Abstract
The purpose of this study is to reveal the chemical and biochemical characteristics and the potential aromatherapy applications of the essential oil (EO) of Salvia officinalis (common sage) within a hospital environment. The chemical composition was determined by gas chromatography with mass spectrometry and ATR-FTIR spectroscopy. Three types of sage EOs were included in this study: two commercial oils and one oil obtained by in-house hydrodistillation. Based on the findings, these EOs were included in different chemotypes. The first two samples were similar to the most common chemotype (α-thujone > camphor > 1,8-cineole > β-thujone), while the in-house sage EO revealed a high content of 1,8-cineole, borneol, α-thujone, similar to the Dalmatian type. The latter sample was selected to be evaluated for its antioxidant and medical effects, as borneol, a bicyclic monoterpene, is known as a substance with anesthetic and analgesic effects in traditional Asian medicine. The study suggests that the antioxidant capacity of the sage EO is modest (33.61% and 84.50% inhibition was determined by DPPH and ABTS assays, respectively), but also that the inhalation of sage EO with high borneol content by hospitalized patients could improve these patients’ satisfaction.
Keywords: Salvia officinalis, essential oil, chemical analysis, antioxidant activity, aromatherapy, inhalation
1. Introduction
Essential oils (EOs) have proven their specific applications in different areas, including in human health, as a treatment for various disorders (migraine [1], skin disorders [2], fatigue [3,4], stroke [5], sleep disorders [6,7], endocrine disorders [8], depressive disorders [9], etc.). In medicine, EOs are considered good candidates as complementary and alternative treatment components due to their antimicrobial properties [10], possible anesthetic [11], or immunostimulatory effects [12]. An EO can be used alone and/or in synergetic associations with other EOs [13,14].
The qualitative composition of essential oils is determined by the genome of the particular plant and the variation caused by several environmental conditions such as daylight, temperature, and light. The conditions mentioned above select a pattern of seasonal fluctuations in plant metabolites. These conditions vary predictably and significantly throughout the vegetation interval, usually recurrent each year [15].
Salvia officinalis L. is one of the most widely used sources for EOs in traditional medicine. Even though the chemical composition of Salvia officinalis EOs has already been determined, the composition of EOs is very complex, depending on the plant part, time of harvesting, season, genetic diversity, climate, and meteorological conditions. Different studies have used gas-chromatography techniques [16,17,18,19] or FTIR [20] to determine Salvia officinalis L. EOs’ chemical composition. It is claimed that the antimicrobial properties of EOs are related to an extended alcoholic profile. Also, it was assessed that skin injury restoration is enhanced in the case of using plant extracts containing increased amounts of ketones [2,21]. EOs are rapidly absorbed via the skin into the bloodstream and thus travel to the brain and other organs [1,4,5,9,22,23,24,25,26,27,28]. A reduction of migraine discomfort is the result of the combination of the following factors: neurogenic inflammation, as well as a decrease in distress perception, along with blood vessel dilation [1]. The same processes may be responsible for the positive response to aromatherapy and synergic musical therapy obtained for anxiousness and/or suffering states recorded in different post-procedural phases of adult patients [29,30]. For insomnia improvement, the olfactory route is considered based on the comparative results regarding inhalation versus the transdermal pathway [31]. The inhalation of EOs may stimulate the immune and limbic systems responsible for the body’s state of well-being and emotional integration. The quality of life in burnout cases, determined by different diseases, such as chronic hemodialysis [32] or long periods of hard work, could be improved through aromatherapy [33,34]. Several reports are focused on evaluating essential oils’ toxicity profile [35,36,37]. For example, sage essential oil toxicity has been assessed on rat hepatocytes [36], and it is not toxic at concentrations below 200 nL mL−1. A report presents an accidental exposure to a newborn and a toddler to sage EO that lead to generalized tonic-clonic seizures [38]. Not all medical aspects are fully elucidated so far, as relevant elements such as the toxicological profile and different interactions still need to be clarified and studied.
It is used to treat colds, tuberculosis, bronchitis, gastrointestinal diseases, inflammation, and presents antibacterial, antifungal [39], antitumor [37,40,41,42], and antioxidant properties [43]. With respect to Salvia officinalis, different mechanisms of action have been proposed. Most of the research has been focused on the influence of Salvia officinalis on mental functions. Memory enhancement is thought to be a consequence of Salvia officinalis’ stimulating effect on the nicotinic and muscarinic receptors. Studies have also been conducted on the inhibitory effect on acetylcholinesterase, a compound considered to have a major role in Alzheimer-specific symptom progress [44]. At 100 mg/mL concentration, Salvia officinalis extract revealed high activity towards Escherichia coli, which is ordinary with respect to Staphylococcus aureus and Pseudomonas aeruginosa, and low in the case of Bacillus subtilis [45]. The use of the extracts in the presence of Hp-2-Minh ligand improved their biocidal characteristic even at lower concentrations. Research has proven the antimicrobial potential of EOs. This finding has medical significance, as it justifies reducing the use of synthetic medicinal products and utilizing EOs as a viable alternative in cases of drug-resistant pathogens.
Aromatherapy refers to the use of volatile compounds or fragrances from EOs obtained from plants, typically by inhalation, to prevent or cure diseases, infections, and indispositions. For alleviating pain, nausea, and anxiety, an ancient practice—clinical aromatherapy—is gaining attention in contemporary health services, where the intention of both the consumers and clinicians is to minimize the usage of medications. Thus, using integrative and complementary therapies is increasing, and scientific proof for the integrative therapies continues to flourish [46].
Different EOs were included in complementary and alternative treatment (such as aromatherapy) and were evaluated in a hospital setting in the following instances: peppermint EO for nausea and vomiting in women [25], lavender EO [47], Salvia officinalis EO for reducing nausea and vomiting in patients with cancer undergoing chemotherapy [48], Rosa damascene EO to decrease anxiety and increase sleep quality in cardiac patients [49], and Citrus aurantium to reduce anxiety in patients with acute coronary syndrome [50]. In the case of hospitalized patients, one of the main issues that arise is the decrease of peace of mind and the increase in anxiety during the hospitalization period [51].
Therefore, the present investigation aimed to assess the chemical composition and antioxidant activity of the essential oil of S. officinalis obtained from aerial parts of the plant and evaluate aromatherapy’s influence on patients in a hospital setup. The results of this study can be a starting point for the development of Aromatherapy Programs in Patient Care Settings in Romania.
2. Materials and Methods
All the reagents and solvents used in the experimental part were of adequate analytical or chromatographical grade (Sigma Aldrich, Fluka, Switzerland, and Merck, Darmstadt, Germany).
2.1. Sample Collection and Preparation
The EO named L-SEO was obtained by hydro-distillation from the dried aerial parts of Salvia officinalis plants grown in Arad County, Romania (coordinates 46°10′30″ N 21°18′45″ E, sample harvested in June 2019). The EO was stored in glass vials at +4 °C until further analysis. The samples B-SEO and EG-SEO are sage EOs commercially available on the Romanian market.
2.2. GC-MS Determination of the Chemical Composition of the Essential Oil from Salvia officinalis
The constituents of the EOs were determined by the gas chromatography method, using a gas chromatograph (GC) (Shimadzu2010, Kyoto, Japan) coupled with a triple quadruple mass spectrometer (MS) (TQ 8040, Shimadzu, Kyoto, Japan). The column used was an Optima 1 MS (30 m × 0.25 mm i.d., with a film thickness of 0.25 mm). At a flow rate of 1 mL/min, Helium was used as a carrier gas. The procedure for separating and quantifying EO components is described above [52]. The compounds from the analyzed samples were identified based on their mass spectra using the NIST 14 and Wiley 09 mass spectrum libraries (Scientific Instrument Services, Palmer, MA, USA). The Kovats retention indices for the identified compounds were calculated using the C8-C40 alkane standard. All analyses were performed in triplicate.
2.3. ATR-FTIR Spectroscopy
The ATR-FTIR spectra of Salvia officinalis L. EOs (L-SEO, B-SEO, and EG-SEO) were obtained on the wavelength range between 600 and 4000 cm−1 with a Bruker Vertex 70 (Bremen, Germany) spectrophotometer equipped with a Pike Miracle ATR cell. A sample volume of ~10 µL from each EO was placed directly on the surface of the ZnSe ATR crystal in the Teflon depression and covered with a metal cover. To avoid evaporation of the sample, a black outer ring was screwed on to press the Teflon depression tightly on the crystal. At the same time, the upper handle of the ATR cell was rotated so that its slightly concave stainless steel tip applied pressure against the metal cover.
The experimental spectra of the samples were recorded with a resolution of 4 cm−1, and 32 scans were accumulated per spectrum. The spectra were obtained in duplicate for each sample, and the average spectrum of the two measurements was calculated. Before each ATR measurement, the ZnSe crystal was carefully cleaned with isopropyl alcohol, and an air background spectrum was performed.
The OPUS software, version 6.5 from Bruker (Germany), was employed for spectra acquisition, minimum–maximum normalization, baseline correction, and identifying the wavelength value corresponding to the maximum absorbance of the recorded FT-IR bands.
2.4. Antioxidant Activity (DPPH and ABTS Assays)
The L-SEO sample’s antioxidant capacity was evaluated using two spectrophotometric assays (DPPH assay and ABTS assay), as reported earlier [22]. A UV-VIS spectrophotometer Specord 200 (Analytik Jena, Jena, Germany) and a 10 mm quartz cuvette were used in the procedure described above. Briefly, for the DPPH assay, we mixed a 0.1 mL control sample with 3 mL of 0.2 mM ethanolic DPPH• solution. The absorbance was recorded after 60 min of reaction in the dark at λ = 517 nm. Positive controls containing 0.02–4 mM Trolox solutions were prepared. The data are expressed in mg Trolox/L and inhibition (%).
For the ABTS assay, we mixed 0.5 mL sample or control with 1 mL ABTS* solution, prepared 16 h before from ABTS reagent and 2.45 mM aqueous solution of sodium persulfate. The absorbance was recorded at λ = 734 nm, after 10 min of reaction time in the dark. Positive controls containing 0.02–1.0 mM Trolox solutions were prepared. The data are expressed in mmol TEAC/L (TEAC: Trolox Equivalent Antioxidant Capacities) and inhibition (%). All the experiments were performed in triplicate.
2.5. Aromatherapy Effects: Clinical Application
This study was performed between August 2019 and August 2020, as a randomized, single-blind study involving adult patients at the Lipova City Hospital, Arad County, Romania.
In total, 174 hospitalized patients aged 23–85 years were enrolled, meeting the following inclusion criteria: adult patients with chronic conditions who have had at least one hospitalization in the same section in the past, an unimpaired sense of smell, no psychiatric pathologies, ability to communicate, and minimum 4 days and maximum 5 days hospital stay. Exclusion criteria included surgery intervention and unwillingness to participate.
Patients were randomized into two groups: 50 in the control group and 124 in the aromatherapy group; no patients were excluded, and no patients quit the study. Random allocation of the patients was made by hospital personnel who were not involved in the data collection or data analysis, depending on the number of patients hospitalized in each room. During the hospitalization period, patients admitted to the same ward were included in either the aromatherapy group or the control group (there were no patients from both groups at the same time in a ward). For the patients enrolled in the aromatherapy group, on a daily basis, a hospital staff member prepared the cotton disc with two drops of sage EO (L-SEO sample) that was kept on the patient’s pillow for a minimum of 30 min as the patient inhaled the volatile compounds. The patients from the control group received only routine care.
Only one EO (L-SEO) was used in aromatherapy, which was selected after determining the chemical composition.
Descriptive information form: A questionnaire was prepared by the authors of this study, including the patients’ related characteristics such as gender, age, weight, educational level, habits, personal evaluation, and health status. The questionnaire was completed by respondents on the last day of the hospitalization and was used to determine the following: (a) the demographic profile of the participants in terms of age, gender, weight, educational level, social status, health status in the last year, marital status, the use of aroma indoor and of perfumes, the use of sedatives/anxiolytics and the presence of some allergies; (b) the evaluation on the quality of in-hospital services in the present hospitalization stay, with seven possible answers: very weak, weak, acceptable, improved, good, very good, and excellent.
The study was approved by the Ethics Committee of Lipova City Hospital (no. 62/1 July 2019) and was conducted following the ethical principles of the Declaration of Helsinki [53]. The patients provided their written informed consent after a verbal and written explanation of the study protocol.
2.6. Statistical Analysis
Data were processed via GraphPad Prism (version 5.0 for Windows, GraphPad Software, San Diego, CA, USA), and F values (at p < 0.05) were considered statistically significant. The t-test, chi-squared test, Kruskal-Wallis test, Fisher’s exact test, Friedman test, and the Mann-Whitney test were used to compare qualitative variables in two groups.
3. Results
3.1. Chemical Analyses
3.1.1. GC-MS Analyses
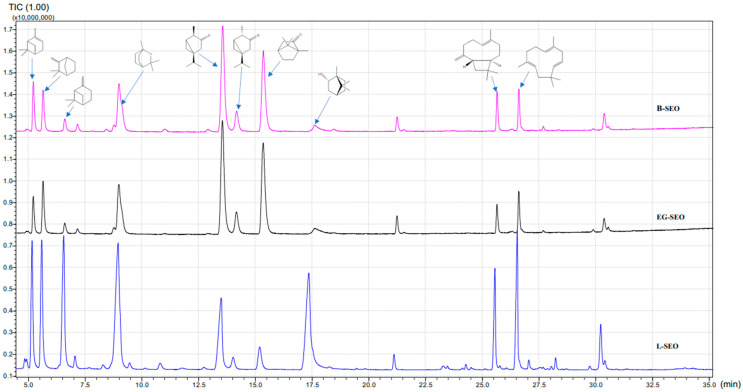
Figure 1 depicts the chromatograms obtained for the three Salvia officinalis essential oils samples investigated in this study, and Table 1 shows their composition as determined by GC-MS method. The EOs’ constitutive elements are listed according to the elution time. In the liquid phase, 47 compounds were separated.
Figure 1.
GC-MS chromatograms obtained for the investigated Salvia officinalis essential oil samples: B-SEO, pink; EG-SEO, black; L-SEO, blue.
Table 1.
Salvia officinalis L. essential oil chemical composition (% TIC) determined by GC-MS analyses.
| No. | KI | Compound/Class | Present Study | Turkey [17] |
Poland [18] |
Morocco [37] |
Sudan [19] |
Brazil [16] |
||
|---|---|---|---|---|---|---|---|---|---|---|
| B-SEO | EG-SEO | L-SEO | ||||||||
| 1 | 925 | Unidentified | 0.21 ± 0.01 | 0.24 ± 0.01 | ||||||
| 2 | 926 | Tricyclene/MH | 0.42 ± 0.01 | |||||||
| 3 | 930 | α-Thujene/MH | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.45 ± 0.01 | 0.31 | ||||
| 4 | 939 | α-Pinene/MH | 5.96 ± 0.03 | 4.23 ± 0.02 | 7.8 ± 0.05 | 7.17 | 0.02 | 3.18 | 8.96 | 3.07 |
| 5 | 954 | Camphene/MH | 5.59 ± 0.07 | 6.92 ± 0.10 | 8.73 ± 0.12 | 8.40 | 3.67 | 5.09 | 4.40 | |
| 6 | 975 | Sabinene/MH | 1.70 ± 0.01 | 1.55 ± 0.03 | 0.14 ± 0.01 | 0.41 | 0.15 | |||
| 7 | 979 | β-Pinene/MH | 0.78 ± 0.03 | 0.55 ± 0.02 | 10.52 ± 0.15 | 2.92 | 0.40 | 2.57 | ||
| 8 | 990 | β-Myrcene/MH | 0.72 ± 0.06 | 1.16 | 1.94 | 3.65 | ||||
| 9 | 1012 | 4-Carene/MH | 0.42 ± 0.02 | 0.07 ± 0.01 | ||||||
| 10 | 1024 | p-Cymene/MH | 0.85 ± 0.02 | 1.01 ± 0.03 | 1.33 | |||||
| 11 | α-Terpinene/MH | 0.34 ± 0.02 | 0.18 | 0.2 | 0.22 | |||||
| 12 | 1029 | 1,8-Cineole/MH | 13.39 ± 0.21 | 14.22 ± 0.19 | 17.98 ± 0.23 | 18.54 | 23.72 | 17.52 | 14.8 | |
| 13 | 1050 | trans-β-Ocimene/MH | 0.52 ± 0.06 | |||||||
| 14 | 1051 | D-Limonene/MH | 2.46 | 1.7 | 0.37 | |||||
| 15 | 1056 | Linalool/MH | 0.79 | |||||||
| 16 | 1059 | γ-Terpinene/MH | 0.60 ± 0.05 | 0.15 ± 0.06 | 0.62 ± 0.08 | 0.18 | 0.42 | |||
| 17 | 1088 | α-Terpinolene/MH | 0.60 ± 0.10 | 0.20 ± 0.05 | 0.12 | |||||
| 18 | 1089 | 2-Carene/MH | 0.19 ± 0.01 | |||||||
| 19 | 1102 | α-Thujone/MO | 26.03 ± 0.25 | 26.73 ± 0.26 | 8.74 ± 0.12 | 22.30 | 21.22 | 0.91 | 24.8 | |
| 20 | 1109 | β-Thujone/MO | 4.65 ± 0.11 | 4.19 ± 0.12 | 1.34 ± 0.11 | 14.28 | 13.45 | 3.97 | ||
| 21 | 1146 | Camphor/MO | 20.09 ± 0.24 | 22.64 ± 0.27 | 2.56 ± 0.12 | 14.40 | 18.22 | 21.23 | 11.57 | 10.9 |
| 22 | 1169 | Borneol/MO | 3.1 ± 0.20 | 3.31 ± 0.19 | 15.86 ± 0.23 | 0.37 | 2.42 | 1.67 | 0.81 | 11.1 |
| 23 | 1188 | α-Terpineol/MO | 0.50 ± 0.07 | 0.46 ± 0.08 | ||||||
| 24 | 1287 | Bornyl acetate/MO | 1.93 ± 0.11 | 2.27 ± 0.13 | 0.88 ± 0.06 | 0.32 | 0.22 | |||
| 25 | 1290 | Thymol/MO | 0.33 ± 0.01 | |||||||
| 26 | 1351 | α-Cubebene/SH | 0.2 ± 0.06 | |||||||
| 27 | 1380 | α-Copaene/SH | 0.08 ± 0.01 | 0.13 | ||||||
| 28 | 1381 | α-Ylangene/SH | 0.26 ± 0.11 | |||||||
| 29 | 1389 | β-Bourbonene/SH | 0.15 ± 0.03 | |||||||
| 30 | 1393 | Unidentified | 0.21 ± 0.03 | |||||||
| 31 | 1402 | Unidentified | 0.23 ± 0.07 | |||||||
| 32 | 1419 | β-Caryophyllene/SH | 4.54 ± 0.23 | 3.28 ± 0.21 | 5.66 ± 0.22 | 0.58 | 3.76 | 2.89 | ||
| 33 | 1429 | Unidentified | 0.39 ± 0.01 | 0.29 ± 0.3 | ||||||
| 34 | 1432 | γ-Cadinen/SH | 0.24 ± 0.09 | |||||||
| 35 | 1439 | α-Guaiene/SH | 0.14 ± 0.01 | |||||||
| 36 | 1456 | α-Humulene/SH | 4.89 ± 021 | 4.29 ± 0.21 | 8.64 ± 0.25 | 0.94 | 1.45 | 1.47 | ||
| 37 | 1479 | γ-Muurolene/SH | 0.40 ± 0.08 | 0.26 ± 0.02 | 0.63 ± 0.06 | |||||
| 38 | 1500 | α-Muurolene/SH | 0.43 ± 0.07 | |||||||
| 39 | 1512 | Unidentified | 0.06 ± 0.01 | |||||||
| 40 | 1523 | δ-Cadinene/SH | 0.10 ± 0.01 | 0.03 ± 0.01 | 0.17 ± 0.01 | |||||
| 41 | 1576 | Isoledene/SH | 0.61 ± 0.05 | |||||||
| 42 | 1583 | Caryophyllene oxide/SO | 0.19 ± 0.01 | 0.29 ± 0.03 | 0.22 ± 0.07 | |||||
| 43 | 1592 | Viridiflorol/SO | 3.09 ± 0.19 | 0.6 | ||||||
| 44 | 1593 | Unidentified | 2.34 ± 0.19 | 1.88 ± 0.18 | ||||||
| 45 | 1594 | Unidentified | 0.35 ± 0.03 | 0.61 ± 0.08 | 0.6 ± 0.09 | |||||
| 46 | 1603 | Unidentified | 0.08 ± 0.01 | |||||||
| 47 | 1607 | Unidentified | 0.6 ± 0.08 | |||||||
MH—monoterpene hydrocarbons; MO—oxygenate monoterpene; SH—sesquiterpene hydrocarbons; SO—oxygenated sesquiterpene, KI—Kovats retention index.
The results obtained follow the data presented in the literature [43]. The monoterpenes are the most represented, whilst the differences between the hydrocarbons (1,8-cineole, 17.98%; β-pinene, 10.52%; camphene, 8.73%) and the oxygenated (borneol, 15.86%; β-thujone, 1.34%) ones are modest. The sesquiterpenes have a prevalence lower than 9% (α-humulene, 8.64%; β-caryophyllene, 5.66%; caryophyllene oxide, 0.22%). The analyzed samples have a different composition in comparison to major compounds. B-SEO and EG-SEO have two major compounds (namely α-thujone and camphor), whilst L-SEO has four major compounds (namely 1,8-cineol, borneol, β-pinene, and α-thujone). L-SEO has a closer composition to the marocain one, possibly due to the plant growing conditions. B-SEO and EG-SEO have a composition similar to the EO obtained in Turkey, as shown in Table 1.
The differences in the chemical composition of the analyzed EOs could be attributed to several factors such as environmental factors, growth conditions, and time of harvesting [19,43,54]. The results obtained in this present study agree with previous research that concluded that 1,8-cineole, camphor, and α- and β-thujone are the main components of Salvia officinalis EOs [17,18,19,37]. As shown earlier, (+)-borneol is the only enantiomer found in the EO of Salvia species [55]; this compound is associated with analgesic and sedative effects [56].
3.1.2. ATR-FTIR Spectroscopy
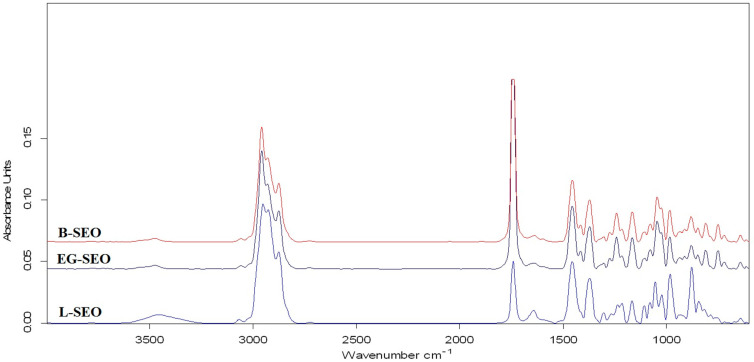
The obtained Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectra for the investigated Salvia officinalis L. EOs are depicted in Figure 2, and the wavelengths values (in the 600–4000 cm−1 range) for all recorded peaks are presented in Table 2. The different chemical compositions of the investigated EOs samples led to obtaining major differences in the intensity of the peaks located at the following wavelengths (L-SEO/B-SEO/ED-SEO): 3459/3474/3474 cm−1, 1741/1741/1741 cm−1, 1642/1637/1638 cm−1, 1054/-/- cm−1, -/1045/1045 cm−1, 982/983/983 cm−1, and 877/880/879 cm−1.
Figure 2.
The ATR-FTIR spectra of the Salvia officinalis L. essential oil samples.
Table 2.
The ATR-FTIR absorption band for SEO samples and vibrational assignments.
| Wavenumber (cm−1) of ATR-FTIR Recorded Bands | Vibrational Assignment | ||
|---|---|---|---|
| L-SEO | B-SEO | EG-SEO | |
| 3459 | 3474 | 3474 | (O-H) stretching vibration [57] |
| 3066 | 3058 | 3058 | (CH) stretching vibration of CH3 and CH2 (Csp3 and Csp2) [57] |
| 2951 | 2958 | 2958 | |
| 2926 | 2929 | 2930 | |
| 2875 | 2875 | 2875 | |
| 1741 | 1741 | 1741 | (C=O) stretching vibration in carbonyl group; (C=C) stretching vibration in (>C=CH2), (-CH=CH-), and (-CH=C<) alkyl groups [20,56,57,58,59] |
| 1642 | 1637 | 1638 | |
| 1456 | 1455 | 1455 | (C-H) symmetric and asymmetric bending vibration of (CH3) and (CH2) groups, (C-H) in-plane bending, (C-O) symmetric and asymmetric stretching vibration, (O-H) in-plane bending, (CH3(CO)) symmetric bending, (C-O-C) symmetric and asymmetric stretching [20,57,59,60] |
| - | 1415 | 1415 | |
| 1371 | 1371 | 1371 | |
| 1303 | 1303 | 1303 | |
| 1266 | 1274 | 1274 | |
| 1236 | 1241 | 1241 | |
| 1214 | 1215 | 1215 | |
| 1166 | 1165 | 1165 | |
| 1106 | 1104 | 1104 | |
| 1080 | 1077 | 1078 | |
| 1054 | - | - | |
| - | 1045 | 1045 | |
| 1022 | 1022 | 1022 | |
| 982 | 983 | 983 | (CH2) and (CH) out-of-plane wagging, (O-H) out-of-plane banding vibration [57,59,60,61] |
| 877 | 880 | 879 | |
| 844 | 847 | 848 | |
| 817 | 810 | 810 | |
| 759 | 750 | 750 | |
| 642 | 642 | 642 | |
3.1.3. Antioxidant Capacity
The antioxidant capacity of the sage EO (sample L-SEO) was evaluated by using two methods: DPPH assay (inhibition 33.61 ± 2.12%, antioxidant activity 0.81 ± 0.11 mg Trolox/L) and ABTS assay (inhibition 84.50 ± 2.23%, antioxidant activity 0.81 ± 0.03 mmol TEAC/L). All analyses were performed in triplicate. The data are expressed as mean ± STDEV.
3.2. Patients’ Characteristics
The mean age of the patients from the control group was 58.74 years whilst that of the salvia EO group was 52.34 years, with a p-value of 0.3530 (t-test). There are no significant differences between the two groups with respect to the following characteristics: gender, weight, educational level, social status, health status in the last year, marital status, the use of aroma indoor and perfumes, the use of sedatives/anxiolytics, and the presence of some allergies (Table 3). Between the groups, there is a significant difference in the variables of the consumption of alcohol, smoking, and the presence of chronic disease.
Table 3.
Patients’ demographic and clinical characteristics.
| Variable | Control Group | Salvia EO Group | Total | p-Value |
|---|---|---|---|---|
| n (%) | ||||
| Sex (Female/Male) | 14/36 | 46/78 | 174 (100) | |
| Weight (Kg) | 76.80 ± 13.74 | 74.98 ± 14.74 | 0.2173 * | |
| Educational level | 0.5877 ** | |||
| Middle school | 7 (14) | 11 (9) | 18 (10) | |
| High school | 25 (50) | 68 (55) | 93 (53) | |
| University | 18 (36) | 45 (36) | 63 (36) | |
| Social status | 0.3032 ** | |||
| Social aid | 3 (6) | 7 (6) | 10 (17) | |
| Active | 24 (48) | 75 (60) | 99 (57) | |
| Retired | 23 (46) | 42 (34) | 65 (37) | |
| Health status in the last year | 0.2839 ** | |||
| Excellent | 13 (26) | 34 (27) | 47 (27) | |
| Very good | 5 (10) | 24 (19) | 29 (17) | |
| Good | 17 (34) | 36 (29) | 53 (30) | |
| Bad | 15 (30) | 26 (21) | 41 (24) | |
| Very bad | 0 (0) | 4 (3) | 4 (2) | |
| Do you use fragrances/aroma in rooms/cars? | 0.1135 ** | |||
| Daily | 22 (44) | 63 (51) | 85 (49) | |
| 1–3 times/week | 5 (10) | 23 (19) | 28 (16) | |
| No | 23 (46) | 38 (31) | 61 (35) | |
| Do you use perfumes? | 0.4354 ** | |||
| Daily | 18 (36) | 57 (46) | 75 (43) | |
| 1–3 times/week | 22 (44) | 49 (40) | 71 (41) | |
| No | 10 (20) | 18 (15) | 28 (16) | |
| Do you smoke? | 0.0046 ** | |||
| Daily | 7 (14) | 46 (37) | 53 (30) | |
| Occasionally | 18 (36) | 42 (34) | 60 (34) | |
| No | 25 (50) | 36 (29) | 61 (35) | |
| Do you drink alcoholic drinks? | 0.1561 *** | |||
| Daily | 0 (0) | 1 (1) | 1 (1) | |
| Occasionally | 10 (20) | 51 (41) | 61 (35) | |
| No | 40 (80) | 72 (58) | 112 (64) | |
| Do you use sedatives/anxiolytics? | 0.0951 *** | |||
| Daily | 0 (0) | 0 (0) | 0 (0) | |
| Occasionally | 15 (30) | 31 (25) | 46 (26) | |
| No | 35 (70) | 93 (75) | 128 (74) | |
| Do you suffer from a chronic disease? | 0.6036 **** | |||
| Yes | 19 (38) | 42 (34) | 61 (35) | |
| No | 31 (62) | 82 (66) | 113 (65) | |
| Do you suffer from any drug/food allergies? | 0.0186 **** | |||
| Yes | 9 (18) | 46 (37) | 55 (32) | |
| No | 41 (82) | 78 (63) | 119 (68) | |
n—number of patients; * t-test; ** chi-squared test; *** Kruskal-Walis test; **** Fisher’s exact test.
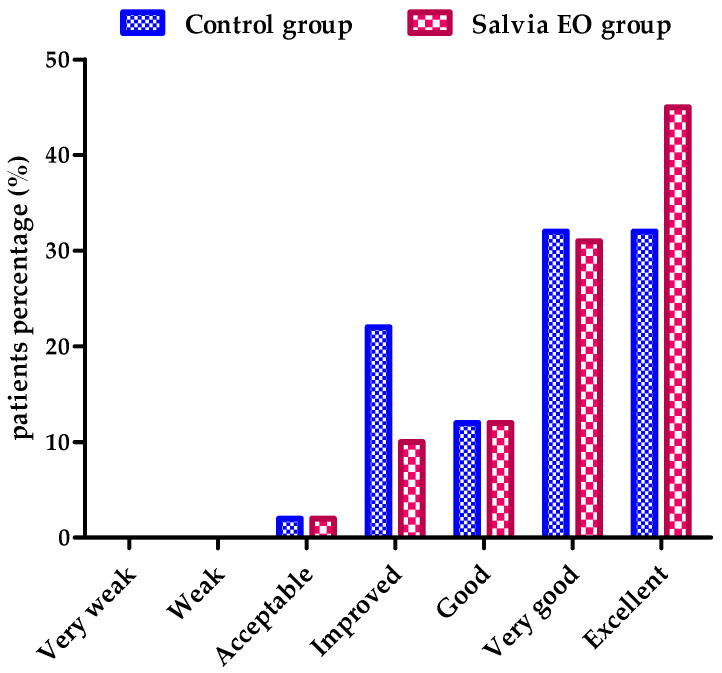
The patients’ perception of the quality of in-hospital services and, thus, their well-being after receiving treatment while hospitalized were assessed by answering questions designed with seven possible answers: very weak, weak, acceptable, improved, good, very good, and excellent. Group comparison was made using the Mann-Whitney U test. No significant differences were observed in the between-group comparison (p = 0.8969, U = 23). Most patients in both groups rated in-hospital services as excellent and very good (64% in the control group and 76% in the aromatherapy group) (Figure 3). No adverse effects were reported by patients or observed by medical staff in this study.
Figure 3.
Patients’ satisfaction with the treatment they received during their current hospitalization (Mann-Whitney U test).
4. Discussion
Much research has focused on investigating sage EO extracted from sage leaves harvested from diverse parts of the world. The percentage of the constituents varies widely due to geographical region, season, environmental conditions, genetic differences, phenological stages, sampling, and extraction methods. The number of detected constituents varies, being around 14–67 (14 with concentrations > 1% [41], 15 [62], 22 [17], 23 [43], 42 [19], 44 [63], 67 [64]). Also, sage EO’s composition is dependent on the growing ecosystem conditions, as determined in an exhaustive study reported by Russo et al., where 18 samples were collected in 2008–2009 in the south-central part of Italy and investigated [41]. The EOs obtained from sage leaves contained α-thujone (7.8–20.1%), camphor (8.4–20.8%), borneol (2.5–16.9%), γ-murolene (2.9–13.8%), and sclareol (5.9–23.1%) as the major compounds. Recently, another study identified oxygenated monoterpenes (67.7%) and monoterpenes hydrocarbons (19.1%) in the EO from sage leaves harvested in June 2020 in Tuscany, Italy. The main components identified in this EO batch were 1,8-cineole (30.3%), camphor (17.1%), α-thujone (9.7%), camphene (7.9%), and chrysanthenone (6.8%) [43].
Due to their chemical composition (shown in Table 1), two of the investigated samples (B-SEO and EG-SEO) were included in the more common C1c chemotype, as classified by Craft et al. [65]: α-thujone > camphor > 1,8-cineole > β-thujone, which was reported in samples from Turkey [17], Brazil [16], Mexico [65], and Croatia [66]. Meanwhile, the L-SEO sample is a chemotype found in the sage EO from the Dalmatian region, where 1,8-cineole is the main component and borneol is in high quantity [67].
The reported FTIR results of the EOs show that the components found in higher concentrations dominate the resulting vibrational spectra, while the components found in low concentrations do not have significant influence [54,68,69]. Ciko et al. [20] showed that FTIR analysis of the EO obtained from Salvia officinalis L. leaves indicated the presence of monoterpenes such as thujones, camphor, 1,8-cineole, and pinene.
The GC-MS results (Table 1) obtained for L-SEO sample indicate 1,8-cineol (17.98%) and borneol (15.86%) as their main components, which can influence the features of the recorded ATR-FTIR spectrum and the intensity of the obtained bands (Figure 2). The 1,8-cineole (eucalyptol) can be distinguished by the following key bands: 1371 cm−1 (δsym(CH3-CO) symmetric in plan bending/scissoring), 1266 cm−1 (ν(C-O) stretching from alkyl ether, 1214 cm−1 (νasym(C-O-C) asymmetric stretching), 1080 cm−1 (νsym(C-O-C) symmetric stretching), and 982 cm−1 (ωasym(CH2) asymmetric out-of-plane bending/wagging vibration). Due to the higher content of 1,8-cineole in L-SEO, the 982 cm−1 band is more intense than in B-SEO and EG-SEO, with 844 cm−1 corresponding to ωasym(C-H) asymmetric out-of-plane bending/wagging vibration [20,70,71].
For borneol, the characteristics bands are 3459 cm−1 (νsym(O-H) stretching from H-bonded alcohols), 1303 cm−1 (δsym(O-H) scissoring vibration), 1166 cm−1 (δsym(C-OH) in-plane bending of alcohol groups), 1054 cm−1 (νasym(C-O) asymmetric stretching from primary alcohols), 1106 cm−1 (νsym(C-O) symmetric stretching deformation), and 642 cm−1 (ω(C-O) out-of-plane bending) [20,30].
Other classes of compounds that occur in high proportions in the composition of L-SEO are bicyclic monoterpenes with =CH2 alkyl groups and sesquiterpenes with C=C bonds (sabinene + camphene, 8.87%; β-caryophyllene + α-humulene, 14.3%), with the key bands located at 3066 cm−1 (νsym(=C-H), Csp2 from the terminal vinyl group) and 1642 cm−1 (ν(C=CH2) stretching vibration). Schultz et. al. reported a band due to the stretching vibration of (C=C) located at 1653 cm−1 for sabinene and 1635 cm−1 for β-caryophyllene [70], while Agatonovik-Kustin et al. mentioned a vinyl group vibration at 1635–1650 cm−1, although the intensity of this peak was very low [70].
Compared to B-SEO and EG-SEO samples, in the case of L-SEO, the 1642 cm−1 band is more evident due to the higher content of compounds with =CH2 and C=C groups, with 1456 cm−1 corresponding to δsym(C-H) and δasym(C-H) in-plane bending of CH3 and -CH2 groups. The C=CH2 in-plane deformation vibration was not recorded as a separate band near ~1410–1420 cm−1 [20], probably being hidden under the -CH3 and -CH2 absorption bands, with 877 cm−1 corresponding to ν(=CH2) methylene out-of-plane deformation due to the strained ring structure with an exocyclic =CH2 group [70]. The higher intensity of the 877 cm−1 band, for the L-SEO compared with B-SEO and EG-ESO, is due to the higher content of compounds with the exocyclic =CH2 group.
In addition, it is mentioned that in the 2850–3000 cm−1 wavelength range, a group of characteristic bands with strong intensity were recorded (2951 cm−1, 2926 cm−1, 2875 cm−1) due to the symmetric and asymmetric stretching vibration of (C-H) bond from CH3, CH2, and CH. The intensities of these bands are higher for L-SEO than for B-SEO and EG-SEO.
For the B-SEO and EG-SEO samples, the obtained GC-MS results (Table 2) reveal that thujone and (+) camphor are the major components (50.77%) that influence the absorption band intensity. These compounds are monoterpenes with a carbonyl group (>C=O), belonging to the ketones class. In the obtained B-SEO and EG-SEO ATR-FTIR spectra (Figure 3), the band positioned at 1741 cm−1 is attributed to thujones and camphor being specific for (ν(C=O)) stretching vibration of 5-membered cyclic ketones [18,23]. Compared with the L-SEO sample, in the case of B-SEO and EG-SEO, the intensity of the 1741 cm−1 band is higher due to the different chemical compositions of the EOs.
Other absorption bands that showed changes in intensity compared to L-SEO sample, due to the difference in the chemical composition of B-SEO and EG-SEO, were observed at 3474 cm−1 due to (ν(O-H)) stretching vibration, weak band; 1415 cm−1 due to (ν(C=CH2) in-plane deformation vibration; 1241 cm−1 due to (ν(C-O)) stretching vibration; and 1045 cm−1 due to (ν(C-O)) stretching vibration from primary alcohols [69].
The main efficient constituents of Salvia essential oils are terpenes and polyphenols, with one major bioactivity being represented by their antioxidant action. The antioxidant capacity of the investigated sage EO (L-SEO) reached 33.61% and 84.50% by DPPH and ABTS analyses, respectively, which are values specific to samples rich in 1,8-cineole (60–70% inhibition in [69,70,71,72]).
The use of plant-derived essential oils for wellness and to sustain human health is called aromatherapy. In British herbal encyclopedias, the species of the Salvia family have long been renowned for being characterized as compounds that improve memory. Salvia essential oils can be helpful for the enhancement of cognition and mood, as demonstrated by various studies [73].
In this randomized, controlled study, the participants were adult men and women undergoing hospitalization who received or did not receive EOs for daily inhalation. The demographic and clinical data (shown in Table 3) revealed no statistical differences between the groups.
Our results revealed that the level of patient perception of the quality of in-hospital services, when sage EO is inhaled daily by patients, during hospitalization, is not statistically significant, as compared to that of the patients from the control group. The aromatherapy confirmed its beneficial action as a complementary treatment to increase the well-being of patients during hospitalization, as it showed a significant effect on hospitalized patients who evaluated their level of satisfaction as excellent (Figure 3).
The main limitations of this study consist of the somewhat limited number of patients in each group and a lack of medium/long-term follow-up. Further studies with a more extended follow-up period and more in-depth research on the use of sage EO in massage are needed to confirm our findings. Additionally, it is worth noting that the study did not include post-surgery patients. Therefore, our future goal is to develop randomized clinical trials to evaluate the large-scale efficacy of aromatherapy as an alternative therapy in patients with diverse pathologies. On the other hand, some strengths of this study must be highlighted, including the use of a chemically characterized EO in aromatherapy and the increase in some of the patients’ satisfaction regarding their hospitalization experience with this approach.
5. Conclusions
The GC-MS analysis of three samples of Salvia officinalis L. EOs indicated the presence of terpenes such as 1,8-cineole, thujones, borneol, camphor, sabinene, camphene, and caryophyllenes as the main components. The FTIR results agree with the GS-MS data of investigated samples, which leads to the conclusion that two commercial samples are part of the α-thujone > camphor > 1,8-cineole > β-thujone chemotype. In contrast, the other, in-house sample is part of the 1,8-cineole, borneol chemotype (Dalmatian type). The chemical analysis of EOs used in aromatherapy mechanism is very important, as different biological outcomes could arise based on the major compounds identified. The present results did not reveal a statistically significant difference between groups that inhaled sage EO during hospitalization versus the group that did not. However, more research is needed to investigate the mechanisms responsible for specific effects attributed to EOs to justify the therapeutic effect of sage EO and identify the side effects related to this product.
Acknowledgments
The authors wish to thank Lisa Craiut for the professional editing of this paper.
Author Contributions
Conceptualization, D.M.T. and D.M.C.; methodology, M.-D.M., A.I.L., C.M., D.C. and D.M.C.; software, A.I.L., C.M., D.C. and L.C.; validation, A.I.L., C.M., D.C., D.M.C. and L.C.; formal analysis, A.I.L., C.M., A.-M.B., D.C. and L.C.; investigation, M.-D.M., A.I.L., C.M., D.C. and D.M.C.; data curation, A.I.L., D.C., A.-M.B. and D.M.C.; writing—original draft preparation, M.-D.M., S.G., M.A.B. and D.M.C.; writing—review and editing, D.M.T., S.G.B. and D.M.C.; supervision, S.G.B. and D.M.C.; project administration, D.M.C.; funding acquisition, A.I.L. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian Ministry of Education and Research, CNCS—UEFISCDI, project number PN-III-P1-1.1-PD-2019-0349, within PNCDI III.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Lipova City Hospital (Spitalul Orasenesc Lipova), Romania (no. 62/1 July 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yuan R., Zhang D., Yang J., Wu Z., Luo C., Han L., Yang F., Lin J., Yang M. Review of Aromatherapy Essential Oils and Their Mechanism of Action Against Migraines. J. Ethnopharmacol. 2021;265:113326. doi: 10.1016/j.jep.2020.113326. [DOI] [PubMed] [Google Scholar]
- 2.Winkelman W.J. Aromatherapy, Botanicals, and Essential Oils in Acne. Clin. Dermatol. 2018;36:299–305. doi: 10.1016/j.clindermatol.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Lizarraga-Valderrama L.R. Effects of Essential Oils on Central Nervous System: Focus on Mental Health. Phytother. Res. 2021;35:657–679. doi: 10.1002/ptr.6854. [DOI] [PubMed] [Google Scholar]
- 4.Zeinalian M., Eshaghi M., Hadian M., Naji H., Marandi S.M.M., Asgary S. Eight Essential Foods in Iranian Traditional Medicine and their Role in Health Promotion and Well-being. Int. J. Prev. Med. 2017;8:2. doi: 10.4103/2008-7802.197688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contrada M., Cerasa A., Tonin P., Bagetta G., Scuteri D. Aromatherapy in Stroke Patients: Is It Time to Begin? Front. Behav. Neurosci. 2021;15:749353. doi: 10.3389/fnbeh.2021.749353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fismer K.L., Pilkington K. Lavender and Sleep: A systematic Review of the Evidence. Eur. J. Integr. Med. 2012;4:E436–E447. doi: 10.1016/j.eujim.2012.08.001. [DOI] [Google Scholar]
- 7.Lillehei A.S., Halcon L.L. A Systematic Review of the Effect of Inhaled Essential Oils on Sleep. J. Altern. Complement. Med. 2014;20:441–451. doi: 10.1089/acm.2013.0311. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins J., Hires C., Dunne E., Baker C. The Relationship between Lavender and Tea Tree Essential Oils and Pediatric Endocrine Disorders: A Systematic Review of the Literature. Complement. Ther. Med. 2020;49:102288. doi: 10.1016/j.ctim.2019.102288. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Long Y., Yu S., Li D., Yang M., Guan Y., Zhang D., Wan J., Liu S., Shi A., et al. Natural Volatile Oils Derived from Herbal Medicines: A Promising Therapy Way for Treating Depressive Disorder. Pharmacol. Res. 2021;164:105376. doi: 10.1016/j.phrs.2020.105376. [DOI] [PubMed] [Google Scholar]
- 10.Almanaa T.N., Alharbi N.S., Ramachandran G., Chelliah C.K., Rajivgandhi G., Manoharan N., Kadaikunnan S., Khaled J.M., Alanzi K.F. Anti-biofilm Effect of Nerium oleander Essential Oils Against Biofilm Forming Pseudomonas aeruginosa on Urinary Tract Infections. J. King Saud Univ. Sci. 2021;33:101340. doi: 10.1016/j.jksus.2021.101340. [DOI] [Google Scholar]
- 11.Brandao F.R., Farias C.F.S., Souza D.C.D., de Oliveira M.I.B., de Matos L.V., Majolo C., de Oliveira M.R., Chaves F.C.M., O’Sullivan F.L.D., Chagas E.C. Anesthetic Potential of the Essential Oils of Aloysia triphylla, Lippia sidoides and Mentha piperita for Colossoma macropomum. Aquaculture. 2021;534:736275. doi: 10.1016/j.aquaculture.2020.736275. [DOI] [Google Scholar]
- 12.Valdivieso-Ugarte M., Plaza-Diaz J., Gomez-Llorente C., Gomez E.L., Sabes-Alsina M., Gil A. In Vitro Examination of Antibacterial and Immunomodulatory Activities of Cinnamon, White Thyme, and Clove Essential Oils. J. Funct. Foods. 2021;81:104436. doi: 10.1016/j.jff.2021.104436. [DOI] [Google Scholar]
- 13.Chraibi M., Fadil M., Farah A., Lebrazi S., Fikri-Benbrahim K. Antimicrobial Combined Action of Mentha pulegium, Ormenis mixta and Mentha piperita Essential Oils Against S. aureus, E. coli and C. tropicalis: Application of Mixture Design Methodology. LWT-Food Sci. Technol. 2021;145:111352. doi: 10.1016/j.lwt.2021.111352. [DOI] [Google Scholar]
- 14.Rocha R.R., Matos M.N.C., Guerrero J.A.P., Cavalcante R.M.B., Melo R.S., Azevedo A.M.A., Pereira A.M.G., Lopes P.H.R., Rodrigues T.H.S., Bandeira P.N., et al. Comparative Study of the Chemical Composition, Antibacterial Activity and Synergic Effects of the Essential Oils of Croton tetradenius Baill. and C. pulegiodorus Baill. Against Staphylococcus aureus Isolates. Microb. Pathog. 2021;156:104934. doi: 10.1016/j.micpath.2021.104934. [DOI] [PubMed] [Google Scholar]
- 15.Bogdan M.A., Bungau S., Tit D.M., Zaha D.C., Nechifor A.C., Behl T., Chambre D., Lupitu A.I., Copolovici L., Copolovici D.M. Chemical Profile, Antioxidant Capacity, and Antimicrobial Activity of Essential Oils Extracted from Three Different Varieties (Moldoveanca 4, Vis Magic 10, and Alba 7) of Lavandula angustifolia. Molecules. 2021;26:4381. doi: 10.3390/molecules26144381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delamare A.P.L., Moschen-Pistorello I.T., Artico L., Atti-Serafini L., Echeverrigaray S. Antibacterial Activity of the Essential Oils of Salvia officinalis L. and Salvia triloba L. Cultivated in South Brazil. Food Chem. 2007;100:603–608. doi: 10.1016/j.foodchem.2005.09.078. [DOI] [Google Scholar]
- 17.Kulak M., Gul F., Sekeroglu N. Changes in Growth Parameter and Essential Oil Composition of Sage (Salvia officinalis L.) Leaves in Response to Various Salt Stresses. Ind. Crops Prod. 2020;145:112078. doi: 10.1016/j.indcrop.2019.112078. [DOI] [Google Scholar]
- 18.Rzepa J., Wojtal L., Staszek D., Grygierczyk G., Labe K., Hajnos M., Kowalska T., Waksmundzka-Hajnos M. Fingerprint of Selected Salvia Species by HS-GC-MS Analysis of Their Volatile Fraction. J. Chromatogr. Sci. 2009;47:575–580. doi: 10.1093/chromsci/47.7.575. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed A., Mustafa A. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oil Salvia officinalis in Sudan. J. Multidis. Res. Rev. 2019;1:43–45. [Google Scholar]
- 20.Ciko L., Andoni A., Ylli F., Plaku E., Taraj K., Armand C. Extraction of Essential Oil from Albanian Salvia officinalis L. and its Characterization by FTIR Spectroscopy. Asian J. Chem. 2016;28:1401–1402. doi: 10.14233/ajchem.2016.19658. [DOI] [Google Scholar]
- 21.Glevitzky I., Dumitrel G.A., Glevitzky M., Pasca B., Otrisal P., Bungau S., Cioca G., Pantis C., Popa M. Statistical Analysis of the Relationship between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. Chim. 2019;70:3103–3107. doi: 10.37358/RC.19.9.7497. [DOI] [Google Scholar]
- 22.Rafi N., Khodadadizadeh A., Nematabad M.S., Sayadi A.R. The Evaluation of the Effect of Aromatherapy with Lavender Essential Oil on the Quality of Sleep of Cardiac Patients Candidate for Angiography. Pak. J. Med. Health Sci. 2020;14:1143–1147. [Google Scholar]
- 23.Russo P., Frustaci A., Del Bufalo A., Fini M., Cesario A. From Traditional European Medicine to Discovery of New Drug Candidates for the Treatment of Dementia and Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Med. Chem. 2013;20:976–983. doi: 10.2174/092986713805288905. [DOI] [PubMed] [Google Scholar]
- 24.Bagheri H., Salmani T., Nourian J., Mirrezaie S.M., Abbasi A., Mardani A., Vlaisavljevic Z. The Effects of Inhalation Aromatherapy Using Lavender Essential Oil on Postoperative Pain of Inguinal Hernia: A Randomized Controlled Trial. J. Perianesthesia Nurs. 2020;35:642–648. doi: 10.1016/j.jopan.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Mohr C., Jensen C., Padden N., Besel J.M., Brant J.M. Peppermint Essential Oil for Nausea and Vomiting in Hospitalized Patients: Incorporating Holistic Patient Decision Making Into the Research Design. J. Holist. Nurs. 2021;39:126–134. doi: 10.1177/0898010120961579. [DOI] [PubMed] [Google Scholar]
- 26.Bertone A.C., Dekker R.L. Aromatherapy in Obstetrics: A Critical Review of the Literature. Clin. Obstet. Gynecol. 2021;64:572–588. doi: 10.1097/GRF.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 27.Karan N.B. Influence of lavender oil inhalation on vital signs and anxiety: A randomized clinical trial. Physiol. Behav. 2019;211:112676. doi: 10.1016/j.physbeh.2019.112676. [DOI] [PubMed] [Google Scholar]
- 28.Lordani T.V.A., de Lara C.E., Ferreira F.B.P., Monich M.D.T., da Silva C.M., Lordani C.R.F., Bueno F.G., Teixeira J.J.V., Lonardoni M.V.C. Therapeutic Effects of Medicinal Plants on Cutaneous Wound Healing in Humans: A Systematic Review. Mediat. Inflamm. 2018;2018:7354250. doi: 10.1155/2018/7354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng C., Xie Y., Liu Y., Li Y., Xiao Y. Aromatherapy Plus Music Therapy Improve Pain Intensity and Anxiety Scores in Patients with Breast Cancer during Perioperative Periods: A Randomized Controlled Trial. Clin. Breast Cancer. 2021;22:115–120. doi: 10.1016/j.clbc.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Huang H., Wang Q., Guan X., Zhang X., Kang J., Zhang Y., Zhang Y., Zhang Q., Li X. Effect of Aromatherapy on Preoperative Anxiety in Adult Patients: A Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Clin. Pract. 2021;42:101302. doi: 10.1016/j.ctcp.2021.101302. [DOI] [PubMed] [Google Scholar]
- 31.Tang Y., Gong M., Qin X., Su H., Wang Z., Dong H. The Therapeutic Effect of Aromatherapy on Insomnia: A Meta-Analysis. J. Affect. Disord. 2021;288:1–9. doi: 10.1016/j.jad.2021.03.066. [DOI] [PubMed] [Google Scholar]
- 32.Mohammadpourhodki R., Sadeghnezhad H., Ebrahimi H., Basirinezhad M.H., Maleki M., Bossola M. The Effect of Aromatherapy Massage with Lavender and Citrus Aurantium Essential Oil on Quality of Life of Patients on Chronic Hemodialysis: A Parallel Randomized Clinical Trial Study. J. Pain Symptom Manag. 2021;61:456–463.e1. doi: 10.1016/j.jpainsymman.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Varney E., Buckle J. Effect of Inhaled Essential Oils on Mental Exhaustion and Moderate Burnout: A Small Pilot Study. J. Altern. Complement. Med. 2013;19:69–71. doi: 10.1089/acm.2012.0089. [DOI] [PubMed] [Google Scholar]
- 34.Wu C.-Y., Lee H.-F., Chang C.W., Chiang H.-C., Tsai Y.-H., Liu H.-E. The Immediate Effects of Lavender Aromatherapy Massage versus Massage in Work Stress, Burnout, and HRV Parameters: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2020;2020:8830083. doi: 10.1155/2020/8830083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesquita K.D., Feitosa B.D., Cruz J.N., Ferreira O.O., Franco C.D., Cascaes M.M., Oliveira M.S., Andrade E.H. Chemical composition and preliminary toxicity evaluation of the essential oil from Peperomia circinnata Link var. circinnata. (Piperaceae) in Artemia salina Leach. Molecules. 2021;26:7359. doi: 10.3390/molecules26237359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lima C.F., Carvalho F., Fernandes E., Bastos M.L., Santos-Gomes P.C., Fernandes-Ferreira M., Pereira-Wilson C. Evaluation of toxic/protective effects of the essential oil of Salvia officinalis on freshly isolated rat hepatocytes. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA. 2004;18:457–465. doi: 10.1016/j.tiv.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 37.El Hadri A., del Rio M.G., Sanz J., Coloma A.G., Idaomar M., Ozonas B.R., González J.B., Reus M.I.S. Cytotoxic Activity of alpha-Humulene and Transcaryophyllene from Salvia officinalis in Animal and Human Tumor Cells. An. R. Acad. Nac. Farm. 2010;76:343–356. [Google Scholar]
- 38.Halicioglu O., Astarcioglu G., Yaprak I., Aydinlioglu H. Toxicity of Salvia officinalis in a newborn and a child: An alarming report. Pediatr. Neurol. 2011;45:259–260. doi: 10.1016/j.pediatrneurol.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Ahangari F., Farshbaf-Khalili A., Javadzadeh Y., Adibpour M., Oskouei B.S. Comparing the Effectiveness of Salvia officinalis, Clotrimazole and their Combination on Vulvovaginal Candidiasis: A Randomized, Controlled Clinical Trial. J. Obstet. Gynaecol. Res. 2019;45:897–907. doi: 10.1111/jog.13918. [DOI] [PubMed] [Google Scholar]
- 40.Luca T., Napoli E., Privitera G., Musso N., Ruberto G., Castorina S. Antiproliferative Effect and Cell Cycle Alterations Induced by Salvia officinalis Essential Oil and Its Three Main Components in Human Colon Cancer Cell Lines. Chem. Biodivers. 2020;17:e2000309. doi: 10.1002/cbdv.202000309. [DOI] [PubMed] [Google Scholar]
- 41.Russo A., Formisano C., Rigano D., Senatore F., Delfine S., Cardile V., Rosselli S., Bruno M. Chemical Composition and Anticancer Activity of Essential Oils of Mediterranean sage (Salvia officinalis L.) Grown in Different Environmental Conditions. Food Chem. Toxicol. 2013;55:42–47. doi: 10.1016/j.fct.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 42.Sertel S., Eichhorn T., Plinkert P.K., Efferth T. Anticancer Activity of Salvia officinalis Essential Oil against HNSCC Cell Line (UMSCC1) Hno. 2011;59:1203–1208. doi: 10.1007/s00106-011-2274-3. [DOI] [PubMed] [Google Scholar]
- 43.Ovidi E., Masci V.L., Zambelli M., Tiezzi A., Vitalini S., Garzoli S. Laurus nobilis, Salvia sclarea and Salvia officinalis Essential Oils and Hydrolates: Evaluation of Liquid and Vapor Phase Chemical Composition and Biological Activities. Plants. 2021;10:707. doi: 10.3390/plants10040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghorbani A., Esmaeilizadeh M. Pharmacological Properties of Salvia officinalis and its Components. J. Tradit. Complement. Med. 2017;7:433–440. doi: 10.1016/j.jtcme.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghaedi M., Naghiha R., Jannesar R., Dehghanian N., Mirtamizdoust B., Pezeshkpour V. Antibacterial and Antifungal Activity of Flower Extracts of Urtica dioica, Chamaemelum nobile and Salvia officinalis: Effects of Zn[OH]2 Nanoparticles and Hp-2-minh on their Property. J. Ind. Eng. Chem. 2015;32:353–359. doi: 10.1016/j.jiec.2015.09.007. [DOI] [Google Scholar]
- 46.Pearson A.C.S., Cutshall S.M., Hooten W.M., Rodgers N.J., Bauer B.A., Bhagra A. Perspectives on the Use of Aromatherapy from Clinicians Attending an Integrative Medicine Continuing Education Event. BMC Complement. Altern. Med. 2019;19:174. doi: 10.1186/s12906-019-2572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho E.H., Lee M.-Y., Hur M.-H. The Effects of Aromatherapy on Intensive Care Unit Patients′ Stress and Sleep Quality: A Nonrandomised Controlled Trial. Evid.-Based Complement. Altern. Med. 2017;2017:2856592. doi: 10.1155/2017/2856592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasab F.R.S., Shahrbabaki P.M., Dehghan M., Tajadini H., Baniasadi H., Sabzevari S. Effect of Abdominal Massage with and without Salvia officinalis on Nausea and Vomiting in Patients with Cancer Undergoing Chemotherapy: A Randomized Clinical Trial. J. Oncol. 2021;2021:9989228. doi: 10.1155/2021/9989228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jodaki K., Abdi K., Mousavi M.S., Mokhtari R., Asayesh H., Vandali V., Golitaleb M. Effect of Rosa damascene Aromatherapy on Anxiety and Sleep Quality in Cardiac Patients: A Randomized Controlled Trial. Complement. Ther. Clin. Pract. 2021;42:101299. doi: 10.1016/j.ctcp.2020.101299. [DOI] [PubMed] [Google Scholar]
- 50.Moslemi F., Alijaniha F., Naseri M., Kazemnejad A., Charkhkar M., Heidari M.R. Citrus aurantium Aroma for Anxiety in Patients with Acute Coronary Syndrome: A Double-Blind Placebo-Controlled Trial. J. Altern. Complement. Med. 2019;25:833–839. doi: 10.1089/acm.2019.0061. [DOI] [PubMed] [Google Scholar]
- 51.Gudmundsson G., Gislason T., Janson C., Lindberg E., Ulrik C.S., Brondum E., Nieminen M.M., Aine T., Hallin R., Bakke P. Depression, Anxiety and Health Htatus after Hospitalisation for COPD: A Multicentre Study in the Nordic Countries. Respir. Med. 2006;100:87–93. doi: 10.1016/j.rmed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Beicu R., Alexa E., Obistioiu D., Cocan I., Imbrea F., Pop G., Circioban D., Moisa C., Lupitu A., Copolovici L., et al. Antimicrobial Potential and Phytochemical Profile of Wild and Cultivated Populations of Thyme (Thymus sp.) Growing in Western Romania. Plants. 2021;10:1833. doi: 10.3390/plants10091833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. [(accessed on 23 June 2019)]. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- 54.Moisa C., Lupitu A., Pop G., Chambre D.R., Copolovici L., Cioca G., Bungau S., Copolovici D.M. Variation of the Chemical Composition of Thymus Vulgaris Essential Oils by Phenological Stages. Rev. De Chim. 2019;70:633–637. doi: 10.37358/RC.19.2.6973. [DOI] [Google Scholar]
- 55.Demirci B., Tabanca N., Baser K.H.C. Enantiomeric Distribution of Some Monoterpenes in the Essential Oils of some Salvia Species. Flavour Fragr. J. 2002;17:54–58. doi: 10.1002/ffj.1039. [DOI] [Google Scholar]
- 56.Granger R.E., Campbell E.L., Johnston G.A.R. (+)- And (−)-Borneol: Efficacious Positive Modulators of GABA Action at Human Recombinant α1β2γ2L GABAA Receptors. Biochem. Pharmacol. 2005;69:1101–1111. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Coates J. Interpretation of Infrared Spectra, A Practical Approach. In: Meyers R.A., McKelvy M.L., editors. Encyclopedia of Analytical Chemistry. John Wiley & Sons, Ltd.; New York, NY, USA: 2006. [DOI] [Google Scholar]
- 58.Schulz H., Baranska M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007;43:13–25. doi: 10.1016/j.vibspec.2006.06.001. [DOI] [Google Scholar]
- 59.Schulz H., Özkan G., Baranska M., Krüger H., Özcan M. Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 2005;39:249–256. doi: 10.1016/j.vibspec.2005.04.009. [DOI] [Google Scholar]
- 60.Chambre D.R., Moisa C., Lupitu A., Copolovici L., Pop G., Copolovici D.M. Chemical composition, antioxidant capacity, and thermal behavior of Satureja hortensis essential oil. Sci. Rep. 2020;10:21322. doi: 10.1038/s41598-020-78263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agatonovic-Kustrin S., Ristivojevic P., Gegechkori V., Litvinova T.M., Morton D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020;10:7294. doi: 10.3390/app10207294. [DOI] [Google Scholar]
- 62.Samani M.R., Pirbalouti A.G., Moattar F., Golparvar A.R. L-Phenylalanine and Bio-Fertilizers Interaction Effects on Growth, Yield and Chemical Compositions and Content of Essential Oil From the Sage (Salvia officinalis L.) Leaves. Ind. Crops Prod. 2019;137:1–8. doi: 10.1016/j.indcrop.2019.05.019. [DOI] [Google Scholar]
- 63.Ed-Dra A., Filali F.R., Lo Presti V., Zekkori B., Nalbone L., Bouymajane A., Trabelsi N., Lamberta F., Bentayeb A., Giuffrida A., et al. Chemical Composition, Antioxidant Capacity and Antibacterial Action of Five Moroccan Essential Oils Against Listeria Monocytogenes and Different Serotypes of Salmonella enterica. Microb. Pathog. 2020;149:104510. doi: 10.1016/j.micpath.2020.104510. [DOI] [PubMed] [Google Scholar]
- 64.Hayouni E.A., Chraief I., Abedrabba M., Bouix M., Leveau J.Y., Mohammed H., Hamdi M. Tunisian Salvia officinalis L. and Schinus molle L. Essential Oils: Their Chemical Compositions an Their Preservative Effects Against Salmonella Inoculated in Minced Beef Meat. Int. J. Food Microbiol. 2008;125:242–251. doi: 10.1016/j.ijfoodmicro.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Craft J.D., Satyal P., Setzer W.N. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines. 2017;4:47. doi: 10.3390/medicines4030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jug-Dujaković M., Ristić M., Pljevljakušić D., Dajić-Stevanović Z., Liber Z., Hančević K., Radić T., Šatović Z. High Diversity of Indigenous Populations of Dalmatian Sage (Salvia officinalis L.) in Essential-Oil Composition. Chem. Biodivers. 2012;9:2309–2323. doi: 10.1002/cbdv.201200131. [DOI] [PubMed] [Google Scholar]
- 67.Kuštrak D., Kuftinec J., Blažević N. Yields and Composition of Sage Oils from Different Regions of the Yugoslavian Adriatic Coast. J. Nat. Prod. 1984;47:520–524. doi: 10.1021/np50033a020. [DOI] [Google Scholar]
- 68.Valderrama A.C.S., Rojas De G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017;8:726–741. doi: 10.4236/ajac.2017.811053. [DOI] [Google Scholar]
- 69.Miguel G., Cruz C., Faleiro M.L., Simoes M.T.F., Figueiredo A.C., Barroso J.G., Pedro L.G. Salvia officinalis L. Essential Oils: Effect of Hydrodistillation Time on the Chemical Composition, Antioxidant and Antimicrobial Activities. Nat. Prod. Res. 2011;25:526–541. doi: 10.1080/14786419.2010.499513. [DOI] [PubMed] [Google Scholar]
- 70.Abou Baker D.H., Amarowicz R., Kandeil A., Ali M.A., Ibrahim E.A. Antiviral Activity of Lavandula angustifolia L. and Salvia officinalis L. Essential Oils Against Avian Influenza H5N1 Virus. J. Agric. Food Res. 2021;4:100135. doi: 10.1016/j.jafr.2021.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ben Farhat M., Jordan M.J., Chaouech-Hamada R., Landoulsi A., Sotomayor J.A. Variations in Essential Oil, Phenolic Compounds, and Antioxidant Activity of Tunisian Cultivated Salvia officinalis L. J. Agric. Food Chem. 2009;57:10349–10356. doi: 10.1021/jf901877x. [DOI] [PubMed] [Google Scholar]
- 72.Bozin B., Mimica-Dukic N., Samojlik I., Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007;55:7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- 73.Fu Z., Wang H., Hu X., Sun Z., Han C. The Pharmacological Properties of Salvia Essential Oils. J. App. Pharm. Sci. 2013;3:122–127. doi: 10.7324/JAPS.2013.3723. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.