Abstract
Acinetobacter baumannii hospital infections are difficult to treat due to the rapid emergence of multidrug-resistant (MDR) strains. In addition, A. baumannii can survive in numerous adverse environments, including in the presence of common hospital antiseptics. We hypothesized that in addition to accumulating drug resistance determinants, MDR A. baumannii strains also accumulate mutations that allow for greater microbicide tolerance when compared to pan-susceptible (PS) strains. To test this hypothesis, we compared the survival of five MDR and five PS patient isolates when exposed to bleach, ethanol, quaternary ammonium compounds, chlorhexidine gluconate, and povidone. We evaluated bacteria in a free-living planktonic state and under biofilm conditions. Each disinfectant eliminated 99.9% of planktonic bacteria, but this was not the case for bacterial biofilms. Next, we characterized strains for the presence of the known microbicide-resistance genes cepA, qacEΔ1, qacE, and qacA. MDR strains did not survive more than PS strains in the presence of microbicides, but microbicide-resistant strains had higher survival rates under some conditions. Interestingly, the PS strains were more likely to possess microbicide-resistance genes. Microbicide resistance remains an important topic in healthcare and may be independent of antimicrobial resistance. Hospitals should consider stricter isolation precautions that take pan-susceptible strains into account.
Keywords: bacteria, multidrug resistance, microbicide resistance, biocide, antiseptic, germicide, hospital-acquired infections, efflux pumps
1. Introduction
Hospital-acquired infections (HAIs), especially those from Gram-negative organisms that tend to become multidrug resistant (MDR), pose serious threats to healthcare systems [1]. Acinetobacter baumannii is a non-spore forming, Gram-negative rod that is ubiquitous in the environment, and an opportunistic human pathogen. A. baumannii infections commonly occur in severely ill patients with extended hospital stays. These bacteria readily acquire antibiotic resistance, resulting in difficult to treat infections which may require higher doses and longer treatment durations of more toxic antibiotics [2,3,4,5]. A. baumannii is one of the five pathogens labeled as an urgent threat by the Centers for Disease Control and Prevention (CDC), due to its ability to become resistant to multiple standard-of-care antibiotics [6]. In fact, roughly 50% of A. baumannii isolates are MDR [7]. The mechanisms of resistance found in this bacterium include the presence of beta lactamases, mutation of target sites, changes in membrane permeability, and overproduction of efflux pumps [8]. First-line treatments for A. baumannii infections typically include cefepime, ampicillin-sulbactam, or meropenem [7]. However, with the emergence of carbapenem-resistant pathogens, newer agents such as cefiderocol, a novel beta-lactam antibiotic that uses a siderophore moiety to bind to bacterial iron transporters, have been used [9,10]. Cefiderocol was shown to be as effective as imipenem-cilastatin for urinary tract infections in a clinical trial [11], but was associated with higher mortality rates compared to other standard treatments in another study [10]. Given this, treatment options for MDR A. baumannii remain limited.
Most infections (greater than 90%) are nosocomial rather than community acquired [12]. Pathogens are classified as urgent, serious, or concerning threats according to the CDC and are monitored through the Multi-site Gram-negative Surveillance Initiative [13]. Carbapenem-resistant Acinetobacter baumannii (CRAB) and carbapenem-resistant Enterobacterales (CRE) are considered urgent threats, while other highly resistant organisms, such as vancomycin-resistant Enterococci (VRE) and multidrug-resistant Pseudomonas aeruginosa, are considered serious threats. Compared to other multidrug-resistant organisms, the number of CRAB infections tends to be lower, but still substantial. In 2017, there were 8500 hospital infections from CRAB that resulted in over 700 deaths, compared to 13,100 hospital infections with 1100 deaths from CRE in the United States. In the same year, there were 54,500 hospital infections with 5400 deaths from MDR P. aeruginosa and 32,600 hospital infections with 2700 deaths from VRE [14].
Globally, there were approximately 1,000,000 A. baumannii HAIs in 2019, about half of which were resistant to carbapenems [15]. The presence of multidrug resistance varies by country. A 2019 metanalysis evaluated the prevalence of A. baumannii hospital and ventilator associated pneumonia (HAP and VAP) and found that Central America, Cuba, Mexico, and Latin America/Caribbean had a lower rate of infections, but 100% of those infections were MDR [16], compared to the United States that had nearly triple the amount of HAPs and VAPs with an MDR prevalence of 69% [16]. During that same year, the prevalence of HAP and VAP infections from MDR A. baumannii was 91% in Greece [16], and a 4-year cohort study conducted in Greece showed that the all-cause hospital mortality rate among 91 patients with a pandrug-resistant (PDR) A. baumannii infection was as high as 68% [17]. Another study conducted on 92 Tunisian ICU patients showed a mortality rate up to 60% among those infected with A. baumannii ventilator associated pneumonia [18]. Furthermore, an analysis of 1423 U.S. patients with A. baumannii infections from 2009 to 2013 revealed that 80% were MDR [19]. Patients infected with MDR strains typically have more severe illness and are associated with higher frequencies of inappropriate antibiotic therapy, further perpetuating the rise of MDR isolates [19].
MDR A. baumannii infections are difficult to treat, which makes controlling hospital outbreaks extremely important, yet it remains a difficult task. This is largely due to A. baumannii’s ability to survive in many different environments and under adverse conditions [20,21,22,23,24]. They have evolved different survival mechanisms, such as resistance to desiccation [12], which allows for survival for extended periods of time on surfaces [4,12]. In fact, one study revealed the presence of A. baumannii on the bedrails of a previously infected patient up to 9 days after discharge [12,25]. This emphasizes the importance of proper decontamination of hospital rooms in preventing the spread of MDR bacteria. Germicides and microbicides are synonymous terms defined by the CDC as agents that destroy microorganisms [26]. Microbicides are routinely used in the hospital environment for disinfection and sterilization to limit the spread of nosocomial infections. A. baumannii has an innate ability to become resistant to microbicides, further contributing to its ability to survive in the harshest conditions [27]. For example, in 283 A. baumannii ICU isolates, the minimum inhibitory concentrations (MICs) increased after treatment with benzalkonium chloride (BAC) and chlorhexidine gluconate (CHG). In addition, the minimum bactericidal concentrations (MBCs) for drug-resistant isolates were also generally higher [27]. Biofilm formation enhances microbicide resistance. A. baumannii biofilms are more resistant than planktonic bacteria to BAC and CHG on ceramic surfaces [28]. Compounding the problem further, Acinetobacter strains that form biofilms are frequently multidrug resistant [29]. As biofilms are also inherently more drug-tolerant, some newer technologies are being explored as alternative therapeutics, including silver nano-particles [30]. In a promising study, nanoparticles were shown to reduce biofilm formation and downregulate the expression of biofilm producing genes among A. baumannii wound isolates [31]. However, evaluation of safety and efficacy is still underway [30]. Acinetobacter biofilms remain challenging to eradicate from surfaces and such infections are still difficult to treat.
The mechanism of action for each antiseptic agent varies. The mechanism of action for ethanol, CHG, and quaternary ammonium compounds (QACs) is the degradation of cell membranes [32], resulting in cell lysis and further leading to the degradation of cellular proteins and enzymes [33]. Bleach is a potent oxidizer and disrupts cellular activities, such as protein synthesis. The skin antiseptic povidone rapidly degrades proteins [32]. Resistance may emerge when an agent is used at a sub-lethal concentration, or for an inappropriate amount of time [34]. The overproduction of certain efflux pumps is a mechanism of microbicide resistance employed by bacterial pathogens [33]. The QAC-specific efflux pumps are members of the small multidrug-resistance (SMR) family [35], and many of the mechanisms that lead to antibiotic resistance, coincide with the mechanisms of microbicide resistance [5,34,36]. For example, the qacE efflux pump gene is commonly found on integrons with the sul1 gene, which confers resistance to sulfonamides [35]. Furthermore, the presence of qacEΔ1 and qacE has been correlated with the presence of certain beta-lactamase genes (blaVIM and blaNDM-1) and has additionally been associated with tolerance to CHG [5]. CepA is an efflux pump that increases the MICs of Klebsiella pneumoniae after exposure to CHG [32,37]. While the qacA gene is frequently seen among Gram-positive pathogens [38], a recent study of 44 A. baumannii isolates in Saudi Arabia revealed that 16.7% of strains contained the qacA gene [39].
The role that microbicide-resistance genes play in hospital outbreaks remains controversial. One study reported that the qacE and qacEΔ1 genes were identified among 100 MDR A. baumannii isolates from an ICU, however no evidence of microbicide tolerance was identified [40]. In agreement, a different study found no differences in bacterial survival among 20 MDR strains and 20 sporadic pan-susceptible strains after exposure to antiseptics [41]. Furthermore, there was no significant correlation between the presence of cepA, qacE, and qacEΔ1 genes and an increased MIC among 44 MDR A. baumannii, Pseudomonas aeruginosa, and K. pneumoniae strains [39]. However, another study found that A. baumannii strains that were resistant to carbapenems, fluoroquinolones, aminoglycosides, and tetracyclines were associated with microbicide tolerance [42].
Hospitals routinely use a variety of disinfecting agents, such as QACs, bleach, and ethanol. These are usually in the form of hospital disinfectant wipes and are used to clean surfaces and medical equipment. Antiseptics such as 10% povidone and 2% CHG are also commonly used for hand hygiene. For antiseptics to be effective against bacteria they must be used at specific contact or dwell times and meet certain Food and Drug Administration (FDA) standards. In general, this requires the germicide to kill 99.9% of bacteria on a hard, non-porous surface after a maximum time of 5 min [43]. Different disinfectant wipes require different dwell times. Sani-cloth® wipes containing bleach are considered bactericidal, viricidal, and fungicidal when a dwell time of 4 min is used. However, the efficacy against certain pathogens varies based on dwell time [44]. At 1 min, it is effective against A. baumannii isolates that are resistant to cefazolin, ampicillin, gentamicin, piperacillin, trimethoprim-sulfamethoxazole, and intermediately resistant to cefotaxime and ceftriaxone, but not necessarily carbapenem-resistant A. baumannii [44]. Furthermore, while it is effective against A. baumannii after 1 min, it is not effective against all pathogens until after 4 min of disinfection [44]. Ethanol typically has a dwell time of 30 s to 1 min [45]. QAC wipes have different dwell times depending on the components. 0.61% dodecyl dimethyl ammonium chloride in 27% ethanol and 25% isopropanol (0.61% DDAC) is used mainly in the ICU and has a dwell time of 1 min [46], whereas the combination QAC wipe of 0.25% alkyl dimethyl ethyl benzyl ammonium chloride, 0.25% alkyl dimethyl benzyl ammonium chloride in 55% isopropanol (0.50% BAC) has a dwell time of 2 min [47].
Most of the research performed so far on microbicide tolerance or resistance among A. baumannii isolates are performed under optimal settings with microbicides used at the proper dwell times. Therefore, a possible reason for hospital outbreaks of multidrug-resistant organisms could be due to improper cleaning techniques. Our goal is to explore the interaction of MDR bacteria with microbicides as potential contributors to hospital outbreaks. We hypothesized that in addition to accumulating drug-resistance determinants, MDR A. baumannii also accumulates mutations that allow for greater antiseptic tolerance when compared to pan-susceptible (PS) strains. We found no significant differences between MDR and PS strain survival among planktonic bacteria. As we did not find significant differences between MDR and PS strains under planktonic conditions, we tested the efficacy of antiseptic agents against bacterial biofilms. Our results demonstrated increased bacterial survival, but no differences between MDR and PS strains. Finally, we determined which strains contained the known microbicide-resistance genes and showed that significantly more microbicide-resistant bacteria survived compared to microbicide-sensitive bacteria after specific treatments. Interestingly, we found that the presence of microbicide-resistance genes was more likely in PS strains. This suggests that PS A. baumannii may be underestimated in the propagation of hospital outbreaks and highlights the need for increased hospital precautions for dealing with the spread of both MDR and PS organisms.
2. Results
2.1. Antiseptic Tolerance of Planktonic Acinetobacter baumannii Isolates
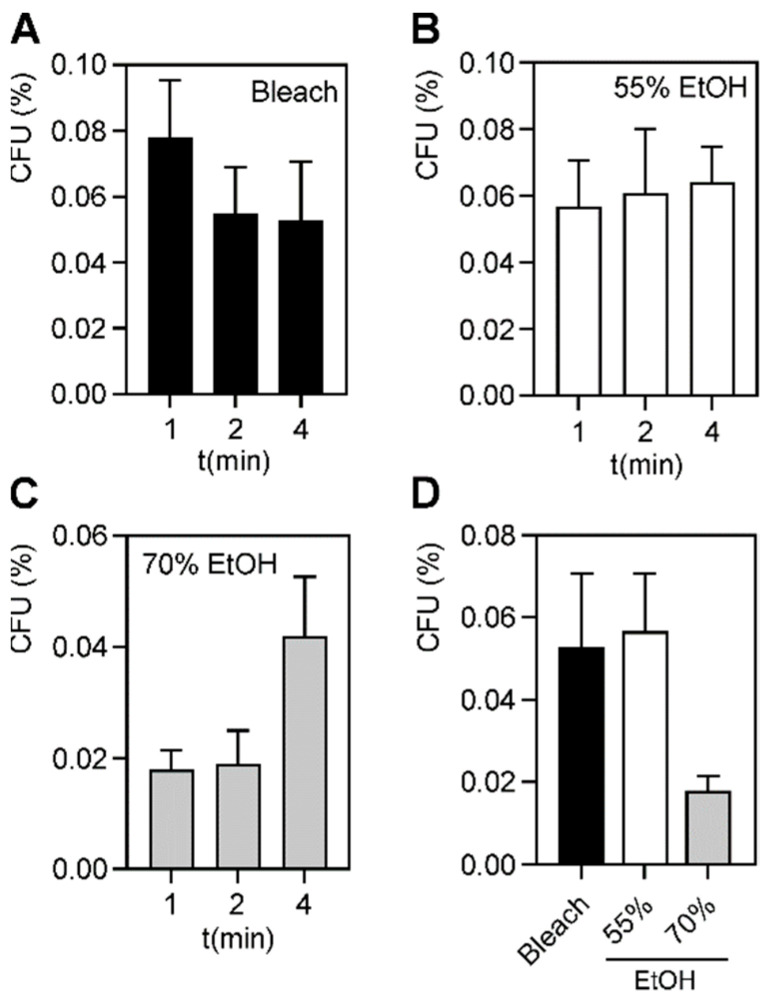
A. baumannii has evolved to survive and persist in harsh environments [4,12,20,21,22,23,24], including in the presence of common hospital antiseptics [5,42]. Hospital outbreaks of A. baumannii are becoming more common, especially among patients in ICUs. We hypothesized that MDR A. baumannii outbreaks occur because these strains are more tolerant or resistant to microbicides than susceptible strains. We thought it possible that some drug-resistance mechanisms may offer cross protection against some antiseptic agents. To test this idea, we performed time kill assays using five MDR and five PS A. baumannii patient isolates, in the presence and absence of various disinfectants and skin antiseptics. Free-living, planktonic A. baumannii strains were exposed to antiseptics for 1, 2 and 4 min, and survivors plated on rich media. Every antiseptic agent tested killed >99% of either MDR or PS bacteria. The most efficacious antiseptics were the QAC’s, and the skin antiseptics CHG and povidone, which completely eradicated all bacteria (Table S1). For 0.63% bleach, 55% and 70% ethanol, a small subpopulation (<1%) of bacteria survived treatment, even beyond the recommended dwell times (Figure 1A–C, Tables S1 and S2).
Figure 1.
Survival of planktonic bacteria following germicide exposure. Average percent survival of ten planktonic A. baumannii strains, both MDR and PS strains combined, after 1-, 2-, and 4-min exposure with (A) 0.63% bleach, (B) 55% ethanol, and (C) 70% ethanol. (D) Comparison of bacterial survival at proper contact times for bleach (4 min), and 55% and 70% ethanol (1 min). A p-value of ≤0.05 was considered significant. The bars show mean survival among all bacterial strains, and error bars represent standard deviation. No significant differences were observed.
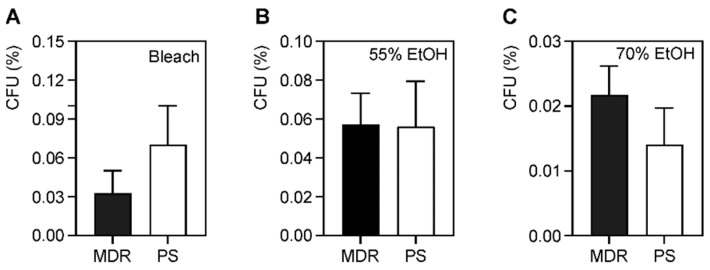
There were no statistical differences among the three treatments (Figure 1D). To determine if the MDR strains survived better than the PS strains, we performed unpaired t-tests among the two separate groups (Figure 2). Again, no statistical differences were observed between any of the three antiseptics, when used at their proper dwell times. Our results suggest that the drug-resistance properties of the bacterial strains do not directly confer increased survival in the presence of common hospital antiseptic agents (Figure 2 and Table S2).
Figure 2.
Comparison of survival of microbicide exposure of multidrug-resistant and pan-susceptible strains. Average percent survival of five MDR and five PS planktonic A. baumannii strains following exposure to (A) 4 min of bleach, (B) 1 min of 55% ethanol, and (C) 1 min of 70% ethanol. The bars show mean survival and error bars represent standard deviation. A p-value of ≤0.05 was considered significant. No significant differences were observed.
2.2. The Presence of Known Microbicide Resistance Genes Confers Higher Tolerance, and Is more Prevalent among Susceptible Strains
Since we did not observe any differences between the survival of MDR and PS strains, we checked the strains for the presence of microbicide-resistance genes. Some known microbicide resistance genes have been characterized in A. baumannii [5]. To test for the presence of some common efflux pump genes, including qacA, qacE, qacEΔ1 and cepA, we amplified each gene by PCR. All ten strains contained the cepA resistance gene, however only four isolates additionally contained both qacE and qacEΔ1. None of the isolates contained the qacA gene (Table 1).
Table 1.
Distribution of known microbicide-resistance genes among A. baumannii strains.
| Strain | Resistance Pattern | qacA | qacEΔ1 | qacE | cepA |
|---|---|---|---|---|---|
| 3 | PS | - | - | - | + |
| 4 | MDR | - | - | - | + |
| 5 | PS | - | - | - | + |
| 9 | MDR | - | - | - | + |
| 49 | MDR | - | - | - | + |
| 51 | MDR | - | - | - | + |
| 54 | PS | - | + | + | + |
| 55 | MDR | - | + | + | + |
| 56 | PS | - | + | + | + |
| 58 | PS | - | + | + | + |
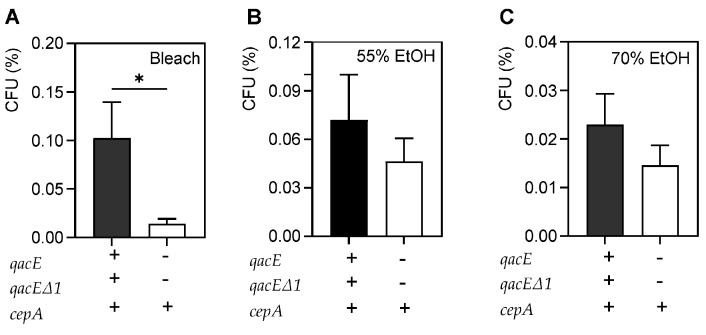
We presumed that the presence of three microbicide-resistance genes would lead to more tolerance to microbicides if they were expressed. The strains were grouped as microbicide-sensitive, containing only cepA, and microbicide-resistant, which contained qacE, qacEΔ1, and cepA. Bacterial survival was evaluated based on microbicide resistance for each antiseptic using unpaired t-tests (Figure 3). Microbicide-resistant bacteria among planktonic bacteria survived more compared to microbicide-sensitive bacteria after treatment with bleach (p = 0.01) but not after 55% and 70% ethanol. This suggests that the presence of these efflux genes may confer microbicide tolerance to bleach, irrespective of their drug-resistance status.
Figure 3.
Comparison of survival of microbicide exposure of microbicide-resistant and -susceptible strains. Average percent survival of four microbicide-resistant, planktonic bacteria vs. six microbicide-sensitive strains after exposure to antiseptics at proper dwell times. (A) Microbicide-resistant strains survived more than microbicide-sensitive bacteria after 4 min of bleach exposure (p = 0.01). No differences were seen after 1 min of 55% ethanol exposure (B), or 1 min of 70% ethanol exposure (C). The bars show mean survival and error bars represent standard deviation. A p-value of ≤0.05 was considered significant. * p-value < 0.05.
2.3. Determination of MICs and MBCs of Components of Antiseptic Wipes
Although the MDR strains were not found to be more resistant to the clinical disinfecting agents than susceptible strains, it is possible that they have an increased MIC or MBC. To test this idea, we determined the MIC and MBC of the A. baumannii strains to each component of the hospital wipes, including bleach, ethanol, and isopropanol. MIC determination for the QACs was not possible, as at higher concentrations, a precipitate formed that led to an artificially high optical density (OD) reading when no bacterial growth was observed by plating. In general, we found that the hospital concentrations used were well above the MICs and MBCs for bleach, ethanol, and isopropanol (Table 2, Table 3 and Table 4).
Table 2.
Average MICs and MBCs of A. baumannii strains following bleach exposure.
| MIC | MBC | MBC/MIC | |
|---|---|---|---|
| All strains | 0.037 | 0.098 | 2.65 |
| MDR | 0.035 | 0.156 | 4.46 |
| PS | 0.039 | 0.039 | 1.00 |
| qacE, qacEΔ1, cepA | 0.039 | 0.186 | 4.77 |
| cepA | 0.036 | 0.137 | 3.81 |
Table 3.
Average MICs and MBCs of A. baumannii strains following ethanol exposure.
| MIC | MBC | MBC/MIC | |
|---|---|---|---|
| All strains | 5.94 | 8.125 | 1.37 |
| MDR | 6.25 | 10 | 1.60 |
| PS | 5.63 | 6.25 | 1.11 |
| qacE, qacEΔ1, cepA | 5.47 | 7.81 | 1.43 |
| cepA | 6.25 | 8.33 | 1.33 |
Table 4.
Average MICs and MBCs of A. baumannii strains following isopropanol exposure.
| MIC | MBC | MBC/MIC | |
|---|---|---|---|
| All strains | 6.25 | 8.33 | 1.33 |
| MDR | 6.25 | 7.81 | 1.25 |
| PS | 6.25 | 8.75 | 1.40 |
| qacE, qacEΔ1, cepA | 6.25 | 10.94 | 1.75 |
| cepA | 6.25 | 6.25 | 1.00 |
As expected, the MBCs for all microbicides were higher than their respective MICs. Generally, an MBC/MIC ratio >4 indicates that the agent has bacteriostatic properties, while a ratio of <4 indicates the agent is bactericidal [48,49]. If looking at the average MBC/MIC ratio for all strains, bleach is bactericidal (Table 2). However, if the strains are separated by drug or microbicide-resistance properties, differences emerge. While the MICs for all groups are very similar, the MBCs for the MDR and microbicide-resistant strains were increased compared to the corresponding sensitive strains (Table 2). The MBC/MIC ratio becomes >4 after bleach treatment for MDR and microbicide-resistant strains, indicating bleach has bacteriostatic properties against these strains. This aligns with our prior time-kill assay results, which showed planktonic microbicide-resistant strains survived more after bleach exposure compared to microbicide-sensitive ones (Figure 3). The MBC for the MDR strains in ethanol was increased compared to the PS strains, however, ethanol was still bactericidal against these strains (Table 3). A similar observation was made for the microbicide-resistant strains with isopropanol (Table 4). The increase in MBC indicates a greater risk for development of microbicide resistance [41].
2.4. Antiseptic Tolerance of A. baumannii Biofilms
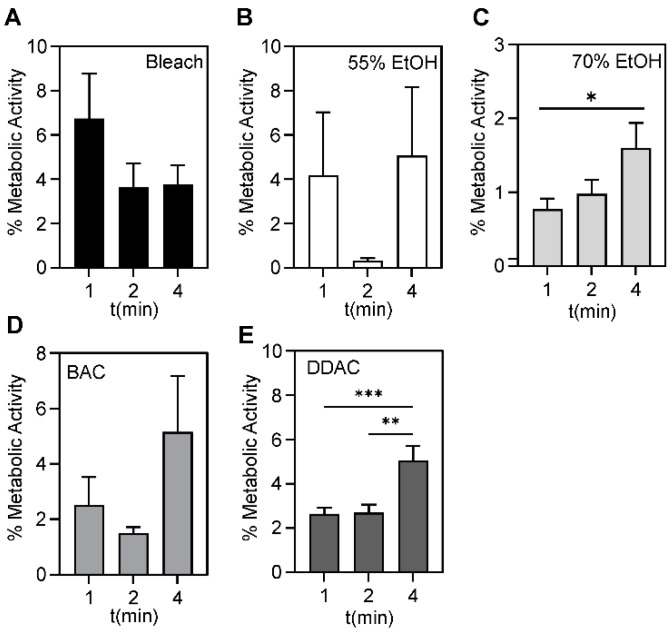
Since we did not observe any differences among MDR and PS A. baumannii strains when grown planktonically, we next determined if bacteria that were present in biofilms would demonstrate any differences in microbicide survival. Biofilm formation in A. baumannii is an important virulence factor that allows for survival in adverse environments, including on polycarbonate, which is often used in the production of medical equipment [50,51,52]. Bacterial biofilms were formed following static growth in 96-well plates for 48 h. Non-adherent cells were removed, and resulting biofilms were exposed to antiseptic agents for 1, 2 and 4 min. Biofilm metabolic activity was determined using an XTT-reduction assay and used as a proxy to determine biofilm viability after exposure to microbicidal agents. Overall, there was significantly more survival among bacteria in biofilms compared to free living (compare Figure 4 to Figure 1D, Table S3). We found that there was 3.7% survival after 4 min of bleach exposure, 6.23% after 1 min of 55% ethanol, and 3.3% after 1 min of 70% ethanol. Whereas previously the QAC formulations led to complete killing of planktonic bacteria, in biofilms there was 2.6% survival after exposure to 1 min of DDAC, and 1.5% after 2 min of exposure of 0.5% BAC (Figure 4). Among the different antiseptic treatments, these differences were not significant (Figure 4 and Table S3).
Figure 4.
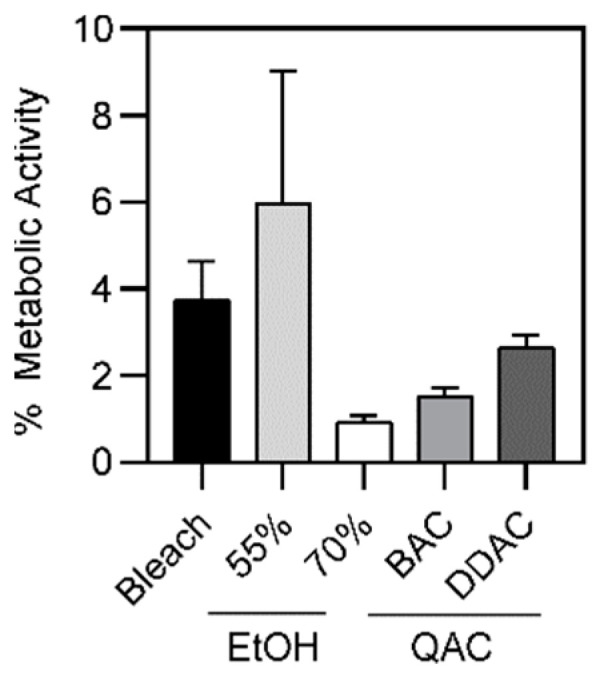
Bacterial biofilm survival when exposed to various microbicides. A comparison of average biofilm survival when exposed to various microbicidal agents. No significant differences were observed between all microbicides at their proper contact times (4 min for bleach, 1 min for 55% and 70% ethanol and DDAC, and 2 min for BAC). The average metabolic activity of all strains is displayed; error bars represent standard deviation.
Increasing the contact time did not significantly affect bacterial survival after treatment with 0.63% bleach, 55% ethanol, or 0.50% DDAC (Figure 5, Table S4). After treatment with 70% ethanol, a small, but significant increase in survival was noted after 2 min (p = 0.04) and 4 min (p = 0.02) compared to 1 min. After treatment with 0.61% BAC, a similar small increase was observed at 2 min (p = 0.0009) and 4 min (p = 0.001) compared to 1 min (Figure 5D,E and Table S4). It is likely that these small observed increases are not biologically significant.
Figure 5.
Bacterial biofilm survival following microbicide exposure. No significant differences were seen in the average metabolic activity for all strains after 1, 2, or 4 min of exposure to (A) 0.63% bleach and (B) 55% ethanol. (C) A small, but significant increase was observed at 4 min compared to 1 min exposure to 70% ethanol (p = 0.04). Of the two QACs tested, no significant differences were seen in (D) 0.5% BAC, and small, but significant differences were observed after (E) 4 min compared to 1 min (p = 0.0009) and 2 min (p = 0.0012) of 0.61% DDAC exposure. The average metabolic activity of all strains is displayed and error bars represent the standard deviation. A p-value of ≤0.05 was considered significant. * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
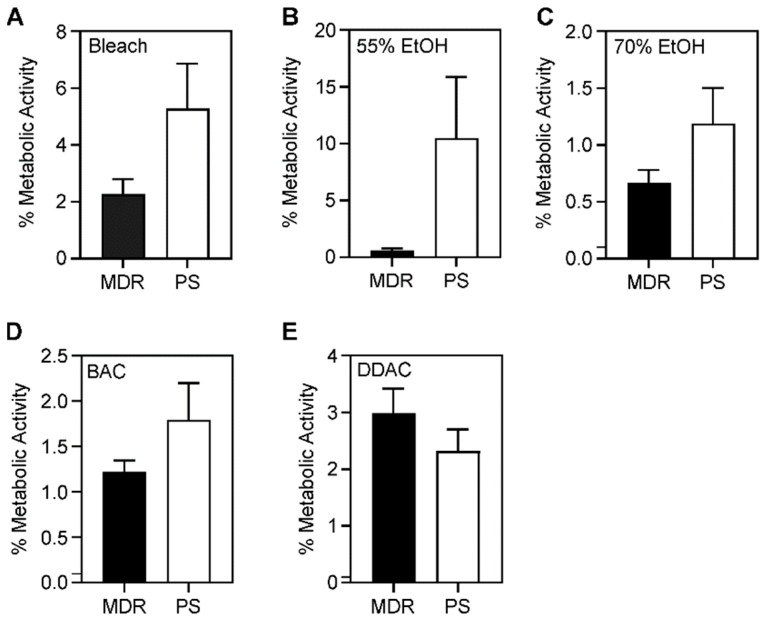
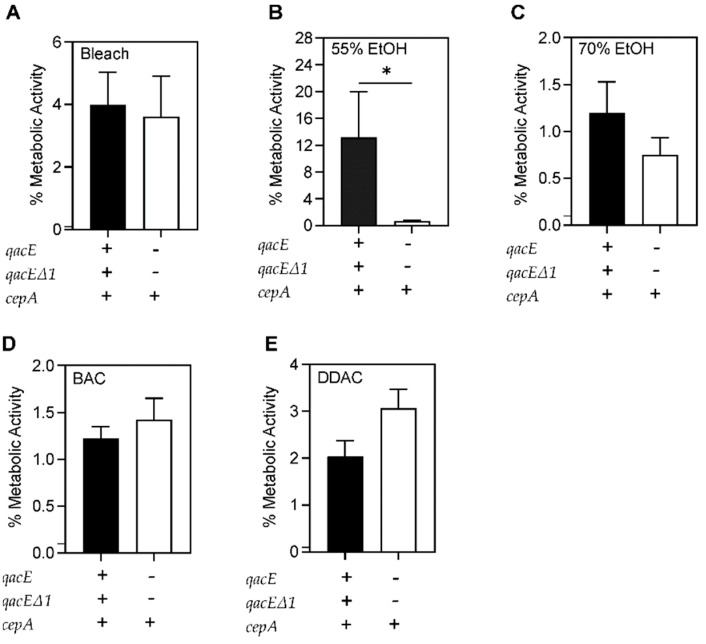
Bacterial biofilm survival was further evaluated based on the resistance pattern in each microbicide (bleach, 55% and 70% ethanol, BAC, and DDAC) using unpaired t-tests. No significant differences were seen between MDR and PS bacterial survival in any of the microbicides after biofilm formation (Figure 6). However, more microbicide-resistant compared to microbicide-sensitive bacteria survived after 55% ethanol treatment (Figure 7B). No significant differences were seen between microbicide-resistant and sensitive isolates in other antiseptics (Figure 7).
Figure 6.
Comparison of survival of bacterial biofilms formed by multidrug-resistant and pan-susceptible strains. Average percentage of metabolic activity of five MDR versus five PS biofilms after (A) 4 min of bleach, (B) 1 min of 55% ethanol, (C) 1 min of 70% ethanol, (D) 2 min of 0.5% BAC, and (E) 1 min of 0.61% DDAC exposure. The average metabolic activity is displayed; error bars represent standard deviations. A p-value of ≤0.05 was considered significant. No significant differences were observed.
Figure 7.
Comparison of survival of bacterial biofilms formed by microbicide-resistant and -susceptible strains. Average percentage of metabolic activity of four microbicide-resistant and six microbicide-sensitive strains. No significant differences were seen between resistant and sensitive strains after (A) 4 min of bleach exposure. (B) Significantly more microbicide-resistant bacteria survived compared to sensitive strains after 1 min of 55% ethanol exposure (p = 0.04). No significant differences between resistant and sensitive bacteria were seen after (C) 1 min of 70% ethanol, (D) 2 min of 0.5% BAC, and (E) 1 min of 0.61% DDAC exposure. The average metabolic activity of is displayed and error bars represent the standard deviation. A p-value of ≤0.05 was considered significant. * p-value < 0.05.
3. Discussion
Hospital-acquired infections can be detrimental to a patient’s health, and outbreaks can be influenced by a multitude of external factors. From 2012–2018, the number of CRAB infections was downtrending [53]. The hospital rates of carbapenem resistance were approximately 20–35% based on antibiograms, but resistance can vary year to year [54]. Recently, the COVID-19 pandemic resulted in increased CRAB hospital outbreaks [55,56,57], which may be attributable to empiric antibiotic overuse [56]. Given the current crisis of antibiotic resistance, preventing and controlling outbreaks has become more important than ever. Besides practicing antimicrobial stewardship, proper decontamination—a term defined by the Occupational Safety and Health Administration (OSHA) to describe the process by which pathogenic organisms are destroyed either physically or chemically—should be employed. This process can be through cleaning, sanitizing, disinfecting, or sterilizing. Cleaning refers to the removal of pathogens using a detergent or surfactant. Sanitizing refers to reducing the number of pathogens within 30 s. Disinfecting refers to the destruction of pathogens, except for sporulating organisms. Sterilizing is the process by which all pathogens are effectively killed, including those that undergo sporulation [26]. The germicidal formulations used in this study mimic hospital disinfectant wipes used by Cooper University Hospital and are approved by the Environmental Protection Agency (EPA) to be effective against specific MDR A. baumannii strains after one a minute exposure of bleach and DDAC, but two minutes for BAC.
The goals of this study were to understand the implications of antibiotic and microbicide-resistance among A. baumannii isolates and to assess their potential contribution to hospital outbreaks. We selected disinfecting wipes and skin antiseptics that are routinely used in Cooper University Hospital. We showed that all wipe formulations were effective disinfectants against free-living, planktonic A. baumannii strains at 1, 2, and 4 min. Since we tested five different hospital wipes and two skin antiseptics, we compared the efficacy of these different microbicides. We found that povidone, CHG, and the QACs killed 100% of planktonic bacteria. However, following exposure to 0.63% bleach, 55% ethanol, and 70% ethanol at their proper dwell times, a small subpopulation (<0.1%) of bacteria repeatedly survived. For disinfectants to properly destroy pathogens, they must be used at the proper dwell time. The standard dwell times for DDAC against A. baumannii is 1 min and 2 min for BAC [46,47]. Bleach is a disinfectant at 1 min, but sterilizes at 4 min, and common hospital practice recommends using a contact time of 4 min [44]. 50% ethanol is generally germicidal after 60 s and 70% ethanol is germicidal after 30 s [45]. Even at an extended dwell time of 4 min, we found that a small subpopulation remained (Figure 1 and Table S2). This suggests that in any population of A. baumannii, microbicide-tolerant persisters may exist, even if the strains were not exposed to these agents prior. Bacterial persistence is a commonly observed phenomenon during drug exposure and is believed to be a factor in recurring bacterial infections [58,59]. Recently, bacterial persisters were found in Escherichia coli in response to QACs [60]. It is possible that these persisters contribute to the spread of A. baumannii infections in hospital settings, as these bacteria survive well on hard surfaces [4,12,20,21,22,23,24,25]. These results were obtained following proper dwell times, so this may become even more significant if improper disinfecting techniques are used.
Antibiotic resistance is selected for when a subpopulation of bacteria survives exposure to a drug, likely by upregulating efflux pumps or obtaining additional resistance genes through plasmid exchange [61]. A similar phenomenon likely exists for microbicide tolerance or resistance. We hypothesized that MDR strains either more easily acquire additional determinants that confer microbicide resistance or contain drug-resistance determinants that confer cross-protection against microbicides. We expected that the MDR strains would be more resistant to some microbicides than PS strains. While we did not find any differences in the percentage of survival between MDR and PS strains during the time-kill assays with bleach (Figure 2), the MDR strains had a higher average MBC. With a higher MBC, this made the MBC/MIC ratio >4, indicating that bleach is not bactericidal against these strains, but is bacteriostatic (Table 2). This was also true for the microbicide-resistant strains. The MDR strains also had an increased MBC to ethanol (Table 3), whereas the microbicide-resistant strains had an increased MBC in isopropanol (Table 4), but both these agents were still bactericidal. The increase in MBC indicates an evolutionary trend and may imply that upon further exposure to the microbicide, it is more likely that they will develop increased tolerance or resistance [41]. These findings highlight the importance of using antiseptics well above the MIC or MBC to ensure proper disinfection [62]. It is also important to note that we only evaluated ten strains; future studies should evaluate more strains to better understand the relationship between antibiotic and microbicidal-resistance genes.
The relationship between microbicidal and drug-resistance is equivocal. Even though some studies identified the presence of microbicidal-resistance genes in MDR pathogens [5,34,36,39], evidence of phenotypic resistance has not been demonstrated in vitro [39,40]. On the other hand, some research has been able to demonstrate such a relationship [42]. It is likely that microbicide tolerance, rather than antibiotic resistance is the significant driver determining bacterial survival in adverse environments [5,32,35,37,42]. We screened our collection for several known microbicidal-resistance genes from the SMR family that included the common efflux pump genes qacA, qacE, and qacEΔ1, and cepA. The qac resistance genes confer resistance to quaternary ammonium compounds, such as BAC and DDAC [35]. They also can confer resistance to specific antimicrobials, such as qacE with sulfonamides [35] and cepA with CHG [5,32,37]. Our data does not demonstrate a relationship between the two types of resistance, as microbicidal-resistance gene distribution in our strain collection was not associated with antimicrobial resistance (Table 1). Only one MDR strain in our collection had the three microbicide-resistance genes whereas and all others were PS. It is possible that MDR strains continue to acquire additional microbicidal-resistance genes during the evolution of a prolonged hospital outbreak. Of course, it is also possible that there are additional, uncharacterized resistance mechanisms in our collection. In addition, we do not know which efflux systems are expressed in these strains, if any. While there was no planktonic bacterial survival after QAC, povidone or CHG exposure among microbicide-resistant strains, there was a significant increase in bacterial survival after bleach treatment compared to microbicide-susceptible strains (Figure 3). This suggests that some of these efflux pumps may be expressed and that their expression confers more tolerance to bleach exposure.
As we did not observe differences among free-living MDR and PS bacteria, we thought that differences might emerge following biofilm growth. The bacterial biofilm encases the cells an extracellular matrix that protects cells in vivo from immune defenses and antibiotics, and protects them from environmental insults such as surface disinfectants [63,64]. In the natural environment, the ability to form biofilms is critical for A. baumannii survival [65]. We assessed microbicide efficacy against A. baumannii following biofilm formation. We found that larger percentages of the population survived, when compared to free-living bacteria. On average, 3.7%, 5.6%, 0.9%, 1.5%, and 2.6% of bacteria survived after exposure to 4 min of bleach, 1 min of 55% ethanol, 1 min of 70% ethanol, 2 min of 0.5% BAC, and 1 min of 0.61% DDAC, respectively (Table S3). Again, increasing the exposure time did not result in an increase in bacterial killing (Table S4). Rather, we observed that longer exposure to 70% ethanol and 0.61% DDAC resulted in statistically significant more bacterial survival compared to the 1-min time point. The small observed increase in survival is likely not biologically relevant. In addition, no differences were observed between MDR and PS strains (Figure 6), however differences were seen between microbicide-resistant and sensitive strains (Figure 7), suggesting that drug resistance is a separate phenomenon from microbicide resistance. This data suggests that the surviving subpopulation is tolerant or resistant to these microbicidal agents. Similar to a prior study which highlighted the connection between biofilm formation and antiseptic resistance [28], our findings of bacterial survival of >0.1% demonstrate that biofilm formation is an innate mechanism of microbicide tolerance. Together, our results suggest the need for a more extensive decontamination process when biofilms might be involved. For instance, a previous study demonstrated that it took up to 10 min to destroy A. baumannii biofilms [66]. Furthermore, if there is extensive grime or other visible soil, additional wipes and pre-cleaning with applied friction is recommended for disinfection [44,46,47]. Our findings, along with prior studies suggest that bacterial biofilms may require pre-cleaning prior to disinfection. As it is difficult to know when bacterial biofilms are present, the safest option is to implement a pre-cleaning step with use of friction as part of standard hospital operating procedures.
In sum, a more holistic approach that extends beyond surface decontamination may be needed to combat HAI’s. Surfaces in hospitals and health care facilities serve as reservoirs for pathogens, and hands are usually the vector transferring those microorganisms to patients. Thus, hand hygiene is important in minimizing transfer of pathogens. Cleaning and disinfecting the environment where the patient resides is fundamental to reduce the microorganism burden in patient’s environment. Most, if not all housekeeping surfaces need to be cleaned with soapy water or a detergent disinfectant, according to product specifications, where an emphasis is placed on scheduling and the technique used to clean and disinfect [67,68]. In addition, to focus on the environmental surfaces, there has been heavy reliance on development of strategies to prevent HAIs, often referred to as “bundles” of infection prevention. A method called the “bundle” approach consists of ongoing hospital staff education with regards to maintaining strict hand hygiene, isolation precautions, environmental cleaning, and surveillance of high-risk areas. In 2009, a hospital in Spain assessed the long-term impact of the bundle approach from 1994 to 2003. Results showed that the rates of colonization and infection significantly decreased from 0.82 cases per 100 admissions in 1994, to 0.46 in 1996–1997, to 0.21 in 1998–2003 [69]. Thus, while proper decontamination is important, a multi-disciplinary approach is essential when attempting to combat this problematic pathogen.
4. Materials and Methods
4.1. Bacterial Strains, Media, and Growth Conditions
Ten A. baumannii patient isolates were collected during a routine workup performed by the clinical microbiology lab at Cooper University Hospital, Camden, NJ, USA. The isolates were collected during a hospital outbreak from 2004–2005 and included strains from sporadic cases from 2007–2012. The bacterial isolates were de-identified of all patient information. Since the goals of the project did not include interactions or interventions with living individuals or their private identifiable data, the Cooper Human Research Protection Program determined that this project did not involve human subjects, and as a result, the present research did not require an IRB review. We selected five MDR and five PS strains, as determined by the susceptibility testing during routine workup. Liquid and agar Mueller-Hinton broth (MHB) and Luria broth (LB) were prepared following standard protocols. For time-kill assays with planktonic bacteria, cells were grown overnight in liquid MHB at 37 °C with aeration. For biofilm assays, starter cultures were grown overnight in liquid LB at 37 °C with aeration. Growth was monitored by measuring the optical density at 600 nm (OD600) when appropriate.
4.2. Time Kill Assays with Planktonic Bacteria
Bacterial strains were grown overnight in MHB at 37 °C with aeration. The following day, 100 μL of stationary phase cells were added to 900 μL of diluted antiseptic in MHB, or MHB alone. The following commonly used hospital disinfectant wipes were recreated as solutions: 55% and 70% ethanol, 0.63% bleach, and two high alcohol QAC’s: (1) 0.61% dodecyl dimethyl ammonium chloride (Millipore Sigma, Burlington, MA, USA) in 27% ethanol and 25% isopropanol, and (2) a combination of 0.25% alkyl dimethyl ethyl benzyl ammonium chloride (Millipore Sigma) and 0.25% alkyl dimethyl benzyl ammonium chloride (Beantown Chemical, Hudson, NH, USA) in 55% isopropanol. Skin antiseptics tested were 2% chlorohexidine gluconate (ThermoFisher Scientific, Waltham, MA, USA), and 10% povidone (ThermoFisher Scientific). The cells were incubated in the presence or absence of microbicides for 1, 2 and 4 min. At each time point, cells were centrifuged, washed twice with phosphate buffered saline (PBS, 10 mM potassium phosphate, pH 7.4, 0.15 M NaCl) and resuspended in 1 mL PBS. Serial dilutions were performed, plated on LB and incubated overnight at 37 °C. Prior to antiseptic treatment, cells were serially diluted and plated to determine initial culture counts. The next day, colony-forming units (CFUs) were counted for each time point, and percent survival determined as antiseptic-resistant CFUs/mL divided by the initial CFUs/mL. Experiments were performed in triplicate if some bacterial survival was noted or in duplicate if there was no bacterial survival (i.e., povidone, CHG, and QAC’s).
4.3. Time Kill Assays with Bacterial Biofilms
Bacterial biofilms were grown as previously described [70]. Briefly, overnight cultures were diluted into fresh LB media at a starting OD600 of 0.1. 200 μL of these solutions were added to a flat-bottomed 96-well plate and grown for 48 h to allow for biofilm formation. Following incubation, non-adherent bacteria were removed by washing three times with an equal volume of PBS. Four wells were used for each strain. MHB alone served as a sterility control. The other three wells were incubated in the presence of an antiseptic for 1, 2 and 4 min. The antiseptics tested were 0.63% bleach, 55% and 70% ethanol, 0.61% DDAC [46], and 0.50% BAC [47]. The wells were washed three times with 100 μL PBS. To estimate survival of cells in the biofilm, XTT reduction assays were performed as described, with minor modifications [71]. A stock solution of 1 mg/mL XTT (Alfa Aesar, Tweksbury, MA, USA) was made in PBS, which required heating to ~50 °C to fully dissolve the XTT. A 0.4 mM stock solution of menadione (MP Biomedicals) was made in dimethyl-formamide (DMF). Both solutions were filter sterilized with a 0.22 μM filter. To make a working solution, 1.5 mL of the XTT stock solution was mixed with 300 μL of the menadione stock solution. To each well, 200 μL of PBS and 12 μL of the XTT-menadione working solution was added. The plates were wrapped in foil and incubated overnight at 37 °C. The OD450 was measured the following day, using a Synergy H1 Microplate reader (Biotek). Prior to analysis, the background was subtracted from each reading. Each experiment was performed three independent times.
4.4. Polymerase Chain Reaction
Colony PCR was performed to detect the presence of the known microbicide-resistance genes qacA, qacE, qacEΔ1, and cepA. The primers used for amplification are listed in Table 5, and conditions used for the reaction were as previously described [5]. The PCR products were resolved on a 1% TAE agarose gel, and bands visualized with EZ Vision dye (Avantor). All PCR reactions were performed two independent times.
Table 5.
Primers used in this study.
| Name | Sequence (5′ → 3′) | Reference |
|---|---|---|
| qacE_For | CCCGAATTCATGAAAGGCTGGCTT | [5] |
| qacE_Rev | TAAGCTTTCACCATGGCGTCGG | [5] |
| qacΔE1_For | TAGCGAGGGCTTTACTAAGC | [5] |
| qacΔE1_Rev | ATTCAGAATGCCGAACACCG | [5] |
| cepA_For | CAACTCCTTCGCCTATCCCG | [5] |
| cepA_Rev | TCAGGTCAGACCAAACGGCG | [5] |
| qacA_For | GCTGCATTTATGACAATGTTTG | [39] |
| qacA_Rev | AATCCCACCTACTAAAGCAG | [39] |
4.5. Minimum Inhibitory Concentration (MIC) and minimum Bactericidal Concentration (MBC) Determinations
The MICs to the microbicides were determined by broth microdilution assay, according to standard protocols [72]. The OD600 of overnight cultures were determined, and cells diluted to a starting OD600 of 0.05. The starting concentration (“X”) for each antiseptic agent is listed in Table 6. Each antiseptic was added at a 2X concentration to the first column of a 96-well plate. Two-fold serial dilutions were performed, and an equal volume of diluted cells was added to each well. The plates were incubated overnight at 37 °C without shaking. The following day, the OD600 values were read using a Synergy H1 Microplate reader (Biotek). All MIC determinations were made at least two independent times. To determine MBCs, 25 μL of cells from each well where no growth was observed were plated on LB. Plates were incubated overnight at 37 °C, and following incubation, plates were observed for bacterial growth. The lowest concentration where no growth was observed, was reported as the MBC. All determinations were made at least two independent times.
Table 6.
Starting concentrations for MIC assays.
| Antiseptic Component | “X” Value |
|---|---|
| Bleach | 5.0% |
| Ethanol | 50% |
| Isopropanol | 50% |
| Dodecyl dimethyl ammonium chloride | 4.88% |
| Alkyl dimethyl ethyl benzyl ammonium chloride | 1.12% |
| Alkyl dimethyl benzyl ammonium chloride | 1.12% |
| Chlorhexidine gluconate | 2.0% |
4.6. Statistical Analyses
Statistical analyses were performed using GraphPad Prism 9. The DUOT outlier test was performed on replicates and removed 5% of outliers. If outliers were removed, the experiment was repeated to produce at least three, biological replicates. For statistical comparisons, strains were grouped based on resistance patterns, i.e., MDR vs. PS and presence of multiple microbicide-resistance genes (microbicide-resistant, cepA, qacE, and qacEΔ1) vs. cepA alone (microbicide-sensitive), for both planktonic and biofilms conditions. One factor ANOVAs with post-hoc Tukey tests were performed to evaluate whether the type of bacterial resistance affected bacterial survival with varying dwell times or varying types of microbicides at proper dwell time. Unpaired t-tests were used to compare MDR and PS strains, and microbicide-resistant and microbicide-sensitive strains for each microbicide. A p value ≤ 0.05 was considered statistically significant.
5. Conclusions
Our findings highlight that antibiotic resistance may occur by mechanisms other than microbicidal resistance and may be expressed independently in some MDR strains. Microbicide-resistance genes were not necessarily associated with MDR strains, as in our collection, it was more likely that the PS strains contained such resistance genes. The presence of such genes does not mean that they are expressed; however, there was increased survival among planktonic bacteria to some antiseptic agents in the presence of these genes, suggesting that one or more might be expressed. Currently, some hospital policies require isolation precautions only if the pathogen is MDR. Given our findings of microbicide resistance even amongst PS strains, hospitals may want to consider stricter contact precautions, even among infections with PS A. baumannii. In addition, while the 0.1% surviving subpopulation represents a small number of bacteria in the population, they may still contribute to hospital outbreaks. It is important to note that our study only evaluated 10 strains, and thus, is a work in progress, requiring further study with a larger sample size to properly evaluate the clinical significance of this subpopulation’s role in perpetuating hospital outbreaks. Finally, and perhaps more pertinent to microbicide resistance, when biofilm formation occurred, a noticeable reduction in disinfectant effectiveness was observed. Therefore, the main form of microbicide tolerance may be due to biofilm formation rather than the expression of microbicide-resistance genes. However, these possibilities are not mutually exclusive, and it may be that the combination of specific resistance genes in bacteria that readily form biofilms is the most problematic in terms of their removal from hospital surfaces. This highlights the need for more thorough decontamination processes that include pre-cleaning in addition to disinfection.
Acknowledgments
We thank Dejan Nikolic for performing mass spectrometry experiments on the collection to confirm the species and for providing susceptibility data.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11050614/s1, Table S1: Percent survival of planktonic bacteria following microbicide exposure at proper contact times, Table S2: Average survival following microbicide exposure of planktonic bacteria, Table S3: Percent survival of bacterial biofilms following microbicide exposure at proper contact times and Table S4: Average survival following microbicide exposure of bacterial biofilms.
Author Contributions
Conceptualization, V.J.C., H.F. and R.N.; Validation, M.B., J.L., M.C. and H.M.G.; Formal Analysis, M.B.; Investigation, M.B., J.L., M.C. and H.M.G.; Resources, H.F., V.J.C., R.N.; Writing—Original Draft Preparation, M.B.; Writing—Review & Editing, M.B., J.L., M.C., H.M.G., V.J.C., R.N. and H.F.; Visualization, M.B. and V.J.C.; Supervision, V.J.C., H.F. and R.N.; Project Administration, V.J.C.; Funding Acquisition, V.J.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This work was supported by Rowan University start-up funds and a Biomedical Sciences Departmental internal award to V.J.C.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fraimow H., Nahra R. Resistant Gram-negative infections. Crit. Care Clin. 2013;29:895–921. doi: 10.1016/j.ccc.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Sunenshine R.H., Wright M.O., Maragakis L.L., Harris A.D., Song X., Hebden J., Cosgrove S.E., Anderson A., Carnell J., Jernigan D.B., et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg. Infect. Dis. 2007;13:97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson R.E., Slayton R.B., Stevens V.W., Jones M.M., Khader K., Rubin M.A., Jernigan J.A., Samore M.H. Attributable mortality of healthcare-associated infections due to multidrug-resistant Gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Infect. Control. Hosp. Epidemiol. 2017;38:848–856. doi: 10.1017/ice.2017.83. [DOI] [PubMed] [Google Scholar]
- 4.Almasaudi S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi J. Biol. Sci. 2018;25:586–596. doi: 10.1016/j.sjbs.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomaa F.A.M., Helal Z.H., Khan M.I. High Prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 genes and their association with decreased susceptibility to antibiotics and common hospital biocides in clinical isolates of Acinetobacter baumannii. Microorganisms. 2017;5:18. doi: 10.3390/microorganisms5020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biggest Threats and Data Antibiotic/Antimicrobial Resistance CDC. [(accessed on 19 March 2022)]; Available online: https://www.cdc.gov/drugresistance/biggest-threats.html#acine.
- 7.Gilbert D.N., Chambers H.F., Saag M.S., Boucher H.W., Black D., Freedman K., Schwartz B.S. The Sanford Guide to Antimicrobial Therapy. 51st ed. Antimicrobial Therapy, Inc.; Sperryville, VA, USA: 2021. [Google Scholar]
- 8.Jamal S., Al Atrouni A., Rafei R., Dabboussi F., Hamze M., Osman M. Molecular mechanisms of antimicrobial resistance in Acinetobacter baumannii, with a special focus on its epidemiology in Lebanon. J. Glob. Antimicrob Resist. 2018;15:154–163. doi: 10.1016/j.jgar.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Noval M., Banoub M., Claeys K.C., Heil E. The battle is on: New beta-lactams for the treatment of multidrug-resistant Gram-negative organisms. Curr Infect. Dis Rep. 2020;22:1. doi: 10.1007/s11908-020-0710-9. [DOI] [PubMed] [Google Scholar]
- 10.Bassetti M., Echols R., Matsunaga Y., Ariyasu M., Doi Y., Ferrer R., Lodise T.P., Naas T., Niki Y., Paterson D.L., et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021;21:226–240. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 11.Portsmouth S., Echols R., Toyoizumi K., Tillotson G., Nagata T.D. Structured patient interview to assess clinical outcomes in complicated urinary tract infections in the APEKS-cUTI study: Pilot investigation. Ther. Adv. Infect. Dis. 2021;8:20499361211058257. doi: 10.1177/20499361211058257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier P.E., Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 2006;42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 13.Multi-Site Gram-negative Surveillance Initiative. [(accessed on 22 April 2022)]; Available online: https://www.cdc.gov/hai/eip/mugsi.html#publications.
- 14.Acinetobacter in Healthcare Settings HAI CDC. [(accessed on 22 April 2022)]; Available online: https://www.cdc.gov/hai/organisms/acinetobacter.html.
- 15.Piperaki E.T., Tzouvelekis L.S., Miriagou V., Daikos G.L. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019;25:951–957. doi: 10.1016/j.cmi.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Mohd Sazlly Lim S., Zainal Abidin A., Liew S.M., Roberts J.A., Sime F.B. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: A systematic review and meta-analysis. J. Infect. 2019;79:593–600. doi: 10.1016/j.jinf.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Karakonstantis S., Gikas A., Astrinaki E., Kritsotakis E.I. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J. Hosp. Infect. 2020;106:447–453. doi: 10.1016/j.jhin.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Chastre J., Fagon J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 19.Zilberberg M.D., Nathanson B.H., Sulham K., Fan W., Shorr A.F. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit. Care. 2016;20:221. doi: 10.1186/s13054-016-1392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Sayed M.H. Multiple heavy metal and antibiotic resistance of Acinetobacter baumannii strain HAF—13 isolated from industrial effluents. Am. J. Microbiol. Res. 2016;4:26–36. [Google Scholar]
- 21.Carvalheira A., Silva J., Teixeira P. Acinetobacter spp. in food and drinking water—A review. Food Microbiol. 2021;95:103675. doi: 10.1016/j.fm.2020.103675. [DOI] [PubMed] [Google Scholar]
- 22.Hrenovic J., Durn G., Music M.S., Dekic S., Troskot-Corbic T., Skoric D. Extensively and multi drug-resistant Acinetobacter baumannii recovered from technosol at a dump site in Croatia. Sci. Total Environ. 2017;607–608:1049–1055. doi: 10.1016/j.scitotenv.2017.07.108. [DOI] [PubMed] [Google Scholar]
- 23.Higgins P.G., Hrenovic J., Seifert H., Dekic S. Characterization of Acinetobacter baumannii from water and sludge line of secondary wastewater treatment plant. Water Res. 2018;140:261–267. doi: 10.1016/j.watres.2018.04.057. [DOI] [PubMed] [Google Scholar]
- 24.Bravo Z., Orruño M., Navascues T., Ogayar E., Ramos-Vivas J., Kaberdin V.R., Arana I. Analysis of Acinetobacter baumannii survival in liquid media and on solid matrices as well as effect of disinfectants. J. Hosp. Infect. 2019;103:e42–e52. doi: 10.1016/j.jhin.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Catalano M., Quelle L.S., Jeric P.E., Di Martino A., Maimone S.M. Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. J. Hosp. Infect. 1999;42:27–35. doi: 10.1053/jhin.1998.0535. [DOI] [PubMed] [Google Scholar]
- 26.Glossary Disinfection & Sterilization Guidelines Library Infection Control CDC. [(accessed on 19 March 2022)]; Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/glossary.html#M.
- 27.Kawamura-Sato K., Wachino J., Kondo T., Ito H., Arakawa Y. Correlation between reduced susceptibility to disinfectants and multidrug resistance among clinical isolates of Acinetobacter species. J. Antimicrob. Chemother. 2010;65:1975–1983. doi: 10.1093/jac/dkq227. [DOI] [PubMed] [Google Scholar]
- 28.Ivanković T., Goić-Barišić I., Hrenović J. Reduced susceptibility to disinfectants of Acinetobacter baumannii biofilms on glass and ceramic. Arh. Hig. Rada I Toksikol. 2017;68:99–107. doi: 10.1515/aiht-2017-68-2946. [DOI] [PubMed] [Google Scholar]
- 29.Yang C.H., Su P.W., Moi S.H., Chuang L.Y. Biofilm formation in Acinetobacter baumannii: Genotype-phenotype correlation. Molecules. 2019;24:1849. doi: 10.3390/molecules24101849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozhin A., Batasheva S., Kruychkova M., Cherednichenko Y., Rozhina E., Fakhrullin R. Biogenic silver nanoparticles: Synthesis and application as antibacterial and antifungal agents. Micromachines. 2021;12:1480. doi: 10.3390/mi12121480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hetta H.F., Al-Kadmy I.M.S., Khazaal S.S., Abbas S., Suhail A., El-Mokhtar M.A., Ellah N.H.A., Ahmed E.A., Abd-Ellatief R.B., El-Masry E.A., et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021;11:10751. doi: 10.1038/s41598-021-90208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonnell G., Russell A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999;12:147–179. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwaśniewska D., Chen Y.-L., Wieczorek D. Biological activity of quaternary ammonium salts and their derivatives. Pathogens. 2020;12:459. doi: 10.3390/pathogens9060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milani E.S., Hasani A., Varschochi M., Sadeghi J., Memar M.Y. Biocide resistance in Acinetobacter baumannii: Appraising the mechanisms. J. Hosp. Infect. 2021;117:135–146. doi: 10.1016/j.jhin.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Coyne S., Courvalin P., Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011;55:947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin F., Xu Y., Chang Y., Liu C., Jia X., Ling B. Molecular characterization of reduced susceptibility to biocides in clinical isolates of Acinetobacter baumannii. Front. Microbiol. 2017;8:1836. doi: 10.3389/fmicb.2017.01836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang C.T., Chen H.C., Chuang Y.P., Chang S.C., Wang J.T. Cloning of a cation efflux pump gene associated with chlorhexidine resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2002;46:2024. doi: 10.1128/AAC.46.6.2024-2028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babaei M., Sulong A., Hamat R., Nordin S., Neela V. Extremely high prevalence of antiseptic resistant quaternary ammonium compound E gene among clinical isolates of multiple drug resistant Acinetobacter baumannii in Malaysia. Ann. Clin. Microbiol. Antimicrob. 2015;14:11. doi: 10.1186/s12941-015-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijayakumar R., Sandle T., Al-Aboody M.S., AlFonaisan M.K., Alturaiki W., Mickymaray S., Premanathan M., Alsagaby S.A. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii—A first report from the Kingdom of Saudi Arabia. J. Infect. Public Health. 2018;11:812–816. doi: 10.1016/j.jiph.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Shirmohammadlou N., Zeighami H., Haghi F., Kashefieh M. Resistance pattern and distribution of carbapenemase and antiseptic resistance genes among multidrug-resistant Acinetobacter baumannii isolated from intensive care unit patients. J. Med. Microbiol. 2018;67:1467–1473. doi: 10.1099/jmm.0.000826. [DOI] [PubMed] [Google Scholar]
- 41.Wisplinghoff H., Schmitt R., Wohrmann A., Stefanik D., Seifert H. Resistance to disinfectants in epidemiologically defined clinical isolates of Acinetobacter baumannii. J. Hosp. Infect. 2007;66:174–181. doi: 10.1016/j.jhin.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Cuenca F., Tomás M., Caballero-Moyano F.-J., Bou G., Martínez-Martínez L., Vila J., Pachón J., Cisneros J.-M., Rodríguez-Baño J., Pascual Á., et al. Reduced susceptibility to biocides in Acinetobacter baumannii: Association with resistance to antimicrobials, epidemiological behaviour, biological cost and effect on the expression of genes encoding porins and efflux pumps. J. Antimicrob. Chemother. 2015;70:3222–3229. doi: 10.1093/jac/dkv262. [DOI] [PubMed] [Google Scholar]
- 43.US EPA . Product Performance Test Guidelines: OCSPP 810.2300 Sanitizers for Use on Hard Surfaces-Efficacy Data Recommendations [EPA 712-C-07-091] US EPA Office of Pesticide Programs; Washington, DC, USA: 2012. p. 13. [Google Scholar]
- 44.US EPA Office of Pesticide Programs, US EPA . Pesticide Product Label, PDI SANI-CLOTH BLEACH WIPES. US EPA Office of Pesticide Programs; Washington, DC, USA: 2020. [Google Scholar]
- 45.Kampf G., Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin. Microbiol. Rev. 2004;17:863–893. doi: 10.1128/CMR.17.4.863-893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US EPA Office of Pesticide Programs, US EPA . Pesticide Product Label, Wonder Woman Formula B Germicidal Disposable Cloth. US EPA Office of Pesticide Programs; Washington, DC, USA: 2016. [Google Scholar]
- 47.US EPA Office of Pesticide Programs, US EPA . Pesticide Product Label, SANI-CLOTH GERMICIDAL WIPES. US EPA Office of Pesticide Programs; Washington, DC, USA: 2020. [Google Scholar]
- 48.Levison M.E. Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. N. Am. 2004;18:451–465. doi: 10.1016/j.idc.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Knapp L., Amezquita A., McClure P., Stewart S., Maillard J.Y. Development of a protocol for predicting bacterial resistance to microbicides. Appl. Environ. Microbiol. 2015;81:2652–2659. doi: 10.1128/AEM.03843-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eze E.C., Chenia H.Y., El Zowalaty M.E. Acinetobacter baumannii biofilms: Effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018;11:2277. doi: 10.2147/IDR.S169894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greene C., Wu J., Rickard A.H., Xi C. Evaluation of the ability of Acinetobacter baumannii to form biofilms on six different biomedical relevant surfaces. Lett. Appl. Microbiol. 2016;63:233–239. doi: 10.1111/lam.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harding C.M., Hennon S.W., Feldman M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018;16:91. doi: 10.1038/nrmicro.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carbapenem Resistant Acinetobacter. [(accessed on 24 April 2022)]; Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/acinetobacter-508.pdf.
- 54.Fraimow H. (Division of Infectious Diseases, Department of Medicine, Cooper University Hospital, Camden, NJ, USA). Cooper University Hospital Antibiogram, 2004–2012. Personal communication.
- 55.Bhargava A., Riederer K., Sharma M., Fukushima E.A., Johnson L., Saravolatz L. High rate of multidrug-resistant organisms (MDROs) among COVID-19 patients presenting with bacteremia upon hospital admission. Am. J. Infect. Control. 2021;49:1441–1442. doi: 10.1016/j.ajic.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silva A.R.O., Salgado D.R., Lopes L.P.N., Castanheira D., Emmerick I.C.M., Lima E.C. Increased use of antibiotics in the intensive care unit during coronavirus disease (COVID-19) pandemic in a brazilian hospital. Front. Pharmacol. 2021;12:778386. doi: 10.3389/fphar.2021.778386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions—New Jersey, February–July 2020. [(accessed on 22 April 2022)]; doi: 10.15585/mmwr.mm6948e1. Available online: https://www.cdc.gov/mmwr/volumes/69/wr/mm6948e1.htm. [DOI] [PMC free article] [PubMed]
- 58.Fisher R.A., Gollan B., Helaine S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017;15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 59.Trastoy R., Manso T., Fernández-García L., Blasco L., Ambroa A., del Molino M.L.P., Bou G., García-Contreras R., Wood T.K., Tomás M. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin. Microbiol. Rev. 2018;31:e00023-18. doi: 10.1128/CMR.00023-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nordholt N., Kanaris O., Schmidt S.B.I., Schreiber F. Persistence against benzalkonium chloride promotes rapid evolution of tolerance during periodic disinfection. Nat. Commun. 2021;12:6792. doi: 10.1038/s41467-021-27019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hooper D.C., Bacterial resistance to antimicrobial agents . In: Harrison’s Principles of Internal Medicine. 20th ed. Jameson J., Fauci A.S., Kasper D.L., Hauser S.L., Longo D.L., Loscalzo J., editors. McGraw Hill; New York, NY, USA: 2018. [(accessed on 22 April 2022)]. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192021126. [Google Scholar]
- 62.Russell A.D. Mechanisms of antimicrobial action of antiseptics and disinfectants: An increasingly important area of investigation. J. Antimicrob. Chemother. 2002;49:597–599. doi: 10.1093/jac/49.4.597. [DOI] [PubMed] [Google Scholar]
- 63.Stewart P. Antimicrobial tolerance in biofilms. Microbiol. Spectr. 2015;3:3. doi: 10.1128/microbiolspec.MB-0010-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dewasthale S., Mani I., Vasdev K. Microbial biofilm: Current challenges in healthcare industry. J. Appl. Biotechnol. Bioeng. 2018;5:160–164. doi: 10.15406/jabb.2018.05.00132. [DOI] [Google Scholar]
- 65.Greene C., Vadlamudi G., Newton D., Foxman B., Xi C. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am. J. Infect. Control. 2016;44:e65–e71. doi: 10.1016/j.ajic.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiang S.R., Jung F., Tang H.J., Chen C.H., Chen C.C., Chou H.Y., Chuang Y.C. Desiccation and ethanol resistances of multidrug resistant Acinetobacter baumannii embedded in biofilm: The favorable antiseptic efficacy of combination chlorhexidine gluconate and ethanol. J. Microbiol. Immunol. Infect. 2018;51:770–777. doi: 10.1016/j.jmii.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Maki D.G., Alvarado C.J., Hassemer C.A., Zilz M.A. Relation of the inanimate hospital environment to endemic nosocomial infection. N. Engl. J. Med. 1982;307:1562–1566. doi: 10.1056/NEJM198212163072507. [DOI] [PubMed] [Google Scholar]
- 68.Danforth D., Nicolle L.E., Hume K., Alfieri N., Sims H. Nosocomial infections on nursing units with floors cleaned with a disinfectant compared with detergent. J. Hosp. Infect. 1987;10:229–235. doi: 10.1016/0195-6701(87)90002-8. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Bano J., Garcia L., Ramirez E., Martinez-Martinez L., Muniain M.A., Fernandez-Cuenca F., Beltran M., Galvez J., Rodriguez J.M., Velasco C., et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive "bundle" approach. Am. J. Infect. Control. 2009;37:715–722. doi: 10.1016/j.ajic.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farshadzadeh Z., Taheri B., Rahimi S., Shoja S., Pourhajibagher M., Haghighi M.A., Bahador A. Growth rate and biofilm formation ability of clinical and laboratory-evolved colistin-resistant strains of Acinetobacter baumannii. Front. Microbiol. 2018;9:153. doi: 10.3389/fmicb.2018.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Z., Liang Y., Lin S., Chen D., Li B., Li L., Deng Y. Crystal violet and XTT assays on Staphylococcus aureus biofilm quantification. Curr. Microbiol. 2016;73:474–482. doi: 10.1007/s00284-016-1081-1. [DOI] [PubMed] [Google Scholar]
- 72.Andrews J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001;48((Suppl. 1)):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.