Abstract
Streptomyces reticuli has an inducible ATP-dependent uptake system specific for cellobiose and cellotriose. By reversed genetics a gene cluster encoding components of a binding protein-dependent cellobiose and cellotriose ABC transporter was cloned and sequenced. The deduced gene products comprise a regulatory protein (CebR), a cellobiose binding lipoprotein (CebE), two integral membrane proteins (CebF and CebG), and the NH2-terminal part of an intracellular β-glucosidase (BglC). The gene for the ATP binding protein MsiK is not linked to the ceb operon. We have shown earlier that MsiK is part of two different ABC transport systems, one for maltose and one for cellobiose and cellotriose, in S. reticuli and Streptomyces lividans. Transcription of polycistronic cebEFG and bglC mRNAs is induced by cellobiose, whereas the cebR gene is transcribed independently. Immunological experiments showed that CebE is synthesized during growth with cellobiose and that MsiK is produced in the presence of several sugars at high or moderate levels. The described ABC transporter is the first one of its kind and is the only specific cellobiose/cellotriose uptake system of S. reticuli, since insertional inactivation of the cebE gene prevents high-affinity uptake of cellobiose.
The ABC superfamily of transporters has been extensively studied, and members have been identified in most bacteria, archaea, and eukaryotes (9). Uptake of a large variety of nutrients seems to be the most obvious task of these systems. Moreover, ABC transporters are involved in the export of drugs or virulence factors, such as hemolysin, extracellular proteases, and toxins; signal transduction; plant host–bacterial-parasite interaction; antigen presentation in immune cells; transport of pheromones; and sporulation of gram-positive bacteria (3, 7).
Members of the family of binding protein-dependent systems have so far been identified only in prokaryotes (3, 7). These multicomponent systems consist of two membrane-inserted subunits, two components inside the cytoplasm that carry the ATP binding site, and the binding protein located outside the cytoplasm. The binding proteins are responsible for the substrate specificity of the ABC transporter. In gram-negative bacteria, the binding protein is a soluble periplasmic protein, whereas in gram-positive bacteria and archaea, it is a lipoprotein exposed to the cell surface (38).
Streptomyces reticuli is a soil bacterium which hydrolyzes crystalline cellulose (Avicel) due to the action of an exoglucanase (Avicelase, Cel1) (32, 33, 44). The generated cellobiose and cellotriose are taken up via an inducible, binding protein-dependent ABC transporter (cellobiose/cellotriose ABC transport system [34]). The corresponding cellobiose/cellotriose binding protein was shown to be a lipoprotein anchored to the cytoplasmic membrane. This protein was extracted from membranes of S. reticuli and purified to homogeneity; it binds to cellobiose and cellotriose with equally high affinities (34). The ATP-hydrolyzing subunit of the cellobiose/cellotriose ABC transporter is MsiK (35). The msiK gene is a homologue of a previously described Streptomyces lividans msiK (15). MsiK from both Streptomyces species assists two different ABC transporters, one for maltose and one for cellobiose and cellotriose. Earlier studies have indicated that the chromosomally located msiK gene of S. reticuli is not situated in the vicinity of genes encoding the other components of the cellobiose/cellotriose ABC transport system (35).
In this report we characterize the additional clustered genes of the cellobiose/cellotriose transport system from S. reticuli. Further physiological, immunological, transcriptional, and mutational experiments elucidate details of the cellobiose/cellotriose ABC transport system.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The wild-type strain S. reticuli Tü45 (DSM 40776; German Collection of Microorganism and Cell Cultures, Braunschweig, Germany) described by Wachinger et al. (44) was obtained from H. Zähner, Tübingen, Germany. The Escherichia coli plasmids pUC18 and pUC19 (47) were used as cloning vectors for DNA sequence analysis and for disruption of the cebE gene. The aminoglycoside 3′-phosphotransferase gene (25) from pUC4K, supplied by Pharmacia, was used for gene disruption experiments. The E. coli K-12 strain DH5α containing the plasmids used in this study was grown in Luria-Bertani medium with 100 μg of ampicillin ml−1 (with pUC derivatives) or 50 μg of kanamycin ml−1 (with pUC4K derivatives) (29). S. reticuli was cultivated in pH-stable minimal medium (45) supplemented with the appropriate carbon source (1%, wt/vol) and 10 mM (NH4)2SO4 as a nitrogen source. For protoplast preparation S. reticuli was grown in complete medium (Oxoid tryptone-soy broth [20 g liter−1], yeast extract [5 g liter−1], sucrose [100 g liter−1], MgCl2 [10 g liter−1]).
DNA preparation and manipulations.
Genomic DNA from S. reticuli was isolated as described previously (12). Plasmids were isolated from E. coli with the aid of a midi plasmid kit (Qiagen GmbH, Hilden, Germany). Restriction enzyme digestions, ligation reactions, and analyses of DNA with nucleases and polymerases were carried out by standard procedures (29). PCR amplification of S. reticuli chromosomal DNA was performed with the oligonucleotides CB1, 5′-GGACATCAACATCAAGGAGAA-3′, and CB2, 5′-CTCCTTSCCSAGGTCSACGAA-3′, the former corresponding to the DINIKEN motif of amino acids located within the signature sequence of CebE (see Fig. 3). PCR was done under standard conditions (1) but in the presence of 5% dimethyl sulfoxide in a total volume of 50 μl. The mixture was covered with 30 μl of mineral oil and subjected to 30 cycles of 1 min at 96°C, 1 min at 52°C, and 1 min at 72°C. PCR products were purified with a QIAquick PCR purification kit (Qiagen GmbH), cloned into plasmid pUC18 with the aid of a Sure Clone ligation kit (Pharmacia, Freiburg, Germany), and subjected to nucleotide sequencing.
FIG. 3.
Alignments of signature sequences from binding proteins. The numbers indicate the positions of the amino acids. The highly conserved lysine (R) residue (according to the work of Tam and Saier [39]) is given in boldface type, and residues conserved in CebE are underlined. Sr, S. reticuli; Sc, S. coelicolor A3(2); Ec, E. coli; Sp, Streptococcus pneumoniae; Sm, Streptococcus mutans; Tt, Thermoanaerobacterium thermosulfurigenes; MalE, MalX, and AmyE, maltose and maltodextrin binding proteins; UgpB, sn-glycerol-3-phosphate binding protein; MsmE, multiple-sugar binding protein.
Preparation and screening of subgenomic DNA libraries.
Total DNA (200 μg) from S. reticuli was cleaved with BamHI, and the resulting fragments were separated on an agarose gel. Fragments of about 1.5 to 2 kb were eluted with a QIAEX II gel extraction kit and ligated to BamHI-digested and dephosphorylated pUC18. The ligation mixtures were transformed to E. coli DH5α by electroporation with an Electroporator II from Invitrogen (NV Leek, The Netherlands). Ampicillin-resistant transformants were tested for the presence of the desired cebE gene by colony hybridization at 54°C overnight with the digoxigenin-labelled PCR fragment (DNA labelling and detection kit; Boehringer, Mannheim, Germany). Three additional subgenomic DNA libraries consisting of SstII (1 to 1.5 kb), ScaI-HindIII (2 to 2.5 kb), and BamHI (2 to 2.5 kb) were generated in pUC18 and screened as described above.
DNA sequence analysis.
DNA sequencing of both strands of the ceb region was performed with double-stranded DNA based on the dideoxy chain termination method (30) with a Cy5 Autoread sequencing kit, Cy5-dATP labelling mix (Pharmacia), and universal or specific primers (MWG-Biotech, Ebersberg, Germany). Sequencing reactions were run on an ALFexpress sequencer from Pharmacia. The DNA and protein sequences were analyzed with the GENMON program (GBF, Braunschweig, Germany), as well as the Genetics Computer Group sequence analysis software package (version 8.0; Biotechnology Center, University of Wisconsin, Madison). Reading frames were determined with the GCWIND program (D. Shields, Dublin, Ireland) on the basis of the codon usage preference in Streptomyces DNA. The predicted proteins were scanned for similarities to sequences in the SWISS-PROT and EMBL databases with the FASTA and BLITZ algorithms (27). Membrane-spanning segments were predicted by the TMpred program according to the method of Hoffman and Stoffel (10).
Determination of amino acid sequences.
Purified CebE protein (2 mg) was incubated overnight at 30°C with 20 mM CNBr in 80% (vol/vol) formic acid and subsequently evaporated to dryness. Resulting peptides were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (15%, wt/vol, acrylamide) and transferred onto a polyvinylidene difluoride membrane (Immobilon P; Millipore GmbH, Eschborn, Germany) as described previously (11). After Coomassie brilliant blue staining, different protein bands were excised and NH2-terminal amino acids were determined by Edman degradation by R. Schmid, University of Osnabrück, Osnabrück, Germany.
Generation of MsiK antibodies and Western blot analysis.
A DNA fragment (372 bp) encoding the C terminus of MsiK was amplified with the primers MsiK1 (CTGTCCAACCTGGACGCCAAG) and MsiK2 (GTGCTCGGGGCGGACGCCGAC) with chromosomal DNA of S. reticuli and cloned into SmaI-restricted pUC18. From this plasmid the PCR fragment was isolated as a BamHI-KpnI fragment (383 bp) and recloned with pQE31 (Qiagen). The resulting construct, pMS131, contained the desired part of msiK with six codons encoding histidines at its 5′ end. Strain SG13009 (Qiagen) transformed with pMS131 produced the His tag MsiK fusion protein in inclusion bodies. Once we obtained and solubilized the inclusion bodies, the fusion protein was purified by affinity chromatography with Ni2+-nitrilotriacetic acid according to the instructions of the manufacturer (Qiagen). Antiserum was obtained by immunization of a rabbit with the purified six-His–MsiK fusion protein (Eurogentec, Seraing, Belgium). Western blot analyses were conducted as described previously (40) with a 1:10,000 dilution of antibodies raised against His-tagged MsiK or a 1:100,000 dilution of antibodies raised against CebE (34).
Construction of a cebE disruption mutant.
The cebE gene in pCB40 was cleaved by NaeI, resulting in an internal cebE deletion of 1,037 bp. Overhangs of the remaining plasmid were filled in with the Klenow enzyme and deoxynucleoside triphosphates and ligated to a PstI fragment from pUC4K (Pharmacia) comprising the kanamycin resistance (aphI) gene (25). The resulting construct was named pCB41. Protoplasts of S. reticuli were generated (12) and transformed with pCB41, which was isolated from the dam and dcm methylation-deficient E. coli strain JM110. Transformants were selected by overlaying regenerating protoplasts with agar (0.75%) containing kanamycin (20 μg/ml).
RNA isolation and Northern blot analysis.
Total RNA was isolated from S. reticuli with acid guanidinium thiocyanate-phenol-chloroform (6). For Northern blot analysis, RNA (15 μg per slot) was separated in formaldehyde gels (1) and transferred onto nylon membranes by vacuum blotting (LKB 2016 VacuGene; Pharmacia). RNA molecular weight marker I (0.3 to 6.9 kb) from Boehringer was used for size determination. The molecular weight markers were stained on the surface of the nylon filter with 0.2% methylene blue in 0.2 M sodium acetate (pH 4.7) (20). Hybridization was performed at 64°C for 20 h in a solution containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% SDS, 100 μg of salmon sperm DNA ml−1, and 5× Denhardt’s reagent (29) with randomly [α-32P]dCTP-labelled DNA fragments (Rediprime DNA labelling system; Amersham, Freiburg, Germany). The membrane was washed twice in 2× SSC–0.1% SDS for 5 min and twice in 0.1× SSC–0.1% SDS for 30 min and subjected to autoradiography at −70°C.
S1 nuclease mapping of transcription start sites.
The probes were generated by PCR amplification of a 396-bp fragment with pCB20 as the template and 10 pmol of the synthetic oligonucleotides E101 (for the cebE probe) and R103 (for the cebR probe) (see Fig. 2). Primers were labelled with [γ-32P]ATP and T4 polynucleotide kinase (1) and used for PCRs with the corresponding unlabelled primer. Labelled PCR products were purified with a QIAquick PCR purification kit (Qiagen). For every S1 nuclease protection reaction, 50 μg of RNA was hybridized to ∼105 Cerenkov counts of the PCR fragment in Na-TCA buffer (23) min−1 at 45°C for 6 h, after denaturation at 65°C for 15 min. Hybridization products were digested with S1 nuclease (Gibco, Life Technologies, Karlsruhe, Germany) as outlined by the manufacturer. Undigested radiolabelled DNA was precipitated with ethanol and run on a 6% polyacrylamide gel. The dried gel was subjected to autoradiography at −70°C. Sequence ladders were generated by the dideoxy chain termination method with the labelled primers R103 and E101 and with pCB20 as a template.
FIG. 2.
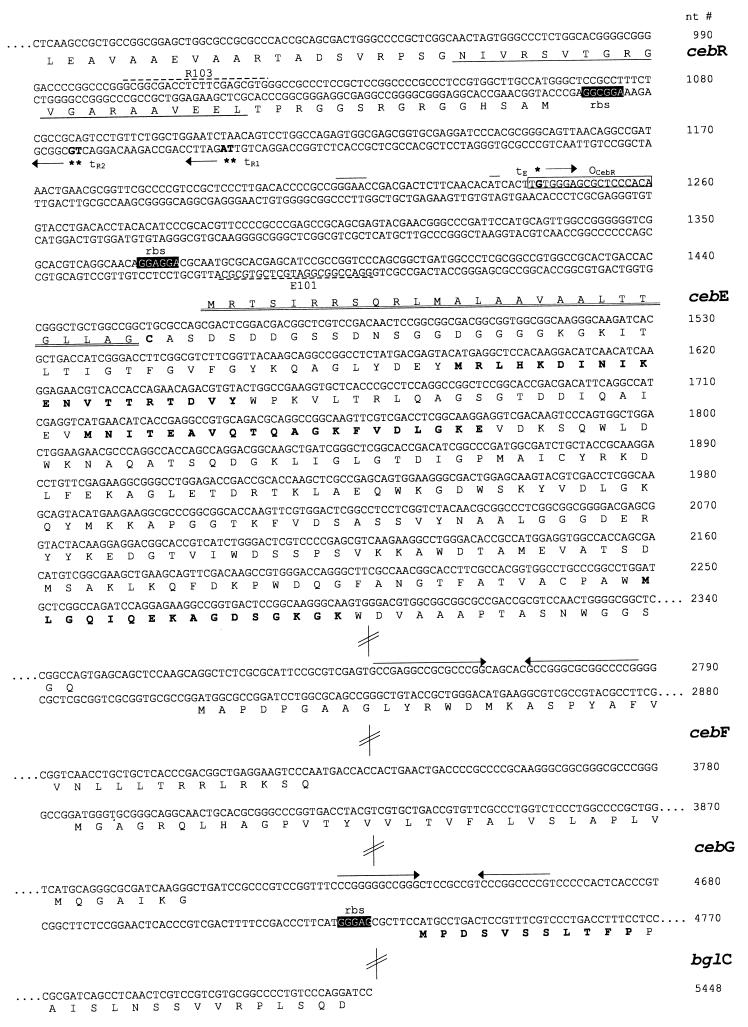
Partial nucleotide sequences and deduced amino acid sequences of cebREFG and bglC. The deduced amino acid sequences are given in the one-letter code below the nucleotide (nt) sequence, and nucleotide numbers are shown on the right. The putative ribosome binding sites (rbs) are in white letters on a black background, and predicted terminators are indicated by arrows above the sequence. The transcriptional start sites are in boldface type and are marked by asterisks followed by arrows indicating the direction of transcription (tE, transcription start site for cebE; tR1 and tR2, transcription start sites for cebR). The sequence similar to that of the Streptomyces class E promoter is marked by a line above the sequence. The oligonucleotides R103 and E101 used for S1 nuclease mapping are represented by broken lines. The predicted HTH motif in the deduced CebR protein is underscored, and the putative operator sequence for CebR binding is boxed (OCebR). The signal peptide of CebE followed by the recognition sequence for the cleavage site of lipoprotein signal peptidase is double underscored. The NH2-terminal amino acids of peptides from the purified CebE and BglC proteins determined by Edman degradation are in boldface type. Most of the sequence within the structural genes has been omitted, as is indicated by dots and double-slashed bars.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this article are stored in the EMBL database under the accession no. AJ009797 and AJ009798.
RESULTS AND DISCUSSION
Cloning of the cebREFG cluster and the bglC gene.
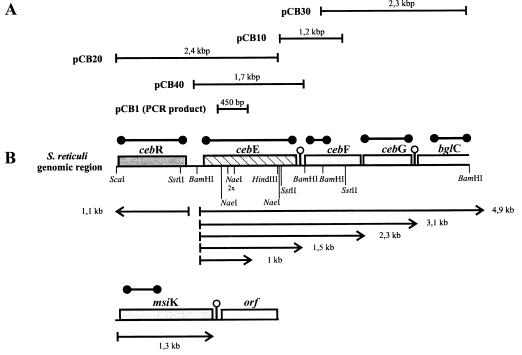
As the NH2-terminal amino acids of the purified CebE protein (34) could not be obtained by Edman degradation, several internal peptides were generated by CNBr treatment. After separation by SDS-PAGE, NH2-terminal amino acids were determined from three peptides (see Fig. 2) and used to deduce corresponding oligonucleotides. These, in turn, were used to generate fragments from total S. reticuli DNA in PCRs. The obtained fragments were cloned into pUC18, and their nucleotide sequences were determined. From the construct which comprises a fragment encoding a portion of the CebE protein (pCB1) (Fig. 1A), the PCR fragment was reisolated, labelled, and used to screen a subgenomic S. reticuli DNA library in E. coli DH5α (containing 1.5- to 2-kb BamHI genomic S. reticuli fragments in pUC18). Several clones were identified by colony hybridization, and their plasmids were analyzed. Sequencing revealed that an inserted 1.7-kb BamHI fragment was the desired one (Fig. 1). As genomic S. reticuli SstII (1.2 kb) and HindIII-ScaI (2.4 kb) fragments hybridized with the 1.7-kb BamHI fragment, corresponding subgenomic libraries were generated in pUC18 and the desired overlapping fragments were gained (Fig. 1). The genomic 2.3-kb BamHI fragment was obtained with the 1.2-kb SstII probe in a manner similar to that described above.
FIG. 1.
Constructs and organization of genes. (A) Initial PCR product and maps of the cloned chromosomal fragments in pUC18. (B) Arrangement of genomic cebREFG and bglC. The restriction sites relevant for cloning are shown. The probes used for transcription analysis are shown above the genes (●——●). Arrows mark the positions of transcripts. Predicted transcription terminators are indicated by stem-loop structures. orf, open reading frame.
Determination of the sequence and its analysis.
The sequences of the cloned overlapping fragments were determined. FRAME analysis (2) of the assembled 5,448-bp DNA fragment revealed the presence of five reading frames, whose codon usage was found to be typical of GC-rich Streptomyces DNA. The sequence of the first reading frame comprises 1,056 bp with a G+C content of 74 mol%. A start codon (ATG), a putative ribosome binding site, and a stop codon (TGA) were identified (Fig. 2). The deduced amino acid sequence of this open reading frame encodes a 39-kDa protein of 351 amino acids (aa). It was named CebR, as within its NH2 terminus a helix-turn-helix (HTH)-containing region characteristic of DNA binding proteins was identified (46). The deduced CebR is most closely related to GalR (38% of its amino acid residues are identical) and RbsR from E. coli (37% of its amino acid residues are identical), the latter representing a repressor of the ribose ABC transport operon (19). GalR and RbsR are both members of the LacI-GalR regulatory family. Interestingly, only 32% of CebR’s amino acid residues are identical to those of the recently identified Streptomyces coelicolor A3(2) MalR regulator of the operon for a maltose ABC transporter (41).
The most conserved portion of HTH motifs from various members of the LacI-GalR family comprises the six residues ATVSRV, which make up the main portion of the recognition helix (GTVSRV in CebR) required for specific binding to the major groove within the operator sequences (36). By sequence alignments it was suggested that the C-terminal domain of GalR is homologous to the E. coli periplasmic d-galactose–d-glucose binding protein (46). Data from the crystal structures of several periplasmatic binding proteins (22, 43) were used for molecular modelling of the binding domain for d-galactose–d-glucose in GalR (14). The amino acid side chains of Phe 73, Arg 194, Asn 245, and Asp 273 from GalR were predicted to be involved in creating the saccharide binding pocket. Three corresponding residues, Phe 73, Arg 194, and Asp 273, are found in the deduced CebR protein, and it appears likely that they interact with cellobiose.
The cebR gene is followed by the reading frame named cebE (1,334 bp), which has an opposite orientation. A start codon (ATG) with a putative Shine-Dalgarno sequence and a stop codon (TGA) were identified (Fig. 2). The deduced CebE protein (47.9 kDa) has 444 aa residues and contains the characterized internal peptides (determined by Edman degradation) of the purified CebE protein. The first 26 aa residues of the deduced CebE include a positively charged NH2-terminal hydrophobic core region and the sequence LLAGCA (the underscore indicates the cleavage site), which corresponds to the consensus (LLAGCS) of the lipoprotein signal peptidase cleavage site (38). This finding is in agreement with those of our previous biochemical experiments, which had revealed that S. reticuli produces CebE as a lipoprotein (34). Only 20% of the deduced amino acids of CebE are identical to those of binding proteins of cluster 1 as defined by Tam and Saier (39). The signature sequence (Fig. 3) comprising the highly conserved lysine residue is, however, conserved in CebE.
The cebE gene is followed by a putative transcription terminator and subsequently by the third (921-bp) and fourth (831-bp) reading frames. Neither reading frame is preceded by putative promoter and ribosome binding sites. The sizes of the deduced proteins for CebF and CebG are 276 and 306 aa, respectively, and both proteins contain six predicted membrane-spanning segments. Between the third and the fourth membrane-spanning segments, both proteins carry an EAA motif that matches the consensus EAAX2DGAX8IXLP sequence characteristic of membrane proteins from binding protein-dependent ABC transporters (31). In the E. coli MalF and MalG proteins the EAA motif has been identified as one site interacting with the predicted α-helical domain of the ATP binding protein MalK (21). Databank searches revealed that the deduced S. reticuli CebF and CebG proteins have highest identities with deduced lactose permeases from Synechocystis sp. (35% identity; EMBL accession no. P73352 and P73854) and deduced lactose permeases from Thermus sp. (36% identity; EMBL accession no. D1029300 and D1029301), all of which are presumed to be subunits of putative ABC transport systems.
The partially sequenced open reading frame following cebG is preceded by a putative transcription terminator and a predicted ribosome binding site and encodes a portion (714 bp) of a protein (238 aa) named BglC. The sequence of the deduced NH2 terminus is MPDSVSSLTFP (Fig. 2) and thus identical with the sequence of amino acids previously determined by Edman degradation for a purified S. reticuli intracellular β-glucosidase of 50 kDa (8). Alignments of the deduced BglC (calculated to correspond to about half of the purified β-glucosidase) have revealed that it has 72% (over 234 aa) and 61% (over 232 aa) identity with β-glucosidases deduced from Streptomyces sp. strain QM-B814 and Microbispora bispora nucleotide sequences, respectively.
The ceb operon lacks a gene encoding an ATP-hydrolyzing protein. Our previous experiments had shown that S. reticuli has a separately located msiK gene (35). In this context it is interesting that the S. coelicolor A3(2) malEFG operon also lacks a gene encoding an ATP binding protein (42). In the thermophilic archaea Thermococcus litoralis and Thermococcus thermosulfurigenes, the malK homologues are also not linked to ABC transport operons for maltose (28) and for maltose and trehalose (13), respectively. Inspection of complete genomic sequences revealed that the msiK homologues msmX from Bacillus subtilis (EMBL accession no. BG 11954) and msiK from Synechocystis sp. strain PCC 6803 (EMBL accession no. slr 0747) and an open reading frame from Methanococcus jannaschii encoding a homologue of the E. coli ATP binding protein UgpC of the sn-glycerol-3-phosphate ABC transporter (EMBL accession no. MJ0121) are located independently of the genes encoding other subunits of binding protein-dependent ABC transporters. Whether the above-cited MsiK homologues assist several ABC transporters as in S. reticuli remains to be elucidated.
Transcriptional experiments.
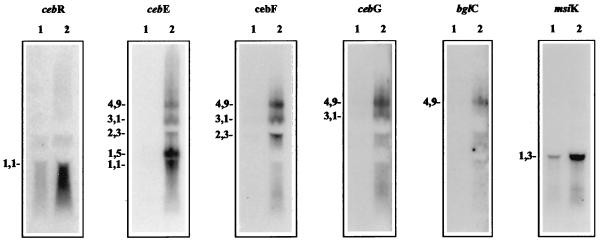
Hybridization experiments with total RNA isolated from cellobiose-grown S. reticuli mycelia revealed the formation of a polycistronic transcript (4.9 kb) comprising cebEFG and bglC. Additionally, shorter transcripts corresponding to cebEFG (3.1 kb), cebEF (2.3 kb), and cebE (1.5 and 1 kb) were detected. The small transcript (1.5 kb) corresponding to the cebE gene attained up to 20-fold higher levels of transcription than the 4.9- and 3.1-kb transcripts. During cultivation with glucose, almost no cebEFG or bglC transcripts were found (Fig. 4). The large amount of the 1.5-kb cebE transcript corresponds to the high levels of CebE protein present within membranes of S. reticuli during cultivation on cellobiose (34). The size of the 1.5-kb cebE transcript is in agreement with the distance from the cebE transcription start site (nucleotide 1244) (Fig. 2) to the predicted terminator following the stop codon of cebE (nucleotides 2749 to 2787). An additionally predicted terminator within the cebE gene corresponds to the 1.0-kb cebE transcript. However, a truncated CebE protein of the corresponding size (30 kDa) was not identified immunologically, suggesting that this small transcript is not translated under the conditions used or that it represents a degraded RNA form.
FIG. 4.
Transcription studies of the ceb operon and msiK gene. Total RNA (15 μg) from mycelia grown in the presence of glucose (lanes 1) and cellobiose (lanes 2) was separated electrophoretically. After transfer of the RNA to a nylon membrane, the strips were hybridized with the indicated 32P-labelled probes and exposed to X-ray films. The X-ray film of the cebR Northern blot analysis was exposed eight times longer than the others. Transcript sizes (in kilobases) are given on the left.
With the cebR-specific DNA probe, a monocistronic transcript of 1.1 kb was detected (Fig. 4). In glucose-grown S. reticuli mycelia, the level of cebR transcript was extremely low but it increased about 10 times during cultivation with cellobiose. A transcript of 1.3 kb corresponded to the monocistronic msiK gene. The quantity of the msiK transcript from cellobiose-grown cultures exceeded that from glucose-grown cultures by about 15-fold.
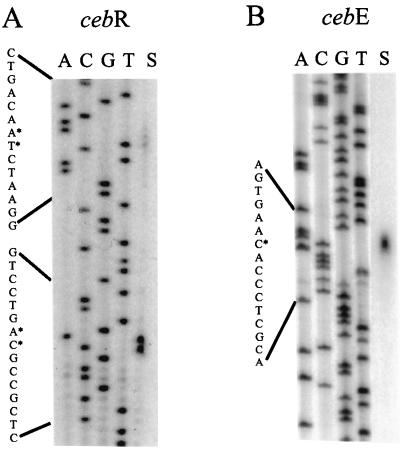
S1 nuclease mapping revealed that the cebE transcript starts 132 nucleotides 5′ upstream of the translational start codon of cebE. The sequence GGAAC is located 24 nucleotides 5′ upstream of the transcription start codon (Fig. 5). This motif matches the previously identified −35 region of the p2 promoter of the agarase gene dagA from S. coelicolor A3(2) (5, 37), a weak promoter of class E lacking a typical −10 region (4). The 5′ upstream region of CebE contains the motif GGAGCGCTCC (Fig. 2), which has similarity to the optimal consensus sequence of the E. coli GalR operator [(G/T)AA(A/C)CGNTT(A/C)] (24, 46). Transcription of cebR starts at the T and G 21 nucleotides 5′ upstream of the ATG start codon. Rarely used transcription start codons of cebR were found 22 nucleotides 5′ upstream.
FIG. 5.
Mapping of the transcription initiation sites of cebE and cebR. RNA (50 μg) prepared from mycelia of S. reticuli grown in the presence of cellobiose was hybridized to 0.1 pmol of the 32P-labelled cebR (A) or cebE (B) probes, and S1 nuclease treatment (lanes S) was done as described in Materials and Methods. ACGT indicates the cebR and cebE nucleotide sequence ladders. The asterisks mark the most probable transcription start sites.
Production of CebE and MsiK.
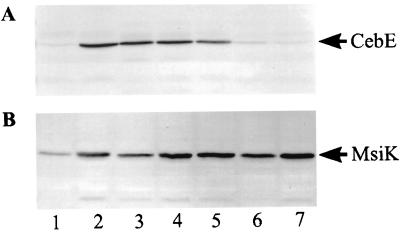
Antibodies raised against the C-terminal part of MsiK and the mature CebE (34) were used to monitor the levels of corresponding proteins during cultivation on minimal media containing different saccharides (Fig. 6). S. reticuli was found to produce CebE only if it was grown in the presence of cellobiose (not with glucose, maltose, or sucrose). When, however, cellobiose and one of the above-mentioned saccharides were added together to the culture medium, the level of CebE attained was nearly that ascertained for mycelia grown only with cellobiose.
FIG. 6.
Synthesis of CebE and MsiK proteins. Wild-type S. reticuli was grown in minimal medium with the following saccharide(s) at 0.5% (wt/vol) each: glucose (lane 1), cellobiose (lane 2), cellobiose plus glucose (lane 3), cellobiose plus maltose (lane 4), cellobiose plus sucrose (lane 5), maltose (lane 6), and sucrose (lane 7). Mycelia were disrupted by sonification, and 20 μg of protein per lane was separated by SDS-PAGE (17). Immunodetection of CebE (A) and MsiK (B) proteins was performed as outlined previously (35).
The quantity of MsiK was highest in mycelia grown with cellobiose, maltose, or sucrose. The addition of glucose to mycelia growing with cellobiose, maltose, or sucrose led to a decrease of MsiK synthesis. The relative amounts of msiK transcripts corresponded to the quantities of MsiK protein obtained from mycelia grown under comparable conditions (data not shown). These data show that MsiK synthesis is regulated differently from that of the CebE protein.
Construction of an S. reticuli cebE mutant.
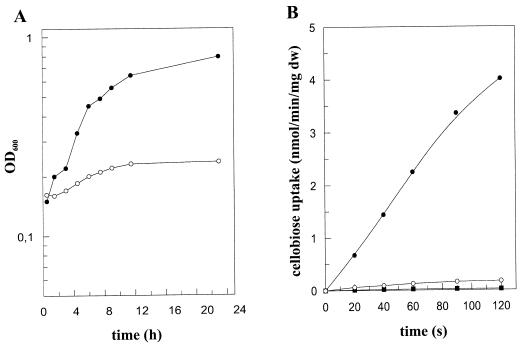
The cebE gene was inactivated by insertion mutagenesis. The aphI gene from Tn903 (25) was inserted into the pUC18-borne cebE gene with an internal deletion of an NaeI fragment (1,037 bp). After the S. reticuli wild type was transformed with the resulting plasmid, pCB41, several resistant colonies growing with 20 μg of kanamycin per ml were found. Following a double crossover between the genomic cebE gene and the cebE-homologous flanking portions of the aphI gene on pCB41, the aphI gene was found to disrupt the reading frame of the cebE gene, which was confirmed by Southern hybridization. The mRNA of this kanamycin-resistant S. reticuli mutant lacks all transcripts corresponding to cebEFG and bglC, showing that the insertion of the aphI gene in cebE had an effect on the transcription of genes located downstream from it. As expected, the CebE protein was not detectable immunologically within cell extracts prepared from the cebE mutant. In minimal medium supplemented with glucose, the cebE mutant grew with the same doubling time as that of the wild-type strain (3 h). In cellobiose-containing minimal medium, the growth rate of the wild-type strain was not affected but the cebE mutant strain grew quite poorly (Fig. 7A). Unlike with the wild type, no or only very little cellobiose was taken up by mycelia of the cebE mutant during short-term uptake experiments (Fig. 7B). However, after prolonged incubation (exceeding 10 min), small amounts of 14C label were detected. Previously (8) we had shown that S. reticuli produces extracellular and intracellular β-glucosidase activities (detected by hydrolysis of p-nitrophenyl-β-d-glucopyranoside). Inspection of the cebE mutant revealed that the β-glucosidase activities were comparable to those of the progenitor strain (data not shown). Thus, we suspect that part of the 14C-labelled cellobiose was cleaved to glucose.
FIG. 7.
Characterization of the cebE mutant. (A) Physiological experiments. Spores (106/ml) of the wild type (●) and the cebE disruption mutant (○) were inoculated into minimal medium containing 0.5% (wt/vol) Casamino Acids and glucose or cellobiose. After 24 h, the mycelia were washed twice with minimal medium lacking Casamino Acids, and after the addition of cellobiose, the growth could be monitored photometrically (optical density at 600 nm [OD600]), due to the finely dispersed hyphae. (B) Cellobiose uptake experiments. Mycelia from the S. reticuli wild type (●) or the cebE mutant (■) were grown in minimal medium containing Casamino Acids (0.5%) and cellobiose for 16 h, and then the mycelia were washed twice with 50 mM potassium phosphate buffer, pH 7.0. Uptake of [14C]cellobiose (5 μM) was determined as described previously (34). As a control, the uptake of cellobiose was determined in wild-type (○) mycelia cultivated with glucose. dw, dry weight.
Unlike with S. reticuli, within E. coli (26) and Bacillus stearothermophilus XL-65-6 (18) cellobiose is taken up via phosphoenolpyruvate-dependent phosphotransferase systems, and phosphorylated cellobiose, in turn, is cleaved by the action of intracellular phospho-β-glucosidases. In the cellulose degrader Trichoderma reesei, cellobiose uptake is mediated by a constitutively synthesized permease that is specific for different β-glucosides such as sophorose, gentiobiose, and cellobiose (16). The newly identified S. reticuli genes are so far the only known genes encoding a functional binding protein-dependent ABC transporter for cellobiose and cellotriose. Thus, this ABC transporter is an excellent model system for more-detailed studies to elucidate its high specificity.
ACKNOWLEDGMENTS
We are grateful to J. Ritz for training J. Jantos to isolate RNA, to T. Aldekamp for performing some of the Western blot analyses, and to R. Schmid, Department of Microbiology, University of Osnabrück, for determining NH2-terminal amino acids of CebE.
M. Lemme supported the writing of the manuscript. The work was initially financed by the SFB (grant 171/C14 to H. Schrempf) and then by the DFG (grant Schl 498/1-1 to A. Schlösser).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates; 1987. [Google Scholar]
- 2.Bibb M J, Findlay P R, Johnson M W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984;30:157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- 3.Boos W, Lucht J M. Periplasmic binding protein-dependent ABC transporters. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1175–1209. [Google Scholar]
- 4.Bourn W R, Babb B. Computer assisted identification and classification of streptomycete promoters. Nucleic Acids Res. 1995;23:3696–3703. doi: 10.1093/nar/23.18.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttner M J, Fearnley I M, Bibb M J. The agarase gene (dagA) of Streptomyces coelicolor A3(2): nucleotide sequence and transcriptional analysis. Mol Gen Genet. 1987;209:101–109. doi: 10.1007/BF00329843. [DOI] [PubMed] [Google Scholar]
- 6.Chromczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Dean M, Allikmets R. Evolution of ATP-binding cassette transporter genes. Curr Opin Genet Dev. 1995;5:779–785. doi: 10.1016/0959-437x(95)80011-s. [DOI] [PubMed] [Google Scholar]
- 8.Heupel C, Schlochtermeier A, Schrempf H. Characterization of an intracellular β-glucosidase from Streptomyces reticuli. Enzyme Microb Technol. 1993;15:127–132. [Google Scholar]
- 9.Higgins C F. ABC transporter: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman K, Stoffel W. TMbase—a database of membrane spanning protein segments. Biol Chem. 1993;347:166. [Google Scholar]
- 11.Höner zu Bentrup K, Schmid R, Schneider E. Maltose transport in Aeromonas hydrophila: purification, biochemical characterization and partial protein sequence analysis of a periplasmic maltose-binding protein. Microbiology. 1994;140:945–951. doi: 10.1099/00221287-140-4-945. [DOI] [PubMed] [Google Scholar]
- 12.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: John Innes Foundation; 1985. [Google Scholar]
- 13.Horlacher R, Xavier K B, Santos H, DiRuggiero J, Kossman M, Boos W. Archaeal binding protein-dependent ABC transporter: molecular and biochemical analysis of the trehalose/maltose transport system of the hyperthermophilic archaeon Thermococcus litoralis. J Bacteriol. 1998;180:680–689. doi: 10.1128/jb.180.3.680-689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh M, Hensley P, Brenowitz M, Fetrow J S. A molecular model of the inducer binding domain of the galactose repressor of Escherichia coli. J Biol Chem. 1994;269:13825–13835. [PubMed] [Google Scholar]
- 15.Hurtubise Y, Shareck F, Kluepfel D, Morosoli R. A cellulase/xylanase-negative mutant of Streptomyces lividans 1326 defective in cellobiose and xylobiose uptake is mutated in a gene encoding a protein homologous to ATP-binding proteins. Mol Microbiol. 1995;17:367–377. doi: 10.1111/j.1365-2958.1995.mmi_17020367.x. [DOI] [PubMed] [Google Scholar]
- 16.Kubicek C P, Messner R, Gruber F, Mandels M, Kubicek-Pranz E M. Triggering of cellulase biosynthesis by cellulose in Trichoderma reesei. J Biol Chem. 1993;268:19364–19368. [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lai X, Ingram L O. Cloning and sequencing of a cellobiose phosphotransferase system operon from Bacillus stearothermophilus XL-65-6 and functional expression in Escherichia coli. J Bacteriol. 1993;175:6441–6450. doi: 10.1128/jb.175.20.6441-6450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopilato J E, Garwin J L, Emr S D, Silhavy T J, Beckwith J R. d-Ribose metabolism in Escherichia coli K-12: genetics, regulation, and transport. J Bacteriol. 1984;158:665–673. doi: 10.1128/jb.158.2.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller K. Gel electrophoresis of RNA. Focus. 1987;9:14–15. [Google Scholar]
- 21.Mourez M, Hofnung M, Dassa E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 1997;16:3066–3077. doi: 10.1093/emboj/16.11.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mowbray S L, Cole L B. 1.7 A X-ray structure of the periplasmic ribose receptor from Escherichia coli. J Mol Biol. 1992;225:155–175. doi: 10.1016/0022-2836(92)91033-l. [DOI] [PubMed] [Google Scholar]
- 23.Murray M. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem. 1986;158:165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- 24.Nieto C, Espinosa M, Puyet A. The maltose/maltodextrin regulon of Streptococcus pneumoniae. J Biol Chem. 1997;272:30860–30865. doi: 10.1074/jbc.272.49.30860. [DOI] [PubMed] [Google Scholar]
- 25.Oka A, Sugisaki H, Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981;147:217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- 26.Parker L L, Hall B G. Characterization and nucleotide sequence of the cryptic cel operon of Escherichia coli K12. Genetics. 1990;124:455–471. doi: 10.1093/genetics/124.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson W R, Lipman D J. Improved tools for biology sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahm K, Matuschek M, Müller H, Mitchell W J, Bahl H. Molecular analysis of the amy gene locus of Thermoanaerobacterium thermosulfurigenes EM1 encoding starch-degrading enzymes and a binding protein-dependent maltose transport system. J Bacteriol. 1996;178:1039–1046. doi: 10.1128/jb.178.4.1039-1046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saurin W, Köster W, Dassa E. Bacterial binding protein-dependent permeases: characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol Microbiol. 1994;12:993–1004. doi: 10.1111/j.1365-2958.1994.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 32.Schlochtermeier A, Niemeyer F, Schrempf H. Biochemical and electron microscopic studies of the Streptomyces reticuli cellulase (Avicelase) in its mycelium-associated and extracellular forms. Appl Environ Microbiol. 1992;58:3240–3248. doi: 10.1128/aem.58.10.3240-3248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlochtermeier A, Walter S, Schröder J, Moormann M, Schrempf H. The gene encoding the cellulase (Avicelase) cel1 from Streptomyces reticuli and analysis of protein domains. Mol Microbiol. 1992;6:3611–3621. doi: 10.1111/j.1365-2958.1992.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 34.Schlösser A, Schrempf H. A lipid-anchored binding protein is a component of an ATP-dependent cellobiose/-triose transport system from the cellulose degrader Streptomyces reticuli. Eur J Biochem. 1996;242:332–338. doi: 10.1111/j.1432-1033.1996.0332r.x. [DOI] [PubMed] [Google Scholar]
- 35.Schlösser A, Kampers T, Schrempf H. The Streptomyces ATP-binding component MsiK assists in cellobiose and maltose transport. J Bacteriol. 1997;179:2092–2095. doi: 10.1128/jb.179.6.2092-2095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher M A, Choi K Y, Zalkin H, Brennan R G. Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by α helices. Nature. 1994;266:763–770. doi: 10.1126/science.7973627. [DOI] [PubMed] [Google Scholar]
- 37.Strohl W R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992;20:961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutcliffe I C, Russell R R B. Lipoproteins of gram-positive bacteria. J Bacteriol. 1995;177:1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam R, Saier M H. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Wezel G P, White J, Young P, Postma P W, Bibb M J. Substrate induction and glucose repression of maltose utilization by Streptomyces coelicolor A3(2) is controlled by malR, a member of the lacI-galR family of regulatory genes. Mol Microbiol. 1997;23:537–549. doi: 10.1046/j.1365-2958.1997.d01-1878.x. [DOI] [PubMed] [Google Scholar]
- 42.van Wezel G P, White J, Bibb M J, Postma P W. The malEFG gene cluster of Streptomyces coelicolor A3(2): characterization, disruption and transcriptional analysis. Mol Gen Genet. 1997;254:604–608. doi: 10.1007/s004380050458. [DOI] [PubMed] [Google Scholar]
- 43.Vyas N K, Vyas M N, Quiocho F A. Comparison of the periplasmic receptors for l-arabinose, d-glucose/d-galactose, and d-ribose. Structural and functional similarity. J Biol Chem. 1991;266:5226–5237. [PubMed] [Google Scholar]
- 44.Wachinger G, Bronnenmeier K, Staudenbauer W L, Schrempf H. Identification of mycelium-associated cellulase from Streptomyces reticuli. Appl Environ Microbiol. 1989;55:2653–2657. doi: 10.1128/aem.55.10.2653-2657.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter S, Schrempf H. Physiological studies of cellulase (Avicelase) synthesis in Streptomyces reticuli. Appl Environ Microbiol. 1996;62:1065–1069. doi: 10.1128/aem.62.3.1065-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weickert M J, Adhya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992;267:15869–15874. [PubMed] [Google Scholar]
- 47.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]