Introduction
The World Health Organization (WHO) has been calling on countries to eliminate asbestos-related diseases (ARDs) by ceasing the consumption of asbestos.1 For public health policy, aggregate-level ecological studies can play an important role from a population perspective. In 2007, we reported clear and plausible ecological associations between deaths from mesothelioma and asbestosis and historical asbestos consumption.2 Since 2007, only a few countries have adopted asbestos bans. Many countries have continued to consume asbestos, and ARDs have continued to take a toll in a range of countries. More data have accumulated, including for countries that had not previously reported data. The present study aimed to assess if and how the associations between deaths from mesothelioma and asbestosis and historical asbestos consumption may have changed since 2007.
Methods
Statistical analyses, including log-transformation of national mortality rates to comply with the assumptions underlying the random errors in the regression model, were carried out as in our 2007 report,2 using R (version 4.1.2) (R Development Core Team). A single resulting visual outlier (Slovenia; unreliable asbestos consumption data) was removed. Number of deaths by sex and 5-y age category was tallied from the WHO mortality database3 following International Classification of Diseases and Related Health Problems, 10th Revision4 classifications as follows: pleural mesothelioma, ICD-10 code C45.0; peritoneal mesothelioma, ICD-10 code C45.1; all mesothelioma, all ICD-10 code C45 subcategories; and asbestosis, ICD-10 code J61. National population data from the United Nations5 and the WHO3 were prioritized for use, in that order. Yearly ARD deaths by sex and 5-y age category were age-standardized to the world standard population6 then averaged for 2010–2014. Raw asbestos consumptions for 1970, 1975, and 1980 were extracted from the U.S. Geological Survey7 and averaged. The R source code and RData are available online (https://github.com/VCCRI/ecological_study_asbestos_ARD_2022).
Results
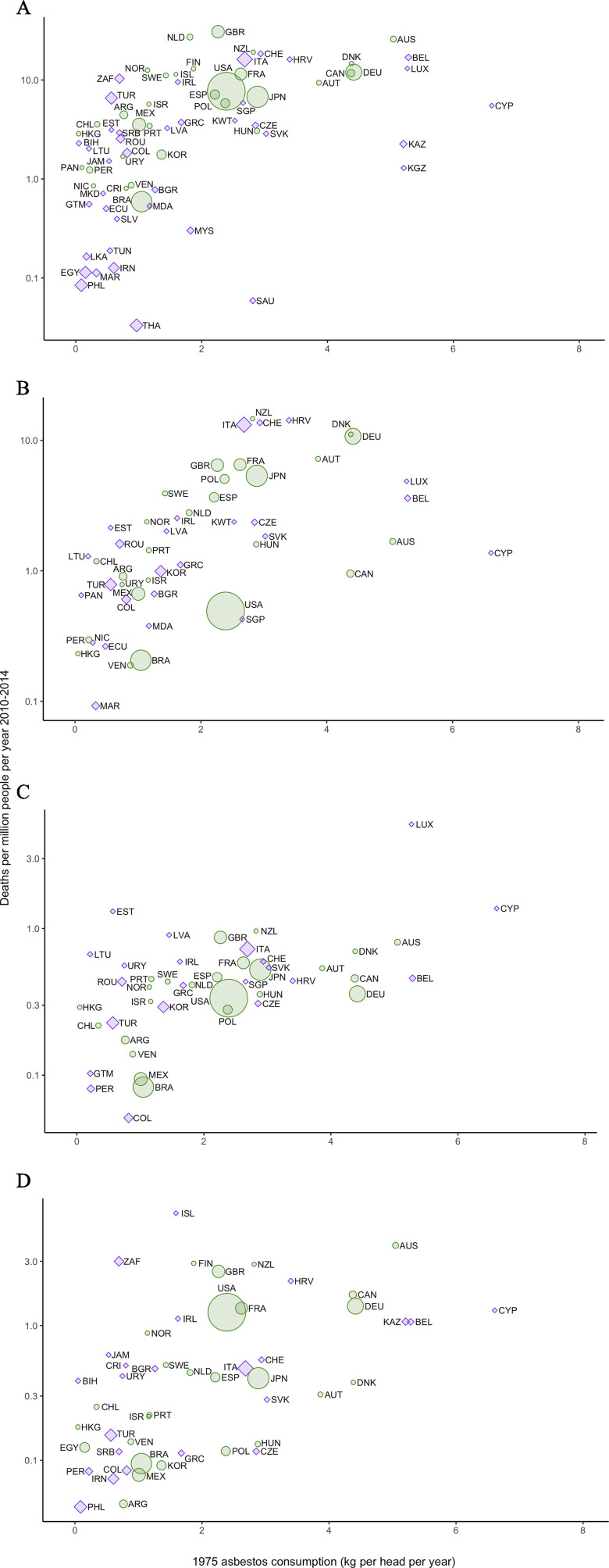
Table 1 shows sex-specific relationships between mortality rates of ARDs and per-capita historical asbestos consumption. The parameters were the intercept (, the expected mortality rate corresponding to zero asbestos consumption or the background mortality rate), and the slope (, the change in ARD mortality rate per unit change in asbestos consumption or the incremental change in mortality rate). Back-transformed values of and were included. All models showed positive slopes and small positive intercepts with variable statistical significances and values. For all mesothelioma, asbestos consumption was a highly significant positive predictor of mortality, with adjusted values of 0.39 () and 0.30 () for men and women, respectively. The slopes, , suggested that for an increment in asbestos consumption of per capita, men had a 2.4-fold [95% confidence interval (CI): 1.8, 3.0] increase and women had a 1.8-fold (95% CI: 1.4, 2.2) increase in deaths. The intercepts, , were small at 0.56 (95% CI: 0.31, 1.01) and 0.35 (95% CI: 0.22, 0.57) deaths per million population for men and women, respectively. Statistically significant positive associations were found for seven of the eight ARD–sex combinations, but not for asbestosis in women. Figure 1 shows the relationship as a scatter plot of countries for men. A positive linear association can clearly be seen between the updated mortality rates and historical asbestos consumption.
Table 1.
Regression analyses for age-adjusted mortality rates (for years 2010–2014) of asbestos-related diseases vs. historical asbestos consumption (in 1970–1979) and log-transformed values back-transformed to the background mortality rate and incremental change in mortality rate.
| Disease categories | Sex | Countries () | Regression parameters (log-transformed)a | Adjusted | -Value | Interpretable regression parameters (inverse log-transformed) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (95% CI) | SE | -Value | (95% CI) | SE | -Value | Background mortality rate (deaths per million) | Incremental change in mortality rate (deaths per million per kg asbestos consumption) | |||||||||
| rate | (95% CI) | rate | (95% CI) | |||||||||||||
| All mesothelioma | Male | 71 | (, 0.004) | 0.128 | 0.0540 | 0.372 | (0.261, 0.483) | 0.056 | 0.385 | 0.561 | (0.311, 1.010) | 2.354 | (1.823, 3.038) | |||
| Female | 69 | (, ) | 0.105 | 0.245 | (0.156, 0.335) | 0.045 | 0.299 | 0.350 | (0.216, 0.567) | 1.760 | (1.432, 2.162) | |||||
| Pleural mesothelioma | Male | 51 | (, ) | 0.139 | 0.0006 | 0.315 | (0.202, 0.429) | 0.057 | 0.376 | 0.312 | (0.164, 0.592) | 2.068 | (1.591, 2.687) | |||
| Female | 49 | (, ) | 0.134 | 0.221 | (0.110, 0.332) | 0.055 | 0.0002 | 0.238 | 0.0002 | 0.135 | (0.072, 0.252) | 1.663 | (1.288, 2.147) | |||
| Peritoneal mesothelioma | Male | 45 | (, ) | 0.081 | 0.167 | (0.101, 0.233) | 0.033 | 0.361 | 0.132 | (0.090, 0.193) | 1.468 | (1.261, 1.710) | ||||
| Female | 46 | (, ) | 0.066 | 0.099 | (0.045, 0.153) | 0.027 | 0.0006 | 0.219 | 0.0006 | 0.109 | (0.080, 0.149) | 1.256 | (1.109, 1.422) | |||
| Asbestosis | Male | 50 | (, ) | 0.112 | 0.295 | (0.201, 0.389) | 0.047 | 0.442 | 0.095 | (0.056, 0.159) | 1.972 | (1.588, 2.448) | ||||
| Female | 30 | (, ) | 0.111 | 0.041 | (, 0.131) | 0.044 | 0.3599 | b | 0.3599 | 0.033 | (0.020, 0.056) | 1.100 | (0.893, 1.351) | |||
Note: , intercept of regression line; , slope of regression line; SE, standard error.
Regression model: (age-adjusted mortality rate of asbestos-related diseases) (deaths per million population per year) = (kilograms per head per year).
Manual inspection of QQ-plots and histograms confirmed that the linear model is appropriate for all data subsets, including the one having negative adjusted .
Figure 1.
Ecological relationship between mortality rates in males for (A) all mesothelioma, (B) pleural mesothelioma, (C) peritoneal mesothelioma, and (D) asbestosis and historical asbestos consumption. Circles and diamonds are proportional to the size of the male population. Color and shape indicate whether countries were included (green circles) or not (purple diamonds) in the previous study.2 Country codes are ISO 3166.
Discussion
Compared with our 2007 study,2 the ecological associations remained consistent, although somewhat reduced for mesothelioma. The associations held when the data were expanded to more countries, including economically developing countries that had started to report ARDs since the initial study: Mortality rates increased exponentially relative to asbestos consumption and countries with little historical asbestos consumption experienced very low background levels of ARDs.
Ecological studies are often underappreciated, mainly due to the “ecological fallacy” of drawing causal inference at the individual level based on population-level associations.8 Although evidence on causality is abundant,9 the world is far from heeding the WHO call to “stop using all types of asbestos.”1 This is particularly true for the many economically developing countries. By providing a population-level perspective that cannot be conveyed by individual-level studies, ecological studies can impact policies by helping policymakers understand health-related phenomena in the context of country experiences.
Using asbestos consumption per capita as the independent variable was a limitation of our study given that we do not know whether and to what extent this indicator represents actual exposure. Other limitations included the lack of distinction between fiber type (e.g., amphiboles, chrysotile) and the preclusion of population attributes that may have confounded the association in question. In addition, lung cancer, an important ARD, was not analyzed because it warranted a separate analytical framework owing to its known causal relationship with smoking.
A strength of our study was the comparability of indicators, for which data were obtained from widely used databases3,7 to calculate age-adjusted mortality rates and year-specific consumption rates. The time frame of our study was advantageous because the global historical peak of asbestos consumption occurred in the 1970s,7 meaning that the 2010–2014 half-decade for mortality rates allowed a 37.5-year lag time to be analyzed in our model.
In the past decade, countries have been slow to adopt new asbestos bans,10 and the global population at risk remains high. Public health policy seeking the ultimate goal of an asbestos ban warrants reinforcement from various perspectives. Findings from our updated ecological analysis support the recommendation that eliminating asbestos consumption must be a priority in efforts to eliminate ARDs.
References
- 1.World Health Organization. 2007. Outline for the Development of National Programmes for Elimination of Asbestos-Related Diseases, https://www.who.int/publications/i/item/WHO-SDE-PHE-07-02 [accessed 24 May 2022].
- 2.Lin RT, Takahashi K, Karjalainen A, Hoshuyama T, Wilson D, Kameda T, et al. 2007. Ecological association between asbestos-related diseases and historical asbestos consumption: an international analysis. Lancet 369(9564):844–849, PMID: , 10.1016/S0140-6736(07)60412-7. [DOI] [PubMed] [Google Scholar]
- 3.WHO (World Health Organization). WHO Mortality Database. https://www.who.int/data/data-collection-tools/who-mortality-database [accessed 1 July 2021].
- 4.WHO. 2004. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision, 2nd ed. https://apps.who.int/iris/handle/10665/42980 [accessed 24 May 2022].
- 5.UN (United Nations). 2019. Population data. https://population.un.org/wpp/Download/Standard/Population/ [accessed 1 June 2021].
- 6.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. 2001. Table 4. WHO World Standard Population Distribution (%), based on world average population between 2000–2025. (Age Standardization of Rates: A New WHO Standard.) GPE Discussion Paper Series, EIP/GPE/EBD, World Health Organization. No. 31.
- 7.Virta RL. 2006. U.S. Geological Survey—Worldwide Asbestos Supply and Consumption Trends from 1900 through 2000. Open-File Report 03-83. 10.3133/ofr0383. [DOI]
- 8.Walter SD. 1991. The ecologic method in the study of environmental health. I. Overview of the method. Environ Health Perspect 94:61–65, PMID: , 10.1289/ehp.94-1567938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IARC (International Agency for Research on Cancer). 2012. Arsenic, Metals, Fibres, and Dusts. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 100C. Lyon, France: IARC. [PMC free article] [PubMed] [Google Scholar]
- 10.Arachi D, Soeberg M, Chimed-Ochir O, Lin RT, Takahashi K. 2021. Trend in the global incidence of mesothelioma: is there any changing trend after asbestos regulation and ban? In: Malignant Pleural Mesothelioma: Advances in Pathogenesis, Diagnosis, and Treatments. Nakano T, Kijima T, eds. Singapore: Springer Singapore, 3–13. [Google Scholar]