The impact of diet on health have been demonstrated by many studies. Revealing these mechanisms of action between homeostasis and diet can enable the use of ingredients with positive effects for therapeutic purposes. In this context, it has become necessary to determine the dietary contents in detail and to reveal their effects on cellular metabolic pathways. New findings draw attention to the existence of ultra-structures that show very different efficacy from food ingredients such as carbohydrates, proteins, fats, minerals, and vitamins. The new players mentioned above were first introduced in 1967.[1] Halperin and Jensen described ultrastructural bodies in carrot cell cultures which we now understand to be extracellular vesicles; mainly exosomes released from plant cells; however, researchers only started isolating and characterizing exosome-like nanoparticles from ginger, carrots, grapefruit, and grapes in 2014.[2] Their data revealed the important role that edible plant-derived exosome-like nanoparticles play in terms of maintaining intestinal homeostasis.
Exosomes are small vesicles with a diameter ranging from 40 to 100 nm. They are formed within endosomal compartments and secreted through the fusion of multivesicular bodies with the plasma membrane.[3] These tiny membrane-coated cargo packages are secreted by prokaryote and eukaryote cells and transport proteins, lipids, and nucleic acids to other cells.[4] Exosomes regulate intercellular communication and affect cellular metabolic activities, even between different species. In an experimental model, mouse exosomes transported to human mast cells induced the expression of mouse proteins in the donor cells.[5] The same interaction mechanism may be valid for plant exosomes. They may enter the cells of different species and affect their metabolic activities. A particularly good example of this interaction is MIR168a. Zhang et al.[6] demonstrated the circulation of MIR168a in the sera of Chinese subjects. This exogenous plant miRNA is mostly found in rice.
Coffee is the most consumed hot beverage globally. Coffee consumption is inversely associated with total and cause-specific mortality.[7] The effects of coffee consumption on the liver are noteworthy. Meta-analyses have revealed the beneficial and protective effects of coffee on hepatic fibrosis and cirrhosis in patients with chronic liver disease[8] and even on long-term survival following liver transplantation.[9] However, studies performed using ingredients of coffee drink, have not been able to elucidate the mechanisms responsible for these beneficial effects. In our study, which we recently completed at the Lösante Hospital/Ankara, for the first time in literature, we identified coffee exosomes. Transmission electron microscope (TEM) characterized coffee exosomes (Fig. 1). As a result of whole exosome RNA sequencing, we identified 15 mature miRNAs. Using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, we investigated the proliferative effects of coffee exosomes on human hepatocellular carcinoma (HCC) cell line; Hep 40 cells. Our MTT results showed us coffee exosomes significantly suppressed hepatocellular carcinoma cell proliferation. This finding was consistent with the reported inverse proportion between coffee consumption and HCC prevalence[10] Our study is at the pre-print stage. Chuang et al.[11] found some differentially methylated CpGs located in the genes of people with diseases that benefit from drinking coffee. Coffee consumption was reported to be associated with DNA methylation levels.
Figure 1.
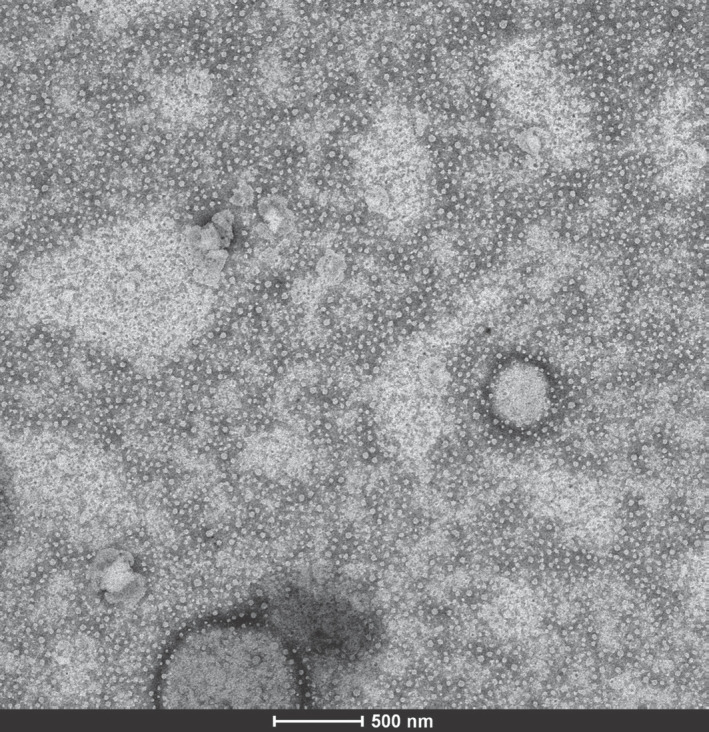
TEM images of isolated exosome vesicles of roasted-ground coffee.
The data in the literature provide clues that plant-derived exosomes may carry out their activities through genetic interaction in the host cells. Coffee exosomes are a new candidate for plant-derived miRNA-based therapies in chronic liver diseases and HCC. These genetic interactions and functional mechanisms should be explored further in future studies. Other edible plants with health benefits should also be investigated in this respect.
References
- 1.Halperin W, Jensen WA. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrastruct Res. 1967;18(3):428–443. doi: 10.1016/s0022-5320(67)80128-x. [DOI] [PubMed] [Google Scholar]
- 2.Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B, et al. Interspecies communication between p lant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22(1):107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;367(3):285. [Google Scholar]
- 8.Liu F, Wang X, Wu G, Chen L, Hu P, Ren H, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10(11) e0142457. [Google Scholar]
- 9.Friedrich K, Smit M, Wannhoff A, Rupp C, Scholl SG, Antoni C, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31(8):1470–1475. doi: 10.1111/jgh.13319. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open. 2017;7(5) e013739. [Google Scholar]
- 11.Chuang YH, Quach A, Absher D, Assimes T, Horvath S, Ritz B. Coffee consumption is associated with DNA methylation levels of human blood. Eur J Hum Genet. 2017;25(5):608–616. doi: 10.1038/ejhg.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


