Abstract
The heme oxygenase (HO) system is believed to be a crucial mechanism for the nervous system under stress conditions. HO degrades heme to carbon monoxide, iron, and biliverdin. These heme degradation products are involved in modulating cellular redox homeostasis. The first identified isoform of the HO system, HO-1, is an inducible protein that is highly expressed in peripheral organs and barely detectable in the brain under normal conditions, whereas HO-2 is a constitutive protein that is highly expressed in the brain. Several lines of evidence indicate that HO-1 dysregulation is associated with brain inflammation and neurodegeneration, including Parkinson’s and Alzheimer’s diseases. In this review, we summarize the essential roles that the HO system plays in ensuring brain health and the molecular mechanism through which HO-1 dysfunction leads to neurodegenerative diseases and disruption of nervous system homeostasis. We also provide a summary of the herbal medicines involved in the regulation of HO-1 expression and explore the current situation regarding herbal remedies and brain disorders.
Keywords: heme oxygenase, neuroinflammation, neurodegenerative diseases, Alzheimer’s disease, Parkinson’s disease
1. Introduction
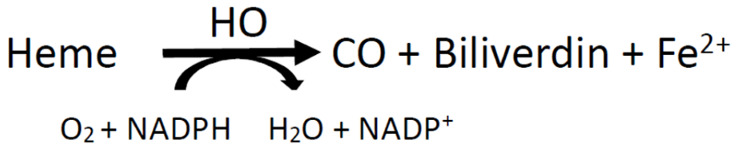
Heme oxygenase (HO) is an evolutionarily conserved enzyme and is involved in many different diseases. HO plays the role of a rate-limiting enzyme in degrading endogenous iron protoporphyrin heme by release of carbon monoxide (CO), biliverdin (BV), and ferrous ions (Fe2+), which could be recycled for heme homeostasis.
 |
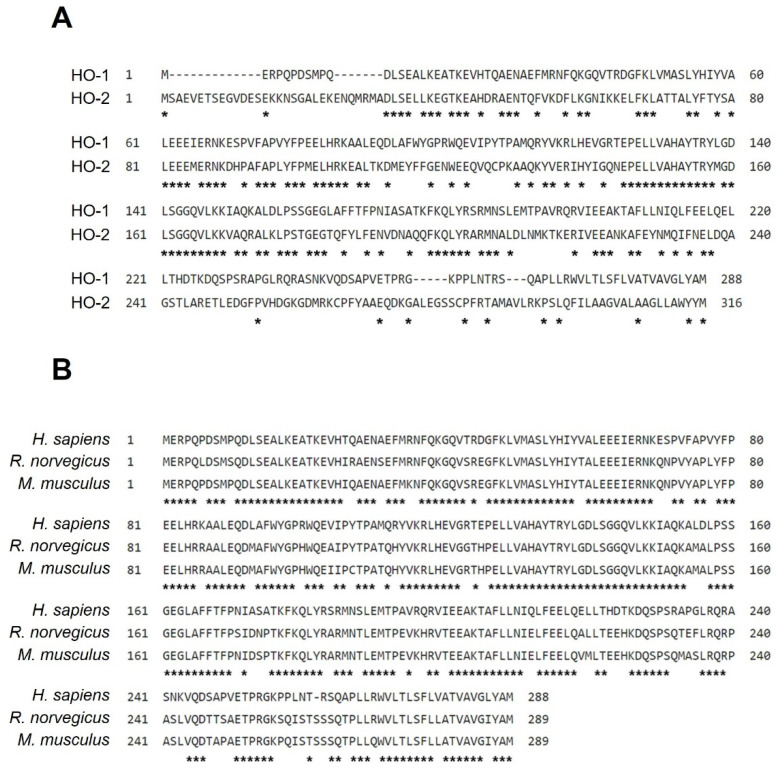
There are three isoforms of the HO system: HO-1, HO-2, and HO-3. Interestingly, HO-3, a pseudogene discovered in rat, is a splice-variant of HO-2 and remains elusive and poorly understood [1,2]. The amino acid alignments of HO-1 and HO-2 are shown in Figure 1A; they demonstrate a 43% homology the amino acid sequence of humans. HO-1, encoded by a gene called HMOX1, is a well-known inducible isoform and can be transcriptionally upregulated as much as 100-fold as a result of stimuli, such as radiation, toxins, infections, and injuries [3]. HO-2, encoded by a gene called HMOX2, is a constitutively expressed protein and is present in high levels in the brain [4].
Figure 1.
The amino acid alignment of HO. (A) Amino acid alignment of HO-1 and HO-2 in human. (B) The amino acid homology of HO-1 in humans, rat, and mice. Asterisks indicate common retention regions, meaning that the amino acids here are identical.
HO is the rate-limiting enzyme of heme degradation, and the end-products, which include CO, Fe2+, and BV (converted into bilirubin (BR) by biliverdin reductase), play important roles in regulating cellular homeostasis. BR is more electrophilic than BV and thereby comparatively increases the reactivity of Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (Keap1) to release Nrf2 [5]. The Keap1–Nrf2 system has been well studied in mammalian cells, especially its protection role against oxidative stress in organisms. CO is well-known for its antioxidant, vasodilator, anti-inflammatory, and anti-apoptotic effects, among others. Therefore, HO and its heme degradation products are potent protective modulators under oxidative stress conditions.
The controversial role of HO-1 is explored in several studies, e.g., they both delineate the importance of its antioxidant activity and also demonstrate its function in the development of diseases. In this review, we summarize the essential roles of HO-1 and its end-products for ensuring brain health and further discuss how HO-1 dysfunction leads to several neural disorders, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD). We also review the ongoing clinical herbal trials aimed at exploring the therapeutic targets derived from HO-1 regulation for the treatment of neural disorders.
2. The Functions of HO-1 in Brain Physiology
2.1. Overview
The brain is the most important organ in the human body and requires sufficient oxygen to maintain its functions, i.e., it needs to consume 20% of the total basal oxygen to support intensive neuronal activity [6]. As a result of the transport and storage of oxygen, heme is necessary for the survival of most organisms. Moreover, in the central nervous system (CNS), redox homeostasis is involved in development, aging, and neural diseases [7]. Since HO is the rate-limiting enzyme in heme degradation and can be modulated by redox status, the role of the HO system is important for maintaining brain function. Current studies demonstrate that dysregulation of the HO system is associated with the pathogenesis of neurodegenerative diseases, such as AD, PD, and multiple sclerosis (MS) [8,9], and is even involved in neurotoxicity and neuroinflammation.
HO-1 was first identified in 1968, and many studies focused on the regulation and function of this protein in heme metabolism [10]. There is a high amino acid homology of HO-1 in humans, mice, and rats (Figure 1B). However, increasingly, amounts of evidence over in recent decades demonstrate that HO-1 could be induced by a variety of inducers other than heme [11,12], such as heat shock, heavy metals, endotoxin, inflammatory cytokines, and even oxidative stress, indicating that HO-1 plays a vital role in modulating cellular homeostasis. Interestingly, HO-1 induction with increased heme degradation products confers antiviral activity by interferon activation against a wide range of viruses, such as HIV, influenza, respiratory syncytial virus, enterovirus 71, human herpes simplex virus, and respiratory syndrome virus, etc. [3]. A current study also indicates that HO-1 activation may be a possible therapeutic strategy against COVID-19-associated complications [3,13]. All these investigations indicate that HO-1 plays a vital role in regulating human physiopathology.
2.2. The Canonical and Non-Canonical Effects of HO-1 in Brain
The by-products of heme degradation by HO-1 include BV, CO, and Fe2+, and the canonical effects of HO in the brain include antioxidant, anti-apoptosis, vasodilation, and anti-inflammatory responses [14,15,16,17]. Due to the direct antioxidant property [18], BV administration in rats can ameliorate damage to the brain by reducing oxidative DNA damage [19]. Furthermore, BV alleviates pro-inflammatory responses through the NF-κB pathway [20] and inhibits toll-like receptor 4 (TLR4) signaling [21], which is the main contributor to neurological disorders [22,23]. Moreover, CO in the brain is an activator of guanylyl cyclase and functions as a neurotransmitter [24,25]. Astrocytic mitochondrial biogenesis can be stimulated by CO through L-type Ca2+ channel-mediated PGC-1α/ERRα signaling [26]. Although it does not directly influence the brain tissue, CO exhibits anti-apoptosis and anti-inflammatory effects in the lungs of brain-dead rats through p38-MAPK signaling [27]. CORM-A1 supplements, i.e., a carbon monoxide donor, offer a novel and effective therapeutic agent against cerebrovascular dysfunction caused by neonatal seizures [28] and experimental allergic encephalomyelitis [29].
Interestingly, aside from the canonical effect, recent studies demonstrate that HO-1 also possesses other physiological functions, which are not correlated with their own enzymatic functions; these are termed “non-canonical functions”. Those non-canonical functions contain protein–protein interaction, intracellular compartmentalization, and extracellular secretion [30]. The protein–protein interaction of HO isoforms was first observed in 1977 [31]. An interaction between HO-1 and HO-2 proteins serves to limit HO activity [32], indicating a possible cytoprotective range of HO expression in brain tissues.
The second non-canonical effect of HO-1 is intracellular compartmentalization. Although studies demonstrated that HO isoforms were localized in the endoplasmic reticulum, HO-1 was also found to be compartmentalized in nuclei, mitochondria, and caveolae [33]. Bioinformatic analysis demonstrates that HO-1 has a nuclear import amino acidic sequence. In the primary astroglial culture system, HO-1 can be induced by excitotoxic injury with concomitant nuclear translocation [34]. HO-1 can translocate into the nucleus under hypoxia or stress conditions with a reduction in HO activity [35]. This nuclear localization of HO-1 may activate certain oxidant-response transcription factors, such as activator protein-1 and NF-κB, and then promote cytoprotection, including cellular proliferation and DNA repair [36,37]. Other subcellular localizations described for HO-1 include mitochondria and caveolae. The localization of HO-1 protein in mitochondria plays an important role in the modulation of mitochondrial heme protein turnover and in protection against pathophysiological condition such as neurodegenerative diseases [38]. Finally, HO-1 has been observed in caveolae exerting a vesicular transport function and involved in receptor signal transduction [39].
Aside from the intracellular compartments, the presence of HO-1 in extracellular compartments and biological fluids has been evaluated. Serum HO-1 is increased in Alzheimer’s disease and exhibits a positive correlation with cognition impairment grade [40]. Schipper HM et al. showed that HO-1 is decreased in the cerebrospinal fluid of patients with AD [41]. HO-1 is increased in the cerebrospinal fluid of children after severe traumatic brain injury [42,43] and patients with Fisher Grade III aneurysmal subarachnoid hemorrhage [44]. These observations demonstrate that HO isoforms, especially HO-1, may influence the physiological functions of the brain via non-canonical effects and serve as a possible biomarker for these diseases. However, there are limited data to this end, and the possible release mechanism(s) of HO-1 in serum or the cerebrospinal fluid remain to be elucidated. In summary, HO-1 is considered to be a survival factor in the brain in response to stress-induced ROS increase.
2.3. HO-1 in Brain Physiology
HO-1 is the inducible isoform of heme oxygenase. Under normal conditions, the expression of HO-1 protein in the brain is low and restricted to localized parts [45]. However, rat model studies indicated that HO-1 mRNA is detectable at high levels in the hippocampus and cerebellum, indicating a cellular reserve of HO-1 for quick protein synthesis [46]. Although HO-1 is present at low levels in most mammalian tissues, it can be upregulated by a number of stimuli [47]. In order to study the effect of the enzyme on human physiology, a gene-knockout animal model or a study of human HO-1 deficiency would represent a good way to delineate the role of this protein in various organs.
The important role of HO-1 has been demonstrated in studies on HMOX1 knockout (HO-1-null) mice. The first HO-1-null mice were established by Poss and Tonegawa in 1997 [48,49]. HO-1-null mice are characterized as an animal model of human hemochromatosis and present with several similar symptoms, such as splenomegaly, iron deposition in tissues, fibrosis and hepatic injury, a mobility decrease, and premature mortality. As compared to cells from wild-type embryos, the embryonic fibroblasts from HO-1-null mice exhibited an increased production of free radicals and reduced survival rate under exposure to several oxidants [49]. Moreover, the first human case of HO-1 deficiency was described in a 6-year-old boy in 1999 by Yachie et al. [50,51] and the second in 2009 by Radhakrishnan et al. [52]. The symptoms in these cases were far more severe under oxidative stress than in HO-1 knockout mice (comparison data in [52] and [50]). The symptoms observed in HO-1-deficiency patients include abnormalities of the fibrinolysis/coagulation system, enhanced systemic inflammation, iron-deficiency anemia/intravascular hemolysis, nephropathy, vascular endothelial injury, and developmental failure [52]. These data demonstrate that HO-1 deficiency is associated with many dangerous side effects, and this accounts for the early death of patients with severe HO-1 deficiency. Interestingly, amyloid deposition, the central neuropathological abnormality in AD and in many neurodegenerative diseases [53], was also observed in severe HO-1 deficiency. These observations indicate that the HO-1 signal plays a crucial anti-oxidative and anti-inflammatory function in modulating human physiology. Thus, how to modulate the HO-1 activity in the brain and what the role of HO-1 is in the development of neurodegenerative diseases are critical to brain pathophysiology.
3. Epigenetic Regulation of HO-1
3.1. Polymorphisms of HO-1 Promoter
Since HO-1 is an inducible isoform of the HO system, the epigenetic regulations need to be discussed. To date, there are three important polymorphisms of the HMOX1 promoter to have been identified, including a (GT)n dinucleotide length polymorphism and two single-nucleotide polymorphisms, G(−1135)A and T(−413)A [54].
The current data demonstrate that the lengths of the (GT)n repeat sequence in the HO-1 gene promoter could range from 12 to 40 [55], where <25 (GT)n repeats increase the transcriptional activity of HMOX1 as compared with >25 (GT)n repeats [56]. In studies on lymphoblastic cell lines, HMOX1 expression was enhanced in cells with shorter repeats concomitant with higher HO-1 activity upon oxidative stress resulting in oxidant-induced apoptosis as compared with cells with longer (GT)n repeats [56]. However, the length of the HO-1 (GT)n promoter varies between different ethnic groups [56]. These observations indicate that the repeat of the (GT)n sequence has a modulating effect on the transcriptional activity of HMOX1.
Two single-nucleotide polymorphisms, G(−1135)A and T(−413)A, were discovered using the PCR method. They were then confirmed by transfection into bovine aortic endothelial cells [57]. The major allele of T(−413)A-(GT)30 polymorphism was shown to have greater promoter activity as compared with another major allele, A(−143)A-(GT)23 [57]. However, the function of the G(−1135)A polymorphism is still not known [58]. Some evidence indicates that the promoter polymorphisms of HMOX1 are associated with certain clinical diseases, such as emphysema in smokers [59], hypertension in women [57], and renal transplantation [60,61]. However, microsatellite polymorphism data do not indicate any association between HMOX1 promoter polymorphism and the development of AD and PD [62].
3.2. Post-Transcriptional Modification by MicroRNA (miRNA)
MiRNAs are a large pool of small non-coding RNAs (approximately 21–23 nucleotides long) for post-transcriptional regulation in animals and plants [63]. In mammals, miRNAs are known to control approximately 30% of all protein-coding genes by mediating mRNA degradation or translational repression. Several studies show that miRNAs are involved in the development of neurological diseases, such as miR-142-5p [64], miR-146a, miR-155 [65], and miR-144 [66]. Furthermore, HO-1 targeting miRNAs were also documented in in vitro and in vivo studies, as is summarized in Table 1.
Table 1.
HO-1 targeting miRNAs and their functions.
| miRNA | Species | Functions | Reference |
|---|---|---|---|
| miR-24-3p | Porcine | Promote Porcine Reproductive and Respiratory Syndrome Virus Replication |
[74] |
| miR-155 | Carp Rodent |
Regulate the immunotoxicity of cadmium in the kidneys Promote T-cell-driven inflammation |
[71,72] |
| miR-181a | Carp | Regulate the immunotoxicity of cadmium in the kidneys | [72] |
| miR-217 & miR-377 | Human | Cytotoxic effect by HO-1 downregulation | [69,70] |
| miR-218-5p | Mouse | Cytotoxic effect in septic mice by HO-1 downregulation | [73] |
| miR-873-5p | Mouse | Cytoprotective effect for suppression of neuron cell apoptosis | [68] |
Senescence-accelerated mouse-prone 8 (SAMP8) is an ideal AD model which is characterized by several behavior disorders, including cognitive function impairment and Aβ accumulation with increased oxidative stress [67]. In SAMP8 mice, the expression of Hmox1 is increased concomitant with decreased expression of miR-873-5p, and a luciferase reporter assay indicated that miR-873-5p directly targets the Hmox1 gene [68]. Through an in silico analysis of the 3′UTR sequence, miR-377 and miR-217 were shown to be the miRNA candidates of HMOX1. Co-transfection of miR-377 and miR-217 into mammalian cells decreases the expression of HMOX1-3′UTR luciferase reporter activity as compared with controls [69,70], indicating that miR-217 together with miR-377 could modulate HMOX1 expression. Moreover, in a rodent model, HO-1 was shown to be a specific target of miR-155, which promoted T-cell-driven inflammation [71]. In C. carpio, miR-155 and miR-181a are involved in regulating immune-cytotoxicity of cadmium by targeting HO-1 [72]. However, miR-218-5p was demonstrated to have a cytotoxic effect in septic mice resulting from HO-1 downregulation [73]. Furthermore, the replication of porcine reproductive and respiratory syndrome virus may also be enhanced by miR-24-3p through the downregulation of HO-1 expression [74].
3.3. Post-Translational Modification
HO-1 was first identified with one consensus sequence for Akt phosphorylation at Ser188 in an isotopic 32P-labeling assay [75]. In HEK293T cells, the phosphorylation level of HO-1 is increased with Akt1 activation. Furthermore, phosphorylated HO-1(S188D) protein showed a 1.7-fold increase in activity as compared with wild-type HO-1 [75]. Salinasa et al. first reported that the protein kinase Akt plays a vital role in the regulation of HO-1 activity. Interestingly, in AD subjects, HO-1 protein activity was significantly increased in the hippocampus concomitant with an increase in Ser-residue phosphorylation [76]. This Ser-residue phosphorylation seems to be correlated with oxidative post-translational modifications in the hippocampus, indicating that HO-1 has a role in the development of AD. These studies demonstrate that HO-1 activity could be modulated by phosphorylation through oxidative post-translational modification.
4. The Redox-Mediated HO-1 Induction in the CNS
Aside from the epigenetic regulation of HMOX1, the promoter region of HMOX1 consists of one proximal and two or more distal enhancers [47]. The promoter region has different binding sequences for many transcription factors, such as nuclear factor-erythroid factor 2-related factor 2 (Nrf2), nuclear factor kappa B (NF-κB), hypoxia-inducible factor 1 (HIF-1), activator protein 1 (AP-1), etc. As described in various studies, Nrf2 plays an important role in redox homeostasis of the brain and nervous system [77]. However, the most well-known transactivation of HMOX1 by oxidative stress in the brain is the binding of transcription factor Nrf2 to cis-acting antioxidant response element (ARE) enhancers [78].
Nrf2 is a redox-related transcription factor and is responsible for the activation of several antioxidant enzymes [79]. Nrf2 is retained in the cytoplasm under a basal condition by its negative regulator Keap1 (Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1) to undergo ubiquitination and proteasomal degradation [80]. However, under oxidative stress, Keap-1 is modified and releases Nrf2 into the nucleus, binding to the ARE sequences before activating HMOX1 expression [81]. As a result of Nrf2 binding to the ARE sequence in the presence of small Maf (sMaf) proteins in the nucleus, the BTB domain and CNC homolog 1 (Bach1) protein are other negative regulators of HO-1 activation [82]. Bach1 is a heme-binding protein and can dimerize with sMafs, which prevents the binding of Nrf2 to ARE sequences [83]. These studies demonstrate that, under stress condition such as an increase in the heme group or oxidative stress, Keap1 and Bach1 are modified and then improve Nrf2-sMafs dimerization, thus promoting binding to ARE sequences and activating HMOX1 expression.
Interestingly, aside from Nrf2-dependent signaling, previous studies demonstrated that there is another pathway to induce HO-1 expression in brain astrocytes [84]. Activation of ERK/NF-κB and JNK/c-Jun cascades as the result of a Nox/ROS-dependent event enhances c-Fos/AP-1 activity and is essential for HO-1 upregulation and the activation induced by bradykinin (BK) in brain astrocytes. Moreover, ROS-dependent Nrf2 activation also contributes to HO-1 induction by BK in astrocytes [84]. Furthermore, the high-glucose-derived oxidative stress-dependent HO-1 expression from astrocytes contributes to the neuronal apoptosis, and the induction of HO-1 is mediated by MAPK-mediated NF-κB and AP-1 cascades [85]. However, these studies suggest that the upregulation of HO-1 may have neurotoxic effects in addition to its protective effects in the CNS [86].
5. The Beneficial and Detrimental Role of HO-1 Induction in Neurodegenerative Disorders
As HO-1 is an inducible enzyme in the nervous system’s response to damage, the effect of HO-1 induction in neurodegenerative diseases needs to be further elucidated. Human neurodegenerative disorders are complicated and vary with many factors, such as onset age, sex predilections, neurological and behavioral symptoms, etc. Among these differences, the most common risk for neurodegenerative disorders is age-related factors. There are many general neuropathological features in neurodegenerative diseases, such as oxidative damage resulting from modification to biological molecules, excessive deposition of non-transferrin-bound iron, and macroautophagy in affected neural regions. The evidence indicates that the number of HO-1-immunoreactive neuron cells increases with age, indicating that HO-1 plays a Janus-faced role in brain physiology. Here, we use AD and PD to illustrate how HO-1 is involved in the pathogenesis of CNS degenerative disorders.
An extensive literature attests to the protective roles of HO-1 in the nervous system under various oxidative stress conditions. AD is a neurodegenerative disease characterized by a set of hallmark brain lesions, such as aggregation of the hyperphosphorylated MAPT (tau) protein in neurofibrillary tangles, β-amyloid aggregation in fibrillary plaques, and a neuro-inflammatory response [87]. HO-1 overexpression reduced tau expression and β-amyloid toxicity in neuroblastoma cells and increase neuronal survival in cell and rat models [88,89,90,91]. Furthermore, the protective role of HO-1 in AD brains may also be related to the ability to convert heme, which has a pro-oxidant effect, into its degradation products, which have an antioxidant effect, creating a suitable redox microenvironment [92]. PD is a common neurodegenerative disorder with an unknown etiology. The typical clinical features of PD involve bradykinesia, resting tremor, and rigidity, and in the later stages, postural instability. The development of this movement disorder is due to the loss of dopaminergic neurons in the substantia nigra pars compacta with intracellular aggregation of α-synuclein and the formation of Lewy bodies and Lewy neurites [93]. In vivo and in vitro research indicates that HO-1 induction increases α-synuclein proteasomal degradation [94], prevents dopaminergic neuronal death by enhancing neurotrophic factor generation [95,96], and promotes the antioxidant response [97]. However, these types of HO-1 induction seem to be highly associated with the Nrf2/ARE signal, demonstrating the impact of the Nrf2/HO-1 pathway on neuroprotection function.
Although previously proposed as a protective effect in AD and PD development, the physiological feature of HO-1 in these neurodegenerative diseases is still under debate. Interestingly, HO-1 is overexpressed in the brain of AD patients by co-localization with neurons, astrocytes, ependymal, corpora amylacea, neurofibrillary tangles, and senile plaques [98,99]. It is also overexpressed in nigral astroglia and in dopaminergic neuronal Lewy bodies of the PD brain [98,99]. HO-1 overexpression in astroglia promotes the oxidation of cholesterol to oxysterols in humans and increases oxysterol levels with a decrease in the intracellular cholesterol content in rat [100,101]. The status of plasma HO-1/biliverdin reductase-A has been proposed as a potential biomarker to detect the earliest stages of AD [102]. Moreover, high glucose-induced HO-1 expression is mediated through the NF-κB and AP-1 pathways in brain astrocytes [85]. All these data support the detrimental role of HO-1 induction in the development of neurodegenerative diseases, especially via an astrocytes-mediated event.
Since HO-1 induction plays a dual role in neuropathogenesis, the function of HO-1 in neuronal cells and in astrocytes, oligodendrocytes, and microglia needs to be considered at the stage of neurodegenerative disorders. Indeed, the role of HO-1 expression is highly complicated and not fully elucidated. However, whether HO-1 induction plays cytoprotective or cytotoxic effect in neuropathogenesis may be related to different signaling pathways [103]. That is to say, Nrf2-dependent activation of HO-1 exerts a cytoprotective effect, in which AP-1- or NF-κB-induced HO-1 activation seems to exert cytotoxic effects in the CNS.
6. Herbal Medicine Induces HO-1 Expression
Since HO-1 induction via Nrf-2 pathway in brain plays main functions for preventing brain damage, there are several HO-1 inducers/modulators for therapy or potential therapeutic functions, such as herbal medicine, hemin [104], edavarone [105], cobalt protoporphirin [9], and adenoviral vector transferring system [106]. However, due to the adjuvant functions of herbal compounds and easy supplement from food, we here only summarized those herb medicines as HO-1 inducers. The herbal medicine data for this review were obtained from the ClinicalTrials.gov (accessed on 29 March 2022) database and include resveratrol, curcumin, coenzyme Q10, sulforaphane, niacin, propolis, atorvastatin, and dimethyl fumarate, which could be involved in HO-1 induction.
6.1. Resveratrol
Resveratrol, 3,5,4′-trihydroxy-trans-stilbene, belongs to the phytoalexin family and is produced by red grapes, red cherries, peanuts, and berries. It is popular as a dietary supplement and the studies demonstrate that it has various health-promoting properties including anti-inflammatory, antioxidant, and neuroprotective effects [107]. However, resveratrol exhibits poor bioavailability due to its instability and poor lipophilic properties. Resveratrol exerts therapeutic effects on neurodegenerative diseases. Resveratrol treatment was shown to improve autonomic dysfunction and motor function in a rat model of spinal cord injury [108]. Furthermore, resveratrol improved BBB integrity as a result of anti-oxidation by upregulating the Nrf2/HO-1 and PI3K/Akt signaling pathways and anti-inflammation by attenuating the activity of NF-κB and JNK/MAPK signals [109,110]. In addition, its major neuroprotective function in AD results from its anti-protein aggregation and anti-amyloidogenesis properties through the abolishment of neurofibrillary tau protein tangles or Aβ protein formation and deposition; thus, it is able to improve brain cognition function [111,112]. Resveratrol could protect dopaminergic SH-SY5Y neuron cells from rotenone-induced cell death in a HO-1-dependent autophagy manner [113]. Although resveratrol’s protective function for cognition is mediated by AMPK/SIRT1 signaling, the network between those anti-inflammatory responses needs to be further elucidated. Hence, resveratrol can improve cognitive function in patients with neurodegenerative diseases and further clinical trials are required to delineate its neuroprotective role.
6.2. Curcumin
Curcumin, 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, is a pigment and active polyphenol found in turmeric (in the ginger family) [114]. Curcumin is the main compound contributing to the biological functions of turmeric, and it is common as a food supplement. Curcumin has many biological functions, such as antioxidant, anti-inflammatory, anti-diabetic, anti-microbial, and neuroprotective properties, due to its ability to pass through the BBB effectively. Curcumin is denoted as “Generally Recognized As Safe” by the US Food and Drug Administration [115] with good safety and tolerability in clinical trials [116,117]. The neuroprotection properties of curcumin are mediated through improving the Nrf2/HO-1 pathway (antioxidant response) and by inhibiting the NF-κB, TLR4/RAGE, and MAPKs (ERK, p38, and JNK) signaling pathways (anti-inflammatory response) in microglial and astrocytes [118]. As a result of the anti-amyloidogenesis and anti-protein aggregation/misfolding properties, curcumin has demonstrated positive effects against neurodegenerative disorders, especially AD [119]. However, like resveratrol, curcumin exhibits poor bioavailability, and increasing curcumin’s bioavailability should be a focus of future research. Further trials are required concerning curcumin’s neuroprotective functions against other neurodegenerative diseases.
6.3. Coenzyme Q10 (CoQ10)
Coenzyme Q10 (CoQ10) plays the role of an electron acceptor in energy metabolism to produce ATP. It is found in food sources such as organ meat, fatty fish, and broccoli. As a result of its lipophilic capacity, CoQ10 also acts as a potent antioxidant and possesses a wide range of therapeutic effects. Moreover, it is effective against various neurodegenerative diseases as it passes through the BBB [120]. Its potent neuroprotective properties are mediated by activating the endogenous antioxidant system via the Nrf2/HO-1 signaling pathway and attenuating the NF-κB-mediated inflammatory pathway to protect the dopaminergic neuron system. In addition, ubiquinol-10, the reduced form of CoQ10, was shown to be safe and improve PD by lowering total Unified Parkinson’s Disease Rating Scale (UPDRS) scores. CoQ10 supplementation was shown to improve PD symptoms in various clinical studies and it has potential as a complementary therapy [121].
6.4. Sulforaphane
Sulforaphane, 1-isothiocyanato-4-(methylsulfinyl) butane, is an aliphatic isothiocyanate found in glucoraphanin in cruciferous vegetables such as broccoli, cauliflower, and cabbage [122]. Sulforaphane is characterized as having antioxidant, anti-inflammatory, and anti-apoptosis properties. It was shown to inhibit oxidative stress via the Keap1/Nrf2/ARE pathway by modulating the expression of GSH peroxidase 1, NQO-1, HO-1, and gamma-glutamylcysteine synthetase [123]. Furthermore, sulforaphane can also reduce neuronal damage upon microglial activation and inhibit the expression of inflammatory mediators, such as TNF-α, IL-1β, inducible nitric oxide synthetase (iNOS), cyclooxygenase-2 (COX-2) and macrophage migration inhibitory factor [124,125,126,127,128,129,130]. As a result of its good oral bioavailability and its ease of crossing through the BBB [131], an increasing number of studies demonstrate the efficacy of sulforaphane as a therapeutic strategy in neurodegenerative disease [132]. Therefore, sulforaphane could be used as a supplement for treating neurodegenerative diseases.
6.5. Niacin
The brain is the most cholesterol-rich organ, and cholesterol content may regulate synaptic function and neuronal cell plasticity [133]. Current studies demonstrate that there is a significant correlation between total cholesterol and pathologically defined AD [134,135]. Niacin is the most potent agent for increasing HDL cholesterol, inhibiting inflammation, and promoting vascular remodeling. Niacin inhibits vascular inflammation via the induction of HO-1 by Nrf2/p38 MAPK signaling [136]. However, whether niacin could be used as a therapy for AD needs further elucidated.
6.6. Propolis
Propolis, a mixture of bee saliva, beeswax, and substances from plants and trees, is a natural product found in beehives that possesses a therapeutic role in PD treatment. Several lines of evidence indicate that flavonoids in propolis demonstrate neuroprotective properties in dopaminergic neurons through the inhibition of oxidative stress [137]. The flavonoids in propolis include caffeic acid phenethyl ester, chrysin (5,7-dihydroxyflavone), and pinocembrin, which easily pass through the BBB and exert antioxidant and anti-inflammatory activities [138]. Pinocembrin treatment was shown to induce the expression of the HO-1 by Nrf2/ARE pathway, significantly reducing MPP+-induced neurotoxicity, ROS production, and the rate of apoptosis and neuron cell death [139,140]. Furthermore, caffeic acid phenethyl ester also exerts protective effects in nigral dopaminergic neurons from 6-hydroxydopamine hemiparkinsonian mice through HO-1 and brain-derived neurotrophic factor signals.
6.7. Atorvastatin
Statin is a common therapeutic strategy for hypercholesterolaemia; however, as previous discussed in the section describing niacin, a significant link between cholesterol and the development of AD has been observed, thus statin therapy might be of benefit for AD pathogenesis [141]. Cholesterol-lowering statins have several biological functions, such as anti-inflammatory, antioxidative, anti-thrombogenic, and immunological effects. Among these statins, atorvastatin has been demonstrated to have benefits in terms of improving AD outcomes. It significantly improves depressive symptoms and cognitive functions at 6 months, and improves cognitive function and psychiatric symptoms at 12 months [141,142]. Furthermore, in a dog preclinical AD model, atorvastatin treatment induced HO-1 expression, providing neuroprotection by modulating oxidative stress [143].
6.8. Dimethyl Fumarate
Among fumaric acid esters, dimethyl fumarate, the methyl ester of fumaric acid, has effective pharmacological functions and exerts anti-inflammatory and antioxidant properties [144]. Dimethyl fumarate is able to cross the BBB and exhibit beneficial effects in the brain via differing mechanisms [145]. Dimethyl fumarate plays the role of an Nrf2 inducer and exerts a neuroprotective role in several neurodegenerative diseases, such as AD, PD, and Huntington’s disease [146].
Although the herbal medicines mentioned above may provide neuroprotective effects through the modulation of HO-1 expression, the bioavailability and lipophilic properties of these medicine must be explored in order to assess their stability and how affective they are at permeating the blood brain barrier (BBB). Nanocarriers represent an interesting solution as a potential drug delivery candidate for passing through the BBB [147]. Thus, how these medicines can be utilized as treatments or preventatives for the development of neurodegenerative disorders is an important issue, and further clinical trials are required.
7. Conclusions
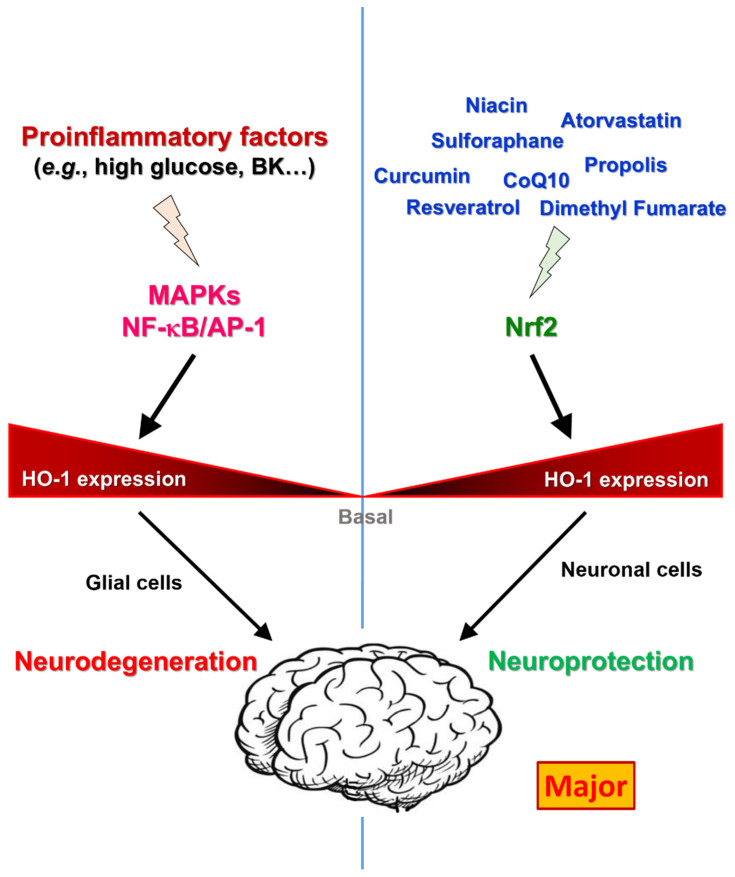
Although HO-1 has been observed to have cytoprotective and cytotoxic effects in the development of neurodegenerative diseases, HO-1 activity needs to be maintained in a well-defined reaction which involves the generation and degradation of heme. Heme metabolism or Nrf2-mediated HO-1 induction in neuronal cells exerts protective effects against many stressors; however, excessive activation of HO-1 by the NF-κB/AP-1 pathway may produce cytopathic effects, depending on the complex of cell–cell interactions or the type of brain tissue. Furthermore, the dysregulation of the heme degradation pathway may alter iron metabolism, leading to neurodegeneration in neurons and glial cells. Neurodegenerative diseases are complex and multifactorial diseases, and interventions should be considered during the long preclinical phase. The currently available drugs have symptomatic effects, with the majority playing the role of an Nrf2 inducer and increasing the expression of HO-1 in order to modulate oxidative stress, as shown in Figure 2. Taken together, these reports indicate that HO-1 induction, especially through Nrf2 pathway, may alleviate the brain damage and plays important therapeutic functions in neurodegenerative diseases. However, the detail mechanism of HO-1 on the cytotoxic effect of glial cells needs to be further elucidated.
Figure 2.
Schematic representation of the HO-1 regulation and its role in the procession of neurodegeneration and neuroprotection. In neurodegeneration, proinflammatory factors induce HO-1 expression via MAPKs, NF-κB, and AP-1 in glial cells. However, several herbal medicines induce HO-1 expression via Nrf2-dependent pathway in neuronal cells, indicating the major route of HO-1 induction for neuroprotective function. Thus, in brain, the final impact of upregulated HO-1 is depend on the stimulatory factors, the activated signaling pathways, and the stimulated cell-type.
Author Contributions
Y.-H.W. and H.-L.H. were involved in drafting the article or revising it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that they have no competing interest.
Funding Statement
This work was supported by the Ministry of Science and Technology, Taiwan, Grant number: MOST108-2320-B-255-002-MY3; Chang Gung Medical Research Foundation, Grant number: CMRPF1L0041, CMRPF1L0021; Chang Gung University of Science and Technology, Grant number: ZRRPF3K0111, ZRRPF3L0091.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCoubrey W.K., Jr., Huang T.J., Maines M.D. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi S., Omata Y., Sakamoto H., Higashimoto Y., Hara T., Sagara Y., Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Singh D., Wasan H., Reeta K.H. Heme oxygenase-1 modulation: A potential therapeutic target for COVID-19 and associated complications. Free Radic. Biol. Med. 2020;161:263–271. doi: 10.1016/j.freeradbiomed.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewing J.F., Maines M.D. Distribution of constitutive (HO-2) and heat-inducible (HO-1) heme oxygenase isozymes in rat testes: HO-2 displays stage-specific expression in germ cells. Endocrinology. 1995;136:2294–2302. doi: 10.1210/endo.136.5.7720678. [DOI] [PubMed] [Google Scholar]
- 5.Nam J., Lee Y., Yang Y., Jeong S., Kim W., Yoo J.W., Moon J.O., Lee C., Chung H.Y., Kim M.S., et al. Is it worth expending energy to convert biliverdin into bilirubin? Free Radic. Biol. Med. 2018;124:232–240. doi: 10.1016/j.freeradbiomed.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franco R., Vargas M.R. Redox Biology in Neurological Function, Dysfunction, and Aging. Antioxid. Redox Signal. 2018;28:1583–1586. doi: 10.1089/ars.2018.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagone P., Patti F., Mangano K., Mammana S., Coco M., Touil-Boukoffa C., Chikovani T., Di Marco R., Nicoletti F. Heme oxygenase-1 expression in peripheral blood mononuclear cells correlates with disease activity in multiple sclerosis. J. Neuroimmunol. 2013;261:82–86. doi: 10.1016/j.jneuroim.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Chora A.A., Fontoura P., Cunha A., Pais T.F., Cardoso S., Ho P.P., Lee L.Y., Sobel R.A., Steinman L., Soares M.P. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J. Clin. Investig. 2007;117:438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi A.M., Alam J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald H.K., O’Rourke S.A., Desmond E., Neto N.G.B., Monaghan M.G., Tosetto M., Doherty J., Ryan E.J., Doherty G.A., Nolan D.P., et al. The Trypanosoma brucei-Derived Ketoacids, Indole Pyruvate and Hydroxyphenylpyruvate, Induce HO-1 Expression and Suppress Inflammatory Responses in Human Dendritic Cells. Antioxidants. 2022;11:164. doi: 10.3390/antiox11010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardallo R.G., Company-Marin I., Folch-Puy E., Rosello-Catafau J., Panisello-Rosello A., Carbonell T. PEG35 and Glutathione Improve Mitochondrial Function and Reduce Oxidative Stress in Cold Fatty Liver Graft Preservation. Antioxidants. 2022;11:158. doi: 10.3390/antiox11010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toro A., Ruiz M.S., Lage-Vickers S., Sanchis P., Sabater A., Pascual G., Seniuk R., Cascardo F., Ledesma-Bazan S., Vilicich F., et al. A Journey into the Clinical Relevance of Heme Oxygenase 1 for Human Inflammatory Disease and Viral Clearance: Why Does It Matter on the COVID-19 Scene? Antioxidants. 2022;11:276. doi: 10.3390/antiox11020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bortolussi G., Shi X., Ten Bloemendaal L., Banerjee B., De Waart D.R., Baj G., Chen W., Oude Elferink R.P., Beuers U., Paulusma C.C., et al. Long-Term Effects of Biliverdin Reductase a Deficiency in Ugt1(−/−) Mice: Impact on Redox Status and Metabolism. Antioxidants. 2021;10:2029. doi: 10.3390/antiox10122029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canesin G., Hejazi S.M., Swanson K.D., Wegiel B. Heme-Derived Metabolic Signals Dictate Immune Responses. Front. Immunol. 2020;11:66. doi: 10.3389/fimmu.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H.P., Ryter S.W., Choi A.M. CO as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 17.Bauer I., Pannen B.H. Bench-to-bedside review: Carbon monoxide--from mitochondrial poisoning to therapeutic use. Crit. Care. 2009;13:220. doi: 10.1186/cc7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayanti S., Vitek L., Tiribelli C., Gazzin S. The Role of Bilirubin and the Other “Yellow Players” in Neurodegenerative Diseases. Antioxidants. 2020;9:900. doi: 10.3390/antiox9090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deguchi K., Hayashi T., Nagotani S., Sehara Y., Zhang H., Tsuchiya A., Ohta Y., Tomiyama K., Morimoto N., Miyazaki M., et al. Reduction of cerebral infarction in rats by biliverdin associated with amelioration of oxidative stress. Brain Res. 2008;1188:1–8. doi: 10.1016/j.brainres.2007.07.104. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs P.E., Maines M.D. Biliverdin inhibits activation of NF-kappaB: Reversal of inhibition by human biliverdin reductase. Int. J. Cancer. 2007;121:2567–2574. doi: 10.1002/ijc.22978. [DOI] [PubMed] [Google Scholar]
- 21.Wegiel B., Gallo D., Csizmadia E., Roger T., Kaczmarek E., Harris C., Zuckerbraun B.S., Otterbein L.E. Biliverdin inhibits Toll-like receptor-4 (TLR4) expression through nitric oxide-dependent nuclear translocation of biliverdin reductase. Proc. Natl. Acad. Sci. USA. 2011;108:18849–18854. doi: 10.1073/pnas.1108571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter S., Letiembre M., Liu Y., Heine H., Penke B., Hao W., Bode B., Manietta N., Walter J., Schulz-Schuffer W., et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol. Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan M.M., Hutchinson M., Watkins L.R., Yin H. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma A., Hirsch D.J., Glatt C.E., Ronnett G.V., Snyder S.H. Carbon monoxide: A putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 25.Wang B., Huang C., Chen L., Xu D., Zheng G., Zhou Y., Wang X., Zhang X. The Emerging Roles of the Gaseous Signaling Molecules NO, H2S, and CO in the Regulation of Stem Cells. ACS Biomater. Sci. Eng. 2020;6:798–812. doi: 10.1021/acsbiomaterials.9b01681. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y.K., Park J.H., Baek Y.Y., Won M.H., Jeoung D., Lee H., Ha K.S., Kwon Y.G., Kim Y.M. Carbon monoxide stimulates astrocytic mitochondrial biogenesis via L-type Ca(2+) channel-mediated PGC-1alpha/ERRalpha activation. Biochem. Biophys. Res. Commun. 2016;479:297–304. doi: 10.1016/j.bbrc.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H., Liu J., Pan P., Jin D., Ding W., Li W. Carbon monoxide inhalation decreased lung injury via anti-inflammatory and anti-apoptotic effects in brain death rats. Exp. Biol. Med. 2010;235:1236–1243. doi: 10.1258/ebm.2010.010147. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Fedinec A.L., Leffler C.W., Parfenova H. Enteral supplements of a carbon monoxide donor CORM-A1 protect against cerebrovascular dysfunction caused by neonatal seizures. J. Cereb. Blood Flow Metab. 2015;35:193–199. doi: 10.1038/jcbfm.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagone P., Mangano K., Quattrocchi C., Motterlini R., Di Marco R., Magro G., Penacho N., Romao C.C., Nicoletti F. Prevention of clinical and histological signs of proteolipid protein (PLP)-induced experimental allergic encephalomyelitis (EAE) in mice by the water-soluble carbon monoxide-releasing molecule (CORM)-A1. Clin. Exp. Immunol. 2011;163:368–374. doi: 10.1111/j.1365-2249.2010.04303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanella L., Barbagallo I., Tibullo D., Forte S., Zappala A., Li Volti G. The non-canonical functions of the heme oxygenases. Oncotarget. 2016;7:69075–69086. doi: 10.18632/oncotarget.11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maines M.D., Ibrahim N.G., Kappas A. Solubilization and partial purification of heme oxygenase from rat liver. J. Biol. Chem. 1977;252:5900–5903. doi: 10.1016/S0021-9258(17)40109-8. [DOI] [PubMed] [Google Scholar]
- 32.Weng Y.H., Yang G., Weiss S., Dennery P.A. Interaction between heme oxygenase-1 and -2 proteins. J. Biol. Chem. 2003;278:50999–51005. doi: 10.1074/jbc.M307644200. [DOI] [PubMed] [Google Scholar]
- 33.Dunn L.L., Midwinter R.G., Ni J., Hamid H.A., Parish C.R., Stocker R. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid. Redox Signal. 2014;20:1723–1742. doi: 10.1089/ars.2013.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Volti G., Ientile R., Abraham N.G., Vanella A., Cannavo G., Mazza F., Curro M., Raciti G., Avola R., Campisi A. Immunocytochemical localization and expression of heme oxygenase-1 in primary astroglial cell cultures during differentiation: Effect of glutamate. Biochem. Biophys. Res. Commun. 2004;315:517–524. doi: 10.1016/j.bbrc.2004.01.090. [DOI] [PubMed] [Google Scholar]
- 35.Lin Q., Weis S., Yang G., Weng Y.H., Helston R., Rish K., Smith A., Bordner J., Polte T., Gaunitz F., et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 36.Costa Silva R.C.M., Correa L.H.T. Correction: Heme Oxygenase 1 in Vertebrates: Friend and Foe. Cell Biochem. Biophys. 2022;80:261. doi: 10.1007/s12013-021-01053-1. [DOI] [PubMed] [Google Scholar]
- 37.Namba F., Go H., Murphy J.A., La P., Yang G., Sengupta S., Fernando A.P., Yohannes M., Biswas C., Wehrli S.L., et al. Expression level and subcellular localization of heme oxygenase-1 modulates its cytoprotective properties in response to lung injury: A mouse model. PLoS ONE. 2014;9:e90936. doi: 10.1371/journal.pone.0090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Converso D.P., Taille C., Carreras M.C., Jaitovich A., Poderoso J.J., Boczkowski J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006;20:1236–1238. doi: 10.1096/fj.05-4204fje. [DOI] [PubMed] [Google Scholar]
- 39.Wang X.M., Kim H.P., Nakahira K., Ryter S.W., Choi A.M. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J. Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 40.Mueller C., Zhou W., Vanmeter A., Heiby M., Magaki S., Ross M.M., Espina V., Schrag M., Dickson C., Liotta L.A., et al. The heme degradation pathway is a promising serum biomarker source for the early detection of Alzheimer’s disease. J. Alzheimers Dis. 2010;19:1081–1091. doi: 10.3233/JAD-2010-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schipper H.M., Chertkow H., Mehindate K., Frankel D., Melmed C., Bergman H. Evaluation of heme oxygenase-1 as a systemic biological marker of sporadic AD. Neurology. 2000;54:1297–1304. doi: 10.1212/WNL.54.6.1297. [DOI] [PubMed] [Google Scholar]
- 42.Aronowski J., Zhao X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke. 2011;42:1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cousar J.L., Lai Y., Marco C.D., Bayir H., Adelson P.D., Janesko-Feldman K.L., Kochanek P.M., Clark R.S. Heme oxygenase 1 in cerebrospinal fluid from infants and children after severe traumatic brain injury. Dev. Neurosci. 2006;28:342–347. doi: 10.1159/000094160. [DOI] [PubMed] [Google Scholar]
- 44.Wang K.C., Tang S.C., Lee J.E., Lai D.M., Huang S.J., Hsieh S.T., Jeng J.S., Tu Y.K. Prognostic value of intrathecal heme oxygenase-1 concentration in patients with Fisher Grade III aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2014;121:1388–1393. doi: 10.3171/2014.7.JNS131704. [DOI] [PubMed] [Google Scholar]
- 45.Turner C.P., Bergeron M., Matz P., Zegna A., Noble L.J., Panter S.S., Sharp F.R. Heme oxygenase-1 is induced in glia throughout brain by subarachnoid hemoglobin. J. Cereb. Blood Flow Metab. 1998;18:257–273. doi: 10.1097/00004647-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Scapagnini G., D’Agata V., Calabrese V., Pascale A., Colombrita C., Alkon D., Cavallaro S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002;954:51–59. doi: 10.1016/S0006-8993(02)03338-3. [DOI] [PubMed] [Google Scholar]
- 47.Keyse S.M., Tyrrell R.M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poss K.D., Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poss K.D., Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawashima A., Oda Y., Yachie A., Koizumi S., Nakanishi I. Heme oxygenase-1 deficiency: The first autopsy case. Hum. Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 51.Yachie A., Niida Y., Wada T., Igarashi N., Kaneda H., Toma T., Ohta K., Kasahara Y., Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radhakrishnan N., Yadav S.P., Sachdeva A., Pruthi P.K., Sawhney S., Piplani T., Wada T., Yachie A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J. Pediatr. Hematol. Oncol. 2011;33:74–78. doi: 10.1097/MPH.0b013e3181fd2aae. [DOI] [PubMed] [Google Scholar]
- 53.Mena M.A., Rodriguez-Navarro J.A., de Yebenes J.G. The multiple mechanisms of amyloid deposition: The role of parkin. Prion. 2009;3:5–11. doi: 10.4161/pri.3.1.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Exner M., Minar E., Wagner O., Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic. Biol. Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan M., Wong R.J., Stevenson D.K. Heme oxygenase-1 promoter polymorphisms: Do they modulate neonatal hyperbilirubinemia? J. Perinatol. 2017;37:901–905. doi: 10.1038/jp.2017.6. [DOI] [PubMed] [Google Scholar]
- 56.Hirai H., Kubo H., Yamaya M., Nakayama K., Numasaki M., Kobayashi S., Suzuki S., Shibahara S., Sasaki H. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102:1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- 57.Ono K., Mannami T., Iwai N. Association of a promoter variant of the haeme oxygenase-1 gene with hypertension in women. J. Hypertens. 2003;21:1497–1503. doi: 10.1097/00004872-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 58.Hansson H.H., Maretty L., Balle C., Goka B.Q., Luzon E., Nkrumah F.N., Schousboe M.L., Rodrigues O.P., Bygbjerg I.C., Kurtzhals J.A., et al. Polymorphisms in the Haem Oxygenase-1 promoter are not associated with severity of Plasmodium falciparum malaria in Ghanaian children. Malar. J. 2015;14:153. doi: 10.1186/s12936-015-0668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada N., Yamaya M., Okinaga S., Nakayama K., Sekizawa K., Shibahara S., Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am. J. Hum. Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Exner M., Bohmig G.A., Schillinger M., Regele H., Watschinger B., Horl W.H., Raith M., Mannhalter C., Wagner O.F. Donor heme oxygenase-1 genotype is associated with renal allograft function. Transplantation. 2004;77:538–542. doi: 10.1097/01.TP.0000113467.36269.F8. [DOI] [PubMed] [Google Scholar]
- 61.Baan C., Peeters A., Lemos F., Uitterlinden A., Doxiadis I., Claas F., Ijzermans J., Roodnat J., Weimar W. Fundamental role for HO-1 in the self-protection of renal allografts. Am. J. Transplant. 2004;4:811–818. doi: 10.1111/j.1600-6143.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 62.Kimpara T., Takeda A., Watanabe K., Itoyama Y., Ikawa S., Watanabe M., Arai H., Sasaki H., Higuchi S., Okita N., et al. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum. Genet. 1997;100:145–147. doi: 10.1007/s004390050480. [DOI] [PubMed] [Google Scholar]
- 63.Michlewski G., Caceres J.F. Post-transcriptional control of miRNA biogenesis. RNA. 2019;25:1–16. doi: 10.1261/rna.068692.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song J., Kim Y.K. Identification of the Role of miR-142-5p in Alzheimer’s Disease by Comparative Bioinformatics and Cellular Analysis. Front. Mol. Neurosci. 2017;10:227. doi: 10.3389/fnmol.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arena A., Iyer A.M., Milenkovic I., Kovacs G.G., Ferrer I., Perluigi M., Aronica E. Developmental Expression and Dysregulation of miR-146a and miR-155 in Down’s Syndrome and Mouse Models of Down’s Syndrome and Alzheimer’s Disease. Curr. Alzheimer Res. 2017;14:1305–1317. doi: 10.2174/1567205014666170706112701. [DOI] [PubMed] [Google Scholar]
- 66.Zhou C., Zhao L., Zheng J., Wang K., Deng H., Liu P., Chen L., Mu H. MicroRNA-144 modulates oxidative stress tolerance in SH-SY5Y cells by regulating nuclear factor erythroid 2-related factor 2-glutathione axis. Neurosci. Lett. 2017;655:21–27. doi: 10.1016/j.neulet.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 67.Liu B., Liu J., Shi J.S. SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer’s Disease. J. Alzheimers Dis. 2020;75:385–395. doi: 10.3233/JAD-200063. [DOI] [PubMed] [Google Scholar]
- 68.Shi R., Zhang S., Cheng G., Yang X., Zhao N., Chen C. Ginsenoside Rg1 and Acori Graminei Rhizoma Attenuates Neuron Cell Apoptosis by Promoting the Expression of miR-873-5p in Alzheimer’s Disease. Neurochem. Res. 2018;43:1529–1538. doi: 10.1007/s11064-018-2567-y. [DOI] [PubMed] [Google Scholar]
- 69.Beckman J.D., Chen C., Nguyen J., Thayanithy V., Subramanian S., Steer C.J., Vercellotti G.M. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J. Biol. Chem. 2011;286:3194–3202. doi: 10.1074/jbc.M110.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aayadi H., Mittal S.P.K., Deshpande A., Gore M., Ghaskadbi S.S. Cytoprotective effect exerted by geraniin in HepG2 cells is through microRNA mediated regulation of BACH-1 and HO-1. BMB Rep. 2017;50:560–565. doi: 10.5483/BMBRep.2017.50.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J., Vandevenne P., Hamdi H., Van Puyvelde M., Zucchi A., Bettonville M., Weatherly K., Braun M.Y. Micro-RNA-155-mediated control of heme oxygenase 1 (HO-1) is required for restoring adaptively tolerant CD4+ T-cell function in rodents. Eur. J. Immunol. 2015;45:829–842. doi: 10.1002/eji.201445066. [DOI] [PubMed] [Google Scholar]
- 72.Li H., Di G., Zhang Y., Xue R., Zhang J., Liang J. MicroRNA-155 and microRNA-181a, via HO-1, participate in regulating the immunotoxicity of cadmium in the kidneys of exposed Cyprinus carpio. Fish Shellfish Immunol. 2019;95:473–480. doi: 10.1016/j.fsi.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Zhang T., Xiang L. Honokiol alleviates sepsis-induced acute kidney injury in mice by targeting the miR-218-5p/heme oxygenase-1 signaling pathway. Cell Mol. Biol. Lett. 2019;24:15. doi: 10.1186/s11658-019-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao S., Wang X., Ni H., Li N., Zhang A., Liu H., Pu F., Xu L., Gao J., Zhao Q., et al. MicroRNA miR-24-3p promotes porcine reproductive and respiratory syndrome virus replication through suppression of heme oxygenase-1 expression. J. Virol. 2015;89:4494–4503. doi: 10.1128/JVI.02810-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salinas M., Wang J., Rosa de Sagarra M., Martin D., Rojo A.I., Martin-Perez J., Ortiz de Montellano P.R., Cuadrado A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett. 2004;578:90–94. doi: 10.1016/j.febslet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 76.Barone E., Di Domenico F., Sultana R., Coccia R., Mancuso C., Perluigi M., Butterfield D.A. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic. Biol. Med. 2012;52:2292–2301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson J.A., Johnson D.A., Kraft A.D., Calkins M.J., Jakel R.J., Vargas M.R., Chen P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y Acad. Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reichard J.F., Motz G.T., Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic. Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 81.Ishii T., Itoh K., Takahashi S., Sato H., Yanagawa T., Katoh Y., Bannai S., Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 82.Sun J., Hoshino H., Takaku K., Nakajima O., Muto A., Suzuki H., Tashiro S., Takahashi S., Shibahara S., Alam J., et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kitamuro T., Takahashi K., Ogawa K., Udono-Fujimori R., Takeda K., Furuyama K., Nakayama M., Sun J., Fujita H., Hida W., et al. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J. Biol. Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 84.Hsieh H.L., Wang H.H., Wu C.Y., Yang C.M. Reactive Oxygen Species-Dependent c-Fos/Activator Protein 1 Induction Upregulates Heme Oxygenase-1 Expression by Bradykinin in Brain Astrocytes. Antioxid. Redox Signal. 2010;13:1829–1844. doi: 10.1089/ars.2009.2957. [DOI] [PubMed] [Google Scholar]
- 85.Yang C.M., Lin C.C., Hsieh H.L. High-Glucose-Derived Oxidative Stress-Dependent Heme Oxygenase-1 Expression from Astrocytes Contributes to the Neuronal Apoptosis. Mol. Neurobiol. 2017;54:470–483. doi: 10.1007/s12035-015-9666-4. [DOI] [PubMed] [Google Scholar]
- 86.Schipper H.M., Song W., Tavitian A., Cressatti M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019;172:40–70. doi: 10.1016/j.pneurobio.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Metaxas A., Thygesen C., Kempf S.J., Anzalone M., Vaitheeswaran R., Petersen S., Landau A.M., Audrain H., Teeling J.L., Darvesh S., et al. Ageing and amyloidosis underlie the molecular and pathological alterations of tau in a mouse model of familial Alzheimer’s disease. Sci. Rep. 2019;9:15758. doi: 10.1038/s41598-019-52357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takeda A., Perry G., Abraham N.G., Dwyer B.E., Kutty R.K., Laitinen J.T., Petersen R.B., Smith M.A. Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. J. Biol. Chem. 2000;275:5395–5399. doi: 10.1074/jbc.275.8.5395. [DOI] [PubMed] [Google Scholar]
- 89.Schipper H.M., Song W., Zukor H., Hascalovici J.R., Zeligman D. Heme oxygenase-1 and neurodegeneration: Expanding frontiers of engagement. J. Neurochem. 2009;110:469–485. doi: 10.1111/j.1471-4159.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- 90.Jiao W., Wang Y., Kong L., Ou-Yang T., Meng Q., Fu Q., Hu Z. CART peptide activates the Nrf2/HO-1 antioxidant pathway and protects hippocampal neurons in a rat model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2018;501:1016–1022. doi: 10.1016/j.bbrc.2018.05.101. [DOI] [PubMed] [Google Scholar]
- 91.Jiao C., Gao F., Ou L., Yu J., Li M., Wei P., Miao F. Tetrahydroxy stilbene glycoside (TSG) antagonizes Abeta-induced hippocampal neuron injury by suppressing mitochondrial dysfunction via Nrf2-dependent HO-1 pathway. Biomed. Pharmacother. 2017;96:222–228. doi: 10.1016/j.biopha.2017.09.134. [DOI] [PubMed] [Google Scholar]
- 92.Hettiarachchi N., Dallas M., Al-Owais M., Griffiths H., Hooper N., Scragg J., Boyle J., Peers C. Heme oxygenase-1 protects against Alzheimer’s amyloid-beta(1-42)-induced toxicity via carbon monoxide production. Cell Death Dis. 2014;5:e1569. doi: 10.1038/cddis.2014.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kouli A., Torsney K.M., Kuan W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Brisbane, QLD, Australia, 2018. [PubMed]
- 94.Song W., Patel A., Qureshi H.Y., Han D., Schipper H.M., Paudel H.K. The Parkinson disease-associated A30P mutation stabilizes alpha-synuclein against proteasomal degradation triggered by heme oxygenase-1 over-expression in human neuroblastoma cells. J. Neurochem. 2009;110:719–733. doi: 10.1111/j.1471-4159.2009.06165.x. [DOI] [PubMed] [Google Scholar]
- 95.Hung S.Y., Liou H.C., Fu W.M. The mechanism of heme oxygenase-1 action involved in the enhancement of neurotrophic factor expression. Neuropharmacology. 2010;58:321–329. doi: 10.1016/j.neuropharm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Masaki Y., Izumi Y., Matsumura A., Akaike A., Kume T. Protective effect of Nrf2-ARE activator isolated from green perilla leaves on dopaminergic neuronal loss in a Parkinson’s disease model. Eur. J. Pharmacol. 2017;798:26–34. doi: 10.1016/j.ejphar.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Tong H., Zhang X., Meng X., Lu L., Mai D., Qu S. Simvastatin Inhibits Activation of NADPH Oxidase/p38 MAPK Pathway and Enhances Expression of Antioxidant Protein in Parkinson Disease Models. Front. Mol. Neurosci. 2018;11:165. doi: 10.3389/fnmol.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schipper H.M., Cisse S., Stopa E.G. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann. Neurol. 1995;37:758–768. doi: 10.1002/ana.410370609. [DOI] [PubMed] [Google Scholar]
- 99.Schipper H.M., Bennett D.A., Liberman A., Bienias J.L., Schneider J.A., Kelly J., Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol. Aging. 2006;27:252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 100.Vaya J., Schipper H.M. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J. Neurochem. 2007;102:1727–1737. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- 101.Vaya J., Song W., Khatib S., Geng G., Schipper H.M. Effects of heme oxygenase-1 expression on sterol homeostasis in rat astroglia. Free Radic. Biol. Med. 2007;42:864–871. doi: 10.1016/j.freeradbiomed.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 102.Di Domenico F., Barone E., Mancuso C., Perluigi M., Cocciolo A., Mecocci P., Butterfield D.A., Coccia R. HO-1/BVR-a system analysis in plasma from probable Alzheimer’s disease and mild cognitive impairment subjects: A potential biochemical marker for the prediction of the disease. J. Alzheimers Dis. 2012;32:277–289. doi: 10.3233/JAD-2012-121045. [DOI] [PubMed] [Google Scholar]
- 103.Waza A.A., Hamid Z., Ali S., Bhat S.A., Bhat M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018;67:579–588. doi: 10.1007/s00011-018-1151-x. [DOI] [PubMed] [Google Scholar]
- 104.Takeda Y., Takeno M., Iwasaki M., Kobayashi H., Kirino Y., Ueda A., Nagahama K., Aoki I., Ishigatsubo Y. Chemical induction of HO-1 suppresses lupus nephritis by reducing local iNOS expression and synthesis of anti-dsDNA antibody. Clin. Exp. Immunol. 2004;138:237–244. doi: 10.1111/j.1365-2249.2004.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Michalickova D., Kubra Ozturk H., Hroudova J., Luptak M., Kucera T., Hrncir T., Kutinova Canova N., Sima M., Slanar O. Edaravone attenuates disease severity of experimental auto-immune encephalomyelitis and increases gene expression of Nrf2 and HO-1. Physiol. Res. 2022;71:147–157. doi: 10.33549/physiolres.934800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inoue S., Suzuki M., Nagashima Y., Suzuki S., Hashiba T., Tsuburai T., Ikehara K., Matsuse T., Ishigatsubo Y. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Hum. Gene Ther. 2001;12:967–979. doi: 10.1089/104303401750195926. [DOI] [PubMed] [Google Scholar]
- 107.Wang J., Hu J., Chen X., Lei X., Feng H., Wan F., Tan L. Traditional Chinese Medicine Monomers: Novel Strategy for Endogenous Neural Stem Cells Activation After Stroke. Front. Cell Neurosci. 2021;15:628115. doi: 10.3389/fncel.2021.628115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu C., Shi Z., Fan L., Zhang C., Wang K., Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 109.Moussa C., Hebron M., Huang X., Ahn J., Rissman R.A., Aisen P.S., Turner R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation. 2017;14:1. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hui Y., Chengyong T., Cheng L., Haixia H., Yuanda Z., Weihua Y. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-beta1-42 in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem. Res. 2018;43:297–305. doi: 10.1007/s11064-017-2421-7. [DOI] [PubMed] [Google Scholar]
- 111.Gomes B.A.Q., Silva J.P.B., Romeiro C.F.R., Dos Santos S.M., Rodrigues C.A., Goncalves P.R., Sakai J.T., Mendes P.F.S., Varela E.L.P., Monteiro M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell Longev. 2018;2018:8152373. doi: 10.1155/2018/8152373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pasinetti G.M., Wang J., Ho L., Zhao W., Dubner L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophys. Acta. 2015;1852:1202–1208. doi: 10.1016/j.bbadis.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin T.K., Chen S.D., Chuang Y.C., Lin H.Y., Huang C.R., Chuang J.H., Wang P.W., Huang S.T., Tiao M.M., Chen J.B., et al. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014;15:1625–1646. doi: 10.3390/ijms15011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Priyadarsini K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules. 2014;19:20091–20112. doi: 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Basnet P., Skalko-Basnet N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lao C.D., Ruffin M.T.t., Normolle D., Heath D.D., Murray S.I., Bailey J.M., Boggs M.E., Crowell J., Rock C.L., Brenner D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teter B., Morihara T., Lim G.P., Chu T., Jones M.R., Zuo X., Paul R.M., Frautschy S.A., Cole G.M. Curcumin restores innate immune Alzheimer’s disease risk gene expression to ameliorate Alzheimer pathogenesis. Neurobiol. Dis. 2019;127:432–448. doi: 10.1016/j.nbd.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Torres-Acosta N., O’Keefe J.H., O’Keefe E.L., Isaacson R., Small G. Therapeutic Potential of TNF-alpha Inhibition for Alzheimer’s Disease Prevention. J. Alzheimers Dis. 2020;78:619–626. doi: 10.3233/JAD-200711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee H.J., Jeong H.R., Park J.H., Hoe H.S. Idebenone Decreases Abeta Pathology by Modulating RAGE/Caspase-3 Signaling and the Abeta Degradation Enzyme NEP in a Mouse Model of AD. Biology. 2021;10:938. doi: 10.3390/biology10090938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gutierrez-Mariscal F.M., Arenas-de Larriva A.P., Limia-Perez L., Romero-Cabrera J.L., Yubero-Serrano E.M., Lopez-Miranda J. Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2020;21:7870. doi: 10.3390/ijms21217870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Panjwani A.A., Liu H., Fahey J.W. Crucifers and related vegetables and supplements for neurologic disorders: What is the evidence? Curr. Opin. Clin. Nutr. Metab. Care. 2018;21:451–457. doi: 10.1097/MCO.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 123.Bergstrom P., Andersson H.C., Gao Y., Karlsson J.O., Nodin C., Anderson M.F., Nilsson M., Hammarsten O. Repeated transient sulforaphane stimulation in astrocytes leads to prolonged Nrf2-mediated gene expression and protection from superoxide-induced damage. Neuropharmacology. 2011;60:343–353. doi: 10.1016/j.neuropharm.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 124.Wang W., Wei C., Quan M., Li T., Jia J. Sulforaphane Reverses the Amyloid-beta Oligomers Induced Depressive-Like Behavior. J. Alzheimers Dis. 2020;78:127–137. doi: 10.3233/JAD-200397. [DOI] [PubMed] [Google Scholar]
- 125.Klomparens E.A., Ding Y. The neuroprotective mechanisms and effects of sulforaphane. Brain Circ. 2019;5:74–83. doi: 10.4103/bc.bc_7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Innamorato N.G., Rojo A.I., Garcia-Yague A.J., Yamamoto M., de Ceballos M.L., Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 127.Healy Z.R., Liu H., Holtzclaw W.D., Talalay P. Inactivation of tautomerase activity of macrophage migration inhibitory factor by sulforaphane: A potential biomarker for anti-inflammatory intervention. Cancer Epidemiol. Biomark. Prev. 2011;20:1516–1523. doi: 10.1158/1055-9965.EPI-11-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gunther S., Fagone P., Jalce G., Atanasov A.G., Guignabert C., Nicoletti F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: From pathogenic factors to therapeutic targets. Drug Discov. Today. 2019;24:428–439. doi: 10.1016/j.drudis.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 129.Nasiri E., Sankowski R., Dietrich H., Oikonomidi A., Huerta P.T., Popp J., Al-Abed Y., Bacher M. Key role of MIF-related neuroinflammation in neurodegeneration and cognitive impairment in Alzheimer’s disease. Mol. Med. 2020;26:34. doi: 10.1186/s10020-020-00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Petralia M.C., Battaglia G., Bruno V., Pennisi M., Mangano K., Lombardo S.D., Fagone P., Cavalli E., Saraceno A., Nicoletti F., et al. The Role of Macrophage Migration Inhibitory Factor in Alzheimer’s Disease: Conventionally Pathogenetic or Unconventionally Protective? Molecules. 2020;25:291. doi: 10.3390/molecules25020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tavakkoli A., Iranshahi M., Hasheminezhad S.H., Hayes A.W., Karimi G. The neuroprotective activities of natural products through the Nrf2 upregulation. Phytother. Res. 2019;33:2256–2273. doi: 10.1002/ptr.6427. [DOI] [PubMed] [Google Scholar]
- 132.Schepici G., Bramanti P., Mazzon E. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020;21:8637. doi: 10.3390/ijms21228637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mauch D.H., Nagler K., Schumacher S., Goritz C., Muller E.C., Otto A., Pfrieger F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 134.Lesser G.T., Haroutunian V., Purohit D.P., Schnaider Beeri M., Schmeidler J., Honkanen L., Neufeld R., Libow L.S. Serum lipids are related to Alzheimer’s pathology in nursing home residents. Dement. Geriatr. Cogn. Disord. 2009;27:42–49. doi: 10.1159/000189268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sabbagh M., Zahiri H.R., Ceimo J., Cooper K., Gaul W., Connor D., Sparks D.L. Is there a characteristic lipid profile in Alzheimer’s disease? J. Alzheimers Dis. 2004;6:585–589; discussion 673–681. doi: 10.3233/JAD-2004-6602. [DOI] [PubMed] [Google Scholar]
- 136.Wu B.J., Chen K., Barter P.J., Rye K.A. Niacin inhibits vascular inflammation via the induction of heme oxygenase-1. Circulation. 2012;125:150–158. doi: 10.1161/CIRCULATIONAHA.111.053108. [DOI] [PubMed] [Google Scholar]
- 137.Ali A.M., Kunugi H. Apitherapy for Parkinson’s Disease: A Focus on the Effects of Propolis and Royal Jelly. Oxid. Med. Cell Longev. 2020;2020:1727142. doi: 10.1155/2020/1727142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shen X., Liu Y., Luo X., Yang Z. Advances in Biosynthesis, Pharmacology, and Pharmacokinetics of Pinocembrin, a Promising Natural Small-Molecule Drug. Molecules. 2019;24:2323. doi: 10.3390/molecules24122323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jin X., Liu Q., Jia L., Li M., Wang X. Pinocembrin attenuates 6-OHDA-induced neuronal cell death through Nrf2/ARE pathway in SH-SY5Y cells. Cell Mol. Neurobiol. 2015;35:323–333. doi: 10.1007/s10571-014-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y., Gao J., Miao Y., Cui Q., Zhao W., Zhang J., Wang H. Pinocembrin protects SH-SY5Y cells against MPP+-induced neurotoxicity through the mitochondrial apoptotic pathway. J. Mol. Neurosci. 2014;53:537–545. doi: 10.1007/s12031-013-0219-x. [DOI] [PubMed] [Google Scholar]
- 141.Sparks D.L., Sabbagh M.N., Connor D.J., Lopez J., Launer L.J., Browne P., Wasser D., Johnson-Traver S., Lochhead J., Ziolwolski C. Atorvastatin for the treatment of mild to moderate Alzheimer disease: Preliminary results. Arch. Neurol. 2005;62:753–757. doi: 10.1001/archneur.62.5.753. [DOI] [PubMed] [Google Scholar]
- 142.Kivipelto M., Solomon A., Winblad B. Statin therapy in Alzheimer’s disease. Lancet Neurol. 2005;4:521–522. doi: 10.1016/S1474-4422(05)70150-2. [DOI] [PubMed] [Google Scholar]
- 143.Butterfield D.A., Barone E., Di Domenico F., Cenini G., Sultana R., Murphy M.P., Mancuso C., Head E. Atorvastatin treatment in a dog preclinical model of Alzheimer’s disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int. J. Neuropsychopharmacol. 2012;15:981–987. doi: 10.1017/S1461145711001118. [DOI] [PubMed] [Google Scholar]
- 144.Albrecht P., Bouchachia I., Goebels N., Henke N., Hofstetter H.H., Issberner A., Kovacs Z., Lewerenz J., Lisak D., Maher P., et al. Effects of dimethyl fumarate on neuroprotection and immunomodulation. J. Neuroinflammation. 2012;9:163. doi: 10.1186/1742-2094-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Balasa R., Barcutean L., Mosora O., Manu D. Reviewing the Significance of Blood-Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021;22:8370. doi: 10.3390/ijms22168370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scuderi S.A., Ardizzone A., Paterniti I., Esposito E., Campolo M. Antioxidant and Anti-inflammatory Effect of Nrf2 Inducer Dimethyl Fumarate in Neurodegenerative Diseases. Antioxidants. 2020;9:630. doi: 10.3390/antiox9070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahlawat J., Guillama Barroso G., Masoudi Asil S., Alvarado M., Armendariz I., Bernal J., Carabaza X., Chavez S., Cruz P., Escalante V., et al. Nanocarriers as Potential Drug Delivery Candidates for Overcoming the Blood-Brain Barrier: Challenges and Possibilities. ACS Omega. 2020;5:12583–12595. doi: 10.1021/acsomega.0c01592. [DOI] [PMC free article] [PubMed] [Google Scholar]