Abstract
Introduction
Medication-related osteonecrosis of the jaws (MRONJ) is a known adverse event related to the use of antiresorptive (AR) drugs. More recently, an association between antiangiogenic (AA) drugs and MRONJ has been suggested. This review aimed to investigate the overall prevalence and relative risk of MRONJ in patients treated concurrently with AA and AR agents in comparison with a single AA or AR drug.
Methods
A review protocol was registered with PROSPERO (ID: CRD42020214244). A systematic literature search, study selection, quality assessment, and data extraction were carried out following PRISMA guidelines. Random-effects meta-analysis models were used to summarize relative estimates for the outcomes, namely prevalence and relative risk of MRONJ. Exposure variable included type of drug, specifically AA and AR agents administered either concurrently or individually.
Results
Eleven studies were included in the final qualitative and quantitative syntheses. The overall pooled weighted prevalence of MRONJ with concurrent AA-AR drugs was 6% (95% CI: 3–8%), compared with 0% (95% CI: 0–0%) for AA only and 5% (95% CI: 0–10%) for AR only. However, high heterogeneity was noted among included studies. Retrospective cohort studies showed a higher pooled prevalence of 13% (95% CI: 10–17%) for concurrent AA-AR therapy. The pooled risk ratio for MRONJ revealed a risk with concurrent AA-AR drugs 2.57 times as high as with AR only (95% CI: 0.84–7.87); however, this difference was not statistically significant. Concurrent AA-AR drugs had a risk for MRONJ 23.74 times as high as with AA only (95% CI: 3.71–151.92).
Conclusions
High-quality, representative studies are needed for accurate estimation of relative risk of MRONJ with concurrent AA and AR therapy.
Keywords: osteonecrosis, bisphosphonates, denosumab, antiangiogenic drugs, antiresorptive drugs
INTRODUCTION
Targeted antiangiogenic (AA) drugs, which are commonly used in conjunction with other chemotherapy agents, have become an integral component of many cancer therapies, including breast, prostrate, colorectal, non-small cell lung (NSCL), and renal cell carcinomas.[1,2] These cancers are also marked by a high prevalence of metastasis to the bone. For instance, more than 50% of renal cell carcinoma patients present with metastasis, predominantly lung and bone metastasis, at initial diagnosis and nearly 40% of initially nonmetastatic patients treated with nephrectomy with a curative intent ultimately develop metastases.[2,3] Similarly, cumulative incidence of bone metastases in advanced stage disease at diagnosis is more than 70% in prostate, 61% in breast, 26% in NSCL, and 7% in colorectal cancer.[4]
Antiresorptive (AR) drugs, including denosumab and bisphosphonates, are used to prevent and treat skeletal-related events, such as pathologic fractures, bone pain, hypercalcemia, and spinal cord compression, resulting from osteolytic destruction in patients with bone metastasis.[5–7] Denosumab (Dmab) is a fully humanized monoclonal antibody that prevents RANK ligand from binding to its receptor, thereby inhibiting osteoclast development, function, and survival.[8] Nitrogen-containing bisphosphonates (BP), such as zoledronate (zoledronic acid), pamidronate, ibandronate, alendronate, and risedronate are pyrophosphate analogs that bind to mature osteoclasts and disable their resorptive function at sites of bone resorption.[8] Nonnitrogen-containing bisphosphonates, such as clodronate, tiludronate and etidronate, induce osteoclast apoptosis, thereby reducing osteolytic activity.[8] Nitrogen-containing bisphosphonates have also been reported to suppress angiogenesis by interfering with cell migration and proliferation in endothelial cells as well as reducing circulating levels of vascular endothelial growth factor.[9–11] Similar effects have not been noted for nonnitrogen-containing BP and Dmab.[11]
A known adverse event correlated with AR drugs is medication-related osteonecrosis of the jaw (MRONJ), which has an overall reported prevalence ranging from 0.3% to 6.7%.[12–15] A similar incidence of MRONJ, ranging from 0.9% to 3.1%, has been reported for BP and Dmab in systematic reviews.[16–19] Furthermore, an association between non-antiresorptive medications, including AA drugs, and MRONJ has been suggested by a recent systematic review.[20]
It has been hypothesized based on clinical impressions that the increase in concurrent administration of AA and AR therapies may present an increased risk for development of MRONJ. We aimed to systematically review the literature, and conduct a meta-analysis when possible, to answer the focused review question: What is the prevalence and risk ratio of MRONJ in oncology patients concurrently treated with two or more AR and AA drugs compared to patients treated with a single AR or AA drug?
METHODS
Protocol and Eligibility Criteria
A review protocol was devised, approved by our multidisciplinary team and registered with PROSPERO database (CRD42020214244). The systematic review was conducted according to the Preferred Reporting Items for Systematic Review (PRISMA) reporting guideline. The search strategies were planned according to the following PICO[21] question:
-
P:
Adults diagnosed with cancer
-
I:
Administration of two or more AR and AA drugs concurrently
-
C:
Administration of a single AR or AA drug
-
O:
Prevalence and relative risk of MRONJ
Definition of outcome
MRONJ, by consensus of the American Association of Oral and Maxillofacial Surgeons (AAOMS), is characterized as exposed bone or bone that is palpable by probing an intra- or extraoral fistula, which is persistent for more than 8 weeks in the maxillofacial region.[12]
Types of studies included
Cohort studies (prospective/retrospective) or clinical trials (phase 1, 2, 3, randomized, nonrandomized).
Study eligibility criteria
Studies were included if they (1) evaluated incidence/prevalence of MRONJ following administration of AR and AA drugs concurrently for cancer, (2) were published in English language, and (3) full texts were available. Studies were excluded if they only included case series data, or if relevant clinical data was not clearly reported (see data extraction section).
Information Sources and Literature Search
Systematic electronic literature searches were conducted on June 5, 2019, and updated on January 12, 2021, to include Medline (Ovid), EMBASE (Ovid), and Cochrane Central Register of Controlled Trials (CENTRAL) using database-specific search strategies (Supplemental Tables S1–S3). The search was limited to studies of human participants (not animals). Electronic search was also supplemented by manual bibliography screening of previously published reviews and retrieved full-text articles.
Study Selection and Data Extraction
Two authors (AS, MC) independently screened titles and abstracts of identified publications based on the predefined eligibility criteria. Differences in abstract selections were resolved through discussion. Full texts of accepted titles were retrieved, and two authors (AS, MC) screened the studies for final inclusion in qualitative and quantitative synthesis.
One review author (AS) extracted data into a predesigned excel sheet, which was reviewed by a second author (GMNG) for any discrepancies. The following data were recorded, if available: (1) general study characteristics, such as authors, year of publication, journal, study aim; (2) study design; (3) details of participants, including age, sex, disease characteristics; (4) exposure to AR and AA therapies, including additional chemotherapy agents; (5) details of MRONJ outcome, including definition, method of assessment, and grading criteria; (6) evaluation period; (7) total sample size and number of MRONJ cases in the concurrent AR-AA and comparator AR/AA groups; (8) standardized dosage of AR and AA therapy; (8) time of exposure to event, dosage/treatment cycles to event; (9) location of MRONJ lesion; and (10) any orodental diagnosis or procedure associated with development of MRONJ. Median time to event was computed from patient-level data when summary measures were not reported in the included studies, where applicable. Additional study, participant, and exposure details were retrieved from earlier reports on the same trial if they were missing in the included full texts. Studies that reported data from two or more trials or cohorts were separated by study design, when applicable.
We previously published another systematic review using the same methodology to estimate the prevalence of MRONJ with sequential AR therapy.[22] The study revealed a higher prevalence of MRONJ when two or more AR drugs were administered sequentially compared with a single AR drug. Within this study,[22] the effect of addition of AA drugs was not evaluated. In this present systematic review, the effect of combination of AR and AA drugs on prevalence and relative risk of MRONJ is evaluated. AR drugs include different types of bisphosphonates, denosumab, as well as sequential AR therapy, which could not be separated for subgroup analyses due to limited data.
Quality Assessment
Methodological and reporting quality were assessed for individual studies using an adapted quality grading criteria (Supplemental Table S4) developed by the Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) group for incidence and outcome evaluation of oral complications from cancer therapies.[23] Quality scores were not used for exclusion of studies or data syntheses in order to avoid bias from applying quality weights for effect size estimation.[24,25] Quality scores were used for qualitative evaluation of included publications and to establish the level of certainty in the evidence used for effect estimation.
Each full text included in the final synthesis was appraised by two authors (AS, MC) using quality points pertaining to representativeness and sources of bias in measurement of MRONJ, including misclassification bias, examiner bias, and outcome measure assessment validity. Maximum permissible quality score was 6 for each included study, ranging from 0 to 2 for representativeness and oral complication validity, and from 0 to 1 for misclassification and examiner bias. Consensus was achieved through discussion in any ambiguous domain grading or disagreements. Outcome measure assessment validity was appraised as high quality if standard validated criteria for MRONJ, such as the AAOMS position paper,[12,26] or Common Terminology Criteria for Adverse Events (CTCAE) grading was used in the study. If studies included another independent diagnostic criterion for MRONJ evaluation, quality points were assigned for study-specific scale/criteria.
Summary Measures and Synthesis of Results
We plotted the prevalence/proportion of MRONJ along with 95% confidence intervals (CIs) using forest plots. We assessed the statistical heterogeneity using the I2 statistic as described by Higgins et al, [27] which measures the percentage of total variation that is due to heterogeneity rather than chance. The I2 statistic measures whether the studies are estimating the same effect and the observed value of the I2 depends on the magnitude and direction of the effect as well as the strength of evidence for heterogeneity. I2 values can range from 0% to 100%; values less than 25% represent low heterogeneity, whereas those between 25% and 50% represent moderate heterogeneity.[27] If I2 was statistically significant, then random effects models were used in which each study was weighted equally in the meta-analysis of the combined prevalence/proportion of MRONJ.[28] A fixed continuity correction of a count of 0.5 was added to both success and failures in the case where a study has 0% or 100% success rate.[29] We used the inverse variance-weighted average method for carrying out the meta-analysis.[25]
We visually examined funnel plots to assess possible publication bias, that is, the tendency to publish either positive or negative results (Appendices 4–6).[30] The vertical line in the funnel plot indicates a fixed-effects summary estimate and the other dotted lines represent the 95% CI; symmetry across the vertical line implies absence of publication bias. Potential sources for the asymmetry include selection bias, including publication bias or selective outcome reporting, and poor methodological quality leading to spuriously inflated effects in smaller studies, true heterogeneity, artifact, and chance.[31] Smaller studies result in increased scatter in the funnel plot, whereas larger studies are represented more in the center of the funnel. In addition to the meta-analyses, we also computed unweighted prevalence of MRONJ by type of malignancy by combining total number of MRONJ cases and total number of cases reported for each type of malignancy. All statistical analyses were done in Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, 2020).
RESULTS
Study Selection and Characteristics
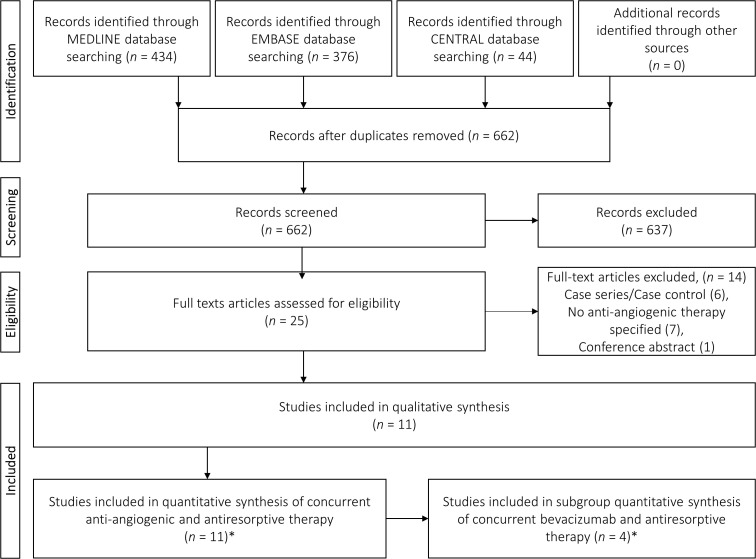
The data search, identification, and screening process for inclusion as per the PRISMA guidelines are depicted in Figure 1. Initial database search yielded 854 records before deduplication. No additional studies were identified from manual bibliography searching. After deduplication, 662 publications were screened based on titles and abstracts; 25 publications were included for full-text analysis. The primary reasons for exclusion of the remaining 637 records were study design (case reports and reviews), overall irrelevance to topic, and lack of concurrent AR-AA administration.
Figure 1.
PRISMA flow diagram of literature search and screening process (*Guarneri et al[52] was split into “a” and “b” resulting in a total of 12 studies for quantitative synthesis and 5 studies for bevacizumab subgroup synthesis). PRISMA: Preferred Reporting Items for Systematic Review.
From the 25 studies that were included for full-text analysis, 14 were excluded; six studies only reported a single arm MRONJ case series or a case control study from which prevalence could not be assessed,[32–37] seven studies did not include/specify any AA therapy,[38–44] and one record was a conference abstract for which a full text was included from the original search.[45] Finally, a total of 11 full texts with extractable data were included in the qualitative and quantitative syntheses.
The vast majority of the included studies were retrospective in methodology including six retrospective cohorts,[2,46–50] and three retrospective trials.[51–53] Additionally, one prospective randomized clinical trial (RCT)[3] and one prospective cohort[54] were included. Of three retrospective trials, one study included data from a Phase II trial,[51] one study included combined data from five Phase II trials and three Phase III trials that could not be separated,[53] and one study included data from two RCTs and one non-RCT with individual data published for the two study designs.[52] The latter study by Guarneri et al[52] was separated as “a” for RCT data and “b” for non-RCT data, respectively, leading to 12 items presented in tables and forest plots; henceforth referred to as 12 studies.
Tables 1 and 2 present detailed study characteristics, patient characteristics, and outcome measures including the following: (1) study design; (2) total study sample size; (3) study duration; (4) study arms, including type of AR drug, sequence of therapy, dose, route, and frequency of drug administration; (5) number of MRONJ cases, location of MRONJ lesion, number of MRONJ cases associated with dental extractions; and (6) sample characteristics in terms of sex, age, and type of malignancy.
Table 1.
Characteristics of included studies reporting on concurrent antiangiogenic-antiresorptive therapy, MRONJ, and summary of outcome parameters
|
Study ID (Last name, year of publication)
|
Study design
|
Total study sample
|
Study duration
|
AA drug
|
Dose, route, frequency (AA drug)
|
MRONJ cases/
total sample
(AA only) |
Exposure time to MRONJ, median (range)
|
AR drug
|
Dose, route frequency (AR only)
|
MRONJ cases/ total sample (AR onlu)
|
Exposure time to MRONJ, median (range)
|
MRONJ cases/ total sample (AA+AR)
|
Exposure time to MRONJ, median (range)
|
Additional chemotherapy
|
| Aragon-Ching (2009)51 | Retrospective (Phase II trial) | 60 | Apr 2005– Sep 2007 | BEV | 15 mg/kg IVI Q3W | – | NA | BP (ZOL) | 3.5–4 mg/ Q3–Q4W IVI | – | NA | 11/55 | AR- 19 (3–36)* AA- 10 (3–17)* | THAL, TXT, PDN |
| Beuselinck (2012)2 | Retrospective cohort | 76 | Nov 2005– May 2012 | SUN, SOR | NR | – | NA | BP (ZOL, PAM, IBAN) | NR Q4W | – | NA | 5/52 | AR- 18 (4–60) AA- 6 (2–39) | NR |
| Broom (2015)3 | Prospective RCT | 30 | Feb 2010– Oct 2011 | EVE | 10 mg daily | 0/15 | NA | BP (ZOL) | 4 mg/Q4W IVI | – | NA | 0/15 | NA | NR |
| Christodoulou (2009)46 | Retrospective cohort | 116 | Jun 2007– Jun 2008 | BEV, SUN, SOR | NR | – | NA | BP (ZOL, IBAN) | ZOL: 4 mg/ Q4W 15-– min IVI | 1/91 | 18 | 4/25 | AR- 2 9(15–78) AA-11.5 (1–20)* | NR |
| Francini (2011)54 | Prospective cohort | 59 | Jul 2007– Dec 2009 | BEV | 15 mg/kg IVI Q3W | – | NA | BP (ZOL) | 4 mg/Q4W 15-min IVI | – | NA | 0/59 | NA | MBC: TXT/T/EC NSCLC: GC/EP |
| Guarneri (2010a)52† | Retrospective RCTs (AVADO, RIBBON–1) | 1309 | NR | BEV | 7.5 or 15 mg/kg IVI Q3W | 2/1075 | 4.5 (2–7) | BP | NR | 0/99 | NA | 2/233 | AR- 3.2 (0.5–6) AAD- 3.5 (3–6) | AVADO:TXT RIBBON-1: CAPE/ TXT/T/EC/AC ± 5–FU |
| Guarneri (2010b)52† | Retrospective non– RCT (ATHENA) | 2251 | NR | BEV | NR | 0/1826 | NA | BP (ZOL, PAM, CLOD, IBAN) | NR | – | NA | 10/425 | AR- NR AA- 9 (5–15) | TXT/PTX ±5-FU/ VNB |
| Guillot (2018)47 | Retrospective cohort | 41 | Jan 2013– Nov 2016 | SUN, PAZ, EVE, TEM, AXT | NR | – | NA | Dmab | NR | – | NA | 7/41 | AR- 19.9 (NR) AA- NR | NR |
| McKay (2014)53 | Retrospective Phase II (n = 5), Phase III (n = 3) | 2749 | Jan 2003– Nov 2011 | SUN, SOR AXT, TEM | NR | 1/2464 | NR | BP | NR | – | NA | 6/285 | NR | NR |
| Pilanci (2015)48 | Retrospective cohort | 97 | Mar 2006– Dec 2013 | Tmab | NR | – | NA | BP (ZOL) | NR | 5/66 | 38 (13–55)* | 8/31 | AR- 42(26–78)* AA- NR | AI, CT |
| Smidt-Hansen (2013)49 | Retrospective cohort | 46 | Aug 2010– Dec 2011 | SUN, SOR, BEV, PAZ, EVE, TEM | SUN: 50 mg/d 4W on/2W off; SOR: 400 mg BD; BEV: 10 mg/kg Q2W; PAZ: 800 mg/d; EVE: 10 mg/d; TEM: 25 mg/wk IV | – | NA | BP (ZOL) | 4 mg/Q6W IVI | – | NA | 7/46 | AR- NR (3.7–26.2) AA-NR (3.7–17.7) | NR |
| van Cann (2018)50 | Retrospective cohort | 623 | Jan 2005– Jan 2017 | SUN, PAZ, AXT, SOR VAN, EG, TIVO | NR | – | NA | BP, Dmab | NR | 58/533 | 19 (1–144) | 10/90 | AR- 5 (1–55) AA- 4 (1–48) | NR |
Published as a combination study; raw data retrieved for analysis.
Computed from raw data.
MRONJ: medication-related osteonecrosis of the jaw; AA: antiangiogenic; AR: antiresorptive; ZOL: zoledronate; IBAN: ibandronate; PAM: pamidronate; CLOD: clodronate; BEV: bevacizumab; EVE: everolimus; SUN: sunitinib; SOR: sorafenib; PAZ: pazopanib; TEM: temsirolimus; Tmab: trastuzumab; AXT: axitinib; VAN: vandetanib; REG: regorafenib; TIVO: tivozanib; TXT: docetaxel; PTX: paclitaxel; THAL: thalidomide; PDN: prednisolone; VNB: vinorelbine; PBO: placebo; SC: subcutaneous; IVI: intravenous infusion; NR: not reported/not retrievable; MBC: metastatic breast cancer; NSCLC: non-small lung cell cancer; EC: epirubicin and cyclophosphamine; GC: gemcitabine and cisplatin: EP: etoposide and cisplatin; CAPE: capecitabine; AC: doxorubicin and cyclophosphamide; 5-FU: 5-fluorouracil; AI: aromatase inhibitor; CT: chemotherapy; W: week.
Table 2.
Summary of sample and disease characteristics in participants diagnosed with MRONJ
|
Study ID (Last name, year of publication)
|
Patient population
|
Total MRONJ cases*
|
Females n (%)
|
Age, y, median (range)
|
Location of MRONJ lesion
|
MRONJ cases associated with dental extractions,
n
(%) |
Spontaneous MRONJ cases,
n
(%) |
Pretherapy oral examination and preventive tx
|
||
|
Mandible,
n
(%) |
Maxilla,
n
(%) |
Both,
n
(%) |
||||||||
| Aragon-Ching (2009)51 | Metastatic castration resistant prostate CA | 11 | 0 (0) | 64 (49–79) | 9 (82) | 1 (9) | 1 (8) | NR | NR | No |
| Beuselinck (2012)2 | Metastatic renal cell CA | 5 | NR | NR | NR | NR | NR | 1 (20) | NR | No |
| Broom (2015)3 | Metastatic renal cell CA | 0 | NA | NA | NA | NA | NA | NA | NA | Yes |
| Christodoulou (2009)46 | Metastasis from Breast, lung, prostate, colorectal, renal cell, non-Hodgkin, gastric, hepatoma, bladder CA | 5 | 2 (40) | 48 (44–77)* | NR | NR | NR | 2 (40) | 3 (60) | NR |
| Francini (2011)54 | Metastatic breast CA, NSCLC | 0 | NA | NA | NA | NA | NA | NA | NA | Yes |
| Guarneri (2010a)52† | Metastatic breast CA | 4 | 4 (100)* | NR | NR | NR | NR | 1 (25) | 3 (75) | NR |
| Guarneri (2010b)52† | Metastatic breast CA | 10 | 10 (100)* | 56 (40–73)* | NR | NR | NR | 2 (20) | NR | NR |
| Guillot (2018)47 | Metastatic renal cell CA | 7 | NR | 62 (54.5–65)† | NR | NR | NR | 3 (42.9) | NR | No‡ |
| McKay (2014)53 | Metastatic renal cell CA | 7 | NR | NR | NR | NR | NR | NR | NR | No |
| Pilanci (2015)48 | Metastatic breast CA | 13 | 13 (100)* | Mean 57 SD 10 | 6 (46) | 5 (39) | 2 (15) | 13 (100) | NA | NR |
| Smidt-Hansen (2013)49 | Metastatic renal cell CA | 7 | NR | NR | NR | NR | NR | 2 (28.5) | 2 (28.5) | No‡ |
| van Cann (2018)50 | Metastasis from renal cell, sarcoma (including gastrointestinal stromal tumors), thyroid, neuroendocrine carcinoma, paraganglioma, breast, prostate, lung, malignant melanoma, bladder, HNSCC, others | 68 | 41 (60.3) | (31–89) | NR | NR | NR | 25 (36.8) | NR | Yes |
Estimated from raw data.
Median (IQR).
Partial sample had oral examination.
MRONJ: medication-related osteonecrosis of the jaw; CA: cancer; NSCLC: non-small cell lung cancer; HNSCC: head and neck squamous cell carcinoma; NR: not reported/not retrievable; tx: treatment.
Of 12 studies, 10 included concurrent AA therapy with BP,[2,3,46,48,49,51–54] one study included only Dmab[47] and another study included both BP and Dmab.[50] Among these, four studies[51,52,54] only included administration of bevacizumab and BP, and we conducted subgroup meta-analysis for this specific combination. Concurrent administration of everolimus[3] and trastazumab[48] with BP were reported in one study each. All other studies (n = 6) included combined data for AA drugs including bevacizumab (n = 2), sunitinib (n = 6), sorafenib (n = 5), everolimus (n = 2), 0azopanib (n = 3), axitinib (n = 3), temsirolimus (n = 3), vandetanib (n = 1), regorafenib (n = 1), and tivozanib (n = 1). Of six combinations studies, two reported ONJ occurrence only in individuals treated with sunitinib and BP;[49,53] however, subgroup analysis was not conducted due to the small number of studies.
A total of 7457 participants were studied in the included 12 samples, while sample size of individual studies ranged from 30 to 2749. Sample size of participants administered concurrent AA and AR therapy ranged from 15 to 425. Only four sample sizes for AA only and AR only were reported and ranged from 15 to 2464 and 66 to 99 participants, respectively. Study duration or follow-up periods ranged from 1 to 12 years for retrospective cohorts, 1.5 to 10 years for retrospective trials, approximately 1.5 years for the prospective trial, and 2.5 years for the prospective cohort study.
Five studies[3,46,48,51,54] reported standardized dose and route of drug administration of BP, specifically zoledronate, which was consistently administered at a dose of 4 mg via intravenous infusion; frequency of administration ranged from every 3 to 4 weeks in one study, 4 weeks on three studies and 6 weeks in one study. Dose, route, and frequency of administration was not reported for any other AR drug. Bevacizumab dosage was reported in four studies; the dosage ranged from 7.5 to 15 mg/kg via intravenous infusion for a period of 2 or 3 weeks.[51,52,54] Standardized dosage and frequency of 10-mg daily everolimus was reported in two studies. No other AA drug dosages were consistently reported in more than one study. Furthermore, only five studies[48,51,52,54] reported additional chemotherapy that was administered concurrently with AA and AR therapy, including thalidomide, taxanes, epirubicin and cyclophosphamine, gemcitabine and cisplatin, capecitabine, doxorubicin and cyclophosphamide, 5-fluorouracil, aromatase inhibitor, and unspecified chemotherapy.
Five of 12 studies only included individuals with metastatic renal cell cancer,[2,3,47,49,53] three included only metastatic breast cancer,[48,52] and one included metastatic castration-resistant prostate cancer.[32] The remaining three studies included a combination of study participants with metastasis from breast, prostate, colorectal, renal cell, non-Hodgkin, gastric, hepatoma, bladder, sarcoma (including gastrointestinal stromal tumors), thyroid, neuroendocrine carcinoma, paraganglioma, malignant melanoma, head and neck, and other cancers.[46,50,54]
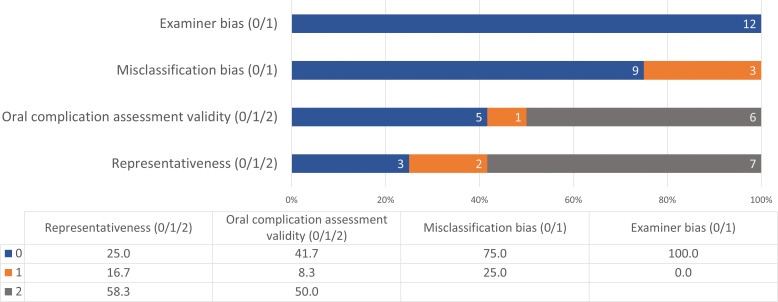
Quality Assessment
Methodological and reporting quality assessment based on the modified MASCC/ISOO quality grading strategy for incidence and outcome evaluation[23] is presented in Figure 2 and Supplemental Table S5. None of the included studies reported having a blinded examiner to evaluate AA and AR drug exposure. Nearly 67% studies scored less than 3 total quality points2,3,46–49,51,54]; of these, two studies[46,8] scored 0 points across all domains. The remaining four studies (33%) had a total of 4 points.[50,52,53]
Figure 2.
Summary of methodological and reporting quality assessment.
Five of 12 studies used standardized validated criteria for ONJ diagnosis; Aragon-Ching et al[51] and Guarneri et al (a and b)[52] reported using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0, whereas Smidt-Hansen et al[49] and van Cann et al[50] used the 2009 and 2014 AAOMS diagnostic criteria, respectively. Francini et al[54] used a study-specific diagnostic criterion including exposed/nonhealing necrotic bone or extraction socket, while the remaining six studies did not report any specific criteria used for diagnosis of ONJ.[2,3,46–48,53] Of these six studies, two reported referral to oral and maxillofacial surgeons or dentists specializing in cancer care for diagnosis of ONJ.[46,48]
Summary of Results
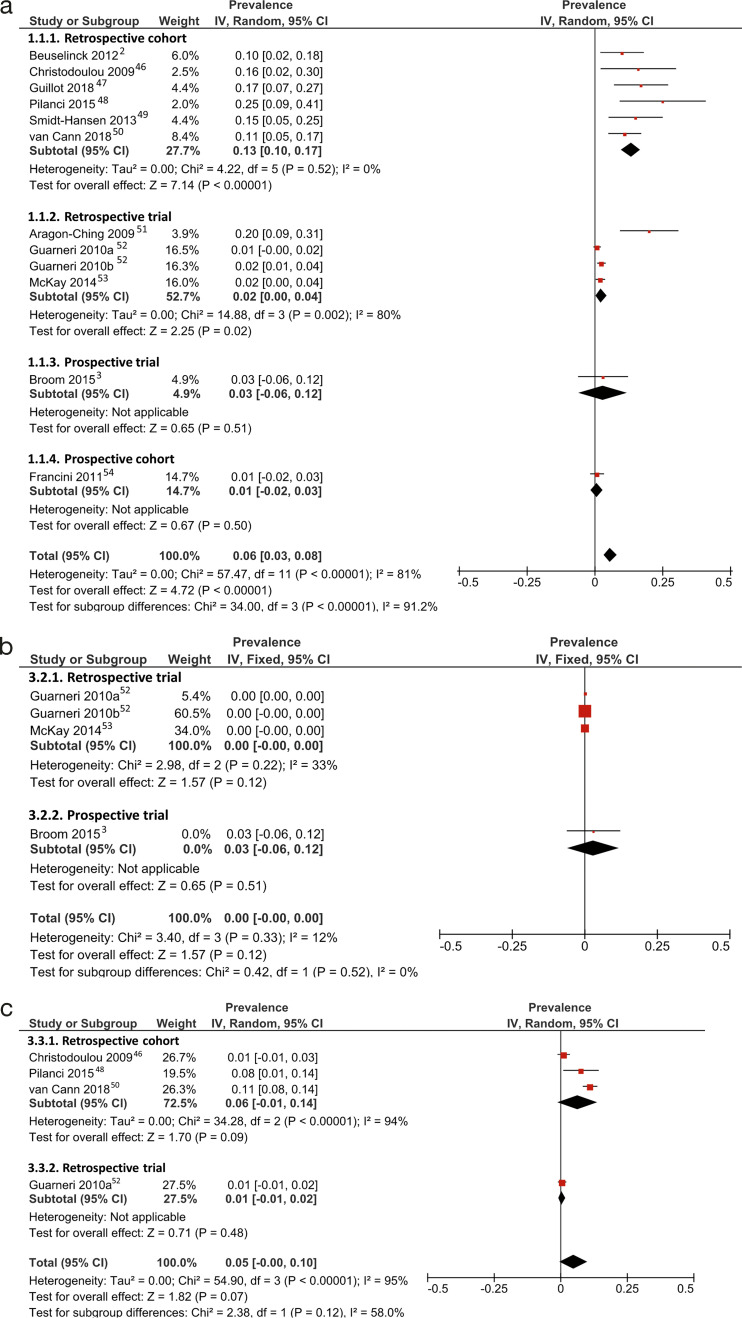
Prevalence of MRONJ
Forest plots for prevalence of MRONJ with effect estimates and weights for each included study and pooled prevalence by study designs is shown in Figures 3 and 4. Weighted prevalence of MRONJ ranged from 1% to 25% for concurrent AA and AR drugs, 0% to 3% for AA drugs only, and 1% to 11% for AR drugs only (Fig. 3). For concurrent AA and AR drugs, a wide variance in weighted prevalence was noted based on study designs; the prevalence ranged from 1% to 3%, 1% to 20%, and 10% to 25%, for the two prospective studies, retrospective trials, and retrospective cohorts, respectively (Fig. 3a).
Figure 3.
Forrest plots for meta-analysis of prevalence of medication-related osteonecrosis of the jaw in patients administered (a) concurrent antiresorptive and anti-angiogenic drugs, (b) antiangiogenic drugs only, and (c) antiresorptive drugs only. Red square: weighted prevalence estimate of individual study; horizontal black line: 95% CI of individual study result; diamond: pooled prevalence estimate.
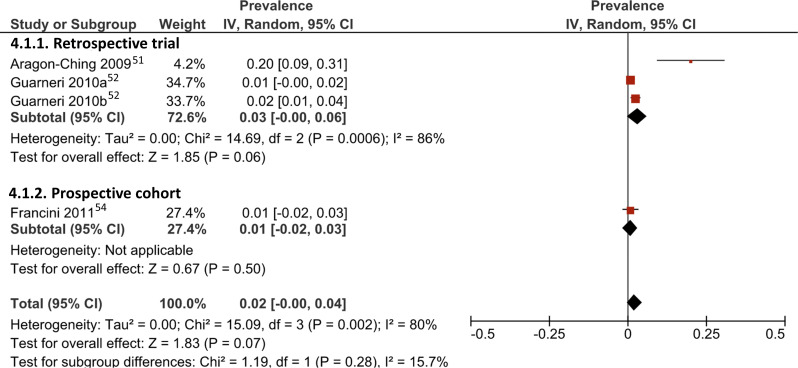
Figure 4.
Forrest plot for meta-analysis of prevalence of medication-related osteonecrosis of the jaw in patients administered concurrent bevacizumab and antiresorptive drugs. Red square: weighted prevalence estimate of individual study; horizontal black line: 95% CI of individual study result; diamond: pooled prevalence estimate.
Weighted prevalence for concurrent administration of bevacizumab and BP ranged from 1% to 20% in three retrospective trials and one prospective cohort (Fig. 4). Furthermore, two studies administering various AA-AR drugs reported that all observed ONJ cases occurred in individuals receiving sunitinib, with unweighted prevalence of 2% and 15%; these were not included in a separate subgroup meta-analysis due to the low number of studies.[49,53]
Unweighted prevalence of MRONJ by type of malignancy revealed 16.9% prevalence among patients with prostate cancer, 0.92% in renal cancer group, 0.77% in breast cancer group, and 3.7% for all other malignancies combined (Supplemental Table S6).
Relative risk
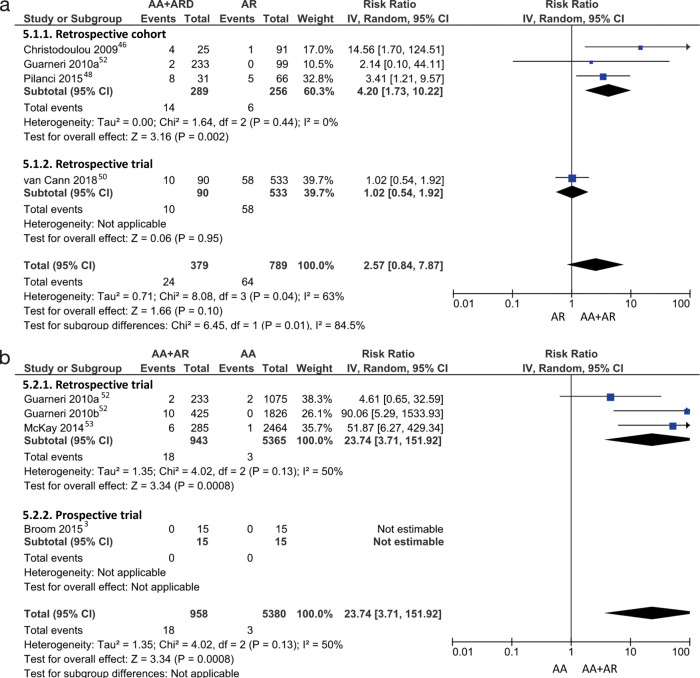
Four studies reported both AA-AR and AR only groups.[46,48,50,52] Two of these studies revealed a significantly higher relative risk of MRONJ with AA-AR, from 3.41 to 14.56 (Figure 5a). The relative risks in the other two studies were also higher for AA-AR, from 1.02 to 2.14, but were not statistically significant. Similarly, four studies included both AA-AR and AA only groups,[3,52,53] with significantly higher risk of MRONJ with AA-AR drugs, ranging from 4.61 to 90.06 (Figure 5b).
Figure 5.
Forrest plot for meta-analysis of risk ratio of medication-related osteonecrosis of the jaw in patients administered concurrent antiresorptive and anti-angiogenic drugs versus (a) antiresorptive drugs only and (b) antiangiogenic drugs only. Blue square: risk ratio estimate of individual study; horizontal black line: 95% CI of individual study result; diamond: pooled risk ratio estimate; vertical black line: line of null effect.
Time to MRONJ event
Median and range for time duration of AA and AR drug administration to diagnosis of MRONJ reported in included studies is presented in Table 1. In the concurrent AA-AR therapy group, the median time to MRONJ for ranged from 3.5[52] to 11.5 months[52] for AA drugs and from 3.2[52] to 42 months[48] for AR drugs, as reported in 9 of 10 studies with MRONJ cases. Meta-analysis of the exposure time to MRONJ could not be carried out due to the heterogeneity of data and summary measures reported in the included studies full texts. Furthermore, time-to-MRONJ data was reported in one and three studies for AA and AR drugs only, respectively; therefore, a comparison between the groups was not conducted.
Sample characteristics and risk factors for MRONJ
Table 2 reports summaries from individual studies on sample characteristics. A total 137 ONJ cases were reported in the 12 included studies, including AA and AR drugs only and concurrent AA-AR groups. Three breast cancer studies[48,52] with 27 MRONJ cases included only females and one prostate cancer study included only 10 males with diagnosed MRONJ.[51] Of the remaining six mixed-sex studies with MRONJ cases, only two reported the sex distribution. Of the total 73 cases, 43 (58.9%) were noted in females.[46,50] The median age of individuals who were diagnosed with MRONJ was reported in four studies and ranged from 48 to 64 years; within these individual studies no significant difference was noted in the median age between MRONJ and non-MRONJ cases.[55–62]
Only two studies reported the location of the MRONJ lesions; 62.5% cases occurred in the mandible, 25% in the maxilla, and 12.5% in both the mandible and maxilla.[55–61,63] Eight of 10 studies reported dental extractions preceding MRONJ lesions in a total of 41.2% cases.
Prevention of MRONJ
Pretherapy oral examination and preventive care was consistently provided only in three studies.[3,50,54] Broom et al[3] mandated oral examination and preventive dental work by a trained dental professional for all study participants before inclusion in the study. Francini et al[54] conducted oral and radiographic baseline examinations, treatment of dental caries and periodontal disease, and extraction of teeth at least 4 weeks before AA-AR therapy. They additionally implemented patient counselling, periodic dental, and radiographic examination during treatment every 6 months, chlorhexidine mouthwash and local antibiotic agents before baseline oral hygiene, avoidance of any invasive dental procedures and a drug holiday of at least 4 weeks if any invasive dental procedure was needed during AA-AR treatment. In both these prospective studies no MRONJ cases were recorded.[3,54] Conversely, in a retrospective cohort study van Cann et al[50] reported that despite pretherapy examination by a family dentist, 25 of 68 patients with MRONJ went through extractions during AR therapy.
In two other studies,[47,49] pretherapy oral examination was received by a fraction of the sample. Guillot et al[47] reported that nearly 83% patients had oral examinations but only 54% received simultaneous radiographic examination; no details of preventive dental treatment or patient counseling were reported. In this study population, dental extractions preceded 60% MRONJ lesions but its association with pretherapy examination was not reported. Smidt-Hansen et al[49] noted a decrease in MRONJ lesions in a group that received pretherapy oral and maxillofacial examination. In this study, 6 of 21 patients with no pretherapy examination and one of nine patients with pretherapy examination developed MRONJ lesions; however, the decrease in MRONJ lesions was not statistically significant, given the small sample size.
In three other studies, pretherapy oral examination and preventive measures were either not mandated or left at the discretion of the treating physician.[2,51,53] Three other studies did not report specific MRONJ prevention measures.[46,48,52]
Synthesis of Results
Pooled weighted prevalence of MRONJ for concurrent AA-AR drugs was 6% (95% CI: 3–8%; Fig. 3a) estimated using a random-effects model. However, significant heterogeneity was present among included studies of all study designs (I2 = 81%, p < 0.01). Retrospective cohort studies showed low heterogeneity (I2 = 0.0%, p < 0.52) and a higher pooled MRONJ prevalence of 13% (95% CI: 10–17%; Fig. 3a), compared with retrospective trials and prospective studies.
In contrast, pooled weighted prevalence of AA drugs only was 0% (95% CI: 0–0%; I2 = 12.0%, p = 0.33; Fig. 3b) and AR drugs only was 5% (95% CI: 0–10%; I2 = 95%, p < 0.01; Fig. 3c). Included studies for bevacizumab and BP therapy also had high heterogeneity (I2 = 80%, p < 0.01) and revealed a pooled weighted MRONJ prevalence of 2% (95% CI: 0–4%; Fig. 4).
Pooled risk ratio for MRONJ revealed a risk with concurrent AA-AR drugs 2.57 times as high as with AR only (95% CI: 0.84–7.87). However, this was not statistically significant. Concurrent AA-AR drugs had a risk for MRONJ 23.74 times as high as with AA drugs only (95% CI: 3.71–151.92).
DISCUSSION
The objective of this systematic review and meta-analysis was to focus on the impact of concurrent antiresorptive and AA therapy on the risk and prevalence of MRONJ. The available literature revealed significant methodological and statistical heterogeneity; therefore, findings of the study should be interpreted with caution. Subgroups based on study designs were added to meta-analyses to elucidate methodological variations in the estimates for MRONJ.
A higher prevalence of MRONJ was observed in retrospective cohort studies than in other study designs. The two prospective studies revealed zero MRONJ cases. Both of these studies included strict protocols for pretherapy oral examination and preventive treatment, periodic maintenance, and emergency dental procedures during AA-AR treatment.[3,54] In contrast, a higher MRONJ prevalence was noted within retrospective studies in patients who underwent invasive dental procedures during or after AA-AR or AR only treatment, irrespective of pretherapy preventive oral treatment.[47,48,50] These findings highlight the importance of preventive and routine oral care as well as patient and dental professional awareness. Invasive dental procedures carried out during AR therapy may represent lack of access to oncologic and medication history, lack of awareness of half-life of drugs and concept of drug holiday, oncologist–dental professional communication issues, and patients' and dental professionals' salience bias.
While data from included retrospective cohort studies represent longer study durations than in prospective studies, the lack of clear documentation of patient follow-up data and differences in vigilance of recognition and reporting of MRONJ may represent an inaccurate estimate of its prevalence in either direction. Of note, all retrospective trial studies except for Aragon-Ching et al[51] revealed low prevalence estimates similar to prospective studies. MRONJ cases observed in Aragon-Ching et al[51] received a combination of bevacizumab, thalidomide, and zoledronic acid in addition to other first-line chemotherapy agents. While both bevacizumab and thalidomide represent AA activity, the etiologic correlation between thalidomide and MRONJ in the literature is not conclusive. No other studies in this review reported concurrent thalidomide administration for estimation of its effects.
Within this review, weighted prevalence of MRONJ with concurrent AA-AR drugs, ranging from 1% to 25%, appeared to be higher than AR drugs only (1–11%). However, similar variance in prevalence estimates for bisphosphonates-related osteonecrosis, ranging from 0.7% to 24.5%, based on differences in study designs, follow-up data and type of bisphosphonates was previously reported.[13]
Across all included studies, we noted a trend for increased relative risk of MRONJ with concurrent AA-AR drugs when compared with only AR or only AA drugs. However, the availability of high-quality comparable data between concurrent AA-AR drugs and AR drugs for risk ratio calculations was largely unsatisfactory. The included studies were undermined by bias in MRONJ measurement due to misclassification, lack of representativeness, examiner bias, and lack standardized criteria used for diagnosis of MRONJ.
For meta-analysis of risk ratios, studies with zero MRONJ events in both arms were excluded as dictated by standard practice.[64] These studies do not provide any direction of the risk of MRONJ between AA-AR in comparison to AA or AR drugs; therefore, any statistical correction of events would be ineffectual in a relative risk calculation. In contrast, studies with zero MRONJ events are relevant in calculation of proportions; therefore, a fixed continuity correction of 0.5 was added to such studies for meta-analysis of MRONJ prevalence. Exclusion of such studies from pooled prevalence calculations would result in an inflated estimate. Furthermore, due to a lack of patient-level exposure-to-event data, we could not calculate cumulative incidence estimates.
CONCLUSION
The overall weighted prevalence of MRONJ with concurrent AR and AA drugs was 6%. Estimates only from retrospective cohort studies revealed a pooled weighted prevalence of 13%. Variations in MRONJ prevalence were also noted based on pretherapy oral examination and preventive treatment delivery, with strict protocols resulting in no MRONJ cases.
Within limitations of methodological and statistical heterogeneity of included studies, a trend toward increased risk of MRONJ with concurrent AA and AR therapy compared with a single AR or AA drug was noted. High-quality, representative studies with larger sample sizes are needed for accurate estimation of relative risk of MRONJ with concurrent AA compared with AR therapy.
Supplemental Material
Supplemental data are available online with the article.
Supplementary Material
Funding Statement
Source of Support: The salary for Graciela M. Nogueras Gonzalez is partly supported by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Conflict of Interest: None.
References
- 1.Al-Husein B, Abdalla M, Trepte M, et al. Antiangiogenic therapy for cancer: an update. Pharmacotherapy . 2012;32:1095–111. doi: 10.1002/phar.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beuselinck B, Wolter P, Karadimou A, et al. Concomitant oral tyrosine kinase inhibitors and bisphosphonates in advanced renal cell carcinoma with bone metastases. Br J Cancer . 2012;107:1665–1671. doi: 10.1038/bjc.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broom RJ, Hinder V, Sharples K, et al. Everolimus and zoledronic acid in patients with renal cell carcinoma with bone metastases: a randomized first-line phase II trial. Clin Genitourin Cancer . 2015;13:50–58. doi: 10.1016/j.clgc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez RK, Wade SW, Reich A, et al. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer . 2018;18:44. doi: 10.1186/s12885-017-3922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Huang W, Zhou R, et al. Bisphosphonates in the treatment of patients with metastatic breast, lung, and prostate cancer: a meta-analysis. Medicine (Baltimore) . 2015;94:e2014. doi: 10.1097/MD.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peddi P, Lopez-Olivo MA, Pratt GF, Suarez-Almazor ME. Denosumab in patients with cancer and skeletal metastases: a systematic review and meta-analysis. Cancer Treat Rev . 2013;39:97–104. doi: 10.1016/j.ctrv.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Pu F, Shao Z. The skeletal-related events of denosumab versus zoledronic acid in patients with bone metastases: a meta-analysis of randomized controlled trials. J Bone Oncol . 2017;9:21–24. doi: 10.1016/j.jbo.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone . 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Gao SY, Zheng GS, Wang L, et al. Zoledronate suppressed angiogenesis and osteogenesis by inhibiting osteoclasts formation and secretion of PDGF-BB. PLoS One . 2017;12:e0179248. doi: 10.1371/journal.pone.0179248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guevarra CS, Borke JL, Stevens MR, et al. Vascular alterations in the sprague-dawley rat mandible during intravenous bisphosphonate therapy. J Oral Implantol . 2015;41:e24–e29. doi: 10.1563/AAID-JOI-D-13-00074. [DOI] [PubMed] [Google Scholar]
- 11.Misso G, Porru M, Stoppacciaro A, et al. Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid. Cancer Biol Ther . 2012;13:1491–500. doi: 10.4161/cbt.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg . 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Migliorati CA, Woo SB, Hewson I, et al. A systematic review of bisphosphonate osteonecrosis (BON) in cancer. Support Care Cancer . 2010;18:1099–1106. doi: 10.1007/s00520-010-0882-1. [DOI] [PubMed] [Google Scholar]
- 14.Rugani P, Walter C, Kirnbauer B, et al. Prevalence of medication-related osteonecrosis of the jaw in patients with breast cancer, prostate cancer, and multiple myeloma. Dent J (Basel) . 2016;4 doi: 10.3390/dj4040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol . 2009;27:5356–5362. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 16.Chen F, Pu F. Safety of denosumab versus zoledronic acid in patients with bone metastases: a meta-analysis of randomized controlled trials. Oncol Res Treat . 2016;39:453–459. doi: 10.1159/000447372. [DOI] [PubMed] [Google Scholar]
- 17.Qi WX, Tang LN, He AN, et al. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol . 2014;19:403–410. doi: 10.1007/s10147-013-0561-6. [DOI] [PubMed] [Google Scholar]
- 18.Boquete-Castro A, Gomez-Moreno G, Calvo-Guirado JL, Aguilar-Salvatierra A, Delgado-Ruiz RA. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin Oral Implants Res . 2016;27:367–375. doi: 10.1111/clr.12556. [DOI] [PubMed] [Google Scholar]
- 19.Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol . 2012;23:1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 20.Nicolatou-Galitis O, Kouri M, Papadopoulou E, et al. Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review. Support Care Cancer . 2019;27:383–394. doi: 10.1007/s00520-018-4501-x. [DOI] [PubMed] [Google Scholar]
- 21.Stone PW. Popping the (PICO) question in research and evidence-based practice. Appl Nurs Res . 2002;15:197–198. doi: 10.1053/apnr.2002.34181. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava A, Nogueras Gonzalez GM, Geng Y, et al. Prevalence of medication related osteonecrosis of the jaw in patients treated with sequential antiresorptive drugs: systematic review and meta-analysis. Support Care Cancer . 2021;29:2305–2317. doi: 10.1007/s00520-020-05882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan MT, Elting LS, Spijkervet FK. Systematic reviews of oral complications from cancer therapies, Oral Care Study Group, MASCC/ISOO: methodology and quality of the literature. Support Care Cancer . 2010;18:979–984. doi: 10.1007/s00520-010-0856-3. [DOI] [PubMed] [Google Scholar]
- 24.Ahn S, Becker BJ. Incorporating quality scores in meta-analysis. J Educ Behav Stat . 2011;36:555–585. [Google Scholar]
- 25.Greenland S, O'Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics . 2001;2:463–471. doi: 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- 26.Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J Oral Maxillofac Surg . 2009;67(5 Suppl):2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ . 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris R, Bradburn M, Deeks J, et al. Metan: fixed- and random-effects meta-analysis. Stata J . 2008;8:3–28. [Google Scholar]
- 29.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health . 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol . 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ . 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abel Mahedi Mohamed H, Nielsen CEN, Schiodt M. Medication related osteonecrosis of the jaws associated with targeted therapy as monotherapy and in combination with antiresorptives. A report of 7 cases from the Copenhagen Cohort. Oral Surg Oral Med Oral Pathol Oral Radiol . 2018;125:157–163. doi: 10.1016/j.oooo.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Fusco V, Porta C, Saia G, et al. Osteonecrosis of the jaw in patients with metastatic renal cell cancer treated with bisphosphonates and targeted agents: results of an Italian multicenter study and review of the literature. Clin Genitourin Cancer . 2015;13:287–294. doi: 10.1016/j.clgc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Lescaille G, Coudert AE, Baaroun V, et al. Clinical study evaluating the effect of bevacizumab on the severity of zoledronic acid-related osteonecrosis of the jaw in cancer patients. Bone . 2014;58:103–107. doi: 10.1016/j.bone.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Ngamphaiboon N, Frustino JL, Kossoff EB, et al. Osteonecrosis of the jaw: dental outcomes in metastatic breast cancer patients treated with bisphosphonates with/without bevacizumab. Clin Breast Cancer . 2011 Aug;11:252–257. doi: 10.1016/j.clbc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Van Poznak C, Reynolds EL, Estilo CL, et al. Osteonecrosis of the jaw risk factors in bisphosphonate-treated patients with metastatic cancer. Oral Dis . 2020. Published online December 4. [DOI] [PMC free article] [PubMed]
- 37.Fusco V, Cabras M, Erovigni F, et al. A multicenter observational study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in advanced cancer and myeloma patients of a cancer network in North-Western Italy. Med Oral Patol Oral Cir Bucal . 2021;26:e466–e473. doi: 10.4317/medoral.24318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margaix-Munoz M, Bagan J, Poveda-Roda R. Intravenous bisphosphonate-related osteonecrosis of the jaws: influence of coadjuvant antineoplastic treatment and study of buccodental condition. Med Oral Patol Oral Cir Bucal . 2013;18:e194–e200. doi: 10.4317/medoral.18604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ria R, Reale A, Moschetta M, et al. A retrospective study of skeletal and disease-free survival benefits of zoledronic acid therapy in patients with multiple myeloma treated with novel agents. Int J Clin Exp Med . 2013;6:30–38. [PMC free article] [PubMed] [Google Scholar]
- 40.Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med . 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 41.Coleman R, Finkelstein DM, Barrios C, et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol . 2020;21:60–72. doi: 10.1016/S1470-2045(19)30687-4. [DOI] [PubMed] [Google Scholar]
- 42.Gnant M, Pfeiler G, Steger GG, et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol . 2019;20:339–351. doi: 10.1016/S1470-2045(18)30862-3. [DOI] [PubMed] [Google Scholar]
- 43.Kemp APT, Ferreira VHC, Mobile RZ, et al. Risk factors for medication-related osteonecrosis of the jaw and salivary IL-6 IN cancer patients. Braz J Otorhinolaryngol . 2020. Published online October 25. 2020.09.010. [DOI] [PMC free article] [PubMed]
- 44.Okuma S, Matsuda Y, Nariai Y, et al. A retrospective observational study of risk factors for denosumab-related osteonecrosis of the jaw in patients with bone metastases from solid cancers. Cancers (Basel) . 2020;12 doi: 10.3390/cancers12051209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillot A, Joly C, Barthelemy P, et al. Denosumab in patients with bone metastases from renal-cell carcinoma treated with anti-angiogenic therapy: a retrospective study from the GETUG (Groupe Etude Des Tumeurs Uro Genitales) Clin Genitourin Cancer . 2016;27 doi: 10.1093/annonc/mdw373.56. [DOI] [Google Scholar]
- 46.Christodoulou C, Pervena A, Klouvas G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology . 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 47.Guillot A, Joly C, Barthelemy P, et al. Denosumab toxicity when combined with anti-angiogenic therapies on patients with metastatic renal cell carcinoma: a GETUG study. Clin Genitourin Cancer . 2017;17:e38–e43. doi: 10.1016/j.clgc.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Pilanci KN, Alco G, Ordu C, et al. Is administration of trastuzumab an independent risk factor for developing osteonecrosis of the jaw among metastatic breast cancer patients under zoledronic acid treatment? Medicine (Baltimore) . 2015;94:e671. doi: 10.1097/MD.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smidt-Hansen T, Folkmar TB, Fode K, et al. Combination of zoledronic Acid and targeted therapy is active but may induce osteonecrosis of the jaw in patients with metastatic renal cell carcinoma. J Oral Maxillofac Surg . 2013;71:1532–1540. doi: 10.1016/j.joms.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 50.van Cann T, Loyson T, Verbiest A, et al. Incidence of medication-related osteonecrosis of the jaw in patients treated with both bone resorption inhibitors and vascular endothelial growth factor receptor tyrosine kinase inhibitors. Support Care Cancer . 2018;26:869–878. doi: 10.1007/s00520-017-3903-5. [DOI] [PubMed] [Google Scholar]
- 51.Aragon-Ching JB, Ning YM, Chen CC, et al. Higher incidence of osteonecrosis of the jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Invest . 2009;27:221–226. doi: 10.1080/07357900802208608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guarneri V, Miles D, Robert N, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat . 2010;122:181–188. doi: 10.1007/s10549-010-0866-3. [DOI] [PubMed] [Google Scholar]
- 53.McKay RR, Lin X, Perkins JJ, et al. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur Urol . 2014;66:502–509. doi: 10.1016/j.eururo.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francini F, Pascucci A, Francini E, et al. Osteonecrosis of the jaw in patients with cancer who received zoledronic acid and bevacizumab. J Am Dent Assoc . 2011;142:506–513. doi: 10.14219/jada.archive.2011.0220. [DOI] [PubMed] [Google Scholar]
- 55.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol . 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 56.Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol . 2006;24:945–952. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 57.Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica . 2006;91:968–971. [PubMed] [Google Scholar]
- 58.Zervas K, Verrou E, Teleioudis Z, et al. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol . 2006;134:620–623. doi: 10.1111/j.1365-2141.2006.06230.x. [DOI] [PubMed] [Google Scholar]
- 59.Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol . 2008;44:857–869. doi: 10.1016/j.oraloncology.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 60.Cafro AM. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: definition and management of the risk related to zoledronic acid. Clin Lymphoma Myeloma . 2008;8:111–116. doi: 10.3816/clm.2008.n.013. [DOI] [PubMed] [Google Scholar]
- 61.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res . 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higuchi T, Soga Y, Muro M, et al. Replacing zoledronic acid with denosumab is a risk factor for developing osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol . 2018;125:547–551. doi: 10.1016/j.oooo.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist . 2008;13:911–920. doi: 10.1634/theoncologist.2008-0091. [DOI] [PubMed] [Google Scholar]
- 64.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.