there were 30 cycles of amplification that included denaturing at 95°C for 30 s, annealing at 46°C for 30 s, and an extension at 72°C for 2 min. There was a final extension at 72°C for 10 min. The 850-bp DNA fragment generated in this manner was digested with BamHI and cloned into the corresponding site of the E. coli/C. coli shuttle vector pUOA13 (20). The resulting vector, pSOD13, was introduced into C. coli CCSD1 by natural transformation, and the transformants were recovered after 48 h, after microaerobic incubation at 37°C on MHA containing kanamycin (60 μg ml−1).
DNA-DNA hybridizations.
Genomic DNA isolated from both the wild-type and the SOD-deficient mutant C. coli CCSD1 was digested with ClaI and EcoRV and electrophoresed on a 0.7% agarose gel. After depurination with 250 mM HCl, the DNA was transferred from the gel to a Hybond N+ nylon membrane (Amersham, Slough, United Kingdom) by using 0.4 M NaOH. Hybridizations were performed with a nonradioactive enhanced chemiluminescence gene detection kit (Amersham) according to the manufacturer’s instructions by using the purified insert from pSOD1 (18) as a gene probe.
Protein extraction and enzymatic analysis.
Crude cell lysates of various strains of C. coli grown on MHA at 37°C under microaerobic conditions were prepared as follows. Harvested cells were washed twice in 0.15 M Tris-HCl (pH 7.0) and resuspended in 0.15 M Tris-HCl (pH 7.0) containing 0.2 mg of lysozyme ml−1 and 0.1 mM EDTA. Five cycles of freeze-thawing, with a combination of liquid N2 and a temperature of 42°C, were used to lyse the cells. The lysates were then cleared by centrifugation at 20,000 × g for 20 min. Soluble cellular proteins were extracted in the supernatant. To demonstrate the expression of SOD activity in the lysates, protein samples were resolved on 7.5% polyacrylamide gels under nondenaturing conditions, and the gels stained for SOD activity by the method of Beauchamp and Fridovich (1).
H2O2 sensitivity assay.
Cells suspensions, prepared from overnight cultures of C. coli UA585, CCSD1, CCSD1 containing pSOD13 or the catalase-deficient mutant CK100 (8) and grown on MHA under microaerobic conditions at 37°C, were inoculated into prewarmed MHB to an OD600 of 0.3 (ca. 5 × 108 CFU ml−1). H2O2 was added to a final concentration of 5 mM, and the cells were incubated microaerobically at 37°C. During the course of the experiment the ability of cells to replicate was assessed by plating diluted aliquots onto MHA plates containing bovine catalase (100 U ml−1). The plates were incubated microaerobically at 37°C for 48 h.
Survival studies.
The survival of the various strains of C. coli during incubation at nongrowth temperatures was assessed in MHB, in commercial skim milk, and on fresh chicken skin. Campylobacter cells grown microaerobically to confluence on MHA at 37°C were used to inoculate flasks (500 ml) containing either 100 ml of MHB or 100 ml of skim milk. The flasks were then incubated aerobically with shaking (150 rpm) at 25°C. Aliquots were removed at regular intervals, and recoverable cell numbers were assessed by plate count by using either MHA or CCDA Campylobacter selective agar (Oxoid). When survival on chicken skin was being studied, 100-μl aliquots of the Campylobacter suspensions were inoculated onto discs of chicken skin (diameter, 2 cm), which had been sterilized by gamma irradiation (20 kGy; Isotron PLC, Swindon, United Kingdom). The samples were then placed into sterile petri dishes and incubated with the lid on at 25°C. At intervals the cells were recovered, after brief vortexing in MHB, and the numbers of recoverable cells were assessed as described above.
Chick colonization model.
Eggs from specific-pathogen-free chickens (Torbay flock 9; Wickham Labs, Wickham, United Kingdom) were hatched in isolators. The hatchlings were randomly divided into groups of 10 birds. Each group of chicks was maintained in a separate isolator with unlimited food and water. At day 1 of age chicks were dosed orally by gavage with 0.1 ml of phosphate-buffered saline (PBS) containing 101 to 106 CFU of either C. coli UA585, the SOD-deficient mutant CCSD1, CCSD1 mutant containing the plasmid pSOD13, or the catalase-deficient mutant CK100 (8). At 5 days postinoculation, the chicks were killed by cervical dislocation, and the cecal contents were cultured as described previously (21), except that the bacteria were cultured on blood agar plates containing tetracycline (10 μg ml−1) when necessary. The degree of colonization was determined as the CFU per gram of cecal contents. The minimum level of detection was 100 CFU g−1 of cecal contents. The data were analyzed by using a nonparametric Mann-Whitney test to assess statistical significance.
To assess the inhibitory action of the resident cecal community on Campylobacter cells, lawns of the various strains were prepared in Brucella agar plates (Oxoid). The cecal contents from five 1-day-old chicks were then pooled and applied to wells punched into the center of the lawned plates. The plates were then incubated at 42°C for 24 h and examined for growth inhibition around the wells.
RESULTS
Construction of an SOD-deficient mutant of C. coli by gene replacement.
In order to evaluate the role of SOD in the oxidative stress resistance and the aerotolerance of Campylobacter species, the sodB gene of C. coli UA585 was inactivated by allelic exchange (14). One tetracycline-resistant transformant generated by this procedure was shown by Southern hybridization to have occurred via a double crossing-over event resulting in the replacement of the native gene with an inactivated copy of the sodB gene (data not shown) and was designated CCSD1. To eliminate the possibility that the phenotype of CCSD1 was a result of a polar effect, following the insertion of the tetO marker, it was necessary to construct a strain in which the disrupted chromosomal sodB gene was complemented by an extrachromosomal copy of this locus. The appropriate strain was generated by introducing the plasmid pSOD13, which contains a recombinant copy of sodB, into C. coli CCSD1.
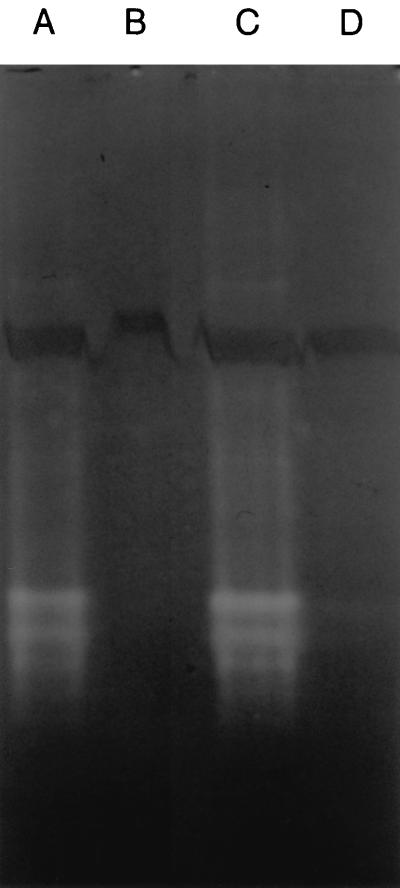
To confirm the absence of SOD activity in the mutant CCSD1, whole-cell crude protein extracts were analyzed by nondenaturing polyacrylamide gel electrophoresis with specific staining for SOD activity (1). Achromatic bands of SOD activity were apparent in extracts derived from the parental strain and CCSD1 containing pSOD13 (Fig. 1), thus demonstrating the expression of a functional sodB gene in these strains. The differing electromorphic forms of SOD apparent in these lysates correspond to multimeric forms of the protein and is a consequence of the nondenaturing conditions used (18). In contrast, no bands of SOD activity were present in extracts from CCSD1, confirming the expected phenotype of the SOD-deficient mutant.
FIG. 1.
The absence of SOD activity in the C. coli mutant CCSD1. Total soluble proteins were analyzed by electrophoresis on 7.5% polyacrylamide gels run under nondenaturing conditions and were visualized after specific staining for SOD activity. SOD activity appears as achromatic bands against a dark background. Lanes: A, lysate from C. coli UA585; B, lysate from C. coli CCSD1; C, lysate from C. coli CCSD1 containing the plasmid pSOD13; D, lysate from C. coli CCDSD1 containing the parental plasmid pUOA13.
Growth and oxidative-stress resistance of the SOD-deficient mutant.
It has been suggested that C. jejuni is able to grow freely in air (13). In order to assess the contribution of SOD to the oxidative-stress tolerance of C. coli, the growth of the parental strain and the sodB mutant CCSD1 was compared in shake flasks incubated in aerobic atmospheres. The presence of the SOD-deficient phenotype did not affect the ability of C. coli to grow under these conditions since the mutant had a growth rate equivalent to that of the parental strain (Fig. 2). The initial description of the adaptation of C. jejuni to aerobic metabolism in (13) was based upon observations of the growth of this bacterium on solid agar in humid environments. We therefore assessed the ability of the parental strain, the SOD-deficient mutant, and the catalase-deficient CK100 mutant (8) to grow aerobically under these conditions. The parental strain and both mutants grew under these conditions, the growth rate and the size of the colony formation being broadly equivalent for all cell types (data not shown).
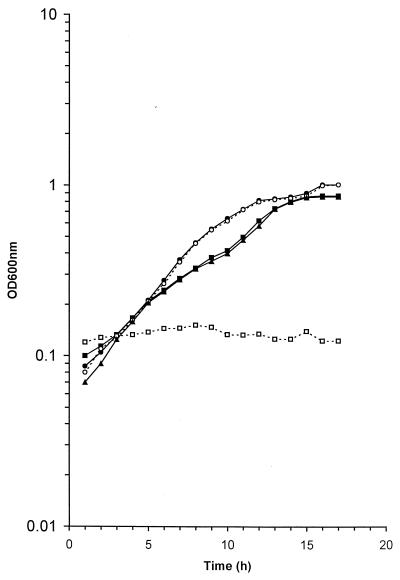
FIG. 2.
The inhibition of the growth of the SOD-deficient mutant CCSD1 by methyl viologen. Cells of C. coli strains UA585 (● and ■), CCSD1 (○ and □), and CCSD1 containing pSOD13 (▴) were grown under aerobic conditions in MHB (○ and ●) and in the presence of 10 μM methyl viologen (□, ■, and ▴). Growth was assessed by taking OD600 readings. Similar results were reproducibly obtained in at least three separate experiments.
The growth of SOD-deficient cells was next assessed in the presence of methyl viologen, an effective superoxide-generating agent. The addition of 1 μM methyl viologen to the growth medium had little effect on the growth rate of either the parental strain or the CCSD1 mutant (data not shown). In contrast, wild-type cells grew in the presence of 10 μM methyl viologen, but SOD-deficient cells did not (Fig. 2). Furthermore, the presence of the plasmid pSOD13 in the CCSD1 cells restored the ability of this strain to grow in the presence of 10 μM methyl viologen. This confirms that the failure of cells containing the sodB mutation to grow in the presence of this concentration of methyl viologen was due to the loss of SOD activity alone.
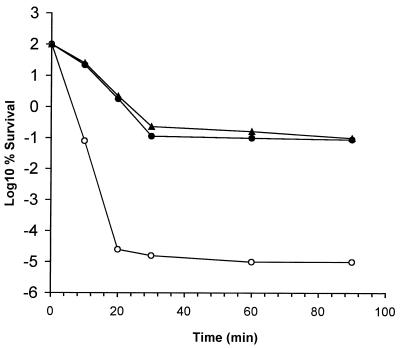
Carlioz and Touati (3) have demonstrated the increased sensitivity of an E. coli sodA sodB double mutant toward the toxic effects of H2O2 and have suggested that this may be due to Fenton-mediated killing. A three-log decline in the percentage of survivors of wild-type cells and CCSD1 cells containing pSOD13 occurred within 30 min of the challenge (Fig. 3). The number of surviving cells remained constant thereafter, suggesting that the available H2O2 had been removed by catalase as demonstrated previously for KatA+ cells of C. coli (8). In comparison, SOD-deficient cells of C. coli exhibited a seven-log reduction in viability in the same time period and were, therefore, considerably more sensitive to H2O2 than the parental strain or CCSD1 cells in which the chromosomal sodB lesion had been complemented.
FIG. 3.
The sensitivity of SOD-deficient cells of C. coli to H2O2. Cells of C. coli strains UA585 (●), CCSD1 (○), and CCSD1 containing pSOD13 (▴) were challenged with 5 mM H2O2, and viability was assessed by plate counting. Similar results were reproducibly obtained in at least three separate experiments.
Survival characteristics of the SOD-deficient mutant.
Catalase, which catalyzes the conversion of H2O2 to water and oxygen is, like SOD, considered to be part of the cellular defense against oxidative stress. Accordingly, catalase is also present in most aerobic organisms. To determine the relative roles of catalase and SOD during the exposure of campylobacters to aerobic conditions, survival studies were conducted at a nongrowth temperature (25°C). The thermophilic campylobacters C. coli and C. jejuni are unable to grow below 30°C and, accordingly, will not grow in most foods if they are stored at room temperature or below.
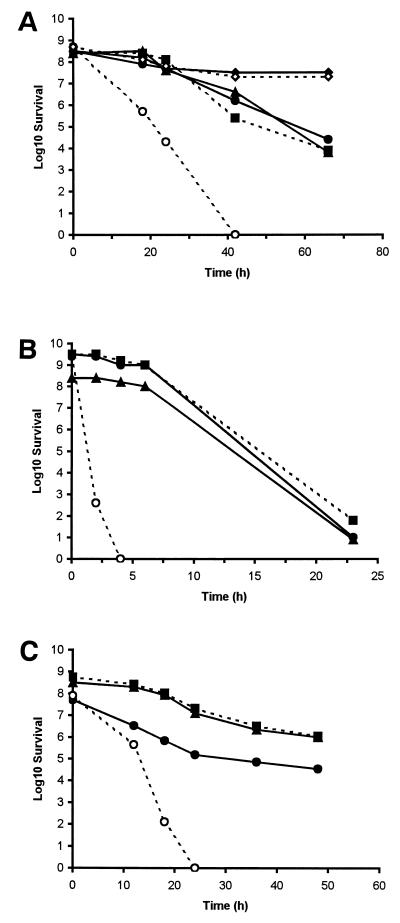
When cell survival was assessed under aerobic conditions in MHB, the number of recoverable wild-type cells declined steadily and had fallen by a factor of 1.2 × 104 by 66 h of incubation at 25°C (Fig. 4A). The isogenic, catalase-deficient mutant CK100, which is derived from C. coli UA585, has been described previously (8). This mutant possessed survival characteristics similar to those of the parental strain. In contrast, bacterial counts of the SOD-deficient mutant decreased by a factor of 2.5 × 104 within the first 24 h of incubation, and viable counts became undetectable shortly afterwards. When the plasmid pSOD13 was introduced into CCSD1, the resulting cells again exhibited levels of survival comparable to those observed for the wild-type strain. This confirmed that the decreased viability of cells containing the sodB mutation was due to the loss of SOD activity alone. Furthermore, survival rates of the parental strain and of the SOD-deficient mutant under microaerobic conditions was similar (Fig. 4A), suggesting that the role of SOD is of primary importance for the survival of C. coli during exposure to aerobic stress.
FIG. 4.
The survival of C. coli UA585 and mutants in MHB and model food systems. Cells of C. coli strains UA585 (●), CK100 (■), CCSD1 (○), and CCSD1 containing pSOD13 (▴) were introduced into MHB (A), into skim milk (B), or onto chicken skin (C) and then incubated aerobically at 25°C. Cells of C. coli UA585 and CCSD1 were also incubated under microaerobic conditions in MHB (⧫ and ◊). Survival was determined from viable counts. Similar results were reproducibly obtained in at least three separate experiments.
To assess whether the SOD deficiency compromised the survival of C. coli in foods, the survival of the various strains of C. coli over time was monitored in milk or on chicken skin. These foodstuffs are considered important vectors for the transmission of campylobacters. In skim milk, levels of the parental strain of C. coli fell by a factor of 3 × 108 within 23 h (Fig. 4B). In parallel with survival studies carried out in MHB, the catalase-deficient mutant (CK100) displayed a pattern of survival similar to that of the wild-type strain, whereas the survival of the SOD-deficient mutant was severely compromised. Viable counts of the SOD-deficient mutant fell to undetectable levels after just 4 h. Chicken skin represents an entirely different environment from milk and, therefore, presents alternative survival challenges to campylobacters. In general, the survival of all of the strains and mutants of C. coli was more prolonged on chicken skin than in other media (Fig. 4C). However, the relative sensitivities of the individual C. coli strains and mutants in this environment was similar to that seen in milk and MHB. Cells of both the wild type and the catalase-deficient mutant showed similar survival characteristics, with plate counts declining steadily until they had decreased by 1,500- and 500-fold, respectively, after 48 h. In contrast, numbers of recoverable cells of the SOD-deficient mutant fell by 6.3 × 105-fold during the first 18 h of incubation and to undetectable levels soon afterwards. Again, when the plasmid pSOD13 containing the cloned sodB gene was introduced into CCSD1 the viability of the resulting cells became broadly equivalent to that of the wild type whether growth was monitored in skim milk or on chicken skin.
Colonization studies.
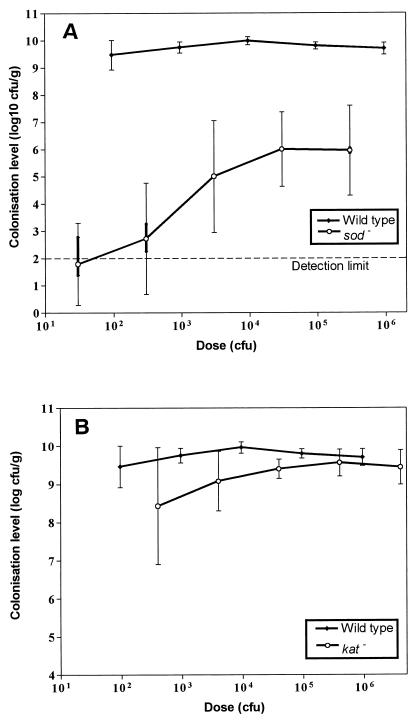
The value of the chicken model to assess the role of campylobacter colonization factors in vivo has been established previously (21). These studies showed that C. jejuni 81116 colonized 1-day-old chicks in a dose-dependent manner, such that a dose of 6 × 105 CFU is needed for maximum colonization (109 CFU g−1 of cecal contents) in 100% of birds. In contrast, C. coli UA585 had a much higher colonization potential, achieving a maximum colonization at a dose of only 95 CFU (Fig. 5A). The catalase-deficient mutant, CK100, had the same colonization potential as the parental strain (Fig. 5B), whereas the SOD-deficient mutant was significantly less efficient at colonizing the chicks than the parental strain at each dose level (P < 0.001); only 30 and 50% of birds were detectably colonized by the SOD-deficient mutant strain at doses of 3 × 101 CFU and 3 × 102 CFU, respectively, and one bird appeared uncolonized even with a dose of 3 × 105 CFU. In addition, a dose of 3 × 105 CFU gave only a maximum colonization level of about 106 CFU g−1 of cecal contents.
FIG. 5.
The colonization of 1-day-old chicks by C. coli UA585 and mutants. The SOD-deficient mutant CCSD1 (A) and the catalase-deficient mutant CK100 (B) were introduced into chicks, and the degree of colonization was determined as the CFU per gram as described previously (21). The lines flow through the geometric mean colonization levels of each group. The minimum level of detection was 100 CFU g−1; hence, for some birds there are vertical bars representing a range of means since birds from which no campylobacters were recovered could be colonized with between 0 and 99 CFU g−1 of cecal contents.
It was possible that the reduction in colonization potential of the SOD-deficient mutant was due to the inhibitory action of the resident avian cecal microflora, such as, for example, after the production of oxidative-stress-generating agents such as H2O2. The sensitivity of the wild-type and mutant cells to this microflora was therefore assessed by using a growth inhibition assay. Under these conditions, no evidence of growth inhibition was observed for wild-type, catalase-deficient, or SOD-deficient cells.
To determine whether the reduced colonization of the SOD-deficient mutant strain was due to the sodB mutation or to polar effects, strain CCSD1 containing pSOD13 was introduced into the chick model. To confirm the stability of pSOD13 during the growth of C. coli in vivo, plasmids were isolated from cells recovered from the chicken ceca after colonization by using a standard preparative technique (20). Unfortunately, when these plasmids were analyzed by restriction analysis and gel electrophoresis they were found not to contain copies of the recombinant sodB gene (data not shown). These results indicate that during in vivo passage, pSOD13 had undergone genetic rearrangements. It was therefore not possible to demonstrate the effects of complementation on the inactivated copy of the sodB gene in CCSD1. Conversely, when plasmids were isolated from cells of CCSD1 incubated under the nongrowth conditions of the survival experiments, no rearrangements resulting in the loss of the sodB sequence in pSOD13 were detected (data not shown).
DISCUSSION
Campylobacters, such as C. jejuni and C. coli, survive in a wide range of environments, including water, meat products, and milk. Under such conditions they are exposed to a variety of stresses which they must be able to tolerate in order to permit their transmission to suitable environments for growth, such as the avian gastrointestinal tract. Campylobacters, like other bacteria, possess a number of inherent mechanisms which confer resistance to such environmental stresses.
Given the microaerophilic nature of Campylobacter species, the ability of these organisms to survive in air and under natural environmental conditions of oxidative stress must be profoundly influenced by the presence of cellular mechanisms which can eliminate reactive oxygen intermediates. The enzyme SOD, which catalyzes the conversion of superoxide radicals to H2O2 and dioxygen, is present in most aerobic organisms and is considered to be an inherent part of the defense against oxidative stress (3, 7). Consequently, SOD is likely to play a crucial role in the survival of campylobacters in environments in which reactive oxygen intermediates are generated. In addition, it has been postulated that SOD is an important mechanism by which bacterial pathogens survive the oxidative bursts generated by eukaryotic inflammatory cells. Thus, SOD may also play a role in virulence (10) and may, therefore, have a potential role in the intracellular survival of C. jejuni (17). In order to investigate the role of SOD in the physiology and oxidative-stress resistance of campylobacters, an isogenic mutant has been generated by allelic exchange.
A number of reports have suggested that campylobacters can adapt to aerobic metabolism in a moist environment (13). In this study, we have demonstrated that the ability of an SOD-deficient strain of C. coli to grow in shake flasks incubated in aerobic atmospheres and to grow in the aerobic conditions defined by Jones et al. (13) was unchanged relative to the parental strain. This indicates that SOD is not essential for aerobic growth in laboratory media. The SOD-deficient strain, however, was more sensitive to the growth-inhibitory effects of methyl viologen. This is likely to be a consequence of its failure to detoxify the superoxide radicals generated by this agent. The SOD-deficient mutant also showed an increased sensitivity to the bactericidal activity of H2O2. The heightened killing effect of this agent on the SOD-deficient mutant may result from the interaction of H2O2 with the elevated levels of endogenous O2− present within SOD-deficient cells (9). Cell damage and accelerated death would then occur from the subsequent generation of highly reactive OH· radicals via an iron-catalyzed Fenton reaction (2).
While the growth of the SOD-deficient mutant under aerobic conditions was comparable to that of the parental strain, the survival of the mutant in a nongrowth environment was severely compromised under a range of conditions, including aerobic storage in MHB, in skim milk, and on chicken skin. Thus, it appears that the contribution of SOD to the aerotolerance of C. coli is dependent on the growth state of the cells. In particular, SOD plays a significant role in the protection of nongrowing cells from aerobic stress. The reason for this is unclear at present, but it may reflect the generation of increased levels of superoxide radicals in cells which are not actively growing.
Catalase is also thought to provide protection against oxidative stress by converting H2O2 to H2O and O2. However, when the catalase-deficient mutant was grown in aerobic atmospheres and in the presence of methyl viologen it behaved like the wild-type strain (data not shown). It also displayed survival characteristics like those of the parental strain when its viability was assessed at nongrowth temperatures. These results indicate that while it is important for protection against the toxic effects of H2O2 (8), catalase, unlike SOD, is not important for the growth of C. coli cells during oxidative stress and for the survival of nongrowing cells after exposure to air.
The avian gut is generally considered to be the natural environmental niche of the thermophilic campylobacters. Although the majority of campylobacters colonizing poultry are C. jejuni strains, C. coli strains are frequently found in broiler flocks (16a). The value of the chick model to assess the role of campylobacter colonization factors has been established previously by using flagellin gene mutants of C. jejuni 81116 (21). Because it gives a dose response curve, C. jejuni 81116 is considered to be an appropriate parent strain for such studies (4). In contrast, C. coli UA585, like many other campylobacter strains now tested, gives an “all or nothing” type of colonization dose response such that low numbers (usually <102) do not colonize, whereas higher numbers give a maximum colonization. Nevertheless, this colonization profile did not preclude observations of major changes in colonization potential. The inactivation of the sodB gene clearly reduced the colonization of the chicken gut by campylobacters. A dose of 102 CFU of the parental strain colonized at a 1,000-fold-higher level than a dose of 105 CFU of the SOD-deficient mutant. Unfortunately, plasmid pSOD13, with which we were able to complement the sodB mutant in the survival studies, proved to be unstable when cells containing it were introduced into the chick model. This is probably a consequence of the absence of antibiotic selection. However, preliminary experiments have demonstrated that maintaining sufficient levels of antibiotics in chicken gastrointestinal tracts is extremely difficult by oral application flocks (16a). We therefore cannot rule out the possibility that the disrupted sodB sequence has a polar effect on the expression of downstream genes. It seems likely, however, that the differential in the colonization potential of the mutant compared to the wild-type strain is attributable solely to SOD since a terminator sequence located downstream of the structural sodB gene is likely to uncouple transcription of the gene from those genes downstream (17, 18).
Campylobacters largely colonize the small intestine and cecum which, given the presence of anaerobic organisms, would tend not to be an aerobic environment. Nevertheless, the reduced colonization potential of the SOD-deficient mutant suggests that these bacteria encounter some form of oxidative stress and that SOD is important in the resistance of the bacterial cells to this stress. It seems unlikely that the reduction in colonization potential of the SOD-deficient mutant is mediated by growth-inhibitory substances, such as H2O2, which are produced by the resident microflora, since the presence of cecal contents did not inhibit the growth of either mutant or parental cells. Furthermore, a catalase-deficient mutant which is hypersensitive to hydrogen peroxide (8) had the same colonization potential as the wild-type cells.
In conclusion SOD, but not catalase, is an important determinant in the ability of nongrowing cells of C. coli to survive in the environment and in food during exposure to aerobic conditions. Furthermore, the inactivation of the sodB gene decreased colonization potential in an experimental oral infection of 1-day-old chicks. Finally, the dependence of environmental survival and of the gastrointestinal colonization of chicks on detectable factors such as SOD suggests that intervention strategies could be designed to target such factors and thereby reduce or eliminate campylobacter contamination in the food chain.
ACKNOWLEDGMENT
We thank the Ministry of Agriculture, Fisheries and Foods (United Kingdom) for financial support for this work.
REFERENCES
- 1.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 2.Beyer W, Imlay J, Fridovich I. Superoxide dismutases. Prog Nucleic Acid Res. 1991;40:221–253. doi: 10.1016/s0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- 3.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cawthraw S, Park S F, Wren B, Ketley J M, Ayling R, Newell D G. The usefulness of the chick colonization model to investigate potential colonization factors of campylobacters. In: Newell D G, Ketley J M, Feldman R, editors. Campylobacters, helicobacters and related organisms. New York, N.Y: Plenum Press, Inc.; 1996. pp. 649–652. [Google Scholar]
- 5.Dickinson J H, Grant K A, Park S F. Targeted and random mutagenesis of the Campylobacter coli chromosome using integrational plasmid vectors. Curr Microbiol. 1995;31:92–96. doi: 10.1007/BF00294282. [DOI] [PubMed] [Google Scholar]
- 6.Farr S B, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridovich I. Superoxide dismutases. Adv Enzymol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- 8.Grant K A, Park S F. Molecular characterization of katA from Campylobacter jejuni and generation of a catalase-deficient mutant of Campylobacter coli by intraspecific allelic exchange. Microbiology. 1995;141:1369–1376. doi: 10.1099/13500872-141-6-1369. [DOI] [PubMed] [Google Scholar]
- 9.Haas A, Brehm K. Superoxide dismutases and catalases: biochemistry, molecular biology and some biomedical aspects. Genet Eng Biotechnol. 1993;13:243–269. [Google Scholar]
- 10.Haas A, Goebel W. Microbial strategies to prevent oxygen dependent killing. Free Radical Res Commun. 1992;16:137–157. doi: 10.3109/10715769209049167. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman P S, George H A, Kreig N R, Smibert R M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. I. Physiological aspects of enhanced aerotolerance. Can J Microbiol. 1979;25:1–7. doi: 10.1139/m79-001. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman P S, George H A, Kreig N R, Smibert R M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979;25:8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- 13.Jones D M, Sutcliffe E M, Rios R, Fox A J, Curry A. Campylobacter jejuni adapts to aerobic metabolism in the environment. J Med Microbiol. 1993;38:145–150. doi: 10.1099/00222615-38-2-145. [DOI] [PubMed] [Google Scholar]
- 14.Labigne-Roussel A, Courcoux P, Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama K. Rapid viability loss on exposure to air in a superoxide dismutase-deficient mutant of Porphyromonas gingivalis. J Bacteriol. 1994;176:1939–1943. doi: 10.1128/jb.176.7.1939-1943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama K. Nucleotide sequence of the Streptococcus mutans superoxide dismutase gene and isolation of insertion mutants. J Bacteriol. 1992;174:4928–4934. doi: 10.1128/jb.174.15.4928-4934.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Newell, D. G. Unpublished data.
- 17.Pesci E C, Cottle D L, Picket C L. Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect Immun. 1994;62:2687–2695. doi: 10.1128/iai.62.7.2687-2694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purdy D, Park S F. Cloning, nucleotide sequence, and characterization of a gene encoding superoxide dismutase from Campylobacter jejuni and Campylobacter coli. Microbiology. 1994;140:1203–1208. doi: 10.1099/13500872-140-5-1203. [DOI] [PubMed] [Google Scholar]
- 18a.Sanger Centre. 26 January 1999, posting date. C. jejuni page. [Online.] http://www.sanger.ac.uk/Projects/C_jejuni. [27 January 1999, last date accessed.]
- 19.Tauxe R V. Epidemiology of Campylobacter jejuni in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 20.Wang Y, Taylor D E. Natural transformation in Campylobacter species. J Bacteriol. 1990;172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wassenaar T M, van der Zeijst B A M, Ayling R, Newell D G. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J Gen Microbiol. 1993;139:1171–1175. doi: 10.1099/00221287-139-6-1171. [DOI] [PubMed] [Google Scholar]