Abstract
Nitrate utilization and ammonium utilization were studied by using three algal isolates, six bacterial isolates, and a range of temperatures in chemostat and batch cultures. We quantified affinities for both substrates by determining specific affinities (specific affinity = maximum growth rate/half-saturation constant) based on estimates of kinetic parameters obtained from chemostat experiments. At suboptimal temperatures, the residual concentrations of nitrate in batch cultures and the steady-state concentrations of nitrate in chemostat cultures both increased. The specific affinity for nitrate was strongly dependent on temperature (Q10 ≈ 3, where Q10 is the proportional change with a 10°C temperature increase) and consistently decreased at temperatures below the optimum temperature. In contrast, the steady-state concentrations of ammonium remained relatively constant over the same temperature range, and the specific affinity for ammonium exhibited no clear temperature dependence. This is the first time that a consistent effect of low temperature on affinity for nitrate has been identified for psychrophilic, mesophilic, and thermophilic bacteria and algae. The different responses of nitrate uptake and ammonium uptake to temperature imply that there is increasing dependence on ammonium as an inorganic nitrogen source at low temperatures.
The forms of inorganic nitrogen used most commonly by bacteria and algae are nitrate and ammonium (82). In many estuarine and marine systems the nitrogen concentration is limiting (14, 23), and nitrate concentrations are generally much higher than ammonium concentrations, although ammonium is invariably the preferred nitrogen source when it is available (46, 82). Despite the relatively low concentrations of ammonium, the great importance of ammonium to the global nitrogen cycle has been increasingly recognized (60, 61). Information concerning the mechanisms of uptake of the two forms of nitrogen is limited (19), and most data have been derived from measurements of uptake systems in higher plants (12, 42, 43) rather than measurements of uptake systems in algae and bacteria.
Previous work has shown that the affinity of bacteria for organic substrates can be highly temperature sensitive (51) and that a low temperature exacerbates any effect of nutrient limitation by making it increasingly difficult to sequester substrates (83). It has been pointed out that decreased affinity for inorganic substrates, such as nitrate, at low temperatures could have profound effects on the productivity of low-temperature environments, such as the Southern Ocean (51). Relatively little is known about the temperature sensitivity of inorganic nitrogen uptake by microorganisms, but it has been suggested that there is a difference between the temperature dependence of the uptake system for nitrate and the temperature dependence of the uptake system for ammonium (13).
A low affinity for inorganic nutrients, as indicated by a high half-saturation constant (Ks) for uptake of silicate and nitrate, has been reported previously for Southern Ocean phytoplankton at low temperatures (36, 69). However, no consistent trend of changing affinity for inorganic nitrogen with temperature has been identified previously by using Ks values alone. The use of Ks to measure affinity can be misleading as this parameter does not necessarily reveal changes in substrate affinity at low concentrations (9). On the other hand, specific affinity (aAo) is a more robust measure of substrate affinity. This parameter is the initial slope of the rectangular hyperbola (Michaelis-Menten or Monod) function relating growth rate (μ) to substrate concentration and is given by aAo = μmax/Ks, where μmax is the maximum growth rate and Ks is the concentration at which μ = 0.5μmax. aAo is the slope of the hyperbola at zero concentration and thus provides an unambiguous measure of the ability of cells to accumulate substrate and grow at very low concentrations, and this parameter is independent of the uptake mechanism (7, 8, 30). Because aAo for growth is a rate divided by a concentration, it has the dimensions time−1 · (mass · liter−3)−1 and in our calculations has the units liters per micromole per hour. Such a measurement of affinity is related to growth through the cell yield.
In this study we investigated the influence of temperature on affinity for nitrate and ammonium in a range of algae and bacteria by using aAo to describe changes in the affinity of an organism for inorganic nitrogen. Note that ammonium is used below to indicate both ammonia (NH3) and ammonium (NH4+), except where a distinction between the two is required, when the appropriate chemical formulae are used.
MATERIALS AND METHODS
Bacterial and algal isolates were chosen so that a wide range of physiological and taxonomic types was represented (Table 1). All cultures were monospecific and axenic and were checked regularly for contamination (6).
TABLE 1.
Isolates used in this study and incubation temperature ranges used for batch culture experiments
| Taxon | Straina | Source | Incubation temp range
|
Person who isolated or reference | |
|---|---|---|---|---|---|
| Minimum temp (°C) | Maximum temp (°C) | ||||
| Vibrio logei | NCIMB 1143 | Seawater, Puget Sound, Southern Ocean | 0.4 | 23 | R. R. Colwell |
| Hydrogenophaga pseudoflava | NCIMB 13125 | Lake sediment, Signy Island, Antarctica | 1.7 | 26.5 | 51 |
| Brevibacterium sp. | NCIMB 13126 | Lake sediment, Signy Island, Antarctica | 1.7 | 26.5 | 51 |
| Klebsiella oxytocab | Freshwater sample, Colne River Estuary, England | 2.4 | 27 | 52 | |
| Escherichia coli | NCIMB 09001 | Unknown | 15 | 35 | Unknown |
| Bacillus stearothermophilus | NCIMB 11401 | Hot water supply, Porton Down, Wiltshire, England | 12.9 | 46.1 | M. J. Cromer |
| Chaetoceros sp.c | CCAP 1010/10 | Seawater sample, Southern Ocean | 2 | 13.6 | Unknown |
| Chaetoceros curvisetumbc | Seawater sample, North Sea | 10.1 | 22.9 | A. Clarke | |
| Dinaliella tertiolectac | CCAP 19/6B | Seawater sample, Oslo Fjord, Norway | 3.3 | 23.9 | 6 |
NCIMB, National Collections of Industrial and Marine Bacteria Ltd., Aberdeen, Scotland; CCAP, Culture Collection of Algae and Protozoa, Dunstaffanage Marine Laboratory, Oban, Scotland.
The isolate was not obtained from a culture collection.
Microalgal isolate.
The μmax values for both bacterial and algal isolates were determined by using a temperature gradient block incubator (51, 77). The temperature range was adjusted so that it was suitable for each organism (Table 1). For algal isolates a temperature gradient block with illuminated wells was used. Illumination was provided by a bank of Triton fluorescent tubes (38 W; 400 to 710 nm, with peaks at 450, 550, and 620 nm; Interpet Ltd., Dorking, England.) placed immediately below the temperature gradient block (well illumination, 200 μmol of quanta · m−2 · s−1). Optically standardized test tubes containing 10 ml of sterile FC2 medium for bacterial isolates (51, 52) or modified f/2 medium for algae (27) were prepared with either 80 μM NH4Cl or 80 μM NaNO3 as the nitrogen source. Two diatoms, Chaetoceros curvisetum and Chaetoceros sp., were not grown on NH4Cl in batch cultures. Preliminary experiments showed that at a concentration of 80 μM, nitrogen was the first nutrient to be depleted, which induced the stationary phase. Subsequent aseptic addition of either a sterile nitrate solution or a sterile ammonium solution resulted in a further increase in the optical density, which confirmed that N limitation occurred. Tubes were placed in the wells of the temperature gradient block, and each tube was aerated continuously; the airstream was humidified to prevent evaporation. After all of the tubes had become equilibrated to the temperature in the block, each tube was inoculated with 0.2 ml of an exponential-phase culture grown on the same medium at the optimum growth temperature of the organism.
After inoculation, growth at each temperature was monitored by periodic measurement of turbidity with a nephelometer (model EEL Unigalvo DS29; Diffusion Systems, London, United Kingdom). The μmax at each temperature was obtained from a first-order linear regression analysis which determined the slope of the linear part of the semilogarithmic plot of optical density versus time.
Batch cultures were regarded as being in the stationary phase when the variation (standard error) in the optical densities at four successive times over a 24-h period was <2% of the mean optical density for the same period. The residual concentration of the limiting nutrient (nitrate or ammonium) during the stationary phase in batch cultures reflected the affinity of the uptake system for the substrate (55). Changes in the residual substrate concentration with temperature provide an indication of changes in affinity (51), although Ks values cannot be calculated directly. When cultures reached the stationary phase, they were removed from the temperature gradient block and centrifuged at 6,000 × g for 15 min. Supernatant (triplicate 1-ml samples) was then removed and analyzed to determine either the residual nitrate content or the residual ammonium content. Nitrate contents were determined colorimetrically (72). The method used was linear for concentrations ranging from 1 to 20 μM, and the limit of detection was 0.25 μM. Ammonium contents were analyzed by the indophenol blue method (29), which was modified by substituting sodium dichloroisocyanuric acid for the original unstable chlorine donor, hypochlorite (37). The ammonium values were linear for concentrations between 1 and 40 μM, and the limit of detection was 0.5 μM. Samples were diluted when necessary in order to obtain concentrations within the detection ranges of the colorimetric methods used.
Chemostat cultures were used to measure μmax independent of batch cultures for all isolates. The use of chemostats also allowed us to measure the Ks and aAo values over a range of temperatures. The chemostat incubation temperatures were set to give a range up to and including the optimum temperature for each isolate. Bacterial (FC2) and algal (f/2) media containing either 80 μM NH4Cl or 80 μM NaNO3 were used for all chemostat experiments so that nitrogen was the growth rate-limiting nutrient. Dilution rates were set at 0.018 h−1 for all chemostats, which were continuously mixed and aerated. All algal cultures were also continuously illuminated (200 μmol of quanta · m−2 · s−1) with twin banks of Triton fluorescent tubes.
Each chemostat was inoculated with 1 ml of an exponential-phase culture grown on the same medium at the optimum growth temperature for the isolate. After inoculation, the growth of each isolate to the steady state was monitored by periodically aseptically removing a 1-ml subsample, whose optical density at 550 nm (OD550) was then determined with a spectrophotometer. A chemostat was considered to be in a steady state when the variation in the standard error for at least six optical densities determined over a period of at least 60 h was <2% of the mean OD550 (51). Nitrogen limitation was confirmed by aseptically adding to a chemostat either 5 ml of a sterile 10 mM nitrate solution or 5 ml of a sterile 10 mM ammonium solution. A subsequent increase in the optical density confirmed that N limitation occurred under steady-state conditions.
Under steady-state conditions Ks values were determined by removing 5-ml subsamples from the chemostats. Each subsample was filtered through a Whatman cellulose acetate filter (nominal particle retention size, 0.2 μm), and residual nitrate or ammonium content in the filtrate was measured. Ks values were calculated by using the following equation: Ks = s(μmax − D)/D, where s is the residual substrate concentration and D is the dilution rate (per hour) (55, 69). Note that the Ks values derived in this way were Ks values for growth and thus are not always equivalent to Ks values determined for uptake (25).
μmax values in the chemostats were determined by increasing the dilution rate to values that were higher than the critical dilution rate in order to induce washout. μmax was calculated from the slope of a plot of ln OD550 versus time during washout. aAo values were then calculated by using μmax values determined for the same chemostat cultures rather than μmax values determined for batch cultures.
Data were analyzed by performing box plots, F tests, one-way analyses of variance (ANOVAs), and first-order linear regression analyses (LRAs) (21). Statistical analysis and data plotting were performed by using the data analysis packages supplied in Systat version 5.04 (Systat Inc.), Excel version 7.0 (Microsoft), and SigmaPlot version 3.0. (Jandel Scientific).
RESULTS
Batch cultures.
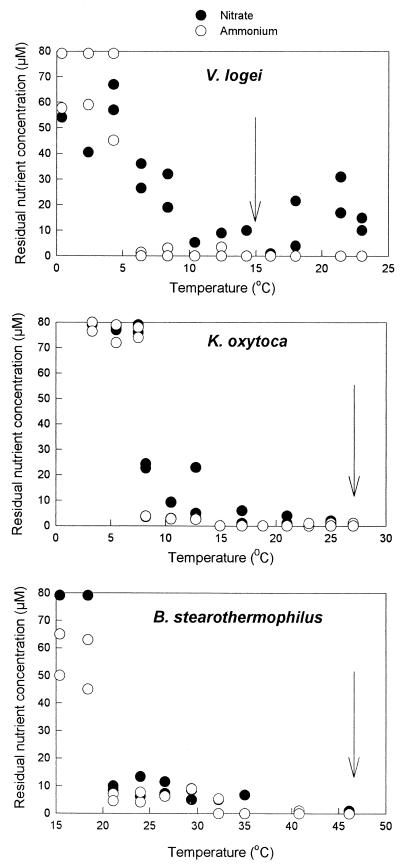
The bacterial and algal isolates used grew at a wide range of temperatures and, consequently, there was a wide range of optimum incubation temperatures (Table 2). The residual concentrations of nitrate and ammonium at the stationary phase in batch cultures exhibited a similar trend with all of the bacterial and algal species investigated (Fig. 1). At or near the optimum temperatures for growth the residual concentrations of either nitrate or ammonium were below the sensitivity of the analyses. As the temperature deviated from the optimum temperature, the residual concentration of either nitrate or ammonium generally increased, although the residual nitrate concentrations tended to increase more than the residual ammonium concentrations increased. As the incubation temperature approached the minimum growth temperature of an organism, the residual concentrations of both N sources increased rapidly. The maximum concentrations (80 μM, equivalent to the starting N concentration in the medium) were observed at temperatures at which growth ceased.
TABLE 2.
Temperature ranges at which isolates grew and optimum temperatures for growth
| Taxon | Growth temp range (°C) | μmax range (h−1) | Optimum incubation temp (°C) |
|---|---|---|---|
| Vibrio logei | 0.4–23 | 0.031–0.077 | 15 |
| Brevibacterium sp. | 1.7–26.5 | 0.055–0.11 | 20 |
| Hygdrogenophaga pseudoflava | 1.7–26.5 | 0.037–0.074 | 24 |
| Klebsiella oxytoca | 8.2–27 | 0.033–0.21 | 27 |
| Escherichia coli | 15–35 | 0.04–0.21 | 35 |
| Bacillus stearothermophilus | 18.4–46.1 | 0.023–0.161 | 46.1 |
| Chaetoceros sp.a | 2–9.2 | 0.017–0.028 | 6 |
| Chaetoceros curvisetuma | 10.1–22.9 | 0.003–0.02 | 22.9 |
| Dunaliella tertiolectaa | 9.1–23.9 | 0.05–0.104 | 23.9 |
Microalgal isolate.
FIG. 1.
Residual concentrations of nitrate (●) and ammonium (○) in batch cultures of three bacterial isolates grown at a range of temperatures. The arrows indicate the optimum growth temperature for each of the isolates.
μmax values exhibited considerable interspecies variation. Psychrotolerant bacteria (Hydrogenophaga pseudoflava, Brevibacterium sp., Vibrio logei) had lower μmax values over their growth temperature ranges than meso- and thermophilic bacteria (Klebsiella oxytoca, Escherichia coli, Bacillus stearothermophilus) had. The flagellate alga Dunaliella tertiolecta had μmax values that were significantly higher than the μmax values obtained for the diatoms C. curvisetum and Chaetoceros sp. The μmax values for all of the bacterial and algal isolates were significantly dependent on temperature (P < 0.05, as determined by LRA). We observed no significant difference (P > 0.2, as determined by ANOVA) between the μmax values for nitrate-limited and ammonium-limited batch cultures of the isolates grown on both substrates.
Chemostat cultures.
There were no significant differences between the μmax values measured in batch cultures and the μmax values measured in chemostat cultures for any of the bacterial or algal cultures, as determined by ANOVA (P > 0.5). The steady-state nitrate concentrations exhibited a significant negative correlation with temperature for all of the isolates (Tables 3 and 4), which reflected the substantial increases in the residual nitrate concentrations that occurred with decreasing temperature in batch cultures. The half-saturation constant for nitrate (Knit) increased as the incubation temperature decreased for most of the bacterial and algal isolates, although no consistent trend was identified for E. coli or B. stearothermophilus. The half-saturation constant for ammonium (Kamm) did not consistently increase as the temperature decreased for any isolate.
TABLE 3.
Growth kinetics for bacterial isolates grown to steady state in nitrogen-limited chemostat cultures
| Taxon | Temp (°C) | Steady-state NO3− concn (μM) | μmax with NO3− (h−1) | Knit (μM) | anito (liter · μmol−1 · h−1) | Steady-state NH4+ concn (μM) | μmax with NH4+ (h−1) | Kamm (μM) | aammo (liter · μmol−1 · h−1) |
|---|---|---|---|---|---|---|---|---|---|
| Vibrio logei | 15 | 2.5 (0.1)a | 0.0677 (0.0003) | 6.9 | 0.0098 | 9.3 (0.1) | 0.0614 (0.002) | 22.4 | 0.0027 |
| 10 | 8.5 (0.1) | 0.053 (0.001) | 16.5 | 0.0032 | 10.8 (0.5) | 0.053 (0.003) | 20.8 | 0.0025 | |
| 8 | 8.5 (0.3) | 0.0375 (0.005) | 9.2 | 0.0041 | 9.1 (0.01) | 0.048 (0.001) | 15.1 | 0.0032 | |
| 6 | 11.0 (0.1) | 0.0373 (0.0001) | 11.8 | 0.0032 | 4.3 (0.2) | 0.049 (0.005) | 7.4 | 0.0066 | |
| 4 | 13.5 (0.1) | 0.0381 (0.001) | 15.1 | 0.0025 | 11.4 (0.2) | 0.0391 (0.002) | 13.4 | 0.0029 | |
| Hydrogenophaga pseudoflava | 15 | NGb | NG | NG | NG | 1.6 (0.02) | 0.074 (0.001) | 5.0 | 0.0148 |
| 10 | NG | NG | NG | NG | 4.5 (0.4) | 0.06 (0.009) | 10.5 | 0.0057 | |
| 5 | NG | NG | NG | NG | 1.6 (0.03) | 0.06 (0.002) | 3.7 | 0.016 | |
| Brevibacterium sp. | 15 | NG | NG | NG | NG | 2.7 (0.7) | 0.12 (0.02) | 13.2 | 0.0091 |
| 10 | NG | NG | NG | NG | 4.5 (0.04) | 0.08 (0.01) | 15.5 | 0.0052 | |
| 5 | NG | NG | NG | NG | 6.4 (0.1) | 0.055 (0.004) | 15.4 | 0.0036 | |
| Klebsiella oxytoca | 20 | 2.5 (0.1) | 0.143 (0.005) | 17.3 | 0.0083 | 2.7 (0.02) | 0.128 (0.008) | 16.5 | 0.0078 |
| 15 | 5.0 (0.1) | 0.116 (0.007) | 27.2 | 0.0043 | 2.8 (0.03) | 0.105 (0.002) | 13.6 | 0.0077 | |
| 10 | 9.5 (0.5) | 0.087 (0.01) | 36.6 | 0.0024 | 3.1 (0.03) | 0.092 (0.003) | 12.8 | 0.0072 | |
| Escherichia coli | 35 | 11 (0.05) | 0.212 (0.004) | 118.6 | 0.0018 | 8.7 (0.1) | 0.172 (0.0001) | 74.4 | 0.0023 |
| 25 | 12 (1) | 0.15 (0.009) | 88.0 | 0.0017 | 6.2 (0.04) | 0.064 (0.001) | 15.8 | 0.0041 | |
| 20 | 14.5 (0.1) | 0.17 (0.011) | 122.4 | 0.0014 | NMc | NM | NM | NM | |
| 15 | 26 (0.3) | 0.08 (0.0001) | 89.6 | 0.0009 | 8.1 (0.2) | 0.037 (0.001) | 8.6 | 0.0043 | |
| Bacillus stearothermophilus | 45 | 5.5 (0.1) | 0.144 (0.007) | 38.4 | 0.0038 | 5.5 (0.1) | 0.144 (0.01) | 38.4 | 0.0038 |
| 40 | 6.0 (0.2) | 0.098 (0.007) | 26.6 | 0.0037 | 7.1 (0.06) | 0.096 (0.005) | 30.7 | 0.0031 | |
| 35 | 8.5 (0.02) | 0.071 (0.012) | 24.9 | 0.0029 | 3.4 (0.04) | 0.078 (0.007) | 11.3 | 0.0069 | |
| 30 | 13.5 (0.1) | 0.058 (0.003) | 29.9 | 0.0019 | 6.3 (0.3) | 0.067 (0.0001) | 17.0 | 0.0039 | |
| 25 | NG | NG | NG | NG | 5.3 (0.05) | 0.058 (0.005) | 11.61 | 0.0050 |
The values in parentheses are standard errors of the means (n = 3).
NG, no growth.
NM, not measured.
TABLE 4.
Growth kinetics for microalgal isolates grown to steady state in nitrogen-limited chemostat cultures
| Taxon | Temp (°C) | Steady-state NO3− concn (μM) | μmax with NO3− (h−1) | Knit (μM) | anito (liter · μmol−1 · h−1) | Steady-state NH4+ concn (μM) | μmax with NH4+ (h−1) | Kamm (μM) | aammo (liter · μmol−1 · h−1) |
|---|---|---|---|---|---|---|---|---|---|
| Chaetoceros sp. | 5 | 3 (0.1)a | 0.033 (0.001) | 2.5 | 0.0132 | 3 (0.02) | 0.033 (0.002) | 2.5 | 0.0132 |
| 3 | 14 (0.02) | 0.029 (0.005) | 8.2 | 0.0035 | 7 (0.02) | 0.029 (0.001) | 4.2 | 0.0069 | |
| 1 | 21.5 (0.2) | 0.022 (0.0001) | 4.7 | 0.0047 | NMb | 0.022 (0.004) | NM | NM | |
| Chaetoceros curvisetum | 22 | 3 (0.1) | 0.028 (0.0001) | 1.7 | 0.0165 | 28.7 (0.1) | 0.0284 (0.009) | 16.5 | 0.0017 |
| 17 | 6.5 (0.01) | 0.027 (0.003) | 3.3 | 0.0082 | 38.6 (0.3) | 0.0252 (0.001) | 14 | 0.0018 | |
| 12 | 42.4 (0.3) | 0.02 (0.001) | 5.7 | 0.0035 | 47.4 (0.5) | 0.019 (0.001) | 5.3 | 0.0036 | |
| Dunaliella tertiolecta | 25 | 5.5 (0.04) | 0.078 (0.01) | 19.1 | 0.0041 | 9 (0.05) | 0.068 (0.007) | 25 | 0.0027 |
| 20 | 14 (0.05) | 0.1 (0.001) | 63.4 | 0.0016 | 7.8 (0.3) | 0.087 (0.001) | 30 | 0.0029 | |
| 15 | 18 (0.2) | 0.095 (0.001) | 77 | 0.0012 | 8 (0.4) | 0.059 (0.0001) | 18 | 0.0033 | |
| 10 | 47 (0.1) | 0.045 (0.005) | 71 | 0.0006 | 13.4 (0.4) | 0.058 (0.002) | 30 | 0.0019 |
The values in parentheses are standard errors of the means (n = 3).
NM, not measured.
The specific affinity for nitrate (anito) values calculated for the temperatures by using the relevant μmax and Ks values consistently decreased as the temperature decreased. An LRA showed that there was a significant linear relationship between anito and temperature for all isolates (P < 0.05, as determined by LRA), although there were large interspecies variations among anito values. The response of anito to temperature change was greater in the psychrotolerant species (whose Q10 values ranged from 3 to 5, where Q10 is the proportional change with a 10°C temperature increase) than in the thermotolerant species (whose Q10 values ranged from 1.8 to 2.5). The specific affinity for ammonium (aammo) values also varied greatly among species but generally showed little temperature dependence (the Q10 values for individual species ranged from 0.75 to 2.1). Only one isolate (a Brevibacterium sp. isolate) showed any significant dependence on temperature (P < 0.05, as determined by LRA; Q10, ≈2). It must be noted that in some cases steady-state concentrations of ammonium approached the limit of detection, and the specific affinity values in such cases must be regarded as lower limits.
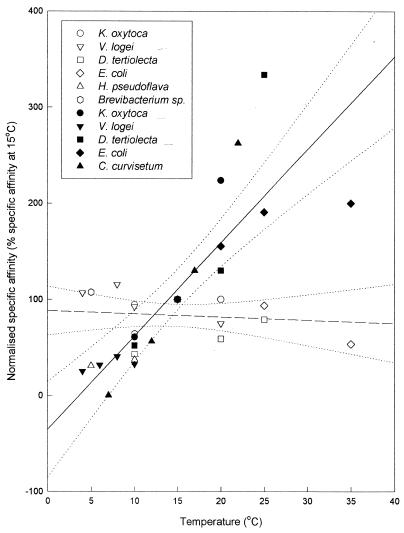
To illustrate the consistent response of aAo to temperature change, aAo data for nitrate and ammonium were normalized to express the aAo value at a given temperature as a percentage of the value at 15°C (Fig. 2). (The psychrophilic diatom Chaetoceros sp. and the thermophilic bacterium B. stearothermophilus did not grow at 15°C, so these organisms were omitted from this comparison.) The normalized plot showed that there was significant dependence of anito on temperature (P < 0.001, as determined by LRA) but no significant temperature dependence of aammo (P = 0.73, as determined by LRA). The Q10 values for normalized aAo were 2.9 ± 0.43 (mean ± standard error; n = 21) and 0.98 ± 0.6 (n = 25) for nitrate and ammonium, respectively. These normalized data again indicated that the temperature response of affinity for nitrate was consistently greater than the temperature response of affinity for ammonium in all of the bacterial and algal isolates examined.
FIG. 2.
Plot of normalized (to 15°C) aammo and anito values versus incubation temperature for a range bacteria and microalgae. The dashed line is the first-order linear regression line through normalized ammonium data (n = 25; r2 = 0.01; P = 0.73). The solid line is the first-order linear regression line through normalized nitrate data (n = 21; r2 = 0.71; P <0.001). The dotted lines are the 95% confidence limits for the regression lines.
DISCUSSION
Relationship between affinity and temperature.
The mechanisms that limit growth at high temperatures (protein denaturation, etc.) are well-documented (16, 64), but the mechanisms responsible for limiting μ at low temperatures are still a source of contention. Our data provide consistent evidence that growth of both bacteria and marine algae under N-limited conditions at low temperatures is restricted by the reduced ability of the organisms to sequester inorganic nitrogen. (A similar decreased affinity for uptake of organic substrates at low temperatures has been reported previously [51].) Although inhibition of membrane transport at low temperatures that leads to growth limitation has been suggested before (35, 49), consistent evidence which supports the hypothesis has not been presented previously.
In the N-limited batch cultures that were grown at or near the optimum growth temperatures of species, the residual concentrations of both nitrate and ammonium were below the analytical limits, indicating that utilization of both N sources is most effective at these temperatures. However, at suboptimal temperatures the increases in the residual concentrations of nitrate and ammonium during the stationary phase illustrated that bacteria and algae became less able to take up inorganic nitrogen as the temperature decreased, whether the isolate was psychrophilic, mesophilic, or thermophilic. This implied that the efficiency of the uptake system for both nitrate and ammonium decreased with temperature and so reduced the ability of the organisms to sequester inorganic nitrogen from the surrounding medium (55). However, the greater effect of suboptimal temperatures on residual nitrate concentrations than on residual ammonium concentrations indicated that low temperatures had different effects on nitrate utilization and ammonium utilization.
The μmax values varied with temperature, as reported previously for N-limited cultures (62, 73). The generally lower μmax values for the psychrotrophic and psychrophilic bacteria (H. pseudoflava, Brevibacterium sp.) than for mesotrophic isolates (e.g., K. oxytoca and E. coli) and the greater μmax values for the microflagellate alga D. tertiolecta than for the two diatoms are both previously identified trends (7).
Nitrate uptake and temperature.
An examination of the measured Ks values indicated that affinity for nitrate was reduced to a greater extent by low temperature than affinity for ammonium was, but when Ks was used alone as a measure of affinity, this trend was by no means consistent. Such inconsistency is common when Ks alone is used as a measure of substrate affinity (15, 48). Such use of Ks has been criticized as it does not adequately reflect changes in the ability of cells to sequester substrate at low concentrations (8), which depends on both Ks and μmax. Alternatively, aAo, approximated by μmax/Ks, has been employed (9, 25, 30, 40, 51). aAo provides an unambiguous measure of the ability of an organism to sequester substrate at low concentrations and is independent of uptake mechanism (26). Measurements of aAo, therefore, allow examination of the effect of temperature on affinity for a substrate and identify temperature effects on uptake alone (8). Using aAo, we demonstrated for the first time that a temperature below the optimum growth temperature consistently resulted in decreased affinity for nitrate in a number of bacteria and marine algae. This trend was consistent for psychrophilic, mesophilic, and thermophilic species despite the different growth temperature ranges of the organisms. These findings suggest that there is a common mechanism that is responsible for the decrease in affinity for nitrate that occurs as the temperature decreases.
Our Ks values for the algae were high compared to the few other values reported previously for algal chemostat cultures (7). Many of the Ks values reported previously for algal growth were determined with fed-batch cultures (17, 44, 81) and cannot be compared with our data because they are Ks values for uptake rather than Ks values for growth and do not represent steady-state conditions (25). It must also be asked whether Ks values by themselves provide any useful information about affinity for a substrate, which might be why coherent trends of aAo with temperature are not seen with Ks alone. The anito values that we determined for algae are similar to the few other values that are available in the literature with comparable units (we have not been able to find any such values for bacteria). For example, our values for anito near the optimal growth temperatures for D. tertiolecta (0.004 μmol liter−1 h−1 at 25°C), C. curvisetum (0.0165 μmol liter−1 h−1 at 22°C), and Chaetoceros sp. (0.013 μmol liter−1 h−1 at 5°C) are similar to the values reported for Scenedesmus sp. (0.02 μmol liter−1 h−1 at 20°C [62]) and Chaetoceros neglectum (0.015 μmol−1 liter−1 h−1 at 0°C [69]).
Despite the interspecies variations in actual anito values, the Q10 values were similar (mean, 3.1; standard error, ±0.48; n = 8), and this finding corroborated the idea that the temperature responses for nitrate uptake by algae and bacteria are similar. Although no previously published Q10 values for anito were found, our Q10 value for anito normalized to 15°C agrees well with the relatively high Q10 values reported for nitrate uptake by microorganisms (39, 59, 74, 75), a feature which is characteristic of active, carrier-mediated transport systems (39). An important consequence of the decrease in affinity for nitrate that occurs as the temperature decreases is the fact that at a low temperature there is an increase in the growth rate-limiting concentration of nitrate (55) and therefore an increased degree of nitrate limitation imposed by the low temperature. In other words, at a low temperature there is exacerbation of nitrate limitation for both algae and bacteria. A corollary of this is the hypothesis that addition of more nitrate should reverse the nitrate limitation imposed by low affinity at a low temperature, as demonstrated for organic substrates by Wiebe et al. (83). This implies that nutrient limitation bioassays must be carried out at the in situ temperature or the effective availability of external substrate pools may change.
The temperature dependence of nitrate use agrees with what is known about the nitrate uptake system. Nitrate is apparently taken up by an active transport system in bacteria, algae, and higher plants, and this transport system appears to be ATP driven rather than directly dependent on an electrochemical gradient (12, 18, 71, 79). There is additional evidence that active nitrate uptake is strictly Na+ dependent (39), is highly sensitive to metabolic inhibitors (78), and may be strongly inhibited by the presence of ammonium (13, 39). Such active uptake systems can be very responsive to temperature changes (66). Although the temperature dependence of nitrate uptake has been demonstrated most clearly in higher plants (22, 42), it seems increasingly apparent from our studies and other studies (39, 41, 74) that nitrate uptake by bacteria and microalgae is also highly temperature dependent. Furthermore, uptake of other inorganic algal nutrients which are primarily sequestered by active transport is also likely to be adversely affected by low temperatures because of decreased affinity.
The data in the literature for μmax and Ks values in chemostats at different temperatures is extremely restricted, but the data which is available tends to support our paradigm. Uptake of nitrate at different temperatures in nitrate-limited cultures of Scenedesmus sp. (62), uptake of phosphate in phosphate-limited cultures of Scenedesmus sp. (1), and silicate uptake in silicate-limited cultures of the ice alga Pseudonitzschia seriata (70) all indicate that decreases in affinity occur at temperatures below the optimum temperature when affinity is measured by determining aAo. It has been pointed out (5) that phosphate, a nutrient primarily acquired through active uptake (4), is not utilized efficiently in cold high-latitude waters.
Ammonium uptake, N preference, and temperature.
The more constant concentrations of ammonium than of nitrate in steady-state chemostats at a range of incubation temperatures indicated that ammonium uptake is less temperature dependent than nitrate uptake is. The fact that low temperature has a greater inhibitory effect on nitrate uptake than on ammonium uptake has been documented previously in higher plant roots by several workers (42, 43), but until now this difference has not been established for phytoplankton and bacteria (53). The fact that the response of affinity for ammonium to decreased temperature is consistently less than the response of affinity for nitrate in bacteria, algae, and higher plants suggests that there are fundamental differences in the ammonium and nitrate uptake mechanisms across a broad phylogenetic range. This is not surprising since the differing biochemical requirements for assimilation of nitrate compared with that of ammonium apply to all organisms and are therefore likely to be evolutionarily highly conserved.
The low Q10 value (≈1) of normalized aammo agrees with data from previous studies of ammonium uptake in higher plants (42, 43). However, higher Q10 values have been reported for ammonium uptake by phytoplankton in the field (56, 68). The low Q10 values found for aammo are characteristic of channel-mediated ion fluxes (66), but the exact mechanisms involved in ammonium uptake and control of uptake are poorly understood. In the last 20 years several uptake pathways, including pathways for both active and passive uptake of ammonium, have been suggested (2, 12, 34, 38, 79, 80). Ammonia (NH3) can diffuse freely through cell membranes (32), but at neutral pH >99% of NH3 is protonated as NH4+ (38). In slightly alkaline environments, such as marine systems, the proportion of NH3 may increase to >10% of the total ammonium (NH3 and NH4+ combined) (67). The concentration gradient of NH3 across a cell membrane may be maintained by NH3 protonation within the cell and by equilibration between ammonium and NH3 outside the cell (31). Passive uptake of NH3 may therefore make a significant contribution to the N requirements of bacteria and algae (particularly organisms with large surface area/volume ratios). Several studies have confirmed that there is a preference for, or selection by, small phytoplankton for ammonium (28, 41, 57). In a comparison of ammonium preference in diatoms, dinoflagellates, cyanobacteria, chlorophytes, and other organisms (13), it was found that the greatest contrast was between the diatoms and the other organism category, which consisted mainly of small flagellates. The microflagellate preference for ammonium was much greater than the preference in diatoms, while the larger diatoms showed greater preference for nitrate (45, 54).
Low temperature probably affects nutrient uptake by causing alterations in physical characteristics of the cell membrane. Such changes associated with low temperature may control active nutrient uptake across cell membranes in several ways (10, 58). Low temperature may hinder conformational changes in membrane transport proteins and thus prevent solute molecules from combining with their carrier proteins, or it may result in reduction in the substrate supply to a transport protein and inactivation of carrier proteins. Low temperature may also result in a reduction in membrane fluidity; it is known that reductions in membrane fluidity decrease the activity of transporter and respiratory proteins (3, 20, 76) embedded in the membrane phospholipids, and indeed the embedded proteins may reciprocally influence the membrane fluidity (47). Thus, while different species may adapt their membranes to be functional over different ranges of temperature by changing the ratios of saturated, unsaturated, or branched-chain membrane lipids (see references 63 and 65 for reviews), we hypothesize that within the range of temperature for each species there is decreased affinity for substrates taken up by active transport as the temperature decreases below the optimum temperature for growth (50). As passive uptake is less affected by temperature than active uptake is (38), any significant passive component should make overall ammonium sequestration less dependent on temperature than active nitrate uptake is.
Nitrogen nutrition at low temperature and its ecological implications.
We demonstrated that a reduction in temperature results in reduced affinity for nitrate and decreased utilization in several algal and bacterial isolates representing a wide range of physiological types. Ammonium uptake and affinity in the same isolates did not appear to be affected by a reduction in temperature to the same extent. This suggests that in low-temperature aquatic plankton ecosystems, microbial nitrogen utilization tends to be biased away from nitrate and that ammonium is a more important substrate.
In the Southern Ocean, surface water temperatures reach as low as −1.8°C, and the maximum summer temperature is around 4.0°C (33). In such a low-temperature environment, we would expect that nitrate uptake by phytoplankton would be reduced and that ammonium would be increasingly important as a nitrogen source. This hypothesis is consistent with the generally low f ratios, 0.2 to 0.6 (13, 53), reported for the Southern Ocean (the f ratio is the uptake of nitrate expressed as a proportion of the total inorganic nitrogen uptake). The low f ratios occur despite nitrate concentrations which are often more than 40 times the ammonium concentrations (11). The importance of ammonium may be further increased by the competitive effects of ammonium and nitrate; even relatively low concentrations of ammonium may inhibit nitrate uptake by microorganisms (13, 24, 82).
The temperature dependence of nitrogen preference in microbial plankton, especially in marine phytoplankton, could have far-reaching implications for biogeochemical nutrient cycling on a global scale. Several large regions of the World Ocean have been characterized as high-nutrient–low-chlorophyll regions, where annual primary production is too low to exhaust the supply of inorganic nutrients. Any changes in the environmental controls which result in suboptimal use of nitrate in these regions could result in major alterations in the pattern of primary production and thus accumulation of organic material.
ACKNOWLEDGMENTS
We thank Peter J. le B. Williams of the University of Bangor for his constructive comments on early drafts of the manuscript.
This work was carried out during research studentship GT4/94/339/L from the Natural Environment Research Council, United Kingdom, to David S. Reay. This studentship was CASE funded in conjunction with the British Antarctic Survey, Cambridge, United Kingdom.
REFERENCES
- 1.Ahlgren G. Temperature functions in biology and their application to algal growth constants. Oikos. 1987;49:177–190. [Google Scholar]
- 2.Balch W M. Exploring the mechanism of ammonium uptake in phytoplankton with an ammonium analogue, methylamine. Mar Biol. 1986;92:163–171. [Google Scholar]
- 3.Baldassare J, Brenckle G, Hoffman M, Silbert D. Modification of membrane lipid: functional properties of membrane in relation to fatty acid structure. J Biol Chem. 1977;252:8797–8803. [PubMed] [Google Scholar]
- 4.Bieleski R L, Ferguson I B. Physiology and metabolism of phosphate and its compounds. In: Läuacli A, Bieleski R L, editors. Inorganic plant nutrition, new series. Berlin, Germany: Springer-Verlag; 1983. pp. 422–445. [Google Scholar]
- 5.Broecker W, Peng T-H. What caused the glacial to intergalcial CO2 change? In: Heimann M, editor. The global carbon cycle. Berlin, Germany: Springer-Verlag; 1993. pp. 95–115. [Google Scholar]
- 6.Butcher R W. Plymouth algal collection no. 83. Fish Invest London Ser. 1959;4:22–23. [Google Scholar]
- 7.Button D K. Kinetics of nutrient-limited transport and microbial growth. Microb Rev. 1985;49:270–297. doi: 10.1128/mr.49.3.270-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Button D K. Nutrient-limited microbial growth kinetics: overview and recent advances. Antonie Leeuwenhoek. 1993;63:225–235. doi: 10.1007/BF00871220. [DOI] [PubMed] [Google Scholar]
- 9.Button D K A. Affinity of organisms for substrate. Limnol Oceanogr. 1986;31:453–456. [Google Scholar]
- 10.Clarkson D T, Earnshaw M J, White P J, Cooper H D. Temperature dependent factors influencing nutrient uptake: an analysis of responses at different levels of organisation. Symp Soc Exp Biol. 1988;42:281–309. [PubMed] [Google Scholar]
- 11.Cota G F, Smith W O, Nelson D M, Muench R D, Gordon L I. Nutrient and biogenic particulate distributions, primary productivity and nitrogen uptake in the Weddell-Scotia Sea marginal ice zone during winter. J Mar Res. 1992;50:155–181. [Google Scholar]
- 12.Cruz C, Lips S H, Martins-Loução M A. Uptake of ammonium and nitrate by carob (Ceratonia siliqua) as affected by root temperature and inhibitors. Physiol Plant. 1993;89:532–543. [Google Scholar]
- 13.Dortch Q. The interaction between ammonium and nitrate uptake in phytoplankton. Mar Ecol Prog Ser. 1990;61:183–201. [Google Scholar]
- 14.Dugdale R C, Goering J J. Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol Oceanogr. 1967;12:196–206. [Google Scholar]
- 15.Ellis-Evans J, Wynn-Williams D. The interaction of soil and lake microflora at Signy Island. In: Siegfried W, Condy P, Laws R, editors. Antarctic nutrient cycles. Berlin, Germany: Springer-Verlag; 1985. pp. 662–668. [Google Scholar]
- 16.Eppley R W. Temperature and phytoplankton growth in the sea. Fish Bull (Dublin) 1972;70:1063–1085. [Google Scholar]
- 17.Eppley R W, Rogers J N, McCarthy J J. Half-saturation constants for uptake of nitrate and ammonium by marine phytoplankton. Limnol Oceanogr. 1969;14:912–920. [Google Scholar]
- 18.Falkowski P G. Nitrate uptake in marine phytoplankton (nitrate, chloride) activated adenosine triphosphate from Skeletonema costatum (Bacillariophyceae) J Phycol. 1975;11:323–326. [Google Scholar]
- 19.Flynn K J, Fasham M J R, Hipkin C R. Modelling the interactions between ammonium and nitrate uptake in marine phytoplankton. Phil Trans R Soc London B Biol Sci. 1997;352:1–22. [Google Scholar]
- 20.Foot M, Jeffcoat R, Barratt M, Russell N. The effect of growth temperature on the membrane lipid environment of the psychrophilic bacterium Micrococcus cryophilus. Arch Biochem Biophys. 1983;224:718–727. doi: 10.1016/0003-9861(83)90260-6. [DOI] [PubMed] [Google Scholar]
- 21.Fry J C. Biological data analysis: a practical approach. Oxford, United Kingdom: IRL Press; 1993. [Google Scholar]
- 22.Glass A D M, Siddiqim M Y, Ruth T J, Rufty T W J. Studies in the uptake of nitrate in barley. II. Energetics. Plant Physiol. 1990;93:1585–1589. doi: 10.1104/pp.93.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glibert P M. Primary productivity and pelagic nitrogen cycling. In: Blackburn T H, Sørensen J, editors. Nitrogen cycling in coastal marine environments. New York, N.Y: John Wiley and Sons Ltd.; 1988. pp. 3–31. [Google Scholar]
- 24.Glibert P M, Biggs D C, McCarthy J J. Utilization of ammonium and nitrate during austral summer in the Scotia Sea. Deep Sea Res. 1982;29:837–850. [Google Scholar]
- 25.Goldman J C, Glibert P M. Kinetics of inorganic nitrogen uptake. In: Carpenter E J, Capone D G, editors. Nitrogen in the marine environment. New York, N.Y: Academic Press; 1983. pp. 233–275. [Google Scholar]
- 26.Gottschal J C. Some reflections on microbial competitiveness among heterotrophic bacteria. Antonie Leeuwenhoek. 1985;51:473–494. doi: 10.1007/BF00404494. [DOI] [PubMed] [Google Scholar]
- 27.Guillard R R L, Ryther J H. Studies of marine planktonic diatoms. I. Cyclotella nana (Hustedt) and Detonula confervacla (Cleve) Gran. Can J Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 28.Harrison W G. The time-course of uptake of inorganic and organic nitrogen compounds by phytoplankton from the Eastern Canadian Arctic: a comparison of temperate and tropical populations. Limnol Oceanogr. 1983;28:1231–1237. [Google Scholar]
- 29.Harwood J E, Kuhn A L. A colorimetric method for ammonia in natural waters. Water Res. 1970;4:805–811. [Google Scholar]
- 30.Healey F P. Slope of the Monod equation as an indicator of advantage in nutrient competition. Microb Ecol. 1980;5:281–286. doi: 10.1007/BF02020335. [DOI] [PubMed] [Google Scholar]
- 31.Henderson P J F. Ion transport by energy-conserving biological membranes. Annu Rev Microbiol. 1971;25:393–428. doi: 10.1146/annurev.mi.25.100171.002141. [DOI] [PubMed] [Google Scholar]
- 32.Henderson P J F. Studies of translocation analysis. Biosci Rep. 1991;11:477–525. doi: 10.1007/BF01130216. [DOI] [PubMed] [Google Scholar]
- 33.Herbert R A, Bhakoo M. Microbial growth at low temperatures. In: Russell A D, Fuller R, editors. Cold tolerant microbes in spoilage and the environment. London, United Kingdom: Academic Press; 1979. pp. 1–16. [Google Scholar]
- 34.Hoch M P, Fogel M L, Kirchman D L. Isotope fractionation associated with ammonium uptake by a marine bacterium. Limnol Oceanogr. 1992;37:1447–1459. [Google Scholar]
- 35.Ingraham J L, Bailey G F. Comparative effect of temperature on metabolism of mesophilic and psychrophilic bacteria. J Bacteriol. 1959;77:609–613. doi: 10.1128/jb.77.5.609-613.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacques G. Some ecophysiological aspects of Antarctic phytoplankton. Polar Biol. 1983;2:27–33. [Google Scholar]
- 37.Kirkwood D S. Nutrients: practical notes on their determination in seawater. Copenhagen, Denmark: ICES; 1996. [Google Scholar]
- 38.Kleiner D. The transport of NH3 and NH4+ across biological membranes. Biochim Biophys Acta. 1981;639:41–52. doi: 10.1016/0304-4173(81)90004-5. [DOI] [PubMed] [Google Scholar]
- 39.Lara C, Rodriguez R, Guerrero M G. Nitrate transport in the cyanobacterium Anacystis nidulans. Physiol Plant. 1993;89:582–587. [Google Scholar]
- 40.Law A T, Button D K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977;129:115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Bouteiller A. Environmental control of nitrate and ammonium uptake by phytoplankton in the Equatorial Atlantic Ocean. Mar Ecol Prog Ser. 1986;30:167–179. [Google Scholar]
- 42.Macduff J H, Hopper M J, Wild A. The effect of root temperature on growth and uptake of ammonium and nitrate by Brassica napus L. cv. Bien venu in flowing solution culture. J Exp Bot. 1987;38:53–66. [Google Scholar]
- 43.Macduff J H, Hopper M J, Wild A, Trim F E. Comparison of the effects of root temperature on nitrate and ammonium nutrition of oilseed rape (Brassica napus L.) in flowing solution culture. J Exp Bot. 1987;38:1104–1120. [Google Scholar]
- 44.MacIsaac J J, Dugdale R C. Interactions of light and inorganic nitrogen in controlling nitrogen uptake in the sea. Deep Sea Res. 1972;19:209–232. [Google Scholar]
- 45.Malone T C. Algal size. In: Morris I, editor. The physiological ecology of phytoplankton. London, United Kingdom: Blackwell; 1980. pp. 433–464. [Google Scholar]
- 46.McCarthy J J, Carpenter E J. Nitrogen cycling in near-surface waters of the open ocean. In: Carpenter E J, Capone D G, editors. Nitrogen in the marine environment. New York, N.Y: Academic Press; 1983. pp. 487–512. [Google Scholar]
- 47.McGibbon L, Cossins A, Quinn P, Russell N. A differential scanning calorimetry and fluorescence polarisation study of membrane lipid fluidity in a psychrophilic bacterium. Biochim Biophys Acta. 1985;820:115–121. [Google Scholar]
- 48.Mechling J A, Kilham S S. Temperature effects on silicon limited growth of the Lake Michigan diatom Stephanodiscus minultus (Bacillariophyceae) J Phycol. 1983;18:119–205. [Google Scholar]
- 49.Morita R Y, Buck G E. Low temperature inhibition of substrate uptake. In: Colwell R R, Morita R Y, editors. Effects of the ocean environment on microbial activities. Baltimore, Md: University Park Press; 1974. pp. 124–129. [Google Scholar]
- 50.Nedwell, D. Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. Submitted for publication. [DOI] [PubMed]
- 51.Nedwell D B, Rutter M. Influence of temperature on growth rate and competition between two psychrotolerant Antarctic bacteria: low temperature diminishes affinity for substrate uptake. Appl Environ Microbiol. 1994;60:1984–1992. doi: 10.1128/aem.60.6.1984-1992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogilvie B G, Rutter M, Nedwell D B. Selection by temperature of nitrate-reducing bacteria from estuarine sediments: species composition and competition for nitrate. FEMS Microb Ecol. 1997;23:11–22. [Google Scholar]
- 53.Olson R J. Nitrate and ammonium uptake in Antarctic waters. Limnol Oceanogr. 1980;25:1064–1074. [Google Scholar]
- 54.Owens N J P, Priddle J, Whitehouse M J. Variations in phytoplanktonic nitrogen assimilation around South Georgia and in the Bransfield Strait (Southern Ocean) Mar Chem. 1991;35:287–304. [Google Scholar]
- 55.Pirt S J. Principles of microbe and cell cultivation. Oxford, United Kingdom: Blackwell Scientific Publications; 1975. [Google Scholar]
- 56.Priscu J C, Palmisano A C, Priscu L R, Sullivan C W. Temperature dependence of inorganic nitrogen uptake and assimilation in Antarctic sea-ice microalgae. Polar Biol. 1989;9:443–446. [Google Scholar]
- 57.Probyn T A. Nitrogen uptake by size-fractionated phytoplankton populations in the southern Benguela upwelling system. Mar Ecol Prog Ser. 1985;22:249–258. [Google Scholar]
- 58.Quinn P J. Effects of temperature on cell membranes. In: Long S P, Woodward F I, editors. Plants and temperature. Cambridge, United Kingdom: Company of Biologists; 1988. pp. 237–258. [Google Scholar]
- 59.Raimbault P. Influence of temperature on transient-response in nitrate uptake and reduction by 4 marine diatoms. J Exp Mar Biol Ecol. 1984;84:37–53. [Google Scholar]
- 60.Raven J A, Wolenweber B, Handley L L. Ammonia and ammonium fluxes between photolithotrophs and the environment in relation to the global nitrogen cycle. New Phytol. 1992;121:5–18. [Google Scholar]
- 61.Raven J A, Wollenweber B, Handley L L. The quantitative role of ammonia/ammonium transport and metabolism by plants in the global nitrogen cycle. Physiol Plant. 1993;89:512–518. [Google Scholar]
- 62.Rhee G-Y, Gotham I J. The effect of environmental factors on phytoplankton growth: temperature and the interactions of temperature with nutrient limitation. Limnol Oceanogr. 1981;26:635–648. [Google Scholar]
- 63.Russell N. Psychrophilic microorganisms. In: Herbert R, Sharp R, editors. Molecular biology and biotechnology of extremophiles. Glasgow, United Kingdom: Blackie; 1992. pp. 203–224. [Google Scholar]
- 64.Russell N J. Cold adaptation of microorganisms. Phil Trans R Soc London B Biol Sci. 1990;326:595–611. doi: 10.1098/rstb.1990.0034. [DOI] [PubMed] [Google Scholar]
- 65.Russell N J, Fukunaga N. A comparison of thermal adaptation of membrane lipids in psychrophilic and thermophilic bacteria. FEMS Microbiol Rev. 1990;75:171–182. [Google Scholar]
- 66.Serrano R. Transport across yeast vacuolar and plasma membranes. In: Broach J R, Pringle J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccaromyces: genome dynamics, protein synthesis, and energetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 523–585. [Google Scholar]
- 67.Sillen L C, Martell A E. Stability constants of metal ion complexes. London, England: Chemical Society; 1964. [Google Scholar]
- 68.Smith W O, Harrison W G. New production in polar regions: the role of environmental controls. Deep Sea Res. 1991;38:1463–1479. [Google Scholar]
- 69.Sommer U. Nitrate and silicate competition among Antarctic phytoplankton. Mar Biol. 1986;91:345–351. [Google Scholar]
- 70.Stapleford L S, Smith R E H. The interactive effects of temperature and silicon limitation on the psychrophilic ice diatom Pseudonitszchia seriata. Polar Biol. 1996;16:589–594. [Google Scholar]
- 71.Stewart W D P. Transport and utilization of nitrogen by algae. In: Payne J W, editor. Microorganisms and nitrogen sources. New York, N.Y: Wiley and Sons Ltd.; 1980. pp. 577–606. [Google Scholar]
- 72.Strickland J D H, Parsons T R. A practical handbook of seawater analysis. Ottawa, Canada: Fisheries Research Board of Canada; 1972. [Google Scholar]
- 73.Thomas W H, Dodson A N. Effect of interactions between temperature and nitrate supply on the cell division rates of two marine phytoflagellates. Mar Biol. 1974;24:213–217. [Google Scholar]
- 74.Tischner R. Interaction between chloroplast-cytoplasm vacuoles with respect to the regulation of nitrogen metabolism in Chlorella. In: Weissner W, Robinson D, Starr R C, editors. Compartments in algal cells and their interaction. Berlin, Germany: Springer-Verlag; 1984. pp. 157–163. [Google Scholar]
- 75.Tischner R, Lorenzen H. Nitrate uptake and reduction in Chlorella. Characterisation of nitrate uptake in nitrate grown and nitrogen-starved Chlorella sorokiniana. In: Bothe H, Trebst A, editors. Biology of inorganic nitrogen and sulfur. Berlin, Germany: Springer-Verlag; 1981. pp. 252–259. [Google Scholar]
- 76.Tsukagoshi N, Fox C. Transport system assembly and the mobility of membrane lipids in Escherichia coli. Biochemistry. 1973;12:2822–2829. doi: 10.1021/bi00739a008. [DOI] [PubMed] [Google Scholar]
- 77.Upton A C, Nedwell D B, Wynn-Williams D D. The selection of microbial communities by constant or fluctuating temperatures. FEMS Microbiol Ecol. 1990;74:243–252. [Google Scholar]
- 78.Watt D A, Amory A M, Cresswell C F. Constitutive and inducible aspects of nitrate-nitrogen uptake by Chlamydomonas reinhardtii. Physiol Plant. 1993;89:507–511. [Google Scholar]
- 79.Wheeler P A. Phytoplankton nitrogen metabolism. In: Carpenter E J, Capone D G, editors. Nitrogen in the marine environment. New York, N.Y: Academic Press; 1983. pp. 309–346. [Google Scholar]
- 80.Wheeler P A. Uptake of methylamine (an ammonium analogue) by Macrocystis pyrifera (Phaeophyta) J Phycol. 1979;15:12–17. [Google Scholar]
- 81.Wheeler P A, Glibert P M, McCarthy J J. Ammonium uptake and incorporation by Chesapeake Bay phytoplankton: short term uptake kinetics. Limnol Oceanogr. 1982;27:1113–1128. [Google Scholar]
- 82.Wheeler P A, Kokkinakis S A. Ammonium recycling limits nitrate use in oceanic subarctic Pacific. Limnol Oceanogr. 1990;35:1267–1278. [Google Scholar]
- 83.Wiebe W J, Sheldon W M J, Pomeroy L R. Evidence for an enhanced substrate requirement by marine mesophilic isolates at minimal growth temperatures. Microb Ecol. 1993;25:151–159. doi: 10.1007/BF00177192. [DOI] [PubMed] [Google Scholar]