Abstract
Several bacteriological surveys were performed from 1994 to 1996 at different Litopenaeus vannamei hatcheries (in Ecuador) and shrimp farms (in Mexico). Samples were taken from routine productions of healthy and diseased L. vannamei larvae, postlarvae, and their culture environment and from healthy and diseased juveniles and broodstock. In Ecuador, the dominant bacterial flora associated with shrimp larvae showing symptoms of zoea 2 syndrome, mysis mold syndrome, and bolitas syndrome has been determined. Strains were characterized by Biolog metabolic fingerprinting and identified by comparison to a database of 850 Vibrio type and reference strains. A selection of strains was further genotypically fine typed by AFLP. Vibrio alginolyticus is predominantly present in all larval stages and is associated with healthy nauplius and zoea stages. AFLP genetic fingerprinting shows high genetic heterogeneity among V. alginolyticus strains, and the results suggest that putative probiotic and pathogenic strains each have specific genotypes. V. alginolyticus was found to be associated with larvae with the zoea 2 syndrome and the mysis mold syndrome, while different Vibrio species (V. alginolyticus and V. harveyi) are associated with the bolitas syndrome. V. harveyi is associated with diseased postlarvae, juveniles, and broodstock. The identities of the strains identified as V. harveyi by the Biolog system could not be unambiguously confirmed by AFLP genomic fingerprinting. Vibrio strain STD3-988 and one unidentified strain (STD3-959) are suspected pathogens of only juvenile and adult stages. V. parahaemolyticus, Photobacterium damselae, and V. mimicus are associated with juvenile and adult stages.
In Ecuador and Mexico, aquaculture of white shrimp, Litopenaeus vannamei, has become an important economic activity. Most of the Ecuadorian larvae are hatchery reared, and bacteriosis has become a source of major economic losses (8, 9, 11). Bacterial diseases of penaeid shrimp have been described by Lightner (9) and others, although it is not always clear whether the bacteria involved are the causal agents. Vibrios, which occur in the dominant flora during larval developmental stages (6), have been described as the causal pathogens of bacteriosis (9). Since many of these vibrios have also been isolated from healthy penaeid shrimp, the hypothesis of the opportunistic nature of vibrios associated with penaeid shrimp has become widely accepted (6, 9, 19, 25). According to Pizzutto and Hirst (15), however, virulent strains of, e.g., Vibrio harveyi are rare and virulence may be explained by transfer of virulence factors. Since the early 1990s, epizootics like the zoea 2 syndrome, the mysis mold syndrome, and the bolitas syndrome have caused mass mortalities in hatchery-reared L. vannamei larvae in Ecuador and Mexico. Typical symptoms of the zoea 2 syndrome, affecting larvae in the zoea 2 stage, are inflammation of the walls of the hepatopancreas and shrinkage of the hepatopancreatic lobes (starting at their tops), absence of lipids, an empty digestive tract, delayed larval growth, the presence of “balls” in the hepatopancreas and the digestive tract, and mass larval mortality. Symptoms of the mysis mold syndrome are an incomplete molting process, followed by mass mortality. In Ecuadorian hatcheries, symptoms of the bolitas syndrome are desquamation of the epithelial cells of the digestive tract, followed by an accumulation of balls which descend through the digestive tract and, eventually, mass mortality. Robertson et al. (17) demonstrated a bacterial cause for the bolitas syndrome, since the disease can be successfully treated with antibiotics, but no information is available on the causal agents of the zoea 2 syndrome and the mysis mold syndrome.
In the present paper, we report on the identification of (i) the dominant microflora associated with cultured healthy and diseased L. vannamei shrimp in Ecuador and Mexico, (ii) the associated microflora of larvae with the zoea 2 syndrome, the mysis mold syndrome, and the bolitas syndrome, and (iii) probionts used in Ecuadorian hatcheries.
MATERIALS AND METHODS
Sample collection. (i) Larviculture samples.
In 1994, diseased larvae at different development stages were collected at three different hatcheries (CENAIM, Larfico SA, and Opumarsa), located near the Gulf of Guayaquil, Ecuador. Animals were considered diseased when they showed symptoms such as abnormal swimming behavior, lack of appetite and empty digestive tracts, color changes of the body, low activity of the larvae at the bottoms of the tanks, or abnormally high mortality.
In 1995, healthy and diseased larvae at different developmental stages, together with samples of nearshore seawater, inlet water, tank water, reservoir water, and live and artificial food, were collected at four different hatcheries (Granjas Marinas, Macrobio, Opumarsa, and Playaespec) located near the Gulf of Guayaquil, Ecuador. Diseased postlarvae and samples of their tank water and samples of the tank water of healthy postlarvae were collected at the Artemia Reference Center, Ghent, Belgium.
In 1994 and 1995, the tank water of healthy larvae was sampled at two L. vannamei hatcheries located in the Pacific states of Nayarit, Sinaloa, and Sonora, Mexico.
In 1996, larvae with the mysis mold syndrome, the bolitas syndrome, and the zoea 2 syndrome were collected in different Ecuadorian hatcheries, whereas diseased postlarvae were collected at the Artemia Reference Center.
(ii) Juvenile and broodstock samples.
Healthy and diseased juveniles and diseased broodstock were sampled at L. vannamei farms and maturation facilities in the Pacific states of Nayarit, Sinaloa, and Sonora, Mexico. Putative pathogens were isolated from the hemolymph, hepatopancreas, and external lesions of diseased shrimp.
(iii) Probiont samples.
In hatchery practice, probionts are single-strain cultures which are gradually scaled up for mass production. Numerically dominant yellow colonies, selected from thiosulfate citrate bile sucrose agar (TCBS; Oxoid, Hampshire, England) and originating from successful larval productions, are used as starter cultures for this purpose (20). For this mass production of probionts, a self-made liquid medium, varying in composition from hatchery to hatchery, is used. After 24 h of growth at room temperature (between 25 and 30°C), samples are plated on marine agar (MA) to measure cell densities. At the same time, cell densities are estimated visually, relying on technician experience, and adequate amounts (of probionts and their growth medium) are added daily to the larval culture tanks. Probiont densities in the larval culture tanks are normally between 103 and 105 CFU/ml. Samples of mass probiont cultures used in various commercial hatcheries in Ecuador were collected from 1994 to 1996.
Isolation and storage of isolates.
From water and probiont samples, serial dilutions were made in 0.85% sterile NaCl solution and duplicate 0.1-ml aliquots were plated on MA containing marine broth 2216 (Difco, Detroit, Mich.) supplemented with 1.5% (wt/vol) bacteriological agar no. 1 (Oxoid) or on TCBS.
Larvae were washed three times with 100 ml of sterile seawater on sterile filters and transferred to sterile 0.85% NaCl solution. For the nauplius, zoea, mysis, and postlarval stages, respectively, 50, 20, 15, and 10 larvae were collected, homogenized in a sterile potter blender, and diluted serially using a 0.85% NaCl solution. In Mexico, samples of infected tissue of juveniles and broodstock were homogenized in a sterile 2.5% NaCl solution. Duplicate aliquots (0.1 ml) of the dilutions were plated on MA and on TCBS medium. All plates were incubated between 28 and 30°C.
After 2 to 7 days of growth, 1 to 3 numerically dominant colonies, originating from plates containing 30 to 300 colonies per plate, were selected based on their morphological appearance and further purified on MA. Suspensions of pure cultures were stored in a freezer at −80°C or in a nitrogen container at −140°C after addition of marine broth or Trypticase soy broth (Becton Dickinson, Cockeysville, Md.) supplemented with 2.0% (wt/vol) NaCl and 15% (wt/vol) glycerol. All strains were stored at the BCCM/LMG Culture Collection (University of Ghent, Ghent, Belgium). STD3 culture numbers refer to the isolates from European Commission project TS3-CT94-0269. A list containing all of the strain information can be obtained from J.V.
Isolate characterization.
Gram staining was performed as described by Smibert and Krieg (21). Further phenotypic characterization was performed by using a modified form of the Biolog GN technique described by Austin et al. (1). Strains were grown on brain heart infusion (Difco, Detroit, Mich.) supplemented with 1.5% (wt/vol) bacteriological agar no. 1 (Oxoid) and 1.5% (wt/vol) NaCl or on MA for 24 h at 25°C. Inocula were prepared in 1.5% (wt/vol) NaCl solution, and cell optical densities were photometrically standardized between 0.261 and 0.300 by using a photometer at 590 nm. The wells of the Biolog GN microplates (Biolog Inc., Hayward, Calif.) were inoculated with the cell suspension, and the microplates were incubated for 24 h at 25°C. Changes in color were measured by using a Multiscan Multisoft filter photometer (Labsystems, Helsinki, Finland) at 550 nm. For identification, the metabolic fingerprints of the strains grown on brain heart infusion agar were compared to a database containing Biolog fingerprints of 850 Vibrio reference strains, including all of the Vibrio type strains. Comparison of the strains was performed by numerical analysis using the Pearson product moment correlation coefficient and the unweighted-pair group method using average linkages (UPGMA) (22) clustering method. Clusters were delineated at 80% relatedness, and isolates clustering with type strains were considered to belong to the same species.
Fatty acid methyl ester (FAME) gas chromatographic analysis was used to identify nonvibrios as described by Vandamme et al. (24). Strains were grown on Trypticase soy broth (Becton Dickinson) supplemented with 1.5% (wt/vol) bacteriological agar no. 1 at 28°C for 24 h. FAME fingerprints were identified by using the Microbial Identification System software package, version 3.9 (Microbial ID, Inc., Newark, Del.).
Genotypic characterization of the representative isolates was performed by AFLP fingerprinting of whole genomes employing oligonucleotide primers H02 and T02 in accordance with the protocol described by Janssen et al. (7). For comparison of the AFLP profiles obtained, the Pearson product moment correlation coefficient was used. The isolates were grouped by UPGMA using the software package GelCompar 4.0 (Applied Maths, Kortrijk, Belgium). The principle of the AFLP method basically involves (i) digestion of total genomic DNA by two restriction enzymes, (ii) ligation of restriction half-side-specific adapters to all restriction fragments, and (iii) selective amplification of a subset of these fragments by using two specific PCR primers. After electrophoretic separation of the amplification products on a polyacrylamide gel, the AFLP patterns are visualized by autoradiography. The resulting banding patterns are captured by high-resolution densitometric scanning and further processed for computer-assisted analysis.
DNA-DNA hybridization experiments were performed on representative isolates. Mean G+C contents were determined by the thermal denaturation method and were calculated by using the equation of Marmur and Doty (10) as modified by De Ley (3). The degrees of DNA-DNA binding, expressed as percentages, were determined spectrophotometrically by the initial renaturation rate method of De Ley et al. (4). Renaturations were performed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate, pH 7.0) at optimal renaturation temperatures of 70.5°C for V. alginolyticus and 70.9°C for V. harveyi, with a total DNA concentration of 51.6 μg/ml.
RESULTS AND DISCUSSION
Isolate identification.
A total of 351 dominant gram-negative isolates were collected. Cell shapes did not show variations in the expected rod, coccoid, and curved cell forms.
Biolog GN phenotypic identification.
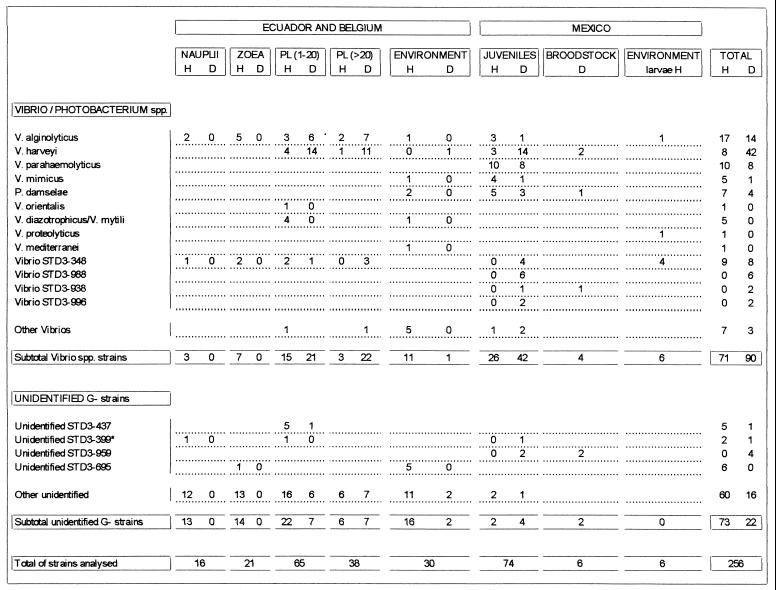
The 11 major Vibrio species and one Photobacterium sp. identified by Biolog fingerprinting (Fig. 1) were V. alginolyticus (84 strains), V. harveyi (57 strains), V. parahaemolyticus (18 strains), V. hollisae (9 strains), V. mimicus (6 strains), V. diazotrophicus/V. mytili (6 strains), V. fluvialis (2), V. piscium (1 strain), V. orientalis (1 strain), V. proteolyticus (1 strain), V. mediterranei (1 strain), Vibrio sp. (5 strains), and Photobacterium damselae (11 strains). Seven other Vibrio groups were characterized and labeled with the code of a reference strain belonging to the cluster, i.e., Vibrio strains STD3-348 (18 strains), STD3-988 (6 strains), STD3-139 (4 strains), STD3-338 (4 strains), STD3-1247 (3 strains), STD3-938 (2 strains), STD3-996 (2 strains), STD3-1058 (1 strain), and STD3-1071 (1 strain).
FIG. 1.
Distribution of bacteria isolated from healthy and diseased L. vannamei larvae, juveniles, and broodstock and their environment at different hatcheries in Ecuador and Mexico. H, isolates from healthy L. vannamei; D, isolates from diseased L. vannamei (isolates from shrimp with bolitas negricans, mysis mold syndrome, and zoea 2 syndrome are not included); environment H and D, isolates from inlet water, wastewater, tank water, nearshore seawater, and food of healthy and diseased larvae, respectively.
FAME analysis.
The remaining 108 non-Vibrio strains remained unidentified. FAME analysis did not allow further identification of these strains.
AFLP genotypic fingerprinting.
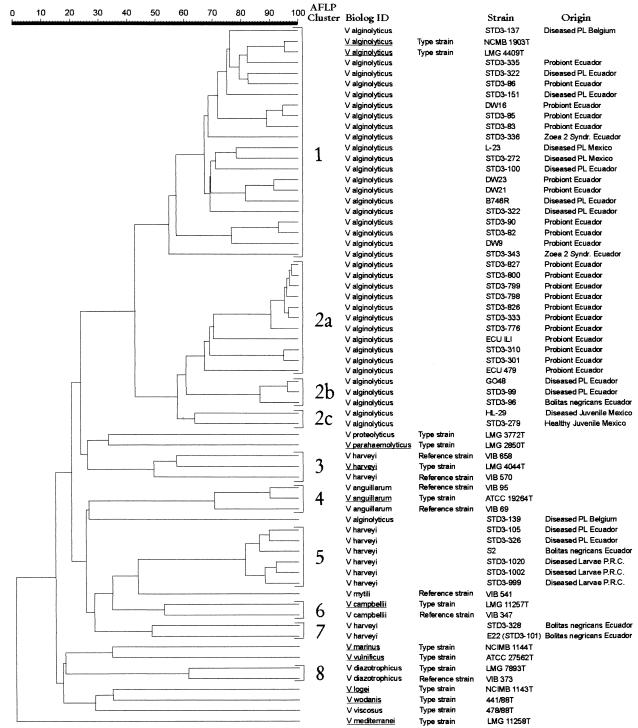
AFLP genotypic fingerprinting was performed on representative V. alginolyticus and V. harveyi strains. Clusters were delineated at 45% similarity (Fig. 2). AFLP fingerprints (Fig. 2) show a high degree of heterogeneity among V. alginolyticus strains (clusters 1 and 2) which was not revealed by the Biolog system. Cluster 1 contains V. alginolyticus reference and type strains, one strain isolated in Belgium from diseased postlarvae, and Ecuadorian strains isolated from probiont cultures, from diseased postlarvae, and from larvae with the zoea 2 syndrome. Cluster 1 strains were identified as V. alginolyticus by the Biolog system. Cluster 2 is highly related to the V. alginolyticus cluster (cluster 1) and contains strains which were also identified as V. alginolyticus by the Biolog system. According to the origin of the strains, cluster 2 can be further subdivided into cluster 2a, containing probiont cultures from Ecuador; cluster 2b, containing strains isolated from diseased postlarvae from Ecuador; and cluster 2c, containing strains from healthy and diseased juveniles from Mexico. Clusters 3 and 4, respectively, represent V. harveyi and V. anguillarum reference and type strains. The strains in clusters 5 and 7 and strain STD3-139, identified as V. harveyi by using the Biolog system, could not be unambiguously identified by AFLP. Pedersen et al. (14), who did an extensive taxonomic study on V. harveyi and V. carchariae, were not able to delineate the V. harveyi species and suggested the delineation of a core of V. harveyi strains which contains the type strains of both V. harveyi and V. carchariae by AFLP analyses. Therefore, the identities of many phenotypically characterized V. harveyi strains should be questioned. These V. harveyi strains were isolated from diseased postlarvae from Ecuador, from larvae with the bolitas syndrome from Ecuador, and from diseased Penaeus chinensis larvae from the People’s Republic of China (25). Clusters 6 and 8 represent the V. campbellii and V. diazotrophicus reference and type strains, respectively. Although the identification results of Biolog and AFLP are similar, except for many phenotypically identified V. harveyi strains, we observed a higher degree of heterogeneity when using AFLP.
FIG. 2.
AFLP fingerprints of whole genomes of representative V. alginolyticus and V. harveyi strains (previously identified by Biolog) isolated from diseased and healthy L. vannamei larvae, postlarvae, juveniles, and hatchery probionts and from Vibrio reference strains. The Pearson product moment correlation coefficient was used for comparison of the AFLP fingerprints. The isolates were grouped by UPGMA using the software package GelCompar 4.0.
DNA-DNA hybridization.
In order to clarify the taxonomic position of the strains in cluster 2 (Fig. 2), DNA-DNA hybridizations of representative strains of clusters 1 (V. alginolyticus) and 2 were performed. The percentage of DNA-DNA binding was 70% ± 3% between the type strain V. alginolyticus NCMB 1903 (Fig. 2, cluster 1) and strain STD3-301 (Fig. 2, cluster 2a) and 99% ± 4% between the type strain and strain STD3-272 (Fig. 2, cluster 1). Due to the high levels of DNA binding (>70%) and the similar G+C contents of 44 to 46 mol%, we must conclude that the strains in clusters 1 and 2 belong to the same species.
Microflora associated with the different larval and postlarval stages. (i) V. alginolyticus.
V. alginolyticus is the dominant Vibrio species in the larval stages of healthy and diseased L. vannamei (Fig. 1). The main bacterial flora associated with healthy nauplii and zoeae is diverse and remained largely unidentified. When V. alginolyticus is present during these early stages, it is associated with healthy larvae. Also, during routine productions of P. chinensis larvae in the People’s Republic of China, V. alginolyticus was found to be a dominant Vibrio species associated with healthy larvae (25). These findings are in contrast with those of Hameed (6), who found that vibrios were dominant during the development of Penaeus indicus eggs to postlarvae. In a previous study on P. chinensis larvae (25), it was also found that vibrios are not the dominant bacteria of nauplii. During the nauplius and zoea stages, an association of V. alginolyticus with healthy L. vannamei larvae was noticed, while during postlarval stages, V. alginolyticus was isolated more often from diseased animals.
(ii) V. harveyi.
V. harveyi was predominantly isolated from diseased postlarvae but was not recovered from the nauplius and zoea stages (Fig. 1), although this species was isolated many times from diseased larvae in Ecuador during other surveys (unpublished results). In 60% of the vibriosis outbreaks in postlarval stages, V. harveyi was isolated as the dominant bacterium. Robles-Arozarena et al. (18) have demonstrated the pathogenicity of V. harveyi STD3-131 by injecting L. vannamei postlarvae with 103 bacteria/animal.
(iii) Other vibrios and unidentified strains.
An association similar to that of V. alginolyticus was found for Vibrio strain STD3-348 (Fig. 1). In Mexico, this strain was dominantly present in the tank water of healthy larvae and in the hemolymph and in lesions in the exoskeleton of diseased juveniles. Vibrio strain STD3-348 challenge infection trials did not induce disease during the zoea and mysis stages (unpublished results). In the People’s Republic of China, this strain was isolated from healthy larvae and postlarvae (25). It is not known if this strain is pathogenic for P. chinensis larvae or grow-out stages. Vibrio strain STD3-348 may be an opportunistic pathogen of L. vannamei from the postlarval stage on, but further challenge infection trials are needed to confirm this.
V. diazotrophicus/V. mytili and unidentified strains STD3-437 and STD3-695 were frequently isolated from healthy larvae or from their environment (Fig. 1), suggesting that their presence might be beneficial for larval health. Further research is needed to clarify their potential beneficial character.
Microflora associated with healthy and diseased juveniles and broodstock.
V. harveyi was the main bacterium associated with diseased juveniles and broodstock (Fig. 1), although other bacteria, such as V. parahaemolyticus, P. damselae, Vibrio strains STD3-348 and STD3-988, and unidentified strain STD3-959, were also frequently isolated from diseased juveniles or broodstock. The data in Fig. 1 suggest that Vibrio strain STD3-988 and unidentified strain STD3-959 might be typical pathogens of the juvenile or adult stages. Further pathogenicity tests are needed.
(i) V. harveyi.
V. harveyi infections are the most important Vibrio infections during the postlarval stage until the adult stage but not during earlier stages. V. harveyi infections in Penaeus esculentus broodstock have been described by Owens et al. (13). Our results show that vibriosis is caused less frequently by V. harveyi during grow-out stages than during postlarval stages. This seems to confirm the data of Prayitno and Latchford (16), who found that older penaeid shrimp were more resistant to V. harveyi infections than were larvae. Sung and Song (23) have found that the systemic, rather than the local, defense system of Penaeus monodon can be enhanced by Vibrio antigen, indicating increased resistance of older shrimp. Since postlarvae more often have V. harveyi infections than do juveniles or adults, the selection of the strongest larvae for grow-out could be an explanation for fewer V. harveyi infections during grow-out. It is not known whether specific V. harveyi genotypes are associated with infections in juvenile or adult shrimp.
(ii) V. parahaemolyticus, V. mimicus, and P. damselae.
V. parahaemolyticus and P. damselae (previously V. damsela) have been mentioned as pathogens of larvae (9). Although some of these vibrios were also isolated from the environment of healthy larvae, they were never found as part of the associated flora of healthy and diseased larvae during the present survey (Fig. 1) or during a previous survey on P. chinensis (25). Our results suggest that V. parahaemolyticus infections (2, 12, 19, 26) and P. damselae infections (12) typically occur in juveniles and adults. V. mimicus was previously isolated from seawater, oysters, and shrimp (5) and was frequently isolated from healthy L. vannamei juveniles during this survey.
Hatchery probionts in Ecuador.
Of the 39 mass cultures of probionts sampled, 37 consisted of single-strain cultures. Strains were identified by Biolog patterns as V. alginolyticus (23 strains), V. hollisae (4 strains), V. fluvialis (2 strains), V. piscium (1 strain), and Vibrio sp. (1 strain). Six strains remained unidentified. Twenty-one V. alginolyticus probiont strains were further genotypically characterized by AFLP (Fig. 2). Ten strains cluster together with a variety of V. alginolyticus strains isolated from diseased larvae or postlarvae (Fig. 2, cluster 1). The 11 remaining V. alginolyticus probionts are found in cluster 2a.
Based on these results, careful use of V. alginolyticus as a probiont should be advocated. Because this species is commonly used as a probiont for L. vannamei larvae in Ecuador (20), special attention should be given to the selection of V. alginolyticus strains to be used as probionts. Until now, these selection criteria were highly empirical; i.e., yellow colonies on TCBS, isolated from successful larval productions, were selected and used as presumed probionts (20), but only 60% of the sampled probionts actually are V. alginolyticus strains. The danger that pathogenic V. alginolyticus strains are used as probionts is realistic, and therefore, we suggest the use of only genotypically well-characterized strains. One V. alginolyticus probiont (strain ILI) which was intensively studied at the CENAIM hatchery and proved to be effective in preventing V. harveyi-related diseases in larvae (20) shows a high level of similarity to probiotic V. alginolyticus strains used at the Playaespec hatchery (Fig. 2, cluster 2a). Further laboratory testing is needed to verify whether all of the strains in cluster 2a can prevent V. harveyi-related diseases in shrimp larvae and whether AFLP can reliably distinguish beneficial from pathogenic V. alginolyticus strains.
Bacteria associated with the zoea 2 syndrome.
Thirty-five dominant isolates were obtained from moribund larvae affected by the zoea 2 syndrome. Twenty-three strains were identified by Biolog as V. alginolyticus. Two representative V. alginolyticus strains (STD3-336 and STD3-343) were genotypically characterized by AFLP and belong to V. alginolyticus cluster 1 (Fig. 2). Our results do not confirm those of analysis by the use of randomly amplified polymorphic DNA, which shows that the causal agent of the zoea 2 syndrome is related to V. harveyi (20).
Bacteria associated with the mysis mold syndrome.
Eight dominant strains were isolated from moribund larvae with the mysis mold syndrome. Six strains were identified by Biolog as V. alginolyticus. Pathogenicity trials are needed to clarify their role during mysis mold syndrome outbreaks.
Bacteria associated with the bolitas syndrome.
Eleven dominant strains were isolated from larvae showing symptoms of the bolitas syndrome. Five strains were identified by Biolog as V. harveyi (strains S2, E22, and STD3-328), V. alginolyticus (STD3-96), and Vibrio strain STD3-348, and six strains remained unidentified. V. harveyi S2, E22, and STD3-328 and V. alginolyticus STD3-96 were further genotypically characterized by AFLP (Fig. 2). The strains phenotypically identified as V. harveyi show three different genotypes in AFLP analysis (Fig. 2, clusters 3, 5, and 7): the type strain genotype (cluster 3), the S2 genotype (cluster 5), and the E22 genotype (cluster 7). V. harveyi S2, which caused the bolitas syndrome in larvae during infection trials at the CENAIM hatchery (unpublished results), shows a high level of genetic similarity to V. harveyi strains from cluster 5 isolated from diseased larvae and postlarvae in the People’s Republic of China and Ecuador, respectively. V. harveyi E22 (also referred to as strain STD3-101), which was able to induce bolitas syndrome symptoms during infection trials (17), shows a low level of genetic similarity to strain S2 (Fig. 2). Also, V. alginolyticus STD3-96 was able to cause low levels of bolitas syndrome symptoms during challenge infection trials (unpublished results) and shows a high level of genetic similarity to two V. alginolyticus strains isolated from diseased postlarvae (Fig. 2, cluster 2b). Based on these results, we can conclude that the bolitas syndrome can be caused by different Vibrio species.
Dominant nonvibrios associated with penaeid shrimp.
Strain STD3-437, isolated in a previous study (25) from P. chinensis larvae and their environment, showed a strong correlation with healthy larvae and was also frequently isolated in Ecuador and Mexico from healthy L. vannamei larvae (Fig. 1). This species might be part of the normal associated flora of penaeid shrimp. Also, strain STD3-695 is associated with healthy larvae in Ecuador (Fig. 1). Since this strain was isolated frequently from healthy P. chinensis and/or L. vannamei larvae, its presence might have a beneficial effect on the health status of the larvae.
From our studies, it is clear that more-predictable shrimp production will be made possible by a better understanding of microbial dynamics during the different production cycles.
ACKNOWLEDGMENTS
We thank Sylvie Van Eygen, from the Laboratory of Microbiology of the University of Ghent, for excellent technical assistance.
We thank the European Commission (EC), which supported this study through EC project grant TS3-CT94-0269.
REFERENCES
- 1.Austin B, Alsina M, Austin D A, Blanch A R, Grimont P A D, Jofre J, Koblavi S, Larsen J L, Pedersen K, Tiainen T, Verdonck L, Swings J. Identification and typing of Vibrio anguillarum: a comparison of methods. Syst Appl Microbiol. 1995;18:285–302. [Google Scholar]
- 2.Chanratchakool P, Pearson M, Limsuwan C, Roberts R J. Oxytetracycline sensitivity of Vibrio species isolated from diseased black tiger shrimp, Penaeus monodon Fabricius. J Fish Dis. 1995;18:79–82. [Google Scholar]
- 3.De Ley J. Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol. 1970;101:738–754. doi: 10.1128/jb.101.3.738-754.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Ley J, Cattoir H, Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970;12:133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 5.Farmer J J, III, Hickman-Brenner F W. The genera Vibrio and Photobacterium. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. III. New York, N.Y: Springer-Verlag; 1992. pp. 2990–2991. [Google Scholar]
- 6.Hameed A S. A study of the aerobic heterotrophic bacterial flora of hatchery-reared eggs, larvae and post-larvae of Penaeus indicus. Aquaculture. 1993;117:195–204. [Google Scholar]
- 7.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP® as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 8.Lightner D V, Redman R M. An epizootic necrotizing hepatopancreatitis in cultured penaeid shrimp (Crustacea: Decapoda) in northwestern Peru. Aquaculture. 1994;122:9–18. [Google Scholar]
- 9.Lightner D V. Diseases of cultured shrimp. In: McVey P V, editor. CRC handbook of mariculture. Boca Raton, Fla: CRC Press; 1993. pp. 393–486. [Google Scholar]
- 10.Marmur J, Doty P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- 11.Mohney L L, Lightner D V, Bell T A. An epizootic of vibriosis in Ecuadorian pond-reared Penaeus vannamei Boone (Crustacea: Decapoda) J World Aquaculture Soc. 1994;25:116–125. [Google Scholar]
- 12.Nash G, Nithimathachoke C, Tungmandi C, Arkarjamorn A, Prathanpipat P, Ruamthaveesub P. Vibriosis and its control in pond-reared Penaeus monodon in Thailand. In: Shariff M, Subasinghe R P, Arthur J R, editors. Diseases in Asian aquaculture I. Fish Health Section. Manila, The Philippines: Asian Fisheries Society; 1992. pp. 143–155. [Google Scholar]
- 13.Owens L, Muir P, Sutton D, Wingfield M. The pathology of microbial diseases in tropical Australian Crustacea. In: Shariff M, Subasinghe R P, Arthur J R, editors. Diseases in Asian aquaculture I. Fish Health Section. Manila, The Philippines: Asian Fisheries Society; 1992. pp. 165–172. [Google Scholar]
- 14.Pedersen K, Verdonck L, Austin B, Austin D A, Blanch A R, Grimont P A D, Jofre J, Koblavi S, Larsen J L, Tiainen T, Vigneulle M, Swings J. Taxonomic evidence that Vibrio carchariae Grimes et al. 1985 is a junior synonym of Vibrio harveyi (Johnson and Shunk 1936) Baumann et al. 1981. Int J Syst Bacteriol. 1998;48:749–758. [Google Scholar]
- 15.Pizzutto M, Hirst R G. Classification of isolates of Vibrio harveyi virulent to Penaeus monodon larvae by protein profile analysis and M13 DNA fingerprinting. Dis Aquat Org. 1995;21:61–68. [Google Scholar]
- 16.Prayitno S B, Latchford J W. Experimental infections of crustaceans with luminous bacteria related to Photobacterium and Vibrio. Effect of salinity and pH on infectiosity. Aquaculture. 1995;132:105–112. [Google Scholar]
- 17.Robertson P A W, Calderon J, Carrera L, Stark J R, Zherdmant M, Austin B. Experimental Vibrio harveyi infections in Penaeus vannamei larvae. Dis Aquat Org. 1998;32:151–155. [Google Scholar]
- 18.Robles-Arozarena, R., J. Vandenberghe, P. Sorgeloos, and J. Swings. Pathogenicity test using Vibrio harveyi injected in Penaeus vannamei post-larvae. Submitted for publication.
- 19.Ruangpan L, Kitao T. Vibrio bacteria isolated from black tiger shrimp, Penaeus monodon Fabricius. J Fish Dis. 1991;14:383–388. [Google Scholar]
- 20.San Miguel, L., M. T. Zherdmant, J. Serrano, E. Donoso, S. Mendoza, E. Motte, L. Carrera, I. Morales, and E. Mialhe. A strain of Vibrio alginolyticus as a candidate for prevention of vibriosis in Penaeus vannamei shrimp larvae. Submitted for publication.
- 21.Smibert R M, Krieg N R. General characterization. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. pp. 409–443. [Google Scholar]
- 22.Sneath P H A, Sokal R R. The principles and practice of numerical classification. W. H. San Francisco, Calif: Freeman & Co.; 1973. [Google Scholar]
- 23.Sung H H, Song Y L. Tissue location of Vibrio antigen delivered by immersion to tiger shrimp (Penaeus monodon) Aquaculture. 1996;145:41–54. [Google Scholar]
- 24.Vandamme P, Vancanneyt M, Pot B, Mels L, Hoste B, Dewettinck D, Vlaes L, Van Den Borre C, Higgins R, Hommez J, Kersters K, Butzler J-P, Goossens H. Polyphasic taxonomic study of the emended genus Arcobacter with Arcobacter butzleri comb. nov. and Acrobacter skirrowii sp. nov., an aerotolerant bacterium isolated from veterinary specimens. Int J Syst Bacteriol. 1992;42:344–356. doi: 10.1099/00207713-42-3-344. [DOI] [PubMed] [Google Scholar]
- 25.Vandenberghe J, Li Y, Verdonck L, Li J, Xu H S, Swings J. Vibrios associated with Penaeus chinensis (Crustacea: Decapoda) larvae and post-larvae in Chinese shrimp hatcheries. Aquaculture. 1998;169:121–132. [Google Scholar]
- 26.Xu H, Xu B, Ji W, Shi J. Pathogens and pathogenicity to Penaeus orientalis Kishinouye. Acta Oceanol Sin. 1994;13:297–304. [Google Scholar]