Abstract
Because of their medicinal characteristics, effectiveness, and importance, plant-derived flavonoids have been a possible subject of research for many years, particularly in the last decade. Plants contain a huge number of flavonoids, and Diosmin, a flavone glycoside, is one of them. Numerous in-vitro and in-vivo studies have validated Diosmin’s extensive range of biological capabilities which present antioxidative, antihyperglycemic, anti-inflammatory, antimutagenic, and antiulcer properties. We have presented this review work because of the greater biological properties and influences of Diosmin. We have provided a brief overview of Diosmin, its pharmacology, major biological properties, such as anti-cancer, anti-diabetic, antibacterial, anticardiovascular, liver protection, and neuroprotection, therapeutic approach, potential Diosmin targets, and pathways that are known to be associated with it.
Keywords: Diosmin, target pathways, anti-inflammatory, anti-oxidant, anti-cardiovascular, anti-diabetic, anti-cancer
1. Introduction
Diosmin is a flavonoid found in citrus fruits. Flavonoids are anti-inflammatory plant compounds that protect your body from free radicals and other unstable molecules. The most prevalent uses for Diosmin include hemorrhoids and leg sores caused by poor blood flow. It is also claimed to heal a variety of diseases, albeit there is no hard evidence to back up these claims [1,2,3]. Hesperidin is frequently used with Diosmin which is another plant chemical. Diosmin may work by reducing swelling and restoring normal vein function. It also appears to have antioxidative effects [1,4,5,6]. Diosmin was first found in 1925 in the wort plant and has since been used as a natural treatment for hemorrhoids, varicose veins, venous insufficiency, leg ulcers, and other circulatory issues [7]. It may help people with venous insufficiency, a condition in which blood flow is restricted, reduce inflammation, and restore normal blood flow [8,9,10,11].
Due to its beneficial effects in a number of major human organ systems, such as the cardiovascular system, Diosmin is now one of the most sought-after natural compounds in the treatment of a variety of human diseases, including chronic venous insufficiency (CVI), a progressive disorder affecting an increasing number of people (Figure 1). Diosmin’s effects in combination with other flavonoids, as well as its delivery routes, have also been investigated in details [4,12,13,14]. Diosmin was shown to have anti-oxidant characteristics, allowing it to successfully modulate the activities of a range of variables (including enzymes and biomarkers) connected to oxidative imbalance in a variety of illnesses. Induced apoptosis has been shown to have anti-cancer characteristics and activity in a variety of cell types. In MCF-7, MDA-MB-231, and SKBR-3 breast cancer cells, as well as DU145 prostate cancer cells, A431 skin cancer cells, and colon, oral, urinary bladder, and esophageal carcinogenesis, Diosmin has been found to trigger apoptosis [15,16,17,18]. Diosmin has also been found to have anti-diabetic properties. Diosmin has therapeutic effects in the event of diabetes and associated complications, such as neuropathy and dyslipidemia. Some evidence of a weak antimicrobial action was also found. A combination of Diosmin and hesperidin has been shown to be particularly effective in the treatment of chronic venous insufficiency and hemorrhoids. Diosmin is a great medical medication that can be used alone or in conjunction with other flavonoids [1,14,17].
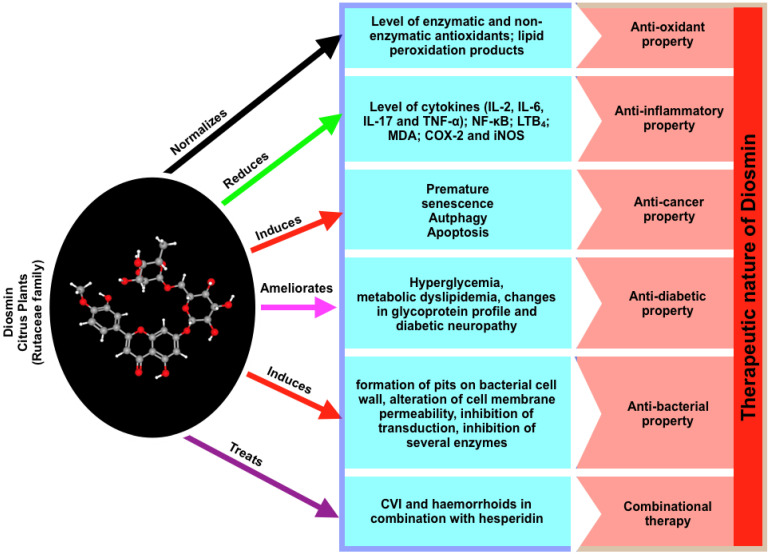
Figure 1.
A summary of Diosmin, the associated diseases and potential properties in terms of disease protection.
Intestinal microflora enzymes rapidly hydrolyze Diosmin into its aglycone form, diosmetin, which is easily absorbed and dispersed throughout the body, according to previous pharmacokinetic studies. In addition, Diosmin is broken down into phenolic acids or glycine-conjugated derivatives, which are excreted in the urine. The presence of break down products such as alkyl-phenolic acids demonstrated that the flavonoids followed a similar metabolic path to other flavonoids [12,18,19,20].
Diosmin’s anti-oxidant properties were discovered to have beneficial effects in a number of illnesses, where it successfully reduced the activities of several components associated to oxidative imbalance, such as enzymes/biomarkers. Induced apoptosis has been shown to have anti-cancer characteristics and activity in a variety of cell types [7,11,21]. In cancer cell lines, such as MCF-7, MDA-MB-231, SKBR-3; DU145; A431; colon, oral, urinary bladder, and esophageal carcinogenesis, Diosmin promotes apoptosis. Diosmin has also been found to have anti-diabetic properties. Diosmin has therapeutic effects in the event of diabetes and associated complications, such as neuropathy and dyslipidemia. Some evidence of a weak antimicrobial action was also found. A combination of Diosmin and hesperidin has been shown to be particularly effective in the treatment of chronic venous insufficiency and hemorrhoids. Diosmin is a great medical medication that can be used alone or in conjunction with other flavonoids [12,18,19,20].
Oxidative stress has been linked to the development of various diseases, including myocardial ischemia, cerebral ischemia–reperfusion injury, diabetes, neuronal cell injury, hypoxia, and cancer [16,22]. In rat models, Diosmin was found to exhibit anti-oxidant effects and to stimulate human neutrophils. Because of its anti-oxidant properties, Diosmin has been shown to offer a variety of therapeutic effects for illnesses characterized by oxidative stress. Many diseases, including arthritis, allergies, asthma, autoimmune diseases, atherosclerosis, diabetes, and cancer, are caused by inflammation. Inflammatory indicators, or biomarkers with elevated levels, can be used to detect inflammation [18,23]. The most common inflammatory markers include immune system cells, such as neutrophils, basophils, eosinophils, platelets, macrophages, and others, cell surface receptors and adhesion molecules such as selectins (L-selectin, P-selectin, and E-selectin), and soluble mediators such as cytokines (IL-1, IL-2, IL-6, TNF-α, TGF-ß, and IFN-γ) (complement factors, C-reactive protein and the coagulation factor fibrinogen) [8,10,15,18].
Recently published studies have shown that Diosmin has dose-dependent pro-apoptotic effects on a range of animal cancers, including breast, prostate, colon, oral, and urinary bladder tumors. Diosmin has been reported to trigger premature senescence in a variety of cancer cell types [23,24]. Other breast cancer cell lines, including MDA-MB-231 and SKBR-3, responded to Diosmin, but MCF-7 was found to be the most responsive. At lower doses, Diosmin produced G2/M cell cycle arrest, elevated p53, p21, and p27 levels, increased SA—gal activity, oxidative stress, and DNA damage in MCF-7 cells, all of which are associated with ageing. Apoptosis can be triggered by increased levels of nitric oxide, total ROS, total superoxide, mitochondrial production, and protein carbonylation. In the treatment of diabetes and its complications, Diosmin has been proven to have therapeutic effects. Diabetes mellitus is a chronic disease characterized by abnormal glucose, protein, and lipid metabolism due to a lack of or reduced insulin action. Diosmin possesses anti-hyperglycemic effects, according to numerous studies [13,14,17,22,25].
Plant-based bioactive components that are effective against fungus, yeasts, and bacteria, as well as insects, nematodes, and other plants, are known as phytochemicals. They can inhibit the synthesis of peptidoglycans, damage microbial membrane structures, alter the hydrophobicity of bacterial membrane surfaces, and interfere with quorum sensing (QS). The antibacterial activities of Diosmin were previously investigated by synthesizing silver nanoparticles from it (AgNPs). Flavonoids-rich diets aid in the improvement of cardiovascular parameters. Anti-platelet activity has been demonstrated for Diosmin. Overall binding in the heparin binding region, which includes helix A, D, and N-terminal residues, is expected to improve with Diosmin sulfation, potentially leading to the development of new anti-thrombin candidates. Diosmin’s anti-thrombotic properties are confirmed by changes in the protein composition of rats with venous thrombosis. Diosmin may stimulate endothelial cell growth by targeting the centrosome-associated protein 350 (CEP350) [5,13,26,27].
Hypoxia, angiogenesis, inflammation, and intrapulmonary vasodilation characterize hepatopulmonary syndrome (HPS), a severe consequence of hepatic cirrhosis. TNF/VEGF, IGF-1/PI3K/AKT, and FGF-1/ANG-2 signaling pathways may be involved in HPS development and can be restored by Diosmin therapy in a chronic bile duct ligation (CBDL)-induced rat model. In the central nervous system, many flavone derivatives have been found as potential GABAA receptor ligands, where they interact with the benzodiazepine binding site to generate depressive effects. Flavonoid glycosides cannot directly regulate the activity of the GABAA receptor. Diosmin contains sedative and sleep-inducing characteristics that have nothing to do with GABAA receptor regulation. 6-C-glycoside-diosmetin has been shown to have memory-enhancing and anxiolytic-like effects by activating the GABAA receptor, which is controversial [7,12,23].
2. Pharmacology of Diosmin
Flavonoids are phytochemicals that have been proven to have medicinal properties [28,29,30]. Diosmin, a flavone glycoside, is also found in hesperidin [9,12]. Daflon is an oral phlebotonic flavonoid combination based on Diosmin and Hesperidin that is widely used to treat and protect blood vessel disorders. After oral administration, Diosmin is converted to diosmetin, which is subsequently absorbed and esterified into glucuronide conjugates that are excreted in the urine [11,23,24,31]. Diosmin’s pharmacological properties have been investigated in a number of in vitro and in vivo studies, and it has been found to have anti-inflammatory, antioxidant, antidiabetic, antibacterial, antihyperlipidemic, and antifibrotic properties in a variety of disease models, as described in the previous section. In a number of clinical studies, Diosmin has also been proven to have a number of positive properties. According to toxicological investigations, Diosmin has a favorable safety profile. As a result, Diosmin has the potential to be an effective and safe treatment for a wide range of conditions. Diosmin, on the other hand, inhibits a variety of metabolic enzymes, encouraging clinical trials to look into its potential therapeutic efficacy and safety in a variety of illnesses while taking into consideration potential interactions. Diosmin is a venoactive drug that works on blood vessels in a variety of ways to improve circulation. It improves lymphatic drainage and microcirculation, as well as venous tone and suppleness. Diosmin is often used to improve vascular health in people with chronic venous disease for these reasons, and it has been demonstrated to improve quality of life. Diosmin also has antioxidant characteristics and scavenges oxygen free radicals, lowering oxidative stress levels that can be detected by biomarkers such as prostaglandin isoprostane precursors [2,11,12,31,32].
3. Critical Properties of Diosmin
3.1. Anti-Oxidant Property
Oxidative stress has been linked to the development of various diseases, including myocardial ischemia, cerebral ischemia–reperfusion injury, diabetes, neuronal cell injury, hypoxia, and cancer. In rat models, Diosmin was found to exhibit anti-oxidant effects and to stimulate human neutrophils. Because of its anti-oxidant properties, Diosmin has been shown to offer a variety of therapeutic effects for illnesses characterized by oxidative stress [22,33,34,35,36,37,38,39,40]. Diosmin was found to have significant anti-diabetic effects in diabetic rats fed streptozotocin nicotinamide (STZ-NA). When diabetic control rats were compared to normal control rats, anti-oxidant enzyme activities, such as glutathione-S-transferase (GST), glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT), as well as levels of low molecular weight antioxidants, such as vitamin C, vitamin E, and reduced glutathione (GSH), were found to be low, whereas lipid peroxidation Hyperglycemia with a significant drop in plasma insulin levels was also seen [7,11,27].
Oral administration of Diosmin enhanced the glycemic and anti-oxidant status of diabetic rats, according to previous studies. Diosmin treatment was also found to reduce lipid peroxidation [21,22,41]. Due to its anti-oxidant properties, Diosmin functions as an anti-hypertensive drug in deoxycorticosterone acetate (DOCA)-salt-induced hypertensive rats. Hypertension is characterized by an excessive production of reactive oxygen species. In DOCA-salt-treated rats, non-enzymatic and enzymatic antioxidants were found to be lower, while lipid peroxidation products (thiobarbituric acid reactive substances, lipid hydroperoxides, and conjugated dienes) were found to be significantly higher in blood plasma and tissues, such as the liver, kidney, heart, and aorta. Antioxidant levels were restored to near-normal levels, and lipid peroxidation products were reduced as a result of the Diosmin therapy. These findings were confirmed by histopathological examinations of the kidney and heart [1,11,31,42] Diosmin was found to have a hepatoprotective effect against ferrous sulfate-induced liver damage in adult male albino rats. Too much iron causes oxidative stress, reactive oxygen species (ROS), lipid peroxidation, inflammation, and tissue necrosis. Elevated ALT, AST, ALP, GGT, LDH activity, and bilirubin levels indicate hepatocyte membrane damage [21]. After Diosmin treatment, these values were considerably normalized. Because it maintains membrane integrity, decreases oxidative stress, and aids in the correction of dyslipidemia, Diosmin is a good hepatoprotective drug. The antioxidant and anti-inflammatory properties of Diosmin are primarily responsible for its hepatoprotective effects [11]. According to Senthamizhselvan et al., pre-treatment with Diosmin reduces oxidative stress in the rat heart after ischemia/reperfusion [43]. Ischemia–reperfusion injury occurs when ischemic tissue is reperfused, causing oxidative stress and cellular damage. The activities of enzyme antioxidants (SOD, CAT, and GPx) and GSH levels declined when hearts of control rats (no Diosmin pre-treatment) were subjected to an ischemia/reperfusion regimen, although the levels of lipid peroxidation products rose [44,45]. After 7 days of oral therapy with Diosmin (50 and 100 mg/kg), the activities of enzymatic antioxidants and GSH levels were found to be increased, while the levels of lipid peroxidation products were found to be reduced [6,43]. Ischemia–reperfusion injury produces edema and tissue destruction in the retina, resulting in vision loss. Diosmin was discovered to help male Wistar rats with retinal edema by maintaining the blood-retinal barrier and lowering vascular permeability. Diosmin’s ability to change the VEGF/PEDF ratio could explain its protective properties. The levels of malondialdehyde (MDA) and the activity of total-superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in retinal tissues that had been altered by ischemia–reperfusion injury, recovered to normal after Diosmin administration (Tong et al., 2013). Diosmin protects male CD-1 mice from brain ischemia–reperfusion injury by stimulating the JAK2/STAT3 signaling pathway [3,13]. In human polymorphonuclear neutrophils stimulated in vitro by phorbol myristate acetate, Cypriani et al. discovered that S5682 (Daflon 500 mg), a purified flavonoid fraction including Diosmin and hesperidin in a 9:1 ratio, has anti-oxidative effects (Cypriani et al., 1993). Diosmin’s free-radical scavenging activity is one of the reasons for its protection against myocardial infarction [42,46].
3.2. Anti-Inflammatory Property
Many diseases, including arthritis, allergies, asthma, autoimmune diseases, atherosclerosis, diabetes, and cancer, are caused by inflammation. Inflammation can be detected by measuring the levels of certain biomarkers known as inflammatory markers. Immune cells, such as neutrophils, basophils, eosinophils, platelets, macrophages, and others, cell surface receptors and adhesion molecules such as selectins (L-selectin, P-selectin, and E-selectin), and soluble mediators, such as cytokines (IL-1, IL-2, IL-6, TNF-α, TGF-ß, and IFN-γ), chemokines, and NF-kB (complement factors, C-reactive protein and the coagulation factor fibrinogen) [8,9,10,15,18].
Diosmin has been shown in multiple studies to lower these markers due to its anti-inflammatory effects. In lung damage produced by lipopolysaccharide (LPS) therapy, pro-inflammatory cytokines (IL-2, IL-6, IL-17, and TNF-α) and NF-kB were shown to be increased. After a few days of Diosmin pre-treatment, the levels of these indicators were dramatically lowered. In this study, male adult Balb/c mice were employed (Imam et al., 2015). In rat colitis caused by trinitrobenzenesulfonic acid, Diosmin was found to decrease the production of LTB4 (eicosanoid) and colonic MDA (TNBS). In the TNBS-treated colon, LTB4 improves neutrophil chemotaxis, adhesion, and degranulation, whereas MDA is a lipid peroxidation product (Crespo et al., 1999). According to Tahir et al., Diosmin reduces COX-2 and iNOS levels in chemically induced hepatocarcinogenesis (inflammatory markers). In female Wistar rats, diethylnitrosamine (DEN) was utilized to induce hepatocarcinogenesis, while 2-acetylaminofluorene was employed to promote it (2-AAF). In a long-term experiment, Diosmin was given orally at doses of 10 and 20 mg/kg b.wt for 9 weeks (Tahir et al., 2013). Diosmin treatment was found to alter the levels of tumor necrosis factor (TNF-α) and cyclooxygenase-2 in acetic acid-induced ulcerative colitis (COX-II). TNF-α and COX-II levels were lowered in a dose-dependent manner in Diosmin-treated rats [10].
3.3. Anti-Cancer Property
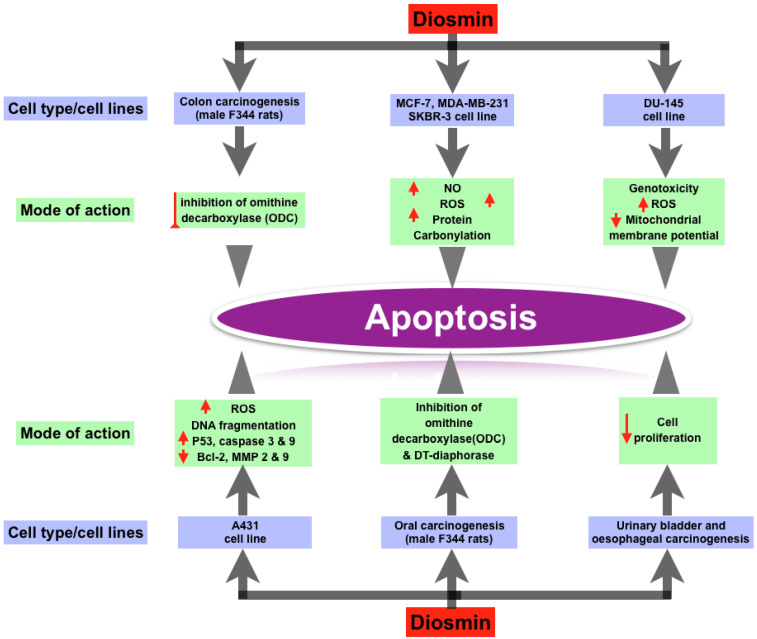
Recent study has discovered that Diosmin has dose-dependent pro-apoptotic effects on a range of animal cancers, including breast, prostate, colon, oral, and urinary bladder tumors. Diosmin causes premature senescence and apoptosis in MCF-7 cells at various doses. Other breast cancer cell lines, including MDA-MB-231 and SKBR-3, responded to Diosmin, but MCF-7 was found to be the most responsive. At lower doses, Diosmin produced G2/M cell cycle arrest, elevated p53, p21, and p27 levels, increased SA—gal activity, oxidative stress, and DNA damage in MCF-7 cells, all of which are associated with ageing. Apoptosis can be triggered by increased levels of nitric oxide, total ROS, total superoxide, mitochondrial production, and protein carbonylation. In research on the androgen-independent prostate cancer cell line DU145, Diosmin’s pro-apoptotic activity was confirmed. The genome and cytotoxicity of three flavonoid glycosides (Diosmin, naringin, and hesperidin) were studied in the DU145 prostate cancer cell line. The most genotoxicity was caused by Diosmin. In DU145 cells, these flavonoids caused oxidative stress or intracellular redox disequilibrium, leading to changes in mitochondrial membrane potential and apoptotic cell death. Diosmin dramatically boosted overall ROS production. Diosmin therapy also increased the amount of double stranded breaks in DNA and the formation of micronuclei (genotoxicity) [16]. Diosmin has anti-proliferative effect in human colon cancer cell lines, according to Kuntz et al. [29]. Tanaka et al. discovered a chemopreventive effect of Diosmin on colon carcinogenesis produced by azoxymethane in male F344 rats [19,47]. The treatment of Diosmin orally reduced colon carcinogenesis, as evidenced by lower rates of colon cancers. They hypothesized that inhibiting ornithine decarboxylase (ODC), a rate-limiting enzyme in polyamine production, was responsible for the decrease in colonic cancers [47]. Cell apoptosis is produced by DNA damage when ODC is inhibited [48]. When exposed to carcinogens, ODC levels have been reported to rise in numerous tissues. ODC activity was likewise elevated in the colonic mucosa of azoxymethane-treated rats. Diosmin has a dose-dependent cytotoxic capability for A431 skin cancer cells, according to Buddhan et al. It inhibits the invasive capacity of A431 cells by inducing apoptosis through a ROS-mediated mechanism [21,27]. DNA fragmentation, overexpression of p53, caspase 3 and caspase 9 genes, and downregulation of Bcl-2, matrix metalloproteinases-2 and 9 genes were all observed in A431 cells after Diosmin treatment. Diosmin’s IC50 value was discovered to be 45 g/mL, at which point it produced significant ROS [49]. Diosmin was found to prevent oral carcinogenesis produced by 4-nitroquinoline 1-oxide in male F344 rats (4-NQO). They theorized that Diosmin had anti-cancer capabilities via a number of mechanisms. One of these mechanisms was the inhibition of ornithine decarboxylase (ODC). Agents that inhibit ODC activity have been shown to be effective in slowing tumor growth. Another hypothesized mechanism is the inhibition of DT-diaphorase activity, which is required for 4-NQO to have its carcinogenic effect [19,47]. Inhibiting oral carcinogenesis, Diosmin is more efficient than diosmentin (the aglycone version of Diosmin) [50]. Oral treatment of 1000 ppm Diosmin suppressed urinary-bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl) nitrosamine in male ICR mice. These findings were supported by a count of silver-stained nucleolar-organizer-region-associated proteins (AgNORs) and a 5-bromodeoxyuridine (BUdR)-labeling index. Diosmin’s anti-carcinogenic effects may be due to a decrease in cell proliferation [13,51]. In male Wistar rats, Diosmin had a similar effect on esophageal carcinogenesis induced with N-methyl-N-amylnitrosamine (MNAN). Diosmin’s chemopreventive effects on several cancer kinds or cell lines are summarized in Figure 2.
Figure 2.
Effect of Diosmin on different types of cancer cell lines and their mode of actions.
Diosmin has also been shown to have anti-metastatic properties in metastatic lung melanoma (B16F10) [52]. Diosmin lowers the number of metastatic nodules, implant percentage, and invasion index in both micro- and macroscopic investigations. In the treatment of metastatic pulmonary melanoma, Diosmin acts in tandem with IFN-γ [53,54]. By disrupting the PI3K–Akt–MDM2 signaling pathway, Diosmin decreased HA22T cell growth (human hepatocellular cancer) in nude mice models and triggered G2/M cell cycle arrest through p53 activation [24,55].
3.4. Anti-Diabetic Property
In the treatment of diabetes and its complications, Diosmin has been proven to have therapeutic effects. Diabetes mellitus is a chronic disease characterized by abnormal glucose, protein, and lipid metabolism due to a lack of or decreased insulin activity. Diosmin possesses anti-hyperglycemic effects, according to numerous studies. According to Pari et al., Diosmin (in various doses) taken orally for 45 days can improve glycemic control. In the study, male albino Wistar strain rats were given streptozotocin-nicotinamide to develop diabetes (STZ-NA) [22,41]. The effect of Diosmin on plasma glucose levels was shown to be dose-dependent. In addition, oral Diosmin (100 mg/kg b.w.) treatment decreased glycosylated hemoglobin while raising hemoglobin and plasma insulin. Hexokinase and glucose-6-phosphate dehydrogenase, two essential liver enzymes, were also suppressed. In addition, there was a rise in body weight [22,41].
Diosmin has also been linked to improved lipid metabolism in people with diabetes. Hypercholesterolemia, lipid buildup in hepatic organs, and alterations in plasma lipid and lipoprotein profiles are all symptoms of metabolic dyslipidemia in people with type-2 diabetes [56,57]. Plasma lipids, tissue lipids (cholesterol, TGs, FFAs, and PLs), and plasma lipoproteins can all be efficiently normalized with Diosmin therapy (LDL, VLDL) [22,41]. Diosmin also corrects changes in glycoprotein profile caused by type-2 diabetes. When glucose is utilized by insulin-independent processes in diabetic people, glycoproteins build up. In STZ-NA-induced diabetic rats, the level of plasma glycoproteins increased dramatically. In diabetic rats’ liver and kidneys, hexose, hexosamine, and fucose levels were much greater, but sialic acid levels were significantly lower. Diosmin, when taken orally, has been shown to counteract these changes in glycoprotein profile [22,41].
Neuropathy is one of the most common diabetic consequences, affecting more than half of those who have the illness. Chronic untreated and uncontrolled hyperglycemia causes pain, tingling, and numbness in the periphery, as well as delayed nerve conduction. Hyperglycemia-induced oxidative stress results in the accumulation of polyols and advanced glycation end products, as well as impairment of (Na+/K+)-ATPase activity and endothelial function. Apoptosis is the death of neurons as a result of oxidative stress. Jain et al. employed streptozotocin and a high-fat diet to induce type-2 diabetes in male Sprague-Dawley rats to assess the effect of Diosmin on diabetic neuropathy. In rats, four weeks of treatment with Diosmin (50 and 100 mg/kg, p.o.) reduced the development of early diabetic neuropathy. It reduced oxidative stress by restoring GSH, NO, and SOD activity that had been changed [27,31,42,58]. Diosmin treatment resulted in considerable NF-kB normalization in alloxan-induced diabetic Wistar mice. NF-kB is important in the pathogenesis of diabetic neuropathy and other inflammatory illnesses [8]. In male Swiss mice, Diosmin was discovered to alleviate neuropathic pain produced by chronic constriction injury (CCI). Diosmin (1 or 10 mg/kg) was given intraperitoneally to reduce CCI-induced mechanical and thermal hyperalgesia. The role of the NO/cGMP/PKG/KATP channel signaling pathway was verified using inhibitors, such as L-NAME (an inhibitor of NOS), ODQ (an inhibitor of soluble guanylate cyclase), KT5823 (an inhibitor of PKG), or glibenclamide (an ATP-sensitive K+ channel blocker). Diosmin treatment also inhibited the production of cytokines (IL-1 and IL-33/St2) and reduced the activation of glial cells [5,31,59].
3.5. Anti-Bacterial Property
In bacteria, the majority of medications have developed resistance. Plants are being researched in the hope of discovering new and effective antibacterial agents. Plant-based bioactive components that are effective against fungus, yeasts, and bacteria, as well as insects, nematodes, and other plants, are known as phytochemicals. They can inhibit the synthesis of peptidoglycans, damage microbial membrane structures, alter the hydrophobicity of bacterial membrane surfaces, and interfere with quorum sensing (QS). By synthesizing silver nanoparticles of Diosmin, it has been shown the antibacterial properties (AgNPs) where they have tested its antimicrobial activity against Escherichia coli, Pseudomonas putida, and Staphylococcus aureus using the disc diffusion method. Diosmin produced hexagonal AgNPs that were slightly antimicrobial and had a size of roughly 5–40 nm. Diosmin inhibited E. coli, P. putida, and S. aureus with zones of inhibition of 6, 6, and 7 mm, respectively. Pits on the bacterial cell wall, changes in cell membrane permeability, obstruction of transduction, suppression of respiratory enzyme function due to free radical generation, and inactivation of various thiol-containing enzymes have all been proposed as antibacterial mechanisms [13,17,51].
Diosmin with amoxicillin-clavulanic acid (AMC) has been found to have mycobactericidal efficacy against Mycobacterium marinum. After treatment with a combination of AMC and Diosmin, the survival of M. marinum-infected Drosophila melanogaster fly models improved by 60%, providing in vitro proof. Its antibacterial activity was also proven against Mtb H37Ra and an MDR clinical isolate. The AMC-Diosmin combination was discovered to target L, D-transpeptidase (LDT) enzymes involved in Mtb cell wall synthesis, resulting in cellular leakage in M. marinum cells [14].
3.6. Cardiovascular Protection
Diets high in flavonoids help promote cardiovascular health. Diosmin has been shown to have anti-platelet action. Sulfation of Diosmin will very certainly improve overall binding at the heparin binding site, which contains helix A, D, and N-terminal residues, resulting in the development of novel anti-thrombin candidates. Diosmin’s anti-thrombotic properties are confirmed by changes in the protein composition of rats with venous thrombosis. Diosmin, which targets centrosome-associated protein 350, may increase endothelial cell proliferation (CEP350). In a rat model produced by the nitric oxide production inhibitor L-NAME, Diosmin’s antihypertensive effects are demonstrated [4,7,11,60,61,62,63,64]. Diosmin appears to protect rats from myocardial infarctions, hyaline arteriopathy, and fibrinoid necrosis caused by L-NAME. The elimination of superoxide anions by Diosmin could be the underlying mechanism for its anti-hypertensive actions. Diosmin has been shown to lower serum cardiac marker enzyme production, reduce plasma lipid peroxidation, and restrict lipid metabolism abnormalities in isoproterenol-induced myocardial-infarcted rats, resulting in anti-hyperlipidemia and cardio-protection. Isoproterenol has been reported to raise cardiac diagnostic markers, heart mitochondrial lipid peroxidation, and calcium ions while lowering anti-oxidant enzyme expression. Diosmin has been shown to be useful in preventing these traits. Left ventricular hypertrophy (LVH), ATPase dysfunction, and electrolyte imbalance all play a role in the etiology of isoproterenol-induced myocardial infarction. Pretreatment with Diosmin may attenuate the degenerative effects of isoproterenol in rats [4,5,7,11,31,65].
Reperfusion of ischemic tissues typically causes the generation of free radicals. Heart function recovery, anti-oxidant enzyme expression, and lipid peroxidation are all protected by Diosmin. In the presence of reperfusion stress, Diosmin successfully preserves TCA cycle enzyme activity. Diosmin reduces metabolic syndrome-related cardiovascular issues in rats, as demonstrated by improvements in systolic and diastolic blood pressure (BP) and ECG parameters. The anti-oxidant and anti-inflammatory effects of Diosmin in rats could explain these findings. Diosmetin protects mice from damage caused by ISO by upregulating AKT and NRF2 signaling while suppressing the NF-kB pathway. In venous smooth muscle action, Ca2+ is a key mediator. The contraction of the inferior vena cava (IVC) in normal Krebs and Ca2+-free Krebs has been shown to be unaffected by Diosmin. Diosmin, on the other hand, enhances the contractile response generated by KCl [23,24].
Sclerotherapy is a telangiectasia and varicose veins treatment that might cause irreversible endothelial damage. An exaggerated inflammatory response is usually induced during the sclerotherapy technique. MPFF has been shown to be useful in lowering inflammatory stress during sclerotherapy. By increasing venular diameter, preserving functional capillary density, reducing the number of leaky sites, and binding leukocytes, MPFF reduces post-sclerotherapy inflammation in a microvascular network. MPFF has been shown to lower the production of metalloproteinase-2 (MMP-2) and MMP-9 in rats while boosting MDA, which helps with varicose vein therapy. Linfadren, a combination of Diosmin, coumarin, and arbutin, has been shown in a randomized controlled trial to treat chronic hand edema in patients with post-trauma/surgery. Linfadren has been demonstrated to assist patients with breast cancer-related lymphedema when used in conjunction with extensive decongestive therapy [1,21,42,66].
Primary reflux from primary valve incompetence and venous thrombosis induces chronic venous illness, which includes pain, edema, skin damage, and ulceration. Two possible explanations are venous hypertension and microcirculation problems. Inflammation is both the beginning and the end of primary valve incompetence, as well as venous hypertension. Daflon 500 mg relieves clinical symptoms by reducing inflammatory reactions not only in the microcirculation but also in the vein wall and valve cusps. According to randomized trials, Daflon 500 mg for 60 days of therapy is also effective in elastic compression and speeding up the healing process in venous ulcers. A meta-analysis of Daflon 500 mg’s effects on venous leg ulcers found that it can be a valuable addition to standard therapy in big and long-standing ulcers. In randomized, double-blind, controlled trials, the therapeutic efficacy of Daflon 500 mg on chronic venous disease symptoms and edema was also studied. Diosmin may significantly reduce angiogenesis and inflammation, as evidenced by downregulation of TNF-α, IL-6, VEGF-C, VEGF-A, and FGF2 expression and overexpression of angiostatin expression in individuals with chronic venous issues. After six months of Daflon treatment, however, air plethysmography revealed no changes in venous hemodynamics. By magnifying adrenergic impact on microcirculation, Diosmin may cause adverse effects by raising creatine phosphokinase and serum lactic dehydrogenase levels [4,67,68,69].
Finally, Diosmin may protect against myocardial infarctions, hyaline arteriopathy, and fibrinoid necrosis caused by L-NAME by reducing serum cardiac marker enzyme production, plasma lipid peroxidation, and lipid metabolism alterations. By correcting isoproterenol-induced LVH, ATPase failure, and electrolyte imbalance, it improves metabolic syndrome-related cardiovascular issues. MPFF decreases MMP-2 and MMP-9 expression while increasing MDA, improving venular diameter, maintaining functional capillary density, reducing leaky sites, and sticking leukocytes. Inflammatory responses in the microcirculation, vein wall, and valve cusps are reduced with Daflon 500 mg. Daflon 500 mg has also been shown to be effective in the treatment of chronic venous disease symptoms and edema. TNF, IL-6, VEGF-C, VEGF-A, and FGF2 expression were all downregulated, whilst angiostatin expression was upregulated, showing that Diosmin reduces angiogenesis and inflammation in chronic venous disease patients. As evidenced by increases in SOD, CAT, GSH, and NO, as well as a decrease in MDA, Diosmin maintains redox equilibrium and downregulates the NF-B signaling pathway. Diosmin also lowers kidney weight, pH, total protein, calcium, and phosphorus in the urine, as well as potassium, sodium, magnesium, creatinine, and uric acid in the blood.
3.7. Liver Protection
Hypoxia, angiogenesis, inflammation, and intrapulmonary vasodilation characterize hepatopulmonary syndrome (HPS), a severe consequence of hepatic cirrhosis. TNF/VEGF, IGF-1/PI3K/AKT, and FGF-1/ANG-2 signaling pathways may be involved in HPS development and can be restored by Diosmin therapy in a chronic bile duct ligation (CBDL)-induced rat model. According to another study, Diosmin decreases BDL-induced liver abnormalities through modifying the Keap-1/NRF2 and p38-MAPK/NF-B/iNOS signaling pathways. Diosmin, pentoxifylline, and their combination have been shown to reduce BDL-induced liver cirrhosis via modulating the Keap-1/Nrf-2/GSH and NF-kB/p65/p38-MAPK signaling pathways. Diosmin also stimulates the production of cytoglobin, which contributes to the compound’s anti-oxidant, anti-inflammatory, and anti-fibrotic properties. Diosmin promotes the NRF2/Keap-1 pathway while suppressing the ROS-induced p38 MAPK pathways and activating the eNOS gene. By binding to the ARE sequence and upregulating the production of target genes, such as HO-1 and SOD, active NRF2 may enter the nucleus to promote transcriptional activity. The activity of the NF-kB, p53, and iNOS signaling pathways may be boosted when the p38 MAPK is triggered [6,7,13,27,41].
3.8. Neuroprotection
Diosmin performs a variety of actions that are linked to neuroprotection. Diosmin is a sedative that induces sleep and decreases the expression of IL-1, TNF, and IL-33/St2, as well as activating glial cells. It lowers mechanical and thermal hyperalgesia by activating D2, GABAA, and opioid receptors but not 5-HT1A receptors, reduces neuropathic pain by activating the NO/cGMP/PKG/KATP pathway, and suppresses IL-1, TNF-a, and other inflammatory mediators. Diosmin has been associated to thermal hyperalgesia, cold allodynia, and walking dysfunctions, as well as oxidative stress. It also helps with scopolamine-induced neural plasticity disruption and cognitive deficits by inhibiting TNF expression. Diosmin has an IC50 of 12.24 0.54 g mL and interacts with AChE enzyme via Tyr72, Tyr124, Trp286, Phe295, and Tyr341. Many flavone derivatives have been discovered as possible GABAA receptor ligands in the central nervous system, where they interact with the benzodiazepine binding site to produce depressive effects. The activity of the GABAA receptor cannot be controlled directly by flavonoid glycosides. Diosmin has sedative and sleep-inducing properties that are unrelated to GABAA receptor modulation. 6-C-glycoside-diosmetin activation of the GABAA receptor has been shown to have memory-enhancing and anxiolytic-like effects, which are controversial. As a result, more research into flavone derivatives’ interactions with the GABAA receptor is required. A range of harmful compounds in the peripheral and central nerve systems induces neuropathic pain. Diosmin coupled with hesperidin effectively reduces mechanical or thermal hyperalgesia in the chronic constriction injury (CCI) rat model. D2, GABAA, and opioid receptor antagonists may prevent these effects, while the 5-HT1A receptor inhibitor does not. In a CCI rat model, Diosmin alone has been shown to have anti-hyperalgesic effects via D2 and opioid receptors, as well as a reduction in pro-inflammatory cytokine (IL-1, TNF, and IL-6) expression [5,7].
Diosmin was found to reduce CCI-induced neuropathic pain by activating the NO/cGMP/PKG/KATP channel signaling pathway, lowering spinal cord cytokine (IL-1, TNF, and IL-33/St2), and activating glial cells in a separate investigation. Thermal hyperalgesia, cold allodynia, and walking dysfunctions, as well as oxidative stress, may be caused by STZ-induced diabetes in rats. The diabetes abnormalities in the brain system can be successfully corrected with Diosmin. Furthermore, diosmetin inhibits nociception in mice via antagonizing the transient receptor potential vanilloid 1 (TRPV1) receptor [12].
Misfolded proteins have been linked to neurodegenerative disorders. Diosmin was discovered to bind to hen egg white lysozyme (HEWL) in a sheet-shape, in an in vitro research, minimizing HEWL aggregation. These may play a role in treating amyloid-related illnesses. The synthesis and aggregation of amyloid-(A) peptide aids the degenerative course of Alzheimer’s disease (AD). Inhibiting GSK-3 using Diosmin and its aglycone derivative diosmetin has been demonstrated to reduce brain soluble A and A oligomer formation, as well as tau hyperphosphorylation, and improve cognitive impairment in rats. Diosmin may aid to ameliorate scopolamine-induced synaptic plasticity disruption and cognitive impairments by reducing the expression of the pro-inflammatory cytokine TNF in the rat hippocampus. Traumatic brain injury (TBI) can cause mental and cognitive disability. Diosmin inhibits the pro-inflammatory cytokine TNF-α, protecting against TBI-induced declines in neurological scores, memory, and long-term potentiation (Yizhou Zheng, 2020a).
Diosmin appears to protect PC12 cells from LPS-induced TNF production and apoptosis, as seen by decreased DNA fragmentation, decreased Bad and caspase-3 expression, and enhanced Bcl-2 expression. Diosmin interacts with critical residues Tyr72, Tyr124, Trp286, Phe295, and Tyr341 in the acetylcholinesterase (AChE) enzyme and has an IC50 value of 12.24 0.54 g mL−1. This has a similar binding orientation to Donepezil. Diosmin’s inhibitory impact on the AChE enzyme has been confirmed in silico and in vitro experiments. In an ELISA experiment using the Ellman method, Diosmin had no effect on the activity of AChE and butyrylcholinesterase (BChE).
3.9. Additional Roles
During ischemia–reperfusion injury, Diosmin protects against retinal edema by maintaining tight junction integrity and lowering capillary permeability [37,41,56]. With an IC50 value of 24 M, Diosmin suppresses AR activity. Diosmin reduces phosphorylation levels of JNK and p38-MAPK, reducing oxidative stress and apoptosis. Diosmin mediates fast current low-voltage-activated K+ channels, voltage-independent K+ channels, and the nitric oxide pathway, acting as a myorelaxant. Diosmin’s anti-oxidative, anti-inflammatory, and anti-apoptotic activities reduce MTX-induced histological changes and restore tissue architecture. By enhancing anti-oxidant enzyme expression and lowering inflammatory cytokine production, Diosmin protects against alcohol-induced abnormalities. Diosmin protects against changes in antioxidant expression, body and organ weight, and histopathology caused by cadmium.
4. Potential Diosmin Target Proteins and the Pathways
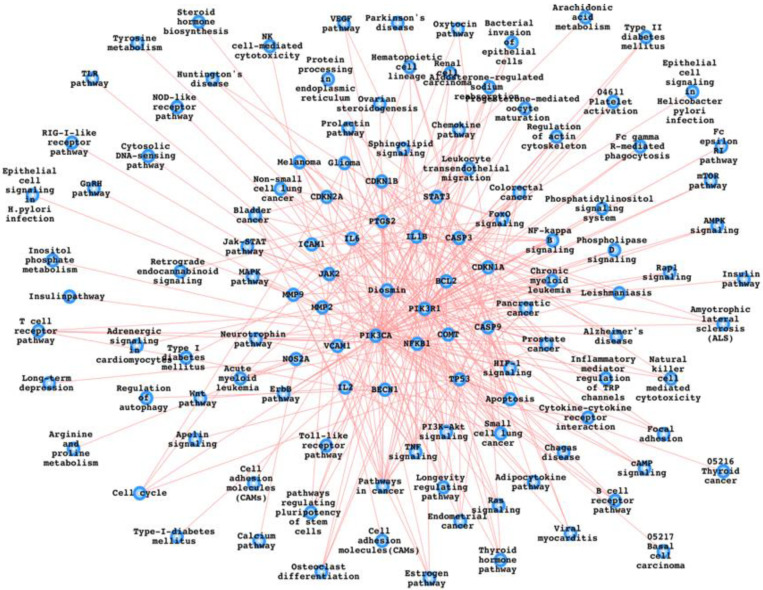
This article has gone over the proteins and pathways that have been identified as possible Diosmin targets. GSH and CAT are increased, while ROS, MDA, and lipid peroxidation are decreased. Diosmin is known to reduce the inflammatory proteins NF-kB, TNF-α, IL-1B, COX2, iNOS, and MAPKs. Diosmin controls the up (B-endorphin and glucose uptake) and down (gluconeogenesis and insulin resistance) of many diabetes-related pathways/pathway components [6,13,14,21]. Diosmin has been demonstrated to reduce proliferation, viability, and activate autophagy, all of which are known to play a role in various types of cancers. Platelet activity suppression, blood pressure, reperfusion stress, MMP-2/9, angiogenesis, and diagnostic markers are only a few of the cardiovascular parameters that Diosmin influences. Other pathways and their components linked to liver protection, neuroprotection, and antimicrobials include Keap-1, NRF2, p38-MAPK, iNOS, I/R-induced damage, EOF, RN4220, pUL5054, Ltd.Mt1, and Ltd.Mt2 [12,13,17,70,71]. Furthermore, we have mapped out the Diosmin target proteins and the respective pathways associated with them as shown in Figure 3. Furthermore, we have also presented a list of pathways which might be affected as a result of Diosmin intake (Table 1).
Figure 3.
Diosmin-associated proteins and the biological pathways. We have used FunCoup database for PPI (protein–protein interactions) and integrated it with the KEGG pathways list, and the same has been mentioned in the main text also and the same has been done for Table 1 [72,73,74,75,76,77,78,79].
Table 1.
Diosmin and its potential interactors followed by their pathways.
| Diosmin | NOS2A | Arginine_and_proline_metabolism |
| Diosmin | NOS2A | Calcium pathway |
| Diosmin | NOS2A | Long-term_depression |
| Diosmin | NOS2A | Small_cell_lung_cancer |
| Diosmin | PTGS2 | Arachidonic_acid_metabolism |
| Diosmin | PTGS2 | VEGF pathway |
| Diosmin | PTGS2 | Leishmaniasis |
| Diosmin | PTGS2 | Pathways_in_cancer |
| Diosmin | PTGS2 | Small_cell_lung_cancer |
| Diosmin | MMP2 | Leukocyte_transendothelial_migration |
| Diosmin | MMP2 | GnRH pathway |
| Diosmin | MMP2 | Pathways_in_cancer |
| Diosmin | MMP2 | Bladder_cancer |
| Diosmin | ICAM1 | Cell_adhesion_molecules_(CAMs) |
| Diosmin | ICAM1 | Natural_killer_cell_mediated_cytotoxicity |
| Diosmin | ICAM1 | Leukocyte_transendothelial_migration |
| Diosmin | ICAM1 | Viral_myocarditis |
| Diosmin | COMT | Steroid_hormone_biosynthesis |
| Diosmin | COMT | Tyrosine_metabolism |
| Diosmin | JAK2 | Chemokine pathway |
| Diosmin | JAK2 | Jak-STAT pathway |
| Diosmin | JAK2 | Adipocytokine pathway |
| Diosmin | JAK2 | Leishmaniasis |
| Diosmin | MMP9 | Leukocyte_transendothelial_migration |
| Diosmin | MMP9 | Pathways_in_cancer |
| Diosmin | MMP9 | Bladder_cancer |
| Diosmin | NFKB1 | MAPK pathway |
| Diosmin | NFKB1 | Chemokine pathway |
| Diosmin | NFKB1 | Apoptosis |
| Diosmin | NFKB1 | Toll-like_receptor pathway |
| Diosmin | NFKB1 | NOD-like_receptor pathway |
| Diosmin | NFKB1 | RIG-I-like_receptor pathway |
| Diosmin | NFKB1 | Cytosolic_DNA-sensing_pathway |
| Diosmin | NFKB1 | T_cell_receptorpathway |
| Diosmin | NFKB1 | B_cell_receptorpathway |
| Diosmin | NFKB1 | Neurotrophin pathway |
| Diosmin | NFKB1 | Adipocytokine pathway |
| Diosmin | NFKB1 | Epithelial_cell_signaling_in_Helicobacter_pylori_infection |
| Diosmin | NFKB1 | Leishmaniasis |
| Diosmin | NFKB1 | Chagas_disease |
| Diosmin | NFKB1 | Pathways_in_cancer |
| Diosmin | NFKB1 | Pancreatic_cancer |
| Diosmin | NFKB1 | Prostate_cancer |
| Diosmin | NFKB1 | Chronic_myeloid_leukemia |
| Diosmin | NFKB1 | Acute_myeloid_leukemia |
| Diosmin | NFKB1 | Small_cell_lung_cancer |
| Diosmin | IL2 | Cytokine-cytokine_receptor_interaction |
| Diosmin | IL2 | T_cell_receptor pathway |
| Diosmin | IL2 | Type_I_diabetes_mellitus |
| Diosmin | CDKN1B | ErbB pathway |
| Diosmin | CDKN1B | Cell_cycle |
| Diosmin | CDKN1B | Pathways_in_cancer |
| Diosmin | CDKN1B | Prostate_cancer |
| Diosmin | CDKN1B | Chronic_myeloid_leukemia |
| Diosmin | CDKN1B | Small_cell_lung_cancer |
| Diosmin | PIK3CA | Inositol_phosphate_metabolism |
| Diosmin | PIK3CA | ErbB pathway |
| Diosmin | PIK3CA | Chemokine pathway |
| Diosmin | PIK3CA | Phosphatidylinositol_signaling_system |
| Diosmin | PIK3CA | mTOR pathway |
| Diosmin | PIK3CA | Apoptosis |
| Diosmin | PIK3CA | Focal_adhesion |
| Diosmin | PIK3CA | Toll-like_receptor pathway |
| Diosmin | PIK3CA | Jak-STAT pathway |
| Diosmin | PIK3CA | Natural_killer_cell_mediated_cytotoxicity |
| Diosmin | PIK3CA | T_cell_receptor pathway |
| Diosmin | PIK3CA | B_cell_receptor pathway |
| Diosmin | PIK3CA | Fc_epsilon_RI pathway |
| Diosmin | PIK3CA | Fc_gamma_R-mediated_phagocytosis |
| Diosmin | PIK3CA | Leukocyte_transendothelial_migration |
| Diosmin | PIK3CA | Neurotrophin pathway |
| Diosmin | PIK3CA | Regulation_of_actin_cytoskeleton |
| Diosmin | PIK3CA | Insulinpathway |
| Diosmin | PIK3CA | Progesterone-mediated_oocyte_maturation |
| Diosmin | PIK3CA | Type_II_diabetes_mellitus |
| Diosmin | PIK3CA | Aldosterone-regulated_sodium_reabsorption |
| Diosmin | PIK3CA | Bacterial_invasion_of_epithelial_cells |
| Diosmin | PIK3CA | Chagas_disease |
| Diosmin | PIK3CA | Pathways_in_cancer |
| Diosmin | PIK3CA | Colorectal_cancer |
| Diosmin | PIK3CA | Renal_cell_carcinoma |
| Diosmin | PIK3CA | Pancreatic_cancer |
| Diosmin | PIK3CA | Endometrial_cancer |
| Diosmin | PIK3CA | Glioma |
| Diosmin | PIK3CA | Prostate_cancer |
| Diosmin | PIK3CA | Melanoma |
| Diosmin | PIK3CA | Chronic_myeloid_leukemia |
| Diosmin | PIK3CA | Acute_myeloid_leukemia |
| Diosmin | PIK3CA | Small_cell_lung_cancer |
| Diosmin | PIK3CA | Non-small_cell_lung_cancer |
| Diosmin | CDKN1A | ErbB pathway |
| Diosmin | CDKN1A | Cell_cycle |
| Diosmin | CDKN1A | Pathways_in_cancer |
| Diosmin | CDKN1A | Glioma |
| Diosmin | CDKN1A | Prostate_cancer |
| Diosmin | CDKN1A | Melanoma |
| Diosmin | CDKN1A | Bladder_cancer |
| Diosmin | CDKN1A | Chronic_myeloid_leukemia |
| Diosmin | IL1B | MAPK pathway |
| Diosmin | IL1B | Cytokine-cytokine_receptor_interaction |
| Diosmin | IL1B | Apoptosis |
| Diosmin | IL1B | Toll-like_receptor pathway |
| Diosmin | IL1B | Hematopoietic_cell_lineage |
| Diosmin | IL1B | Type_I_diabetes_mellitus |
| Diosmin | IL1B | Alzheimer’s_disease |
| Diosmin | BECN1 | Regulation_of_autophagy |
| Diosmin | CASP9 | Apoptosis |
| Diosmin | CASP9 | VEGF pathway |
| Diosmin | CASP9 | Alzheimer’s_disease |
| Diosmin | CASP9 | Parkinson’s_disease |
| Diosmin | CASP9 | Amyotrophic_lateral_sclerosis_(ALS) |
| Diosmin | CASP9 | Huntington’s_disease |
| Diosmin | CASP9 | Pathways_in_cancer |
| Diosmin | CASP9 | Colorectal_cancer |
| Diosmin | CASP9 | Pancreatic_cancer |
| Diosmin | CASP9 | Endometrial_cancer |
| Diosmin | CASP9 | Prostate_cancer |
| Diosmin | CASP9 | Small_cell_lung_cancer |
| Diosmin | CASP9 | Non-small_cell_lung_cancer |
| Diosmin | CASP9 | Viral_myocarditis |
| Diosmin | IL6 | Cytokine-cytokine_receptor_interaction |
| Diosmin | IL6 | Toll-like_receptor pathway |
| Diosmin | IL6 | Hematopoietic_cell_lineage |
| Diosmin | IL6 | Pathways_in_cancer |
| Diosmin | TP53 | MAPK pathway |
| Diosmin | TP53 | Cell_cycle |
| Diosmin | TP53 | Apoptosis |
| Diosmin | TP53 | Wnt pathway |
| Diosmin | TP53 | Neurotrophin pathway |
| Diosmin | TP53 | Pathways_in_cancer |
| Diosmin | TP53 | Colorectal_cancer |
| Diosmin | TP53 | Pancreatic_cancer |
| Diosmin | TP53 | Endometrial_cancer |
| Diosmin | TP53 | Glioma |
| Diosmin | TP53 | Prostate_cancer |
| Diosmin | TP53 | 05216_Thyroid_cancer |
| Diosmin | TP53 | 05217_Basal_cell_carcinoma |
| Diosmin | TP53 | Melanoma |
| Diosmin | TP53 | Bladder_cancer |
| Diosmin | TP53 | Chronic_myeloid_leukemia |
| Diosmin | TP53 | Small_cell_lung_cancer |
| Diosmin | TP53 | Non-small_cell_lung_cancer |
| Diosmin | PIK3R1 | ErbB pathway |
| Diosmin | PIK3R1 | Chemokine pathway |
| Diosmin | PIK3R1 | Phosphatidylinositol_signaling_system |
| Diosmin | PIK3R1 | mTOR pathway |
| Diosmin | PIK3R1 | Apoptosis |
| Diosmin | PIK3R1 | Focal_adhesion |
| Diosmin | PIK3R1 | Toll-like_receptor pathway |
| Diosmin | PIK3R1 | Jak-STATpathway |
| Diosmin | PIK3R1 | Natural_killer_cell_mediated_cytotoxicity |
| Diosmin | PIK3R1 | T_cell_receptor pathway |
| Diosmin | PIK3R1 | B_cell_receptor pathway |
| Diosmin | PIK3R1 | Fc_epsilon_RI pathway |
| Diosmin | PIK3R1 | Fc_gamma_R-mediated_phagocytosis |
| Diosmin | PIK3R1 | Leukocyte_transendothelial_migration |
| Diosmin | PIK3R1 | Neurotrophinpathway |
| Diosmin | PIK3R1 | Regulation_of_actin_cytoskeleton |
| Diosmin | PIK3R1 | Insulinpathway |
| Diosmin | PIK3R1 | Progesterone-mediated_oocyte_maturation |
| Diosmin | PIK3R1 | Type_II_diabetes_mellitus |
| Diosmin | PIK3R1 | Aldosterone-regulated_sodium_reabsorption |
| Diosmin | PIK3R1 | Bacterial_invasion_of_epithelial_cells |
| Diosmin | PIK3R1 | Chagas_disease |
| Diosmin | PIK3R1 | Pathways_in_cancer |
| Diosmin | PIK3R1 | Colorectal_cancer |
| Diosmin | PIK3R1 | Renal_cell_carcinoma |
| Diosmin | PIK3R1 | Pancreatic_cancer |
| Diosmin | PIK3R1 | Endometrial_cancer |
| Diosmin | PIK3R1 | Glioma |
| Diosmin | PIK3R1 | Prostate_cancer |
| Diosmin | PIK3R1 | Melanoma |
| Diosmin | PIK3R1 | Chronic_myeloid_leukemia |
| Diosmin | PIK3R1 | Acute_myeloid_leukemia |
| Diosmin | PIK3R1 | Small_cell_lung_cancer |
| Diosmin | PIK3R1 | Non-small_cell_lung_cancer |
| Diosmin | CDKN2A | Cell_cycle |
| Diosmin | CDKN2A | Pathways_in_cancer |
| Diosmin | CDKN2A | Pancreatic_cancer |
| Diosmin | CDKN2A | Glioma |
| Diosmin | CDKN2A | Melanoma |
| Diosmin | CDKN2A | Bladder_cancer |
| Diosmin | CDKN2A | Chronic_myeloid_leukemia |
| Diosmin | CDKN2A | Non-small_cell_lung_cancer |
| Diosmin | VCAM1 | Cell_adhesion_molecules_(CAMs) |
| Diosmin | VCAM1 | Leukocyte_transendothelial_migration |
| Diosmin | CASP3 | MAPK pathway |
| Diosmin | CASP3 | Apoptosis |
| Diosmin | CASP3 | Natural_killer_cell_mediated_cytotoxicity |
| Diosmin | CASP3 | Alzheimer’s_disease |
| Diosmin | CASP3 | Parkinson’s_disease |
| Diosmin | CASP3 | Amyotrophic_lateral_sclerosis_(ALS) |
| Diosmin | CASP3 | Huntington’s_disease |
| Diosmin | CASP3 | Epithelial_cell_signaling_in_Helicobacter_pylori_infection |
| Diosmin | CASP3 | Pathways_in_cancer |
| Diosmin | CASP3 | Colorectal_cancer |
| Diosmin | CASP3 | Viral_myocarditis |
| Diosmin | STAT3 | Chemokine pathway |
| Diosmin | STAT3 | Jak-STAT pathway |
| Diosmin | STAT3 | Adipocytokine pathway |
| Diosmin | STAT3 | Pathways_in_cancer |
| Diosmin | STAT3 | Pancreatic_cancer |
| Diosmin | STAT3 | Acute_myeloid_leukemia |
| Diosmin | BCL2 | Apoptosis |
| Diosmin | BCL2 | Focal_adhesion |
| Diosmin | BCL2 | Amyotrophic_lateral_sclerosis_(ALS) |
| Diosmin | BCL2 | Pathways_in_cancer |
| Diosmin | BCL2 | Colorectal_cancer |
| Diosmin | BCL2 | Prostate_cancer |
| Diosmin | BCL2 | Small_cell_lung_cancer |
| Diosmin | BCL2 | Protein_processing_in_endoplasmic_reticulum |
| Diosmin | NFKB1 | Ras_signaling |
| Diosmin | PIK3CA | Ras_signaling |
| Diosmin | PIK3R1 | Ras_signaling |
| Diosmin | PIK3CA | Rap1_signaling |
| Diosmin | PIK3R1 | Rap1_signaling |
| Diosmin | BECN1 | Apelin_signaling |
| Diosmin | ICAM1 | NF-kappa_B_signaling |
| Diosmin | IL1B | NF-kappa_B_signaling |
| Diosmin | NFKB1 | NF-kappa_B_signaling |
| Diosmin | PTGS2 | NF-kappa_B_signaling |
| Diosmin | BCL2 | NF-kappa_B_signaling |
| Diosmin | VCAM1 | NF-kappa_B_signaling |
| Diosmin | ICAM1 | TNF_signaling |
| Diosmin | IL1B | TNF_signaling |
| Diosmin | IL6 | TNF_signaling |
| Diosmin | MMP9 | TNF_signaling |
| Diosmin | NFKB1 | TNF_signaling |
| Diosmin | PIK3CA | TNF_signaling |
| Diosmin | PIK3R1 | TNF_signaling |
| Diosmin | PTGS2 | TNF_signaling |
| Diosmin | VCAM1 | TNF_signaling |
| Diosmin | CASP3 | TNF_signaling |
| Diosmin | CDKN1A | HIF-1_signaling |
| Diosmin | CDKN1B | HIF-1_signaling |
| Diosmin | IL6 | HIF-1_signaling |
| Diosmin | NFKB1 | HIF-1_signaling |
| Diosmin | PIK3CA | HIF-1_signaling |
| Diosmin | PIK3R1 | HIF-1_signaling |
| Diosmin | BCL2 | HIF-1_signaling |
| Diosmin | STAT3 | HIF-1_signaling |
| Diosmin | CDKN1A | FoxO_signaling |
| Diosmin | CDKN1B | FoxO_signaling |
| Diosmin | IL6 | FoxO_signaling |
| Diosmin | PIK3CA | FoxO_signaling |
| Diosmin | PIK3R1 | FoxO_signaling |
| Diosmin | STAT3 | FoxO_signaling |
| Diosmin | PIK3CA | Phospholipase_D_signaling |
| Diosmin | PIK3R1 | Phospholipase_D_signaling |
| Diosmin | NFKB1 | Sphingolipid_signaling |
| Diosmin | PIK3CA | Sphingolipid_signaling |
| Diosmin | PIK3R1 | Sphingolipid_signaling |
| Diosmin | BCL2 | Sphingolipid_signaling |
| Diosmin | TP53 | Sphingolipid_signaling |
| Diosmin | NFKB1 | cAMP_signaling |
| Diosmin | PIK3CA | cAMP_signaling |
| Diosmin | PIK3R1 | cAMP_signaling |
| Diosmin | CDKN1A | PI3K-Akt_signaling |
| Diosmin | CDKN1B | PI3K-Akt_signaling |
| Diosmin | IL2 | PI3K-Akt_signaling |
| Diosmin | IL6 | PI3K-Akt_signaling |
| Diosmin | JAK2 | PI3K-Akt_signaling |
| Diosmin | NFKB1 | PI3K-Akt_signaling |
| Diosmin | BCL2 | PI3K-Akt_signaling |
| Diosmin | TP53 | PI3K-Akt_signaling |
| Diosmin | CASP9 | PI3K-Akt_signaling |
| Diosmin | PIK3CA | AMPK_signaling |
| Diosmin | PIK3R1 | AMPK_signaling |
| Diosmin | JAK2 | pathways_regulating_pluripotency_of_stem_cells |
| Diosmin | PIK3CA | pathways_regulating_pluripotency_of_stem_cells |
| Diosmin | PIK3R1 | pathways_regulating_pluripotency_of_stem_cells |
| Diosmin | STAT3 | pathways_regulating_pluripotency_of_stem_cells |
| Diosmin | PTGS2 | Retrograde_endocannabinoid_signaling |
| Diosmin | IL1B | Inflammatory_mediator_regulation_of_TRP_channels |
| Diosmin | PIK3CA | Inflammatory_mediator_regulation_of_TRP_channels |
| Diosmin | PIK3R1 | Inflammatory_mediator_regulation_of_TRP_channels |
| Diosmin | IL1B | Osteoclast_differentiation |
| Diosmin | NFKB1 | Osteoclast_differentiation |
| Diosmin | PIK3CA | Osteoclast_differentiation |
| Diosmin | PIK3R1 | Osteoclast_differentiation |
| Diosmin | NFKB1 | Longevity_regulating_pathway |
| Diosmin | PIK3CA | Longevity_regulating_pathway |
| Diosmin | PIK3R1 | Longevity_regulating_pathway |
| Diosmin | TP53 | Longevity_regulating_pathway |
| Diosmin | IL1B | Hematopoietic_cell_lineage |
| Diosmin | IL6 | Hematopoietic_cell_lineage |
| Diosmin | PIK3CA | 04611_Platelet_activation |
| Diosmin | PIK3R1 | 04611_Platelet_activation |
| Diosmin | PTGS2 | Ovarian_steroidogenesis |
| Diosmin | MMP2 | Estrogen pathway |
| Diosmin | MMP9 | Estrogen pathway |
| Diosmin | PIK3CA | Estrogen pathway |
| Diosmin | PIK3R1 | Estrogen pathway |
| Diosmin | JAK2 | Prolactin pathway |
| Diosmin | NFKB1 | Prolactin pathway |
| Diosmin | PIK3CA | Prolactin pathway |
| Diosmin | PIK3R1 | Prolactin pathway |
| Diosmin | STAT3 | Prolactin pathway |
| Diosmin | CDKN1A | Oxytocin pathway |
| Diosmin | PTGS2 | Oxytocin pathway |
| Diosmin | PIK3CA | Thyroid_hormone pathway |
| Diosmin | PIK3R1 | Thyroid_hormone pathway |
| Diosmin | TP53 | Thyroid_hormone pathway |
| Diosmin | CASP9 | Thyroid_hormone pathway |
| Diosmin | BCL2 | Adrenergic_signaling_in_cardiomyocytes |
To prepare this pathways list, we have first used SwissTargetPrediction (http://www.swisstargetprediction.ch/predict.php) (accessed on 21 December 2021) to generate the proteins which could bind with Diosmin and then mapped out the KEGG pathways from the KEGG database (https://www.genome.jp/kegg/pathway.html) (accessed on 21 December 2021) for these Diosmin interactors (proteins). Table 1 contains the overall pathways and the respective proteins while similar information is presented in Figure 3 as a network of Diosmin, its interactors, and the pathways.
5. Combinational Therapy, Side Effects, and the Administrative Route
Diosmin is a venoactive drug administered orally for the treatment of chronic venous insufficiency (CVI). In CVI, veins have trouble sending blood from limbs back to the heart as a result of which blood gets pooled in the veins of legs. Reflux of the venous valves is the most common cause of CVI [80,81,82]. Edema and symptoms of chronic venous disease (CVD), particularly so-called venous pain, are treated with venoactive medicines (VAD). The usefulness of VAD is contested on a regular basis, despite the fact that it is well documented. Our goal was to gather all randomized controlled trials (RCTs) and meta-analyses devoted to VAD and symptoms in CVD, present them to a panel of international CVD experts, and have them vote by secret ballot on the level of efficacy of each drug, using EBM (Evidence-Based Medicine) rules and critical analysis [82,83,84]. Diosmin in combination with hesperidin (Daflon 500 mg) has been found more effective than Diosmin alone on venous symptoms [4,82]. The most common symptoms of CVI include leg ache, sensation of heaviness or tension, nocturnal cramps, sensation of swelling, restless legs, and itching. Burning, heaviness, weakness, and functional pain are reduced after two months of Daflon treatment. It also enhances the skin microcirculation’s blood velocity. Daflon is not considered a cure for CVI because it does not address the underlying cause of the condition; rather, it is used to alleviate the symptoms of the disease. Diosmin and Micronized Purified Flavonoid Fraction (MPFF) have been found to affect venous tone, lymphatic drainage, and microcirculation in CVI patients. They improve venous tone by blocking COMT (catechol-O-methyltransferase) from breaking down norepinephrine (noradrenaline) and so prolonging noradrenergic action. They enhance the number of functional lymphatics, lymphatic flow, capillary hematocrit, and red cell velocity while decreasing lymphatic channel width and intra-lymphatic pressure. They also prevent leukocyte adherence, intra-tissue movement, and the release of leukocyte (L-selectin) and endothelial (ICAM-1, VCAM-1) adhesion molecules, which protect microvascular permeability (Inflammatory mediators) [21,66].
Daflon treatment for four weeks (four tablets per day, in two divided doses) has also shown improvement in the symptoms associated with hemorrhoids (pain, heaviness, bleeding, pruritus and anal discharge) [85,86,87]. However, purified Diosmin has also been shown to reduce pain and bleeding. Daflon has also been reported to exhibit anti-oxidative property. Combination of Diosmin with amoxicillin-clavulanic acid (AMC) has been found to possess mycobactericidal activity against Mycobacterium Marinum [14,20,88].
6. Future Perspectives
Diosmin is an anti-oxidant, anti-cancer, anti-diabetic, and mild anti-bacterial flavone glycoside derived from citrus trees. Because of its characteristics, it is a good therapeutic treatment for a variety of disorders. It reduces oxidative stress by altering the activity of particular enzymes and promotes apoptosis in a variety of cancer cell lines via several ways. Its anti-inflammatory properties are due to its ability to lower the levels of numerous inflammation markers. It also helps alleviate the consequences of diabetes, such as neuropathy and dyslipidemia. It has been found to be quite useful in the treatment of chronic venous insufficiency and hemorrhoids when combined with other flavonoids, particularly hesperidin. Treatment with Diosmin appears to be promising in the treatment of many malignancies, diabetes, and disorders linked to oxidative stress and inflammation. Diosmin is an anti-hyperglycemic drug that also helps with the issues that come with it. Its ability to modulate the VEGF/PEDF ratio could be investigated further to determine if it is a pro- or anti-angiogenic factor. Its combination with hesperidin has been proven to be extremely helpful in the treatment of CVI and hemorrhoids, making it a good example of drug synergism. Its interaction with other flavonoids or phytochemicals could be investigated in the future using various methodologies, including system-level knowledge [77,89,90,91,92]. Furthermore, it could also be explored by generating, analyzing, exploring, and integrating large-scale datasets to understand the potentials of Diosmin in case of human diseases [72,73,74,76,77,78,79,90,93,94,95,96,97,98,99,100].
7. Conclusions
We have presented a review work that summarizes Diosmin’s pharmacology, significant biological features, such as anti-oxidant, anti-inflammatory, anti-cancer, anti-diabetic, antibacterial, anti-cardiovascular, liver protection, and neuroprotection, therapeutic strategy, possible Diosmin targets, and pathways connected with Diosmin (Figure 1) due to the diverse and potential biological and pharmacological properties and influences of Diosmin (Figure 1). Finally, we have summarized our review work where Diosmin actively plays critical roles in controlling well-known signaling components and the pathways. In this table, we clearly see that there are a number of proteins and these proteins are dominantly associated with inflammatory processes, cancer-associated pathways, antidiabetic, antioxidant, and antibacterial properties.
Acknowledgments
We are thankful to K.A.U., K.I. and J.M.I. and for providing us the resources and the facility to carry out the work.
Author Contributions
Conceptualization, E.H. and M.M.; methodology, E.H. and M.M.; software, E.H. and M.M.; validation, E.H. and M.M.; formal analysis, E.H. and M.M.; investigation, E.H. and M.M.; resources, E.H. and M.M.; data curation, E.H. and M.M.; writing—original draft preparation, E.H. and M.M.; writing—review and editing, E.H. and M.M.; visualization, E.H. and M.M.; supervision, E.H. and M.M.; project administration, E.H. and M.M.; funding acquisition, E.H. and M.M.; funding acquisition, E.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bogucka-Kocka A., Woźniak M., Feldo M., Kockic J., Szewczyk K. Diosmin—Isolation techniques, determination in plant material and pharmaceutical formulations, and clinical use. Nat. Prod. Commun. 2013;8:545–550. doi: 10.1177/1934578X1300800435. [DOI] [PubMed] [Google Scholar]
- 2.Gerges S.H., Wahdan S.A., Elsherbiny D.A., El-Demerdash E. Pharmacology of Diosmin, a Citrus Flavone Glycoside: An Updated Review. Eur. J. Drug Metab. Pharmacokinet. 2021;47:1–18. doi: 10.1007/s13318-021-00731-y. [DOI] [PubMed] [Google Scholar]
- 3.Liu W.Y., Liou S.-S., Hong T.-Y., Liu I.-M. The Benefits of the Citrus Flavonoid Diosmin on Human Retinal Pigment Epithelial Cells under High-Glucose Conditions. Molecules. 2017;22:2251. doi: 10.3390/molecules22122251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldo M., Wójciak-Kosior M., Sowa I., Kocki J., Bogucki J., Zubilewicz T., Kęsik J., Bogucka-Kocka A. Effect of Diosmin Administration in Patients with Chronic Venous Disorders on Selected Factors Affecting Angiogenesis. Molecules. 2019;24:3316. doi: 10.3390/molecules24183316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fattori V., Rasquel-Oliveira F.S., Artero N.A., Ferraz C.R., Borghi S.M., Casagrande R., Verri W.A. Diosmin Treats Lipopolysaccharide-Induced Inflammatory Pain and Peritonitis by Blocking NF-κB Activation in Mice. J. Nat. Prod. 2020;83:1018–1026. doi: 10.1021/acs.jnatprod.9b00887. [DOI] [PubMed] [Google Scholar]
- 6.Imam F., Al-Harbi N.O., Al-Harbi M.M., Ansari M.A., Zoheir K.M.A., Iqbal M., Anwer M.K., Hoshani A.A.R., Attia S.M., Ahmad S.F. Pharmacological Research. Pharmacol. Res. 2015;102:1–11. doi: 10.1016/j.phrs.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Liu Q., Yang J., Yao H., Fan R., Cao C., Liu C., Zhang S., Lei X., Xu S. The proteomic profiling of multiple tissue damage in chickens for a selenium deficiency biomarker discovery. Food Funct. 2020;11:1312–1321. doi: 10.1039/C9FO02861G. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed S., Mundhe N., Borgohain M., Chowdhury L., Kwatra M., Bolshette N., Ahmed A., Lahkar M. Diosmin Modulates the NF-kB Signal Transduction Pathways and Downregulation of Various Oxidative Stress Markers in Alloxan-Induced Diabetic Nephropathy. Inflammation. 2016;39:1783–1797. doi: 10.1007/s10753-016-0413-4. [DOI] [PubMed] [Google Scholar]
- 9.Crespo M.E., Gálvez J., Cruz T., Ocete M.A., Zarzuelo A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999;65:651–653. doi: 10.1055/s-2006-960838. [DOI] [PubMed] [Google Scholar]
- 10.Shalkami A.S., Hassan M., Bakr A.G. Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 2017;37:78–86. doi: 10.1177/0960327117694075. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Reheim M.A., Messiha B.A.S., Abo-Saif A.A. Hepatoprotective Effect of Diosmin on Iron-induced Liver Damage. Int. J. Pharmacol. 2017;13:529–540. doi: 10.3923/ijp.2017.529.540. [DOI] [Google Scholar]
- 12.Carballo-Villalobos A.I., González-Trujano M.-E., Pellicer F., López-Muñoz F.J. Antihyperalgesic Effect of Hesperidin Improves with Diosmin in Experimental Neuropathic Pain. BioMed Res. Int. 2016;2016:8263463. doi: 10.1155/2016/8263463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilit A.C., Kose E.O., Imir N.G., Aydemir E. Anticancer and antimicrobial activities of diosmin. Genet. Mol. Res. 2021;20:GMR18752. doi: 10.4238/gmr18752. [DOI] [Google Scholar]
- 14.Pushkaran A.C., Vinod V., Vanuopadath M., Nair S.S., Nair S.V., Vasudevan A.K., Biswas R., Mohan C.G. Combination of Repurposed Drug Diosmin with Amoxicillin-Clavulanic acid Causes Synergistic Inhibition of Mycobacterial Growth. Sci. Rep. 2019;9:6800. doi: 10.1038/s41598-019-43201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali N., AlAsmari A.F., Imam F., Ahmed M.Z., Alqahtani F., Alharbi M., AlSwayyed M., AlAsmari F., Alasmari M., Alshammari A., et al. Protective effect of diosmin against doxorubicin-induced nephrotoxicity. Saudi J. Biol. Sci. 2021;28:4375–4383. doi: 10.1016/j.sjbs.2021.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewinska A., Adamczyk-Grochala J., Kwasniewicz E., Deregowska A., Wnuk M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017;265:117–130. doi: 10.1016/j.toxlet.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Patel K., Gadewar M., Tahilyani V., Patel D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013;19:792–800. doi: 10.1007/s11655-013-1595-3. [DOI] [PubMed] [Google Scholar]
- 18.Yao X., Gu X., Jin S., Shi K., Gao X., Wang Q., Zhao J., Zhang H., Lai X. Anticancer and Anti-inflammatory Effect of Diosmin against Dalton Ascitic Lymphoma Induced Leukemia. J. Oleo Sci. 2021;70:665–673. doi: 10.5650/jos.ess21022. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka T., Makita H., Kawabata K., Mori H., Kakumoto M., Satoh K., Hara A., Sumida T., Fukutani K., Ogawa H. Modulation of N-methyl-N-amylnitrosamine-induced rat oesophageal tumourigenesis by dietary feeding of diosmin and hesperidin, both alone and in combination. Carcinogenesis. 1997;18:761–769. doi: 10.1093/carcin/18.4.761. [DOI] [PubMed] [Google Scholar]
- 20.Corsale I., Carrieri P., Martellucci J., Piccolomini A., Verre L., Rigutini M., Panicucci S. Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I–III degree hemorroidal disease: A double-blind multicenter prospective comparative study. Int. J. Colorectal Dis. 2018;33:1595–1600. doi: 10.1007/s00384-018-3102-y. [DOI] [PubMed] [Google Scholar]
- 21.Perumal S. Effect of diosmin on apoptotic signaling molecules in N- nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Mol. Cell. Biochem. 2018;449:27–37. doi: 10.1007/s11010-018-3339-3. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan S., Pari L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem.-Biol. Interact. 2012;195:43–51. doi: 10.1016/j.cbi.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Soares J.M., Faria B.M.D., Ascari L.M., Souza J.M.D., Soares A.G., Cordeiro Y., Romão L.F. Diosmin induces caspase-dependent apoptosis in human glioblastoma cells. An. Acad. Bras. Ciências. 2019;91:e20191031. doi: 10.1590/0001-3765201920191031. [DOI] [PubMed] [Google Scholar]
- 24.Dung T., Lin C.-H., Việt Bình T., Hsu H.-H., Su C.-C., Lin Y.-M., Tsai C.-H., Tsai F.-J., Kuo W.-W., Chen L.-M., et al. Diosmin induces cell apoptosis through protein phosphatase 2A activation in HA22T human hepatocellular carcinoma cells and blocks tumour growth in xenografted nude mice. Food Chem. 2012;132:2065–2073. doi: 10.1016/j.foodchem.2011.11.149. [DOI] [Google Scholar]
- 25.Dubey K., Dubey R., Gupta R., Gupta A. Exploration of Diosmin to Control Diabetes and Its Complications-an In Vitro and In Silico Approach. Curr. Comput. Aided-Drug Des. 2021;17:307–313. doi: 10.2174/1573409916666200324135734. [DOI] [PubMed] [Google Scholar]
- 26.Li T., Zhu W., Liu G., Fang C., Quan S. Diosmin for the prevention of ovarian hyperstimulation syndrome. Int. J. Gynecol. Obstet. 2020;149:166–170. doi: 10.1002/ijgo.13100. [DOI] [PubMed] [Google Scholar]
- 27.Eraslan G., Sarıca Z.S., Bayram L.Ç., Tekeli M.Y., Kanbur M., Karabacak M. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ. Sci. Pollut. Res. Int. 2017;24:27931–27941. doi: 10.1007/s11356-017-0232-7. [DOI] [PubMed] [Google Scholar]
- 28.Caltagirone S., Rossi C., Poggi A., Ranelletti F.O., Natali P.G., Brunetti M., Aiello F.B., Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::AID-IJC21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Kuntz S., Wenzel U., Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S., Pandey A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozdağ M., Eraslan G. The effect of diosmin against lead exposure in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020;393:639–649. doi: 10.1007/s00210-019-01758-4. [DOI] [PubMed] [Google Scholar]
- 32.Waring M.J., Arrowsmith J., Leach A.R., Leeson P.D., Mandrell S., Owen R.M., Pairaudeau G., Pennie W.D., Pickett S.D., Wang J., et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Publ. Group. 2015;14:475–486. doi: 10.1038/nrd4609. [DOI] [PubMed] [Google Scholar]
- 33.Chikara S., Nagaprashantha L.D., Singhal J., Horne D., Awasthi S., Singhal S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–134. doi: 10.1016/j.canlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Pfeffer P.E., Lu H., Mann E.H., Chen Y.-H., Ho T.-R., Cousins D.J., Corrigan C., Kelly F.J., Mudway I.S., Hawrylowicz C.M. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS ONE. 2018;13:e0200040. doi: 10.1371/journal.pone.0200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zraika S., Hull R.L., Udayasankar J., Aston-Mourney K., Subramanian S.L., Kisilevsky R., Szarek W.A., Kahn S.E. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khosravi M., Poursaleh A., Ghasempour G., Farhad S., Najafi M. The effects of oxidative stress on the development of atherosclerosis. Biol. Chem. 2019;400:711–732. doi: 10.1515/hsz-2018-0397. [DOI] [PubMed] [Google Scholar]
- 37.Sepidarkish M., Farsi F., Akbari-Fakhrabadi M., Namazi N., Almasi-Hashiani A., Maleki Hagiagha A., Heshmati J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019;139:141–152. doi: 10.1016/j.phrs.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Hallak M., Vazana L., Shpilberg O., Levy I., Mazar J., Nathan I. A molecular mechanism for mimosine-induced apoptosis involving oxidative stress and mitochondrial activation. Apoptosis. 2008;13:147–155. doi: 10.1007/s10495-007-0156-7. [DOI] [PubMed] [Google Scholar]
- 39.De Las Heras N., Martín Giménez V.M., Ferder L., Manucha W., Lahera V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants. 2020;9:897. doi: 10.3390/antiox9090897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poznyak A., Grechko A.V., Poggio P., Myasoedova V.A., Alfieri V., Orekhov A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020;21:1835. doi: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pari L., Srinivasan S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2010;64:477–481. doi: 10.1016/j.biopha.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Adouani I., Qureshi A.S., Hang T.-J. Preparation, evaluation and pharmacokinetics of diosmin herbosomein beagle dogs. Pak. J. Pharm. Sci. 2019;33:033–040. [PubMed] [Google Scholar]
- 43.Senthamizhselvan O., Manivannan J., Silambarasan T., Raja B. Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur. J. Pharmacol. 2014;736:131–137. doi: 10.1016/j.ejphar.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 44.Tong N., Zhang Z., Zhang W., Qiu Y., Gong Y., Yin L., Qiu Q., Wu X. Diosmin Alleviates Retinal Edema by Protecting the Blood-Retinal Barrier and Reducing Retinal Vascular Permeability during Ischemia/Reperfusion Injury. PLoS ONE. 2013;8:e61794. doi: 10.1371/journal.pone.0061794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calvert J.W. Chapter 5—Ischemic Heart Disease and its Consequences. In: Willis M.S., Homeister J.W., Stone J.R., editors. Cellular and Molecular Pathobiology of Cardiovascular Disease. Academic Press; San Diego, CA, USA: 2014. pp. 79–100. Cellular and Molecular Pathobiology of Cardiovascular Disease. [Google Scholar]
- 46.Queenthy S.S., John B. Diosmin exhibits anti-hyperlipidemic effects in isoproterenol induced myocardial infarcted rats. Eur. J. Pharmacol. 2013;718:213–218. doi: 10.1016/j.ejphar.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka T., Makita H., Kawabata K., Mori H., Kakumoto M., Satoh K., Hara A., Sumida T., Tanaka T., Ogawa H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis. 1997;18:957–965. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- 48.Pendeville H., Carpino N., Marine J.C., Takahashi Y., Muller M., Martial J.A., Cleveland J.L. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 2001;21:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baskar R., Dai J., Wenlong N., Yeo R., Yeoh K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014;1:24. doi: 10.3389/fmolb.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Browning A.M., Walle U.K., Walle T. Flavonoid glycosides inhibit oral cancer cell proliferation—role of cellular uptake and hydrolysis to the aglycones. J. Pharm. Pharmacol. 2005;57:1037–1041. doi: 10.1211/0022357056514. [DOI] [PubMed] [Google Scholar]
- 51.Sahu N., Soni D., Chandrashekhar B., Satpute D.B., Saravanadevi S., Sarangi B.K., Pandey R.A. Synthesis of silver nanoparticles using flavonoids: Hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016;6:173–181. doi: 10.1007/s40089-016-0184-9. [DOI] [Google Scholar]
- 52.Martínez C., Vicente V., Yáñez J., Alcaraz M., Castells M.T., Canteras M., Benavente-García O., Castillo J. The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histol. Histopathol. 2005;20:1121–1129. doi: 10.14670/HH-20.1121. [DOI] [PubMed] [Google Scholar]
- 53.Álvarez N., Vicente V., Martínez C. Synergistic Effect of Diosmin and Interferon-α on Metastatic Pulmonary Melanoma. Cancer Biother. Radiopharm. 2009;24:347–352. doi: 10.1089/cbr.2008.0565. [DOI] [PubMed] [Google Scholar]
- 54.Martínez Conesa C., Vicente Ortega V., Yáñez Gascón M.J., Alcaraz Baños M., Canteras Jordana M., Benavente-García O., Castillo J. Treatment of Metastatic Melanoma B16F10 by the Flavonoids Tangeretin, Rutin, and Diosmin. J. Agric. Food Chem. 2005;53:6791–6797. doi: 10.1021/jf058050g. [DOI] [PubMed] [Google Scholar]
- 55.Dung T.D., Day C.H., Binh T.V., Lin C.-H., Hsu H.-H., Su C.-C., Lin Y.-M., Tsai F.-J., Kuo W.-W., Chen L.-M., et al. PP2A mediates diosmin p53 activation to block HA22T cell proliferation and tumor growth in xenografted nude mice through PI3K–Akt–MDM2 signaling suppression. Food Chem. Toxicol. 2012;50:1802–1810. doi: 10.1016/j.fct.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Farmer J.A. Diabetic dyslipidemia and atherosclerosis: Evidence from clinical trials. Curr. Diabetes Rep. 2008;8:71–77. doi: 10.1007/s11892-008-0013-2. [DOI] [PubMed] [Google Scholar]
- 57.Shepherd J. Does statin monotherapy address the multiple lipid abnormalities in type 2 diabetes? Atheroscler. Suppl. 2005;6:15–19. doi: 10.1016/j.atherosclerosissup.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Jain D., Bansal M.K., Dalvi R., Upganlawar A., Somani R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014;12:35–41. doi: 10.1016/S2095-4964(14)60001-7. [DOI] [PubMed] [Google Scholar]
- 59.Zielinska D.F., Gnad F., Schropp K., Wiśniewski J.R., Mann M. Short Article. MOLCEL. 2012;46:542–548. doi: 10.1016/j.molcel.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 60.Luepker R.V. Cardiovascular disease: Rise, fall, and future prospects. Annu. Rev. Public Health. 2011;32:1–3. doi: 10.1146/annurev-publhealth-112810-151726. [DOI] [PubMed] [Google Scholar]
- 61.Nabel E.G. Cardiovascular disease. N. Engl. J. Med. 2003;349:60–72. doi: 10.1056/NEJMra035098. [DOI] [PubMed] [Google Scholar]
- 62.Iafisco M., Alogna A., Miragoli M., Catalucci D. Cardiovascular nanomedicine: The route ahead. Nanomedicine. 2019;14:2391–2394. doi: 10.2217/nnm-2019-0228. [DOI] [PubMed] [Google Scholar]
- 63.Martín Giménez V.M., Kassuha D.E., Manucha W. Nanomedicine applied to cardiovascular diseases: Latest developments. Ther. Adv. Cardiovasc. Dis. 2017;11:133–142. doi: 10.1177/1753944717692293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mashour N.H., Lin G.I., Frishman W.H. Herbal medicine for the treatment of cardiovascular disease: Clinical considerations. Arch. Intern. Med. 1998;158:2225–2234. doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- 65.Stansfield W.E., Ranek M., Pendse A., Schisler J.C., Wang S., Pulinilkunnil T., Willis M.S. Chapter 4—The Pathophysiology of Cardiac Hypertrophy and Heart Failure. In: Willis M.S., Homeister J.W., Stone J.R., editors. Cellular and Molecular Pathobiology of Cardiovascular Disease. Academic Press; San Diego, CA, USA: 2014. pp. 51–78. Cellular and Molecular Pathobiology of Cardiovascular Disease. [Google Scholar]
- 66.Katsenis K. Micronized purified flavonoid fraction (MPFF): A review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr. Vasc. Pharmacol. 2005;3:1–9. doi: 10.2174/1570161052773870. [DOI] [PubMed] [Google Scholar]
- 67.Manning B.D., Cantley L.C. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munjal A., Khandia R. Atherosclerosis: Orchestrating Cells and Biomolecules Involved in Its Activation and Inhibition. Elsevier Ltd.; Amsterdam, The Netherlands: 2019. pp. 1–38. [DOI] [PubMed] [Google Scholar]
- 69.Renehan A.G., Zwahlen M., Egger M. Adiposity and cancer risk:new mechanistic insightsfrom epidemiology. Nat. Rev. Cancer. 2015;15:484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 70.Lee J., Song K.-M., Jung C.H. Diosmin restores the skin barrier by targeting the aryl hydrocarbon receptor in atopic dermatitis. Phytomedicine. 2021;81:153418. doi: 10.1016/j.phymed.2020.153418. [DOI] [PubMed] [Google Scholar]
- 71.Singh S., Sharma B., Kanwar S.S., Kumar A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016;7:1667. doi: 10.3389/fpls.2016.01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warsi M.K., Kamal M.A., Baeshen M.N., Izhari M.A., Mobashir A.F.A.M. Comparative Study of Gene Expression Profiling Unravels Functions associated with Pathogenesis of Dengue Infection. Curr. Pharm. Des. 2020;26:5293–5299. doi: 10.2174/1381612826666201106093148. [DOI] [PubMed] [Google Scholar]
- 73.Kamal M.A., Warsi M.K., Alnajeebi A., Ali H.A., Helmi N., Izhari M.A., Mustafa S., Mobashir M. Gene expression profiling and clinical relevance unravel the role hypoxia and immune signaling genes and pathways in breast cancer: Role of hypoxia and immune signaling genes in breast cancer. J. Intern. Med. Sci. Art. 2020;1:2–10. doi: 10.36013/jimsa.v1i1.3. [DOI] [Google Scholar]
- 74.Bajrai L., Sohrab S.S., Alandijany T.A., Mobashir M., Parveen S., Kamal M.A., Azhar E.I. Gene expression profiling of early acute febrile stage of dengue infection and its comparative analysis with Streptococcus pneumoniae infection. Front. Cell. Infect. Microbiol. 2021;11:1018. doi: 10.3389/fcimb.2021.707905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexeyenko A., Sonnhammer E.L.L. Global networks of functional coupling in eukaryotes from comprehensive data integration. Genome Res. 2009;19:1107–1116. doi: 10.1101/gr.087528.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mobashir M., Schraven B., Beyer T. Simulated evolution of signal transduction networks. PLoS ONE. 2012;7:e50905. doi: 10.1371/journal.pone.0050905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mobashir M., Madhusudhan T., Isermann B., Beyer T., Schraven B. Negative Interactions and Feedback Regulations Are Required for Transient Cellular Response. Sci. Rep. 2014;4:3718. doi: 10.1038/srep03718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eldakhakhny B.M., Sadoun A.H., Choudhry H., Mobashir M. In-Silico Study of Immune System Associated Genes in Case of Type-2 Diabetes with Insulin Action and Resistance, and/or Obesity. Front. Endocrinol. 2021;12:281. doi: 10.3389/fendo.2021.641888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krishnamoorthy P.K.P., Kamal M.A., Warsi M.K., Alnajeebi A., Ali H.A., Helmi N., Izhari M.A., Mustafa S., Firoz A., Mobashir M. In-silico study reveals immunological signaling pathways, their genes, and potential herbal drug targets in ovarian cancer. Inform. Med. Unlocked. 2020;20:100422. doi: 10.1016/j.imu.2020.100422. [DOI] [Google Scholar]
- 80.Christopoulos D., Nicolaides A.N., Szendro G. Venous reflux: Quantification and correlation with the clinical severity of chronic venous disease. Br. J. Surg. 1988;75:352–356. doi: 10.1002/bjs.1800750419. [DOI] [PubMed] [Google Scholar]
- 81.De Smet F., Christopoulos A., Carmeliet P. Allosteric targeting of receptor tyrosine kinases. Nat. Biotechnol. 2014;32:1113–1120. doi: 10.1038/nbt.3028. [DOI] [PubMed] [Google Scholar]
- 82.Ramelet A.A., Boisseau M.R., Allegra C., Nicolaides A., Jaeger K., Carpentier P., Cappelli R., Forconi S. Veno-active drugs in the management of chronic venous disease. An international consensus statement: Current medical position, prospective views and final resolution. Clin. Hemorheol. Microcirc. 2005;33:309–319. [PubMed] [Google Scholar]
- 83.Cazaubon M., Benigni J.-P., Steinbruch M., Jabbour V., Gouhier-Kodas C. Is There a Difference in the Clinical Efficacy of Diosmin and Micronized Purified Flavonoid Fractio.on for the Treatment of Chronic Venous Disorders? Review of Available Evidence. Vasc. Health Risk Manag. 2021;17:591–600. doi: 10.2147/VHRM.S324112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serra R., Ielapi N., Bitonti A., Candido S., Fregola S., Gallo A., Loria A., Muraca L., Raimondo L., Velcean L., et al. Efficacy of a Low-Dose Diosmin Therapy on Improving Symptoms and Quality of Life in Patients with Chronic Venous Disease: Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021;13:999. doi: 10.3390/nu13030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meshikhes A.W.N. Daflon for haemorrhoids: A prospective, multi-centre observational study. Surgeon. 2004;2 doi: 10.1016/S1479-666X(04)80032-5. [DOI] [PubMed] [Google Scholar]
- 86.Cypriani B., Limasset B., Carrié M.L., Le Doucen C., Roussie M., de Paulet A.C., Damon M. Antioxidant activity of micronized diosmin on oxygen species from stimulated human neutrophils. Biochem. Pharmacol. 1993;45:1531–1535. doi: 10.1016/0006-2952(93)90056-3. [DOI] [PubMed] [Google Scholar]
- 87.Oh-hora M., Komatsu N., Pishyareh M., Feske S., Hori S., Taniguchi M., Rao A., Takayanagi H. Agonist-Selected T Cell Development Requires Strong T Cell Receptor Signaling and Store-Operated Calcium Entry. Immunity. 2013;38:881–895. doi: 10.1016/j.immuni.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castle A.R., Gill A.C. Physiological Functions of the Cellular Prion Protein. Front. Mol. Biosci. 2017;4:19. doi: 10.3389/fmolb.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borisov N., Aksamitiene E., Kiyatkin A., Legewie S., Berkhout J., Maiwald T., Kaimachnikov N.P., Timmer J., Hoek J.B., Kholodenko B.N. Systems-level interactions between insulin–EGF networks amplify mitogenic signaling. Mol. Syst. Biol. 2009;5:256. doi: 10.1038/msb.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar J., Umar T., Kausar T., Mobashir M., Nayeem S.M., Hoda N. Identification of lead BAY60-7550 analogues as potential inhibitors that utilize the hydrophobic groove in PDE2A: A molecular dynamics simulation study. J. Mol. Modeling. 2017;23:7. doi: 10.1007/s00894-016-3171-1. [DOI] [PubMed] [Google Scholar]
- 91.Pinu F.R., Beale D.J., Paten A.M., Kouremenos K., Swarup S., Schirra H.J., Wishart D. Systems Biology and Multi-Omics Integration: Viewpoints from the Metabolomics Research Community. Metabolites. 2019;9:76. doi: 10.3390/metabo9040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rekhi R., Qutub A.A. Systems approaches for synthetic biology: A pathway toward mammalian design. Front. Physiol. 2013;4:285. doi: 10.3389/fphys.2013.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mustafa S., Mobashir M. LC–MS and docking profiling reveals potential difference between the pure and crude fucoidan metabolites. Int. J. Biol. Macromol. 2020;143:11–29. doi: 10.1016/j.ijbiomac.2019.11.232. [DOI] [PubMed] [Google Scholar]
- 94.Mobashir M. Ph.D. Thesis. Magdeburg University; Magdeburg, Germany: 2013. Mathematical Modeling and Evolution of Signal Transduction Pathways and Networks. [Google Scholar]
- 95.Singh A., Maqbool M., Mobashir M., Hoda N. Dihydroorotate dehydrogenase: A drug target for the development of antimalarials. Eur. J. Med. Chem. 2017;125:640–651. doi: 10.1016/j.ejmech.2016.09.085. [DOI] [PubMed] [Google Scholar]
- 96.Maqbool M., Mobashir M., Hoda N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s. Eur. J. Med. Chem. 2015;107:63–81. doi: 10.1016/j.ejmech.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 97.Helmi N., Alammari D., Mobashir M. Role of Potential COVID-19 Immune System Associated Genes and the Potential Pathwayslinkage with Type-2 Diabetes. Comb. Chem. High. Throughput Screen. 2021;24:1–11. doi: 10.2174/1386207324666210804124416. [DOI] [PubMed] [Google Scholar]
- 98.Kumar J., Meena P., Singh A., Jameel E., Maqbool M., Mobashir M., Shandilya A., Tiwari M., Hoda N., Jayaram B. Synthesis and screening of triazolopyrimidine scaffold as multi-functional agents for Alzheimer’s disease therapies. Eur. J. Med. Chem. 2016;119:260–277. doi: 10.1016/j.ejmech.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 99.Krishnamoorthy P.K.P., Subasree S., Arthi U., Mobashir M., Gowda C., Revanasiddappa P.D. T-cell Epitope-based Vaccine Design for Nipah Virus by Reverse Vaccinology Approach. Comb. Chem. High. Throughput Screen. 2020;23:788–796. doi: 10.2174/1386207323666200427114343. [DOI] [PubMed] [Google Scholar]
- 100.Bajrai L.H., Sohrab S.S., Mobashir M., Kamal M.A., Rizvi M.A., Azhar E.I. Understanding the role of potential pathways and its components including hypoxia and immune system in case of oral cancer. Sci. Rep. 2021;11:19576. doi: 10.1038/s41598-021-98031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.