Abstract
Purpose
The aim of this study is to analyze SMAD 3, integrin and VEGF expressions in the periodontal ligament during orthodontic tooth movement induced by hyperbaric oxygen therapy and Stichopus hermanii.
Materials and Methods
Thirty Cavia cobaya were divided into 5 groups, namely, a normal control group (KN) without installation of helical springs or administration of HBOT and Stichopus hermanii gel. The negative control K(-) had helical spring without administration of HBOT and Stichopus hermanii gel for 14 days, while P1 had helical spring for 14 days then on day 3–14, Stichopus hermanii gel was added. Also, the helical spring was installed in P2 for 14 days then on day 8–14, HBOT 2.4 ATA was added 3 × 30 minutes a day, while P3 had helical spring for 14 days then on day 3–14, the gel was applied, and on day 8–14, HBOT 2.4 ATA was administered 3 × 30 minutes a day. Furthermore, SMAD3, integrin, and VEGF expressions were examined using immunohistochemical staining.
Results
SMAD3, integrins, and VEGF expressions showed significant differences within the groups. The combination of HBOT and Stichopus hermanii increased the expression of SMAD3 and VEGF compared to the single administration of Stichopus hermanii. The combination treatment also decreased integrin expression compared to a single HBOT administration.
Conclusion
The combination of HBOT and Stichopus hermanii increases the expression of SMAD3, integrins, and VEGF compared to control but did not show significant differences compared to single HBOT treatment.
Keywords: collagen, vascularization, periodontal ligament fibers, orthodontic treatment
Introduction
The global prevalence of malocclusion is 56%, with no differences in gender.1,2 It is defined as an irregularity in teeth alignment during dental occlusion beyond the normal range, thereby affecting several oral functions, such as chewing, swallowing, speaking,3 and temporomandibular dysfunction. It also influences dentofacial aesthetic and psychosocial self-reliance.4,5
Treatment for correcting malocclusion using orthodontic appliance aims to achieve an aesthetic dentofacial appearance by adjusting the crowding of teeth, rotational and apical deviations, as well as creating good occlusion within a period of ± 1–2 years. Accelerating orthodontic treatment can reduce the duration of the active phase of therapy and also increase patient satisfaction.6 There are many methods regarding accelerating tooth movement using pharmacological agent, physical stimuli and also surgical.
Orthodontic appliance pressure such as helical spring appliance when applied to the tooth causes movement that leads to the remodeling of the periodontal ligament and alveolar bone in areas of stress and tension.6 Helical spring, in which a wire is wrapped in a coil that appears as a screw thread, is the most commonly used mechanical spring. It can be designed to press or pull the mechanical force. The tissues usually affected during Orthodontic Tooth Movement (OTM) include the periodontal ligament, capillaries and nerves, alveolar bone, as well as the cementum.7 Meanwhile, several cellular changes commonly observed during this procedure include modifications in cells, cell-matrix, and gingival crevicular fluid (GCF), which is a biomarker of the periodontium.8 Vascularization is also important in remodeling,9 considering that Vascular Endothelial Growth Factor (VEGF) plays a role in collagen synthesis.10 Collagen is a constituent of the periodontal ligament, while SMAD3 is a protein transcription factor needed for its proliferation.11 Also, integrin protein plays a vital role in the binding of the periodontal ligament to the alveolar bone.12
Oxygen is important in periodontal tissue remodeling,8,10 while Hyperbaric Oxygen Therapy (HBOT) is a treatment method for breathing pure oxygen (100%) at a pressure greater than the normal range. It is usually given within the range of 1 to 3 (1.5 and 2.4) ATA with a duration of 90–120 minutes.8,10 Previous studies showed that hyperbaric oxygen stimulates repair and growth of blood vessels, prevents the inflammatory phase, reduces free radicals, and increases osteoblast activity in tooth movement.8 Furthermore, Stichopus hermanii is a marine biota that plays a role in inflammation, wound healing, and also contains active ingredients of flavonoids, chondroitin sulfate, hyaluronic acid, and arginine, which accelerate the remodeling process of orthodontic tooth by 60%.12,13
The combination of HBOT and Stichopus hermanii is thought to play a role in the remodeling of the periodontal ligament and alveolar bone to accelerate orthodontic tooth movement. Stichopus hermanii functions locally when administered topically, while HBOT acts systemically through inhalation.14,15 Local treatment of Stichopus hermanii and systemic treatment of hyperbaric oxygen yield favorable outcomes in orthodontic tooth movement due to the consideration variability of periodontal ligament remodeling during orthodontic tooth movement. The combination of these two agents with a logical approach suggests that Stichopus hermanii can increase periodontal ligament remodeling due to active ingredients such as collagen, flavonoid, chondroitin sulphate, and hyaluronic acid, in line with hyperbaric oxygen, which can increase periodontal ligament remodeling through increased vascularization. However, studies on the combination of the two treatments for accelerating tooth movement have not been carried out. This study aims to analyze SMAD 3, integrin and VEGF expressions in a periodontal ligament during orthodontic tooth movement induced by hyperbaric oxygen therapy and Stichopushermanii.
Materials and Methods
Experimental Design and Ethics Approval
This is a true experimental study with a single post-test control group design. Meanwhile, ethical permission and clearance was approved and issued by The Ethical Clearance of Health Experiment Committee, Faculty of Dentistry Hang Tuah University, registration number 055/KEPK/I/2020. All Cavia cobaya were healthy and housed in accordance with Government Regulation of The Republic of Indonesia Number 95 of 2012 Concerning Veterinary Public Health And Animal Welfare. Research and testing using animal should comply with ethical principles standard means a technical specification or standardized including procedures and methods prepared based on consensus of all- parties concerned with due regard to the requirements for safety, security, health, environment, development of science and technology, and experience of current and future developments to obtain maximum benefit.
Preparation of Animal Model
This study used experimental animals, namely, male Cavia cobaya aged between ± 2 and 3 months, which is categorized as young adults given that the married age ranges from 3 to 4 months. Also, the bodyweight ranged from 300 to 400 grams, and all had good physical health, while the randomization technique used was simple random allocation. Thirty samples were divided into 5 groups, namely a normal control (KN) without helical springs installation or administration of HBOT and Stichopus hermanii gel as well as a negative control K (-) with helical spring installation but without HBOT and Stichopus hermanii gel for 14 days. Furthermore, P1 was the treatment group with the helical spring installed for 14 days and then on days 3–14, the gel was added. Similarly, P2 was a treatment group with helical spring installation for 14 days then on day 8–14, HBOT 2.4 ATA was added 3 × 30 minutes a day, while P3 group had helical spring installed for 14 days, then on day 3–14, Stichopus hermanii gel was applied, and on day 8–14, HBOT 2.4 ATA was given 3 × 30 minutes a day.11,13 Cavia cobaya was prepared according to pre-determined criteria, the samples were kept in a cage and placed in a space with sufficient air and light, while food was provided by placing it in a container with drinking water ad libitum. Furthermore, the samples were adapted for 1 week to obtain good general health and adjustment to the environment before treatment, while the parameter measurements were carried out routinely to meet sample criteria.
Preparation of Stichopus hermanii Gel
Stichopus hermanii purchased from the Madura (Sumenep) island in wet conditions was cleaned for the stomach contents and washed under running water. Furthermore, it was placed in a plastic bag, weighed up to 1 kg, and then blended using a Waring Commercial Model HGBTWT laboratory blender until smooth and placed into an Erlenmeyer flask with a spatula and dried (freeze dryer) at −85°C with a pressure of 5 m Torr for approximately 72 hours. The dried sample was then weighed and blended again using a laboratory blender until 31 grams of powder was obtained.
Moreover, Stichopus hermanii gel with a dose of 3.5% was prepared by mixing 0.35 g of 100% powder with 0.2 g of NaCMC. The 2% NaCMC gel was made by mixing 0.2 g of the powder with 10 mL of distilled water. Each of these mixtures was then stirred using a mortar and pestle on a hot plate.
Stichopus hermanii Gel Administration to Cavia cobaya
The experimental animals were prepared as follows: the insulin syringe needle was blunt using scissors to avoid damaging the gingival sulcus. Afterward, incisive bands and helical springs were installed on the left and right maxillary incisors using a plier for 14 days in groups K(-), P1, P2, and P3. The administration of Stichopus hermanii gel started from the third day after the orthodontic appliance was installed and removed until the 14th day 2 times daily with 0.025 mL in the morning and afternoon consecutively. Stichopus hermanii gel was made with a concentration of 3.5% by mixing 0.35 g of 100% powder with 0.2 g of NaCMC, dissolved in 10 mL of distilled water. Meanwhile, HBOT was applied with a pressure of 2.4 ATA for 3 × 30 minutes a day with 5-minute intervals inhaling normal air was for 7 consecutive days.
Preparation of Hyperbaric Oxygen Therapy in Cavia cobaya
The hyperbaric oxygen therapy in groups P2 and P3 was carried out on days 8–14 without releasing the maxillary expansion in experimental animals. Meanwhile, in the chamber, the animal felt uncomfortable due to changes in air pressure, which tend to cause ear pain. This pain was overcome by giving food/drink; therefore, the swallowing process occurs which then reduced the pain. After the P2 and P3 groups were put into the multiplace chamber, the pressure was increased to 2.4 ATA and 100% pure oxygen flowed for 3 × 30 minutes per day with 5-minute intervals breathing with normal air, then the process was stopped and lowered to its original condition, namely 1 ATA. The samples were then removed from the chamber and brought to the original cage; this treatment was carried out on days 8–14.
Immunohistochemical Staining for SMAD, Integrin and VEGF Expression in Periodontal Ligament
On the 14th day, groups K(N), K(-), P1, P2, and P3 were sacrificed to measure the distance of tooth movement using overdose anesthesia, namely, Overdose of Chemical Anesthetic and ketamine-acepromazine, then the samples were decapitated to take the maxilla and the teeth. Furthermore, the maxilla was fixed in a buffered solution of formalin and Ethylene Diamine Tetra Acid (EDTA). The next step was deparaffinization and staining process of IHC (Immunohistochemical) with SMAD3, Integrin, and VEGF monoclonal antibodies. Positive SMAD3, integrin and VEGF expressions were examined with CX22 Binocular, Olympus (×200). Expressions in each sample were assessed semiquantitatively based on the modified Kaemmerer method.15 Immunoreactive Score (IRS) is scored by multiplication percentage of positive cells score with the intensity of staining score produced in that cell. The percentage of positive cells score is based on: 0 = no positive cells, 1 = positive cells less than 30%, 2 = positive cells 30%–60%, 3 = positive cells more than 60%. The intensity of staining score produced in that cell indicator is based on: 0 = no color reaction, 1 = low intensity of the color reaction, 2 = medium intensity, and 3 = intense intensity. Positive cells with anti-SMAD3, integrin and VEGF indicated brown color. Data obtained from the calculated expressions of SMAD3, integrin and VEGF in tension area were tabulated and were analyzed by Kruskal–Wallis test and continued with Mann–Whitney test.
Results
This study aims to SMAD 3, integrin and VEGF expression in a periodontal ligament during orthodontic tooth movement induced by hyperbaric oxygen therapy 2.4 ATA and Stichopus Hermanii gel 3.5%. Data on the expression of SMAD3 (Figure 1), integrins (Figure 2), and VEGF (Figure 3) were obtained from calculations on immunohistochemical images of the periodontal ligament in the tension area with 400x magnification. Positive cells with anti-SMAD3, integrin and VEGF indicated brown color. The ordinal data obtained were analyzed using descriptive statistical tests as well as Kruskal–Wallis and Mann–Whitney tests with a significance level of 95% (P = 0.05) using the SPSS version 22 program. The immunohistochemical scoring parameters for SMAD3 expression, Integrin, and VEGF are presented using a point score (Table 1).
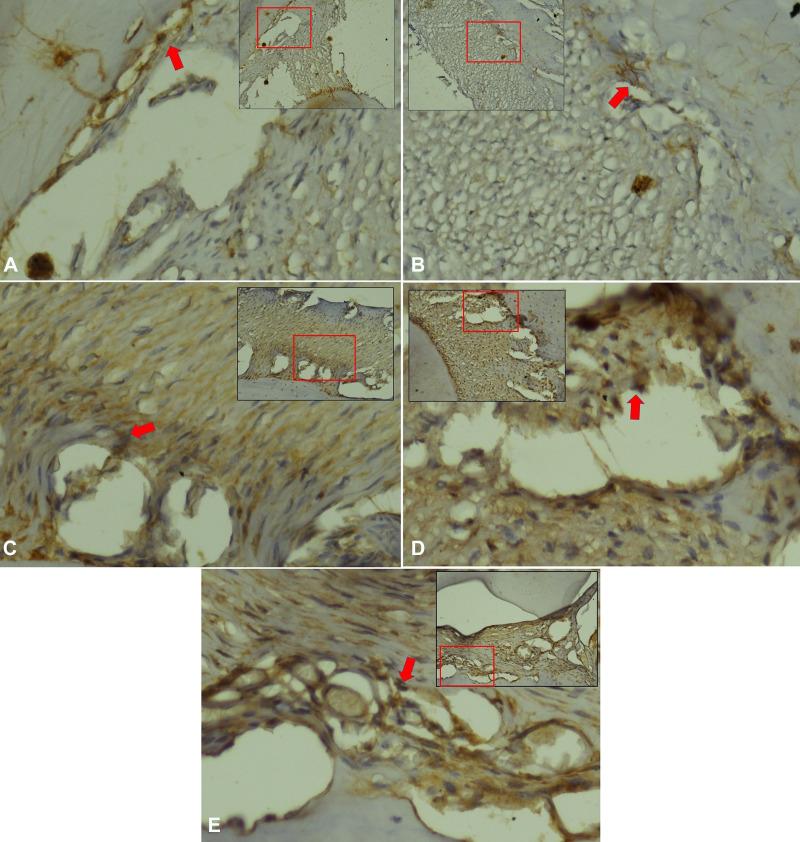
Figure 1.
SMAD3 expression in periodontal ligament Cavia cobaya. (A) SMAD3 expression in negative control group. (B) SMAD3 expression in normal control group. (C) SMAD3 expression in treatment group with Stichopus hermanii gel (D) SMAD3 expression in treatment group with HBOT (E) SMAD3 expression in treatment group with HBOT and Stichopus hermanii gel. Positive SMAD expression marked in brown (red arrow) examination with CX22 Binocular, Olympus (×200).
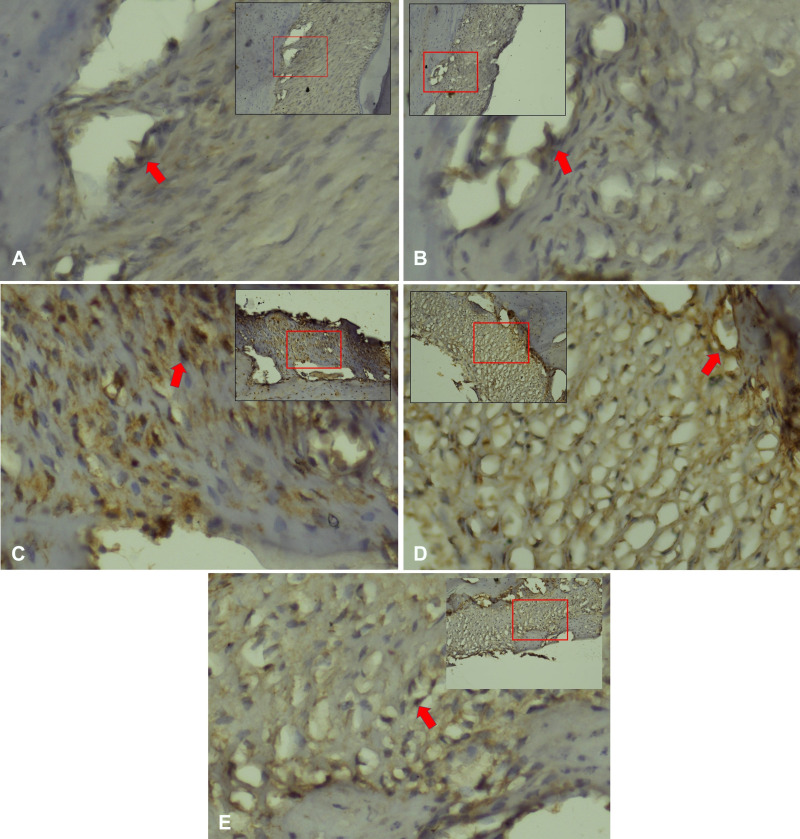
Figure 2.
Integrin expression in periodontal ligament Cavia cobaya. (A) Integrin expression in negative control group. (B) Integrin expression in normal control group. (C) SMAD3 expression in treatment group with Stichopus hermanii gel (D) Integrin expression in treatment group with HBOT (E) Integrin expression in treatment group with HBOT and Stichopus hermanii gel. Positive Integrin expression marked in brown (red arrow) examination with CX22 Binocular, Olympus (×200).
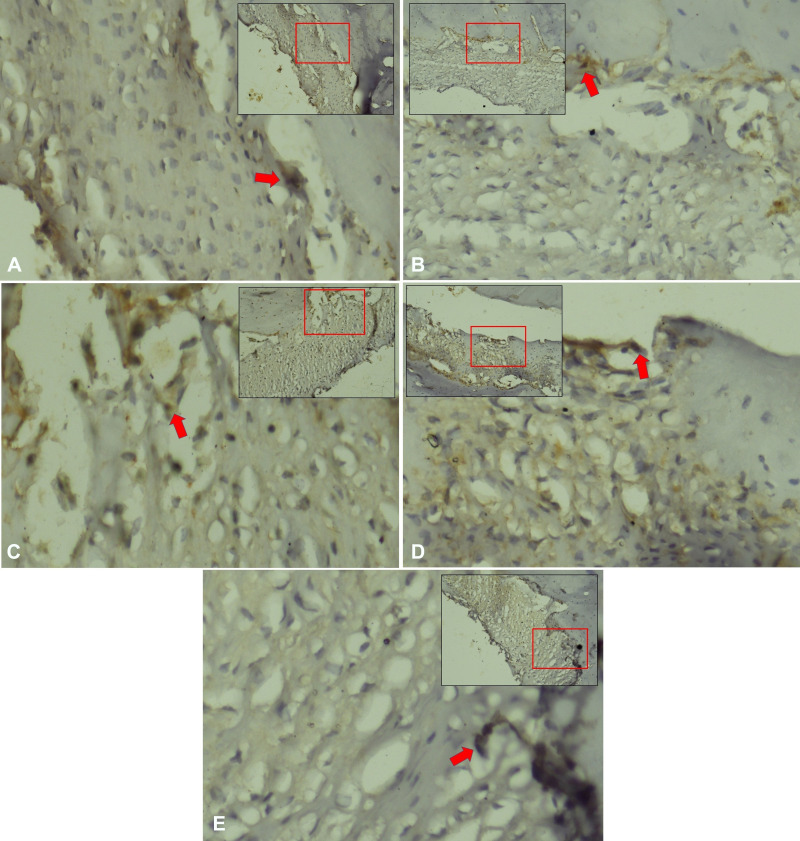
Figure 3.
VEGF expression in periodontal ligament Cavia cobaya. (A) VEGF expression in negative control group. (B) VEGF expression in normal control group. (C) VEGF expression in treatment group with Stichopus hermanii gel (D) VEGF expression in treatment group with HBOT (E) VEGF expression in treatment group with HBOT and Stichopus hermanii gel. Positive VEGF expression marked in brown (red arrow) examination with CX22 Binocular, Olympus (×200).
Table 1.
Immunohistochemical Scoring Parameters
| Point Score | A | B | AXB |
|---|---|---|---|
| 0 | No cells with a positive reaction. | No color reaction. | 0–1 = negatif |
| 1 | ≤10% cells with a positive reaction. | The low intensity of the color reaction. | 2–3 = low |
| 2 | 11%-50% cells with a positive reaction. | The medium intensity of the color reaction. | 4–8 = medium |
| 3 | 51%-80% of cells with a positive reaction. | Intense color reaction. | 9–12 = strong |
| 4 | 80% cells with a positive reaction. |
Notes: The IRS semiquantitative scale is a product of the percentage of the positive cells score (A) with the intensity of staining score (B), becoming an immunoreactive score (IRS) = (A x B).
Immunohistochemical Overview of SMAD3, Integrin, VEGF Expression in the Periodontal Ligament
SMAD3 expression was examined using antibody staining from Sigma Aldrich, while integrin and VEGF used Abcam. The three components were assessed semi-quantitatively to calculate the expression score.
Examination of SMAD3, integrins, and VEGF expression scores used immunohistochemical methods to observe the differences between the groups, namely, K (N), K (-), P1, P2, and P3. The mean data show varying expression scores for the five groups. The combination of HBOT and Stichopus hermanii increased the expression of SMAD3, integrins, and VEGF compared to the control group. Based on the Kruskal–Wallis test, there was a significant difference in the SMAD3 (P ≤ 0.05), integrin (P ≤ 0.05), and VEGF expression (P ≤ 0.05) for the periodontal ligament during orthodontic tooth movement accelerated by the combination treatment (Table 2).
Table 2.
Mean and Median of SMAD3, Integrin, and VEGF Expression as Periodontal Ligament Parameters with the Administration of HBOT and Stichopus hermanii to Accelerate Tooth Movement
| Group | N | SMAD3 | Integrin | VEGF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | p-value | Mean | Median | p-value | Mean | Median | P-value | ||
| K(N) | 6 | 2 | 2 | 0.001* | 1.17±0.4 | 1 | 0.001* | 1 | 1 | 0.001* |
| K(-) | 6 | 5±1.1 | 5 | 1.33±0.5 | 1 | 1.83±1.1 | 1.5 | |||
| P1 | 6 | 5.83±1.8 | 6 | 2.83±1.1 | 3 | 1.83±0.4 | 2 | |||
| P2 | 6 | 8.67±2.6 | 9 | 3.5±0.8 | 4 | 2.67±0.8 | 2.5 | |||
| P3 | 6 | 8.5±1.2 | 9 | 2 | 2 | 3 | 3 | |||
Note: *significantly different on P ≤ 0.05.
The Mann–Whitney analysis results showed significant differences within groups for the three parameters. SMAD3 expression (Figure 1) in all treatment groups showed a significant increase compared to the normal control (P ≤ 0.05), but only the HBOT treatment group and its combination with Stichopus hermanii increased significantly compared to the control (-) (P ≤ 0.05). Among the treatment groups, the combined treatment showed a significant increase compared to the single administration of Stichopus hermanii with P = 0.023 (Table 3).
Table 3.
Association Among Expressions of SMAD3, Integrin, and VEGF as Parameters of the Periodontal Ligament with the Administration of a Combination of HBOT and Stichopus hermanii to Accelerate Tooth Movement Using Mann Whitney Test
| Groups | Mann Whitney Test (p-value) | ||
|---|---|---|---|
| K(N) vs K(-) | 0.002* | 0.523 | 0.058 |
| K(N) vs P1 | 0.002* | 0.016* | 0.005* |
| K(N) vs P2 | 0.002* | 0.003* | 0.002* |
| K(N) vs P3 | 0.001* | 0.005* | 0.001* |
| K(-) vs P1 | 0.423 | 0.029* | 0.523 |
| K(-) vs P2 | 0.024* | 0.004* | 0.068 |
| K(-) vs P3 | 0.005* | 0.019* | 0.038* |
| P1 vs P2 | 0.064 | 0.262 | 0.020* |
| P1 vs P3 | 0.023* | 0.107 | 0.001* |
| P2 vs P3 | 0.674 | 0.006* | 1.000 |
Note: *significantly different on P ≤ 0.05.
Integrin expression (Figure 2) in all treatment groups showed a significant increase compared to the normal control and the control group (-) with P ≤ 0.05. Among the treatment groups, the combination of HBOT and Stichopus hermanii decreased significantly compared to a single HBOT treatment (P = 0.006) (Table 3). VEGF expression (Figure 3) in all treatment groups showed a significant increase in the normal control group (P ≤ 0.05), but only the combined treatment showed a significant increase compared to the control group (-) (P = 0.038). Furthermore, the treatment group using HBOT and the combination with Stichopus hermanii showed a significant increase compared to the single administration of Stichopus hermanii (P ≤ 0.05) (Table 3).
Discussion
This study used a combination of 3.5% Stichopus hermanii gel on days 3–14 and hyperbaric oxygen therapy with 2.4 ATA on days 8–14 to analyze the expression of SMAD3, integrins, and VEGF of the periodontal ligament in the tension area of orthodontic tooth movement. The experimental animal used as the sample was Cavia cobaya.
Orthodontic tooth movement is characterized by changes in the remodeling of the periodontal ligament tissue (PDL), alveolar bone, pulp, and gingiva. This alters PDL vascularization, leading to a local synthesis of important molecules such as neurotransmitters, cytokines, growth factors, colony-stimulating factors, and arachidonic acid metabolites.16 When pressure is applied adequately to the teeth, it leads to the remodeling of the periodontal structure. In relation to oxygen administration, vascularization performs an important role in the process of remodeling and the oxygen pressure also contributes to a shared role.14 The administration of mechanical force with orthodontic pressure on the periodontal ligament causes changes in blood vessels, both in diameter and in the number of blood vessels, as well as transformations in the endothelium as a signal for periodontal tissue remodeling.10,14
The periodontal ligament is a collagenous structure that supports the tooth architecture separating it from the alveolar bone with a width of approximately 0.5 mm along the tooth root.17 Meanwhile, SMAD3 is a protein that maintains the stability of the periodontal ligament and induces collagen formation.11 The ligament responds to pressure on tooth movement,12,14,17 hence, stretching causes a decrease in blood flow and changes in blood vessels in both diameter and number, as well as changes in the endothelium.18 Furthermore, VEGF (Vascular Endothelial Growth Factor) is a growth factor that regulates vascularization and is released from the endothelium.10,14,17,19
The results showed that orthodontic mechanical stress affects SMAD3, while integrins and VEGF had no effect. This is consistent with a previous study, which reported that mechanical stress causes changes in type 1 collagen mRNA expression, and alkaline phosphatase activity in osteoblasts/cementoblasts.19 Mechanical stimulation causing an increase in type 1 collagen mRNA indicates that strain stress facilitates cellular differentiation from periodontal ligaments to osteoprogenitor cells.11
The use of marine biota for orthodontic treatment has been developed, for example, Stichopus hermanii 3% has been shown to raise tooth movement by 60% through increased bone remodeling.12,13 The compounds contained in Stichopus hermanii include lectins, sterols, saponins/tripertens glycosides, proteins, collagen, mucopolysaccharides, glycosaminoglycans, chondroitin sulfate E and fucosylate, amino acids, fatty acids including eicosapentaenate (EPA) and docosahexesaenate (DHA), vitamins, namely, thiamine, riboflavin, niacin, C, E, and Carotenoids. It also contains minerals such as iron, magnesium, calcium, zinc, chromium, as well as polyphenols, flavonoids, and SOD.20–22
According to Prameswari (2017) and Shahrulazua (2013), the highest content of Stichopus hermanii is 86% protein of which 80% is collagen, and others are minerals, mucopolysaccharides, glucosaminoglycans (GAGs), chondroitin, omega-3, 6, and 9, as well as cell growth factors. There was an increase in the average expression of SMAD3, integrins, and VEGF, but the most significant was integrins with P = 0.029.13,23 Compared with other studies using low-energy laser irradiation to accelerate orthodontic tooth movement, low-energy laser radiation has also been reported to have a role in fibroblast proliferation and collagen synthesis, too.24
Integrins are cell surface receptors composed of α- and β- subunits; it causes cell adhesion both in the cell-matrix and between cells, as well as transduces chemical and mechanical signals. Certain integrins have a function in mediating mechanical stress to induce proliferation and stress shear that activates extracellular regulated-protein kinases (ERKs), and c-Jun kinases (JNKs) pathways referred to as mechanotransduction.25 Furthermore, the expression of integrins in stretched ligaments plays a role in the transduction of mechanical stimuli and activates several signaling pathways.26 Integrin α2β1 is the main receptor of type 1 collagen expressed on Th 17 cells to mediate the attachment to bone27 and increase the synthesis as well as the turnover of type 1 collagen.12 Meanwhile, Stichopus hermanii has been shown to increase integrin α2β1 in previous studies on orthodontic relapse. The use of 2.5% and 3% gel increased integrin 2β1 as type 1 collagen, thereby decreasing orthodontic relapse.12
Based on the results, the administration of HBOT increased SMAD3, integrins, and VEGF expressions compared to normal (P ≤ 0.05) and controls (-) but only SMAD3 with P = 0.024 and integrins P = 0.04 showed significant differences. Anti-inflammatory cytokines, such as IL-4 and IL-10, have been demonstrated to increase expression after treatment with HBOT.28 Besides, HBOT modulates nitric oxide (NO), which contributes importantly to maintaining blood vessel tone, increasing VEGF and bFGF, and transforming growth factor-β1 (TGF-β) expression.10 Furthermore, VEGF, bFGF, TGF-β, together with fibroblasts, stimulate the synthesis of angiogenesis, which is one of the stages in wound healing.29 Angiogenesis involves the formation of new branches from pre-existing blood vessels with the onset of vasodilation in response to NO and the increased permeability by VEGF.30 Blood vessels also contribute significantly to providing oxygen, nutrients, and other materials that are important for orthodontic tooth movement.31 Meanwhile, HBOT increases TGF-β1, which plays a pivotal role in connective tissue regeneration and bone remodeling.32,33 The TGF-β family-induced SMAD pathway involves two different classes of serine/threonine kinase receptors called types I and II. SMAD pathway is a key process through which TGF-β1 modulates osteogenesis. The overexpression of SMAD3 in MC3T3-E1 cells enhances ALP activity and mineralization. Moreover, Borton et al showed that mice with a targeted deletion of SMAD3 had osteopenia and a decreased rate of bone formation.34
The combination of HBOT and Stichopus hermanii increased the expression of SMAD3, integrins, and VEGF compared to the normal (N) and the control group (-) with P ≤ 0.05 because the contents of the two components interact. The content of Stichopus hermanii which acts locally and HBOT systemically has a synergistic effect. HBOT increases SMAD3 through TGF-β,10,34 while Stichopus hermanii through glycosaminoglycans (chondroitin sulfate) also raise TGF-β and integrins levels.12,35
The combination of HBOT and Stichopus hermanii did not significantly increase SMAD3 and VEGF expression compared to single HBOT treatment, but integrin showed a significant decrease with P = 0.004. The interactions of integrins with the extracellular matrix extracellularly and with cytoskeletal components intracellularly are considered to be force-transducing elements in fibroblasts. Furthermore, the periodontal ligament fibroblasts interact with the extracellular matrix via integrin binding of different collagenous and non-collagenous substrates.36 Based on the results, integrin α2β1 decreased, indicating a reduction in the synthesis and the attachment of type 1 collagen as a matrix in the alveolar bone. Integrins as mechanical links act as mechanosensors and generate signals that affect cell physiology through complex intracellular signaling mechanisms, including autocrine and paracrine.37,38 Besides, mechanical tension also increases its affinity,39 therefore, the decrease in integrin indicates that the combination of HBOT and Stichopus hermanii exhibits reduced strain due to orthodontic mechanical stress.
Conclusion
The combination of HBOT and Stichopus hermanii increases the expression of SMAD3, integrins, and VEGF compared to the normal (N) and the control group (-) with P ≤ 0.05. However, the combination did not show significant differences compared to a single HBOT treatment. Dentists can use this combination of HBOT and Stichopus hermanii because this combination can maintain the stability of the periodontal ligament and induce collagen formation, as well as exhibits reduced strain due to orthodontic mechanical stress.
Acknowledgment
The authors gratefully acknowledge that the present research was supported by The Ministry of Education, Culture, Research, and Technology Republic of Indonesia, under research grant PDUPT of the year 2021.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lombardo G, Vena F, Negri P, et al. Worldwide prevalence of malocclusion in the different stages of dentition: a systematic review and meta-analysis. Eur J Paediatr Dent. 2020;21(2):115–122. doi: 10.23804/ejpd.2020.21.02.05 [DOI] [PubMed] [Google Scholar]
- 2.Afrashtehfar KI. Patient and miniscrew implant factors influence the success of orthodontic miniscrew implants. Evid Based Dent. 2016;17(4):109–110. [DOI] [PubMed] [Google Scholar]
- 3.Khan MT, Verma SK, Maheshwari S, Zahid SN, Chaudhary PK. Neuromuscular dentistry: occlusal diseases and posture. J Oral Biol Craniofac Res. 2013;3(3):146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellot-Arcís C, Montiel-Company JM, Almerich-Silla JM. Psychosocial impact of malocclusion in Spanish adolescents. Korean J Orthod. 2013;43(4):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masood Y, Masood M, Zainul NN, et al. Impact of malocclusion on oral health related quality of life in young people. Health Qual Life Outcomes. 2013;26(11):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proffit WR, Fields HW. Contemporary Orthodontics. 5th ed. St Louis: Mosby; 2012:76–82. [Google Scholar]
- 7.Bhalajhi SI. Orthodontic the Art and Science. 3rd ed. New Delhi: Arya (MEDI) Publishing House; 2006. [Google Scholar]
- 8.Brahmanta A, Soetjipto narmada IB. The expression of collagen type-I in the tension area of orthodontic tooth movement with adjuvant of hyperbaric oxygen therapy. Int J ChemTech Res. 2016;9(7):2455–9555. [Google Scholar]
- 9.Gokce S, Bengi AO, Akin E, et al. Effects of hyperbaric oxygen during experimental tooth movement. Angle Orthod. 2008;78(2):304–308. [DOI] [PubMed] [Google Scholar]
- 10.Brahmanta A, Prameswari N. VEGF regulates osteoblast differentiation in tension and pressure regions orthodontic tooth movement administered with hyperbaric oxygen therapy. J Int Dent Med Res. 2019;12(4):13. [Google Scholar]
- 11.Watanabe T, Yasue A, Tanaka E. Inhibition of transforming growth factor β1/smad3 signaling decreases hypoxia‐inducible factor‐1α protein stability by inducing prolyl hydroxylase 2 expression in human periodontal ligament cells. J Periodontol. 2013;84(9):1346–1352. [DOI] [PubMed] [Google Scholar]
- 12.Prameswari N, Prabowo PB. FGF-2, MMP-8 and integrin α2β1 expression in periodontal ligament remodelling tension area with nanopowder stichopus hermanii application to prevent orthodontic relapsing. Int J Mater Sci Appl. 2017a;6(6):284–289. [Google Scholar]
- 13.Prameswari N, Brahmanta A. The role of active ingredients nanopowder Stichopus hermanii gel to bone resorption in tension area of orthodontic tooth movement. Dent J. 2017;50(4):188–192. [Google Scholar]
- 14.Brahmanta A, Sutjipto IB. Histological changes during orthodontic tooth movement due to hyperbaric oxygen therapy. Dent J. 2016;49(2):63–66. [Google Scholar]
- 15.Neumeister M. Hyperbaric Oxygen Therapy. eMedicene. 2005;21:1–9. [Google Scholar]
- 16.Niklas A, Proff P, Gosau M, Romer P. The role of hypoxia in orthodontic tooth movement. Int J Dent. 2013;2013:841840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ariffin SHZ, Yamamoto Z, Abidin IZZ, Wahab RMA, Ariffin ZZ. Cellular and molecular changes in orthodontic tooth movement. Sci World J. 2011;11:1788–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nimeri G, Kau CH, Abou-Kheir NS, Corona R. Acceleration of tooth movement during orthodontic treatment - a frontier in orthodontics. Prog Orthod. 2013;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enokiya Y, Hashimoto S, Muramatsu T, et al. Effect of stretching stress on gene transcription related to early-phase differentiation in rat periodontal ligament cells. Bull Tokyo Dent Coll. 2010;51(3):129–137. [DOI] [PubMed] [Google Scholar]
- 20.Prameswari N, Handayani B. Stichopus hermanii stimulation to Runx2 expression as periodontal remodeling biomarkers to accelerate orthodontic tooth movement. IOP Conf Ser: Earth Environ Sci. 2019;217:012058. [Google Scholar]
- 21.Sarhadizadeh N, Afkhami M, Ehsanpour M. Evaluation bioactivity of a Sea cucumber, Stichopus hermanni from Persian gulf. Europ J Exp Biol. 2014;4(1):254–258. [Google Scholar]
- 22.Althunibat OY, Hashim R, Bakhtiar MT, Mohd DJ, Ikeda MA, Ibrahim Z. In vitro antioxidant and antiproliferative activities of three Malaysian Sea cucumber species. Eur J Sci Res. 2019;37:376–387. [Google Scholar]
- 23.Shahrulazua A, Samsudin AR, Iskandar MA, Amran AS. The in-vitro effects of Sea cucumber (Stichopus sp1) extract on human osteoblast cell line. Malays Orthop J. 2013;7(1):2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasai K, Chou MY, Yamaguchi M. Molecular effects of low-energy laser irradiation during orthodontic tooth movement. Semin Orthod. 2015;21(3):203–209. [Google Scholar]
- 25.Wernig F, Mayr R, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cell is mediated by β-1 integrin signaling pathways hypertension. J Am Heart Assoc. 2003;41(4):903–911. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Yao M, Zhou J, et al. The promotion of neural progenitor cells proliferation by aligned and randomly oriented collagen nanofibers through β1 integrin/MAPK signaling pathway. Biomaterials. 2011;32(28):6737–6744. [DOI] [PubMed] [Google Scholar]
- 27.Boisvert M, Chetoui N, Gendron S, Aoudjit F. Alpha2beta1 integrin is the major collagen-binding integrin expressed on human Th17 cells. Eur J Immunol. 2010;40(10):2710–2719. [DOI] [PubMed] [Google Scholar]
- 28.Shapira R, Solomon B, Efrati S, Frenkel D, Ashery U. Hyperbaric oxygen therapy ameliorates pathophysiology of 3xTg-AD mouse model by attenuating neuroinflammation. Neurobiol Aging. 2018;62:105–119. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129(4):469.e1–32. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell L. Introduction to Orthodontics. Oxford: Oxford University Press; 2013. [Google Scholar]
- 31.Domenico MD, D’Apuzzo F, Feola A, Cito L, Monsurro A, Pierrantoni GM. Cytokines and VEGF induction in orthodontic movement in animal models. J Biomed Biotechnol. 2012;2:201689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alghamdi MYM The effects of hyperbaric oxygen therapy on bone distant from sites of surgery. Thesis. University of Toronto. 2011. [Google Scholar]
- 33.Inokuchi T, Kawamoto T, Aoki K, et al. The effects of hyperbaric oxygen on tooth movement into the regenerated area after distraction osteogenesis. Cleft Palate Craniofac J. 2010;47(4):382–392. [DOI] [PubMed] [Google Scholar]
- 34.Ochiai H, Yamamoto Y, Yokoyama K, et al. Dual Nature of TGF-β1 in osteoblastic differentiation of human periodontal ligament cells. J Hard Tissue Biol. 2010;19(3):187–194. [Google Scholar]
- 35.Alliston T. Chondroitin sulfate and growth factor signaling in the skeleton: possible links to MPS VI. J Pediatr Rehabil Med. 2010;3(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barczyk M, Bolstad AI, Gullberg D. Role of integrins in the periodontal ligament: organizers and facilitators. Periodontol 2000. 2013;63(1):29–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michael KE, Dumbauld DW, Burns KL, Hanks SK, García AJ. FAK modulates cell adhesion strengthening via integrin activation. Mol Biol Cell. 2009;20:2508–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boettiger D. Mechanical control of integrin-mediated adhesion and signaling. Curr Opin Cell Biol. 2012;24:592–599. [DOI] [PubMed] [Google Scholar]