Abstract
The utilization of 1,2,4,5-tetrachloro-, 1,2,4-trichloro-, the three isomeric dichlorobenzenes and fructose as the sole carbon and energy sources at nanomolar concentrations was studied in batch experiments with Burkholderia sp. strain PS14. In liquid culture, all chlorobenzenes were metabolized within 1 h from their initial concentration of 500 nM to below their detection limits of 0.5 nM for 1,2,4,5-tetrachloro- and 1,2,4-trichlorobenzene and 7.5 nM for the three dichlorobenzene isomers, with 63% mineralization of the tetra- and trichloroisomers. Fructose at the same initial concentration was, in contrast, metabolized over a 4-h incubation period down to a residual concentration of approximately 125 nM with 38% mineralization during this time. In soil microcosms, Burkholderia sp. strain PS14 metabolized tetrachlorobenzene present at 64.8 ppb and trichlorobenzene present at 54.4 ppb over a 72-h incubation period to below the detection limits of 0.108 and 0.09 ppb, respectively, with approximately 80% mineralization. A high sorptive capacity of Burkholderia sp. strain PS14 for 1,2,4,5-tetrachlorobenzene was found at very low cell density. The results demonstrate that Burkholderia sp. strain PS14 exhibits a very high affinity for chlorobenzenes at nanomolar concentrations.
Chlorinated benzenes are widely used as solvents in chemical reactions and to dissolve materials, such as oils, waxes, resins, greases, and rubber, and are also employed in the production of herbicides and pesticides. They are highly toxic compounds that cause a wide variety of effects ranging from immunological disorders to adverse effects on the liver, kidney, thyroid, and lung, sometimes accompanied by porphyria (13, 14). Large amounts have been produced (e.g., in 1986 17,000 to 20,000 tons of 1,2-dichlorobenzene [1,2-DCB] and 25,000 tons of 1,4-dichlorobenzene [1,4-DCB] and in 1985 5,000 tons of 1,2,4-trichlorobenzene [1,2,4-TCB] were produced in the Federal Republic of Germany [27]), and much of this has entered the environment. Such compounds also escape in drainage fluids from hazardous waste disposal sites. Although the amounts of chlorinated benzenes present in contaminated soils and sediments can be high, their concentrations in the aqueous phase of such materials are rather low, often in the micro- to nanomolar range, due to their poor water solubility and sorption to colloid particles in the surface waters or to the matrix or organic phase of the soil (45). Only oligotrophic or facultatively oligotrophic microorganisms can grow on substrates at such low concentrations (25, 35), which makes these microorganisms particularly suitable for the degradation of poorly bioavailable pollutants. A number of studies have shown that certain pollutant and nonpollutant substrates are only degraded to some residual concentrations, after which no further metabolism is observed (2, 11, 19, 28, 31, 32, 42, 43). Various explanations of such threshold concentrations have been suggested, including the possibility that they represent the lowest substrate concentration needed to maintain viability of the microorganism and the notion that they are below the level needed to maintain synthesis of the catabolic enzymes. Such thresholds are not absolute but vary with the compound to be degraded and the degrading bacterium and its environment. The existence of threshold concentrations may account for the persistence of degradable organic compounds in the environment and may have implications for the removal of contaminants from soil, sediments, and groundwater.
Burkholderia sp. strain PS14 (formerly known as Pseudomonas sp. strain PS14 (29) but now reclassified by 16S rRNA analysis (21a) is, with Burkholderia sp. strain PS12 (5), the only microorganism presently known to utilize 1,2,4,5-tetrachlorobenzene (1,2,4,5-TeCB) aerobically as the sole source of carbon and energy (29). Burkholderia sp. strain PS14 also degrades 1,2,4-TCB and the three isomeric dichlorobenzenes (1,2-DCB, 1,3-DCB, and 1,4-DCB) as do some other organisms (10, 12, 16, 17, 22, 23, 29, 34, 36–38, 43, 44, 49). Chlorinated benzenes are converted by PS12 and PS14 by an aromatic ring dioxygenase and a dihydrodiol dehydrogenase to chlorocatechols as central intermediates (5, 29), which are degraded via the modified ortho-cleavage pathway to 3-oxoadipate (29).
The novelty of the catabolic ability of strain PS14 and its potential importance for degradation of highly chlorinated benzenes in the environment led us to investigate its ability to degrade chlorinated benzenes at nanomolar concentrations in liquid and in soil cultures to determine possible threshold concentrations in these systems. Fructose was chosen as a control substrate to compare the rate and extent of degradation of hydrophobic chlorobenzenes with those of a hydrophilic compound, since from all the mono- and disaccharides tested, including the pentoses and hexoses of the polysaccharides of the organic soil fraction (1), it was the only one PS14 was able to grow on. In this report we present results which show rapid degradation of chlorobenzene isomers by PS14 and indicate that any threshold concentrations for the metabolism of chlorobenzenes at nanomolar concentrations are below current analytical detection limits.
MATERIALS AND METHODS
Chemicals.
1,2-, 1,3-, and 1,4-DCB were obtained from Merck (Darmstadt, Germany). 1,2,4-TCB was from Fluka (Neu-Ulm, Germany). 1,2,4,5-TeCB was supplied by Aldrich (Steinheim, Germany). Yeast extract and tryptone were purchased from Difco (Detroit, Mich.). Substrates uniformly labelled with 14C had the following specific activities: 1,2,4-TCB, 21 mCi/mmol; d-fructose, 285 mCi/mmol; and 1,2,4,5-TeCB, 13.2 mCi/mmol); 1,2,4-TCB (98.9% purity) and d-fructose (98.1% purity) were from Amersham (Little Chalfont, Buckinghamshire, England), and 1,2,4,5-TeCB (99% purity) was from Sigma (St. Louis, Mo.). All other reagents were analytical grade from commercial sources. The solubilities of these chlorinated benzenes at 20°C in water are as follows: 1,2-DCB, 1,020.0 μM (4); 1,3-DCB, 7,482.5 μM (4); 1,4-DCB, 476.2 μM (4); 1,2,4-TCB, 165.3 μM (27); and 1,2,4,5-TeCB, 2.5 μM (48).
Growth conditions.
Mineral salts medium had the following composition (in grams per liter of distilled water): Na2HPO4 · 2H2O, 7.0; KH2PO4, 2.0; (NH4)2SO4, 0.5; MgCl2 · 6H2O, 0.1; Ca(NO3)2 · 4H2O, 0.05; and 1 ml of a trace element solution (24) but without EDTA (29). Inocula were grown in 500-ml shake flasks containing 100 ml of mineral salts medium on a rotary shaker (120 rpm) at 30°C. Finely mortar-ground 1,2,4,5-TeCB was added after sterilization to an initial concentration equivalent to 1 mM, whereas 1,2,4-TCB and the three isomeric dichlorobenzenes were added via the vapor phase to an initial concentration of 0.2 mM. Fructose, dissolved in mineral salts medium, was filter sterilized and added to a final concentration of 27.8 mM. The inocula were grown, until the cells reached approximately the middle of the logarithmic growth phase. Starved inocula prepared from fructose-grown cultures were obtained by incubating washed cells in mineral salts medium without a carbon source for 24 h. The inocula grown on the different carbon sources were centrifuged at 12,000 × g for 20 min at 4°C. Prior to centrifugation, inocula with 1,2,4,5-TeCB as the carbon source were aseptically freed from undissolved TeCB by filtration. The pellets were aseptically washed two times with mineral salts medium, resuspended in a small volume of mineral salts medium, and incubated for 3 h at ambient temperature to allow the intracellular substrate and any substrate still adhering to the cells to be degraded. After centrifugation, the pellet was resuspended to an optical density at 578 nm of 0.015 (corresponding to approximately 106 CFU/ml or 6.7 mg [dry weight] of bacteria per liter) in 100 ml of fresh mineral salts medium containing the corresponding chlorobenzenes or fructose at a concentration of 500 nM. Prior to use of the culture media, several aliquots were taken and the substrate content was quantitated. For determination of residual fructose concentration, a mineral salts medium with reduced salt content and having the following composition (in grams per liter of distilled water): Na2HPO4 · 2H2O, 0.1; (NH4)2SO4, 0.03; MgCl2 · 6H2O, 0.01; Ca(NO3)2 · 4H2O, 0.01; and 1 ml of a trace element solution as described above was used. Incubations were carried out in tightly closed 500-ml flasks at 30°C with shaking. Controls of uninoculated media were made. Bacteria were enumerated on solid medium composed of 10 g of tryptone, 5 g of yeast extract, 10 g of NaCl, and 15 g of agar per liter.
For analyzing the degradation of chlorobenzenes in soil, a low-contaminated soil (BBA standard soil, Borstel near Neustadt am Rübenberge, Lower Saxonia, Germany) was used; this soil is recommended by the Biological Federal Institute for Agriculture and Forestry (BBA) in Braunschweig, Germany, as a standard soil for testing of herbicides for approval (30). It was composed of 74.2% (wt/wt) sand, 19.5% (wt/wt) silt, and 6.3% (wt/wt) clay. Its organic C content was 1.31% (wt/wt), its pH was 6.0, and its maximal water capacity was 36% (wt/wt). The soil was passed through a 2-mm-pore-diameter sieve. Nonsterile soil was dried in a desiccator with silica gel for 3 days in order to achieve good distribution of the chlorinated benzenes, whereas sterilized soil had been autoclaved three times (45 min at 121°C) and dried. 1,2,4,5-TeCB or 1,2,4-TCB dissolved in 17 ml of mineral salts medium was thoroughly mixed into 83 g of soil with a spatula to give a 1,2,4,5-TeCB concentration of 64.8 ppb or a 1,2,4-TCB concentration of 54.4 ppb. Both concentrations correspond to 300 nmol/kg (wet weight) of soil. The soil was inoculated with amounts of Burkholderia sp. strain PS14 which gave an optical density at 578 nm of 0.015 in a liquid culture of the same weight (corresponding to approximately 106 CFU/ml or 6.7 mg [dry weight] of bacteria per liter). The degradation studies were carried out in tightly closed 500-ml shake flasks at 30°C at 120 rpm. For enumeration of the bacteria, 1 g of sterile soil mixed with 1,2,4,5-TeCB or 1,2,4-TCB and inoculated with Burkholderia sp. strain PS14 was suspended in 99 ml of sodium pyrophosphate solution (2.8 g of Na4P2O7 in 1 liter of water) and stirred at room temperature for 5 min. The viable bacteria were enumerated by plating 1:10 serial dilutions in triplicate.
Analytical methods.
To determine the concentration of chlorobenzenes in liquid cultures, 100-ml shake flask cultures were extracted twice with 50 ml of redistilled n-hexane. Soil was extracted with 130 ml of redistilled n-hexane–acetone (10:3, vol/vol) and twice with 100 ml of redistilled n-hexane. The extracts were pooled, dried over anhydrous sodium sulfate, and concentrated by evaporation to 1 ml at 1.4 × 104 Pa at 30°C. The chlorobenzenes were analyzed by gas chromatography (model 5890; Hewlett-Packard, Wilmington, Del.) using a 63Ni high-temperature electron capture detector and a 30-m fused silica capillary column (PTE-5; Supelco). Helium was used as a carrier gas, and nitrogen was used as a detector-quench gas. The operating temperatures of the injector and detector were 250 and 300°C, respectively. The oven temperature program was as follows: 60°C for 2 min, increase to 170°C at a rate of 4°C/min, increase to 300°C at a rate of 60°C/min, with an isothermal period of 10 min at the end. Retention times and peak areas were determined by using the HP 3365 ChemStation software. Peaks were identified and quantified by comparing injections with authentic external standards prepared by dissolving definite amounts of chlorobenzenes in mineral salts medium. These aqueous solutions were extracted, and the extracts were concentrated in the same way as the samples to be analyzed.
In order to ensure that the residual fructose remained completely dissolved in small amounts of water after lyophilization of the culture supernatant, a mineral salts medium with a reduced salt content was used. A 1,000-ml culture grown on this medium was centrifuged at 12,000 × g for 20 min at 4°C, and the pellet obtained was washed with fresh medium. In order to analyze fructose enzymatically, its binding to proteins during freeze-drying of the culture fluid must be prevented. Therefore, 10 μM mannose was added to the combined culture supernatant and washing fluid. The culture fluid was then stirred for 10 min at ambient temperature and concentrated by lyophilization to 5 ml. The concentration of fructose was then determined enzymatically (6).
For tests of mineralization in liquid cultures, shake flasks containing 100 ml of mineral salts medium and the appropriate chlorobenzene or fructose at a concentration of 500 nM were prepared as described above. Approximately 10 μl of a stock of 14C-labelled chlorinated benzenes dissolved in acetone (approximately 3 × 105 cpm) had previously been added, and the solvent was allowed to evaporate. The same amount of 14C from a stock of 14C-labelled fructose dissolved in water was added. The media were inoculated with 10 to 50 μl of a cell suspension to an optical density at 578 nm of 0.015 (corresponding to approximately 106 CFU/ml or 6.7 mg [dry weight] of bacteria per liter). Duplicate samples of 1 ml were acidified with 7.4 N sulfuric acid to a pH of ≤2. They were stripped with air for 5 min, mixed with 4 ml of Ultima Gold scintillation cocktail (Packard, Frankfurt am Main, Germany) and the radioactivity was measured with a liquid scintillation counter (LS 1801; Beckman, Fullerton, Calif.). The radioactivity measured represents the 14C activity of nonvolatile compounds. Preliminary experiments with a solution of NaH14CO3 of 2,800 cpm/ml which was acidified with 7.4 N sulfuric acid to a pH of ≤2 and stripped with air for various periods demonstrated that 99% of the labelled CO2 was released from the solution within 5 min. Duplicate 1-ml samples were mixed with 10 N NaOH (final pH ≥12). Stripping with air and measuring radioactivity were performed as described above. This measurement represents the sum of 14C activity of CO2 and nonvolatile compounds. The formation of CO2 in liquid cultures was calculated by the difference between the radioactivity of the alkaline and acidic samples. The percentage of 14C incorporation into biomass was assumed to be the difference between the percentage of 14C in the untreated culture broth and the percentage of 14C in the untreated culture supernatant.
For tests of mineralization in soil, approximately 10 μl of a stock of 14C-labelled 1,2,4-TCB or 1,2,4,5-TeCB dissolved in acetone (approximately 3 × 105 cpm) were added to 17 ml of mineral salts medium containing the corresponding unlabelled substrates. This solution was thoroughly mixed into 83 g of soil with a spatula to give a 1,2,4-TCB concentration of 54.4 ppb or a 1,2,4,5-TeCB concentration of 64.8 ppb. Incubation was performed as described above. Distilled water (50 ml) was added to duplicate flasks, the slurry was acidified with 7.4 N sulfuric acid to a pH of ≤2 and stirred for 10 min on a magnetic stirrer. The treated soil slurry was extracted once with 130 ml of redistilled n-hexane–acetone (10:3, vol/vol) and two times with 100 ml of redistilled n-hexane. Radioactivity in 1 ml of the extract was measured as described above. This measurement represents the 14C activity of nonvolatile compounds. Preliminary experiments in which a soil slurry with NaH14CO3 (3,000 cpm/ml) was treated similarly demonstrated that 98% of the labelled CO2 was released from the soil suspension within 5 min. To measure the 14C activity of nonvolatile compounds and CO2, 50 ml of distilled water was added to a second pair of duplicate flasks and mixed with 10 N NaOH so that the pH of the soil slurry was ≥12. The subsequent treatment and the measurement of radioactivity were done as described above. The formation of CO2 in soil was calculated by the difference between the radioactivity of the extract from the alkaline soil slurry and the extract from the acidic soil slurry. The losses of 14C due to volatilization of the substrates during stirring of the soil slurry were very small, since the substrates were strongly adsorbed to cells and soil (see Results and Discussion). Sterile controls were included in all experiments to check for abiotic disappearance of the substrates.
Biosorption of 1,2,4,5-TeCB.
Washed cells of Burkholderia sp. strain PS14 grown on fructose or 1,2,4,5-TeCB were added to 50 ml of mineral salts medium containing 0.4% (wt/vol) sodium azide to a final optical density at 578 nm of 0.03, and the suspension was shaken for 30 min at ambient temperature and 120 rpm. Portions (50 ml) of mineral salts medium containing 0.15 to 1.4 μM 1,2,4,5-TeCB were then added so that the cell suspension obtained had a concentration of 6.7 mg (dry weight) of bacteria per liter. After 1, 3, 5, 10, 20, 30, and 60 min, 100-ml samples of this cell suspension were rapidly centrifuged at 12,000 × g at 4°C and the supernatant was analyzed for 1,2,4,5-TeCB as described above. Controls were uninoculated mineral salts medium also containing 0.2% (wt/vol) sodium azide and 1,2,4,5-TeCB of the corresponding concentration. The recovery efficiency for 1,2,4,5-TeCB from autoclaved cell suspensions and cell suspensions treated with sodium azide ranged from 91 to 98%.
RESULTS
Degradation of chlorobenzenes and fructose in liquid cultures.
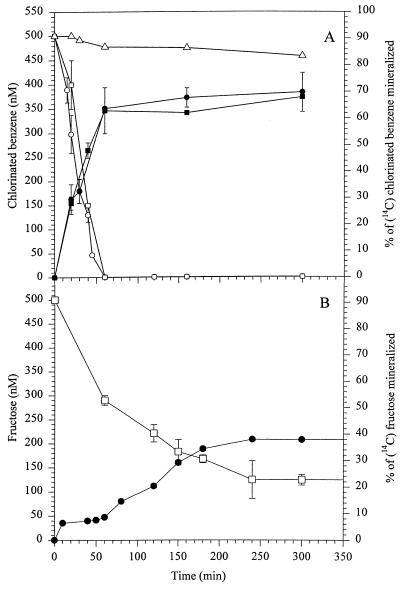
In liquid cultures of Burkholderia sp. strain PS14 with 1,2,4,5-TeCB or 1,2,4-TCB as the only carbon and energy source, the chlorobenzene concentration decreased during 1 h of incubation from 500 nM to below the detection limit of 0.5 nM. In cultures containing radioactive substrate, about 63% of the added radiolabel was converted to CO2 (Fig. 1A). Approximately 26% of 14C from 1,2,4,5-TeCB was incorporated into biomass over a 5-h period of incubation. When 1,2,4,5-TeCB and 1,2,4-TCB were used together at initial concentrations of 250 nM, both substrates were metabolized simultaneously and as rapidly as if they were the sole substrate. Similarly, 1,2-DCB, 1,3-DCB, and 1,4-DCB were metabolized within 1 h from initial concentrations of 500 nM to below their detection limits of 7.5 nM (data not shown).
FIG. 1.
Metabolism and mineralization of 1,2,4,5-TeCB, 1,2,4-TCB, and fructose in liquid cultures of Burkholderia sp. strain PS14 in mineral salts medium. Percentages of mineralization (solid symbols) and nanomolar concentrations (open symbols) of 1,2,4,5-TeCB, 1,2,4-TCB, and fructose are shown. (A) ■, [14C]1,2,4,5-TeCB; ●, [14C]1,2,4-TCB; □, 1,2,4,5-TeCB; ○, 1,2,4-TCB; ▵, 1,2,4-TCB in the sterile control. (B) ●, [14C]fructose; □, fructose. The values are the means of results of two to four independent experiments, and error bars show standard deviations.
In contrast, fructose was metabolized much more slowly and only to a threshold of approximately 125 nM during a 4-h incubation (Fig. 1B). No difference in the residual fructose concentration between cultures inoculated with starved or nonstarved cells was found. Approximately 38% of fructose had been mineralized and approximately 31% had been incorporated into biomass after 4 h of incubation. Cell densities at 578 nm of about 0.015 and the number of viable cells of approximately one million CFU of PS14 per ml on 500 nM chlorinated benzenes or fructose did not increase significantly.
Metabolism and mineralization of 1,2,4,5-TeCB and 1,2,4-TCB in soil.
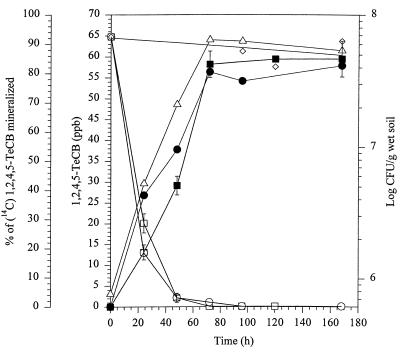
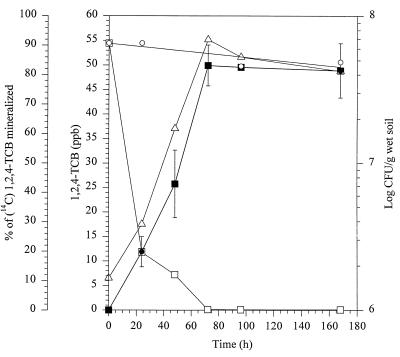
Metabolism and mineralization of 64.8 ppb of 1,2,4,5-TeCB (corresponding to 300 nmol/kg [wet weight] of soil) were measured in both sterilized and nonsterilized soil having a moisture content of 20% (wt/wt) inoculated with Burkholderia sp. strain PS14. In both soils, the concentration of 1,2,4,5-TeCB fell to below the detection limit of 0.108 ppb (corresponding to 0.5 nmol/kg [wet weight] of soil) during 72 to 96 h of incubation. During this period approximately 80% of the substrate was mineralized in both soils and the number of viable cells of PS14 increased from 7.6 × 105 to 647 × 105 per g (wet weight) of nonsterilized soil (Fig. 2). Similar results were obtained with 1,2,4-TCB as the substrate (Fig. 3). No degradation was observed in uninoculated soils. This and the results with 1,2,4,5-TeCB indicate that indigenous soil microorganisms or sterilization (autoclaving) of the soil did not influence the degradation and mineralization of 1,2,4,5-TeCB by PS14.
FIG. 2.
Metabolism and mineralization of 1,2,4,5-TeCB in unsterilized and sterilized BBA standard soil inoculated with Burkholderia sp. strain PS14. Percentages of mineralization of [14C]1,2,4,5-TeCB (solid symbols) and concentrations (in parts per billion) of 1,2,4,5-TeCB (open symbols) in nonsterile (circles) and sterile (squares) soil are shown. ⋄, concentrations (in parts per billion) of 1,2,4,5-TeCB in the uninoculated control; ▵, CFU of Burkholderia sp. strain PS14 per gram (wet weight) of sterile soil. The values are the means of results of two to four independent experiments, and error bars show standard deviations.
FIG. 3.
Metabolism and mineralization of 1,2,4-TCB in sterilized BBA standard soil inoculated with Burkholderia sp. strain PS14. Percentages of mineralization of [14C]1,2,4-TCB (■) and concentrations of 1,2,4-TCB (in parts per billion) (□) in soil are shown. ○, concentration (in parts per billion) of 1,2,4-TCB in the uninoculated control; ▵, CFU per gram (wet weight) of soil. The values are the means of results of four independent experiments, and the error bars show standard deviations.
Sorption of 1,2,4,5-TeCB onto PS14 cells.
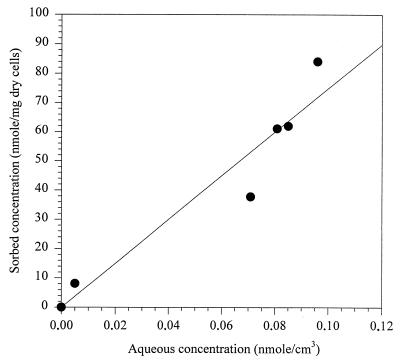
Sorption of 1,2,4,5-TeCB onto cells of Burkholderia sp. strain PS14 was studied with autoclaved and 0.2% (wt/vol) sodium azide-containing cell suspensions. Their concentrations were 6.7 mg (dry weight) of bacteria per liter corresponding to an optical density at 578 nm of 0.015. Sorption reached equilibrium within 3 to 5 min. Eighty-three percent of 0.5 μM 1,2,4,5-TeCB was adsorbed on cells treated with sodium azide. Autoclaved cells showed the same sorptive capacity for 1,2,4,5-TeCB (82.5% ± 2.6%) as those treated with sodium azide. There was no difference in sorption of 1,2,4,5-TeCB between cells of PS14 grown on fructose (82.8% ± 2.5%) or 1,2,4,5-TeCB (83% ± 1.1%). An isotherm was determined for the biosorption of 1,2,4,5-TeCB onto cells of PS14 treated with 0.2% (wt/vol) sodium azide (Fig. 4). Linear regression of these data yielded a partition coefficient (Kp) of 7.18 × 105 ± 0.93 × 105 cm3/g (dry weight) of cells.
FIG. 4.
Linear isotherm for 1,2,4,5-TeCB sorption to Burkholderia sp. strain PS14. The values represent the means of duplicate samples.
DISCUSSION
The ability to aerobically degrade 1,2,4,5-TeCB, in addition to the less-chlorinated congeners (29), makes Burkholderia sp. strain PS14 a potentially important resource for bioremediation of chlorobenzene-contaminated sites. A critical requirement of remediation processes is reduction of pollutants to below prescribed levels. The applicability of bioremediation is sometimes limited by substrate threshold levels which determine the extent to which a pollutant can be degraded. For this reason, we have analyzed the degradation of chlorobenzenes present at very low concentrations by strain PS14. The degradation of 1,2,4,5-TeCB, 1,2,4-TCB, the three dichlorobenzene isomers, and fructose as the sole carbon and energy sources in liquid batch cultures of PS14 was studied at concentrations in the nanomolar range. The concentration of the chlorinated benzenes decreased from 500 nM to below the detection limits within 1 h. This suggests that in batch experiments with PS14 under the described conditions no measurable residual concentrations (threshold values) exist for these compounds. Such a phenomenon was also observed by Tros et al. (40) for the degradation of 3-chlorobenzoate in batch cultures of Pseudomonas sp. strain B13 and by van der Meer et al. (43) during a batch incubation of an effluent from a soil column continuously fed with 1,2-DCB. On the other hand, distinct threshold concentrations for substrate consumption have been observed for many other bacteria (7, 11, 18, 19, 21, 28, 32, 47). Such residual concentrations were correlated with the maintenance needs of the microorganisms (28, 33). Bosma et al. (8) introduced the mass transfer of a chemical to the degrading microorganism as a second parameter determining the occurrence and height of a threshold value and established a relationship in which the threshold concentration of a compound for growth is inversely proportional to the exchange constant k, a measure of mass transfer, and directly proportional to the base-level maintenance flux qm. According to this model, our failure to detect threshold values for chlorinated benzenes above the corresponding detection limits in batch cultures of PS14 implies a very low maintenance requirement of the nongrowing cells and a nonlimiting mass transfer.
In contrast to the metabolism of chlorinated benzenes, fructose at an initial concentration of 500 nM was metabolized more slowly and incompletely, so that a residual concentration of approximately 125 nmol/liter remained after 4 h of incubation, regardless of whether the cells had been starved or not. Only 38% of the fructose was mineralized, and approximately 31% of [14C]fructose was incorporated into biomass during the 4-h incubation (63% of the 1,2,4,5-TeCB was mineralized within 1 h of incubation, and approximately 26% of 14C was incorporated into biomass). The PS14 strain is thus clearly specialized in its metabolism for chlorobenzenes and does not catabolize the hydrophilic control substrate fructose efficiently.
Although 1,2,4,5-TeCB and 1,2,4-TCB were degraded by strain PS14 to below the detection limits in both liquid cultures and soil, they were, as expected, metabolized and mineralized faster in liquid cultures, presumably due to their adsorption to soil particles. In the absence of PS14, 83.5% of 255 nM 1,2,4,5-TeCB adsorbed to 83 g of soil/liter used in the present study within a 30-min incubation, which corresponds to a Kp of 61 cm3/g. Only 14.3% of this sorbed 1,2,4,5-TeCB could be desorbed by exchanging the aqueous phase (20). Clearly, PS14 must enhance desorption of 1,2,4,5-TeCB from soil and/or effectively compete by adsorbing this compound more efficiently than soil. Our experiments suggest that 1,2,4,5-TeCB adsorbs to PS14 with a Kp of 7.18 × 105 cm3/g, i.e., approximately 4 orders of magnitude more strongly than to the soil used in the present study. Using the same low concentration of cells as in the degradation experiments but autoclaved or treated with sodium azide, approximately 83% of 500 nM 1,2,4,5-TeCB adsorbed within 3 to 5 min. There was no difference in sorption between fructose- and 1,2,4,5-TeCB-grown cells. Sorption of 1,2,4,5-TeCB to PS14 seemed to be passive, since dead cells sorbed as well as live cells (data not shown; see also references 41 and 46). The Kp of 1,2,4,5-TeCB in suspensions of PS14 cells was 7.18 × 105 ± 0.93 × 105 cm3/g, namely, about the same order of magnitude as the octanol-water partition coefficient (Kow) of 1,2,4,5-TeCB of 1.12 × 105 (48). The Kp of 1,2,4,5-TeCB in suspensions of PS14 cells was thus about 40 times greater than that predicted by the empirical relation log Kp = 0.907 log Kow − 0.361 established by Baughman and Paris (3) between bacterial Kp and the Kow of organic compounds. This difference may be partly due to the extremely low biomass of 6.7 mg (dry weight) of cells per liter used in our sorption studies, since it has been shown by Brandt et al. (9) that the sorption capacity of a bacterium for a highly chlorinated organic compound significantly increases with decreasing biomass in the concentration range below 0.5 g/liter. In contrast to the degradation of 1,2,4-TCB and 1,2,4,5-TeCB by PS14 to below detection limits in a soil contaminated for 1 or 2 weeks with these chemicals, residual concentrations of these compounds were found in batch cultures of a mixed population and a soil contaminated for some decades with chlorinated benzenes (15). This incomplete degradation may be attributed partly to transformation systems with lower affinities for chlorobenzenes than those in PS14. Nevertheless, the progressive entrapment of 1,2,4-TCB and 1,2,4,5-TeCB in microscopic pores of the soil is almost certainly the most important barrier to complete degradation (39). However, in the case of PS14, the large differences in partition coefficients of soil and PS14 cells for chlorobenzenes will result in steep concentration gradients in soil that promote faster intraparticle mass transfer rates (26) than those which would exist in the absence of PS14 cells. This would obviously be an important property for an organism that is to be used for bioremediation.
ACKNOWLEDGMENTS
We thank M. Sylla for technical assistance and Fritz Homann (Borstel, Neustadt am Rübenberge, Germany) for supplying the BBA standard soil. We are grateful to E. R. B. Moore for the 16S rRNA analysis and to D. H. Pieper and R.-M. Wittich for valuable discussions.
This research was financially supported by the German Federal Ministry for Education, Science, Research and Technology (BMBF grant 0139433). Kenneth N. Timmis expresses gratitude to the Fonds der Chemischen Industrie for generous support.
REFERENCES
- 1.Alexander M. The soil environment. In: Alexander M, editor. Introduction to soil microbiology. New York, N.Y: John Wiley & Sons; 1977. pp. 3–15. [Google Scholar]
- 2.Alexander M. Biodegradation of organic chemicals. Environ Sci Technol. 1985;18:106–111. doi: 10.1021/es00135a601. [DOI] [PubMed] [Google Scholar]
- 3.Baughman G L, Paris D F. Microbial bioconcentration of organic pollutants from aquatic systems—a critical review. Crit Rev Microbiol. 1981;8:205–228. doi: 10.3109/10408418109085079. [DOI] [PubMed] [Google Scholar]
- 4.Beck U. Nucleus-chlorinated aromatic hydrocarbons. In: Campbell F T, Pfefferkorn R, Rounsaville J F, editors. Ullmann’s encyclopedia of industrial chemistry. 5th ed. Weinheim, Germany: VCH Verlagsgesellschaft; 1986. pp. 330–355. [Google Scholar]
- 5.Beil S, Happe B, Timmis K N, Pieper D H. Genetic and biochemical characterization of the broad spectrum chlorobenzene dioxygenase from Burkholderia sp. strain PS12. Dechlorination of 1,2,4,5-tetrachlorobenzene. Eur J Biochem. 1997;247:190–199. doi: 10.1111/j.1432-1033.1997.00190.x. [DOI] [PubMed] [Google Scholar]
- 6.Boehringer Mannheim Biochemica. Methoden der enzymatischen Bioanalytik und Lebensmittelanalytik. Mannheim, Germany: Boehringer Mannheim; 1994. [Google Scholar]
- 7.Boethling R S, Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979;37:1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosma T N P, Middeldorp P J M, Schraa G, Zehnder A J B. Mass transfer limitation of biotransformation: quantifying bioavailability. Environ Sci Technol. 1997;31:248–252. [Google Scholar]
- 9.Brandt S, Zeng A-P, Deckwer W-D. Adsorption and desorption of pentachlorophenol on cells of Mycobacterium chlorophenolicum PCP-1. Biotechnol Bioeng. 1997;55:480–489. doi: 10.1002/(SICI)1097-0290(19970805)55:3<480::AID-BIT3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Brunsbach F R, Reineke W. Degradation of chlorobenzenes in soil slurry by a specialized organism. Appl Microbiol Biotechnol. 1994;42:415–420. doi: 10.1007/BF00902751. [DOI] [PubMed] [Google Scholar]
- 11.Button D K. Kinetics of nutrient-limited transport and microbial growth. Microbiol Rev. 1985;49:270–297. doi: 10.1128/mr.49.3.270-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bont J A M, Vorage M J A W, Hartmans S, van den Tweel W J J. Microbial degradation of 1,3-dichlorobenzene. Appl Environ Microbiol. 1986;52:677–680. doi: 10.1128/aem.52.4.677-680.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Den Besten C, Vet J J R M, Besselink H T, Kiel G S, van Berkel B J M, Beems R, van Bladeren P J. The liver, kidney, and thyroid toxicity of chlorinated benzenes. Toxicol Appl Pharmacol. 1991;111:69–81. doi: 10.1016/0041-008x(91)90135-2. [DOI] [PubMed] [Google Scholar]
- 14.Den Besten C, Brouwer A, Rietjens I M C M, van Bladeren P J. Biotransformation and toxicity of halogenated benzenes. Hum Exp Toxicol. 1994;13:866–875. doi: 10.1177/096032719401301209. [DOI] [PubMed] [Google Scholar]
- 15.Fieseler C, Noll B. Biologischer Abbau von Schadstoffen in mischkontaminierten Böden. EntsorgungsPraxis. 1994;10:16–19. [Google Scholar]
- 16.Haigler B E, Nishino S F, Spain J C. Degradation of 1,2-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1988;54:294–301. doi: 10.1128/aem.54.2.294-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haigler B E, Pettigrew C A, Spain J C. Biodegradation of mixtures of substituted benzenes by Pseudomonas sp. strain JS150. Appl Environ Microbiol. 1992;58:2237–2244. doi: 10.1128/aem.58.7.2237-2244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins B T, McInerney M J, Warikoo V. Evidence for an anaerobic syntrophic benzoate degradation threshold and isolation of the syntrophic benzoate degrader. Appl Environ Microbiol. 1995;61:526–530. doi: 10.1128/aem.61.2.526-530.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jetten M S M, Stams A J M, Zehnder A J B. Acetate threshold values and acetate activating enzymes in methanogenic bacteria. FEMS Microbiol Ecol. 1990;73:339–344. [Google Scholar]
- 20.Keskin M. Ph.D. thesis. Hamburg, Germany: University of Hamburg; 1994. [Google Scholar]
- 21.LaPat-Polasko L T, McCarty P L, Zehnder A J B. Secondary substrate utilization of methylene chloride by an isolated strain of Pseudomonas sp. Appl Environ Microbiol. 1984;47:825–830. doi: 10.1128/aem.47.4.825-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Moore, E. R. B. Personal communication.
- 22.Oldenhuis R, Kuijk L, Lammers A, Janssen D B, Witholt B. Degradation of chlorinated and non-chlorinated aromatic solvents in soil suspensions by pure bacterial cultures. Appl Microbiol Biotechnol. 1989;30:211–217. [Google Scholar]
- 23.Oltmanns R H, Rast H G, Reineke W. Degradation of 1,4-dichlorobenzene by enriched and constructed bacteria. Appl Microbiol Biotechnol. 1988;28:609–616. [Google Scholar]
- 24.Pfennig N, Lippert K D. Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol. 1966;55:245–256. [Google Scholar]
- 25.Poindexter J S. Oligotrophy: fast and famine existence. Adv Microb Ecol. 1981;5:63–89. [Google Scholar]
- 26.Rijnaarts H H M, Bachmann A, Jumelet J C, Zehnder A J B. Effect of desorption and intraparticle mass transfer on the aerobic biomineralization of α-hexachlorocyclohexane in a contaminated calcareous soil. Environ Sci Technol. 1990;24:1349–1354. [Google Scholar]
- 27.Rippen G. Handbuch Umweltchemikalien. Landsberg am Lech, Germany: Ecomed Verlagsgesellschaft AG & Co KG; 1991. [Google Scholar]
- 28.Rittmann B E, McCarty P L. Evaluation of steady-state-biofilm kinetics. Biotechnol Bioeng. 1980;22:2359–2373. doi: 10.1002/bit.260241018. [DOI] [PubMed] [Google Scholar]
- 29.Sander P, Wittich R-M, Fortnagel P, Wilkes H, Francke W. Degradation of 1,2,4-trichloro- and 1,2,4,5-tetrachlorobenzene by Pseudomonas strains. Appl Environ Microbiol. 1991;57:1430–1440. doi: 10.1128/aem.57.5.1430-1440.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schinkel K. Modifizierung der Lysimeterrichtlinie (Richtlinie der Biologischen Bundesanstalt, Teil IV, 4-3) Nachrbl Dtsch Pflanzenschutzd. 1991;43:183. [Google Scholar]
- 31.Schmidt S K, Alexander M, Shuler M L. Predicting threshold concentrations of organic substrates for bacterial growth. J Theor Biol. 1985;114:1–8. [Google Scholar]
- 32.Schmidt S K, Scow K M, Alexander M. Kinetics of p-nitrophenol mineralization by a Pseudomonas sp.: effects of second substrates. Appl Environ Microbiol. 1987;53:2617–2623. doi: 10.1128/aem.53.11.2617-2623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt S K, Simkins S, Alexander M. Models for the kinetics of biodegradation of organic compounds not supporting growth. Appl Environ Microbiol. 1985;50:323–331. doi: 10.1128/aem.50.2.323-331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schraa G, Boone M L, Jetten M S M, van Neerven A R W, Colberg P J, Zehnder A J B. Degradation of 1,4-dichlorobenzene by Alcaligenes sp. strain A175. Appl Environ Microbiol. 1986;52:1374–1381. doi: 10.1128/aem.52.6.1374-1381.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenov A M. Physiological bases of oligotrophy of microorganisms and the concept of microbial community. Microb Ecol. 1991;22:239–247. doi: 10.1007/BF02540226. [DOI] [PubMed] [Google Scholar]
- 36.Spain J C, Nishino S F. Degradation of 1,4-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1987;53:1010–1019. doi: 10.1128/aem.53.5.1010-1019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiess E, Sommer C, Görisch H. Degradation of 1,4-dichlorobenzene by Xanthobacter flavus 14p1. Appl Environ Microbiol. 1995;61:3884–3888. doi: 10.1128/aem.61.11.3884-3888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Springer W, Rast H G. Biologischer Abbau mehrfach halogenierter mono- und polyzyklischer Aromaten. GWF Wasser Abwasser. 1988;129:70–75. [Google Scholar]
- 39.Steinberg S M, Pignatello J J, Sawhney B L. Persistence of 1,2-dibromoethane in soils: entrapment in intraparticle micropores. Environ Sci Technol. 1987;21:1201–1208. [Google Scholar]
- 40.Tros M E, Schraa G, Zehnder A J B. IchemE Second International Symposium on Environmental Biotechnology. London, United Kingdom: Chameleon Press Ltd.; 1994. Biodegradation of 3-chlorobenzoate at low concentrations: transformation kinetics and residual concentrations; pp. 70–72. [Google Scholar]
- 41.Tsezos M, Bell J P. Comparison of the biosorption and desorption of hazardous organic pollutants by live and dead biomass. Water Res. 1989;23:561–568. [Google Scholar]
- 42.van der Kooij D, Hijnen W A M. Nutritional versatility and growth kinetics of an Aeromonas hydrophila strain isolated from drinking water. Appl Environ Microbiol. 1988;54:2842–2851. doi: 10.1128/aem.54.11.2842-2851.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Meer J R, Roelofsen W, Schraa G, Zehnder A J B. Degradation of low concentrations of dichlorobenzenes and 1,2,4-trichlorobenzene by Pseudomonas sp. strain P51 in nonsterile soil columns. FEMS Microbiol Ecol. 1987;45:333–341. [Google Scholar]
- 44.van der Meer J R, van Neerven A R W, de Vries E J, de Vos W M, Zehnder A J B. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J Bacteriol. 1991;173:6–15. doi: 10.1128/jb.173.1.6-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Meer J R, Bosma T N P, De Bruin W P, Harms H, Holliger C, Rijnaarts H H M, Tros M E, Schraa G, Zehnder A J B. Versatility of soil column experiments to study biodegradation of halogenated compounds under environmental conditions. Biodegradation. 1992;3:265–284. [Google Scholar]
- 46.Wang X, Grady C P L., Jr Comparison of biosorption isotherms for di-N-butyl phthalate by live and dead bacteria. Water Res. 1994;28:1247–1251. [Google Scholar]
- 47.Westermann P, Ahring B K, Mah R A. Threshold acetate concentrations for acetate catabolism by aceticlastic methanogenic bacteria. Appl Environ Microbiol. 1989;55:514–515. doi: 10.1128/aem.55.2.514-515.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yalkowsky S H, Valvani S C. Solubility and partitioning I: solubility of nonelectrolytes in water. J Pharm Sci. 1980;69:912–922. doi: 10.1002/jps.2600690814. [DOI] [PubMed] [Google Scholar]
- 49.Zaitsev G M, Uotila J S, Tsitko I V, Lobanok A G, Salkinoja-Salonen M S. Utilization of halogenated benzenes, phenols, and benzoates by Rhodococcus opacus GM-14. Appl Environ Microbiol. 1995;61:4191–4201. doi: 10.1128/aem.61.12.4191-4201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]