Abstract
Solvent-tolerant microorganisms are useful in biotransformations with whole cells in two-phase solvent-water systems. The results presented here describe the effects that organic solvents have on the growth of these organisms. The maximal growth rate of Pseudomonas putida S12, 0.8 h−1, was not affected by toluene in batch cultures, but in chemostat cultures the solvent decreased the maximal growth rate by nearly 50%. Toluene, ethylbenzene, propylbenzene, xylene, hexane, and cyclohexane reduced the biomass yield, and this effect depended on the concentration of the solvent in the bacterial membrane and not on its chemical structure. The dose response to solvents in terms of yield was linear up to an approximately 200 mM concentration of solvent in the bacterial membrane, both in the wild type and in a mutant lacking an active efflux system for toluene. Above this critical concentration the yield of the wild type remained constant at 0.2 g of protein/g of glucose with increasing concentrations of toluene. The reduction of the yield in the presence of solvents is due to a maintenance higher by a factor of three or four as well as to a decrease of the maximum growth yield by 33%. Therefore, energy-consuming adaptation processes as well as the uncoupling effect of the solvents reduce the yield of the tolerant cells.
Many organic solvents are toxic to living organisms because of their devastating effects on biological membranes (31). This toxicity correlates with the hydrophobic character of the solvent, expressed by the logarithm of its partition coefficient between octanol and water (log PO/W value). Solvents with a log PO/W value between 1 and 5, like toluene, are highly toxic to whole cells (30). Due to these toxic effects, the choice of solvents for whole-cell biotransformations in two-phase solvent-water systems is limited. Only less-toxic solvents with higher hydrophobicities (28) can be applied. In the last decade, however, more and more bacterial strains that can adapt to toxic organic solvents have been isolated and characterized (5, 15, 26, 34). These solvent-tolerant strains presumably will become a useful key in the performance of whole-cell biotransformations in the presence of these toxic, more polar solvents.
In recent years, many efforts have been made to uncover the mechanisms behind the solvent tolerance of these strains belonging to the genus Pseudomonas. Up to now different adaptation mechanisms have been found. Alterations at the level of the cell envelope structure, which suppress the effects of the solvents on the membrane stability or limit their rate of diffusion into the cell, have been described (12, 23, 27, 35). Furthermore, enhanced rates of phospholipid biosynthesis, speeding up repairing processes, have been reported (24). Last but not least, active export systems have been shown to exclude the solvent toluene from the cell (6, 16, 19, 27). The active efflux of solvents is an energy-dependent process. Therefore, it should increase the maintenance requirement of the cells in the presence of solvents. To what extent organic solvents enhance the energy requirement of the solvent-tolerant strains must still be determined.
However, although the adaptation mechanisms of some solvent-tolerant Pseudomonas strains have been studied in detail, no studies have been made of the effects of solvents on growth yields and maintenance requirements of these organisms. We now have determined the effects of toluene and other solvents on the growth parameters of Pseudomonas putida S12.
MATERIALS AND METHODS
Microorganisms and media.
P. putida S12 was isolated as a styrene-degrading organism (10). This strain grows in the presence of a second phase of various organic solvents, even if these solvents, like toluene, cannot be metabolized (34). P. putida JK1 is a solvent-sensitive transposon mutant of P. putida S12. This mutant has inactive sprABC genes which code for the energy-dependent solvent efflux system (19). Both strains were cultivated in a minimal medium as described by Hartmans et al. (9) with 1.8 g of glucose per liter as the sole source of carbon and energy. For cultivation on a solidified medium 3.5 g of yeast extract per liter and 15 g of agar per liter were added. For the mutant strain 50 mg of kanamycin per liter was always added to the growth medium.
Batch cultivation.
Batch culture cells were grown in 25-ml shaken cultures in 250-ml bottles sealed with Mininert valves (Phase Separations, Waddinxveen, The Netherlands) to prevent evaporation of the solvent. These valves possess movable Teflon-rubber septa for both sealing with Teflon and sampling. Different concentrations of toluene were added to the medium and equilibrated at 30°C for at least 12 h. The amount of solvent necessary to achieve a certain amount of solvent in the medium was calculated as follows:
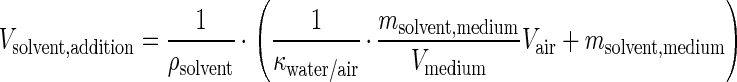 |
where Vsolvent,addition is the volume of solvent (in microliters) necessary to achieve the amount of solvent (msolvent,medium [in milligrams]) in the medium, ρsolvent is the density of solvent (in grams per milliliter), κwater/air is the partition coefficient of solvent between water and air as given by Amoore and Hautala (2), Vmedium is the volume of the medium, and Vair is the volume of the air. Additionally, the concentration of a solvent was controlled by gas chromatography analysis of the headspace.
Cells were precultivated in continuous cultures (dilution rate = 0.2 h−1) in a medium saturated with toluene. After reaching the steady state these cells were used as the inoculum (1% [vol/vol]) for the batch cultures.
Cultivation of these batch cultures took place in a horizontally shaking water bath at 30°C. During growth we monitored the optical density at 560 nm, the CO2 and protein production, and the consumption of glucose. The maximal errors encountered in the determination of protein and other parameters were 20 and 10%, respectively.
Continuous cultivation.
Continuous-culture experiments were performed in a chemostat with a 0.5-liter working volume at 30°C, pH 7.0, 600 rpm, and the dilution rates mentioned. The amount of oxygen in the culture broth was determined with a Clark-type oxygen electrode. Various solvents at different concentrations were supplied to the chemostat via the gas phase by passing a part of the airflow through a column filled with the solvent (at least 15 cm). The total airflow was kept constantly at 400 ml min−1. Headspace samples of the air entering and leaving the chemostat were analyzed. The solvent concentrations in the medium were calculated from the concentration in the headspace by using the partition coefficients given by Amoore and Hautala (2). To achieve the adaptation towards a certain solvent concentration the continuous culture was run first at a dilution rate of 0.05 h−1 for at least 12 h and then switched to the dilution rate of interest. The steady state was reached after five further exchanges of the volume. From the steady state we determined the concentration of protein in quintuplicate and the concentration of glucose remaining in the medium in duplicate. In this continuous approach the errors of the protein and glucose determinations were less than 15 and 5%, respectively.
Analytical methods.
The amount of the metabolized carbon source, glucose, was determined by high-pressure liquid chromatography analysis of the culture supernatant. The supernatant was filtered via a 0.2-μm-pore-size filter prior to high-pressure liquid chromatography analysis performed at 70°C on an ION-300 column (LC-Service, Emmen, The Netherlands) with 5 mM H2SO4 as an eluent and with refractive index detection (7). Headspace analysis of organic solvents was performed by analyzing 100 μl of the gas phase on a model 437A gas chromatograph (Packard, Delft, The Netherlands) with a 10% SE-30 Chromosorb WHP 80-100 mesh column (Chrompack, Middelburg, The Netherlands). The CO2 concentration was measured via headspace analysis in a model 427 gas chromatograph (Packard) with a Hayesep Q column (Chrompack). Dry weights were determined by drying washed cell suspensions at 105°C for 24 h prior to weighing. The method of Lowry et al. (22) was used to determine the protein concentration with bovine serum albumin as the standard.
Determination of yield and maintenance.
We determined the yield by measuring the amount of protein produced per amount of glucose consumed. As protein constitutes 60% of the total cell dry weight in P. putida S12 this value correlates with the amount of biomass produced per amount of glucose consumed.
The maximum growth yields and the maintenance coefficients were determined according to the equation of Pirt (25) from the data determined in the carbon-limited continuous culture as follows: 1/Yobs = (1/Ymax) + (m/μobs). Yobs is the observed growth yield, Ymax is the maximum growth yield, μobs is the observed specific growth rate as set by the dilution rate, and m is the maintenance metabolism rate.
Determination of solvent concentrations in the membrane.
The amount of a solvent accumulating in the bacterial membrane was calculated from its concentration in the water phase and its log PO/W, the logarithm of the partition coefficient of the solvent between octanol and water. For this calibration we made use of the equilibration found by Sikkema et al. (30). This equilibration correlates the log PO/W with the log PM/B, the logarithm of the partition coefficient of the solvent between the membrane and the buffer, as follows: log PM/B = 0.97 × log PO/W − 0.64. The values for the log PO/W were obtained from the list reported by Laane et al. (21).
Chemicals.
Toluene, benzene, ethylbenzene, propylbenzene, xylene, hexane, cyclohexane, and hexadecane were obtained from Janssen Chimia (Tilburg, The Netherlands). All other chemicals were commercially available and used without any further purification.
RESULTS
Growth kinetics of P. putida S12 in batch cultures.
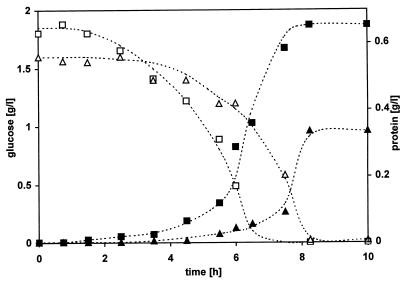
P. putida S12 was precultivated in a continuous culture in a medium saturated with toluene. These adapted cells were used as the inoculum for batch cultures, and their growth was monitored. With this approach cells are already adapted in the beginning of a batch experiment. Therefore, the response of these cells to a new exposure to toluene demonstrates the effect of this solvent alone and not the induction of adaptation mechanisms. The batch growth rate was 0.8 h−1 and was not affected by the presence of toluene. However, variations in the lag phase occurred depending on the concentration of toluene present. Furthermore, the presence of toluene led to a lower yield. In the absence and presence of 6.2 mM toluene the yields observed at the end of the exponential phase were 0.34 and 0.20 g of protein/g of glucose, respectively. Growth kinetics are presented for cultures growing in either the absence or the presence of 6.2 mM toluene (Fig. 1). The yield and lag phase obtained for all concentrations of toluene tested are also shown (see Fig. 4).
FIG. 1.
Growth kinetics of P. putida S12 in batch cultures. Cells were adapted to toluene and transferred into a minimal medium with glucose as the sole source of carbon and energy. The concentrations of protein (filled symbols) and glucose (open symbols) were determined in the absence (squares) or presence (triangles) of 6.2 mM toluene.
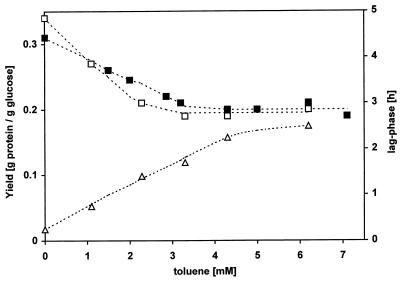
FIG. 4.
Effects of various concentrations of toluene on the lag phase and yield of P. putida S12. The lag phase (▵) is taken as the period of time from the inoculation of batch cultures until an increase in the optical density was observed. Yields were determined in batch cultures at the end of the exponential phase (□) and from cells growing in a glucose-limited chemostat at a dilution rate of 0.2 h−1 (■).
Effect of toluene on growth of P. putida S12 in carbon-limited continuous culture.
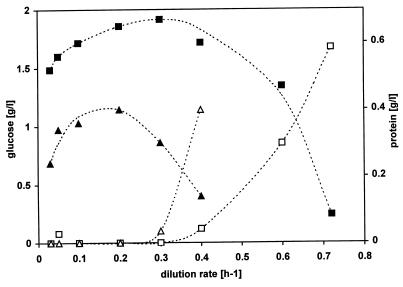
P. putida S12 was cultivated in a carbon-limited chemostat at different dilution rates in the presence and absence of 6.2 mM toluene. After the cells had reached the steady state we determined the protein content and the concentration of glucose as the growth-limiting substrate (Fig. 2). In the absence of toluene the washout occurred at a dilution rate above 0.72 h−1. In the presence of 6.2 mM toluene, however, this washout occurred at a lower growth rate, and it was not possible to obtain a steady state at dilution rates above 0.4 h−1. The presence of toluene also led to smaller amounts of biomass at all dilution rates. It was not possible to detect protein in the culture supernatant at all dilution rates tested.
FIG. 2.
Growth of P. putida S12 in a glucose-limited chemostat. Steady-state values of the concentrations of protein (filled symbols) and glucose (open symbols) were determined at different dilution rates in the absence (squares) and presence (triangles) of 6.2 mM toluene.
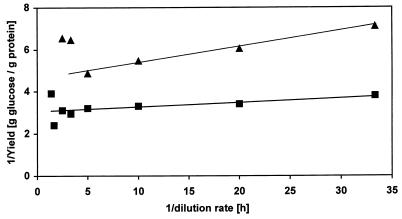
From the data shown in Fig. 2 we calculated the growth parameters of P. putida S12 growing in the absence and presence of 6.2 mM toluene. The reciprocal values of yields were plotted against the reciprocal dilution rates (Fig. 3). From the linear regressions of this plot the maintenance coefficients and the maximum growth yields were determined. In the absence and presence of toluene the maintenance coefficients were 0.023 and 0.076 g of glucose/g of protein · h−1, respectively, and the maximum growth yields were 0.33 and 0.22 g of protein/g of glucose, respectively.
FIG. 3.
Graphic determination of maintenance coefficients and the maximum growth yields of P. putida S12 growing in a carbon-limited chemostat in the absence (■) and presence (▴) of 6.2 mM toluene. The linear regression values (determined before washout occurs) are as follows: 1/Y = (0.023/D) + 3.00 (in the absence of toluene) and 1/Y = (0.076/D) + 4.57 (in the presence of toluene).
P. putida S12 was also cultivated in continuous cultures at a dilution rate of 0.2 h−1 in the presence of various concentrations of toluene. Figure 4 shows the effect of toluene on the yield. Up to a 3 mM concentration of toluene in the medium, the yield decreases linearly with increasing concentrations of toluene. Above this concentration of toluene, the yield remained nearly constant at 0.21 g of protein/g of glucose. This dose response is similar to results obtained in batch cultures.
Effects of different solvents on the yield of P. putida S12.
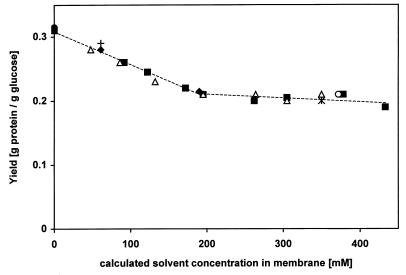
P. putida S12 was grown in continuous cultures at a dilution rate of 0.2 h−1. Various aromatic and aliphatic organic solvents were added at different concentrations. We determined the concentration of the solvent in the medium and calculated the corresponding concentration in the bacterial membrane as described in Materials and Methods. We plotted the yield of P. putida S12 against these membrane concentrations (Fig. 5). The plot shows a direct correlation between the yields observed and the concentrations of solvents in the membrane, irrespective of the solvent tested.
FIG. 5.
Effects of different solvents on the yield of P. putida S12. Cells were cultivated in a glucose-limited chemostat at a dilution rate of 0.2 h−1. Toluene (■), ethylbenzene (▵), propylbenzene (∗), xylene (○), hexane (+), cyclohexane (⧫), and hexadecane (●) were added at different concentrations. The theoretical concentrations of the solvents in the bacterial membrane were calculated (see Materials and Methods), and the yields were plotted against these concentrations.
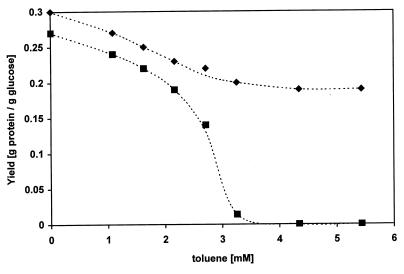
Effect of toluene on the yield of the solvent-sensitive mutant P. putida JK1.
The effects of solvents were also studied in the solvent-sensitive mutant P. putida JK1. This mutant is lacking the operon for the solvent efflux system. The cells were grown in batch cultures in the presence of various concentrations of toluene. The cells were transferred from lower toluene concentrations to higher ones in small concentration steps of about 0.5 mM each at the end of the exponential phase. In this way the growth of the mutant could be obtained at toluene concentrations up to 3.2 mM. Above this concentration no growth of the mutant strain was observed, while the wild-type strain tolerated a 6.2 mM concentration of toluene. For both strains we monitored the protein production and glucose consumption in the presence of various concentrations of toluene. From these data, yields were calculated and plotted against the concentration of toluene (Fig. 6). Up to a 2 mM concentration of toluene the yields of both strains were similar. Higher concentrations reduced the yield of the mutant to zero.
FIG. 6.
Effects of various concentrations of toluene on the yield of P. putida S12 (⧫) and the solvent-sensitive mutant JK1 (■) growing in a minimal medium in batch cultures.
DISCUSSION
The growth-inhibiting effect of organic solvents on microorganisms has been reported repeatedly (28, 31). The presence of solvents may lead to a reduction in the maximum growth rate, but cells which can adapt to solvents can achieve the same maximum growth rate in the presence of solvents (3, 11, 15, 26). These results were obtained by employing batch systems, and we also observed that in batch cultivation no effect of toluene on the growth rate occurred. However, these results in the case of glucose as a carbon source may be misleading, as it was reported previously that gluconic acid transiently accumulates during the cultivation of P. putida on glucose (8). Under more-defined conditions in continuous cultures we observed that the maximal growth rate is strongly affected by the presence of solvents. This reduction was not caused by oxygen limitation, as we had determined that sufficient oxygen was present in all cases. We speculate that the reduction of the maximum growth rate in the presence of toluene is caused by a reduction in the affinity of the cells for glucose. Such a change in the affinity for glucose may be caused by the mechanisms of adaptation to toluene that reduce the permeability and change the structure of the cell envelope (18, 23, 27, 36).
The effects of solvents on the biomass yield have been implied in previous reports (1, 3, 27). From these reports it can be deduced that the presence of toluene leads to reduced yields. We observed similar results. In our batch cultures the yield as affected by toluene dropped by 30%.
In continuous cultures, yields and maintenance coefficients were obtained for P. putida S12 grown under glucose limitation in either the presence or the absence of toluene and other solvents. In the absence of solvents, both the maximal yield and the maintenance coefficient were similar to those reported for other Pseudomonas species growing on glucose under aerobic conditions (8, 25, 32, 33).
The presence of toluene decreased the yield and increased the maintenance coefficient. These effects will be caused both by energy-consuming adaptation mechanisms and by a less effective energy metabolism in the presence of solvents. Specific energy-consuming processes include the active export of solvents in P. putida S12 (16, 19) and possibly an enhanced phospholipid biosynthesis rate as observed in P. putida Idaho (24). Ineffective energy metabolism will occur due to the uncoupling character of organic solvents (4, 13, 29, 30) and by disturbing effects of the solvents on the energy-transducing proteins (29, 30) as observed in nontolerant microorganisms.
The dose-response effect of toluene on the overall yield of P. putida S12 was studied in both batch and chemostat cultures. The yield decreased linearly with increasing concentrations (up to 3 mM) of toluene. Above this concentration, no further drop in the yield occurred. The adaptation of P. putida S12 to toluene has been observed at 3 mM or higher concentrations of toluene. Adaptation mechanisms triggered at this concentration include changes in the fatty acid profiles of membranes (35) and the active efflux of solvents from the membrane (20). Cells precultured at this concentration or higher ones not only survived the presence of a second phase of toluene (34), but they also showed an enhanced resistance towards various antibiotics (17). Consequently, a toluene concentration of 3 mM is critical. Below 3 mM cells do not react to toluene and thus are slightly affected by the solvent. Above this concentration of toluene, various mechanisms come into operation to protect cells from excessive damage.
The constant yield values observed at toluene concentrations of 3 to 6 mM may indicate either that (i) the energy requirement of the adaptation mechanisms acting at these concentrations is very limited or (ii) the systems require substantial energy input but are compensated by the effective removal of toluene from the cell.
The results obtained for the wild type were confirmed in experiments with the solvent-sensitive mutant P. putida JK1. At low concentrations of toluene, the effects of solvents on the yield were similar in the wild type and the solvent-sensitive mutant P. putida JK1. The slightly lower yield of the mutant strain is caused by the presence of kanamycin as the selective marker. The mutant lacks the energy-consuming solvent efflux system. Therefore, the reduction of the yield observed at low concentrations of toluene cannot be caused by the energy requirement of the active efflux system.
Our results on yields of P. putida S12 as affected by various other solvents show that the dose-response effect of solvents is the same when the actual concentration in the bacterial membrane is taken as the dose. Therefore, not the chemical structure but the amount of solvent accumulated in the bacterial membrane determines the effect of a solvent on the yield. Hence, the results found for toluene can be used for other solvents as well. It has been reported earlier that the concentration of solvents in bacterial membranes correlates directly with changes in the fatty acid profile and with the reduction of the maximal growth rate (14). In artificial membranes the same concentration of a solvent in the membrane results in the same expansion of the membrane. This membrane concentration also determines the release of ions and the effect on the proton and electrical gradients (30).
We conclude that, at low concentrations, the effects of solvents on P. putida S12 are not different from their effects on any other cell. The difference between solvent-tolerant and -intolerant cells seems to be that the effects of solvents are counterbalanced at higher concentrations by specific mechanisms. We suggest that these mechanisms keep the actual concentrations of solvents in the membrane constant. We think that this constant concentration of solvent in the membrane is approximately 200 mM, the concentration reached when adaptation starts.
ACKNOWLEDGMENTS
This work was financially supported in the framework of an industrially relevant research program of the Association of Biotechnology Centers in The Netherlands.
REFERENCES
- 1.Abe A, Inoue A, Usami R, Moriya K, Horikoshi K. Degradation of polyaromatic hydrocarbons by organic solvent-tolerant bacteria from deep sea. Biosci Biotechnol Biochem. 1995;59:1154–1156. doi: 10.1271/bbb.59.1154. [DOI] [PubMed] [Google Scholar]
- 2.Amoore J E, Hautala E. Odor as an aid to chemical safety: odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J Appl Toxicol. 1983;3:272–290. doi: 10.1002/jat.2550030603. [DOI] [PubMed] [Google Scholar]
- 3.Aono R, Ito M, Inoue A, Horikoshi K. Isolation of novel toluene-tolerant strain Pseudomonas aeruginosa. Biosci Biotechnol Biochem. 1992;56:145–146. [Google Scholar]
- 4.Cartwright C P, Juroszek J-R, Beavan M J, Ruby F M S, de Morais S M F, Rose A H. Ethanol dissipates the proton motive force across the plasma membrane of Saccharomyces cerevisiae. J Gen Microbiol. 1986;132:369–377. [Google Scholar]
- 5.Cruden D L, Wolfram J H, Rogers R D, Gibson D T. Physiological properties of a Pseudomonas strain which grows with p-xylene in a two-phase (organic-aqueous) medium. Appl Environ Microbiol. 1992;58:2723–2729. doi: 10.1128/aem.58.9.2723-2729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukumori F, Hirayama H, Takami H, Inoue A, Horikoshi K. Isolation and transposon mutagenesis of a Pseudomonas putida KT2442 toluene-resistant variant: involvement of an efflux system in solvent resistance. Extremophiles. 1998;2:395–400. doi: 10.1007/s007920050084. [DOI] [PubMed] [Google Scholar]
- 7.Grobben G J, Sikkema J, Smith M R, de Bont J A M. Production of extracellular polysaccharides by Lactobacillus delbrueckii ssp. bulgaricus NCFB 2772 grown in a chemically defined medium. J Appl Bacteriol. 1995;79:103–107. [Google Scholar]
- 8.Hack C J, Woodley J M, Lilly M D, Liddell J M. The production of Pseudomonas putida for the hydroxylation of toluene to its cis-glycol. Appl Microbiol Biotechnol. 1994;41:495–499. [Google Scholar]
- 9.Hartmans S, Smits J P, van der Werf M J, Volkering F, de Bont J A M. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl Environ Microbiol. 1989;55:2850–2855. doi: 10.1128/aem.55.11.2850-2855.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmans S, van der Werf M J, de Bont J A M. Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl Environ Microbiol. 1990;56:1347–1351. doi: 10.1128/aem.56.5.1347-1351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heipieper H J, de Bont J A M. Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of fatty acid composition of membranes. Appl Environ Microbiol. 1994;60:4440–4444. doi: 10.1128/aem.60.12.4440-4444.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heipieper H-J, Diefenbach R, Keweloh H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol. 1992;58:1847–1852. doi: 10.1128/aem.58.6.1847-1852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heipieper H-J, Keweloh H, Rehm H-J. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl Environ Microbiol. 1991;57:1213–1217. doi: 10.1128/aem.57.4.1213-1217.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heipieper H J, Loffeld B, Keweloh H, de Bont J A M. The cis/trans isomerization of unsaturated fatty acids in Pseudomonas putida S12: an indicator for environmental stress due to organic compounds. Chemosphere. 1995;30:1041–1051. [Google Scholar]
- 15.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentrations of toluene. Nature. 1989;338:264–266. [Google Scholar]
- 16.Isken S, de Bont J A M. Active efflux of toluene in a solvent-resistant bacterium. J Bacteriol. 1996;90:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isken S, Santos P M A C, de Bont J A M. Effect of solvent adaptation on the antibiotic resistance in Pseudomonas putida S12. Appl Microbiol Biotechnol. 1997;48:642–647. [Google Scholar]
- 18.Isken S, de Bont J A M. Solvent-tolerant bacteria. Extremophiles. 1998;2:229–238. doi: 10.1007/s007920050065. [DOI] [PubMed] [Google Scholar]
- 19.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1998;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- 20.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Active efflux of organic solvents in Pseudomonas putida S12 is induced by solvents. J Bacteriol. 1998;180:6769–6772. doi: 10.1128/jb.180.24.6769-6772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laane C, Boeren S, Hilhorst R, Veeger C. Optimization of biocatalysis in organic media. In: Laane C, Tramper J, Lilly M D, editors. Biocatalysis in organic media. Amsterdam, The Netherlands: Elsevier Science Publishers; 1987. [Google Scholar]
- 22.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Pinkart H C, Wolfram J W, Rogers R, White D C. Cell envelope changes in solvent-tolerant and solvent-sensitive Pseudomonas putida strains following exposure to o-xylene. Appl Environ Microbiol. 1996;62:1129–1132. doi: 10.1128/aem.62.3.1129-1132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinkart H C, White D C. Phospholipid biosynthesis and solvent tolerance in Pseudomonas putida strains. J Bacteriol. 1997;179:4219–4226. doi: 10.1128/jb.179.13.4219-4226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirt S J. Principles of microbe and cell cultivation. New York, N.Y: Wiley; 1975. pp. 63–70. [Google Scholar]
- 26.Ramos J L, Duque E, Huertas M-J, Haïdour A. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J Bacteriol. 1995;177:3911–3916. doi: 10.1128/jb.177.14.3911-3916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos J L, Duque E, Rodriguez-Herva J J, Godoy P, Haïdour A, Reyes F, Fernandez-Barrero A. Mechanisms for solvent tolerance in bacteria. J Biol Chem. 1997;272:3887–3890. doi: 10.1074/jbc.272.7.3887. [DOI] [PubMed] [Google Scholar]
- 28.Salter G J, Kell D B. Solvent selection for whole cell biotransformation in organic media. Crit Rev Biotechnol. 1995;15:139–177. doi: 10.3109/07388559509147404. [DOI] [PubMed] [Google Scholar]
- 29.Sikkema J, Poolman B, Konings W N, de Bont J A M. Effects of the membrane action of tetraline on the function and structural properties of artificial and bacterial membranes. J Bacteriol. 1992;174:2986–2992. doi: 10.1128/jb.174.9.2986-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sikkema J, de Bont J A M, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- 31.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdoni N, Aon M A, Lebeault J M. Metabolic and energetic control of Pseudomonas mendocina growth during transitions from aerobic to oxygen-limited conditions in chemostat cultures. Appl Environ Microbiol. 1992;58:3150–3156. doi: 10.1128/aem.58.9.3150-3156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K-W, Baltzis B C, Lewandowski G A. Kinetics of phenol biodegradation in the presence of glucose. Biotechnol Bioeng. 1996;51:87–94. doi: 10.1002/(SICI)1097-0290(19960705)51:1<87::AID-BIT10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Weber F J, Ooijkaas L P, Schemen R M W, Hartmans S, de Bont J A M. Adaptation of Pseudomonas putida S12 to high concentrations of styrene and other organic solvents. Appl Environ Microbiol. 1993;59:3502–3504. doi: 10.1128/aem.59.10.3502-3504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber F J, Isken S, de Bont J A M. Cis/trans isomerization of fatty acids as a defense mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology. 1994;140:2013–2017. doi: 10.1099/13500872-140-8-2013. [DOI] [PubMed] [Google Scholar]
- 36.Weber F J, de Bont J A M. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim Biophys Acta. 1996;1286:225–245. doi: 10.1016/s0304-4157(96)00010-x. [DOI] [PubMed] [Google Scholar]