Abstract
This study investigated the dietary effect of Spirulina platensis phycocyanin (SPC) on growth performance (body weight (BW), body weight gain (BWG), feed intake (FI), feed conversion ratio (FCR)) at starter, grower, and finisher stages, intestinal histomorphology, serum biochemical parameters, inflammatory and antioxidant indices, and proinflammatory cytokines (tumor necrosis factor-α and caspase-3) immune expression in broiler chickens. In total, 250 one-day-old chicks (Ross 308 broiler) were randomly allotted to five experimental groups (5 replicates/group, 10 chicks/replicate) and fed basal diets supplemented with five levels of SPC (0, 0.25, 0.5, 0.75, and 1 g kg–1 diet) for 35 days. Compared with SPC0 treatment, different SPC levels increased the overall BW and BWG without affecting the total feed consumption. However, the FCR decreased linearly with an increase in supplementation level. The serum levels of total proteins, albumin, globulins, and growth hormone increased linearly by increasing levels of SPC supplementation. Further, SPC supplementation increased the thyroxin hormones without affecting serum glucose and leptin levels. Serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) values decreased in broilers fed SPC0.250 and SPC1 diets. Triglycerides (TG) decreased in SPC0.25-, SPC0.75-, and SPC1-treated groups. Though antioxidant enzyme activities (total antioxidant capacity, catalase, and superoxide dismutase) increased linearly and quadratically, malondialdehyde (MDA) decreased linearly by increasing the SPC level. There was no effect on serum proinflammatory cytokines IL1β levels. Immunolabelling index of caspase-3 and tumor necrosis factor-α (TNF-α) were downregulated by SPC supplementation. The intestinal histomorphology is represented by increased villus height, the villus height to crypt depth ratio, and numbers of goblet cells in different sections of the small intestine. In conclusion, SPC supplementation is beneficial in broiler chicken diets due to its growth-promoting, antioxidant, and anti-inflammatory properties.
Keywords: broiler chickens, growth performance, phycocyanin, proinflammatory cytokines, gut histology
1. Introduction
There is a growing need for alternative strategies to replace synthetic components with natural antimicrobial compounds in poultry diets to improve growth performance, enhance immunity, reduce oxidative stress, and improve gut histology and digestibility. The extensive use of synthetic antimicrobials in poultry diets causes drug-resistant microbial growth, impacting human and bird health [1,2,3].
Microalgae are photosynthetic prokaryotic or eukaryotic organisms that convert sunlight, water, and CO2 to algal biomass. Some species are rich in carbohydrates, minerals, proteins, and other essential compounds. The nutrient value is influenced by algae kind, culture media composition, incubation time and temperature, and environmental conditions [4].
Spirulina is a blue-green filamentous photosynthetic alga, containing 55–70% proteins, 15–25% carbohydrates, 18% essential fatty acids, minerals, vitamins, carotenes, chlorophyll-a, and phycobiliprotein pigments (phycocyanin, phycoerythrin, and allophycocyanin). Moreover, it is rich in phenolic acids and gamma-linoleic acid [5]. Spirulina platensis and Spirulina maxima are the most important Spirulina species and are cultured commercially in several countries [6]. S. platensis has several health benefits, including immunity stimulation and growth parameters with hypolipidemic, anti-inflammatory, and antioxidative activities [7,8].
Phycocyanin (PC) is a blue photosynthetic pigment in cyanobacteria and some red algae of the phycobiliprotein family. It is water-soluble, located in the cytoplasmic membrane, and released outside when the thylakoid membrane is destroyed by lysozyme enzyme and EDTA chelate cations [9,10]. PC can be extracted using organic and inorganic solvents, ultrasound, enzyme homogenization, freezing, and thawing from Spirulina [11,12]. PC possesses antioxidant, radical scavenging, anti-inflammatory, antiarthritic, hepatoprotective, antitumor, and immune-enhancing properties [6,13]. The antioxidant activity of PC is attributed to alkyl, hydroxyl, and peroxyl radical scavenging activity due to their hydroxyl and aromatic substituent structures. For their health-stimulating characteristics, natural antioxidants are preferred in broilers production [14] as they reduce reactive oxygen species (ROS) production and subsequent oxidative stress [15]. PC is a safe, natural antimicrobial product [16,17].
To the best of our knowledge, studies on SPC in poultry are limited. Hence, this study aimed to evaluate the effects of dietary supplementation of different SPC levels on growth, intestinal histomorphology (an indicator of gut health), blood biochemical parameters, antioxidant status, and immunoexpression of proinflammatory cytokines and apoptotic proteins in broiler chicken.
2. Material and Methods
2.1. Isolation and Purification of Spirulina Platensis Phycocyanin
2.1.1. Micro-Organism Source
S. platensis was acquired from the Microbiology Department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt.
2.1.2. Culture Reparation
The S. platensis cultures were grown in a nitrogen-free medium (BG011). The fresh S. platensis culture was prepared by inoculating 250 mL of BG011 medium with 10 mL of 10 days old culture in 500 mL Erlenmeyer flasks. Inoculated flasks were incubated at 26 ± 2 °C for 28 days under continuous illumination (600–800 lux) using a 36 W white fluorescent lamp.
2.1.3. Phycocyanin Extraction and Purification
PC was extracted from S. platensis fresh biomass according to Sarada et al. [18] and Salama et al. [19]. S. platensis cells (after 30 days of growth) were harvested by centrifugation at 3000× g for 5 min (Jouan, MR 18-22, Saint-Herblain, France) at 20 °C. Separated cell pellets were washed in 1M Tris–HCl buffer (pH 8.1) (Sigma-Aldrich, Darmstadt, Germany). One volume of washed cell mass was re-suspended in five volumes of the same buffer and treated for the extraction of PC using the freeze–thaw method (freezing at –50 °C and thawing at 25 °C). The resulting suspension was centrifuged for 10 min at 5000× g to separate the supernatant (the crude extract) and kept in the refrigerator. The pigment absorption was measured using UV/VIS Spectrophotometer (JENWAY, Cole-Parmer, Staffordshire, England) at 620, 640, and 650 nm against 0.05 M phosphate buffer as blank. To obtain 20% saturation, powdered ammonium sulfate was gradually added to the crude extract of PC and kept under continuous stirring for 1 h. The resulting solution was stored overnight and centrifuged at 17,000× g for 20 min. As previously described, the resulting supernatant was pooled and subjected to 70% ammonium sulfate saturation. After overnight incubation, the solution was centrifuged at 17,000× g for 20 min, and the resulting pellets were re-suspended in a small quantity of 20 mM Tris–HCl buffer (pH 8.1) and subjected to dialysis for 48 h against the same buffer, with changes of buffer four times. Next, it was lyophilized and stored at –20 °C for further use.
To determine the absorbance of the sample and PC estimation [20], the following equations were used:
2.2. Birds
The experimental trial was conducted in the Poultry Research Unit of the Faculty of Veterinary Medicine, Zagazig University, Egypt. The experiment procedures were approved by the Institutional Animal Care and Use Committee (ZU-IACUC) of Zagazig University, Egypt (Approval No. ZU-IACUC/2/F/16/2022). A total of 250 one-day-old Ross 308 broiler chicks were procured from a local hatchery. They were incubated on neomycin broad-spectrum antibiotic and dehydrated solution for three days, reaching an average initial weight of 89.46 ± 0.85 g. The chicks were raised in an open, ventilated house with suitable litter. During the first week, the room temperature was controlled at 34 °C and was gradually decreased until reaching 25 °C at the end of the rearing stage. Standard health and vaccination programs were conducted against Newcastle and Gumboro diseases. Chicks were observed daily, and notes were taken for any health issues.
2.3. Experimental Design and Diets
The chicks were randomly allotted into five experimental groups (50 chicks each, 5 replicates/group, 10 chicks/replicate). The experimental treatments were as follows: T1, control group (basal diet without SPC addition (SPC0); T2, basal diet + 0.25 g SPC kg–1 diet (SPC0.25); T3, basal diet + 0.5 g SPC kg–1 diet (SPC0.5); T4, basal diet + 0.75 g SPC kg–1 diet (SPC0.75); T5, basal diet + 1 g SPC kg–1 diet (SPC1). Feed and water were added ad libitum for 35 days of trial. As shown in Table 1, rations were provided in the mashed form and were formulated according to Ross’s manual guide, AVIAGEN [21].
Table 1.
Proximate and chemical composition of the experimental diets (%).
| Ingredients | Starter Period (4–10 Day) | Grower Period (11–23 Day) | Finisher Period (24–35 Day) |
|---|---|---|---|
| Yellow corn | 56 | 59.3 | 62 |
| Soybean meal, 48% | 33.33 | 28.1 | 23.825 |
| Corn gluten, 60% | 3.925 | 5.275 | 6.07 |
| Soybean oil | 2.2 | 3 | 4 |
| Calcium carbonate | 1.2 | 1.2 | 1.1 |
| Calcium dibasic phosphate | 1.5 | 1.4 | 1.3 |
| Common salt | 0.15 | 0.15 | 0.15 |
| Premix * | 0.3 | 0.3 | 0.3 |
| DL-methionine, 98% | 0.4 | 0.3 | 0.33 |
| Lysine HCl, 78% | 0.47 | 0.45 | 0.40 |
| Choline | 0.07 | 0.07 | 0.07 |
| Threonine | 0.1 | 0.1 | 0.1 |
| Phytase | 0.005 | 0.005 | 0.005 |
| Na2CO3 | 0.25 | 0.25 | 0.25 |
| Antimycotoxin | 0.1 | 0.1 | 0.1 |
| Chemical composition | |||
| ME (Kcal/kg) | 3003.9 | 3100.4 | 3200.8 |
| Crude protein % | 23.01 | 21.52 | 20.15 |
| Calcium % | 0.941 | 0.904 | 0.833 |
| Available phosphorus % | 0.481 | 0.449 | 0.418 |
| Lysine % | 1.465 | 1.315 | 1.163 |
| Methionine % | 0.721 | 0.610 | 0.626 |
| Threonine % | 0.823 | 0.765 | 0.713 |
* Premix per kg of diet: Vitamin A, 1 500 IU; Vitamin D3, 200 IU; Vitamin E, 10 mg; Vitamin K3, 0.5 mg; thiamine, 1.8 mg; riboflavin, 3.6 mg; pantothenic acid, 10 mg; folic acid, 0.55 mg; pyridoxine, 3.5 mg; niacin, 35 mg; cobalamin, 0.01 mg; biotin, 0.15 mg; Fe, 80 mg; Cu, 8 mg; Mn, 60 mg; Zn, 40 mg; I, 0.35 mg; Se, 0.15 mg.
2.4. Growth Performance
Birds were weighed individually on the 4th day of age to determine the average initial BW and then weighed at 10, 23, and 35 days to calculate the average BW of the birds in each group. At each interval, the BWG was determined as the difference between the final and the initial BW. The feed intake (FI) and feed conversion ratio (FCR) were calculated as per the following equations:
2.5. Sampling
Three birds were randomly selected from each replicate (n = 15/group) and euthanized by cervical dislocation, according to the American Veterinary Medical Association guidelines [22]. Then, blood samples were collected into sterilized tubes without anticoagulant and were at room temperature for clotting. The samples were then centrifuged (at 3500 rpm for 15 min) to separate the serum. The serum samples were kept at −20 °C in Eppendorf tubes until chemical analysis. For histological and immunohistochemical examination, the small intestine and liver samples were collected.
2.6. Blood Biochemical Indices
The total protein serum level was determined according to Grant [23], and the serum albumin level was evaluated as per Doumas et al. [24]. The serum globulin level was calculated by subtracting albumin values from total proteins [25]. The growth hormone (GH) was determined according to the instructions provided in the chicken ELISA kit of MyBioSource Co., San Diego, CA, USA with Cat. No. MBS266317.
According to the procedures of McGowan et al. [26] and Allain et al. [27], the serum triglycerides (TG) and total cholesterol (TC) were determined, respectively, with diagnostic kits of Spectrum BioScience (Egyptian Company for Biotechnology, Cairo, Egypt). Serum high-density lipoprotein cholesterol level (HDL-C) was measured using Vassault et al. [28] methodology. LDL-C was estimated using the following Iranian formula:
2.7. Inflammatory and Antioxidant Indices
To quantify interleukin 1β (IL1β) (Cat. No. MBS2024496), specific ELISA assay kits (MyBioSource, San Diego, CA, USA) were used. According to the methodology of McDonald and Hultin [29], Rice-Evans and Miller [30], Aebi [31], and Nishikimi et al. [32], Malondialdehyde (MDA), the total antioxidant capacity (TAC), catalase (CAT) activity, and superoxide dismutase (SOD) were estimated, respectively, using MyBioSource ELISA Kits (Cat. Nos. MBS2700234, MBS038818, and MBS705758, respectively).
2.8. Histological and Immunohistochemical Examination
For analysis, two-centimeter samples (three/group) were taken from the small intestine (duodenum, jejunum, and ileum) and were fixated in neutral buffered formalin (10%). Briefly, specimens were subjected to ascending grades of ethanol (75–100%) for dehydration. These specimens were placed in xylol I and II, embedded in paraffin, sliced with a microtome (Leica RM 2155, Wetzlar, Germany) into 4 µm cross-sections and longitudinal sections, and stained using hematoxylin and eosin (H&E) [33]. Images of each animal in each group (25 images for each group) were captured with an AmScope 5.0 MP microscope digital camera (AmScope, Irvine, CA, USA) in a low-power field (40× magnification). Intestinal villus height (VH) was measured (µm) tip to the base of the villus, and the crypt depth (CD) was calculated from the villus—crypt junction to the distal limit of the crypt. The VH, villus width (VW), CD, and goblet cell count (GCC) per area of epithelium layer were measured using Mitocam® software (Motic Images plus 2.0, Hong Kong, China).
According to Saber Saber et al. [34], liver samples (three samples/group) were collected to examine the immunoexpression of caspase-3 and TNF-α at the end of the experiment. To incubate the tissues, an endogenous peroxidase blocking reagent containing hydrogen peroxide and sodium azide (DAKO peroxidase blocking reagent, Cat. No. S 2001) was used. Then, 1–2 drops of the supersensitive primary monoclonal antibody against caspase-3 and TNF-α (Cat. Nos. NB100–56708 and NB600–587, respectively, Novus Biologicals, Briarwood Avenue, USA) were added to these sections. The slides were then counterstained using hematoxylin and visualized under the microscope. ImageJ software (ImageJ bundled with 64-bit Java 1.8.0_172) was used to estimate the percentages of immunostaining positive area at high magnification (100×) in five sections per group, according to Rizzardi et al. [35].
2.9. Statistical Analysis
ANOVA was applied based on polynomial orthogonal contrasts. SPSS Version 17 for Windows (SPSS Inc., Chicago, IL, USA) calculated the linear and quadratic regression equations. Duncan’s multiple range test verified the significant difference between mean values, and the significance level was set at p < 0.05.
3. Results
3.1. Phycocyanin Characterization
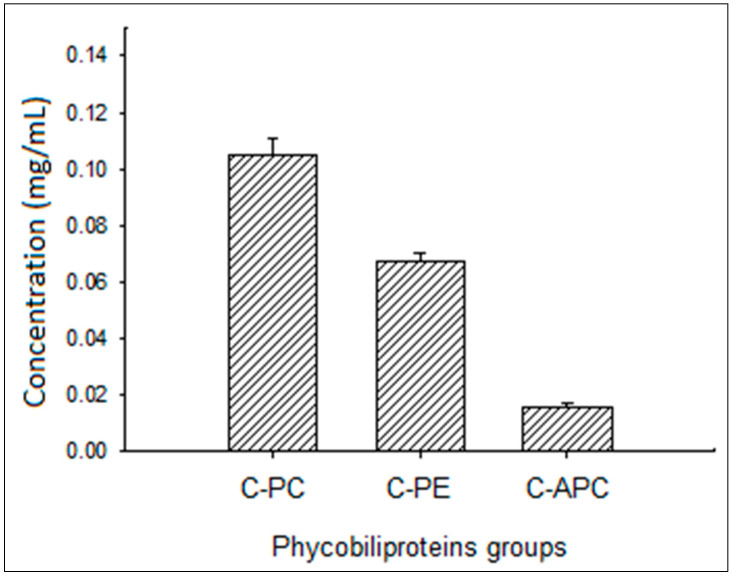
The three PC components (Figure 1) with the highest concentrations were located in the following order: PC (0.105 mg/mL) > phycoerythrin (0.067 mg/mL) > allophycocyanin (0.016 mg/mL).
Figure 1.
Cyanobacterial phycobiliproteins group concentration (mg/mL): phycocyanin (C-PC), phycoerythrin (C-PE), and allophycocyanin (C-APC).
3.2. Growth Performance
Table 2 presents the growth parameter data. Body weight (BW) and body weight gain (BWG) was linearly increased (p < 0.01) throughout the starter period in broilers fed 1 g/kg SPC-supplemented diets. Different SPC levels did not affect the FCR and FI compared to the SPC0 treatment (p > 0.05). BW and BWG increased linearly in chickens given SPC0.75 treatment (p ≤ 0.01) during the grower period. However, those fed SPC0.25, SPC0.5, and SPC1 diets showed nonsignificant improvement compared to SPC0 treatment. FCR linearly decreased in chicken given SPC0.5, SPC0.75, and SPC1 treatments and linearly increased under SPC0.25 treatment (p < 0.01). Moreover, all SPC levels did not affect the average FI (p = 0.10) compared with the SPC0 treatment. Different SPC levels linearly and quadratically increased (p < 0.01) BW and BWG with no effect on the total FI during the final and overall periods. Overall, FCR linearly decreased (p = 0.02) in SPC0.5, SPC0.75, and SPC1 groups. Further, final BW was the highest among broilers fed diets supplemented with SPC at 0.75 g/kg and the least in the SPC0 group.
Table 2.
Effects of dietary Spirulina platensis phycocyanin on the growth performance of broiler chicken.
| Trait Studied | SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Initial BW (g) | 88.96 ± 0.95 | 89.17 ± 1.30 | 90.00 ± 0.63 | 89.59 ± 0.96 | 89.59 ± 0.36 | 0.333 | 0.41 |
| Starter Period (4–10 Days) | |||||||
| BW (g) | 320.60 ± 4.93 bc | 315.61 ± 7.64 c | 333.25 ± 8.39 ab | 331.23 ± 9.78 ab | 337.81 ± 7.59 a | <0.01 | 0.84 |
| BWG (g) | 231.64 ± 5.78 bc | 226.44 ± 6.41 c | 243.26 ± 8.94 ab | 241.65 ± 9.01 ab | 248.23 ± 7.66 a | <0.01 | 0.76 |
| FI (g) | 240.84 ± 6.26 | 236.88 ± 16.16 | 241.25 ± 9.44 | 247.29 ± 12.35 | 238.13 ± 15.27 | 0.83 | 0.75 |
| FCR | 1.04 ± 0.01 | 1.05 ± 0.07 | 0.99 ± 0.02 | 1.02 ± 0.06 | 0.96 ± 0.07 | 0.09 | 0.62 |
| Grower Period (11–23 Days) | |||||||
| BW (g) | 1140.97 ± 0.87 bc | 1100.90 ± 15.89 c | 1211.72 ± 25.68 ab | 1243.33 ± 67.66 a | 1205.17 ± 47.86 ab | <0.01 | 0.40 |
| BWG (g) | 820.37 ± 5.46 bc | 785.30 ± 23.30 c | 878.47 ± 28.36 ab | 912.10 ± 60.87 a | 867.36 ± 40.88 ab | 0.01 | 0.34 |
| FI (g) | 1070.09 ± 16.56 ab | 1170.36 ± 52.13 a | 1042.92 ± 26.25 b | 1091.25 ± 63.36 ab | 1077.08 ± 75.44 ab | 0.51 | 0.65 |
| FCR | 1.30 ± 0.02 b | 1.49 ± 0.05 a | 1.19 ± 0.07 c | 1.20 ± 0.06 c | 1.24 ± 0.05 bc | <0.01 | 0.86 |
| Finisher Period (24–35 Days) | |||||||
| BW (g) | 2126.22 ± 32.50 b | 2363.89 ± 38.08 a | 2487.50 ± 78.70 a | 2499.17 ± 80.55 a | 2455.83 ± 82.17 a | <0.01 | <0.01 |
| BWG (g) | 985.25 ± 31.82 b | 1262.99 ± 127.54 a | 1275.78 ± 53.23 a | 1255.83 ± 80.86 a | 1250.67 ± 54.17 a | <0.01 | <0.01 |
| FI (g) | 1709.82 ± 105.53 | 1710.12 ± 127.23 | 1736.51 ± 233.40 | 1752.08 ± 165.63 | 1748.61 ± 185.14 | 0.71 | 0.96 |
| FCR | 1.73 ± 0.08 | 1.37 ± 0.23 | 1.36 ± 0.13 | 1.40 ± 0.21 | 1.40 ± 0.14 | 0.06 | 0.05 |
| Overall Performance (1–35 Days) | |||||||
| Final BW, g | 2126.22 ± 32.50 b | 2363.89 ± 138.08 a | 2487.50 ± 78.70 a | 2499.17 ± 80.55 a | 2455.83 ± 82.17 a | <0.01 | 0.01 |
| Total BWG, g | 2037.27 ± 32.11 b | 2274.72 ± 138.99 a | 2397.50 ± 78.26 a | 2409.58 ± 80.04 a | 2366.25 ± 81.89 a | <0.01 | 0.01 |
| Total FI, g | 3020.75 ± 85.47 | 3117.35 ± 142.64 | 3020.68 ± 216.28 | 3090.63 ± 238.36 | 3063.82 ± 257.01 | 0.87 | 0.86 |
| FCR | 1.48 ± 0.03 a | 1.38 ± 0.14 ab | 1.26 ± 0.05 b | 1.28 ± 0.11 b | 1.30 ± 0.08 b | 0.02 | 0.09 |
# The regressions were considered significant at p ≤ 0.05. a,b,c Means within the same row carrying different superscripts were significantly different at p ≤ 0.05. SPC0: control diet (basal diet with no additives); SPC0.25: basal diets supplemented with 0.25 g SPC kg–1 diet; SPC0.5: basal diets supplemented with 0.5 g SPC kg–1 diet; SPC0.75: basal diets supplemented with 0.75 g SPC kg–1 diet; SPC1: basal diets supplemented with 1 g SPC kg–1 diet. BW: body weight, BWG: body weight gain, FI: feed intake, and FCR: feed conversion ratio.
3.3. Serum Biochemical Indices
The effect of SPC addition on the serum biochemical parameters of broilers is highlighted in Table 3. Total proteins (p < 0.01), albumin (p < 0.01), total globulins (p = 0.02), and GH levels were linearly increased by different SPC level supplementations (p < 0.01). These changes were greater in broilers fed a 1 g/kg SPC-supplemented diet than those fed a 0.75 g/kg SPC diet. Meanwhile, serum glucose and leptin levels were not significantly changed (p > 0.05). Thyroxin hormones (T3 and T4) were increased linearly and quadratically in all SPC treatment groups compared with the SPC0 treatment group (p < 0.01).
Table 3.
Effect of dietary Spirulina platensis phycocyanin addition on serum biochemical parameters.
| SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | ||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Glucose (mg/dL) | 335.00 ± 2.65 | 337.33 ± 7.57 | 339.33 ± 6.43 | 338.67 ± 6.43 | 340.33 ± 6.11 | 0.30 | 0.77 |
| Total proteins (g/dL) | 3.21 ± 0.25 b | 4.32 ± 0.89 a | 4.69 ± 0.36 a | 4.88 ± 0.53 a | 5.05 ± 0.27 a | <0.01 | 0.10 |
| Albumin (g/dL) | 1.21 ± 0.03 c | 1.42 ± 0.13 bc | 1.54 ± 0.17 b | 1.87 ± 0.31 a | 2.02 ± 0.04 a | <0.01 | 0.79 |
| Total globulins (g/dL) | 2.00 ± 0.24 b | 2.90 ± 0.80 a | 3.15 ± 0.28 a | 3.02 ± 0.25 a | 3.03 ± 0.24 a | 0.02 | 0.05 |
| GH (ng/mL) | 2.90 ± 0.30 c | 4.20 ± 0.30 b | 4.90 ± 0.44 ab | 5.30 ± 0.46 a | 5.60 ± 0.72 a | <0.01 | 0.05 |
| Leptin (ng/mL) | 2.18 ± 0.06 | 2.19 ± 0.02 | 1.65 ± 0.51 | 2.02 ± 0.32 | 2.06 ± 0.52 | 0.54 | 0.24 |
| T3 (ng/mL) | 3.44 ± 0.21 c | 4.42 ± 0.10 b | 4.29 ± 0.11 b | 4.41 ± 0.10 b | 4.85 ± 0.22 a | <0.01 | 0.03 |
| T4 (ng/mL) | 18.55 ± 1.47 c | 23.06 ± 0.57 b | 24.62 ± 0.88 ab | 25.12 ± 1.02 a | 24.41 ± 0.25 ab | <0.01 | <0.01 |
# The regressions were considered significant at p ≤ 0.05. a,b,c Mean values in the same row with different superscripts differ significantly (p < 0.05). SPC0: control diet (basal diet with no additives); SPC0.25: basal diets supplemented with 0.25 g SPC kg–1 diet; SPC0.5: basal diets supplemented with 0.5 g SPC kg–1 diet; SPC0.75: basal diets supplemented with 0.75 g SPC kg–1 diet; SPC1: basal diets supplemented with 1 g SPC kg–1 diet. GH: growth hormone, T3: triiodothyronine, and T4: thyroxine hormone.
3.4. Lipid Profile
Serum TC and LDL values decreased linearly in broilers fed ration supplemented with SPC at 0.25 or 1 g/kg diet. In contrast, serum triglyceride decreased linearly in groups given SPC0.250, SPC0.750, and SPC1 treatments (p < 0.05). All supplementation levels had no significant effect on high-density lipoprotein cholesterol (HDL-C) and very low-density lipoprotein cholesterol (VLDL-C) (p > 0.05) when compared with the control group (Table 4).
Table 4.
Effect of dietary Spirulina platensis phycocyanin addition on serum lipid profile.
| SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | ||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| TC (mmol/L) | 3.54 ± 0.06 a | 3.35 ± 0.01 c | 3.46 ± 0.05 ab | 3.48 ± 0.03 a | 3.39 ± 0.02 bc | 0.04 | 0.23 |
| HDL-C (mmol/L) | 1.98 ± 0.03 | 2.07 ± 0.12 | 2.07 ± 0.13 | 2.08 ± 0.08 | 2.13 ± 0.09 | 0.11 | 0.79 |
| LDL-C (mmol/L) | 1.32 ± 0.03 a | 1.05 ± 0.13 b | 1.17 ± 0.15 ab | 1.16 ± 0.12 ab | 1.03 ± 0.09 b | 0.04 | 0.50 |
| VLDL-C (mmol/L) | 0.23 ± 0.02 | 0.23 ± 0.01 | 0.23 ± 0.02 | 0.24 ± 0.01 | 0.23 ± 0.02 | 0.91 | 0.93 |
| TG (mmol/L) | 1.27 ± 0.04 a | 1.17 ± 0.04 b | 1.19 ± 0.04 ab | 1.15 ± 0.03 b | 1.17 ± 0.07 b | 0.02 | 0.09 |
# The regressions were considered significant at p ≤ 0.05. a,b,c Mean values in the same row with different superscripts differ significantly (p < 0.05). SPC0: control diet (basal diet with no additives); SPC0.25: basal diets supplemented with 0.25 g SPC kg–1 diet; SPC0.5: basal diets supplemented with 0.5 g SPC kg–1 diet; SPC0.75: basal diets supplemented with 0.75 g SPC kg–1 diet; SPC1: basal diets supplemented with 1 g SPC kg–1 diet. TC: total cholesterol, TG: triglycerides, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, and VLD-CL: very low-density lipoprotein cholesterol.
3.5. Inflammatory and Antioxidant Indices
The impact of SPC on the activities of serum antioxidant enzymes of broiler chickens is shown in Table 5. TAC, catalase (CAT), and superoxide dismutase (SOD) activities increased linearly and quadratically in all SPC treatment groups (p < 0.05) in comparison with the SPC0 treatment group. The higher supplementation levels observed the highest results. Moreover, serum MDA linearly decreased in groups fed diets supplemented with SPC at 0.5, 0.75, and 1 g/kg diets. SPC addition did not affect IL1β levels.
Table 5.
Effect of dietary Spirulina platensis phycocyanin (SPC) addition on serum antioxidant and inflammatory indices.
| SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | ||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| TAC (U/mL) | 9.69 ± 0.63 c | 11.59 ± 1.51 b | 13.11 ± 0.81 ab | 13.12 ± 0.46 ab | 13.69 ± 0.41 a | <0.01 | 0.04 |
| CAT (U/mL) | 2.57 ± 0.45 b | 5.03 ± 1.92 a | 6.50 ± 0.57 a | 6.43 ± 0.55 a | 6.57 ± 0.60 a | <0.01 | 0.02 |
| SOD (U/mL) | 135.96 ± 3.34 b | 152.21 ± 8.28 a | 156.32 ± 1.97 a | 162.44 ± 3.39 a | 153.49 ± 9.03 a | 0.01 | 0.02 |
| MDA nmol/mL | 4.59 ± 0.39 a | 4.60 ± 1.18 a | 3.63 ± 0.75 b | 3.17 ± 0.90 b | 3.43 ± 0.71 b | 0.03 | 0.59 |
| Serum IL1β (μg/mL) | 155 ± 11.79 | 157 ± 12.12 | 142 ± 6.08 | 157 ± 15.13 | 165 ± 7.94 | 0.35 | 0.11 |
# The regressions were considered significant at p ≤ 0.05. a,b,c Mean values in the same column with different superscripts differ significantly (p < 0.05). TAC: total antioxidant capacity, CAT: catalase, SOD: superoxide dismutase, MDA: malondialdehyde, and IL1β: interleukin 1β. SPC0: control diet (basal diet with no additives); SPC0.25: basal diets supplemented with 0.25 g SPC kg–1 diet; SPC0.5: basal diets supplemented with 0.5 g SPC kg–1 diet; SPC0.75: basal diets supplemented with 0.75 g SPC kg–1 diet; SPC1: basal diets supplemented with 1 g SPC kg–1 diet.
3.6. Histological Findings and Morphometric Measures
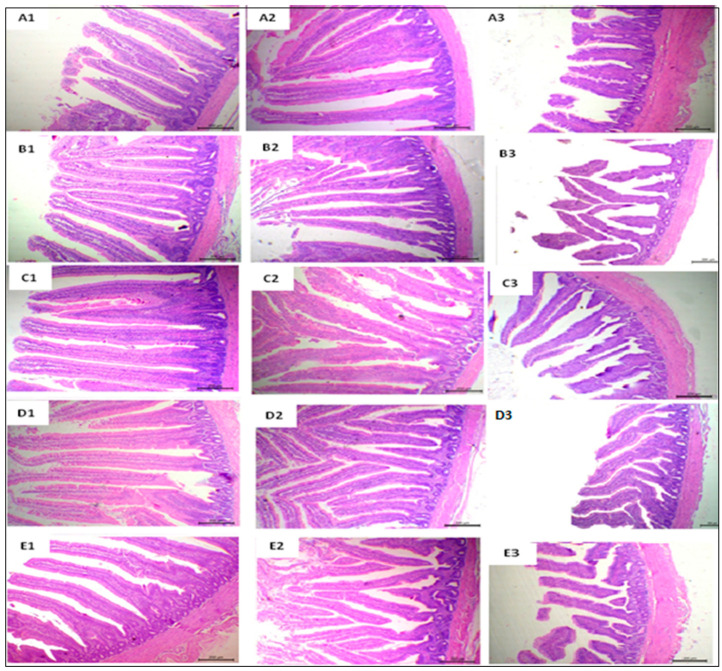
Figure 2 presents a representative photomicrograph of small intestine sections stained with H&E of broilers with 40× magnification. Normal villi with a free lumen were observed in the slices of the duodenal segments from the SPC0 group. A mild increase in villus height was shown in the SPC0.25 group. In contrast, the SPC0.5–1 groups showed prominently tall, thin, and distinct villi with a mild proliferation of goblet cells, along with an enlarged size, and rows of enterocytes with arranged lamina propria. Free lumina with normal villous structures were exhibited in segments from the jejunal sections of the SPC0 group, whereas sections from the SPC0.25 group showed a small increase in villi height. SPC0.5, SPC0.75, and SPC1 jejunum segments showed closely packed villi with a significant increase in length and distinctly serrated surfaces with goblet cell metaplasia. Ileal segment samples from the basal control treatment showed tongue-shaped villi with different heights. The SPC0.25 group, on the other hand, showed normal histology, while the SPC0.5 and SPC0.75 groups had significantly increased villus height, and the SPC1 group showed normal villi.
Figure 2.
Representative photomicrograph of H&E-stained small intestine sections of broiler chickens in 40× magnification. Duodenal sections from SPC0 showed normal intestinal villi with a free lumen (A1); those from SPC0.25 group showed a mild increase in villus height (B1); sections from SPC0.5–1 groups revealed markedly thin, tall, and separate villi with mild goblet cell proliferation, increased sizes, and rows of enterocytes with arranged lamina propria (C1,D1,E1). Jejunal segment sections of SPC0 showed free lumina with nearly normal villus structures (A2), while sections from the SPC0.25 group showed a small increase in villi length (B2). Jejunum segments from SPC0.5−1 groups showed closely packed villi with a marked increase in their length and markedly serrated surfaces with goblet cell metaplasia (C2,D2,E2). Ileal segment sections from basal treatment showed tongue-shaped villi with a different height (A3); sections from the SPC0.25 group exhibited normal histology (B3); the sections from the SPC0.5 and SPC0.075 groups showed relatively increased villus height (C3,D3); those from the SPC1 diet group showed normal villi (E3).
Table 6 shows the results of intestinal morphometric measurements. In comparison to the SPC0 treatment group, there was a linear and quadratic increase in the villus height (VH), crypt depth (CD), VH: CD ratio, and numbers of goblet cells in different sectors of the small intestine observed in all SPC treatment groups (p < 0.01). Different SPC supplementation levels had no effect on the duodenal and jejunal villus width. However, ileal villus width increased linearly in the SPC0.25 and SPC1 treatment groups (p < 0.01).
Table 6.
Effect of dietary Spirulina platensis phycocyanin addition on intestinal histology.
| SPC0 | SPC0.25 | SPC0.5 | SPC0.75 | SPC1 | Regression # | ||
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Duodenum | |||||||
| VH µm | 626.55 ± 36.97 d | 706.20 ± 31.64 c | 921.23 ± 40.00 b | 1042.44 ± 20.14 a | 922.34 ± 29.14 b | <0.01 | <0.01 |
| VW µm | 130.36 ± 1.64 | 82.46 ± 35.09 | 115.57 ± 41.43 | 118.39 ± 9.57 | 65.51 ± 15.68 | 0.07 | 0.64 |
| CD µm | 115.26 ± 13.38 d | 119.01 ± 19.78 cd | 153.78 ± 24.18 b | 232.42 ± 13.51 a | 148.40 ± 7.78 b | <0.01 | <0.01 |
| VH:CD | 4.66 ± 0.44 c | 5.85 ± 0.32 ab | 5.81 ± 0.39 ab | 5.49 ± 0.18 b | 6.29 ± 0.21 a | <0.01 | 0.02 |
| GCC | 100.70 ± 11.84 c | 125.90 ± 11.64 b | 139.23 ± 6.60 ab | 155.59 ± 5.16 a | 131.39 ± 10.24 b | <0.01 | <0.01 |
| Jejunum | |||||||
| VH µm | 854.95 ± 77.77 d | 995.13 ± 66.11 c | 1213.0 ± 38.47 b | 1373.18 ± 104.11 a | 1038.93 ± 56.37 c | <0.01 | <0.01 |
| VW µm | 83.30 ± 14.85 | 98.86 ± 8.94 | 95.14 ± 5.05 | 93.77 ± 8.11 | 90.82 ± 17.21 | 0.36 | 0.17 |
| CD µm | 163.78 ± 6.42 d | 210.36 ± 24.55 c | 254.38 ± 16.90 b | 321.66 ± 17.43 a | 195.31 ± 13.12 c | <0.01 | <0.01 |
| VH:CD | 1.28 ± 0.09 c | 5.18 ± 0.93 a | 4.78 ± 0.19 ab | 4.27 ± 0.11 b | 5.42 ± 0.18 a | <0.01 | <0.01 |
| GCC | 170.45 ± 17.64 c | 210.36 ± 24.55 b | 242.98 ± 22.74 b | 298.98 ± 13.12 a | 210.45 ± 11.48 b | <0.01 | <0.01 |
| Ileum | |||||||
| VH µm | 254.76 ± 44.46 c | 382.31 ± 14.48 b | 392.03 ± 10.37 b | 506.08 ± 41.73 a | 349.00 ± 28.15 b | <0.01 | <0.01 |
| VW µm | 72.72 ± 7.33 c | 107.17 ± 5.56 ab | 74.97 ± 11.78 c | 91.41 ± 9.98 bc | 124.69 ± 16.28 a | <0.01 | 0.07 |
| CD µm | 86.15 ± 8.35 c | 92.34 ± 4.22 bc | 100.49 ± 2.51 ab | 110.11 ± 10.54 a | 87.73 ± 2.56 c | 0.09 | <0.01 |
| VH:CD | 626.55 ± 36.97 d | 3.93 ± 0.22 b | 3.90 ± 0.18 b | 4.60 ± 0.17 a | 3.98 ± 0.24 b | <0.01 | <0.01 |
| GCC | 130.36 ± 1.64 | 71.56 ± 6.23 b | 80.55 ± 2.57 ab | 89.01 ± 4.02 a | 74.01 ± 5.14 b | <0.01 | <0.01 |
# The regressions were considered significant at p ≤ 0.05. a,b,c,d Means within the same row carrying different superscripts were significantly different (p ≤ 0.05). SPC0: control diet (basal diets with no additives); SPC0.250: basal diets supplemented with 0.250 g SPC kg–1 diet; SPC0.5: basal diets supplemented with 0.5 g SPC kg–1 diet; SPC0.75: basal diets supplemented with 0.75 g SPC kg–1 diet; SPC1: basal diets supplemented with 1 g SPC kg–1 diet. VH: villus height, VW: villus width, CD: crypt depth, and GCC: goblet cell count.
3.7. Immunohistochemical Analysis and Morphometric Measures
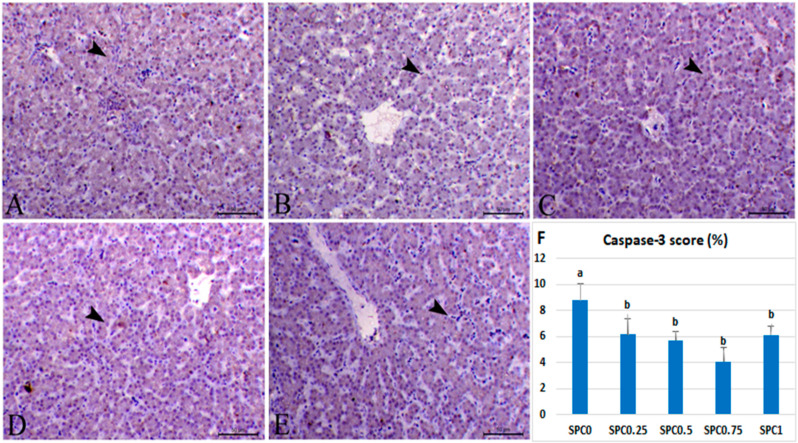
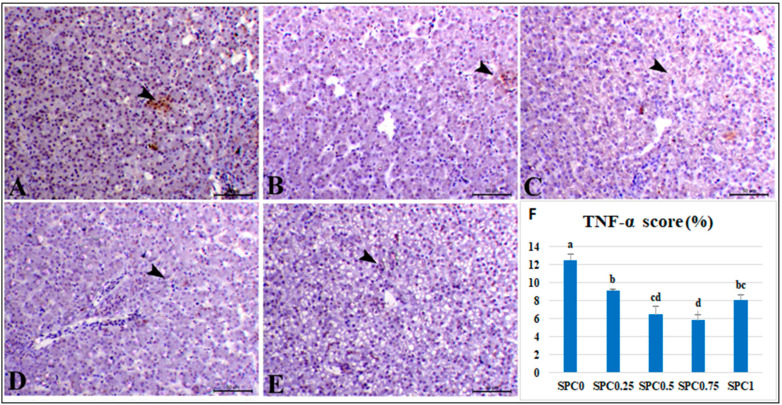
Figure 3F and Figure 4F demonstrate the morphometric scores of the immune staining expression of caspase-3 and TNF-α antibodies in the liver. Immunolabelling index percentage of caspase-3 and TNF-α were lowered linearly and quadratically in all SPC-supplemented groups (p < 0.05), with the lowest value in the SPC0.75 group, followed by the SPC0.5 group.
Figure 3.
Photomicrograph of hepatic tissues immunostained with caspase-3 antibody. Liver of control basal group showing mild cytoplasmic expression of caspase-3 within hepatocytes (A) (arrowhead). Liver of SPC0.25 group showing mild expression of caspase-3 within hepatocytes (B) (arrowhead). The liver of the SPC0.5 group showed a decrease in the cytoplasmic immunostaining of caspase-3 within hepatocytes (C) (arrowhead). Liver of SPC0.75 group showing mild cytoplasmic expression of caspase-3 within hepatocytes (D) (arrowhead). Liver of SPC1 group showing mild cytoplasmic expression of caspase-3 within hepatocytes (E) (arrowhead). Bar = 50 µm. (F) shows morphometric measures of caspase-3 immunostaining expression (%). a,b Means carrying different superscripts were significantly different (p ≤ 0.05).
Figure 4.
Photomicrograph of hepatic tissues immunostained with TNF-α antibody. The liver of the control SPC0 group showed mild expression of TNF-α within a few hepatocytes (A) (arrowhead). The liver of the SPC0.25 group showed scanty expression of TNF-α within hepatocytes (B) (arrowheads). The liver of the SPC0.5 group showed a marked decrease in immunostaining of TNF-α within hepatic tissues (C) (arrowhead). The liver of the SPC0.75 showed a significant decrease in the expression of TNF-α within the blood sinusoids (D) (arrowhead). The liver of SPC1 showed a marked decrease in the expression of TNF-α within hepatocytes (E) (arrowhead). Bar = 50 µm. (F) shows morphometric measures of TNF-α immunostaining expression (%). a,b,c,d Means carrying different superscripts were significantly different (p ≤ 0.05).
Figure 3 shows the photomicrograph of hepatic tissues immunostained with caspase-3 antibody. The liver of the control group revealed the mild cytoplasmic appearance of caspase-3 within the hepatic cells (8.77%). The SPC0.25 group showed mild caspase-3 expressions within hepatocytes (6.17%), and the SPC0.5 group demonstrated a decrease in the cytoplasmic immunostaining of caspase-3 within hepatocytes (5.7%). The SPC0.75 and SPC1 groups exhibited mild cytoplasmic manifestation of caspase-3 within hepatocytes (4.08 and 6.13%, respectively).
Figure 4 shows a photomicrograph of hepatic tissues immunostained with TNF-antibodies. TNF-expression was mild within a few hepatocytes (12.5%) in the liver of the SPC0 group, but TNF-expression was scanty within hepatocytes in the liver of the SPC0.25 group (9.08%). SPC0.5 and SPC1 groups showed a significant decrease in immunostaining of TNF-α within hepatic tissues (6.47 and 8.08%). SPC0.75 group showed a marked reduction in TNF-α expression within the blood sinusoids (5.83%).
4. Discussion
4.1. Growth Performance
The addition of dietary S. platensis PC positively affects the final BW and BWG of broilers chickens without influencing FI and FCR. The SPC0.75 treatment group showed the best outcomes, followed by the SPC0.5 group. This advance in growth parameters may be due to the improvement in birds’ health, evidenced by the small intestine morphology with greater villi length, increasing numbers of goblet cells, and enhancement in their absorption surface, resulting in enhanced nutrient digestibility and absorption. SPC has been shown to increase antioxidant enzyme activity while lowering proinflammatory cytokines (IL1β and IFN-γ) production. It has a positive effect on gastrointestinal flora and increases the activities of digestive enzymes, resulting in enhanced total tract digestibility of dry matter and nitrogen [36]. Furthermore, it improves nutrient digestibility of amino acids and protein synthesis [37] and apparent metabolizable energy digestibility [38], as well as positively modifying the intestinal microbial population by decreasing pathogenic bacteria such as E. coli and increasing lactic acid bacteria [39,40]. PC stimulates short-chain fatty acid production and reduces intestine pathogens, resulting in gut health improvement [41]. High essential amino acid contents of Spirulina are critical for improving health status and BW, as well as decreasing health disorders and effects of heat stress [42].
Furthermore, PC is a hydrophilic protein that regulates vascular colloidal osmotic pressure to maintain equilibrium with bodily fluids [43,44]. PC possess antioxidant, anti-inflammatory, and immune-boosting properties [41,42,45]. Spirulina extracts have antimicrobial effects that restrict the development of pathogens, including Staphylococcus aureus, Escherichia coli, Salmonella typhi, and Klebsiella pneumonia (Kaushik and Chauhan, 2008). Spirulina has immunostimulant properties that improve the second humoral response against SRBC antigens of broilers [46,47].
Abdelnour et al. [39] have shown BW, average daily gain, and FCR improvements in rabbits growing under high ambient temperature with the addition of PC at 50, 100, and 150 mg/kg diet. Moreover, the addition of S. platensis in the diets improved the BW and BWG but decreased FCR of broilers [36,48,49] and weaned piglets [50]. In contrast, Mirzaie et al. [46] found that adding SP at 0.5, 1, or 2% to diets had no effect on BW, average daily BWG, FI, and FCR of broilers grown under high temperature. Furthermore, prior studies have reported that adding Spirulina has a nonsignificant effect on broilers’ performance parameters [51,52,53].
4.2. Blood Biochemical Parameters and Lipid Profile
Our findings showed that SPC had no influence on glucose levels, which resembled the findings of Zahir et al. [54], who demonstrated that dietary supplementation with SP at 0.5, 1, and 1.5% had no effect on glucose levels in broiler chicken. As the amino acid aspartate found in PC is crucial for protein synthesis and hormone liberation, SPC addition enhanced the serum total proteins, albumin, total globulins, and GH, as well as linear and quadratic increase in thyroxin [55]. Thyroid hormones have an important role in lipid homeostasis by transport and β-oxidation of fatty acids and cholesterol clearance [56]. Thyroid hormones increase the hydroxylase levels that transform cholesterol into bile acids, control cholesterol levels, and inhibit LDL-C apolipoprotein expression, reducing their serum levels [57]. Moreover, thyroid hormones adjust the expression of genes in lipogenesis [58]. Furthermore, the findings showed that SPC treatments lowered cholesterol, LDL-C, and triglycerides levels. Safari et al. [10] demonstrated that C-PC is a potent free radical scavenger that inhibits lipid peroxidation in zero time and sixty days later.
Moreover, previous studies in broilers have demonstrated that Spirulina supplementation lowered serum cholesterol levels, triglycerides, total lipids, and LDL compared to the control treatment [46,59,60]. In addition, plasma cholesterol level was reduced with Spirulina supplementation [5]. The hypolipidemic effect of SPC may be attributed to decreasing the lipase activity of the pancreas in a dose-dependent manner [7] and enhancing lipid peroxidation [61]. In contrast, Abdelnour et al. [39] found that adding PC to rabbit diets at 50, 100, and 150 mg/kg diet had no effect on triglyceride levels.
4.3. Antioxidant Activity and Inflammatory Markers
The activity of serum antioxidant enzymes (CAT, SOD, and TAC) increased linearly and quadratically. SPC supplementation reduced MDA linearly, which might be attributed to their potent antioxidant activity [62,63,64], resulting from radical-scavenging and metal chelation [65]. These findings are consistent with those of Abdelnour et al. [39], who discovered that adding 50 or 100 mg/kg PC to a rabbit diet increased TAC. Furthermore, Mirzaie et al. [46] and Moustafa et al. [60] showed that birds fed Spirulina-containing diets had higher SOD and total antioxidant activities and lower MDA values than those fed basal diets. Furthermore, Park et al., 2018, showed a linear increase in enzyme activities of SOD and GPx by Spirulina supplementation at 0.25, 0.5, 0.75, and 1% of broiler diets.
This study showed that SPC supplementation does not affect IL1β. IL1β is a potent proinflammatory cytokine essential for disease and infection responses [66] and is an integral member of the IL-1 family, secreted by different cell types. The anti-inflammatory properties of PC could be due to its potential to inhibit the synthesis of IL-1, IL-6, and TNF-gamma cytokines and inducible nitric oxide synthase activities (iNOS), enzymes of cyclooxygenase 2 (COX-2) [61], and the strong anti-inflammatory effect of C-phycocyanin [67]. However, adding PC at 50 and 100 mg/kg diet decreased the interleukin-4 and interferon-gamma levels of rabbits [39].
4.4. Histological Findings
The duodenum and jejunum play an important role in the digestion and absorption of nutrients in broilers. Well-developed small intestine results in improved nutrient utilization and further improves growth performance [3,68]. This intestinal development is measured using the morphometric measures of the VH and CD, with longer villus and lower CD, resulting in increased mucosal surface area and improved digestive efficacy [68,69]. In addition, the goblet cell count evaluates the small intestine condition [70]. This study showed increased villus height and width, VH: CD ratio, and goblet cells count of small intestine sections by SPC supplementation that suggested its positive effect on gut health, nutrient utilization, and growth. The findings are consistent with previous research on broilers, which stated that SPC supplementation positively affects villi height, CD, and goblet cell numbers of the intestine, improving their nutrients absorption, FCR, and body mass [71,72].
4.5. Immunohistochemical Examination
The anti-inflammatory effect of SPC supplementation at different levels showed a decreased immunolabelling index of caspase-3 and TNF-α. TNF-α is an inflammatory cytokine produced by macrophages/monocytes during acute inflammation and is essential in fighting cancer and infection. Caspase-3 is a lysosomal enzyme that destroys specific proteins and is required for the efficient execution of cell apoptosis. The anti-inflammatory properties of PC could be due to its aptitude to downregulate the expression of IL-1β, IL-2, interferon-γ, and TNF-α and increase IL-4 anti-inflammatory cytokines expression [73]. PC is a COX-2 inhibitor with hepatoprotective and anti-inflammatory activities [74]. Its hepatoprotective property is attributed to its ability to inhibit hepatocyte growth factor and TGF-β1 production, obstructing inflammatory infiltration [75]. Martinez et al. [76] demonstrated that PC preparation can limit TNF-α and interleukin-6 (IL-6) and can inhibit the formation of iNOS, COX-2, TNF-α, and neutrophil infiltration into the inflammation site [77].
5. Conclusions
The study concludes that SPC supplementation in broiler chicken diets could improve their performance, characterized by increased final BW and BWG and decreased FCR without affecting total FI. Dietary SPC increased the serum levels of total proteins, albumin, total globulins, growth, and thyroxin hormones, without affecting serum glucose and leptin levels. It acts as a hypolipidemic substance that decreases TC, LDL-C, and triglyceride levels, improves the intestinal histology, and enhances the antioxidant activity represented by increased TAC, CAT, and SOD activities. SPC supplementation decreases the MDA level and acts as an anti-inflammatory compound by downregulating the percentage of the immunolabelling index of caspase-3 and TNF-α. Therefore, SPC can be used as an alternative natural growth promoter, antioxidant, and anti-inflammatory feed additive for broilers production.
Acknowledgments
The authors extend their appreciation to the Faculty of Veterinary Medicine, Zagazig University, Egypt, and the management of the Kuwait Institute for Scientific Research (KISR) for their technical and financial support. The authors also would like to thank Taif University Research Supporting Project number (TURSP-2020/76), Taif University, Taif, Saudi Arabia, for supporting this research.
Author Contributions
Conceptualization, S.A.A. (Shimaa A. Amer); methodology, S.A.A. (Shimaa A. Amer) A.E.O., A.G., A.O., S.I.S., E.M.R., S.A.A. (Samar A. Abdo), S.A.A. (Sozan A. Ali), A.M.H. and H.S.A.-K.; resources, S.A.A. (Shimaa A. Amer); software, A.E.O.; formal analysis, A.E.O.; investigation, S.A.A. (Shimaa A. Amer); data curation, A.E.O.; visualization, S.A.A. (Shimaa A. Amer); writing—original draft, A.E.O. and S.A.A. (Shimaa A. Amer); writing—review and editing, A.E.O., S.A.A. (Shimaa A. Amer), and A.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All experiment procedures were approved by the Institutional Animal Care and Use Committee (ZU-IACUC) of Zagazig University, Egypt (Approval No. ZU-IACUC/2/F/16/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data contained in the article.
Conflicts of Interest
The authors declared that they have no conflict of interest.
Funding Statement
Taif University Research Supporting Project number (TURSP-2020/76), Taif University, Taif, Saudi Arabia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sørum H., Sunde M. Resistance to antibiotics in the normal flora of animals. Vet. Res. 2001;32:227–241. doi: 10.1051/vetres:2001121. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim R.E., Ahmed S.A., Amer S.A., Al-Gabri N.A., Ahmed A.I., Abdel-Warith A.-W.A., Younis E.-S.M., Metwally A.E. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020;18:100545. doi: 10.1016/j.aqrep.2020.100545. [DOI] [Google Scholar]
- 3.Amer S.A., Mohamed W.A., Gharib H.S., Al-Gabri N.A., Gouda A., Elabbasy M.T., El-Rahman A., Ghada I., Omar A.E. Changes in the growth, ileal digestibility, intestinal histology, behavior, fatty acid composition of the breast muscles, and blood biochemical parameters of broiler chickens by dietary inclusion of safflower oil and vitamin C. BMC Vet. Res. 2021;17:68. doi: 10.1186/s12917-021-02773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muthulakshmi M., Saranya A., Sudha M., Selvakumar G. Extraction, partial purification, and antibacterial activity of phycocyanin from Spirulina isolated from fresh water body against various human pathogens. J. Algal Biomass Util. 2012;3:7–11. [Google Scholar]
- 5.Mariey Y., Samak H., Ibrahem M. Effect of using Spirulina platensis algae as a feed additive for poultry diets: 1-productive and reproductive performances of local laying hens. Poult. Sci. 2012;32:201–215. [Google Scholar]
- 6.Xalxo R.K., Sao S., Sahu P.K. Effect of antibacterial properties of cyanobacterial Spirulina platensis. Int. J. Pharm. Life Sci. 2013;4:7. [Google Scholar]
- 7.Deng R., Chow T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010;28:e33–e45. doi: 10.1111/j.1755-5922.2010.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoseini S.M., Khosravi-Darani K., Mozafari M.R. Nutritional and medical applications of spirulina microalgae. Mini Rev. Med. Chem. 2013;13:1231–1237. doi: 10.2174/1389557511313080009. [DOI] [PubMed] [Google Scholar]
- 9.Minkova K., Tchernov A., Tchorbadjieva M., Fournadjieva S., Antova R., Busheva M.C. Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. J. Biotechnol. 2003;102:55–59. doi: 10.1016/S0168-1656(03)00004-X. [DOI] [PubMed] [Google Scholar]
- 10.Safari R., Amiri Z.R., Kenari R.E. Antioxidant and antibacterial activities of C-phycocyanin from common name Spirulina platensis. Iran. J. Fish. Sci. 2020;19:1911–1927. [Google Scholar]
- 11.Sivasankari S., Ravindran D., Ravindran D. Comparison of different extraction methods for phycocyanin extraction and yield from Spirulina platensis. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:904–909. [Google Scholar]
- 12.Kuddus M., Singh P., Thomas G., Ali A. Biotechnology of Bioactive Compounds: Sources and Applications. Wiley; Hoboken, NJ, USA: 2015. Production of cphycocyanin and its potential applications; pp. 283–299. [Google Scholar]
- 13.Romay C., Gonzalez R., Ledon N., Remirez D., Rimbau V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- 14.Amer S.A., Al-Khalaifah H.S., Gouda A., Osman A., Goda N.I., Mohammed H.A., Darwish M.I., Hassan A.M., Mohamed S.K.A. Potential Effects of Anthocyanin-Rich Roselle (Hibiscus sabdariffa L.) Extract on the Growth, Intestinal Histomorphology, Blood Biochemical Parameters, and the Immune Status of Broiler Chickens. Antioxidants. 2022;11:544. doi: 10.3390/antiox11030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doan H.V., Hoseinifar S.H., Hung T.Q., Lumsangkul C., Jaturasitha S., El-Haroun E., Paolucci M. Dietary inclusion of chestnut (Castanea sativa) polyphenols to Nile tilapia reared in biofloc technology: Impacts on growth, immunity, and disease resistance against Streptococcus agalactiae. Fish Shellfish. Immunol. 2020;105:319–326. doi: 10.1016/j.fsi.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 16.El-Araby D.A., Amer S.A., Attia G.A., Osman A., Fahmy E.M., Altohamy D.E., Alkafafy M., Elakkad H.A., Tolba S.A. Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture. 2022;546:737413. doi: 10.1016/j.aquaculture.2021.737413. [DOI] [Google Scholar]
- 17.Demirel Z., Yilmaz-Koz F.F., Karabay-Yavasoglu U.N., Ozdemir G., Sukatar A. Antimicrobial and antioxidant activity of brown algae from the Aegean Sea. J. Serb. Chem. Soc. 2009;74:619–628. doi: 10.2298/JSC0906619D. [DOI] [Google Scholar]
- 18.Sarada R., Pillai M.G., Ravishankar G. Phycocyanin from Spirulina sp.: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999;34:795–801. doi: 10.1016/S0032-9592(98)00153-8. [DOI] [Google Scholar]
- 19.Salama A., Ghany A.A., Osman A., Sitohy M. Maximising phycocyanin extraction from a newly identified Egyptian cyanobacteria strain: Anabaena oryzae SOS13. Int. Food Res. J. 2015;22:517–525. [Google Scholar]
- 20.Patel A., Mishra S., Pawar R., Ghosh P. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005;40:248–255. doi: 10.1016/j.pep.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Aviagen R. Ross Broiler Management Manual, 2009. Vol. 9. ROSS; Richmond, VA, USA: 2014. pp. 350–364. [Google Scholar]
- 22.American Veterinary Medical Association . AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. American Veterinary Medical Association; Schaumburg, IL, USA: 2013. [Google Scholar]
- 23.Grant G.H., Silverman L.M., Christenson R.H. Amino acids and proteins. In: Tietz N.Z., editor. Fundamentals of Clinical Chemistry. WB Saunders; Philadelphia, PA, USA: 1987. pp. 291–345. [Google Scholar]
- 24.Doumas B., Baysa D., Carter R., Peters T., Schaffer R. Determination of serum total protein. Clin Chem. 1981;27:1642. doi: 10.1093/clinchem/27.10.1642. [DOI] [PubMed] [Google Scholar]
- 25.Doumas B., Biggs H. Determination of serum albumin in standard method of clinical chemistry. Stand. Methods Clin. Chem. 1972;7:175–188. [Google Scholar]
- 26.McGowan M.W., Artiss J.D., Strandbergh D.R., Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 1983;29:538–542. doi: 10.1093/clinchem/29.3.538. [DOI] [PubMed] [Google Scholar]
- 27.Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. doi: 10.1093/clinchem/20.4.470. [DOI] [PubMed] [Google Scholar]
- 28.Vassault A., Grafmeyer D., Naudin C., Dumont G., Bailly M., Henny J., Gerhardt M., Georges P. Protocole de validation de techniques. Ann. Biol. Clin. 1986;44:45. [Google Scholar]
- 29.Mcdonald R.E., Hultin H.O. Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J. Food Sci. 1987;52:15–21. doi: 10.1111/j.1365-2621.1987.tb13964.x. [DOI] [Google Scholar]
- 30.Rice-Evans C., Miller N.J. 241 Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994;234:279–293. doi: 10.1016/0076-6879(94)34095-1. [DOI] [PubMed] [Google Scholar]
- 31.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 32.Nishikimi M., Rao N.A., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 33.Suvarna S., Layton C., Bancroft J. Bancroft’s Theory and Practice of Histological Techniques. 7th ed. Churchill Livingstone; London, UK: 2013. The hematoxylins and eosin; pp. 172–186. [Google Scholar]
- 34.Saber S., Khalil R.M., Abdo W.S., Nassif D., El-Ahwany E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019;364:120–132. doi: 10.1016/j.taap.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Rizzardi A.E., Johnson A.T., Vogel R.I., Pambuccian S.E., Henriksen J., Skubitz A.P., Metzger G.J., Schmechel S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012;7:42. doi: 10.1186/1746-1596-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J., Lee S., Kim I. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult. Sci. 2018;97:2451–2459. doi: 10.3382/ps/pey093. [DOI] [PubMed] [Google Scholar]
- 37.Evans A., Smith D., Moritz J. Effects of algae incorporation into broiler starter diet formulations on nutrient digestibility and 3 to 21 d bird performance. J. Appl. Poult. Res. 2015;24:206–214. doi: 10.3382/japr/pfv027. [DOI] [Google Scholar]
- 38.Tavernari F.D.C., Roza L., Surek D., Sordi C., Silva M., Albino L., Migliorini M., Paiano D., Boiago M. Apparent metabolisable energy and amino acid digestibility of microalgae Spirulina platensis as an ingredient in broiler chicken diets. Br. Poult. Sci. 2018;59:562–567. doi: 10.1080/00071668.2018.1496401. [DOI] [PubMed] [Google Scholar]
- 39.Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y., El-Hack M.E.A., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. doi: 10.1080/1828051X.2020.1815598. [DOI] [Google Scholar]
- 40.Alwaleed E.A., El-Sheekh M., Abdel-Daim M.M., Saber H. Effects of Spirulina platensis and Amphora coffeaeformis as dietary supplements on blood biochemical parameters, intestinal microbial population, and productive performance in broiler chickens. Environ. Sci. Pollut. Res. 2021;28:1801–1811. doi: 10.1007/s11356-020-10597-3. [DOI] [PubMed] [Google Scholar]
- 41.Xie Y., Li W., Zhu L., Zhai S., Qin S., Du Z. Effects of phycocyanin in modulating the intestinal microbiota of mice. MicrobiologyOpen. 2019;8:e00825. doi: 10.1002/mbo3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osman A., Abd-Elaziz S., Salama A., Eita A.A., Sitohy M. Health protective actions of phycocyanin obtained from an Egyptian isolate of Spirulina platensis on albino rats. EurAsian J. BioSciences. 2019;13:105–112. [Google Scholar]
- 43.Wu Q., Liu L., Miron A., Klímová B., Wan D., Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016;90:1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 44.Aladaileh S.H., Khafaga A.F., El-Hack M.E.A., Al-Gabri N.A., Abukhalil M.H., Alfwuaires M.A., Bin-Jumah M., Alkahtani S., Abdel-Daim M.M., Aleya L. Spirulina platensis ameliorates the sub chronic toxicities of lead in rabbits via anti-oxidative, anti-inflammatory, and immune stimulatory properties. Sci. Total Environ. 2020;701:134879. doi: 10.1016/j.scitotenv.2019.134879. [DOI] [PubMed] [Google Scholar]
- 45.Farag M.R., Alagawany M., El-Hack M., Dhama K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2016;12:36–51. doi: 10.3923/ijp.2016.36.51. [DOI] [Google Scholar]
- 46.Mirzaie S., Zirak-Khattab F., Hosseini S.A., Donyaei-Darian H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas. J. Anim. Sci. 2018;31:556. doi: 10.5713/ajas.17.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qureshi M., Garlich J., Kidd M. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacol. Immunotoxicol. 1996;18:465–476. doi: 10.3109/08923979609052748. [DOI] [PubMed] [Google Scholar]
- 48.Kharde S., Shirbhate R., Bahiram K., Nipane S. Effect of Spirulina supplementation on growth performance of broilers. Indian J. Vet. Res. 2012;21:66–69. [Google Scholar]
- 49.Shanmugapriya B., Babu S.S. Supplementary effect of Spirulina platensis on performance, hematology and carcass yield of broiler chicken. Indian Streams Res. J. 2014;4:1–7. [Google Scholar]
- 50.Zhang L., Jin P., Qin S., Liu J., Yang Z., Zhao H., Sheng Q. Effects of dietary supplementation with S. platensis and probiotics on the growth performance, immune response and the fecal Lactobacillus spp. and E. coli contents of weaned piglets. Livest. Sci. 2019;225:32–38. doi: 10.1016/j.livsci.2019.04.013. [DOI] [Google Scholar]
- 51.Bonos E., Kasapidou E., Kargopoulos A., Karampampas A., Nikolakakis I., Christaki E., Florou-Paneri P. Spirulina as a functional ingredient in broiler chicken diets. South Afr. J. Anim. Sci. 2016;46:94–102. doi: 10.4314/sajas.v46i1.12. [DOI] [Google Scholar]
- 52.Gongnet G., Niess E., Rodehutscord M., Pfeffer E. Algae-meal (Spirulina platensis) from lake Chad replacing soybean-meal in broiler diets. Archiv fur Geflugelkunde. 2001;65:265–268. [Google Scholar]
- 53.Toyomizu M., Sato K., Taroda H., Kato T., Akiba Y. Effects of dietary Spirulina on meat colour in muscle of broiler chickens. Br. Poult. Sci. 2001;42:197–202. doi: 10.1080/00071660120048447. [DOI] [PubMed] [Google Scholar]
- 54.Zahir U., AnwarulHaque B., Maksuda B., Mahfuj U.M. Effect of dietary supplement of algae (Spirulina platensis) as an alternative to antibiotics on growth performance and health status of broiler chickens. J. Poult. Sci. 2019;18:576–584. [Google Scholar]
- 55.Ruth M.R., Field C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013;4:27. doi: 10.1186/2049-1891-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha R.A., Singh B.K., Yen P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldberg I.J., Huang L.-S., Huggins L.A., Yu S., Nagareddy P.R., Scanlan T.S., Ehrenkranz J.R. Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway. Endocrinology. 2012;153:5143–5149. doi: 10.1210/en.2012-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., Viscarra J., Kim S.-J., Sul H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdel-Moneim A.-M.E., Shehata A.M., Mohamed N.G., Elbaz A.M., Ibrahim N.S. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol. Trace Elem. Res. 2022;200:768–779. doi: 10.1007/s12011-021-02662-w. [DOI] [PubMed] [Google Scholar]
- 60.Moustafa E.S., Alsanie W.F., Gaber A., Kamel N.N., Alaqil A.A., Abbas A.O. Blue-green algae (Spirulina platensis) alleviates the negative impact of heat stress on broiler production performance and redox status. Animals. 2021;11:1243. doi: 10.3390/ani11051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang J.H., Chen J.C., Yang S.Y., Wang M.F., Liu T.C., Chan Y.C. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus. Laryngoscope. 2011;121:361–364. doi: 10.1002/lary.21283. [DOI] [PubMed] [Google Scholar]
- 62.Bhat V.B., Madyastha K. C-phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Commun. 2000;275:20–25. doi: 10.1006/bbrc.2000.3270. [DOI] [PubMed] [Google Scholar]
- 63.Estrada J.P., Bescós P.B., Fresno A.V.D. Antioxidant activity of different fractions of Spirulina platensis protean extract. Il Farmaco. 2001;56:497–500. doi: 10.1016/S0014-827X(01)01084-9. [DOI] [PubMed] [Google Scholar]
- 64.Khan Z., Bhadouria P., Bisen P. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005;6:373–379. doi: 10.2174/138920105774370607. [DOI] [PubMed] [Google Scholar]
- 65.Bermejo P., Piñero E., Villar Á.M. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chem. 2008;110:436–445. doi: 10.1016/j.foodchem.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 66.Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. doi: 10.1182/blood.V87.6.2095.bloodjournal8762095. [DOI] [PubMed] [Google Scholar]
- 67.Romay C., Ledón N., González R. Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation. Inflamm. Res. 1998;47:334–338. doi: 10.1007/s000110050338. [DOI] [PubMed] [Google Scholar]
- 68.Amer S.A., Beheiry R.R., Fattah D.M.A., Roushdy E.M., Hassan F.A., Ismail T.A., Zaitoun N., Abo-Elmaaty A., Metwally A.E. Effects of different feeding regimens with protease supplementation on growth, amino acid digestibility, economic efficiency, blood biochemical parameters, and intestinal histology in broiler chickens. BMC Vet. Res. 2021;17:283. doi: 10.1186/s12917-021-02946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amer S.A., Naser M.A., Abdel-Wareth A.A., Saleh A.A., Elsayed S.A., Metwally A.E. Effect of dietary supplementation of alpha-galactosidase on the growth performance, ileal digestibility, intestinal morphology, and biochemical parameters in broiler chickens. BMC Vet. Res. 2020;16:144. doi: 10.1186/s12917-020-02359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amer S.A., Tolba S.A., AlSadek D.M., Fattah D.M.A., Hassan A.M., Metwally A.E. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet. Res. 2021;17:312. doi: 10.1186/s12917-021-03022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan S., Mobashar M., Mahsood F.K., Javaid S., Abdel-Wareth A., Ammanullah H., Mahmood A. Spirulina inclusion levels in a broiler ration: Evaluation of growth performance, gut integrity, and immunity. Trop. Anim. Health Prod. 2020;52:3233–3240. doi: 10.1007/s11250-020-02349-9. [DOI] [PubMed] [Google Scholar]
- 72.Shanmugapriya B., Babu S., Hariharan T., Sivaneswaran S., Anusha M., Raja P.U. Synergistic effect of Spirulina platensis on performance and gut microbial load of broiler chicks. Ind. Asian J. Multidiscip. Res. 2015;1:149–155. [Google Scholar]
- 73.Saini M.K., Vaish V., Sanyal S.N. Role of cytokines and Jak3/Stat3 signaling in the 1, 2-Dimethylhydrazine Dihydrochloride-induced rat model of colon carcinogenesis. Eur. J. Cancer Prev. 2013;22:215–228. doi: 10.1097/CEJ.0b013e3283584932. [DOI] [PubMed] [Google Scholar]
- 74.Reddy C.M., Bhat V.B., Kiranmai G., Reddy M.N., Reddanna P., Madyastha K. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000;277:599–603. doi: 10.1006/bbrc.2000.3725. [DOI] [PubMed] [Google Scholar]
- 75.Ou Y., Zheng S., Lin L., Jiang Q., Yang X. Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocyte damage in vitro and in vivo. Chem. Biol. Interact. 2010;185:94–100. doi: 10.1016/j.cbi.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Martinez S.E., Chen Y., Ho E.A., Martinez S.A., Davies N.M. Pharmacological effects of a C-phycocyanin-based multicomponent nutraceutical in an in-vitro canine chondrocyte model of osteoarthritis. Can. J. Vet. Res. 2015;79:241–249. [PMC free article] [PubMed] [Google Scholar]
- 77.Shih C.-M., Cheng S.-N., Wong C.-S., Kuo Y.-L., Chou T.-C. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth. Analg. 2009;108:1303–1310. doi: 10.1213/ane.0b013e318193e919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data contained in the article.