Abstract
Long-term ingestion of arsenicals, a heterogeneous group of toxic compounds, has been associated with a wide spectrum of human pathologies, which include various malignancies. Although their mechanism of toxicity remains largely unknown, it is generally believed that arsenicals mainly produce their effects via direct binding to protein thiols and ROS formation in different subcellular compartments. The generality of these mechanisms most probably accounts for the different effects mediated by different forms of the metalloid in a variety of cells and tissues. In order to learn more about the molecular mechanisms of cyto- and genotoxicity, there is a need to focus on specific arsenic compounds under tightly controlled conditions. This review focuses on the mechanisms regulating the mitochondrial formation of ROS after exposure to low concentrations of a specific arsenic compound, NaAsO2, and their crosstalk with the nuclear factor (erythroid-2 related) factor 2 antioxidant signaling and the endoplasmic reticulum stress response.
Keywords: arsenic, arsenite, mitochondrial ROS, endoplasmic reticulum stress, toxicity, Nrf2
1. Introduction
Arsenicals are natural components of the Earth’s crust, normally present in traces in rocks, soil, water, air, plants, and animals. However, due to natural conditions or human activities [1], their levels are significantly increased in various regions, in particular in Bangladesh and West Bengal (India), in which human exposure to contaminated groundwater sources has been described by the World Health Organization (WHO) as “the largest mass poisoning of a population in history” [2]. Contamination of underground water occurs in other countries as well, including Chile, Argentina, Mexico, the USA, China, Taiwan, and Thailand [3].
Long-term exposure to arsenicals has been associated with an increased risk of skin, bladder, lung, kidney, and liver cancer [4] as well as with other non-cancerous diseases [4] and metabolism-related pathologies [5]. Furthermore, prenatal exposure to arsenicals impairs foetal brain development and cognition and increases the number of deaths in young adults [6].
Arsenic carcinogenesis has been associated with the induction of oxidative stress [7], genotoxicity [8], and epigenetic mechanisms [9].
Arsenic is a metalloid, sharing characteristics of both metals and non-metals. It exhibits a variety of oxidation states and can be found in the environment in different inorganic and organic forms in combination with other elements. The dominant forms are represented by trivalent and pentavalent arsenic, the former being remarkably more toxic than the latter [10]. Arsenic trioxide (As2O3), sodium arsenite (NaAsO2), and arsenic trichloride (AsCl3) are the most common inorganic trivalent compounds. Arsenical is metabolised via biomethylation, normally associated with reduced toxicity, with numerous reported exceptions [11].
NaAsO2, from now on defined as arsenite, avidly binds to -SH groups in proteins, thereby (possibly) altering their conformation, function, and interactions with other proteins. Consistently, various studies have shown that arsenite inactivates key cellular enzymes, especially those involved in the regulation of cellular energy pathways as well as DNA synthesis and repair [12].
Not surprisingly, arsenite also promotes the formation of reactive oxygen species (ROS) [11,13,14,15], commonly believed to account for both the cytotoxic and genotoxic effects of the metalloid [11,13,14,15,16,17], via yet poorly defined mechanisms, which however involve the mitochondrial respiratory chain [18,19,20] and the enzyme NADPH oxidase [21,22,23].
The notion that both the aforementioned mechanisms are Ca2+ dependent [24,25,26] implies that their recruitment is regulated by the relative concentrations of Ca2+ in specific sub-cellular microdomains, i.e., mitochondria for mitochondrial ROS formation or restricted cytosolic/membrane compartments for NADPH oxidase-derived ROS formation.
Thus, the effects of arsenic on Ca2+ homeostasis seem to be pivotal for the activation of a specific mechanism of ROS formation [27,28,29], a consideration bearing two important implications. The first one is that the endoplasmic reticulum (ER) represents a critical target of the metalloid [30,31,32,33,34,35], which is indeed considered a potent ER stressor [30,31,32,33,34,35]. The second implication is that arsenite potentially induces different Ca2+ responses and hence triggers different mechanisms of ROS formation in cell types characterised by a different functional organisation of their ER.
Deregulation of Ca2+ homeostasis also significantly impacts the triggering of specific toxicity mechanisms of the metalloid. An increased mitochondrial concentration of Ca2+ ([Ca2+]m), besides favouring the formation of mitochondrial superoxide (mitoO2•−), also promotes the mitochondrial permeability transition (MPT) and the MPT-dependent apoptosis [36,37,38]. MPT-independent mechanisms might on the other hand, mediate toxicity under conditions in which the metalloid elevates the cytosolic concentration of Ca2+ ([Ca2+]c) in the absence of significant increases in the [Ca2+]m [39,40]. Furthermore, as indicated above, arsenite is an important ER stressor, and ER stress may critically crosstalk with, and contribute to, both MPT-dependent and -independent mechanisms of toxicity [41,42,43,44,45,46,47].
ROS formation is often associated with activation of nuclear factor (erythroid-2 related) factor 2 (Nrf2) antioxidant signaling [48,49]. Arsenite-derived ROS may, therefore, differentially activate this response when generated in different subcellular compartments. In addition, there are other potential mechanisms whereby arsenite may activate Nrf2 and promote survival.
In this review, the points raised above will be analysed and discussed. In particular, we will focus on the mechanism(s) whereby arsenite promotes mitochondrial Ca2+ accumulation and ROS formation, as well as on the crosstalk between these events and the Nrf2 and ER stress responses.
2. Arsenite Promotes the Mitochondrial Formation of Superoxide and Downstream Intra-Mitochondrial and Extra-Mitochondrial Effects
It is well known that mitochondria release significant amounts of ROS after stimulation mediated by various toxins [50,51]. Although multiple mechanisms contribute to this event [51,52], the respiratory chain by far represents the most efficient mechanism of mitoO2•− formation [26,51,53]. Importantly, once released in the matrix, O2•− is promptly converted by superoxide dismutase 2 (SOD2) to H2O2, which can now be degraded by glutathione (GSH) peroxidase, peroxiredoxins 3 and 5 or catalase, thereby preventing further reactions of the oxidant. However, the fraction of H2O2 escaping these interactions may eventually generate mitochondrial lesions through the formation of hydroxyl radicals, requiring the reaction of the oxidant with divalent iron (Fenton reaction). mitoO2•−, besides being a preferential substrate for SOD2-dependent conversion to H2O2, it can also interact with nitric oxide to generate the highly reactive peroxynitrite, thereby contributing to the formation of mitochondrial damage and dysfunction [18].
Importantly, mitoO2•−-derived H2O2 escaping metabolism by H2O2-detoxifying enzymes can also stimulate an array of signalling pathways [52,53] or generate deleterious effects on distal targets, such as various cytosolic proteins, genomic DNA, etc. [53]. These events are associated with the neutral and diffusible nature of the oxidant, which can easily cross the inner and outer mitochondrial membranes [26,51,53]. mitoO2•− is also directly released in the intermembrane space [26], in which conversion to H2O2 is mediated by SOD1 [26], and its interaction with nitric oxide leads to the extra-mitochondrial formation of peroxynitrite [18].
The notion that arsenite under specific conditions generates mitoO2•− is well documented [15,18,19,20], and the general idea is that this event is causally linked to the induction of mitochondrial dysfunction [18,19,41,42], and MPT-dependent apoptosis [41,42,43,44]. Likewise, mitoO2•−-derived H2O2 also mediates extra-mitochondrial effects, such as strand scission of genomic DNA [54], or the activation of the Nrf2 signalling pathway [55].
Our previous work has contributed to defining these events using U937 cells (which do not express nitric oxide synthase and are therefore unable to generate nitric oxide) exposed to low micromolar concentrations of arsenite. Under these conditions, the metalloid uniquely promoted the formation of mitoO2•−, i.e., without affecting ROS formation. The mitochondrial origin of the ROS induced by arsenite was established using various strategies, which include the use of specific fluorescent probes [56] and the inhibition of the activity of mitochondrial aconitase [54], an enzyme particularly sensitive to mitoO2•− [57]. Moreover, ROS formation was suppressed by rotenone, a complex I inhibitor [58], or by the respiration-deficient phenotype [54], as well as by ascorbic acid supplementation [54], which is associated with the accumulation of millimolar mitochondrial concentrations of the vitamin [59]. Under the same conditions, we also observed suppression of early events, including DNA damage [54] and Nrf2 activation [55], as well as delayed mitochondrial dysfunction and apoptosis [60].
Thus, convincing experimental evidence documents the ability of arsenite to promote, under specific conditions, mitoO2•− formation and a cascade of events leading to cyto- and genotoxicity.
3. ER-Mitochondria Crosstalk Regulates Arsenite-Induced mitoO2•− Formation
Various agents have previously been reported to increase the rate of mitoO2•− formation via Ca2+-dependent mechanisms [25,61,62], thereby implying that the onset of the ROS response is critically connected to other events causing an increased [Ca2+]m [25,61]. The notion that arsenite affects Ca2+ homeostasis has been demonstrated in numerous studies [27,28,29,63]. This event, however, is not necessarily associated with the mitochondrial uptake of the cation. It is indeed well established that mitochondrial Ca2+ accumulation takes place via low-affinity transport mechanisms mediated by the mitochondrial Ca2+ uniporter (MCU) [62,64]. High Ca2+ concentrations are thus necessary for MCU-dependent transport, and these conditions are met at contact sites between the ER and mitochondria [65,66].
Recent studies from our laboratory have provided details of the effects of arsenite in the ER. Briefly, in keeping with the findings of others [28,63], we initially found that the inositol-1,4,5-triphosphate receptor (IP3R) is a primary target of the metalloid [67]. However, we also noticed that the resulting release of Ca2+, while limited and mediated by a saturable mechanism, was nevertheless critical to promoting a further release of the cation from the ryanodine receptor (RyR) [67]. Most importantly, the fraction of Ca2+ accumulated by the mitochondria was entirely derived from the RyR [54], thereby implying the relevance of this Ca2+ channel in processes associated with arsenite-induced mitochondrial Ca2+ accumulation. A cause–effect relationship between the increased [Ca2+]m and the mitoO2•− formation was next established by showing remarkable similarities in the time-dependence of these events [68], as well as the suppression of mitoO2•− formation mediated by treatments preventing Ca2+ mobilisation from the IP3R or RyR or the transport of the cation in mitochondria [56]. Consistently, arsenite failed to increase the [Ca2+]m and to promote the formation of mitoO2−. in cell types, which do not express the RyR, such as HeLa cells, differentiated U937 cells, or undifferentiated C2C12 cells [56].
Thus, arsenite causes mitoO2•− formation only in cell types, such as undifferentiated U937 cells or differentiated C2C12 cells, concomitantly expressing the IP3R and the RyR, with the latter channel in a close spatial and functional connection with the mitochondria [56].
We recently determined the mechanism regulating the crosstalk between the IP3R and the RyR, apparently based on the activation/expression of ER oxidoreductin 1α (ERO1α) [69]. More specifically, we found that the fraction of Ca2+ released by the IP3R (and RyR) was critical to activating ERO1α and that ERO1α was critical to promoting Ca2+ release from the RyR in microdomains sensed by the mitochondria [69]. Given the importance of the increased [Ca2+]m in events associated with the mitoO2•− formation, it appears clear that the above positive feedback amplification cycle is pivotal for the regulation of this response in cells exposed to arsenite.
However, the increased [Ca2+]m represents a condition necessary but not sufficient to promote the mitoO2•− formation, which in fact requires additional effects in the mitochondrial respiratory chain [68]. It was therefore interesting to observe that the concentration and time of exposure requirements for the effects of arsenite in the mitochondrial respiratory chain were significantly lower than those necessary for the triggering of the Ca2+ responses described above [68]. Remarkably, a 10 min exposure to RyR or IP3-releasing agonists, which rapidly increase the [Ca2+]m [68], was sufficient to induce maximal mitoO2•− emission in the presence of very low concentrations of arsenite. Thus, the effects of arsenite in the mitochondrial respiratory chain are also necessary but not sufficient and, most importantly, present much lower concentration and time of exposure requirements in comparison with Ca2+ mobilisation and mitochondrial accumulation.
Based on the observed Ca2+-dependence and sensitivity to rotenone, our findings are in keeping with the possibility that arsenite promotes its effects in the mitochondrial respiratory chain via a mechanism involving inhibition of complex III, a notion that should be experimentally established. Other studies have reported that arsenite effects follow a nonlinear dose-response in which low concentrations promote increased expression and activity of complex I and, under the same conditions, elicit cytoprotective signalling [19]. The effects mediated by the high concentrations were instead associated with reduced activity of the electron transport chain. These results, while interesting, are difficult to compare with those obtained in our studies since specific questions related to the concomitant changes in Ca2+ homeostasis were not addressed. In particular, the lack of complex III inhibition could depend on the lack of mitochondrial Ca2+ accumulation.
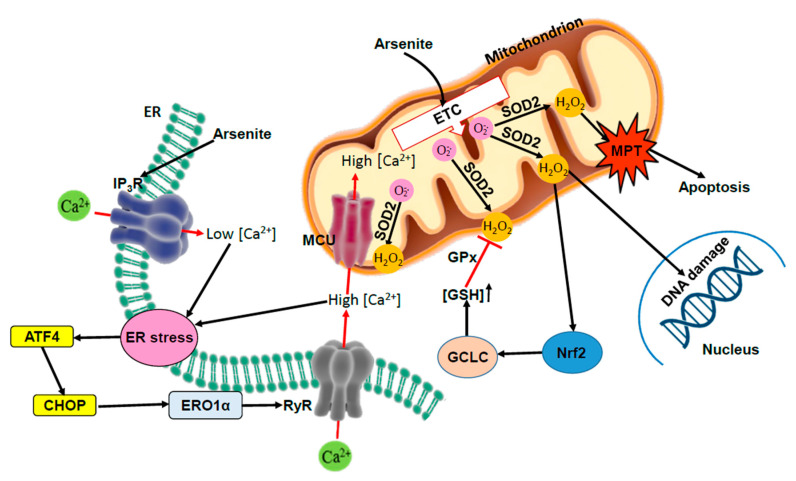
We, therefore, conclude that the process of mitoO2•− formation requires two separate effects induced by arsenite. The first one is rapidly induced by low concentrations of the metalloid at the level of the mitochondrial respiratory chain, whereas the second, much slower and requiring greater arsenite concentrations, is targeted on the ER and associated with Ca2+ mobilisation from the IP3R, the recruitment of the RyR regulated by ERO1α and the mitochondrial accumulation of the cation (Figure 1).
Figure 1.
Crosstalk between arsenite-induced mitochondrial ROS, ER stress, and Nrf2. Arsenite promotes mitoO2•− formation via a mechanism requiring interactions with the mitochondrial respiratory chain and an accumulation of Ca2+ in these organelles. The metalloid initially stimulates Ca2+ release from the IP3R, which, although not directly taken up by the mitochondria, nevertheless contributes to this event via RyR stimulation. Indeed, due to the close apposition with the mitochondria, only the fraction of Ca2+ the RyR can be taken up by the mitochondria. Cells uniquely expressing the IP3R, in which these channels are in close contact with the mitochondria, failed to generate mitoO2•− in response to arsenite. RyR activation was regulated by ERO1α and the resulting mitochondrial accumulation of Ca2+ was critical for the formation of mitoO2•. In this perspective, while the ER stress response appears upstream to mitoO2•− formation, it is nevertheless reasonable to predict that persistent mitoO2•−-derived H2O2 promotes mitochondrial dysfunction and toxicity. The early ER stress response was also critically connected through mitoO2• formation with the triggering of the Nrf2 cytoprotective signaling, which indeed significantly mitigated and delayed the onset of MPT-dependent apoptosis.
4. Effect of Arsenic on Nrf2 and Its Target Genes
Cells respond to potentially toxic compounds with several adaptive mechanisms. One such mechanism is represented by the induction of phase II drug-metabolizing enzymes in response to electrophiles via the electrophile response element, which is present in several genes involved in the detoxification of xenobiotics [70]. This definition was then broadened to that of antioxidant response element (ARE) [71]. Several transcription factors bind ARE sequences, particularly Nrf2 [71,72], which is considered a master regulator of the transcription of several genes coding for enzymes with antioxidant functions.
Nrf2-regulates antioxidant defence genes, including GSH biosynthetic enzymes, antioxidant enzymes, and the GSH-regenerating enzyme, glutathione reductase (GR) [73,74]. Other Nrf2 target genes are important in maintaining protein thiols in the reduced state and include thioredoxin (Trx), Trx reductase, and sulfiredoxin, as well as genes involved in energetic metabolism, iron metabolism, survival, proliferation, autophagy, proteasomal degradation, DNA repair, and mitochondrial physiology [72,75]. In addition, Nrf2 activation is associated with the induction of mitochondrial antioxidant enzymes such as Trx reductase-2, peroxiredoxins 3 and 5, GSH peroxidase, and SOD2, highlighting a role for Nrf2 in the control of mitochondrial redox homeostasis [76]. Nrf2 also regulates mitochondrial biogenesis by influencing the expression levels of coactivators and critical transcription factors [74,77,78].
In the absence of oxidants/electrophiles, Nrf2 associates with Kelch ECH-associated protein 1 (KEAP1), which, in association with cullin 3, targets Nrf2 for ubiquitinylation and proteasomal degradation. In the canonical pathway of Nrf2 activation, electrophiles and ROS react with KEAP1 cysteines, affecting their conformation and thus impeding Nrf2 ubiquitylation [79]. As a result, Nrf2 translocates into the nucleus and activates the transcription of genes containing the ARE. Once the levels of ROS get low, KEAP1 turns again Nrf2 signalling off [80].
The finding of the redox sensitivity of Nrf2 has emphasised its importance in the adaptation to ROS rather than just xenobiotics. Consistent with this, experiments with Nrf2 knockout mice have shown its importance in protecting from pulmonary oxygen toxicity through the induction of ROS-detoxifying enzymes [81] and in hyperoxia-induced retinopathy of prematurity [82]. Nrf2 is also activated in post-ischemic reperfusion injury where it confers protection [83].
Several studies reported activation of the Nrf2 pathway by arsenite, which is consistent with its reactivity towards cysteines, and gene expression profiling of human bronchial epithelial cells exposed to different concentrations of the metalloid showed induction of some Nrf2 target genes [84]. Heme oxygenase induction has been reported in vivo or in vitro in several studies with various arsenical compounds, including arsenite [85].
It should be noted, however, that the results of Nrf2 activation on GSH levels depend on the experimental model used. A recent meta-analysis of 88 studies on the effect of arsenite on Nrf2 [86] has shown that while in vivo administration of the metalloid causes a depletion of cellular GSH, in vitro it increases it. This probably reflects the overall balance between the effect of GSH depletion, either directly by the metalloid or via overproduction of ROS, and the effect of GSH synthesis, which is promoted by Nrf2. This seems supported by another recent meta-analysis of 39 in vivo studies showing that, while arsenite consistently induces Nrf2 target genes, it also induces ROS generation and depletes GSH levels [87].
The mechanism by which arsenite can activate Nrf2 is complex. Mutation of specific cysteines (particularly Cys51) on KEAP1 shows that this is important in the interaction with arsenite [88], but arsenite can also directly bind cysteines of Nrf2, and these are also important in its transcriptional activation, as shown by studies with cysteine mutants [89]. Other pathways contribute to arsenite-induced Nrf2 activation. Arsenite induces p62 accumulation due to dysregulated autophagy flux [90,91,92], and accumulation of p62 then results in the sequestration of KEAP1 in the autophagosomes, impairing Nrf2 degradation [93]. On the other hand, p62 is a downstream gene of Nrf2, therefore implying a positive feedback loop [90]. In addition, arsenite induces acetylation of Nrf2 by p300/CREB (cAMP response element-binding protein), which enhances Nrf2’s binding capacity to promoter-specific DNA [94].
Many studies reported that arsenite increases Nrf2 mRNA levels [87], suggesting additional mechanisms to the classical transcriptional activation mediated by regulation of Nrf2 stability and its degradation. As will be discussed below, arsenite can promote an ER stress response and induce PERK-mediated activation of Nrf2 [95] or activating transcription factor 4 (ATF4)-dependent transcriptional regulation of Nrf2 [96].
The main mechanisms by which arsenite can activate Nrf2 are highlighted in Figure 2, in which the possibility of an indirect mechanism, i.e., via the formation of ROS, is also included. A final consideration is that activation of Nrf2 is, in general, of critical importance to mitigating arsenite toxicity, although its persistent activation associated with prolonged exposure to the metalloid may, in fact, promote deleterious events enhancing its cancerogenicity [97,98].
Figure 2.
Mechanisms of Nrf2 activation by arsenite. The different pathways by which arsenite can promote the transcriptional activation of Nrf2 are highlighted. ① Arsenite stimulates ROS production by NADPH oxidase in the plasma membrane and by mitochondria; ② ROS and arsenite oxidize Keap1 and Nrf2 directly or induce Keap1 sequestration via p62; ③ arsenite causes ER stress which activates Nrf2 via PERK; ④ as a result, Nrf2 is freed, translocates to the nucleus and activates the transcription of several enzymes; ⑤ additionally, arsenite can induce neosynthesis of Nrf2 protein at the transcriptional level.
5. Arsenite-Dependent Regulation of Nrf2 by Mitochondrial ROS: Impact on Survival vs. Apoptotic Signalling
It is well established that ROS can induce Nrf2 activation via a mechanism involving Keap-1 oxidation and suppression of Keap-1-dependent Nrf2 degradation [79,80]. Thus, as indicated in the previous section, arsenite can activate Nrf2 through this indirect mechanism [91,92,99] driven by both NADPH-oxidase- and mitochondria-derived ROS. Although it is at present unclear whether these two mechanisms similarly or differentially activate Nrf2, the diffusible nature of H2O2 is consistent with the possibility of an effective and similar contribution.
The notion that mitochondrial ROS activates Nrf2 is well established [100,101], and it is also clear that Nrf2 target genes can exert beneficial effects in mitochondria through different mechanisms, which include up-regulation of the mitochondrial antioxidant defence [76], mitophagy [78,102], and mitochondriogenesis [74,77,78].
These findings, therefore, imply a beneficial role of mitoO2•−, mediated by different mechanisms converging into Nrf2 activation-dependent mitohormesis [100]. Given the well-established toxic potential of mitoO2•−, it appears reasonable to predict that the protective signalling will prevail under conditions in which mitoO2•− is generated transiently and in limited amounts. While an excess of mitoO2•− promotes toxicity, based on both excessive damage and inhibition of Nrf2 signalling [100,101], it is likely that intermediate levels of mitoO2•− cause both opposing responses. This is consistent with the notion that the Nrf2 signalling mediates an adaptive response aimed at preventing/mitigating, and delaying, the onset of cell death. Clearly, we might also expect the contribution of an array of variables in the regulation of this delicate equilibrium.
Our previous work using well-defined conditions, in which arsenite generates mitoO2•−, established a link between these toxic events and Nrf2 activation and the ensuing enhanced expression of target genes as γ-glutamylcysteine synthase and increased GSH levels [55].
However, as previously discussed, the same species also mediate other events, such as an early DNA strand scission [54] and the delayed induction of mitochondrial dysfunction associated with the triggering of MPT-dependent apoptosis [56]. It was, therefore, interesting to observe that these events were anticipated, with remarkably lower arsenite concentration requirements, under conditions in which the increased GSH biosynthesis was blunted.
Thus, arsenite promotes mitoO2•− formation under conditions in which pro-survival Nrf2-dependent as well as MPT-dependent apoptotic signalling responses are sequentially generated, and the triggering of Nrf2 significantly limits and delays the ensuing apoptosis (Figure 1). A final consideration is based on the fact that, as previously discussed, the possibility that arsenite generates mitoO2•− is heavily conditioned by the specific functional organisation of the ER in different cells [56], thereby implying a cell type dependence also for the Nrf2 response driven by these species.
6. Crosstalk between Arsenite-Induced ER Stress, UPR, and Nrf2-Mediated Antioxidant Responses
ER stress, triggered by the accumulation of unfolded or misfolded proteins, activates a homeostatic response, the unfolded protein response (UPR), which aims at reestablishing the ER homeostasis. As the term UPR suggests, unfolded proteins, resulting from a defect between the load of proteins to be folded into the ER and the capacity of the organelle to fold them, activate the stress in the organelle with the consequent response [103].
UPR is a multifaceted response that improves the ability of ER to fold proteins by increasing from one side its chaperoning and degradation capacity, mostly up-regulating the dedicated chaperones/enzymes, and from the other side by promoting the attenuation of the protein translation.
UPR is initiated by the following three ER membrane receptors: inositol-requiring enzyme (IRE1), protein kinase R-like endoplasmic reticulum kinase (PERK), and activation transcription factor 6 (ATF6), which regulate the three related pathways of signal transduction. The most ancient of the three is IRE, which promotes the unconventional splicing of X-box-binding protein 1 (XBP1) mRNA, resulting in the translation of this transcription factor, which finally regulates the so-called UPR target genes. ATF6 is proteolytically activated, thereby translocating to the nucleus and acting as a transcription factor for chaperones such as BIP (GRP78). PERK is an initiation factor 2 (eIF-2) kinase that promotes a general attenuation of protein translation by phosphorylating eIF-2 and the selective translation of ATF4, which is upstream to the pro-apoptotic C/EBP homologous protein CHOP and its two targets ERO1α and GADD34 [104,105].
Of note, ERO1α is a protein disulfide oxidase that, in virtue of its role in protein oxidative folding, is up-regulated during UPR. However, ERO1α generates a stoichiometric amount of H2O2 in its catalytic reaction of electron relay with PDI aimed at introducing disulfide bonds in client proteins, thereby generating a high amount of this oxidant in highly secretory cells [106]. For this purpose, an analysis with fluorescent probes, which measure ROS in the ER, suggested that this organelle potentially generates an amount of ROS even greater than that generated by mitochondria, further supporting the hypothesis that ER and ERO1α activity may represent an important source of H2O2/ROS [107].
Different proteins/enzymes into the ER were suggested to counteract the ERO1α-generated H2O2 or the effects of the oxidant on protein targets. For example, peroxiredoxin IV, which is a 2-Cys peroxiredoxin, reduces the H2O2 in water, also promoting PDI re-oxidation in the absence of ERO1 and thus participating in the disulfide bond formation of newly synthesised proteins into the ER [108]. SEPN1, a type II selenocysteine-containing membrane protein of the ER, counteracts the ERO1α-mediated hyperoxidation of the sarcoplasmic reticulum calcium pump SERCA2 in a redox-dependent manner, finally promoting the activation of this calcium pump [109,110]. Therefore, the local oxidative activity of ERO1α is counteracted by other proteins/enzymes localised into the ER.
However, ERO1α, by increasing Ca2+ release from the ER, could also promote Ca2+-dependent ROS formation in various subcellular compartments, including the mitochondria [69,111,112], thereby implying that the UPR, by activating PERK and then ERO1α, regulates the ROS-dependent Nrf2 antioxidant response.
Additionally, activated PERK can also promote the direct phosphorylation of Nrf2 and its transcriptional activation [95]. The crosstalk between PERK signalling and Nrf2-mediated antioxidant response is further supported by the evidence pointing that impaired signalling in this branch of the UPR is due to genetic mutations in both PERK and ATF4, which markedly raises the levels of ROS in ER-stressed cells [113].
The UPR signalling network, also converging in the activation of autophagy [114,115], is stimulated by ER stress to restore homeostasis and hence survival [114,115,116]. On the other hand, under conditions of sustained or prolonged ER stress, the UPR and/or autophagy promote apoptosis [115,116,117]. Many of the above pathways converge at different levels, with potentially significant impacts in different toxicity paradigms.
Substantial evidence in the literature documents the ability of arsenite to promote ER stress, eventually associated with the triggering of apoptosis [33,34,35,45,118,119]. The mechanism mediating ER stress activation is, however, poorly defined, and the specific relevance of direct effects of the metalloid remains elusive. The possibility of indirect effects mediated by ROS is also poorly understood, with very few details, if any, on the relative impact of ROS derived from different sources. Limited information is also available in the opposite direction, i.e., on the impact of ER stress on ROS formation, in particular on the mechanisms regulating Ca2+ homeostasis in microdomains, in which ROS formation takes place.
We have previously briefly discussed the close contact existing between the ER and the mitochondria, and the resulting crosstalk between these two organelles [56]. mitoO2•−-derived H2O2 can easily reach the ER and promote effects such as ER stress and Ca2+ release from either the IP3R, RyR, or both, as a consequence of direct oxidation of specific thiols present in both channels [120,121,122,123]. In an opposite direction, IP3R- and/or RyR-derived Ca2+ might instead be taken up by mitochondria, thereby boosting mitochondrial ROS emission [25,124,125].
Our previous work demonstrated that arsenite-induced mitoO2•− formation causes an initial stimulation of Ca2+ release from the IP3R, critically connected with the triggering of further Ca2+ release from the RyR, apparently mediated by ERO1α [69]. On the other hand, ERO1α activation/expression was causally linked to Ca2+ mobilisation from the IP3R and RyR, which leads to the conclusion that ER stress and ERO1α are part of a positive amplification loop leading to Ca2+ mobilisation and accumulation in mitochondria to build-up mitoO2•− formation in these organelles. It is instead still unclear whether mitochondrial ROS contributes to ERO1α activation/expression. In principle, they should, although a specific investigation to provide an answer to this question has yet not been performed.
In any case, ERO1α promotes ROS formation in situ into the ER as well as in mitochondria, possibly linked to RyR sensitization and to the ensuing mitochondrial accumulation of Ca2+ [69]. This crosstalk may, therefore, at the initial stages, promote survival through the activation of the Nrf2 signalling. Likewise, it can be predicted that the persistence of these events will rather lead to toxicity associated with the induction of mitochondrial dysfunction. Under these conditions, the reduced rate of ATP formation may further compromise the Ca2+ buffering capacity of the ER, thereby fueling the amplification loop involved in the regulation of the above crosstalk. This will eventually cause Ca2+ overload in the mitochondria, which in turn, is associated with the induction of MPT and the ensuing apoptotic signalling [126,127]. As a final note, we also reported that arsenite-induced ER stress is associated with autophagy and that inhibition of the autophagic process partially prevents mitochondrial dysfunction and toxicity [60].
Thus, the ER stress response induced by arsenite is critically connected with the formation of mitochondrial ROS and, hence, with the ROS-dependent activation of the Nrf2 protective signalling and ROS-dependent toxicity.
7. Conclusions
The general idea deriving from the analysis of numerous studies, in which the source of ROS was not always determined, is that arsenite promotes an array of effects via multiple mechanisms, which crosstalk at different levels. In particular, numerous mechanisms whereby arsenite induces Nrf2 expression interconnected at various levels with ER stress signalling responses have been thus far proposed. The overall scenario is therefore confusing, most likely as a consequence of the chemical nature of the metalloid, inducing a wide spectrum of effects through its binding to protein thiols and ROS formation.
We tried to connect the above information with our findings obtained with arsenite in well-controlled cellular systems characterised by the unique formation of mitoO2•−. The overall mechanism emerging from these studies is shown in Figure 1.
Arsenite promotes mitoO2•− formation via a mechanism requiring interactions with the mitochondrial respiratory chain and the ER. The crosstalk between the ER and mitochondria is critically influenced by their functional and spatial organisation. Arsenite causes an initial IP3R activation and a successive stimulation of the RyR, critically mediated by an ERO1α-dependent mechanism, leading to the mitochondrial accumulation of Ca2+. MitoO2•−-derived H2O2 mediates mitochondrial dysfunction and toxicity. The early ER stress response is also critically connected through mitoO2•− formation with the triggering of the Nrf2 cytoprotective signalling, which indeed significantly mitigates and delays the onset of MPT-dependent apoptosis.
Author Contributions
Conceptualization O.C., A.G., M.F., E.Z., P.G. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by Ministero dell’Università e della Ricerca Scientifica e Tecnologica, Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale, 2017, (Grant number: 2017FJSM9S, Orazio Cantoni).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Masuda H. Arsenic cycling in the Earth’s crust and hydrosphere: Interaction between naturally occurring arsenic and human activities. Prog. Earth Planet. Sci. 2018;5:68. doi: 10.1186/s40645-018-0224-3. [DOI] [Google Scholar]
- 2.World Health Organization . Guidelines for Drinking-Water Quality [Electronic Resource]: Incorporating First Addendum. Volume 1 World Health Organization; Geneva, Switzerland: 2006. Recommendations. [Google Scholar]
- 3.Shankar S., Shanker U. Shikha Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014;2014:304524. doi: 10.1155/2014/304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong Y.-S., Song K.-H., Chung J.-Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health Yebang Uihakhoe Chi. 2014;47:245–252. doi: 10.3961/jpmph.14.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ro S.-H., Bae J., Jang Y., Myers J.F., Chung S., Yu J., Natarajan S.K., Franco R., Song H.-S. Arsenic Toxicity on Metabolism and Autophagy in Adipose and Muscle Tissues. Antioxidants. 2022;11:689. doi: 10.3390/antiox11040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra M., Rai C.B., Kumari N., Sandhu V.K., Chandra K., Krishna M., Kota S.H., Anand K.S., Oudin A. Air Pollution and Cognitive Impairment across the Life Course in Humans: A Systematic Review with Specific Focus on Income Level of Study Area. Int. J. Environ. Res. Public Health. 2022;19:1405. doi: 10.3390/ijerph19031405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumagai Y., Sumi D. Arsenic: Signal Transduction, Transcription Factor, and Biotransformation Involved in Cellular Response and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2007;47:243–262. doi: 10.1146/annurev.pharmtox.47.120505.105144. [DOI] [PubMed] [Google Scholar]
- 8.Hei T.K., Filipic M. Role of oxidative damage in the genotoxicity of arsenic. Free Radic. Biol. Med. 2004;37:574–581. doi: 10.1016/j.freeradbiomed.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Reichard J.F., Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2:87–104. doi: 10.2217/epi.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen S.M., Arnold L.L., Beck B.D., Lewis A.S., Eldan M. Evaluation of the carcinogenicity of inorganic arsenic. Crit. Rev. Toxicol. 2013;43:711–752. doi: 10.3109/10408444.2013.827152. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y., Li J., Lou B., Wu R., Wang G., Lu C., Wang H., Pi J., Xu Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules. 2020;10:240. doi: 10.3390/biom10020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen S., Li X.-F., Cullen W.R., Weinfeld M., Le X.C. Arsenic Binding to Proteins. Chem. Rev. 2013;113:7769–7792. doi: 10.1021/cr300015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flora S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Jomova K., Jenisova Z., Feszterova M., Baros S., Liska J., Hudecova D., Rhodes C.J., Valko M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 15.Prakash C., Soni M., Kumar V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: A review. J. Appl. Toxicol. 2016;36:179–188. doi: 10.1002/jat.3256. [DOI] [PubMed] [Google Scholar]
- 16.Hughes M.F., Beck B.D., Chen Y., Lewis A.S., Thomas D.J. Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol. Sci. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurchi V.M., Djordjevic A.B., Crisponi G., Alexander J., Bjørklund G., Aaseth J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules. 2020;10:235. doi: 10.3390/biom10020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S.-X., Davidson M.M., Tang X., Walker W.F., Athar M., Ivanov V., Hei T.K. Mitochondrial Damage Mediates Genotoxicity of Arsenic in Mammalian Cells. Cancer Res. 2005;65:3236–3242. doi: 10.1158/0008-5472.CAN-05-0424. [DOI] [PubMed] [Google Scholar]
- 19.Chavan H., Christudoss P., Mickey K., Tessman R., Ni H.-M., Swerdlow R., Krishnamurthy P. Arsenite Effects on Mitochondrial Bioenergetics in Human and Mouse Primary Hepatocytes Follow a Nonlinear Dose Response. Oxidative Med. Cell. Longev. 2017;2017:9251303. doi: 10.1155/2017/9251303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei S., Qiu T., Yao X., Wang N., Jiang L., Jia X., Tao Y., Wang Z., Pei P., Zhang J., et al. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J. Hazard. Mater. 2020;384:121390. doi: 10.1016/j.jhazmat.2019.121390. [DOI] [PubMed] [Google Scholar]
- 21.Chou W.-C., Jie C., Kenedy A.A., Jones R.J., Trush M.A., Dang C.V. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc. Natl. Acad. Sci. USA. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemarie A., Bourdonnay E., Morzadec C., Fardel O., Vernhet L. Inorganic arsenic activates reduced NADPH oxidase in human primary macrophages through a Rho kinase/p38 kinase pathway. J. Immunol. 2008;180:6010–6017. doi: 10.4049/jimmunol.180.9.6010. [DOI] [PubMed] [Google Scholar]
- 23.Straub A.C., Clark K.A., Ross M.A., Chandra A.G., Li S., Gao X., Pagano P.J., Stolz D.B., Barchowsky A. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase–generated superoxide. J. Clin. Investig. 2008;118:3980–3989. doi: 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandes R.P., Weissmann N., Schröder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 25.Bertero E., Maack C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018;122:1460–1478. doi: 10.1161/CIRCRESAHA.118.310082. [DOI] [PubMed] [Google Scholar]
- 26.Angelova P.R., Abramov A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016;100:81–85. doi: 10.1016/j.freeradbiomed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Pachauri V., Mehta A., Mishra D., Flora S.J. Arsenic induced neuronal apoptosis in guinea pigs is Ca2+ dependent and abrogated by chelation therapy: Role of voltage gated calcium channels. NeuroToxicology. 2013;35:137–145. doi: 10.1016/j.neuro.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Chen A., Cao E.-H., Zhang T.-C., Qin J.-F. Arsenite-induced reactive oxygen species and the repression of alpha-tocopherol in the MGC-803 cells. Eur. J. Pharmacol. 2002;448:11–18. doi: 10.1016/S0014-2999(02)01901-5. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee C., Goswami R., Datta S., Rajagopal R., Mazumder S. Arsenic-induced alteration in intracellular calcium homeostasis induces head kidney macrophage apoptosis involving the activation of calpain-2 and ERK in Clarias batrachus. Toxicol. Appl. Pharmacol. 2011;256:44–51. doi: 10.1016/j.taap.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Bolt A.M., Zhao F., Pacheco S., Klimecki W.T. Arsenite-induced autophagy is associated with proteotoxicity in human lymphoblastoid cells. Toxicol. Appl. Pharmacol. 2012;264:255–261. doi: 10.1016/j.taap.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou C.-T., Lin H.-T., Hwang P.-A., Wang S.-T., Hsieh C.-H., Hwang D.-F. Taurine resumed neuronal differentiation in arsenite-treated N2a cells through reducing oxidative stress, endoplasmic reticulum stress, and mitochondrial dysfunction. Amino Acids. 2015;47:735–744. doi: 10.1007/s00726-014-1901-1. [DOI] [PubMed] [Google Scholar]
- 32.Dodson M., de la Vega M.R., Harder B., Castro-Portuguez R., Rodrigues S.D., Wong P.K., Chapman E., Zhang D.D. Low-level arsenic causes proteotoxic stress and not oxidative stress. Toxicol. Appl. Pharmacol. 2018;341:106–113. doi: 10.1016/j.taap.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X., Liu X., Chen Y., Wang H., Zhang R., Zhang Q., Wei Y., Shi S., Li X. Calreticulin regulated intrinsic apoptosis through mitochondria-dependent and independent pathways mediated by ER stress in arsenite exposed HT-22 cells. Chemosphere. 2020;251:126466. doi: 10.1016/j.chemosphere.2020.126466. [DOI] [PubMed] [Google Scholar]
- 34.Liu C., Zhang A. ROS -mediated PERK-eIF2alpha-ATF4 pathway plays an important role in arsenite-induced L-02 cells apoptosis via regulating CHOP-DR5 signaling. Environ. Toxicol. 2020;35:1100–1113. doi: 10.1002/tox.22946. [DOI] [PubMed] [Google Scholar]
- 35.Chen C., Gu S., Jiang X., Zhang Z. Arsenite-induced endoplasmic reticulum-dependent apoptosis through disturbance of calcium homeostasis in HBE cell line. Environ. Toxicol. 2017;32:197–216. doi: 10.1002/tox.22226. [DOI] [PubMed] [Google Scholar]
- 36.Lemasters J.J., Theruvath T.P., Zhong Z., Nieminen A.-L. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroemer G., Galluzzi L., Brenner C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 38.Rasola A., Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukumaran P., Da Conceicao V.N., Sun Y., Ahamad N., Saraiva L.R., Selvaraj S., Singh B.B. Calcium Signaling Regulates Autophagy and Apoptosis. Cells. 2021;10:2125. doi: 10.3390/cells10082125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Xu Y., Wang H., Xue P., Li X., Li B., Zheng Q., Sun G. Arsenic induces mitochondria-dependent apoptosis by reactive oxygen species generation rather than glutathione depletion in Chang human hepatocytes. Arch. Toxicol. 2009;83:899–908. doi: 10.1007/s00204-009-0451-x. [DOI] [PubMed] [Google Scholar]
- 42.Du L., Yu Y., Chen J., Liu Y., Xia Y., Chen Q., Liu X. Arsenic induces caspase- and mitochondria-mediated apoptosis inSaccharomyces cerevisiae. FEMS Yeast Res. 2007;7:860–865. doi: 10.1111/j.1567-1364.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 43.Naranmandura H., Chen X., Tanaka M., Wang W.W., Rehman K., Xu S., Chen Z., Chen S.Q., Suzuki N. Release of Apoptotic Cytochrome C From Mitochondria by Dimethylarsinous Acid Occurs Through Interaction With Voltage-Dependent Anion Channel In Vitro. Toxicol. Sci. 2012;128:137–146. doi: 10.1093/toxsci/kfs154. [DOI] [PubMed] [Google Scholar]
- 44.Peraza M.A., Cromey D.W., Carolus B., Carter D.E., Gandolfi A.J. Morphological and functional alterations in human proximal tubular cell line induced by low level inorganic arsenic: Evidence for targeting of mitochondria and initiated apoptosis. J. Appl. Toxicol. 2006;26:356–367. doi: 10.1002/jat.1149. [DOI] [PubMed] [Google Scholar]
- 45.Sun H., Yang Y., Shao H., Sun W., Gu M., Wang H., Jiang L., Qu L., Sun D., Gao Y. Sodium Arsenite-Induced Learning and Memory Impairment Is Associated with Endoplasmic Reticulum Stress-Mediated Apoptosis in Rat Hippocampus. Front. Mol. Neurosci. 2017;10:286. doi: 10.3389/fnmol.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mu Y.F., Chen Y.H., Chang M.-M., Chen Y.C., Huang B.M. Arsenic compounds induce apoptosis through caspase pathway activation in MA-10 Leydig tumor cells. Oncol. Lett. 2019;18:944–954. doi: 10.3892/ol.2019.10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu C.-W., Lin P.-J., Tsai J.-S., Lin C.-Y., Lin L.-Y. Arsenite-induced apoptosis can be attenuated via depletion of mTOR activity to restore autophagy. Toxicol. Res. 2019;8:101–111. doi: 10.1039/C8TX00238J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 49.He F., Ru X., Wen T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020;21:4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spinelli J.B., Haigis M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018;20:745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kudryavtseva A.V., Krasnov G.S., Dmitriev A.A., Alekseev B.Y., Kardymon O.L., Sadritdinova A.F., Fedorova M.S., Pokrovsky A.V., Melnikova N.V., Kaprin A.D., et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7:44879–44905. doi: 10.18632/oncotarget.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 53.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guidarelli A., Fiorani M., Cerioni L., Cantoni O. Calcium signals between the ryanodine receptor- and mitochondria critically regulate the effects of arsenite on mitochondrial superoxide formation and on the ensuing survival vs apoptotic signaling. Redox Biol. 2019;20:285–295. doi: 10.1016/j.redox.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiorani M., Guidarelli A., Capellacci V., Cerioni L., Crinelli R., Cantoni O. The dual role of mitochondrial superoxide in arsenite toxicity: Signaling at the boundary between apoptotic commitment and cytoprotection. Toxicol. Appl. Pharmacol. 2018;345:26–35. doi: 10.1016/j.taap.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Guidarelli A., Catalani A., Spina A., Varone E., Fumagalli S., Zito E., Fiorani M., Cantoni O. Functional organization of the endoplasmic reticulum dictates the susceptibility of target cells to arsenite-induced mitochondrial superoxide formation, mitochondrial dysfunction and apoptosis. Food Chem. Toxicol. 2021;156:112523. doi: 10.1016/j.fct.2021.112523. [DOI] [PubMed] [Google Scholar]
- 57.Gardner P.R. Aconitase: Sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 58.Esposti M.D. Inhibitors of NADH–ubiquinone reductase: An overview. Biochim. Biophys. Acta. 1998;1364:222–235. doi: 10.1016/S0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 59.Fiorani M., Azzolini C., Cerioni L., Scotti M., Guidarelli A., Ciacci C., Cantoni O. The mitochondrial transporter of ascorbic acid functions with high affinity in the presence of low millimolar concentrations of sodium and in the absence of calcium and magnesium. Biochim. Biophys. Acta. 2015;1848:1393–1401. doi: 10.1016/j.bbamem.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Guidarelli A., Carloni S., Balduini W., Fiorani M., Cantoni O. Mitochondrial ascorbic acid prevents mitochondrial O2.- formation, an event critical for U937 cell apoptosis induced by arsenite through both autophagic-dependent and independent mechanisms. Biofactors. 2016;42:190–200. doi: 10.1002/biof.1263. [DOI] [PubMed] [Google Scholar]
- 61.Raffaello A., Mammucari C., Gherardi G., Rizzuto R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016;41:1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 63.Suriyo T., Watcharasit P., Thiantanawat A., Satayavivad J. Arsenite promotes apoptosis and dysfunction in microvascular endothelial cells via an alteration of intracellular calcium homeostasis. Toxicol. Vitr. 2012;26:386–395. doi: 10.1016/j.tiv.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Kirichok Y., Krapivinsky G., Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 65.Csordás G., Weaver D., Hajnóczky G. Endoplasmic Reticulum–Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018;28:523–540. doi: 10.1016/j.tcb.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giorgi C., Danese A., Missiroli S., Patergnani S., Pinton P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018;28:258–273. doi: 10.1016/j.tcb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Guidarelli A., Fiorani M., Cantoni O. Low Concentrations of Arsenite Target the Intraluminal Inositol 1, 4, 5-Trisphosphate Receptor/Ryanodine Receptor Crosstalk to Significantly Elevate Intracellular Ca(2) J. Pharmacol. Exp. Ther. 2018;367:184–193. doi: 10.1124/jpet.118.250480. [DOI] [PubMed] [Google Scholar]
- 68.Guidarelli A., Cerioni L., Fiorani M., Catalani A., Cantoni O. Arsenite-Induced Mitochondrial Superoxide Formation: Time and Concentration Requirements for the Effects of the Metalloid on the Endoplasmic Reticulum and Mitochondria. J. Pharmacol. Exp. Ther. 2020;373:62–71. doi: 10.1124/jpet.119.262469. [DOI] [PubMed] [Google Scholar]
- 69.Spina A., Guidarelli A., Fiorani M., Varone E., Catalani A., Zito E., Cantoni O. Crosstalk between ERO1alpha and ryanodine receptor in arsenite-dependent mitochondrial ROS formation. Biochem. Pharmacol. 2022;198:114973. doi: 10.1016/j.bcp.2022.114973. [DOI] [PubMed] [Google Scholar]
- 70.Friling R.S., Bensimon A., Tichauer Y., Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc. Natl. Acad. Sci. USA. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu C., Li C.Y.-T., Kong A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharmacal Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 72.Cuadrado A., Manda G., Hassan A., Alcaraz M.J., Barbas C., Daiber A., Ghezzi P., León R., López M.G., Oliva B., et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018;70:348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 73.Jobbagy S., Vitturi D.A., Salvatore S.R., Turell L., Pires M.F., Kansanen E., Batthyany C., Lancaster J.R., Freeman B.A., Schopfer F.J. Electrophiles modulate glutathione reductase activity via alkylation and upregulation of glutathione biosynthesis. Redox Biol. 2019;21:101050. doi: 10.1016/j.redox.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tejo F.V., Quintanilla R. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants. 2021;10:1069. doi: 10.3390/antiox10071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Ryoo I.-G., Kwak M.-K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018;359:24–33. doi: 10.1016/j.taap.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 77.Piantadosi C.A., Withers C.M., Bartz R.R., MacGarvey N.C., Fu P., Sweeney T.E., Welty-Wolf K.E., Suliman H.B. Heme Oxygenase-1 Couples Activation of Mitochondrial Biogenesis to Anti-inflammatory Cytokine Expression. J. Biol. Chem. 2011;286:16374–16385. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baird L., Llères D., Swift S., Dinkova-Kostova A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. USA. 2013;110:15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De La Vega M.R., Chapman E., Zhang D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho H.-Y., Reddy S.P., Debiase A., Yamamoto M., Kleeberger S.R. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic. Biol. Med. 2005;38:325–343. doi: 10.1016/j.freeradbiomed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 82.Uno K., Prow T.W., Bhutto I.A., Yerrapureddy A., McLeod D.S., Yamamoto M., Reddy S.P., Lutty G.A. Role of Nrf2 in retinal vascular development and the vaso-obliterative phase of oxygen-induced retinopathy. Exp. Eye Res. 2010;90:493–500. doi: 10.1016/j.exer.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu M., Grigoryev D.N., Crow M.T., Haas M., Yamamoto M., Reddy S.P., Rabb H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–285. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- 84.Andrew A.S., Warren A.J., Barchowsky A., Temple K.A., Klei L., Soucy N.V., O’Hara K.A., Hamilton J.W. Genomic and proteomic profiling of responses to toxic metals in human lung cells. Environ. Health Perspect. 2003;111:825–835. doi: 10.1289/ehp.111-1241504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Del Razo L.M., Quintanilla-Vega B., Brambila-Colombres E., Calderon-Aranda E.S., Manno M., Albores A. Stress Proteins Induced by Arsenic. Toxicol. Appl. Pharmacol. 2001;177:132–148. doi: 10.1006/taap.2001.9291. [DOI] [PubMed] [Google Scholar]
- 86.Ran S., Liu J., Li S. A Systematic Review of the Various Effect of Arsenic on Glutathione Synthesis In Vitro and In Vivo. BioMed Res. Int. 2020;2020:9414196. doi: 10.1155/2020/9414196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang C., Niu Q., Ma R., Song G., Hu Y., Xu S., Li Y., Wang H., Li S., Ding Y. The Variable Regulatory Effect of Arsenic on Nrf2 Signaling Pathway in Mouse: A Systematic Review and Meta-analysis. Biol. Trace Element Res. 2019;190:362–383. doi: 10.1007/s12011-018-1549-x. [DOI] [PubMed] [Google Scholar]
- 88.He X., Ma Q. Critical Cysteine Residues of Kelch-Like ECH-Associated Protein 1 in Arsenic Sensing and Suppression of Nuclear Factor Erythroid 2-Related Factor 2. J. Pharmacol. Exp. Ther. 2010;332:66–75. doi: 10.1124/jpet.109.160465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He X., Ma Q. NRF2 Cysteine Residues Are Critical for Oxidant/Electrophile-Sensing, Kelch-Like ECH-Associated Protein-1-Dependent Ubiquitination-Proteasomal Degradation, and Transcription Activation. Mol. Pharmacol. 2009;76:1265–1278. doi: 10.1124/mol.109.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jain A., Lamark T., Sjøttem E., Larsen K.B., Awuh J.A., Øvervatn A., McMahon M., Hayes J.D., Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu X., Ling M., Chen C., Luo F., Yang P., Wang D., Chen X., Xu H., Xue J., Yang Q., et al. Impaired autophagic flux and p62-mediated EMT are involved in arsenite-induced transformation of L-02 cells. Toxicol. Appl. Pharmacol. 2017;334:75–87. doi: 10.1016/j.taap.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 92.Shah P., Trinh E., Qiang L., Xie L., Hu W.-Y., Prins G.S., Pi J., He Y.-Y. Arsenic Induces p62 Expression to Form a Positive Feedback Loop with Nrf2 in Human Epidermal Keratinocytes: Implications for Preventing Arsenic-Induced Skin Cancer. Molecules. 2017;22:194. doi: 10.3390/molecules22020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.-S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 94.Sun Z., Chin Y.E., Zhang D.D. Acetylation of Nrf2 by p300/CBP Augments Promoter-Specific DNA Binding of Nrf2 during the Antioxidant Response. Mol. Cell. Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cullinan S.B., Zhang D., Hannink M., Arvisais E., Kaufman R.J., Diehl J.A. Nrf2 Is a Direct PERK Substrate and Effector of PERK-Dependent Cell Survival. Mol. Cell. Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wadgaonkar P., Chen F. Connections between endoplasmic reticulum stress-associated unfolded protein response, mitochondria, and autophagy in arsenic-induced carcinogenesis. Semin. Cancer Biol. 2021;76:258–266. doi: 10.1016/j.semcancer.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sinha D., Biswas J., Bishayee A. Nrf2-mediated redox signaling in arsenic carcinogenesis: A review. Arch. Toxicol. 2013;87:383–396. doi: 10.1007/s00204-012-0920-5. [DOI] [PubMed] [Google Scholar]
- 99.Pi J., Qu W., Reece J.M., Kumagai Y., Waalkes M.P. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: Involvement of hydrogen peroxide. Exp. Cell Res. 2003;290:234–245. doi: 10.1016/S0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- 100.Kasai S., Shimizu S., Tatara Y., Mimura J., Itoh K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules. 2020;10:320. doi: 10.3390/biom10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bjorkman S.H., Pereira R.O. The Interplay Between Mitochondrial Reactive Oxygen Species, Endoplasmic Reticulum Stress, and Nrf2 Signaling in Cardiometabolic Health. Antioxid. Redox Signal. 2021;35:252–269. doi: 10.1089/ars.2020.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim I., Rodriguez-Enriquez S., Lemasters J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walter P., Ron D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 104.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 105.Marciniak S.J., Yun C.Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H.P., Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zito E. ERO1: A protein disulfide oxidase and H2O2 producer. Free Radic. Biol. Med. 2015;83:299–304. doi: 10.1016/j.freeradbiomed.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 107.Malinouski M., Zhou Y., Belousov V.V., Hatfield D.L., Gladyshev V.N. Hydrogen Peroxide Probes Directed to Different Cellular Compartments. PLoS ONE. 2011;6:e14564. doi: 10.1371/journal.pone.0014564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zito E., Melo E.P., Yang Y., Wahlander Å., Neubert T.A., Ron D. Oxidative Protein Folding by an Endoplasmic Reticulum-Localized Peroxiredoxin. Mol. Cell. 2010;40:787–797. doi: 10.1016/j.molcel.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chernorudskiy A.L., Zito E. Regulation of Calcium Homeostasis by ER Redox: A Close-Up of the ER/Mitochondria Connection. J. Mol. Biol. 2017;429:620–632. doi: 10.1016/j.jmb.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 110.Pozzer D., Varone E., Chernorudskiy A., Schiarea S., Missiroli S., Giorgi C., Pinton P., Canato M., Germinario E., Nogara L., et al. A maladaptive ER stress response triggers dysfunction in highly active muscles of mice with SELENON loss. Redox Biol. 2019;20:354–366. doi: 10.1016/j.redox.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li G., Mongillo M., Chin K.-T., Harding H., Ron D., Marks A.R., Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress–induced apoptosis. J. Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anelli T., Bergamelli L., Margittai E., Rimessi A., Fagioli C., Malgaroli A., Pinton P., Ripamonti M., Rizzuto R., Sitia R. Ero1alpha Regulates Ca(2+) Fluxes at the Endoplasmic Reticulum-Mitochondria Interface (MAM) Antioxid. Redox Signal. 2012;16:1077–1087. doi: 10.1089/ars.2011.4004. [DOI] [PubMed] [Google Scholar]
- 113.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M., Sadri N., Yun C., Popko B., Paules R.S., et al. An Integrated Stress Response Regulates Amino Acid Metabolism and Resistance to Oxidative Stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 114.Senft D., Ronai Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015;40:141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z., Zhang L., Zhou L., Lei Y., Zhang Y., Huang C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019;25:101047. doi: 10.1016/j.redox.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen X., Cubillos-Ruiz J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Cancer. 2021;21:71–88. doi: 10.1038/s41568-020-00312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hetz C., Papa F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell. 2018;69:169–181. doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 118.Lin A.M.Y., Chao P.L., Fang S.F., Chi C.W., Yang J.C.-H. Endoplasmic reticulum stress is involved in arsenite-induced oxidative injury in rat brain. Toxicol. Appl. Pharmacol. 2007;224:138–146. doi: 10.1016/j.taap.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 119.Lin A.M.Y., Fang S.F., Chao P.L., Yang J.C.-H. Melatonin attenuates arsenite-induced apoptosis in rat brain: Involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J. Pineal Res. 2007;43:163–171. doi: 10.1111/j.1600-079X.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 120.Berridge M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 121.Joseph S.K. Role of Thiols in the Structure and Function of Inositol Trisphosphate Receptors. Curr. Top. Membr. 2010;66:299–322. doi: 10.1016/s1063-5823(10)66013-9. [DOI] [PubMed] [Google Scholar]
- 122.Anzai K., Ogawa K., Ozawa T., Yamamoto H. Oxidative Modification of Ion Channel Activity of Ryanodine Receptor. Antioxid. Redox Signal. 2000;2:35–40. doi: 10.1089/ars.2000.2.1-35. [DOI] [PubMed] [Google Scholar]
- 123.Sun Q.-A., Wang B., Miyagi M., Hess D.T., Stamler J.S. Oxygen-coupled Redox Regulation of the Skeletal Muscle Ryanodine Receptor/Ca2+ Release Channel (RyR1): Sites and Nature of Oxidative Modification. J. Biol. Chem. 2013;288:22961–22971. doi: 10.1074/jbc.M113.480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Csordás G., Hajnóczky G. SR/ER–mitochondrial local communication: Calcium and ROS. Biochim. Biophys. Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garbincius J.F., Elrod J.W. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022;102:893–992. doi: 10.1152/physrev.00041.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bauer T.M., Murphy E. Role of Mitochondrial Calcium and the Permeability Transition Pore in Regulating Cell Death. Circ. Res. 2020;126:280–293. doi: 10.1161/CIRCRESAHA.119.316306. [DOI] [PMC free article] [PubMed] [Google Scholar]