Abstract
Widespread multidrug-resistant (MDR) and multi-virulent diarrheagenic E. coli create several crises among human and animal populations worldwide. For this reason, we looked forward to a breakthrough with this issue and tried to highlight these emerging threats. A total of 140 diarrheagenic E. coli isolates were recovered from animal and human sources. The O26 serotype, alongside the ampicillin/cefoxitin resistance phenotype, was predominant among both human and animal isolates. Of note, imipenem represented the most effective antibiotic against all the investigated isolates. Unfortunately, 90% and 57.9% of the tested isolates showed MDR and multi-virulent patterns, respectively. The animal isolates were more virulent and showed higher sensitivity to antimicrobial agents. Both animal and human isolates could not be arranged into related clusters. A strong negative correlation between the existence of virulence genes and antimicrobial resistance was clearly detected. A significant correlation between serotypes and antimicrobial resistance was not detected; meanwhile, a significant positive correlation between some serotypes and the presence of certain virulence genes was announced. Finally, our results confirmed the urgent need for restricted guidelines, in addition to new alternative therapies, due to the genetic diversity and wide spreading of MDR side by side with multi-virulent E. coli isolates.
Keywords: E. coli, MDR, multi-virulent, serotypes, genetic diversity
1. Introduction
High morbidity and mortality rates have been detected in the developing world, due to diarrhea, which receives more attention in these countries. Several new phenotypes of enteric pathogens were recorded, which cause severe gastroenteritis [1]. One of the most important etiologic agents of diarrhea is E. coli. Although E. coli is normal commensal flora, it may convert to a full pathogen by the acquisition of mobile genetic materials, through horizontal gene transfer, or bacteriophages, causing several intestinal and extraintestinal infections, which affect both humans and animals [2].
Escherichia coli (E. coli), as a widespread bacterial pathogen, has adaptive ability in diverse ecological niches. E. coli strains could potentially have a serious impact on both humans and animals, including cattle, horses, and birds, since they can induce enteric [3] and extraintestinal infections [4]. Moreover, E. coli is involved in urinary tract infections, sepsis/meningitis and gastrointestinal diseases. There are two major public health crises in terms of E. coli infections. Firstly, widespread pathogenic E. coli in environmental water, such as irrigation and drinking water, causes severe infections in humans and animals [5]. Secondly, the presence of multi-virulent and multidrug-resistant (MDR) E. coli strains, which exhibited resistance to at least three antimicrobial drugs from different classes [6].
According to the disease type, E. coli can be classified into two main types: diarrhoeagenic E. coli (DEC) and extraintestinal pathogenic E. coli (ExPEC). The ExPEC has two pathovars: uropathogenic E. coli and neonatal meningitis E. coli. Moreover, there are six pathovars of DEC, according to the mechanisms of diarrhea, including enteroinvasive E. coli, enteropathogenic E. coli, enteroaggregative E. coli, diffusely adherent E. coli, enterotoxigenic E. coli, and enterohaemorrhagic E. coli [2]. The genetic background of these pathovars is diverse, and their evolution can occur through the acquisition and loss of pathogenicity islands [7]. Many virulence genes of recognized importance are associated with severe infections caused by E. coli strains. They include genes encoding for adhesion (fimH and kpsM), enterotoxin (astA), intimin (eaeA), Vero toxins (vtx), Shiga toxins (stx), hemolysin (hly) and outer membrane proteins (omp). Additionally, transcriptional activators for aggregative adherence fimbriae and invasion were encoded by aggR and invE genes, respectively [8,9,10]. These virulence factors are involved in the pathogenicity of E. coli strains, as they help these organisms to adhere to the host surfaces, invade host cells and tissues, avoid host defense mechanisms, and incite noxious inflammatory responses, thereby causing clinical diseases [11].
Regarding the fact that DEC is widespread throughout the world, high prevalence rates of DEC were found among childhood diarrhea in Kenya (55.9%) [12], Peruvian (43.0%), coastal periurban areas of Peru (31.5%) [13], and rural South Africa (40.8%) [14]. Moreover, moderate prevalence rates were found in Mexico (23%) [15], Vietnam (22.5%) [16], and Indonesia (21%) [12]. On the other hand, the prevalence rate was low in Tanzania (10%) [17]. The variation in prevalence rates may be attributed to climate change adaptation, as the prevalence rate increases by 5% when the temperature increases by one degree [18].
The role of antibiotic misuse in developing the resistance among E. coli strains cannot be ignored. It is caused by the absence of restricted regulation of the administration of antimicrobials, in addition to some bad habits, such as unwarranted and self-prescription [19]. The widespread resistance among E. coli strains might be a result of soil, aquatic, and environmental contamination by untreated manure or sewage coming from animals or humans treated with several antibiotics [20], as most antimicrobial agents are not fully eliminated during the treatment process of sewage [21]. Therefore, soil and aquatic environments could facilitate the dissemination of resistance genes among E. coli strains [22], especially in developing countries.
It is noteworthy that the possibility of the simultaneous transmission of virulence genes with resistance genes in mobile genetic materials may induce the emergence of new pathogenic strains [23]. The infections with these new strains are particularly problematic, due to the lack of susceptibility to antibiotic medications and the extraordinary ability to express a large repertoire of virulence genes. Therefore, next-generation therapies and innovative drug repurposing are urgently needed [24,25,26]. In light of the above-mentioned threats, the present study was designed to elucidate the phylogenetic analysis depending on phenotypic and genotypic characterization of MDR and multi-virulent diarrheagenic E. coli (DEC) isolates from several sources, to gain a clear picture of the epidemic situation of these strains in Egypt.
2. Results
2.1. Phenotypic Characterization of DEC
Out of 350 samples collected from diarrhetic horses, cows, and humans, 140 (40%) DEC isolates were detected on the basis of their biochemical and cultural properties. The results of API 20E system and PCR amplification of the 16SrRNA gene were in agreement with those of conventional methods. The highest prevalence rate of DEC (53.3%, 80/150) was recorded among human samples. A sum of 35 and 25 DEC isolates was recovered from cow and horse diarrheal stool specimens, with isolation percentages of 35 and 25%, respectively. The results of the serogroup analysis using specific polyvalent and monovalent O antisera revealed seven different serotypes (O26, O44, O55, O151, O125, O145 and O1) among 119 E. coli isolates; meanwhile, 21 isolates (15%) were untypeable. Notably, E. coli O26 was the most predominant serotype (25%, 35/140) distinguished from the typable strains. As for the distribution of other serotypes, both O44 and O55 were detected with a high frequency (15% each), followed by O1 and O125 serotypes (10% each). On the other hand, serotypes O151 and O145 were detected with a low percentage (5% each, 7/140). The distribution of various serotypes represented among the 140 DEC isolates is shown in Figure 1, Figure 2A and Figure S1.
Figure 1.
Percentages of antimicrobial resistance, virulence genes and serotypes in diarrheagenic E. coli isolates. AMP: ampicillin, CPZ: cefoperazone, FOX: cefoxitin, AMC: amoxicillin/clavulanic acid, ATM: aztreonam, CPM: cefepime, TZP: piperacillin/tazobactam, IPM: imipenem, C: chloramphenicol, E: erythromycin, CN: gentamycin, CIP: ciprofloxacin, SXT: sulfamethoxazole/trimethoprim, and TE: tetracycline. Genes encoding for adhesion (fimH and kpsM), enterotoxin (astA), intimin (eaeA), Vero toxins (vtx), Shiga toxins (stx), hemolysin (hly), outer membrane protein (omp) and transcriptional activators for aggregative adherence fimbriae (aggR) and invasion (invE) genes.
Figure 2.
Distribution of serotypes (A), antimicrobial resistance (B), virulence genes (C) and multidrug resistance and multi-virulence patterns (D) among diarrheagenic E. coli isolates from human and animal sources. FOX: cefoxitin, AMP: ampicillin, ATM: aztreonam, AMC: amoxicillin/clavulanic acid, CPZ: cefoperazone, CPM: cefepime, TZP: piperacillin/tazobactam, IPM: imipenem, E: erythromycin, CIP: ciprofloxacin, C: chloramphenicol, SXT: sulfamethoxazole/trimethoprim, CN: gentamycin, and TE: tetracycline. Genes encoding for adhesion (fimH and kpsM), enterotoxin (astA), intimin (eaeA), Vero toxins (vtx), Shiga toxins (stx), hemolysin (hly), outer membrane protein (omp) and transcriptional activators for aggregative adherence fimbriae (aggR) and invasion (invE) genes.
2.2. Antimicrobial Susceptibility Patterns
The antimicrobial susceptibility patterns of all the recovered DEC isolates revealed that the highest resistance rates were shown in ampicillin, followed by cefoxitin (89.3 and 85.7%, respectively). On the other hand, imipenem represented the most effective antibiotic against many of the DEC isolates (59.9%), followed by ciprofloxacin (46.4%) (Figure 1 and Figure S2). The analyzed data demonstrated that there were significant differences in the resistance patterns of most of the recovered DEC isolates towards different antimicrobials (p < 0.005). Unreasonably, 90% (126/140) of the investigated E. coli isolates showed the MDR patterns. Taken together, an analysis of our data provided further evidence of the high prevalence of antimicrobial resistance among human isolates, as illustrated in Figure 2B. Confirming this context, 3.7 and 21.7% of the human and animal isolates showed the non-MDR patterns, respectively. Surprisingly, both the human and animal isolates could be treated with imipenem, ciprofloxacin and piperacillin/tazobactam, as shown in Figure 2B. The multiple antibiotic resistance (MAR) index of the isolates ranged from 0.07 to 1 (resistant to all the tested antimicrobials). Five out of eight isolates with an MAR index of one belonged to human isolates. It was demonstrated that the MAR index of the antimicrobials varied from 0.030 to 0.064. The MAR index was found to be the highest for ampicillin (0.064), in contrast to imipenem (0.030).
2.3. Virulence Gene Profiles
Concerning PCR determination of DEC virulence genes, both ompA and fimH genes were detected in all the tested isolates, but none of these isolates carried kpsMTII, hly and vt2e genes (Figure 1 and Figure 2C). Most commonly, the astA gene was detected among 43.6% of the DEC isolates; meanwhile, only 11 isolates (7.9%) harbored the invE gene. The distribution of virulence genes among human and animal DEC isolates is illustrated in Figure 2C and Figure S3. Interestingly, the virulence genes were identified in animal isolates more often than in human isolates. The majority of the human and animal isolates were categorized, depending on the presence of virulence genes, as multi-virulent, as well as being MDR (Figure 2D).
2.4. Correlation between Serotypes, Existence of Virulence Genes and Antimicrobial Resistance
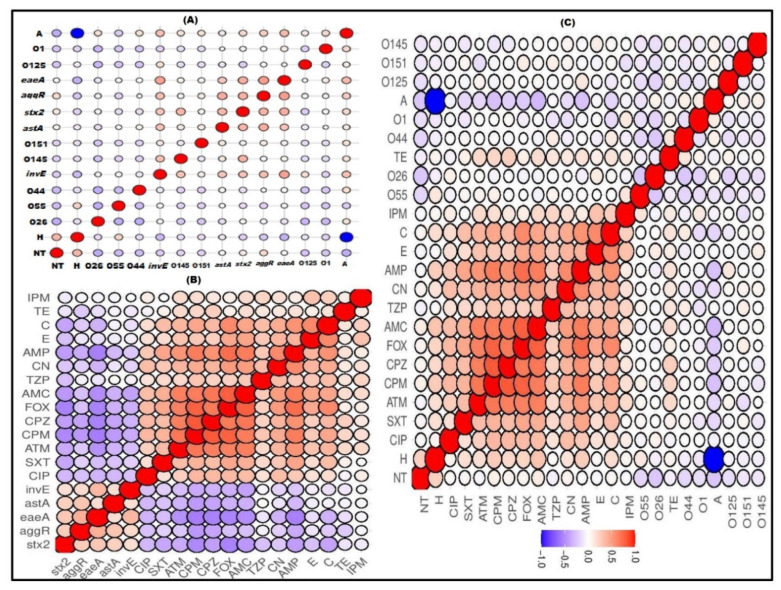
Regarding the correlation between DEC serotypes and their virulence genes, the existence of invE and stx2 genes was observed to be positively correlated with the O145 serotype (R = 0.27 and 0.41, respectively). Meanwhile, their frequency was noted to have a slight negative correlation with the O44 serotype, as shown in Figure 3A and Figure S4A. Interestingly, a significant negative correlation (R = −0.5:−1, p < 0.05) between antimicrobial resistance and the existence of virulence genes was observed (Figure 3B and Figure S4B). The correlation among antimicrobial resistance revealed an overall significant positive correlation (R = 0.5:1, p < 0.05) among ampicillin, amoxicillin/clavulanic acid, aztreonam, cefoxitin, cefoperazone, and cefepime, as observed in Figure 3B,C. According to Figure 3C and Figure S4C, there was no correlation between the DEC serotypes and their antimicrobial resistance.
Figure 3.
Correlation (R) between serotypes and virulence genes (A), virulence genes and antimicrobial resistance (B), and serotypes and antimicrobial resistance (C) of diarrheagenic E. coli. Blue and red colors indicate positive and negative correlation, respectively. The degree of correlation coefficient (R) is represented by the color key. The darker blue and red colors imply stronger negative (R = −0.5:−1) and positive (R = 0.5:1) correlations, respectively. Genes encoding for adhesion (fimH and kpsM), enterotoxin (astA), intimin (eaeA), Vero toxins (vtx), Shiga toxins (stx), hemolysin (hly), outer membrane protein (omp) and transcriptional activators for aggregative adherence fimbriae (aggR) and invasion (invE) genes. H; human, A; animal, AMP: ampicillin, AMC: amoxicillin/clavulanic acid, ATM: aztreonam, FOX: cefoxitin, CPZ: cefoperazone, CPM: cefepime, TZP: piperacillin/tazobactam, IPM: imipenem, C: chloramphenicol, E: erythromycin, CN: gentamycin, CIP: ciprofloxacin, SXT: sulfamethoxazole/trimethoprim, and TE: tetracycline.
Based on serotyping, antimicrobial resistance and virulence gene profiles, all the tested DEC isolates (140), with the exception of 14 isolates (3 pairs of human and equine isolates, 2 pairs of human isolates, and 2 pairs of human and cow isolates), could be typed into different lineages, as shown in Figure 4.
Figure 4.
Heat map for the investigated diarrheagenic E. coli isolates, depending on the existence of virulence genes, antimicrobial resistance, and serotypes. The blue and red colors represent the sensitivity and resistance to particular antimicrobial drugs, and the absence and presence of certain serotype and virulence genes, respectively. The DEC isolates from human (H), cow (C) and equine (E) sources are coded on the right of the heat map. AMP: ampicillin, CPZ: cefoperazone, AMC: amoxicillin/clavulanic acid, CPM: cefepime, ATM: aztreonam, E: erythromycin, FOX: cefoxitin, TZP: piperacillin/tazobactam, IPM: imipenem, C: chloramphenicol, CN: gentamycin, CIP: ciprofloxacin, SXT: sulfamethoxazole/trimethoprim, and TE: tetracycline.
3. Discussion
One of the most important worldwide crises is the wide spreading of infections with resistant microorganisms, especially multidrug-resistant (MDR) bacterial and fungal pathogens [23,27,28,29,30]. These crises were compounded by the evolution of both microbial resistance and virulence [31]. Recently, many reports have announced the increase in foodborne infections, which are attributed to resistant animal pathogens with multi-virulence arrays [32,33,34]. In this context, many health-associated problems were linked to DEC, which can be transmitted through several food chains of animal origin. Unfortunately, there were not enough details about the phylogenetic analysis of animal and human DEC. Scarce reports were available regarding the correlation analysis within and among different DEC serotypes, especially in Egypt. Therefore, this study has focused on the great emergence and heterogeneity of MDR/multi-virulent DEC serotypes among human and animal hosts.
The occurrence of E. coli was recorded in several reports among different countries, depending on the disparity in sample types, sampling schemes, geographic location, detection protocols, and seasonal and environmental factors. In this study, the prevalence rate of E. coli (40%) was in accordance with that observed in Bangladesh (41%) [35]; however, it was much higher in comparison with those recorded in several studies carried out in Egypt (23%) [36] and Zimbabwe (20%) [37]. Regarding the serotyping results, seven different serotypes, O26, O44, O55, O151, O125, O145 and O1, were detected, with the predominance of the O26 serotype (25%). Of note, there is a variation in the serotypes of E. coli among different studies worldwide [30,38]. This variation can be accepted on the basis of the positive correlation between E. coli serotypes and the types of diseases or clinical forms of their infections.
Studying the epidemiology, virulence, and antimicrobial resistance of E. coli serotypes, in addition to their correlation, is essential to improve the control and treatment of this pathogen. In this context, and based on the antimicrobial susceptibility results of E. coli, the maximum antimicrobial resistance was recorded towards ampicillin and cefoxitin. In other studies, higher resistance rates have been announced to tetracycline in Brazil [39], Egypt [37], and Korea [40]. Meanwhile, in India [41] and Egypt [38], higher prevalence rates of E. coli resistance were detected to erythromycin and streptomycin. These variations in antimicrobial resistance may be associated with the type of prescribed antimicrobial agents in various hospitals, alongside different geographical areas. Additionally, the antimicrobial usage in animal production and the variations in legislation that guide the use of antibiotics from one region to another may lead to these variations [42]. Unfortunately, the current study indicated an alarmingly high prevalence of MDR (90%) among the tested E. coli strains, in addition to their high MAR indices, especially for the human isolates. Therefore, continuous surveillance for antimicrobial resistance among E. coli strains is urgently needed to avoid treatment failure.
Virulence factors and antimicrobial resistance are necessary to overcome the host immune response and enhance the ability of bacteria to survive under adverse conditions [43]. The presence of multiple virulence factors, as observed in our isolates, may exacerbate this issue. Alarmingly, the vast majority of our human and animal isolates were MDR, as well as being multi-virulent. This reflects the evolution of the epidemic situation in Egypt. Our results showed high diversity among, and within, animal and human DEC isolates. There were no similarities between the profiles of human and animal strains, as they were clustered in different lineages. Therefore, the findings of this manuscript did not support the possibility of zoonotic transmission among our isolates. The lack of host adaptation, in addition to the chance of contamination between both hosts and the heterogeneous nature of the E. coli genome, may have led to this result [42].
The correlation between antimicrobial resistance and virulence production is difficult to understand, due to the complex phylogenetic background of the bacteria, which depends on several factors, such as the bacterial species, host, ecological niche, and virulence and resistance mechanisms [43]. We observed a strong negative correlation between antimicrobial resistance and virulence gene profiles. Several previous studies announced that the acquisition of antibiotic resistance leads to a fitness cost [44,45]. These results strengthen the hypothesis that the loss of pathogenic fitness and virulence potential has always been associated with the acquisition of antimicrobial resistance [46]. In the same context, there was no absolute correlation between E. coli serotypes and antimicrobial resistance. Meanwhile, a positive correlation between some serotypes and the existence of certain virulence factors was observed. Consistent with our results, several reports announced that there was an association between E. coli serotypes and some virulence genes [47,48]. Moreover, another study demonstrated the diversity of virulence genes and serotypes among E. coli strains [48]. In contrast to our findings, several studies found a significant relationship between certain microbial serotypes and antimicrobial resistance [49,50,51]. This indicates that there is no fixed or absolute correlation between serotypes and antimicrobial resistance and virulence production. Furthermore, there is high genetic variation within each E. coli serotype, as the strains within a single serotype belong to different lineages or genotypes. This supports the evidence that there is no single factor responsible for the virulence and resistance of E. coli strains, and their expression may be affected by several environmental conditions.
4. Materials and Methods
4.1. Study Design and DEC Isolation and Identification
This is a retrospective study that was conducted on 350 stool samples collected from diarrhetic animals (horses (100) and cows (100)) and humans (150) from both university and private hospitals in Port Said and Sharkia Governorates. All the collected samples were initially inoculated into brain heart infusion broth (Oxoid, UK) to enhance the growth of E. coli. A loopful from the cultured broth was inoculated into MacConkey and eosin methylene blue agar media (Oxoid, UK), and then the plates were incubated at 37 °C for 24 h. Suspected E. coli isolates were identified according to their cultural and biochemical characteristics, such as citrate utilization, indole production and reactions on triple sugar iron agar medium (Oxoid, UK) [52]. Confirmation of E. coli isolates was performed using API 20E strips (BioMérieux, Mary l’Etoile, France) and specific polymerase chain reaction (PCR) amplification of the 16S rRNA gene [53]. The following cycle condition was used to amplify ECP79F-ECR620R regions of the 16S rRNA gene: pre-heating at 95 °C for 5 min, then forty cycles of 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 1.5 min, and a final extension for 5 min at 72 °C. E. coli ATCC 25,922 and Staphylococcus aureus ATCC 25,923 were used as positive and negative controls, respectively.
4.2. Serotyping
The serotyping of DEC strains was performed by an agglutination test using polyvalent and monovalent O-specific antisera (Test Sera Enteroclon, Anti–Coli, Germany), according to the manufacturer’s recommendations. Briefly, a clean glass slide was divided into 2 sections by a marker pin, and then one drop of polyvalent O-specific antisera (test) and one drop of physiological saline (control) were added to each section. One drop of DEC suspension was mixed with the test and control drops. The slide was tilted and the agglutination was recorded. This test was repeated using monovalent O-specific antisera instead of the polyvalent serum, which agglutinated.
4.3. Antimicrobial Susceptibility Testing
In vitro determination of the susceptibility patterns of all the recovered DEC isolates to various antimicrobials was conducted, adopting the Kirby–Bauer disc diffusion method using Mueller–Hinton agar and standard antimicrobial discs, according to the Clinical and Laboratory Standards Institute (CLSI) recommendations [54]. The antimicrobial agents tested were ampicillin (AMP), cefoperazone (CPZ), cefoxitin (FOX), amoxicillin/clavulanic acid (AMC), aztreonam (ATM), piperacillin/tazobactam (TZP), chloramphenicol (C), imipenem (IPM), gentamycin (CN), ciprofloxacin (CIP), sulfamethoxazole/trimethoprim (SXT), erythromycin (E), cefepime (CPM), and tetracycline (TE). Susceptibility to antimicrobials using the broth microdilution method [55] was also determined for all DEC isolates, to check the results of disc susceptibility testing. The isolates that showed non-susceptibility patterns to at least one agent in three or more different classes of antimicrobial agents were defined as MDR [56].
4.4. Determination of Multiple Antibiotic Resistance (MAR) Indices
The multiple antibiotic resistance (MAR) index is one of the tools that reveals the spread of resistant pathogens in a given population. It is a good way to identify the relative risk for the source of contamination, as the multiple and abuse of the antibiotic were recorded when the MAR was >0.2 [1]. The MAR index values for each isolate and antimicrobial were calculated [57,58] according to the following formulas: MAR index for the isolate = number of antimicrobials to which the isolate was resistant/number of tested antimicrobials. MAR index for the antimicrobial = number of antimicrobial-resistant isolates/number of antimicrobials x number of isolates.
4.5. Virulence Genotyping of E. coli
The total bacterial genomic DNA was isolated from DEC isolates using QIAamp DNA Mini Kit (QIAGEN GmbH, Hilden, Germany). Specific PCR assays were then performed to screen for the presence of some virulence genes using specific primers and thermal cycling conditions described elsewhere [59,60,61,62,63,64,65]. The gene-specific primer sequences, as well as the amplicon sizes, are listed in Table 1. In each PCR run, appropriate positive and negative controls were included. The amplicons were then analyzed by agarose gel electrophoresis.
Table 1.
Primer sequences and amplicon sizes of the corresponding virulence genes of DEC.
| Target Virulence | Primers Sequences (5′-3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|
| ompA | F: AGCTATCGCGATTGCAGTG | 919 | 59 |
| R: GGTGTTGCCAGTAACCGG | |||
| kpsMTII | F: CAGGTAGCGTCGAACTGTA | 280 | 59 |
| R: CATCCAGACGATAAGCATGAGCA | |||
| hly | F: AACAAGGATAAGCACTGTTCTGGCT | 1177 | 60 |
| R: ACCATATAAGCGGTCATTCCCGTCA | |||
| stx2 | F: CCATGACAACGGACAGCAGTT | 779 | 61 |
| R: CCTGTCAACTGAGCAGCACTTTG | |||
| fimH | F: TGCAGAACGGATAAGCCGTGG | 508 | 62 |
| R: GCAGTCACCTGCCCTCCGGTA | |||
| vt2e | F: CCT TAA CTA AAA GGA ATA TA | 230 | 63 |
| R: CTG GTG GTG TAT GAT TAA TA | |||
| astA | F: TGCCATCAACACAGTATATCC | 116 | 64 |
| R: TCAGGTCGCGAGTGACGGC | |||
| invE | F: CGATAGATGGCGAGAAATTATATCCCG | 766 | 65 |
| R:CGATCAAGAATCCCTAACAGAAGAATCAC |
4.6. Statistical Analysis
The antimicrobial susceptibility data were expressed as percentages. The significant differences in the levels of resistance among the selected antimicrobials were determined depending on two-way analysis of variance (ANOVA), without replication. The P value of <0.05 was considered to be statistically significant. All statistical and correlation analyses were carried out using the R package corrplot, heatmaply, hmisc, ggpubr and GraphPad Prism (version 6; GraphPad Software Inc.; San Diego, CA, USA).
5. Conclusions
The heterogeneity of our DEC isolates among both human and animal hosts was an indication of the need for more restricted guidelines, in order to prevent the evolution of this pathogen. Furthermore, new alternative therapies are urgently needed, due to the wide spreading of MDR phenotypes, which increases the possibility of treatment failure. We found that the human isolates exhibited high resistance patterns; meanwhile, the animal isolates were more virulent. Additionally, high MAR indices were recorded to the prescribed antibiotics within each hospital in this study. These findings were consistent with the hypothesis that the acquisition of antimicrobial resistance is always associated with the loss of bacterial fitness, and the prescribed antibiotics were consistently associated with the microbial resistance patterns. Finally, our results presented an indication of the diversity of E. coli isolates, but we did not support the possibility of its zoonotic transmission. Therefore, further investigations are needed to gather more information about the epidemiological aspects of DEC isolates, to identify the potential sources of their infections and to implement control measures for preventing their further spread.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics11050552/s1: Figure S1: Heat map showing the occurrence of serotypes in the studied diarrheagenic E. coli isolates. Figure S2: Heat map showing the occurrence of antimicrobial resistance in the studied diarrheagenic E. coli isolates. Figure S3: Heat map showing the occurrence of virulence genes in the studied diarrheagenic E. coli isolates. Figure S4: Correlation coefficient values (R) between serotypes and virulence genes (A), antimicrobial resistance and virulence genes (B), and serotypes and antimicrobial resistance (C) of diarrheagenic E. coli.
Author Contributions
Methodology and writing—review and editing, M.M.B., M.I.A.-H.; conceptualization and formal analysis, W.H.M., N.N.O. and M.M.A.-S.; software, W.A.A.; data curation, and validation, N.N.O., M.A., A.S.A. and M.M.B.; writing—original draft preparation and visualization, H.M.A. and R.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
Authors would like to acknowledge Taif University Researchers Supporting project number (TURSP 2020/257), Taif University, Taif, Saudi Arabia.
Institutional Review Board Statement
All the clinical samples from the patients were obtained with the sole aim to undergo antimicrobial susceptibility testing, to allow proper treatment. Therefore, ethical approval for this work was not necessary; however, the informed consents of participants were obtained.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data in this study are presented in the submitted manuscript, and in the supplementary figures.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davis R., Brown P.D. Mltiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J. Med. Microbiol. 2016;65:261–271. doi: 10.1099/jmm.0.000229. [DOI] [PubMed] [Google Scholar]
- 2.Croxen M., Finlay B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 3.Awad N.S., Abd El-Hamid M.I., Hashem Y.M., Erfan A.M., Abdelrahman B.A., Mahmoud H.I. Impact of single and mixed infections with Escherichia coli and Mycoplasma gallisepticum on Newcastle disease virus vaccine performance in broiler chickens: An in vivo perspective. J. Appl. Microbiol. 2019;127:396–405. doi: 10.1111/jam.14303. [DOI] [PubMed] [Google Scholar]
- 4.Jang J., Hur H.G., Sadowsky M.J., Byappanahalli M.N., Yan T., Ishii S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017;123:570–581. doi: 10.1111/jam.13468. [DOI] [PubMed] [Google Scholar]
- 5.Koczura R., Mokracka J., Jablonska L., Gozdecka E., Kubek M., Kaznowski A. Antimicrobial resistance of integron-harboring Escherichia coli isolates from clinical samples, wastewater treatment plant and river water. Sci. Total Environ. 2012;414:680–685. doi: 10.1016/j.scitotenv.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Hamelin K., Bruant G., El-Shaarawi A., Hill S., Edge T.A., Fairbrother J., Harel J., Maynard C., Masson L., Brousseau R. Occurrence of virulence and antimicrobial resistance genes in Escherichia coli isolates from different aquatic ecosystems within the St. Clair River and Detroit River areas. Appl. Environ. Microbiol. 2007;73:477–484. doi: 10.1128/AEM.01445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasko D.A., Rosovitz M.J., Myers G.S., Mongodin E.F., Fricke W.F., Gajer P., Crabtree J., Sebaihia M., Thomson N.R., Chaudhuri R., et al. The pangenome structure of Escherichia coli: Comparative genomic analysis of E. coli commensal and pathogenic isolates. J. Bacteriol. 2008;190:6881–6893. doi: 10.1128/JB.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broach W.H., Egan N., Wing H.J., Payne S.M., Murphy E.R. VirF-independent regulation of Shigella virB transcription is mediated by the small RNA RyhB. PLoS ONE. 2012;7:e33529. doi: 10.1371/journal.pone.0038592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Stanford K., McAllister T.A., Johnson R.P., Chen J., Hou H., Zhang G., Niu Y.D. Biofilm formation, virulence gene profiles, and antimicrobial resistance of nine serogroups of non-O157 Shiga toxin–producing Escherichia coli. Foodborne Pathog Dis. 2016;13:316–324. doi: 10.1089/fpd.2015.2099. [DOI] [PubMed] [Google Scholar]
- 10.Sarowska J., Futoma-Koloch B., Jama-Kmiecik A., Frej-Madrzak M., Ksiazczyk M., Bugla-Ploskonska G., Choroszy-Krol I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019;11:10. doi: 10.1186/s13099-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson J.R., O’Bryan T.T., Kuskowski M., Maslow J.N. Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect Immun. 2001;69:5363–5374. doi: 10.1128/IAI.69.9.5363-5374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima Y., Oundo J.O., Hibino T., Saidi S.M., Hinenoya A., Osawa K., Shirakawa T., Osawa R., Yamasaki S. High Prevalence of Diarrheagenic Escherichia coli among Children with Diarrhea in Kenya. Jpn. J. Infect. Dis. 2017;24:80–83. doi: 10.7883/yoken.JJID.2016.064. [DOI] [PubMed] [Google Scholar]
- 13.Acosta G.J., Vigo N.I., Durand D., Riveros M., Arango S., Zambruni M., Ochoa T.J. Diarrheagenic Escherichia coli: Prevalence and Pathotype Distribution in Children from Peruvian Rural Communities. Am. J. Trop. Med. Hyg. 2016;7:574–579. doi: 10.4269/ajtmh.16-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qato M., Como N., Meta E., Pipero P., Ostreni V., Kraja D. Prevalence of diarrheagenic Escherichia coli in young children from rural South Africa: The Mal-ED cohort. Int. J. Infect. Dis. 2014;21:149. doi: 10.1016/j.ijid.2014.03.734. [DOI] [Google Scholar]
- 15.Canizalez-Roman A., Flores-Villaseñor H.M., Gonzalez-Nuñez E., Velazquez-Roman J., Vidal J.E., Muro-Amador S., Alapizco-Castro G., Díaz-Quiñonez J.A., León-Sicairos N. Surveillance of Diarrheagenic Escherichia coli Strains Isolated from Diarrhea Cases from Children, Adults and Elderly at Northwest of Mexico. Front. Microbiol. 2016;7:1924. doi: 10.3389/fmicb.2016.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen T.V., Le-Van P., Le-Huy C., Gia K.N., Weintraub A. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J. Clin. Microbiol. 2005;43:755–760. doi: 10.1128/JCM.43.2.755-760.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gascón J., Vargas M., Schellenberg D., Urassa H., Casals C., Kahigwa E., Aponte J.J., Mshinda H., Vila J. Diarrhea in children under 5 years of age from Ifakara, Tanzania: A case-control study. J. Clin. Microbiol. 2000;38:4459–4462. doi: 10.1128/JCM.38.12.4459-4462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebecca P., Sharia M., Berry J., Brosi K.L. Climatic Drivers of Diarrheagenic Escherichia coli Incidence: A Systematic Review and Meta-analysis. J. Infect. Dis. 2016;214:6–15. doi: 10.1093/infdis/jiw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaidi M.B., Zamora E., Diaz P., Tollefson L., Fedorka-Cray P.J., Headrick M.L. Risk factors for fecal quinolone-resistant Escherichia coli in Mexican children. Antimicrob. Agents Chemother. 2003;47:1999–2001. doi: 10.1128/AAC.47.6.1999-2001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baquero F., Martinez J.L., Canton R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008;19:260–265. doi: 10.1016/j.copbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Hegstad K., Langsrud S., Lunestad B.T., Scheie A.A., Sunde M., Yazdankhah S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010;16:91–104. doi: 10.1089/mdr.2009.0120. [DOI] [PubMed] [Google Scholar]
- 22.Lupo A., Coyne S., Berendonk T.U. Origin and evolution of antibiotic resistance: The common mechanisms of emergence and spread in water bodies. Front. Microbiol. 2012;3:18. doi: 10.3389/fmicb.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen B., Zheng W., Yu Y., Huang W., Zheng S., Zhang Y., Guan X., Zhuang Y., Chen N., Topp E. Class 1 integrons, selected virulence genes, and antibiotic resistance in Escherichia coli isolates from the Minjiang River, Fujian Province, China. Appl. Environ. Microbiol. 2011;77:148–155. doi: 10.1128/AEM.01676-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegazy W.H., Khayat M.T., Ibrahim T.S., Nassar M.S., Bakhrebah M.A., Abdulaal W.H., Alhakamy N.A., Bendary M.M. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganisms. 2020;22:1285. doi: 10.3390/microorganisms8091285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elfaky M.A., Abdel-Hamid M.I., Khalifa E., Alshareef W.A., Mosbah R.A., Elazab S.T., Ghoneim M.M., Al-Sanea M.M., Bendary M.M. Innovative next-generation therapies in combating multi-drug-resistant and multi-virulent Escherichia coli isolates: Insights from in vitro, in vivo, and molecular docking studies. Appl. Microbiol. Biotechnol. 2022;106:1691–1703. doi: 10.1007/s00253-022-11781-w. [DOI] [PubMed] [Google Scholar]
- 26.Ghaly M.F., Nasr Z.M., Abousaty A.I., Seadawy H.G., Shaheen M.A.A., Albogami S., Al-Sanea M.M., Bendary M.M. Alternative and Complementary Therapies against Foodborne Salmonella Infections. Antibiotics. 2021;10:1453. doi: 10.3390/antibiotics10121453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammar A.M., Abd El-Hamid M.I., El-Malt R.M.S., Azab D.S., Albogami S., Al-Sanea M.M., Soliman W.E., Ghoneim M.M., Bendary M.M. Molecular Detection of Fluoroquinolone Resistance among Multidrug-, Extensively Drug-, and Pan-Drug-Resistant Campylobacter Species in Egypt. Antibiotics. 2021;10:1342. doi: 10.3390/antibiotics10111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ammar A.M., Attia A.M., Abd El-Hamid M.I., El-Shorbagy I.M., Abd El-Kader S.A. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell. Mol. Biol. 2016;62:7–15. [PubMed] [Google Scholar]
- 29.Bendary M.M., Ibrahim D., Mosbah R.A., Mosallam F., Hegazy W.A.H., Awad N.F., Alshareef W.A., Alomar S.Y., Zaitone S.A., Abd El-Hamid M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics. 2020;10:25. doi: 10.3390/antibiotics10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghaly M., Shaheen A., Bouhy A., Bendary M. Alternative therapy to manage otitis media caused by multidrug-resistant fungi. Arch. Microbiol. 2020;202:1231–1240. doi: 10.1007/s00203-020-01832-z. [DOI] [PubMed] [Google Scholar]
- 31.Ghaith D.M., Zafer M.M., Said H.M., Elanwary S., Elsaban S., Al-Agamy M.H., Bohol M.F.F., Bendary M.M., Al-Qahtani A., Al-Ahdal M.N. Genetic diversity of carbapenem-resistant Klebsiella Pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 2019;39:583–591. doi: 10.1007/s10096-019-03761-2. [DOI] [PubMed] [Google Scholar]
- 32.Abd El-Hamid M.I., Bendary M.M., Merwad A.M., Elsohaby I., Ghaith D.M., Alshareef W.A. What is behind phylogenetic analysis of hospital, community and livestock associated methicillin-resistant Staphylococcus aureus? Transbound Emerg. Dis. 2019;66:1506–1517. doi: 10.1111/tbed.13170. [DOI] [PubMed] [Google Scholar]
- 33.Abd El-Aziz N.K., Abd El-Hamid M.I., Bendary M.M., El-Azazy A.A., Ammar A.M. Existence of vancomycin resistance among methicillin resistant S. aurues recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018;55:221–230. [Google Scholar]
- 34.Abd El-Hamid M.I., Abd El-Aziz N.K., Samir M., El-Naenaeey E.Y., Abo Remela E.M., Mosbah R.A., Bendary M.M. Genetic Diversity of Campylobacter jejuni Isolated from Avian and Human Sources in Egypt. Front. Microbiol. 2019;10:2353. doi: 10.3389/fmicb.2019.02353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman M.A., Samad M.A., Kabir M.L. Bacterio-pathological studies on salmonellosis, colibacillosis and pasteurellosis in natural and experimental infections in chickens. Bangladesh J. Vet. Med. 2004;2:1–8. doi: 10.3329/bjvm.v2i1.1926. [DOI] [Google Scholar]
- 36.Ammar A.M., Abdel-hamid M.I., Eid S.E., El-Oks S. Insights into antimicrobial resistance and virulence genes of emergent multidrug resistant avian pathogenic Escherichia coli in Egypt: How closely related are they? Rev. Med. Vet. 2015;166:304–314. [Google Scholar]
- 37.Saidi B., Mafirakureva P., Mbanga J. Antimicrobial resistance of E. coli isolated from chickens with colibacillosis in and around Harare, Zimbabwe. Avian Dis. 2013;57:152–154. doi: 10.1637/10325-081512-Case.1. [DOI] [PubMed] [Google Scholar]
- 38.Eid S.E., Erfan A.M. Characterization of E. coli associated with high mortality of poultry flocks. Assiut Vet. Med. J. 2013;59:51–61. [Google Scholar]
- 39.Lima-filho J.V., Martins L.V., Nascimento D.C., Venturea R.F., Batista J.E., Silva A.F., Ralph M.T., Vazr V., Rabello C.B., Silva M., et al. Zoonotic potential of multidrug-resistant extraintestinal pathogenic E. coli obtained from healthy poultry carcasses in Salvador, Brazil. Braz. J. Infect. Dis. 2013;17:54–61. doi: 10.1016/j.bjid.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T.E., Jeong Y.W., Cho S.H., Kim S.J., Kwon H.J. Chronological study of antibiotic resistances and their relevant genes in Korean avian pathogenic E. coli isolates. J. Clin. Microbiol. 2007;45:3309–3315. doi: 10.1128/JCM.01922-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharada R., Ruban S.W., Thiyageeswaran M. Isolation, characterization and antibiotic resistance pattern of E. coli isolated from poultry. Am.-Eurasian J. Sci. Res. 2010;5:18–22. [Google Scholar]
- 42.Bendary M.M., Solyman S.M., Azab M.M., Mahmoud N.F., Hanora A.M. Characterization of Methicillin Resistant Staphylococcus aureus isolated from human and animal samples in Egypt. Cell. Mol. Biol. 2016;29:94–100. [PubMed] [Google Scholar]
- 43.Beceiro A., Tomás M., Bou G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013;26:185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogwill T., MacLean R.C. The genetic basis of the fitness costs of antimicrobial resistance: A meta-analysis approach. Evol. Appl. 2015;8:284–295. doi: 10.1111/eva.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bendary M.M., Solyman S.M., Azab M.M., Mahmoud N.F., Hanora A.M. Genetic diversity of multidrug resistant Staphylococcus aureus isolated from clinical and non clinical samples in Egypt. Cell. Mol. Biol. 2016;62:55. [PubMed] [Google Scholar]
- 46.Boerlin P., McEwen S.A., Boerlin-Petzold F., Wilson J.B., Johnson R.P., Gyles C.L. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 1999;37:497–503. doi: 10.1128/JCM.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kannika K., Pisuttharachai D., Srisapoome P., Wongtavatchai J., Kondo H., Hirono I., Unajak S., Areechon N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017;122:1497–1507. doi: 10.1111/jam.13447. [DOI] [PubMed] [Google Scholar]
- 48.Cho S.H., Oh K.H., Kim S.H., Oh H.B., Park M.S. Distribution of Virulence Genes and Their Association of Serotypes in Pathogenic Escherichia coli Isolates From Diarrheal Patients in Korea. Osong Public Health Res. Perspect. 2010;1:29–35. doi: 10.1016/j.phrp.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De-Francesco M.A., Caracciolo S., Gargiulo F., Manca N. Phenotypes, genotypes, serotypes and molecular epidemiology of erythromycin-resistant Streptococcus agalactiae in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:1741–1747. doi: 10.1007/s10096-011-1495-4. [DOI] [PubMed] [Google Scholar]
- 50.Schuab R.B., Arêas G.P., Souza V.C., Barros R.R. Molecular epidemiology of Streptococcus agalactiae recovered from significant bacteriuria. Infect. Dis. 2015;47:637–642. doi: 10.3109/23744235.2015.1040446. [DOI] [PubMed] [Google Scholar]
- 51.Yan Y., Hu H., Lu T., Fan H., Hu Y., Li G., Zhang X., Shi Y., Xia R. Investigation of serotype distribution and resistance genes profile in group B Streptococcus isolated from pregnant women: A Chinese multicenter cohort study. APMIS. 2016;124:794–799. doi: 10.1111/apm.12570. [DOI] [PubMed] [Google Scholar]
- 52.Quinn P.J., Markey B.K., Carter M.E., Donnelly W.J.C., Lenard F.C. Veterinary Microbiology and Microbial Diseases. 1st ed. Blackwell Science Ltd.; Oxford, UK: 2002. [Google Scholar]
- 53.Sabat G., Rose P., Hickey W.J., Harkin J.M. Selective and sensitive method for PCR amplification of Escherichia coli 16S rRNA genes in soil. Appl. Environ. Microbiol. 2000;66:844–849. doi: 10.1128/AEM.66.2.844-849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2019. CLSI Document M100-S15; 15th International Supplement. [Google Scholar]
- 55.Clinical and Laboratory Standards Institute . M07: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. CLSI; Wayne, PA, USA: 2018. Approved Standard. [Google Scholar]
- 56.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 57.Krumperman P.H. Multiple Antibiotic Resistance Indexing of Escherichia coli to Identify High-Risk Sources of Fecal Contamination of Foods. Appl. Environ. Microbiol. 1983;46:165–170. doi: 10.1128/aem.46.1.165-170.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tambekar D., Dhanorkar D., Gulhane S., Khandelwal V., Dudhane M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006;5:1562–1565. doi: 10.4314/ajb.v5i17.43162. [DOI] [Google Scholar]
- 59.Ewers C., Li G., Wilking H., Kiebling S., Alt K., Antáo E.M., Laturnus C., Diehl I., Glodde S., Homeier T., et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Inter. J. Med. Microbiol. 2007;297:163–176. doi: 10.1016/j.ijmm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Piva I.C., Pereira A.L., Ferraz L.R., Silva R.S., Vieira A.C., Blanco J.E., Blanco M., Blanco J., Giugliano L.G. Virulence Markers of Enteroaggregative Escherichia coli Isolated from Children and Adults with Diarrhea in Brasília. Brazil. J. Clin. Microbiol. 2003;41:1827–1832. doi: 10.1128/JCM.41.5.1827-1832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dipineto L., Santaniello A., Fontanella M., Lagos K., Fioretti A., Menna L.F. Presence of Shiga toxin-producing Escherichia coli O157:H7 in living layer hens. Let. Appl. Microbiol. 2006;43:293–295. doi: 10.1111/j.1472-765X.2006.01954.x. [DOI] [PubMed] [Google Scholar]
- 62.Ghanbarpour R., Salehi M. Determination of Adhesin Encoding Genes in Escherichia coli Isolates from Omphalitis of Chicks. Am. J. Anim. Vet. Sci. 2010;5:91–96. doi: 10.3844/ajavsp.2010.91.96. [DOI] [Google Scholar]
- 63.Johnson W.M., Pollard D.R., Lior H., Tyler S.D., Rozee K.R. Differentiation of genes coding for Escherichia coli verotoxin 2 and the verotoxin associated with porcine edema disease (VTe) by the polymerase chain reaction. J. Clin. Microbiol. 1990;28:2351–2353. doi: 10.1128/jcm.28.10.2351-2353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ewers C., Janßen T., Kießling S., Philipp H.C., Wieler L.H. Rapid Detection of Virulence-Associated Genes in Avian Pathogenic Escherichia coli by Multiplex Polymerase Chain Reaction. Avian Dis. 2005;49:269–273. doi: 10.1637/7293-102604R. [DOI] [PubMed] [Google Scholar]
- 65.Yun Z., Zeng L., Huang W., Wu Q., Fan Y., Zheng S., Peng L., Han J., Huang Y., Zhou H., et al. Detection and Categorization of Diarrheagenic Escherichia coli with Auto-microfluidic Thin-film Chip Method. Sci. Rep. 2018;8:12926. doi: 10.1038/s41598-018-30765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in this study are presented in the submitted manuscript, and in the supplementary figures.