Abstract
Like bacteria, fungi play an important role in the soil ecosystem. As only a small fraction of the fungi present in soil can be cultured, conventional microbiological techniques yield only limited information on the composition and dynamics of fungal communities in soil. DNA-based methods do not depend on the culturability of microorganisms, and therefore they offer an attractive alternative for the study of complex fungal community structures. For this purpose, we designed various PCR primers that allow the specific amplification of fungal 18S-ribosomal-DNA (rDNA) sequences, even in the presence of nonfungal 18S rDNA. DNA was extracted from the wheat rhizosphere, and 18S rDNA gene banks were constructed in Escherichia coli by cloning PCR products generated with primer pairs EF4-EF3 (1.4 kb) and EF4-fung5 (0.5 kb). Fragments of 0.5 kb from the cloned inserts were sequenced and compared to known rDNA sequences. Sequences from all major fungal taxa were amplified by using both primer pairs. As predicted by computer analysis, primer pair EF4-EF3 appeared slightly biased to amplify Basidiomycota and Zygomycota, whereas EF4-fung5 amplified mainly Ascomycota. The 61 clones that were sequenced matched the sequences of 24 different species in the Ribosomal Database Project (RDP) database. Similarity values ranged from 0.676 to 1. Temperature gradient gel electrophoresis (TGGE) analysis of the fungal community in the wheat rhizosphere of a microcosm experiment was carried out after amplification of total DNA with both primer pairs. This resulted in reproducible, distinctive fingerprints, confirming the difference in amplification specificity. Clear banding patterns were obtained with soil and rhizosphere samples by using both primer sets in combination. By comparing the electrophoretic mobility of community fingerprint bands to that of the bands obtained with separate clones, some could be tentatively identified. While 18S-rDNA sequences do not always provide the taxonomic resolution to identify fungal species and strains, they do provide information on the diversity and dynamics of groups of related species in environmental samples with sufficient resolution to produce discrete bands which can be separated by TGGE. This combination of 18S-rDNA PCR amplification and TGGE community analysis should allow study of the diversity, composition, and dynamics of the fungal community in bulk soil and in the rhizosphere.
Fungi play an important role in the soil ecosystem as major decomposers of plant residues, releasing nutrients that sustain and stimulate plant growth in the process. Some fungi possess properties antagonistic towards plant pathogens (15). In the study of soil fungi, particular attention is given to the rhizosphere; a well-developed and diverse rhizosphere community is thought to play a role in the suppression of pathogens (1, 11). Knowledge of the structure and diversity of the fungal community in the rhizosphere will lead to a better understanding of pathogen-antagonist interactions. In this work, the development and application of molecular techniques for assessing fungal diversity in the rhizosphere are described. As only a certain fraction of the fungi in soil can be cultured, molecular methods are expected to give a more realistic view of species richness and distribution. Several fungal taxa such as the saprophytic basidiomycetes and the arbuscular endomycorrhiza, which belong to the Glomales, are difficult or impossible to isolate from soil by dilution plating (26). Moreover, investigating fungi by estimating their numbers as CFU can be misleading, since most colonies on plates stem from fungal spores (5).
To circumvent the cultivation problem, an array of molecular techniques, such as amplified ribosomal DNA (rDNA) sequencing, amplified rDNA restriction analysis, and temperature and denaturing gradient gel electrophoreses (TGGE and DGGE) of rDNA, has been applied to elucidate microbial population structures in the environment (3, 8, 9, 13, 14, 17, 20, 23, 27, 29). Application of these molecular methods has led to a tremendous increase in knowledge of microbial ecology and has revealed the existence of formerly unknown microorganisms (14).
Several molecular techniques have been applied to study medically important fungi and phytopathogenic strains (22, 28). These methods are generally not suited for soil studies, since the primers described are not specific for fungi and some of the systems are designed to type strains or cultivars, and therefore yield taxonomic resolutions that are too high. The taxonomic resolution of 18S rDNA might not always be sufficient to identify fungal species and strains. However, amplified 18S rDNA will provide information on the fungal diversity and dynamics of related species of fungi in soil by producing discrete bands on TGGE gels. Among the scarce reports on this subject is that of Kowalchuk et al. (13), who obtained interesting results by using PCR and DGGE for the analysis of fungi associated with Marram grass in coastal dunes. These investigators used the primers of Sogin (24) and White et al. (30) and reported the coamplification of plant rDNA with these primer sets (13). However, since these primers also amplify 18S rDNA from plants, algae, and nematodes, they are not suited for direct and specific amplification of fungal 18S rDNA. Bock et al. (2) also reported specificity problems when using these primers for the detection of pathogenic fungi in clinical specimens. When TGGE banding patterns are to be used for studying fungal communities, it is essential to know that all bands are of fungal origin. In complex ecosystems, such as agricultural soil, other 18S-rDNA (i.e., eukaryotic) sequences could obscure the fungal banding pattern.
The objective of this study was to develop primers for the specific amplification of fungal 18S rDNA and to investigate the performance of these primers in the characterization of fungal communities. An important objective was to achieve an amplification range with a broad coverage of all fungal taxa without losing specificity.
A computer-aided prediction of primer specificity was made, based on the fungal 18S-rDNA sequences in the Ribosomal Database Project (RDP) database, and then the range of species that could be amplified by the primers was tested with a collection of different fungal isolates from all major taxa. Subsequently, the primer sets were used to generate PCR products from bulk and rhizosphere microcosm soil samples. Common 0.5-kb fragments of cloned 18S rDNA obtained from both soil samples with both primer sets were sequenced, these sequences were matched to those in the database, and the specificity and bias of the two primer systems were assessed. TGGE analysis of the bulk and rhizosphere samples was done to assess differences in fungal community structure.
MATERIALS AND METHODS
Primer design and computation of primer pair specificity.
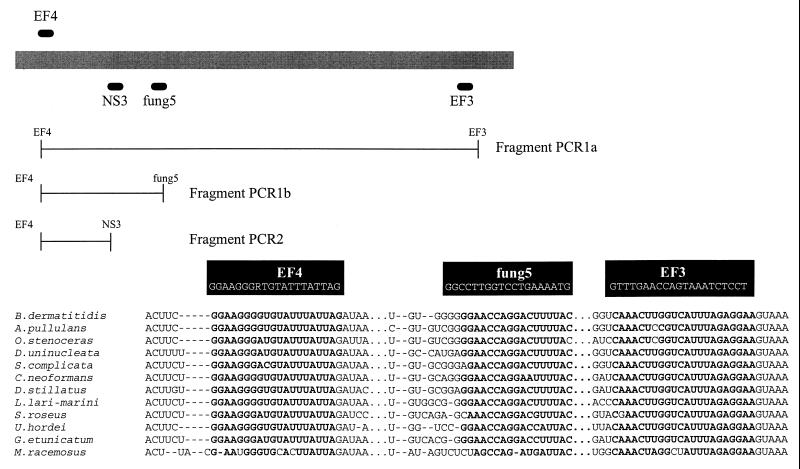
In order to develop primers for the specific amplification of fungi, a number of aligned 18S-rDNA sequences of fungi and other eukaryotes were retrieved from the RDP database (16). Several primers were chosen to allow the amplification of 18S-rDNA sequences from a wide range of fungal taxa (Fig. 1): EF3 (5′-TCCTCTAAATGACCAAGTTTG-3′), EF4 (5′-GGAAGGG[G/A]TGTATTTATTAG-3′), and fung5 (5′-GTAAAAGTCCTGGTTCCCC-3′). The EF4-EF3 primer set was chosen to amplify a major part of the 18S rDNA, and primer set EF4-fung5 was chosen to amplify an approximately 550-bp fragment. Both primer sets were developed for the amplification of fungal 18S rDNA directly from the soil for cloning and sequencing. The primer sequence NS3 was previously described (30), and a G-C tail (NS3-GC) was added for TGGE (20). NS3-GC can be combined with EF4 to amplify a DNA fragment that can then be analyzed by TGGE. NS3 is, however, not specific for fungi and can only be used in a nested amplification approach with the specific primer pairs.
FIG. 1.
Primers for amplification of fungal 18S-rDNA sequences. Primer pair EF4-EF3 (fragment PCR1a) is specific for fungal 18S rDNA, and primer pair EF4-fung5 (fragment PCR1b) amplifies a smaller, 550-bp fragment specific for fungi. Primer pair EF4-NS3 (PCR2) can be used directly on clones or in a nested approach on PCR1 products for community analysis by TGGE. The primers which were developed in this work are given in alignment with 18S-rDNA sequences of species from all major fungal taxa.
To obtain a computer-aided evaluation of the amplification range of the primers, each sequence was compared to 436 aligned eukaryotic 18S-rDNA sequences with the Check Probe program of the RDP database. Fungal sequences with full homology or one mismatch for each primer were selected from the analysis listing and are shown with relation to particular taxa in Table 1.
TABLE 1.
Computer analysis giving the percentage of database sequences showing fewer than two mismatches in both primers of a given pair
| Taxon (n)a | % of species homologous to primer pair:
|
||
|---|---|---|---|
| EF4-EF3 | EF4-fung5 | EF4-NS3 | |
| Ascomycota | |||
| Euascomycetes | |||
| Plectomycetes (17) | 88 | 94 | 100 |
| Loculoascomycetes (2) | 50 | 100 | 100 |
| Pyrenomycetes (11) | 91 | 100 | 91 |
| Hemiascomycetes (28) | 61 | 68 | 86 |
| Archaeascomycetes (7) | 71 | 86 | 100 |
| Basidiomycota | |||
| Hymenomycetes (28) | 95 | 85 | 100 |
| Uredinomycetes (8) | 100 | 25 | 100 |
| Ustilagomycetes (3) | 100 | 33 | 100 |
| Zygomycota (5) | 75 | 40 | 60 |
| Glomales (2) | 100 | 0 | 100 |
| Chytridiomycota (8) | 12 | 14 | 29 |
| All fungib (119) | 78 | 71 | 91 |
| Plants | 0 | 0 | 44c |
| Algae | 0 | 0 | 0 |
| Nematodes | 0 | 0 | 0 |
n, number of sequences of different species in the taxa to which the primers were matched.
Values are not the means of the values above, but are overall percentages of the number of species homologous to the primers.
EF4 has two mismatches and NS3 has none. This does not exclude the amplification of plant DNA.
Fungal strains, culture conditions, and amplification range.
Various fungal species from all major taxa were used to test the primer sets (Table 2). Lyophilized cultures were rehydrated with a sterile 0.85% NaCl solution and applied to potato dextrose agar plates. Plates were incubated at 28°C for several days to 2 weeks, until sufficient hyphal growth was observed.
TABLE 2.
Amplification range of several primer pairs of 18S rDNA from various fungal species
| Taxon | Originb | Result of amplification on tests witha:
|
||
|---|---|---|---|---|
| EF4-EF3c | EF4-NS3d | EF4-fung5c | ||
| Ascomycota | ||||
| Euascomycetes | ||||
| Emericella nidulans | CBS 565.70 | + | + | + |
| Cladosporium herbarum | RIVM | + | + | + |
| Botrytis cinera | RIVM | + | + | + |
| Penicillium digitatum | RIVM | + | + | + |
| Verticillium lecanii | RIVM | + | + | + |
| Aureobasidium pullulans | RIVM | − | + | + |
| Geotrichum candidum | RIVM | + | + | + |
| Fusarium oxysporum | CBS 254.52 | + | + | + |
| Trichoderma hamatum | SI | + | + | + |
| Penicillium type I | SI | + | + | + |
| Penicillium type II | SI | + | + | + |
| Gliocladium roseum | SI | + | + | + |
| Cladosporium sp. | SI | + | + | + |
| Acremonium curvulum | SI | + | + | − |
| Alternaria alternata | CBS 154.31 | + | + | + |
| Fusarium cerealis | CBS 623.85 | + | + | + |
| Hemiascomycetes | ||||
| Candida lipolytica | RIVM | − | − | − |
| Saccharomyces sp. | IPO | + | + | + |
| Zygomycota | ||||
| Zygomycetes | ||||
| Rhizopus stolonifer | SI | + | + | + |
| Absidia sp. | SI | + | + | − |
| Syncephalastrum sp. | SI | + | + | + |
| Mucor sp. | SI | + | + | − |
| Basidiomycota | ||||
| Lycoperdon echinatum | CBS 245.51 | + | + | + |
| Agaricus syvaticus | CBS 354.74 | + | + | + |
| Tremella sp. | CBS 680.93 | + | + | + |
| Ustilago avenae | CBS 340.32 | + | + | − |
| Unknown | ||||
| Monocillium mucidum | SI | + | + | + |
| Coniothyrium sporulosum | SI | − | + | ND |
| Broomella acuta | SI | + | ND | ND |
| Other eukaryotes | ||||
| Plant DNA | ||||
| Phaseolus radiatus | − | + | − | |
ND, not determined.
SI, soil isolate.
Primer set specific for fungi.
Primer set is not specific and can be used in TGGE analysis in nested PCR.
Fungi were also cultured from the soil. Samples of 10 g of soil were shaken in 100 ml of a MgSO4 solution (10 mM) for 15 min. Serial dilutions were plated onto 2% malt extract agar containing 0.33% Solacol and 200 ppm of aureomycine (7). Plates were incubated at 20°C for 1 week. Fungi with different morphologies were then selected and streaked onto cornmeal agar. The plates were incubated for 1 to 2 weeks, and some of the fungi were identified by W. Gams at the Centraal Bureau voor Schimmelcultures, Baarn, The Netherlands. The species that were found are listed in Table 2.
DNA was isolated by scraping hyphae from the agar surface. Cells were disrupted, DNA was released by bead beating, and the resulting lysate was purified as described by Smit et al. (23).
Prior to the examination of the amplification range of the primer sets EF4-EF3, EF4-fung5, and EF4–NS3-GC on a collection of fungal isolates, fungal cell lysis and the quality of the extracted DNA were tested by using the general eukaryotic primer set 106-107 (24). Only 1 strain (Coniothyrium sporulosum) out of 27 was poorly lysed (results not shown). A positive signal obtained by using these primers indicated that the fungal cells were lysed by the bead-beating method and that the quality of the DNA was sufficiently high for PCR amplification.
Soil microcosms and DNA extraction.
To study the fungal community in wheat rhizosphere soil, we collected soil from a small field plot on the campus of the University of Utrecht located on the Uithof, Utrecht, The Netherlands. This is a clay soil containing 4% organic matter with a pH of 5.0. Samples from this soil were used for plating culturable fungi and for setting up the microcosm experiment. The soil was air dried and sieved, and nine small pots (diameter, 10 cm) were filled. Soil was seeded with eight seeds of Triticum aestivum cv. Baldus per pot. Fluctuations in moisture content were minimized by supplying water daily to keep the soil moisture content at 20%. Microcosms were incubated in a climate chamber with a light-dark regimen of 16 and 8 h at 20 and 15°C, respectively. Microcosms were sampled in duplicate on days 5 and 10. Bulk soil samples of 3 g were taken from root-free soil. Rhizosphere soil was obtained by gently shaking the soil from the roots, and roots with adhering soil were added to 50-ml polypropylene tubes with 10 ml of sterile sodium phosphate buffer (120 mM; pH 8) and 1 g of gravel. Tubes were vortexed for 30 s, and the buffer-soil slurry mixture was poured into a new tube, leaving the gravel and roots behind. Total DNA was extracted from the rhizosphere soil slurry by using a bead beater (23). One microliter of the extract was used for PCR amplification.
The extent to which this method lyses fungi present in the soil is not known. However, bead beating has been shown to lyse bacterial spores (18), and all cultured fungal strains in this study could be disrupted by bead beating. Moreover, the Braun bead beater also lyses Saccharomyces cerevisiae cells under the conditions used.
PCR amplification.
Total DNA extracts from duplicate soil samples were pooled before PCR. Primer sets EF4-EF3 and EF4-fung5 were used for direct amplification of 18S-rDNA sequences from extracted wheat rhizosphere DNA.
18S-rDNA clones amplified with both primer sets were also analyzed by TGGE. For this purpose, they were reamplified from Escherichia coli colony material. PCR was performed by using primer set EF4–NS3-GC to generate 18S-rDNA fragments suitable for analysis by TGGE. In order to generate TGGE profiles of the soil fungal community, a nested approach was necessary. An initial PCR was done with either EF4-EF3 or EF4-fung5, as described above. Subsequently, the reaction mixture was diluted 1:500, and a second amplification round was performed with EF4–NS3-GC (Fig. 1). With this approach, corresponding fragments can be analyzed by TGGE, although different primer sets had been initially used for the specific amplification of the fungal community in the wheat rhizosphere. Differences in banding patterns will reflect the differences in specificity of the initial PCR (Fig. 2). 18S-rDNA fragments were obtained from cloned material (see below) for TGGE analysis by the direct use of EF4–NS3-GC on a small amount of a specific E. coli colony as template source.
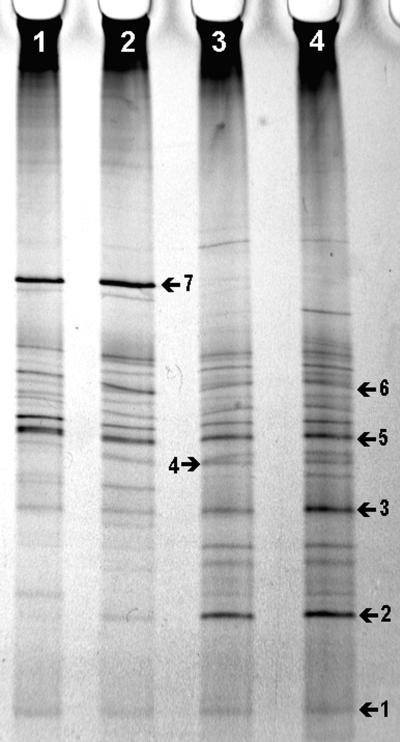
FIG. 2.
TGGE of amplified 18S-rDNA fragments representing the fungal community in bulk and rhizophere soil samples of the microcosm experiment. Banding patterns were obtained by mixing the duplicate samples before PCR and adding PCR products from both primer pairs (EF4-EF3 and EF4-fung5) the same lane. Lane 1, day 5 bulk soil; lane 2, day 5 rhizosphere soil; lane 3, day 10 bulk soil; and lane 4, day 10 rhizosphere soil. Numbered bands are explained in the text.
PCR mixtures for all three primer sets consisted of 5 μl of 10× PCR buffer (Boehringer), 1.7 mM MgCl2, 200 μM concentrations of each deoxynucleoside triphosphate, 300 nM concentrations of each primer, 2.6 U of Expand enzyme mix (Boehringer), 1 μl of 1:10-diluted template DNA, and sterile Millipore water to a final volume of 50 μl. The following thermocycling pattern was used: 94°C for 3 min (1 cycle); 94°C for 1 min, 48°C for 1 min, and 72°C for 3 min (40 cycles); and 72°C for 10 min (1 cycle).
Cloning of EF4-EF3 and EF4-fung5 PCR products.
Only PCR products from the pooled, day 10 rhizosphere samples were cloned and sequenced. PCR products from the soil microbial community were separated on a 1% agarose-Tris-borate-EDTA gel. Bands were excised, and the DNA was purified by centrifuging the gel pieces for 15 min at 16,000 × g in a Wizard column without resin. The flowthrough containing the DNA was collected and used without further purification. DNA fragments were then ligated into the pGEM-T vector, which has 3′ T overhangs to facilitate the cloning of PCR products (Promega, Madison, Wis.). Ligation mixes were used to transform ultracompetent E. coli XL1-Blue cells (Stratagene, Cambridge, United Kingdom) according to the manufacturer’s instructions. White colonies were selected, cultured in 2 ml of Luria broth, and stored at −70°C. Subsequently, a quick clone screening procedure based on PCR amplification was performed directly from colony material. Clones obtained from the rhizosphere soil sample with both primer sets and containing inserts of the correct size were selected for sequencing.
TGGE analysis of the fungal rhizosphere community and the 18S-rDNA clones.
The products amplified from the fungal community of the microcosm experiment and from the clones were analyzed by TGGE. For TGGE analysis, a Diagen electrophoresis unit was used according to the instructions of the manufacturer. The polyacrylamide gel was composed of 8% (wt/vol) acrylamide, 0.21% (wt/vol) bisacrylamide, 8 M urea, 20 mM MOPS (morpholinepropanesulfonic acid), 1 mM EDTA (pH 8), and 20% (vol/vol) formamide. The gel was polymerized onto a gel support film (Gelbond PAG; FMC). Electrophoresis was performed at a constant of 110 V for 17 h with a temperature gradient from 36 to 44°C.
Gels were silver stained with the PlusOne DNA silver staining kit (Pharmacia Biotech) in a Hoefer automated gel stainer (Pharmacia Biotech) according to the standard DNA-staining protocol with increased solution volumes (total volume, 187.5 ml), a developing time of 8 min, an extra washing step with H2O, and a Na2CO3 fixing step.
Stained gels were air dried, and the images were analyzed with Bioprint (version 96.11) and Biogene (version 96.15) software packages (Vilber Lourmat, Marne-la-Vallee, France). Banding patterns were analyzed by cluster analysis using the unweighted pair group method, and genetic similarity was calculated according to the method of Nei and Li (6a) [a = 2nxy/(nx+ny)] by using Biogene software (Vilbert Lourmat).
Partial sequencing of the cloned fungal 18S-rDNA fragments.
A 0.5-kb fragment of each clone obtained from wheat rhizosphere soil was sequenced in both directions on an ABI377 DNA sequencer by cycle sequencing using the dye terminator system (Eurogentec, Seraing, Belgium). EF4-fung5 clones were sequenced with the SP6 and T7 primers. The corresponding region of EF4-EF3 clones, which contains a much longer insert, was sequenced with primers EF4 and FS7 (5′-GCTTTGAACACTCTAATT-3′). Consensus sequences were obtained by using the DNASIS software package (version 2.5; Hitachi Software Engineering America, Ltd., San Francisco, Calif.). Sequences were then manually edited and analyzed with the Sequence Match (version 2.7) program of the RDP database (containing 10,723 unaligned ribosomal sequences) and the Blast 2 Advanced Blast program at the National Center for Biotechnology Information (NCBI) (containing 351,020 sequences). Fungal species sequences in the database most similar to those of the clones are listed in Tables 3 and 4. To check for occurrences of presumptively chimeric sequences, all clones were analyzed by using the Chimera Check program of the RDP database (version 2.7). Since it is impossible to unambiguously determine whether a sequence is chimeric, all clones were designated either N (not likely to be chimeric) or P (possibly chimeric) (Tables 3 and 4).
TABLE 3.
Species of fungi with 18S-rDNA sequences most similar to EF4-fung5 clones from a wheat rhizospherea
| Clone no. | Fungal species with most similar sequence | Fragment size (bp) | Similarity | Result of Chimera Checkb | Accession no. |
|---|---|---|---|---|---|
| Ascomycota | |||||
| Euascomycetes | |||||
| UUF34 | Symbiotaphrina buchneri | 523 | 0.856 | N | AF095665 |
| UUF41 | Symbiotaphrina buchneri | 522 | 0.681 | P | AF095669 |
| UUF46 | Symbiotaphrina buchneri | 534 | 0.676 | N | AF095673 |
| Pyrenomycetes | |||||
| UUF02 | Neocosmospora vasinfecta | 524 | 0.907 | N | AF095648 |
| UUF13 | Neocosmospora vasinfecta | 521 | 0.890 | N | AF095654 |
| UUF18 | Neocosmospora vasinfecta | 524 | 0.927 | N | AF095656 |
| UUF38 | Neocosmospora vasinfecta | 522 | 0.792 | P | AF095666 |
| UUF60 | Neocosmospora vasinfecta | 520 | 0.877 | N | AF095681 |
| UUF63 | Neocosmospora vasinfecta | 521 | 0.897 | P | AF095683 |
| UUF27 | Paecilomyces tenuipes | 515 | 0.900 | N | AF095659 |
| UUF42 | Paecilomyces tenuipes | 529 | 0.756 | N | AF095670 |
| UUF04 | Verticillium dahliae | 529 | 0.893 | N | AF095649 |
| UUF12 | Verticillium dahliae | 529 | 0.848 | N | AF095653 |
| UUF39 | Verticillium dahliae | 521 | 0.693 | P | AF095667 |
| UUF40 | Verticillium dahliae | 522 | 0.897 | N | AF095668 |
| UUF62 | Sordaria fimicola | 529 | 0.720 | N | AF095682 |
| UUF55 | Chaetomium elatum | 520 | 0.758 | P | AF095678 |
| Chaetithyriales | |||||
| UUF05 | Exophiala mansonii | 524 | 0.838 | N | AF095650 |
| Loculoascomycetes | |||||
| UUF22 | Cladosporium cladosporioides | 520 | 0.949 | N | AF095657 |
| UUF23 | Cladosporium cladosporioides | 522 | 0.937 | N | AF095658 |
| UUF33 | Cladosporium cladosporioides | 520 | 0.937 | N | AF095664 |
| UUF50 | Cladosporium cladosporioides | 528 | 0.821 | N | AF095675 |
| UUF66 | Cladosporium cladosporioides | 523 | 0.896 | N | AF095685 |
| UUF10 | Pleospora rudis | 525 | 0.937 | N | AF095652 |
| UUF29 | Pleospora rudis | 518 | 0.866 | N | AF095661 |
| UUF44 | Alternaria alternata | 517 | 0.984 | N | AF095671 |
| UUF47 | Alternaria alternata | 526 | 0.743 | N | AF095674 |
| UUF51 | Alternaria alternata | 521 | 0.911 | N | AF095676 |
| UUF45 | Pleospora herbarum | 528 | 0.868 | N | AF095672 |
| UUF59 | Pleospora herbarum | 521 | 0.772 | N | AF095680 |
| UUF68 | Mycosphaerella mycopappi | 519 | 0.899 | N | AF095686 |
| Erysiphales | |||||
| UUF09 | Blumeria graminis | 535 | 0.859 | P | AF095651 |
| UUF28 | Blumeria graminis | 521 | 0.899 | N | AF095660 |
| Plectomycetes | |||||
| UUF15 | Geosmithia lavendula | 525 | 0.803 | P | AF095655 |
| UUF56 | Geosmithia lavendula | 526 | 0.796 | N | AF095679 |
| UUF30 | Eupenicillium javanicum | 518 | 0.978 | N | AF095662 |
| UUF31 | Eupenicillium javanicum | 515 | 1.000 | N | AF095663 |
| Zygomycota | |||||
| Zygomycetes | |||||
| UUF64 | Mortierella polycephala | 529 | 0.762 | N | AF095684 |
| Chytridiomycota | |||||
| Chytridiomycetes | |||||
| UUF54 | Spizellomyces acuminatus | 522 | 0.691 | N | AF095677 |
Sequences were compared to those in the RDP database. No Basidiomycota clones were detected.
P, possible chimeric sequence; N, not likely a chimeric sequence.
TABLE 4.
Species of fungi with 18S-rDNA sequences most similar to the EF4-EF3 clones from the wheat rhizospherea
| Clone no. | Fungal taxa with most similar sequence | Fragment size (bp) | Identity | Results of Chimera Checkb | Accession no. |
|---|---|---|---|---|---|
| Ascomycota | |||||
| Euascomycetes | |||||
| UUF146 | Symbiotaphrina kochii | 680c | 0.984 | N | |
| Erysiphales | |||||
| UUF115 | Blumeria graminis | 500 | 0.872 | N | AF096354 |
| Basidiomycota | |||||
| Hymenomycetes | |||||
| Stereales | |||||
| UUF101 | Athelia bombacina | 513 | 0.809 | N | AF096351 |
| Tremellales | |||||
| UUF103 | Bullera crocea | 504 | 0.839 | N | AF096352 |
| UUF136 | Bullera crocea | 510 | 0.864 | N | AF096364 |
| UUF121 | Bullera crocea | 510 | 0.820 | N | AF096358 |
| UUF143 | Bullera crocea | 508 | 0.867 | N | AF096366 |
| UUF120 | Filobasidium floriforme | 509 | 0.837 | N | AF096357 |
| UUF124 | Cryptococcus albidus | 510 | 0.825 | N | AF096360 |
| UUF149 | Sclerotium sp. BSC-97 | 728c | 0.902 | N | |
| Zygomycota | |||||
| Zygomycetes | |||||
| Mucorales | |||||
| UUF112 | Mortierella polycephala | 515 | 0.844 | N | AF096353 |
| UUF116 | Mortierella polycephala | 509 | 0.854 | N | AF096355 |
| UUF119 | Mortierella polycephala | 503 | 0.779 | P | AF096356 |
| UUF123 | Mortierella polycephala | 503 | 0.844 | N | AF096359 |
| UUF125 | Mortierella polycephala | 514 | 0.970 | N | AF096361 |
| UUF127 | Mortierella polycephala | 503 | 0.839 | N | AF096362 |
| UUF128 | Mortierella polycephala | 504 | 0.767 | N | AF096363 |
| UUF142 | Mortierella polycephala | 507 | 0.834 | N | AF096365 |
| UUF144 | Mortierella polycephala | 508 | 0.852 | N | AF096367 |
| UUF147 | Mortierella polycephala | 507 | 0.844 | N | AF096368 |
| UUF150 | Mortierella polycephala | 514 | 0.968 | N | AF096369 |
| Chytridiomycota | |||||
| Chytridiomycetes | |||||
| UUF130 | Spizellomyces acuminatus | 739c | 0.935 | N |
Sequences were compared to those in the RDP database, containing 10,723 entries.
P, possible chimeric sequence; N, not likely a chimeric sequence.
Sequence were determined in one direction only and not submitted to NCBI.
Nucleotide sequence accession numbers.
All nucleotide sequences were submitted to NCBI and assigned accession no. AF095648 to AF095686 and AF096351 to AF096369.
RESULTS AND DISCUSSION
Primer design, computation of specificity, and analysis of amplification range.
The heterogeneity of fungal taxa and the homology of fungi to other eukaryotes made it unfeasible to develop a single specific primer set with both a satisfactory fungal amplification range and a sufficiently low 18S homology to other eukaryotes. The target sequences of the primers shown in Fig. 1 demonstrate that the EF4-EF3 primer set exhibited only an occasional mismatch with some fungi. From the computer comparison of EF4-EF3 to the RDP database, in which one mismatch was allowed for each primer, it was predicted that 78% of all fungal 18S-rDNA sequences in the aligned database would be amplified (Table 1). This is only an estimation, since it is not known how many mismatches can be tolerated under the given PCR conditions. Primer set EF4-EF3 exhibits a relatively high coverage of the Basidiomycota (95 to 100%) compared to EF4-fung5. The latter primer pair shows a relatively high coverage of the Ascomycota and a lower coverage of the Basidiomycota. Neither set of primers has full coverage of the Zygomycota; EF4-EF3 will theoretically amplify 75% of the species of this taxon, while EF4-fung5 will amplify only 40%. According to the computer analysis, both sets of primers for the analysis of fungal communities should adequately amplify most species of the different fungal taxa, except for the Chytridiomycota, which are regarded as having little importance for soil ecosystems.
The results in Table 2 demonstrate that all fungi studied, except C. sporulosum, Candida lipolytica, and Aureobasidium pullulans, could be amplified by using primer set EF4-EF3. Although A. pullulans and C. sporulosum could be amplified with EF4-fung5 and EF4–NS3-GC, little difference was found between the amplification ranges of EF4-EF3 and EF4-fung5 for the Ascomycota. Quite a difference can be observed for the Zygomycota, since EF4-fung5 amplified only two of the four species amplified by EF4-EF3. The four species of Basidiomycota tested could be amplified by all three primer sets, except for Ustilago avenae, which failed to be amplified by EF4-fung5. Although the results of the computer prediction (Table 1) are based on the assumption that only one mismatch per primer is allowed, the amplification coverage of the isolates (Table 2) seems to show the same trend. However, the amplification of 18S rDNA from fungal isolates does not necessarily predict the performance of the primer sets in environmental samples which harbor complex communities.
Analysis of the EF4-fung5 clones from wheat rhizosphere soil.
About 70 clones of the 18S-rDNA sequences amplified with EF4-fung5 obtained from the wheat rhizosphere sample were checked for the presence of an insert of the expected size (approximately 0.5 kb) by reamplifying the fragments directly from colony material. Thirty-nine E. coli clones with the inserts of the correct size were further analyzed by sequencing.
Sequences were analyzed by using the Blast 2.0 (advanced) program at the NCBI and by applying the Sequence Match program of the RDP. Both programs yielded identical results for almost all clones. In Table 3, the species of fungus with 18S-rDNA sequences most similar to the clone are listed. In cases where different results were obtained, both alignments were manually checked and the most likely species were chosen. Among the 39 sequences, 17 different species were identified. Some nonidentical cloned sequences matched the sequences of identical species. This is probably due to the incompleteness of the database, although PCR-related amplification errors cannot be excluded. As shown in Table 3, most clones detected by PCR with EF4-fung5 belong to the euascomycetes of the Ascomycota. No Basidiomycota were detected, although the computer-based analysis and isolate tests (Table 2) indicate that EF4-fung5 could amplify some members of the basidiomycetes. Besides PCR bias, cloning bias could also play a role. One sequence of the Zygomycota was detected (UUF64), and a sequence of the Chytridiomycota was found, although the computer analysis predicts that only 14% of the species of this taxon can be amplified. Only one clone, UUF31, completely matched a sequence in the database (Eupenicillium javanicum). All other clones exhibited similarities ranging from 0.676 to 0.978 to various database sequences. Clones with a similarity to a fungal species below 0.95 can be expected to have originated from a fungal species only phylogenetically related to the given name. This suggests that most of the species names are only very approximate indications of the identities of the cloned sequences. Clones with low similarities probably originated from species distantly related to those present in the database and whose 18S-rDNA sequences have not been determined, or they are from fungi yet to be isolated. For instance, several clones matched Neocosmospora vasinfecta, which is not common in the soil of temporate regions (7a). Since N. vasinfecta has 18S sequences similar to those of Fusarium solani, the sequences detected could represent Fusarium species from which no 18S sequences are present in the database. Nevertheless, it is of importance for judging the primer performance that genera matching clones of such organisms as Cladosporium, Eupenicillium, Exophiala, and Pleospora and the potential plant pathogens Alternaria and Verticillium are common in soil (4, 6, 10, 11, 19). Both Spizellomyces- and Mycosphaerella-like sequences (as in clones UUF54 and UUF68) were detected by Kowalchuk et al. (13) in association with Marram grass roots.
Analysis of the EF4-EF3 clones from wheat rhizosphere soil.
The clones obtained by PCR with EF4-EF3 are depicted in Table 4. Of 50 clones, 26 were found to have inserts of the expected size, and out of these 26 clones, only 22 could be sequenced with the use of the internal primer FS7. Nine different species were identified among the twenty-two clones. This primer set obviously is biased to amplifying Basidiomycota, since most of the species that were detected belong to this taxon. Clones resembling the yeast-like symbionts of the genus Symbiotaphrina were detected by using both primer sets. The relatively low similarities suggest that the cloned 18S rDNA could represent distant euascomycete relatives of these symbionts (21). A large number of clone sequences matched the fungus Mortierella polycephala. Obviously these sequences were related only to those of M. polycephala since this species is not a common soil inhabitant, and it might be more likely that the sequences represent fungi such as Mortierella alpina or Mortierella elongata, whose 18S sequences are not present in the database (7a). A thorough phylogenetic analysis of the clones with sequences of related species could give more information; this is, however, beyond the scope of the present report.
Examining the fungal diversity of the wheat rhizosphere with both primer systems revealed that 24 different species were detected among 61 clones. Clone diversity is much greater since no clones were found with similar sequences, and the number of species in the database limits the potential matches that can be found. The high sequence diversity and the broad taxonomic range of the clones are indicative of the importance of using both primer sets in a complementary fashion in studies of rhizosphere fungal communities.
TGGE analysis of the fungal community and clones from a wheat rhizosphere.
TGGE analysis of amplified fungal 18S-rDNA fragments from wheat rhizosphere samples was done by using a nested PCR approach in two amplification rounds, the first with EF4-EF3 or EF4-fung5 and the second with EF4–NS3-GC. Analysis of the fungal community profiles from soil samples of the microcosm experiment by using either EF4-EF3 or EF4-fung5 in the first selective amplification round produced different banding patterns when the different primer sets were used (not shown). This result supported the biases of both primer systems which were found by using the clone libraries. This confirms that, in order to visualize as many bands as possible, both primer systems should be used in combination to study fungal diversity in soil. Repeated analyses of the TGGE patterns of duplicate soil samples, with either primer pair, showed little variation, which suggests good reproducibility of the DNA extraction procedure, the PCR amplification, and the TGGE analysis.
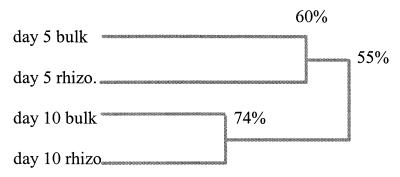
The soil samples from the microcosm experiment could be adequately analyzed by mixing the PCR products of both primer systems before electrophoresis. TGGE analysis of mixed, duplicate samples revealed differences between both the day 5 and 10 fungal communities and the bulk and rhizosphere samples (Fig. 2 and 3). Banding patterns of the bulk and rhizosphere samples at days 5 and 10 revealed considerable differences: in the bulk soil sample at day 5, 24 bands were present, while in the rhizosphere sample, 20 bands could be visualized. On day 10, 26 bands could be produced from the bulk sample, and 25 bands from the rhizosphere sample could be visualized. A dendrogram representing the differences between the profiles indicates 60% similarity between the bulk and rhizosphere samples at day 5 and 75% at day 10 and a similarity of 55% between day 5 and 10 profiles (Fig. 3). Results suggest that both the presence of plant roots and the time of sampling of the microcosms affects fungal diversity and composition in soil. Considering the number of bands, diversity seems to be somewhat lower in the rhizosphere than in bulk soil.
FIG. 3.
A dendrogram based on the presence or absence of bands (Fig. 2) was constructed to represent the percentages of genetic identity between the profiles of the microcosm samples of bulk and rhizosphere (rhizo) soils.
By comparing the single-clone bands with those of the community profiles, the presumptive identities of some of the bands in the community profile were obtained (Fig. 2). It should be stated that this method provides only circumstantial evidence of identity. For more exact identification, bands should be excised and sequenced. Some bands, such as band 1, which might be UUF10, band 3, which could be UUF30-31, and band 5, of unknown identity (Fig. 2), appear in all sample profiles (Fig. 2). Clones UUF30 and UUF31 have high similarities to E. javanicum, which is common in both soil and rhizosphere (6). Other bands appear in specific samples, such as band 2, which is of high intensity in day 10 samples, and band 4, which matches clone UUF103, which is mainly found in day 10 samples. Band 7, of unknown origin, is found only in day 5 samples. The group of bands around the position of band 6 matched some bands of the clones with Mortierella-like sequences, which appeared to have different mobilities (not shown).
Primer performance and resolution.
The results of this study show that primer sets enabled us to detect sequences of all major fungal taxa, except the hemiascomycetes (yeasts) and archaeascomycetes in wheat rhizosphere soil samples. Analysis of the fungal community by TGGE allows the study of the differences between samples and the dynamics of certain species. Comparison of cultured isolates from soil to 18S-rDNA sequences was not feasible, since this study did not thoroughly isolate fungi. Nevertheless, any overlap in species identification due to the use of both methods is expected to be small, since plate count techniques favor the isolation of fast-growing, low-substrate-specific, spore-producing fungi (25), while molecular techniques probably favor numerically dominant fungi with relatively high amounts of vegetative mycelium. Moreover, the sequences should be efficiently amplified and cloned in order to be detected. Fusaria are often found in soil and especially in the wheat rhizosphere by plate techniques (11, 12). One striking observation of this study is that no Fusarium species were identified. This is in line with the work of Kowalchuk et al. (13), who studied Marram grass roots and also did not detect fusaria with their PCR method. However, no matches can be found, since a screening of the database showed that no 18S-rDNA sequences from Fusarium species were present (only 28S and internal transcribed spacer sequences were present in the database). This clearly illustrates that a shortcoming of the use of 18S rDNA to detect and identify fungi lies in the incompleteness of existing databases and the taxonomic resolution of the sequence. For certain fungi, either the ITS region or the 28S rDNA gives a higher resolution which enables discrimination between closely related species. At the moment, a major disadvantage of using these sequences to develop primers for all fungi in soil is the relatively low number of sequences present in the 28S-rDNA database. However, for the amplification of specific fungal groups, such as Fusarium, development of primers for 28S-rDNA sequences will probably be much more suitable and feasible.
The combination of cloning and sequencing with whole-community TGGE analysis, although still in its infancy for fungi, has been shown to be a powerful technique for elucidating the differences and dynamics of the fungal community in the rhizosphere.
ACKNOWLEDGMENTS
This investigation was performed for the Dutch Ministry of Housing, Spatial Planning and Environment, Directorate General for Environmental Protection.
We are indebted to K. Smalla and coworkers for their help and instructions with the TGGE technique. We also thank W. Gams and G. Kowalchuk for critically reading the manuscript and for their valuable advice.
REFERENCES
- 1.Alabouvette C. Biological control of Fusarium wilt pathogens in suppressive soils. In: Hornby D, editor. Biological control of soil-borne pathogens. Wallingford, United Kingdom: CAB International; 1990. pp. 27–34. [Google Scholar]
- 2.Bock M, Maiwald M, Kappe R, Näher H. Polymerase chain reaction-based detection of dermatophyte DNA with a fungus-specific primer system. Mycoses. 1994;37:79–84. doi: 10.1111/j.1439-0507.1994.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 3.Borneman J, Skroch P W, O’Sullivan K M, Palus J A, Rumjanek N G, Jansen J L, Nienhuis J, Triplett E W. Molecular microbial diversity of an agricultural soil in Wisconsin. Appl Environ Microbiol. 1996;62:1935–1943. doi: 10.1128/aem.62.6.1935-1943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen M. Species diversity and dominance in fungal communities. In: Wicklow D T, Carroll G C, editors. The fungal community. New York, N.Y: Marcel Dekker, Inc.; 1981. pp. 201–232. [Google Scholar]
- 5.De Ley F A A M, Lynch J M. Functional diversity of the rhizosphere. In: Ogoshi A, Kobayashi K, Homma Y, Kadoma F, Kondo N, Akino S, editors. Plant growth-promoting rhizobacteria. Proceedings of the Fourth International Workshop on plant-growth promoting rhizobacteria. Paris, France: OECD; 1997. pp. 38–43. [Google Scholar]
- 6.Domsch K H, Gams W, Anderson T-H. Compendium of soil fungi. Vol. 1. London, England: Academic Press; 1980. [Google Scholar]
- 6a.Ferguson A. Biochemical systematics and evolution. Glasgow, United Kingdom: Blackie; 1980. [Google Scholar]
- 7.Gams W, Van Laar W. The use of solacol (validamycin) as a growth retardant in the isolation of soil fungi. Neth J Plant Pathol. 1982;88:39–45. [Google Scholar]
- 7a.Gams, W. Personal communication.
- 8.Heuer H, Krsek M, Baker P, Smalla K, Wellington E M H. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol. 1997;63:3233–3241. doi: 10.1128/aem.63.8.3233-3241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiorns W D, Hastings R C, Head I M, McCarthy A J, Saunders J R, Pickup R W, Hall G H. Amplification of 16S ribosomal RNA genes of autotrophic ammonia-oxidizing bacteria demonstrates the ubiquity of Nitrospiras in the environment. Microbiology. 1995;141:2793–2800. doi: 10.1099/13500872-141-11-2793. [DOI] [PubMed] [Google Scholar]
- 10.Hurek T, Wagner B, Reinhold-Hurek B. Identification of N2-fixing plant- and fungus-associated Azoarcus species by PCR-based genomic fingerprints. Appl Environ Microbiol. 1997;63:4331–4339. doi: 10.1128/aem.63.11.4331-4339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarosik V, Kovacikova E, Maslowska H. The influence of planting location, plant growth stage and cultivars on microflora of winter wheat roots. Microbiol Res. 1996;151:177–182. [Google Scholar]
- 12.Khanna R, Chandra S, Khanna K K. Rhizosphere microflora of Triticale. Biol Mem. 1993;19:111–121. [Google Scholar]
- 13.Kowalchuk G A, Gerards S, Woldendorp J W. Detection and characterization of fungal infections of Ammophila arenaria (Marram grass) roots by denaturing gradient gel electrophoresis of specifically amplified 18S rDNA. Appl Environ Microbiol. 1997;63:3858–3865. doi: 10.1128/aem.63.10.3858-3865.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liesack W, Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992;174:5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumsden R D. Ecology of mycoparasitism. In: Wicklow D T, Carroll G C, editors. The fungal community. New York, N.Y: Marcel Dekker, Inc.; 1981. pp. 295–328. [Google Scholar]
- 16.Maidak B L, Olsen G J, Larson N, Overbeek R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:106–111. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Murcia A J, Acinas S G, Rodriguez-Valera F. Evaluation of prokaryotic diversity by restrictase digestion of 16S rDNA directly amplified from hypersaline environments. FEMS Microbiol Ecol. 1995;17:247–256. [Google Scholar]
- 18.Moré M I, Herrick J B, Silva M C, Ghiorse W C, Madsen E L. Quantitative cell lysis of indigenous microorganisms and rapid extraction of microbial DNA from sediment. Appl Environ Microbiol. 1994;60:1572–1580. doi: 10.1128/aem.60.5.1572-1580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller-Dombois D. Ecological measurements and microbial populations. In: Wicklow D T, Carroll G C, editors. The fungal community. New York, N.Y: Marcel Dekker, Inc.; 1981. pp. 173–184. [Google Scholar]
- 20.Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noda H, Kodama K. Phylogenetic position of yeastlike endosymbionts of Anobiid beetles. Appl Environ Microbiol. 1996;62:162–167. doi: 10.1128/aem.62.1.162-167.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rand K H, Houck H, Wolf M. Detection of candidemia by polymerase chain reaction. Mol Cell Probes. 1994;8:215–222. doi: 10.1006/mcpr.1994.1030. [DOI] [PubMed] [Google Scholar]
- 23.Smit E, Leeflang P, Wernars K. Detection of shifts in microbial community structure and diversity in soil caused by copper contamination using amplified ribosomal DNA restriction analysis. FEMS Microbiol Ecol. 1997;23:249–261. [Google Scholar]
- 24.Sogin M L. Amplification of ribosomal RNA genes for molecular evolution studies. In: Innes M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols. San Diego, Calif: Academic Press; 1990. pp. 307–320. [Google Scholar]
- 25.States J S. Useful criteria in the description of fungal communities. In: Wicklow D T, Carroll G C, editors. The fungal community. New York, N.Y: Marcel Dekker, Inc.; 1981. pp. 185–200. [Google Scholar]
- 26.Thorn R G, Reddy C A, Harris D, Paul E A. Isolation of saprophytic basidiomycetes from soil. Appl Environ Microbiol. 1996;62:4288–4292. doi: 10.1128/aem.62.11.4288-4292.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torsvik V, Sørheim R, Goksør J. Total bacterial diversity in soil and sediment communities—a review. J Ind Microbiol. 1996;17:170–178. [Google Scholar]
- 28.Walsh T J, Francesconi A, Kasai M, Chanock S J. PCR and single-strand conformational polymorphism for recognition of medically important opportunistic fungi. J Clin Microbiol. 1995;33:3216–3220. doi: 10.1128/jcm.33.12.3216-3220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weidner S, Arnold W, Pühler A. Diversity of uncultured microorganisms associated with the seagrass Halophila stipulacea estimated by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes. Appl Environ Microbiol. 1996;62:766–771. doi: 10.1128/aem.62.3.766-771.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White T J, Burns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phyologenetics. In: Innes M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols. San Diego, Calif: Academic Press; 1990. pp. 315–322. [Google Scholar]