Abstract
Simple Summary
Systemic mastocytosis (SM) is a clonal haematopoietic stem cell disease typically characterized by the expansion and accumulation of neoplastic mast cells carrying the activating KIT D816V as a driver mutation. Multilineage involvement of haematopoiesis by this KIT mutation, particularly in a multi-mutated context, also involving other genes (e.g., SRSF2, ASXL1, DNMT3A, RUNX1, EZH2, CBL and NRAS) found to be frequently mutated in other myeloid neoplasms, have recently emerged as a genetic background associated with malignant transformation of SM. Therefore, assessment of multilineage involvement of haematopoiesis by KIT D816V and additional mutations in genes known to be associated with the prognosis of SM have become of great help to identify good vs. poor-prognosis SM patients who could benefit from a closer follow-up and, eventually, also early cytoreductive treatment.
Abstract
Systemic mastocytosis (SM) is a rare clonal haematopoietic stem cell disease in which activating KIT mutations (most commonly KIT D816V) are present in virtually every (>90%) adult patient at similar frequencies among non-advanced and advanced forms of SM. The KIT D816V mutation is considered the most common pathogenic driver of SM. Acquisition of this mutation early during haematopoiesis may cause multilineage involvement of haematopoiesis by KIT D816V, which has been associated with higher tumour burden and additional mutations in other genes, leading to an increased rate of transformation to advanced SM. Thus, among other mutations, alterations in around 30 genes that are also frequently mutated in other myeloid neoplasms have been reported in SM cases. From these genes, 12 (i.e., ASXL1, CBL, DNMT3A, EZH2, JAK2, KRAS, NRAS, SF3B1, RUNX1, SF3B1, SRSF2, TET2) have been recurrently reported to be mutated in SM. Because of all the above, assessment of multilineage involvement of haematopoiesis by the KIT D816V mutation, in the setting of multi-mutated haematopoiesis as revealed by a limited panel of genes (i.e., ASXL1, CBL, DNMT3A, EZH2, NRAS, RUNX1 and SRSF2) and associated with a poorer patient outcome, has become of great help to identify SM patients at higher risk of disease progression and/or poor survival who could benefit from closer follow-up and eventually also early cytoreductive treatment.
Keywords: systemic mastocytosis, prognostic, mutations, KIT, D816V, ASXL1, DNMT3A, EZH2, RUNX1, SRSF2
1. Introduction
Systemic mastocytosis (SM) is a rare hematologic disease characterized by an abnormal expansion and accumulation of pathological mast cells (MCs) in skin and/or other several extracutaneous tissues such as bone marrow (BM) and the gastro-intestinal tract. Currently, SM is divided into five different diagnostic subtypes according to the World Health Organization (WHO) 2016 classification [1]. These include indolent SM (ISM), smouldering SM (SSM), aggressive SM (ASM), SM with associated haematological neoplasms (SM-AHN) and MC leukaemia (MCL). Additionally, the inclusion of two new subtypes of SM into the classification of the disease is currently under consideration: a variant of ISM known as BM mastocytosis (BMM) [2,3], which is characterized by a low BM MC burden in the absence of skin lesions, and a very rare (<5%) variant of mastocytosis, which shows tumour mast cells (MCs) with a well-differentiated morphology together with a CD25− CD2− immunophenotype and unique clinical, biological and molecular features, termed well-differentiated SM (WDSM) [4]. From a prognostic point of view, all these diagnostic subtypes of SM can be grouped into (i) non-advanced forms of SM (Non-AdvSM), which include BMM, ISM and SSM, typically characterized by a more stable and indolent course of the disease and a life expectancy similar or close to that of a sex- and age-matched population; and (ii) advanced SM (AdvSM) including ASM, SM-AHN and MCL, which typically display an adverse prognosis associated with a significantly shortened life expectancy requiring cytoreductive therapy [1]. Despite this, some ISM patients (<5%) can eventually evolve to SSM and AdvSM [5]. Conversely, a small proportion of AdvSM patients may also show a relatively stable disease course over years or even decades [6,7].
Currently, the aetiopathogenic mechanisms involved in malignant transformation of SM remain largely unknown. However, from an ontogenetic point of view, it is known that pathological MCs from the vast majority of patients (>90%) carries the D816V mutation in the KIT protooncogene [8,9] regardless of the diagnostic subtype of SM and its clinical course (e.g., Non-AdvSM or AdvSM). Despite this, multilineage involvement of hematopoietic cells other than MCs by KIT D816V, together with the existence of additional mutations in genes other than KIT (e.g., SRSF2, ASXL1, RUNX1, EZH2) have been demonstrated in recent years to mostly affect (but not only) AdvSM patients [10,11,12]. It is noteworthy that most of the latter mutations involve genes that are also recurrently mutated in other (myeloid) haematological malignancies [10,11,13], suggesting a tendency for their acquisition in haematopoietic cells that have an appropriate altered genetic background (i.e., KIT D816V-positive cells) that could favour malignant transformation and a more aggressive disease behaviour.
2. KIT Mutations in Systemic Mastocytosis
The KIT gene is a proto-oncogene encoding for a trans-membrane receptor (mast/stem cell growth factor receptor (KIT)) with tyrosine kinase (TK) activity located on the long arm of human chromosome 4 [14]. When the KIT ligand—stem cell growth factor (SCF)—binds to KIT, conformational changes occur that lead to dimerization of the receptor and its activation by autophosphorylation [15]. Of note, intracellular signalling triggered upon activation of the KIT receptor is key to the normal development of haematopoiesis and the survival of haematopoietic stem cells (HSC) [16]. Except for MCs and some natural killer (NK) cells, KIT is no longer expressed by other mature myeloid and lymphoid haematopoietic cells [17]. In MCs, KIT expression remains at high levels throughout maturation [18,19], playing a critical role in MC proliferation, differentiation and survival [15,20]. Therefore, the acquisition of mutations that could impair the normal function of KIT (e.g., activating KIT mutations) has pro-oncogenic effects associated with inhibition of apoptosis and increased MC proliferation and survival [21,22].
2.1. KIT D816V Mutation
The D816V mutation of KIT is located at exon 17 within the tyrosine kinase (TK) 2 domain of the KIT gene. This mutation causes constitutive activation of the KIT receptor in the absence of SCF binding and represents the most frequent genetic alteration in SM (>90% of adult SM patients) [9,15]. In fact, constitutive activation of KIT causes preferential differentiation of HSC toward cell lines regulated by KIT expression and signalling (mainly MCs and to a large extent also other myeloid lineages). The fact that MCs are the only haematopoietic cells that express KIT throughout their maturation [18,19] would explain why this KIT-activating mutation induces the expansion and accumulation of pathological MCs in different organs and tissues, as typically observed in SM and other KIT-mutated MC diseases [23]. Of note, the prevalence of the KIT D816V mutation is very similar among adult patients diagnosed with Non-AdvSM and AdvSM [9]. Therefore, the KIT D816V mutation is considered as a (specific) diagnostic marker of SM, regardless of the subtype of the disease, its presence being one of the four minor criteria required by WHO for the diagnosis of SM [1,24,25]. However, the presence of this mutation cannot explain by itself the wide spectrum of disease behaviour observed among SM patients, ranging from stable and even pauci-symptomatic to progressive and even highly-aggressive disease [5].
2.2. Other KIT Mutations
Overall, KIT mutations other than KIT D816V can be found in up to 4–5% adults and one third of children with mastocytosis [9]. In adults, these mutations are mostly located at codons 814–822 within exon 17 [9,26,27,28,29,30,31], including several mutant variants at codon 816 [9,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] (Table 1). KIT mutations located outside exon 17 include rare mutations that mostly affect exons 2 [29], 5 [40], 7–11 [29,32,40,46,47,48,49,50,51,52,53,54,55,56,57,58], 13 [29,59] and 18 [29]. Of note, most mutations other than KIT D816V correspond to isolated cases of SM-AHN, MCL or WDSM (Table 1). Interestingly, MCL patients with KIT mutations other than D816V often lack additional somatic high-risk mutations [46]. Although the vast majority of KIT mutations defined above are acquired (somatic) genetic variants, a few mutations typically located in exons 8 to 10 of KIT (e.g., delD419 [60], S451C [61], K509I [62,63] or F522C [54]) correspond to germinal mutations that frequently show a familial aggregation pattern.
Table 1.
KIT mutations other than D816V described in adult patients with systemic mastocytosis (SM).
| Domain | Exon | Mutation | Subtype SM |
|---|---|---|---|
| Extracellular: Ligand (SCF) binding domain | 2 | R49C | SM-u [29] |
| 5 | Y269C | SM-AHN [40] | |
| Extracellular: Dimerization domain | 7 | V399I | SM-u [29] |
| 8 | D419del | ISM [47] | |
| 9 | S451C | SM-u [61] | |
| S476I | MCL [48] | ||
| S501_A502dup | ASM [50] MCL [49] | ||
| A502_Y503dup | SM-u [29] MCL [51,52] | ||
| Y503_F504InsAY | ASM [40] | ||
| F504_N505delIns5 | SM-AHN [51] | ||
| K509R | SM-u [29] | ||
| K509I | ISM [62] ASM [53] MCL [62] WDSM [63] | ||
| Transmembrane domain |
10 | F522C | WDSM [32,54,55] MCL [46] |
| Juxta-membrane domain |
11 | V559I | ASM [56] |
| V560G | SM-u [40] ISM [57] MCL [58] | ||
| TK1 domain |
13 | K642E | ASM [29,40] |
| V654A | MCL [59] | ||
| TK2 domain |
17 | A814S | SM-AHN [26] |
| A814T | SM-AHN [27] | ||
| I815-V816Ins | ISM [9] | ||
| D816H | AdvSM [32,33] SM-AHN [26,34,35,36] MCL [46,67,68] | ||
| D816Y | ISM [37] AdvSM [32,33] SM-AHN [26,27,36,38,39] MCL [9,46] | ||
| D816I | SM-AHN [40] | ||
| D816A | SM-AHN [41,45] ASM [42] | ||
| D816G | MCL [43] | ||
| D816T | SM-u [44] | ||
| I817V | WDSM [9] | ||
| D820G | ASM [28] | ||
| N822K | SM-u [30] SM-AHN [31] | ||
| 18 | V852G | SM-u [29] |
Abbreviations: AdvSM: advanced systemic mastocytosis (SM); ASM: aggressive SM; ISM: indolent SM; MCL: mast cell leukaemia; SM-AHN: SM with an associated haematological neoplasm; SM-u: SM unclassified; WDSM: well-differentiated SM.
From a clinical point of view, the exact location of the mutations in the KIT gene is of great relevance, since those mutations that occur within the transmembrane or juxtamembrane domains of the KIT gene (exons 9–11) induce spontaneous receptor dimerization, making pathological MCs sensitive to conventional TK inhibitor therapies (e.g., imatinib) [52,53,54,55,62,64], while KIT mutations involving the catalytic domain (exons 13–18) cause a conformational change of the protein, which confers intrinsic resistance to imatinib and other TK inhibitors commonly used to treat other human tumours [65,66].
3. Clonal Haematopoiesis in Systemic Mastocytosis
SM is considered a clonal HSC disease characterized by the expansion and accumulation of neoplastic MCs [69,70,71]. As a neoplasm involving the HSC compartment, the KIT D816V (and other KIT) mutations can be found in both neoplastic MCs and CD34+ BM HSC, as well as in other myeloids (e.g., neutrophils [9,72,73,74], monocytes [9,70,72,73,74], basophils [70,72,74] and/or eosinophils [9,74]) and/or lymphoid (e.g., T and B lymphocytes [9,70,73,74]) cells. In such cases presenting multilineage involvement of haematopoiesis, clonal myeloid (MM) or myeloid plus lymphoid (MML) cells are found, which derive from the expansion and differentiation of D816V-mutated HSCs to different myeloid and/or lymphoid cell lineages [5,75]. Moreover, KIT D816V-mutated BM mesenchymal stem cells (MSCs) are also frequently detected in MML-mutated cases [36,76,77]. Overall, multilineage involvement of haematopoiesis by the KIT D816V mutation is found in virtually all ASM and SSM patients, in around one third of ISM cases and in a small proportion (≤10%) of BMM patients [9,78]. In SM-AHN, the frequency of patients that show a multilineage KIT D816V mutation may vary significantly [36] depending on the specific subtypes of SM and AHN [9]. Thus, KIT D816V-mutated AHN cells have been found in 89% of SM associated with chronic myelomonocytic leukaemia (SM-CMML), while this would only occur in 20% of SM associated with myeloproliferative neoplasms (MPN) and 30% of SM associated with acute myeloblastic leukaemia (AML); in turn, the KIT mutation is almost systematically restricted to the MC compartment in patients with SM associated with lymphoid neoplasms [36].
4. Mutations in Genes Other Than KIT
Emergence of the KIT D816V mutation in an HSC during the development of haematopoietic cells would potentially lead to multilineage involvement of haematopoiesis [77]. This would favour the expansion of neoplastic MCs and an increasing tumour burden; in addition, it might also lead to an increased genomic instability that may facilitate acquisition and accumulation of additional genetic alterations (Table 2 and Table S1) in the KIT-mutated or unmutated HSC and contribute to the malignant transformation of the disease via distinct molecular mechanisms, e.g., activation/repression of anti-/pro-apoptotic mechanisms [79].
Table 2.
Mutations in genes other than KIT reported in systemic mastocytosis.
| Gene | Exon | Gene Mutations | ||||
|---|---|---|---|---|---|---|
| ASXL1 | 6 | S135C [12] | ||||
| 8 | G219V [12] | |||||
| 12 | Y591* [80] | I641fs [13] | P698Afs* [32] | I919Yfs* [29] | H1008Tfs* [29] | |
| E602* [29] | G642fs [12] | R786Efs* [29] | P920Tfs* [80] | G1026Dfs* [29] | ||
| A611T [29] | G643Wfs* [13] | D820Mfs* [29] | R965_G966del [12] | I1220F [80] | ||
| I617Pfs* [32] | G646Wfs* [29,81] | T844fs [12] | Y974* [32] | G1397S [29] | ||
| H630fs [12]/Gfs* [29] | G646Afs* [29] | S846Vfs* [81] | I980Kfs* [32] | A1521S [12] | ||
| E635Rfs* [13,29,32] | R693* [13] | P849Lfs* [29] | E997* [29] | *1542fs [13] | ||
| CBL | 8 | Q367dup [80] | Y371C [29]/H [29]/S [29] | M374K [29] | L380P [10,29,82] | |
| C384R [12]/Y [29] | M400K [10,32] | C404Y [10,29] | W408C [10,32] | |||
| 9 | G413D [29] | R420Q [10,29] | I423N [29] | |||
| 11 | R550W [12] | |||||
| DNMT3A | 3 | E30A [80] | ||||
| 4 | N90S [12] | |||||
| 8 | R320* [12,29] | |||||
| 10 | A380V [12] | K420* [12] | ||||
| 15 | W581C [12] | L594Cfs* [29] | R598* [29] | D600Afs* [29] | ||
| 16 | S638C [12] | |||||
| 17 | S663L [12] | |||||
| 18 | S714F [12] | R720L [12] | ||||
| 19 | E733G [29] | F755S [12] | R771G [29]/Q [80] | |||
| 23 | N879D [13] | R882C [12,13]/H [12,13,80] | ||||
| EZH2 | 3 | L50Wfs* [13] | ||||
| 5 | I146T [13] | |||||
| 7 | S220F [32] | |||||
| 8 | R288* [32] | |||||
| 14 | Q545* [13] | |||||
| 15 | R583Q [13] | N608K [13] | ||||
| 17 | F672L [29] | |||||
| 18 | R690C [29] | H694R [40] | ||||
| JAK2 | 14 | V617F [12,29,32] | S605Y [12] | |||
| KRAS | 2 | V14I [83] | ||||
| 3 | Q70H [12] | |||||
| 5 | I187N [12] | |||||
| NRAS | 2 | G12S [29] | G12D [29,84] | G13D [84] | ||
| 3 | Q61L [29] | |||||
| RUNX1 | 4 | L56S [29] | P86fs [13] | E88Rfs* [13] | S94I [29] | |
| D96Gfs [85] | R107C [13] | N109T [13]/del [29] | F116L [12] | |||
| 5 | S141L [29] | A142T [13] | N146K [12,13] | R162K [13,29] | ||
| 7 | V238Gfs* [13] | |||||
| SF3B1 | 5 | Y141C [12] | ||||
| 14 | R625C [29] | W658C [29] | T663I [29] | K666N [29]/T [13,32] | ||
| 15 | K700E [13,29,83] | A711D [86] | ||||
| SRSF2 | 1 | V18L [10] | P95 A [13]/H [10,32,85]/L [10]/R [12,13]/T [87] | |||
| TET2 | 3 | L34F [29] | Q321* [83] | P562Tfs* [29] | Q729* [32] | Q933* [51] |
| H192Y [13] | E368* [13] | N595Ifs* [51] | Q731* [51] | Q939* [29] | ||
| V218Wfs* [32] | Q373Rfs* [51] | P612fs [12] | Q734* [51] | K948Nfs* [83] | ||
| Y234* [51] | P409Lfs* [80] | L615Sfs* [29] | Q752_fs* [29] | W954* [29] | ||
| R248Q [29] | G429R [29] | Y620fs [12] | L757Tfs* [29] | Q958Tfs* [29] | ||
| S254Rfs* [51] | L431* [51] | Y634* [29] | L806Rfs* [29] | Q963* [29] | ||
| E259Gfs* [29] | E452Rfs* [29,88] | Q652* [29] | Q810* [29,88] | S972Ffs* [29] | ||
| N275Ifs* [12,13] | D527Gfs* [29] | Q652Sfs* [80] | N837Yfs* [32] | C1016Wfs* [29,88] | ||
| Q278* [29] | Q530* [29] | Q659Rfs* [29] | L840* [51] | Q1020* [80] | ||
| T279fs [12] | E537Sfs* [29] | Q684Nfs* [29] | T849Hfs* [89] | I1024Qfs* [51] | ||
| N281* [29] | R550* [29] | Q705Sfs* [29] | Y867H [29] | P1061Qfs* [51] | ||
| R282G [29] | H558Lfs* [51] | A727S [12] | V872Cfs* [29] | Q1084P [29] | ||
| 4 | D1143Mfs* [32,51] | |||||
| 5 | Q1170* [88] | |||||
| 6 | H1219D [32] | Y1245Lfs [85] | S1246* [51] | Y1255fs [12] | ||
| 8 | Y1337* [80] | A1341E [85] | ||||
| 9 | Y1351* [51] | R1359 C [88]/H [29] | S1369L [29] | H1380Y [29] | D1384V [29] | |
| Q1389* [51] | T1393I [29] | |||||
| 10 | R1465* [29] | R1467G_fs* [29] | K1493fs [12] | |||
| 11 | L1515Ffs* [51] | M1615* [89] | V1718L [29] | L1819* [29] | N1890S [12] | |
| L1531A_fs* [29,88] | Q1652Hfs* [29] | P1723S [29] | I1873T [80] | R1891G [29] | ||
| K1533* [29] | Y1679L_fs* [29] | D1750Efs* [51] | E1879* [29] | F1901Lfs* [51] | ||
| E1555R_fs* [29] | Q1680* [29] | N1765* [29] | H1881L [29]/R [29,51,88] | Y1902C [29] | ||
| Y1598Sfs* [29] | S1688_fs* [29,51,88] | M1800Dfs* [51] | T1884A [29] | H1904R [80] | ||
| S1611Y [12] | M1701I [29] | H1817Pfs* [29] | L1886S [29] | H1912Y [13] | ||
*: Stop codon resulting in an incomplete protein.
In line with this hypothesis, mutations in genes which are also frequently mutated in other myeloid malignancies are also present at relatively high frequencies in AdvSM patients [10,11,13,90,91,92] (Figure 1). In this regard, it has been recently described that certain DNA methylation patterns may be relevant in the pathogenesis of systemic diseases associated with MC activation [93]. Moreover, a significant number of somatic mutations has been identified in a broad number of genes involved in epigenetic regulatory mechanisms, which have been associated, at least in part, with the pathogenesis, clinical behaviour and evolution of different myeloid neoplasms, including SM [94,95]. Thus, around 30–40% of AdvSM present with an associated myeloid haematological neoplasm already at diagnosis [69], suggesting a close relationship between both malignancies. In line with this, next generation sequencing (NGS) studies have confirmed the presence of recurrent mutations in genes involved in post-transcriptional mRNA processing, epigenetic modification of DNA and transcription and signal transduction factors, in both SM and other myeloid neoplasms [10,11,51,81,87]. Among others, mutations have been recurrently reported in AdvSM in the ASXL1, CBL, DNMT3A, NRAS, RUNX1, SRSF2 and TET2 genes in AdvSM [10,12,13,29,32,46,50,51,68,80,81,87,96,97]. In contrast, the presence of these additional mutations is a relatively infrequent finding in BMM and ISM patients [10,29,51,80,87].
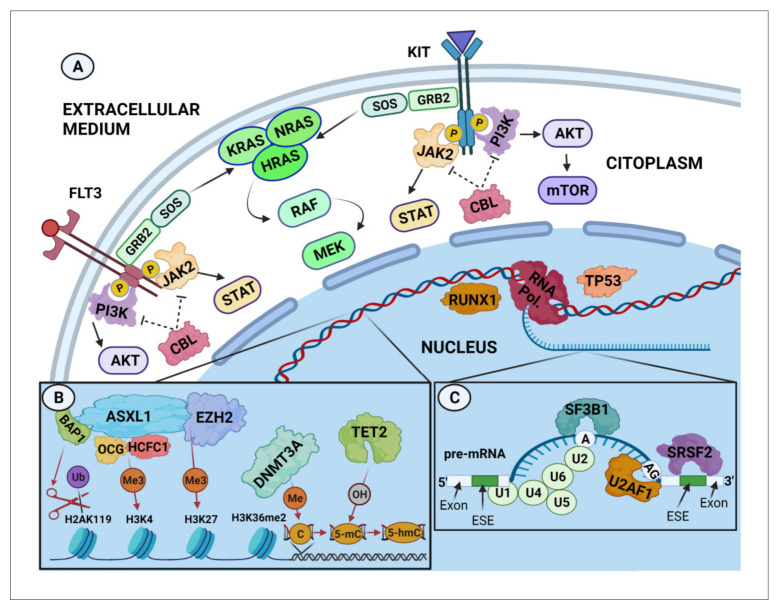
Figure 1.
Genes recurrently mutated in systemic mastocytosis categorized by cellular functions. (A) Signal transduction and transcription regulation. Extracellular signals are received and transmitted effectively into the cell by activation of cell-surface receptors such as tyrosine kinase receptors TKR (e.g., FLT3 or KIT), resulting in the activation of intracellular signalling cascades, including the MAPK (i.e., RAS), STATs (i.e., JAK2) and PI3K pathways, which promote cell proliferation, survival and apoptosis by inducing gene transcription and/or DNA epigenetic modifications [98]. Activation/repression of these pathways require appropriate regulation of the activity and/or quantity of specific proteins. As an example, CBL proteins negatively regulate TKR (e.g., FLT3, KIT) and non-TKR (e.g., PI3K, JAK2) proteins through their ubiquitination and proteasomal degradation [99]. (B) Epigenetic regulation. ASXL1 and EZH2 are members of the Polycomb group (PcG) of proteins, which are considered necessary to disrupt chromatin compaction in localized areas by activating/repressing specific histone markers. EZH2 is involved in transferring methyl groups to histone H3 lysine 27 (H3K27), whereas ASXL1, associated with BAP1, is involved in de-ubiquitinating mono-ubiquitinated histone H2AK119 and, when associated with the OGT/HCFC1 complex, in the methylation (Me3) of H3K4 [100]. The DNMT3A protein is recruited by the histone mark H3K36me2 [101] to be involved in the methylation of cytosines (5mC), whereas the TET protein family is involved in active demethylation through oxidation of 5mC to 5hmC [102]. Overall, this mechanism results in enhancing transcription of certain genes while repressing the transcription of other genes. (C) RNA splicing. At the pre-mRNA level, the SF3B1, SRSF2 and U2AF1 proteins cooperate with U1–U6 small nuclear ribonucleoproteins (sn-RNPs), forming the U2-dependent splicing complex that brings the two intronic ends together by attaching the two exons and removing the intron [103]. This process transforms the pre-mRNA into mRNA, which can be transduced into a protein by ribosomes. Abbreviations: A, branch site; AG, splice receptor site; BAP1, BRCA-1-associated protein 1; ESE, exonic splicing enhancer; 5mC, 5 methyl cytosine; H, histone; HCFC1, host cell factor C1; Me, methylation; OH, hydroxylation; OGT, O-linked N-acetylglucosamine (GlcNAc) transferase; pre-mRNA, precursor messenger RNA; PcG, polycomb group; RTKs, receptor tyrosine kinases; Ub, ubiquitin; U1-U6, small nuclear ribonucleoproteins (snRNPs). Created using BioRender.
4.1. Mutations Affecting Transcription Factors and Signalling Pathways
The correct function and development of the human organism strongly relies on the precise regulation and appropriate production of specific sets of proteins. Gene expression is largely regulated by transcription factors and the activation of processes involved in various intracellular signalling pathways. In this regard, alterations in genes involved in these processes, such as the CBL, JAK2, K/NRAS and/or RUNX1 genes [104], have been associated with several haematological malignancies. To date, mutations in a total of 11 genes related to transcription factors and signalling pathways have been described in patients with different subtypes of SM; of note, while some of these genes have been sporadically reported to be mutated in SM (EPHA7 [12,13], FLT3 [29], IKZF1 [13], PIK3CD [12,13], ROS1 [12,13] and TP53 [29]) (Tables S1 and S2), others (e.g., CBL, JAK2, K/NRAS and RUNX1) are recurrently found to be altered in SM, particularly among SM-AHN patients (Table 3).
Table 3.
Frequency of mutations in genes affecting transcription factors and signalling pathways found to be recurrently altered in systemic mastocytosis.
| Gene | SM Diagnostic Subgroup | Mutated Cases/Total Cases (%) | Overall Frequency |
WHO Subtype |
Mutated Cases/ Total Cases (%) |
Overall Frequency |
|
|---|---|---|---|---|---|---|---|
| CBL | Non-AdvSM | 1/12 (0) [10] 1/216 (0.5) [12] 0/6 (0) [13] 0/44 (0) [29] 0/1 (0) [50] 1/29 (3.4) [51] 0/26 (0) [68] 0/15 (0) [80] 0/6 (0) [85] |
1% | BMM | 0/65 (0) [12] | 0% | |
| ISM | 1/10 (10) [10] 0/3 (0) [13] 0/1 (0) [50] 0/26 (0) [68] 0/4 (0) [85] |
1/144 (1) [12] 0/44 (0) [29] 1/28 (4) [51] 0/15 (0) [80] |
1% | ||||
| SSM | 0/2 (0) [10] 0/3 (0) [13] 0/2 (0) [85] |
0/7 (0) [12] 0/1 (0) [51] |
0% | ||||
| AdvSM | 7/27 (26) [10] 1/13 (8) [12] 0/14 (0) [13] 16/106 (15) [29] 25/272 (9) [32] 1/25 (4) [50] 0/35 (0) [51] 10/83 (12) [68] 1/10 (10) [80] 3/26 (12) [81] 2/13 (15) [85] 4/19 (21) [90] |
11% | ASM | 0/1 (0) [10] 0/9 (0) [13] 0/2 (0) [50] 0/3 (0) [68] 0/1 (0) [85] |
0/9 (0) [12] 1/25 (4) [29] 0/9 (0) [51] 0/2 (0) [80] 0/6 (0) [90] |
2% | |
| SM-AHN | 6/23 (26) [10] 0/5 (0) [13] 1/21 (5) [50] 10/72 (14) [68] 2/12 (17) [85] |
1/4 (25) [12] 15/80 (19) [29] 0/23 (0) [51] 3/26 (12) [81] 4/13 (31) [90] |
15% | ||||
| MCL | 2/7 (29) [10] 0/2 (0) [50] 0/8 (0) [68] |
0/1 (0) [29] 0/3 (0) [51] |
10% | ||||
| JAK2 | Non-AdvSM | 0/12 (0) [10] 2/97 (2) [12] 0/6 (0) [13] 0/44 (0) [29] 0/1 (0) [50] 0/29 (0) [51] 2/26 (8) [68] 0/6 (0) [85] 0/13 (0) [88] |
2% | BMM | 1/23 (4) [12] | 4% | |
| ISM | 0/10 (0) [10] 0/3 (0) [13] 0/1 (0) [50] 2/26 (8) [68] 0/13 (0) [88] |
1/70 (1) [12] 0/44 (0) [29] 0/28 (0) [51] 0/4 (0) [85] |
2% | ||||
| SSM | 0/2 (0) [10] 0/3 (0) [13] 0/2 (0) [85] |
0/4 (0) [12] 0/1 (0) [51] |
0% | ||||
| AdvSM | 2/27 (7) [10] 0/14 (0) [13] 9/106 (9) [29] 25/213 (12) [32] 3/25 (12) [50] 3/35 (9) [51] 12/83 (15) [68] 3/47 (6) [81] 1/13 (8) [85] 2/29 (7) [88] |
10% | ASM | 0/1 (0) [10] 0/9 (0) [13] 0/2 (0) [50] 0/3 (0) [68] 0/5 (0) [88] |
0/7 (0) [12] 0/25 (0) [29] 0/9 (0) [51] 0/1 (0) [85] |
0% | |
| SM-AHN | 2/23 (9) [10] 0/5 (0) [13] 3/21 (14) [50] 12/72 (17) [68] 1/12 (8) [85] |
0/3 (0) [12] 9/80 (11) [29] 3/23 (13) [51] 3/47 (6) [81] 2/23 (9) [88] |
11% | ||||
| MCL | 0/3 (0) [10] 0/1 (0) [50] 0/8 (0) [68] |
0/1 (0) [29] 0/3 (0) [51] 0/1 (0) [88] |
0% | ||||
| KRAS | Non-AdvSM | 0/12 (0) [10] 2/97 (2) [12] 0/6 (0) [13] 0/29 (0) [51] 0/36 (0) [84] |
1% | BMM | 0/23 (0) [12] | 0% | |
| ISM | 0/10 (0) [10] 0/3 (0) [13] 0/27 (0) [84] |
2/70 (3) [12] 0/28 (0) [51] |
1% | ||||
| SSM | 0/2 (0) [10] 0/3 (0) [13] 0/9 (0) [84] |
0/4 (0) [12] 0/1 (0) [51] |
0% | ||||
| AdvSM | 4/27 (15) [10] 0/10 (0) [12] 0/14 (0) [13] 0/35 (0) [51] 2/16 (13) [68] |
6% | ASM | 0/1 (0) [10] 0/9 (0) [13] | 0/7 (0) [12] 0/9 (0) [51] |
0% | |
| SM-AHN | 4/23 (17) [10] 0/5 (0) [13] 2/16 (13) [68] |
0/3 (0) [12] 0/23 (0) [51] |
9% | ||||
| MCL | 0/3 (0) [10] | 0/2 (0) [51] | 0% | ||||
| NRAS | Non-AdvSM | 0/12 (0) [10] 0/6 (0) [13] 0/44 (0) [29] 0/1 (0) [50] 0/23 (0) [51] 0/36 (0) [84] 1/298 (0.3) [97] |
0.2% | BMM | |||
| ISM | 0/10 (0) [10] 0/44 (0) [29] 0/22 (0) [51] |
0/3 (0) [13] 0/1 (0) [50] 0/27 (0) [84] |
0% | ||||
| SSM | 0/2 (0) [10] 0/1 (0) [51] |
0/3 (0) [13] 0/9 (0) [84] |
0% | ||||
| AdvSM | 2/27 (7) [10] 0/14 (0) [13] 3/105 (3) [29] 1/25 (4) [50] 1/25 (4) [51] 2/16 (13) [68] 3/173 (2) [97] |
3% | ASM | 0/1 (0) [10] 0/25 (0) [29] 0/7 (0) [51] |
0/9 (0) [13] 0/2 (0) [50] |
0% | |
| SM-AHN | 2/23 (9) [10] 3/80 (4) [29] 1/16 (6) [51] |
0/5 (0) [13] 1/21 (5) [50] 2/16 (13) [68] |
6% | ||||
| MCL | 0/3 (0) [10] 0/2 (0) [50] | 1/1 (100) [29] 0/2 (0) [51] |
13% | ||||
| RUNX1 | Non-AdvSM | 0/12 (0) [10] 1/309 (0.3) [12] 2/10 (20) [13] 0/44 (0) [29] 0/26 (0) [68] 0/6 (0) [85] 1/530 (0.2) [97] |
0.4% | BMM | 1/90 (1) [12] | 1% | |
| ISM | 0/10 (0) [10] 0/3 (0) [13] 0/26 (0) [68] | 0/211 (0) [12] 0/44 (0) [29] 0/4 (0) [85] |
0% | ||||
| SSM | 0/2 (0) [10] 2/7 (29) [13] |
0/8 (0) [12] 0/2 (0) [85] |
11% | ||||
| AdvSM | 9/27 (33) [10] 0/13 (0) [12] 7/24 (29) [13] 5/106 (5) [29] 66/329 (20) [32] 15/83 (18) [68] 1/13 (8) [85] 38/210 (18) [97] |
18% | ASM | 1/1 (100) [10] 2/11 (18) [13] 1/3 (33) [68] | 0/9 (0) [12] 0/25 (0) [29] 0/1 (0) [85] |
8% | |
| SM-AHN | 8/23 (35) [10] 5/13 (39) [13] 14/72 (19) [68] | 0/4 (0) [12] 5/80 (6) [29] 1/12 (8) [85] |
16% | ||||
| MCL | 0/3 (0) [10] 0/8 (0) [68] |
0/1 (0) [29] | 0% | ||||
Overall frequencies represent the weighted average of the percentage of patients with at least one mutation in that gene. out of the total number of patients studied within the different cohorts, for each SM subgroup. Abbreviations: AdvSM: advanced systemic mastocytosis (SM); ASM: aggressive SM; BMM: bone marrow mastocytosis; ISM: indolent SM; MCL: mast cell leukaemia; Non-AdvSM: non-advanced SM; SM-AHN: SM with an associated haematological neoplasm; SSM: smouldering SM.
The CBL (Casitas B-lineage lymphoma proto-oncogene) gene is located on chromosome 11 and encodes for a protein involved in the functional regulation (via competitive blockade) of tyrosine kinase (TK) receptors; in addition, the CBL product also acts in ubiquitination-mediated protein degradation in the proteasome [105,106]. Overall, mutations affecting the CBL gene in myeloid malignancies show a predominance of deletions involving the exon 8 of this gene [107] at frequencies that vary from 15% of patients diagnosed with juvenile myelomonocytic leukaemia, to 13% of CMML (mostly the CBL Y371 mutation) [108,109], 10% of AML and 8% of atypical chronic myeloid leukaemia cases [109,110,111]. Similarly, CBL mutations are found in a variable percentage of SM patients [10,11,29,46,68,80,81], where they are predominantly located at exon 8 (frequently also at codon Y371) (Table 2), their frequency ranging from <1% in Non-AdvSM patients to >10% of AdvSM cases [10,29,80,81,90], including >25% of SM-AHN patients in some cohorts [10,90] (average of 15%) (Table 3). In contrast to other myeloid neoplasms in which the impact of CBL mutations remains unclear [106,109,110,112], their presence in SM has been associated with poorer outcomes [90].
The JAK2 (Janus Kinase 2) gene is located on human chromosome 9 and encodes a protein that acts as an intracellular (non-receptor) TK that is associated with various cell surface receptors for transducing activating signals through relevant pathways such as the mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription (STATs) pathways [98,113,114]. The most common JAK2 activating mutation, the JAK2 V617F mutation, has been reported in several diagnostic subtypes of MPN [115], which can explain its high incidence (about 11%) in SM-AHN patients [29,50,51,81,88] as compared to other diagnostic subtypes of SM [10,12,29,51,88] (Table 3 and Table S3). A recent study in SM-AHN patients showed that KIT D816V and JAK2 V617F mutations probably arise in two independent clones in most patients, in which the presence of JAK2 mutations appears to have a low prognostic impact [116].
The KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) and NRAS (Neuroblastoma RAS Viral Oncogene Homolog) genes are both located on chromosome 12, and they encode proteins involved in signalling pathways associated with growth factor membrane receptors through their interaction with membrane GTPases. A large number of somatic mutations involving the KRAS/NRAS genes have been identified, mostly associated with solid tumours such as lung cancer, pancreatic cancer and colorectal cancer, among other prevalent tumours [117,118]; in some of these tumours such as metastatic colorectal cancer, KRAS and NRAS mutations have also been associated with a poorer prognosis [119]. In myeloid neoplasms, NRAS mutations have been associated with the development of AML (7–13%) secondary to different subtypes of MPN; however, it remains unclear whether these mutations directly promote progression to leukaemia [111]. With regards to SM, KRAS and/or NRAS mutations have been sporadically reported in ISM [12,97] and MCL cases [10,29], while they are more frequently found among SM-AHN patients, particularly in cases associated with poor-prognosis myeloid neoplasms (i.e., AML) [10,29,46,50,51,68,84] (Table 3 and Table S3); in this setting, some authors have suggested that these mutations might have an adverse prognostic impact [120].
RUNX1 (Runt-Related Transcription Factor 1) is a gene located on human chromosome 21 that encodes a functional protein that acts as a transcription factor involved in the development of HSC [121]. The most frequent RUNX1 mutations have been associated with progression from MPN to AML [122], which could explain the high frequency of these mutations (up to 37%) among patients with secondary AML [105,111]. In line with these findings, the presence of RUNX1 mutations in patients with MDS is associated with resistance to specific chemotherapeutic drugs and shortened survival [123,124]. In SM, RUNX1 mutations are preferentially located at exons 4 and 5 of the gene [10,11,12,13,29,32,68,97,125] (Table 2), with a frequency that ranges from <1% of Non-AdvSM patients to up to 18% of AdvSM cases, the highest frequency being detected in SM-AHN patients [10,13] (Table 3). From a prognostic point of view, RUNX1-mutated cases have been associated with an adverse outcome, both among Non-AdvSM and AdvSM patients [11,12,13,97,126].
4.2. Mutations in Genes Involved in Epigenetic Regulatory Mechanisms
Although the specific role of each individual epigenetic alteration detected in SM remains unknown [127,128,129,130], recurrent mutations in genes involved in epigenetic modifications of DNA (i.e., ASXL1, CILK1, DNMT3A, EZH2, IDH1, IDH2, KAT6B, NPM1, SETBP1 and TET2 genes) have been recurrently identified (Table 2, Tables S1 and S2); among these, mutations involving the ASXL1, DNMT3A, EZH2 and TET2 genes are the most commonly reported ones (Table 4).
Table 4.
Frequency of mutations in genes involved in epigenetic regulatory mechanisms found to be recurrently altered in systemic mastocytosis.
| Gene | SM Diagnostic Subgroup | Mutated Cases/Total Cases (%) | Overall Frequency | WHO Subtype |
Mutated Cases/ Total Cases (%) |
Overall Frequency |
|
|---|---|---|---|---|---|---|---|
| ASXL1 | Non-AdvSM | 0/12 (0) [10] 6/309 (2) [12] 0/10 (0) [13] 0/44 (0) [29] 0/1 (0) [50] 0/26 (0) [68] 1/15 (7) [80] 0/6 (0) [85] 6/530 (1) [97] |
1% | BMM | 1/90 (1) [12] | 1% | |
| ISM | 0/10 (0) [10] 0/3 (0) [13] 0/1 (0) [50] 1/15 (7) [80] 6/530 (1) [97] |
4/211 (2) [12] 0/44 (0) [29] 0/26 (0) [68] 0/4 (0) [85] |
1% | ||||
| SSM | 0/2 (0) [10] 0/7 (0) [13] | 1/8 (13) [12] 0/2 (0) [85] |
5% | ||||
| AdvSM | 8/27 (30) [10] 2/13 (15) [12] 2/24 (8) [13] 25/106 (24) [29] 66/229 (29) [32] 12/25 (48) [50] 21/83 (25) [68] 2/10 (20) [80] 6/43 (14) [81] 5/13 (39) [85] 5/19 (26) [90] 35/210 (17) [97] |
24% | ASM | 0/1 (0) [10] 1/11 (9) [13] 0/2 (0) [50] 0/2 (0) [80] 1/6 (17) [90] |
1/9 (11) [12] 4/25 (16) [29] 0/3 (0) [68] 0/1 (0) [85] |
9% | |
| SM-AHN | 8/23 (35) [10] 1/13 (8) [13] 4/21 (19) [50] 6/43 (14) [81] 4/13 (31) [90] | 1/4 (25) [12] 21/80 (26) [29] 21/72 (29) [68] 5/12 (42) [85] |
25% | ||||
| MCL | 0/3 (0) [10] 0/2 (0) [50] |
0/1 (0) [29] 0/8 (0) [68] |
0% | ||||
| DNMT3A | Non-AdvSM | 14/309 (5) [12] 0/10 (0) [13] 2/44 (5) [29] 0/26 (0) [68] 2/15 (13) [80] 20/530 (4) [97] |
4% | BMM | 2/90 (2) [12] | 2% | |
| ISM | 10/211 (0.5) [12] 2/44 (5) [29] 2/15 (13) [80] | 0/3 (0) [13] 0/26 (0) [68] |
5% | ||||
| SSM | 2/8 (25) [12] | 0/7 (0) [13] | 13% | ||||
| AdvSM | 4/13 (31) [12] 3/24 (13) [13] 7/106 (7) [29] 1/83 (1) [68] 1/10 (10) [80] 2/19 (11) [90] 9/210 (4) [97] |
6% | ASM | 3/9 (33) [12] 0/25 (0) [29] 0/2 (0) [80] |
3/11 (27) [13] 0/3 (0) [68] 0/6 (0) [90] |
11% | |
| SM-AHN | 1/4 (25) [12] 7/80 (9) [29] 1/8 (13) [80] |
0/13 (0) [13] 1/72 (1) [68] 2/13 (15) [90] |
6% | ||||
| MCL | 0/1 (0) [29] | 0/8 (0) [68] | 0% | ||||
| EZH2 | Non-AdvSM | 0/12 (0) [10] 0/309 (0) [12] 1/10 (10) [13] 0/44 (0) [29] 0/26 (0) [68] 0/15 (0) [80] 0/6 (0) [85] |
0.2% | BMM | 0/90 (0) [12] | 0% | |
| ISM | 0/10 (0) [10] 0/3 (0) [13] 0/26 (0) [68] 0/4 (0) [85] |
0/211 (0) [12] 0/44 (0) [29] 0/15 (0) [80] |
0% | ||||
| SSM | 0/2 (0) [10] 1/7 (14) [13] | 0/8 (0) [12] 0/2 (0) [85] |
5% | ||||
| AdvSM | 2/27 (7) [10] 0/13 (0) [12] 4/24 (17) [13] 3/106 (3) [29] 17/305 (6) [32] 2/83 (2) [68] 0/10 (0) [80] 2/13 (15) [85] |
5% | ASM | 0/1 (0) [10] 2/11 (18) [13] 0/3 (0) [68] 0/1 (0) [85] |
0/9 (0) [12] 1/25 (4) [29] 0/2 (0) [80] |
6% | |
| SM-AHN | 2/23 (9) [10] 2/13 (15) [13] 2/72 (3) [68] 2/12 (17) [85] | 0/4 (0) [12] 2/80 (3) [29] 0/8 (0) [80] |
5% | ||||
| MCL | 0/3 (0) [10] 0/8 (0) [68] | 0/1 (0) [29] | 0% | ||||
| TET2 | Non-AdvSM | 0/12 (0) [10] 7/309 (2) [12] 0/10 (0) [13] 0/1 (0) [50] 2/29 (7) [51] 1/26 (4) [68] 1/15 (7) [80] 0/6 (0) [85] 2/13 (15) [88] |
3% | BMM | 2/90 (2) [12] | 2% | |
| ISM | 0/10 (0) [10] 0/3 (0) [13] 1/28 (4) [51] 1/15 (7) [80] 2/13 (15) [88] | 5/211 (2) [12] 0/1 (0) [50] 1/26 (4) [68] 0/4 (0) [85] |
3% | ||||
| SSM | 0/2 (0) [10] 0/7 (0) [13] 0/2 (0) [85] |
0/8 (0) [12] 1/1 (100) [51] |
5% | ||||
| AdvSM | 15/27 (56) [10] 2/13 (15) [12] 3/24 (13) [13] 137/329 (42) [32] 12/25 (48) [50] 12/35 (34) [51] 33/83 (40) [68] 5/10 (50) [80] 12/32 (38) [81] 6/13 (46) [85] 10/29 (35) [88] 5/19 (26) [90] |
39% | ASM | 0/1 (0) [10] 0/11 (0) [13] 3/9 (33) [51] 0/2 (0) [80] 2/5 (40) [88] |
1/9 (11) [12] 2/2 (100) [50] 0/3 (0) [68] 0/1 (0) [85] 1/6 (17) [90] |
21% | |
| SM-AHN | 15/23 (65) [10] 3/13 (23) [13] 9/23 (39) [51] 5/8 (63) [80] 6/12 (50) [85] 4/13 (31) [90] | 1/4 (25) [12] 9/21 (43) [50] 33/72 (46) [68] 12/32 (38) [81] 8/23 (35) [88] |
43% | ||||
| MCL | 0/3 (0) [10] 0/3 (0) [51] 0/1 (0) [88] | 1/2 (50) [50] 0/8 (0) [68] |
6% | ||||
Overall frequencies represent the weighted average of the percentage of patients with at least one mutation in that gene out of the total number of patients studied in the different cohorts for each SM subgroup. Abbreviations: AdvSM: advanced systemic mastocytosis (SM); ASM: aggressive SM; BMM: bone marrow mastocytosis; ISM: indolent SM; MCL: mast cell leukaemia; Non-AdvSM: non-advanced SM; SM-AHN: SM with an associated haematological neoplasm; SSM: smouldering SM.
The ASXL1 (ASXL transcriptional regulator 1) gene encodes for a protein that interacts with the retinoic acid receptor involved in chromatin remodelling, although its precise function remains largely unknown [131]. The most frequent ASXL1 mutations found in myeloid neoplasms are located at exon 12 [132], with an overall incidence that ranges from <7% of patients with essential thrombocytopenia (ET) or polycythaemia vera (PV), to almost 40% of primary myelofibrosis cases [133]. ASXL1 is also the second most frequently mutated gene in MDS and CMML, and it is altered in up to 30% of AML patients [132,134]. Most reported ASXL1 mutations in SM are also located at exon 12 [12,13,29,32,80,81] (Table 2) with a highly variable frequency that ranges from 1% of BMM cases to >20% of AdvSM patients, particularly of SM-AHN cases (Table 4). Similarly to other myeloid neoplasms [124,133,135], ASXL1 mutations have been also (recurrently) associated with a worse prognosis in SM [11,29,68,80,81,85].
The DNMT3A (DNA Methyltransferase 3 Alpha) gene located on chromosome 2, encodes for an enzyme responsible for the methylation of CpG islands, which is critical in various physiological processes during embryogenesis and/or in the inactivation of the X chromosome [136]. The most frequently described mutation in the DNMT3A gene occurs at codon R882 [137], being present in 8–13% of MDS, 26% of AML secondary to MDS and 2% of CMML patients [137,138]. In general, the presence of DNMT3A mutations in patients with myeloid malignancies has been associated with a higher number of blasts in BM and greater leukocyte counts in blood [124,134] in the absence of a clear prognostic impact [134,137,138,139]. Although DNMT3A mutations have been described at relatively similarly low frequencies in Non-AdvSM and AdvSM (4% vs. 6%, respectively) (Table 4), their presence has been associated with a significantly poorer prognosis in some patient cohorts [12,80].
The EZH2 (Enhancer of Zeste 2 polycomb repressive complex 2 subunit) gene encodes a protein of the PRC2 complex involved in proliferation, differentiation, ageing and maintenance of the chromatin structure through methylation, acting as both a tumour suppressor gene and an oncogene [105]. The EZH2 gene is coded in chromosome 7, and its mutations have been described in both myeloid and lymphoid malignancies, as well as in solid tumours, where they have been recurrently associated with more advanced tumour stages and metastatic disease [140]. In myeloid neoplasms, EZH2 mutations have been described in patients with PV (3%), myelofibrosis (13%), CMML (6%), AML (6%) and MDS (10%) [105,134,139,141,142]; in MDS they have been associated with a worse prognosis [124,142]. In SM, EZH2 mutations have been reported almost exclusively within AdvSM patients [10,13,29,32,85], particularly among ASM and SM-AHN cases (Table 4).
The TET2 (Ten–eleven translocation methylcytosine dioxygenase 2) gene is located on chromosome 4 and encodes for a protein that catalyses the conversion of 5-methylcytosine (5-mc) to 5-hydroxymethylcytosine (5-hmc) in the DNA [143]. It is believed that 5-hmc may initiate DNA demethylation by preventing binding to the CpG islands of DNA methyltransferases characteristic of these sequences [144]. To date, TET2 mutations have been described in every exon of the gene, and sometimes mutations involving both alleles coexist in the same cell [13,145]. TET2 mutations are considered to be early events in the development of haematological malignancies such as MPN, MDS, CMML and different subtypes of leukaemia and lymphoma, as well as in SM [145]. Overall, TET2 mutations have been described in about 14% of MPN, 23% of MDS (in which they usually occur together with mutations in SF3B1, U2AF1, ASXL1, SRSF2 and/or DNMT3A and also a normal karyotype [124]) and 30% of CMML patients (often associated with mutations in the SRSF2 and U2AF1 genes) [10,91,124,134,146]. In SM, TET2 is the most frequently mutated gene other than KIT. In these later patients, TET2 mutations have been reported along the entire gene sequence but more frequently at exons 3, 9 and 11 (Table 2). As found also in MDS, the coexistence of TET2 and SRSF2 gene mutations has also been reported in SM [10,85]. Of note, in vitro studies suggest that in a significant proportion of patients with SM-AHN, TET2 mutations may precede the KIT D816V mutation [85], similarly to what would also occur with ASXL1 and SRSF2 mutations. However, despite TET2 mutations being significantly more frequently detected in AdvSM vs. Non-AdvSM patients (39% vs. 3% of the cases, respectively) [10,12,13,32,50,51,68,80,81,85,88,90] (Table 4), and their being associated with the presence of C-findings [51], they do not seem to have any prognostic impact in SM [10,11,12,13,29,68,80,81,97,139].
4.3. Mutations in Genes Involved in Alternative mRNA Splicing
The presence of mutations in genes associated with the spliceosome, responsible for alternative RNA processing, has been linked to different diagnostic subtypes of haematopoietic malignancies (e.g., MDS) and some solid tumours (e.g., ocular uveal melanoma or pulmonary fibrosis) [86,147]. These include mutations in the SF3B1, SRSF2 and U2AF1 genes, from which mutations in the former two genes have been described in SM at relatively high frequencies in SM (Table 5) and/or (i.e., SRSF2) in association with poorer outcomes [11,96].
Table 5.
Frequency of mutations in genes involved in alternative mRNA splicing recurrently found in systemic mastocytosis.
| Gene | SM Prognostic Subgroup | Mutated Cases/ Total Cases (%) |
Overall Frequency |
WHO Subtype |
Mutated Cases/ Total Cases (%) |
Overall Frequency |
|
|---|---|---|---|---|---|---|---|
| SF3B1 | Non-AdvSM | 2/309 (0.6) [12] 0/10 (0) [13] 0/44 (0) [29] 0/26 (0) [68] 0/6 (0) [85] |
0.5% | BMM | 1/90 (1) [12] | 1% | |
| ISM | 1/211 (0.5) [12] 0/44 (0) [29] 10/4 (0) [85] |
0/3 (0) [13] 0/26 (0) [68] | 0.3% | ||||
| SSM | 0/8 (0) [12] 0/2 (0) [85] |
0/7 (0) [13] | 0% | ||||
| AdvSM | 0/13 (0) [12] 3/24 (13) [13] 9/106 (9) [29] 18/305 (6) [32] 2/83 (2) [68] 1/13 (8) [85] |
7% | ASM | 0/9 (0) [12] 1/25 (4) [29] 0/1 (0) [85] |
2/11 (18) [13] 0/3 (0) [68] |
6% | |
| SM-AHN | 0/4 (0) [12] 7/80 (9) [29] 1/12 (8) [85] | 1/13 (8) [13] 2/72 (3) [68] |
6% | ||||
| MCL | 1/1 (100) [29] | 0/8 (0) [68] | 13% | ||||
| SRSF2 | Non-AdvSM | 0/12 (0) [10] 0/309 (0) [12] 0/10 (0) [13] 0/44 (0) [29] 0/1 (0) [50] 0/26 (0) [68] 0/6 (0) [85] 7/530 (1) [97] |
0.7% | BMM | 0/90 (0) [12] | 0% | |
| ISM | 0/10 (0) [10] 0/3 (0) [13] 0/1 (0) [50] 0/4 (0) [85] | 0/211 (0) [12] 0/44 (0) [29] 0/26 (0) [68] | 0% | ||||
| SSM | 0/2 (0) [10] 0/7 (0) [13] |
0/8 (0) [12] 0/2 (0) [85] |
0% | ||||
| AdvSM | 14/27 (52) [10] 2/13 (15) [12] 3/24 (13) [13] 1/106 (1) [29] 120/329 (37) [32] 8/25 (32) [50] 31/83 (37) [68] 4/13 (31) [85] 79/210 (38) [97] |
32% | ASM | 0/1 (0) [10] 0/11 (0) [13] 1/2 (50) [50] 0/1 (0) [85] |
1/9 (11) [12] 0/25 (0) [29] 0/3 (0) [68] |
4% | |
| SM-AHN | 13/23 (57) [10] 3/13 (23) [13] 7/21 (33) [50] 4/12 (33) [85] |
1/4 (25) [12] 1/80 (1) [29] 31/72 (43) [68] |
27% | ||||
| MCL | 1/3 (33) [10] 0/2 (0) [50] | 0/1 (0) [29] 0/8 (0) [68] | 7% | ||||
Overall frequencies represent the weighted average of the percentage of patients with at least one mutation in that gene out of the total number of patients studied within the different cohorts for each subgroup of SM. Abbreviations: AdvSM: advanced systemic mastocytosis (SM); ASM: aggressive SM; BMM: bone marrow mastocytosis; ISM: indolent SM; MCL: mast cell leukaemia; Non-AdvSM: non-advanced SM; SM-AHN: SM with an associated haematological neoplasm; SSM: smouldering SM.
The SRSF2 (serine and arginine rich splicing factor 2) gene encodes for a protein that is critical for alternative mRNA processing at the post-transcriptional level [148], which also acts as an important regulator of DNA stability, being a key player in the DNA acetylation/phosphorylation network [149]. The most frequent somatic mutations of SRSF2 found in SM patients are located at codon P95 [10,12,13,32,87] (Table 2). Among patients with other myeloid haematological neoplasms, SRSF2 mutations are particularly frequent (28–30%) among CMML cases [150] and, to a less extent, MDS (11%) and AML (6%) patients [124,134,150]. Recent studies in SM patients show the presence of SRSF2 mutations in a variable percentage of cases ranging from <1% of Non-AdvSM cases to around one third of AdvSM patients (Table 5), being one of the most frequently mutated genes in SM, particularly in SM-AHN cases [10,11,12,13,29,32,46,50,68,80,87,97]. In contrast to other haematological neoplasms [134,151,152,153], the presence of SRSF2 mutations has been consistently associated with an adverse prognosis in patients with SM [13,97], particularly among AdvSM cases [11,46,68].
The SF3B1 (splicing factor 3b subunit 1) gene is located in chromosome 2, and it encodes for the largest subunit of the SF3B complex, a core component of the U2 small nuclear ribonucleoprotein of the U2-dependent spliceosome [154]. SF3B1 is the most commonly mutated splicing factor gene in MDS patients [155], in whom it is associated with a more favourable outcome [156]. In contrast to SRSF2, SF3B1 mutations have been less frequently described in SM [12,13,29,32,83,86], with only the K666 codon found to be mutated in more than two patient series. Actually, SF3B1 mutations are detected in <7% of AdvSM patients (most frequently in SM-MDS cases [13,68,85]) (Table S3), while they are rarely found in Non-AdvSM patients [10,12,29,68] (Table 5). Likewise, U2AF1 mutations are also relatively rare in SM, with a higher incidence in AdvSM [10,29,50] vs. Non-AdvSM cases (6% vs. 1%, respectively) (Table S2); these mutations are mostly located at codons S34 [29,157,158] and Q157 [29] of the U2AF1 gene (Table S1).
5. Prognostic Impact of Acquired Gene Mutations in Systemic Mastocytosis
Acquisition of the KIT D816V mutation in HSC during haematopoiesis leads to multilineage involvement by the KIT mutation [77], which is associated with a poor prognosis of Non-AdvSM cases due to an increased risk of progression to AdvSM [5,77]. Despite the relatively early onset of the KIT D816V mutation throughout life, in at least a fraction of (i.e., multilineal) SM patients [159], the most common clinical manifestations of the disease (e.g., urticaria pigmentosa and/or anaphylaxis) usually emerge at the third or fourth decades of life in the majority of SM cases [9,159,160]. Thus, from the constitutive activation of KIT in HSC until the development of an advanced form of SM, progressive expansion and accumulation of mutated cells is required to occur, probably in association with the acquisition of secondary genetic lesions, an increased capacity to maintain them (e.g., activation/repression of anti-/pro-apoptotic mechanisms) [79] and/or the cooperation with a specific genetic background [161]. Studies performed in murine models and in patients with SM have shown that the coexistence of the KIT D816V mutation and mutation(s) in genes other than KIT are probably necessary for the progression and transformation from pauci-symptomatic Non-AdvSM to advanced forms of the disease [10,51,159,162]. However, neither a specific mutation (or mutation profile) nor a specific genetic background shared by all AdvSM patients have been identified so far [10,11,13,51,80,81,85,87,88,137]. Instead, the number of mutated genes (other than KIT) significantly increases from ISM to ASM [13,46] and other subtypes of AdvSM [10,29,163]. Of note, the acquisition of these additional (somatic) mutations in ISM patients who present with the multilineage KIT D816V mutation is usually associated with progression of the disease to, e.g., SSM and/or ASM [12,32]. In fact, demonstration of multilineage involvement of haematopoiesis by the KIT D816V mutation has been shown to be an independent prognostic factor for predicting progression of ISM [5]. In line with these findings, most AdvSM cases carry the multilineage KIT D816V mutation associated with involvement of CD34+ HSC, except for a minor fraction of SM-AHN patients that have the KIT D816V mutation restricted to the MC compartment in BM. Interestingly, in these latter cases, the SM and AHN components of the disease appear to derive from independent clones that coexist in the same individual [36]. Despite the prognostic relevance of the multilineage KIT mutation, access to BM cell purification techniques required to investigate the presence of the KIT mutation in different myeloid and lymphoid compartments of BM cells is still restricted to a limited number of diagnostic laboratories, which has hampered the use of the multilineage KIT mutation as a predictor for the progression of ISM to SSM and AdvSM in routine diagnostics [7,32,120]. In line with this, the use of high sensitivity (quantitative) methods for the identification of the KIT D816V mutation [164,165,166] has proven in recent years to be of great utility to identify ISM patients that present the multilineage KIT as those that display a high KIT D816V variant allele frequency (VAF) in unfractionated BM (i.e., VAF ≥ 1–2%) [12,167] and/or blood (VAF ≥ 6%). [78] In fact, these later ISM cases also carry a significantly higher probability of undergoing disease progression associated with a significantly shortened life expectancy [167]. These findings support the use of a high KIT D816V VAF (as assessed by allele-specific qPCR) as a surrogate marker of multilineage involvement of haematopoiesis by the KIT D816V mutation [168].
In parallel, several studies on the general adult population have shown that the presence of mutations in genes that are considered to be initiators (or “drivers”) of clonal expansions of HSC [169,170], while exceptional among individuals <40 years of age [170], progressively increases from the fifth decade of life onwards [171,172], being recognized as age-related clonal haematopoiesis (ARCH). Of note, ARCH is characterized by the presence of somatic mutations in genes that are also frequently mutated in SM (e.g., ASXL1, DNMT3A, EZH2, RUNX1, SF3B1, SRSF2 and TET2) and other myeloid neoplasms [92,173,174]. Some of these ARCH-related genetic mutations that are frequently reported in AdvSM cases have been confirmed to be directly associated with the development of haematopoietic neoplasms and are considered clonal haematopoiesis of oncogenic potential (CHOP) mutations [175] (e.g., SRSF2 [11], ASXL1 [11], DNMT3A [80], RUNX1 [11], EZH2 [13], CBL [90]). These findings may contribute to explain the higher prevalence of myeloid neoplasms among older individuals and would be in agreement with the observation that age ≥60 years at diagnosis of SM predicts an increased risk of (primary and secondary) AdvSM [7,32,120]. Therefore, acquisition of ARCH-related gene mutations is currently considered to be closely associated with (a higher risk for) more advanced forms of SM. Among AdvSM patients, mutations in most of these genes (e.g., ASXL1, CBL, JAK2, KRAS, NRAS, RUNX1, SRSF2 and TET2) have been reported to be more frequently associated with SM-AHN than ASM, with only a few exceptions that involve genes that show similarly mutated frequencies in both subtypes of SM (i.e., DNMT3A, EZH2 and SF3B1) (Table 4 and Table 5). Moreover, for most of these mutated genes (e.g., ASLXL1, DNMT3A, EZH2, IKZF1, RUNX1, SF3B1, SRSF2 and TET2) [12], a high VAF is usually detected in the BM of AdvSM patients, which might also reflect the presence of multilineage involvement of haematopoiesis by these mutations, similarly to what has been described above for KIT D816V [9,13,74]. In these multi-mutated SM patients, the exact sequence of acquisition of genetic mutations remains unclear; thus, in some patients, the KIT D816V appears to be the first acquired mutation [13], while another subgroup of SM cases carries the KIT D816V mutation and mutations in genes other than KIT in different cell clones [13,36,85], and in a third subgroup of SM patients, the KIT mutation appears to be a secondary event. Of note, the two later subgroups of patients are usually diagnosed with SM associated with another myeloid neoplasm (i.e., SM-AHN) [13,36,85].
Altogether, these observations suggest that in patients with Non-AdvSM, the disease is mostly driven by the KIT D816V mutation, while the occurrence of additional mutations in other genes would be required (prior to or after the KIT mutation) for the development of AdvSM. In order to elucidate whether any of these mutated genes confers an adverse prognosis, multiple studies have been conducted in SM [11,12,13,29,32,68,80,90,120,160,176], from which a few include medium to large patient cohorts (n ≥ 100) [12,29,32,68,97]. Of note, the number of genes screened in these studies is highly variable, ranging from 9 genes [80] to 410 genes [13], with a few patients being investigated by whole-genome [13] or whole-exome [42,177,178,179] sequencing. Overall, these studies found a total of 30 different genes to be mutated in SM (Table 2 and Table S1), from which 11 (i.e., ASXL1, CBL, DNMT3A, EZH2, JAK2, KRAS, NRAS, RUNX1, SF3B1, SRSF2, TET2) are recurrently mutated genes in several SM cohorts (Table 3, Table 4 and Table 5). From these later 11 mutated genes, a few have (independent) prognostic implications as regards disease progression and/or overall patient survival (i.e., SRSF2 [11,32], ASXL1 [11,32], DNMT3A [12], RUNX1 [11,32], EZH2 [13], CBL [90] and NRAS [120]), particularly when mutation/s are present at high VAF [12]. Because of this, the presence of mutations in limited sets of genes has been included in several recently proposed risk stratification models for both AdvSM (i.e., SRSF2/ASXL1/RUNX1 [11], SRSF2/ASXL1/RUNX1/EZH2 [13], ASXL1/RUNX1/NRAS [120]) and Non-AdvSM (e.g., ASXL1/RUNX1/DNMT3A [12]). The recent development of SM-induced pluripotent stem cells (iPSCs) positive for KIT D816V and other concurrent mutations [180], which accurately reflect the genetic background of SM patients’ multi-mutated pathological cells, may become a powerful tool to dissect the impact of these mutations on the aetiopathogenic mechanisms involved in disease progression [181]. Therefore, molecular characterization of the genetic background of AdvSM patients, including NGS as described above, VAF assessment of somatic mutations [125,182] and drug screening in patient-derived iPSCs [180,183], may lead to improved molecularly targeted treatment options in a context of personalized precision medicine.
6. Conclusions
At present it is well-established that SM is a clonal HSC disease characterized by the expansion and accumulation of neoplastic MCs, where the presence of activating KIT mutations (most commonly KIT D816V) is a hallmark of the disease, being present in most (>90%) adult patients, at similar frequencies in Non-AdvSM and AdvSM. Despite the KIT D816V mutation being currently considered the pathogenic driver of SM, it cannot explain by itself the heterogeneous clinical behaviour of this disease. In this regard, the presence of multilineage involvement of haematopoiesis by the KIT D816V mutation, particularly in the context of a multi-mutated disease in which additional myeloid-neoplasm-associated genes other than KIT are also mutated, emerges as the altered genetic background that might contribute to explain malignant transformation of SM. Because of this, assessment of multilineage involvement of haematopoiesis by the KIT D816V mutation should be performed in newly diagnosed SM patients to identify those cases at high risk of progression to AdvSM. In addition, identification of other pathogenic mutations in genes with known prognostic impacts in SM (i.e., SRSF2, ASXL1, DNMT3A, RUNX1, EZH2, CBL and NRAS) should also be performed in SM patients with multilineage involvement of haematopoiesis by KIT D816V for further identification of patients at higher risk of death who may benefit from a closer follow-up and eventually, also, early cytoreductive treatment. Just as nowadays the measurement of allele burden of the KIT D816V mutation has become an important predictor of treatment response assessment [182] and survival [125], further analysis of the VAF for these later genes might provide a more reliable marker for assessing tumour burden as compared to other clinical and/or laboratory parameters, which can be also altered by medication or intercurrent processes (e.g., infectious and/or allergic diseases) [184,185,186]. Importantly, all above molecular markers should be used in combination with other disease features for accurate risk stratification of SM patients [12,32,97,120].
Acknowledgments
Figure 1 was created with BioRender.com software (BioRender, Toronto, Canada).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14102487/s1, Table S1: Sporadic gene mutations reported in systemic mastocytosis patients; Table S2: Frequency of mutations involving genes other than KIT found to be sporadically mutated in systemic mastocytosis patients; Table S3: Frequency of mutations involving genes other than KIT in patients with systemic mastocytosis associated to another haematological neoplasm.
Author Contributions
Conceptualization: A.C.G.-M.; data curation and construction of tables and figure: O.G.-L. and A.C.G.-M.; writing, editing and reviewing of the manuscript: O.G.-L., J.I.M.-G., A.O., I.Á.-T. and A.C.G.-M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Our laboratory is supported by grants from the Instituto de Salud Carlos III (ISCIII) and co-founded by the European Union through the European Regional Development Fund (FEDER; grant number PI19/01166), as well as from the Centro de Investigación Biomédica en Red en Cáncer (CIBERONC) programme (grant number CB16/12/00400). O.G.-L. was supported by a grant from ISCIII-FEDER (grant number FI20/00116).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valent P., Akin C., Metcalfe D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanotti R., Bonifacio M., Lucchini G., Sperr W.R., Scaffidi L., van Anrooij B., Oude Elberink H.N., Rossignol J., Hermine O., Gorska A., et al. Refined diagnostic criteria for bone marrow mastocytosis: A proposal of the European competence network on mastocytosis. Leukemia. 2022;36:516–524. doi: 10.1038/s41375-021-01406-y. [DOI] [PubMed] [Google Scholar]
- 3.Escribano L., Alvarez-Twose I., Garcia-Montero A., Sanchez-Munoz L., Jara-Acevedo M., Orfao A. Indolent systemic mastocytosis without skin involvement vs. isolated bone marrow mastocytosis. Haematologica. 2011;96:e26; author reply e28. doi: 10.3324/haematol.2011.040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Álvarez-Twose I., Jara-Acevedo M., Morgado J.M., García-Montero A., Sánchez-Muñoz L., Teodósio C., Matito A., Mayado A., Caldas C., Mollejo M., et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J. Allergy Clin. Immunol. 2016;137:168–178.e161. doi: 10.1016/j.jaci.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Escribano L., Alvarez-Twose I., Sanchez-Munoz L., Garcia-Montero A., Nunez R., Almeida J., Jara-Acevedo M., Teodosio C., Garcia-Cosio M., Bellas C., et al. Prognosis in adult indolent systemic mastocytosis: A long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J. Allergy Clin. Immunol. 2009;124:514–521. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Valent P., Akin C., Hartmann K., Nilsson G., Reiter A., Hermine O., Sotlar K., Sperr W.R., Escribano L., George T.I., et al. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017;77:1261–1270. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperr W.R., Kundi M., Alvarez-Twose I., van Anrooij B., Oude Elberink J.N.G., Gorska A., Niedoszytko M., Gleixner K.V., Hadzijusufovic E., Zanotti R., et al. International prognostic scoring system for mastocytosis (IPSM): A retrospective cohort study. Lancet Haematol. 2019;6:e638–e649. doi: 10.1016/S2352-3026(19)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longley B.J., Tyrrell L., Lu S.Z., Ma Y.S., Langley K., Ding T.G., Duffy T., Jacobs P., Tang L.H., Modlin I. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: Establishment of clonality in a human mast cell neoplasm. Nat. Genet. 1996;12:312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Montero A.C., Jara-Acevedo M., Teodosio C., Sanchez M.L., Nunez R., Prados A., Aldanondo I., Sanchez L., Dominguez M., Botana L.M., et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: A prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 10.Schwaab J., Schnittger S., Sotlar K., Walz C., Fabarius A., Pfirrmann M., Kohlmann A., Grossmann V., Meggendorfer M., Horny H.P., et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 11.Jawhar M., Schwaab J., Schnittger S., Meggendorfer M., Pfirrmann M., Sotlar K., Horny H.P., Metzgeroth G., Kluger S., Naumann N., et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia. 2016;30:136–143. doi: 10.1038/leu.2015.284. [DOI] [PubMed] [Google Scholar]
- 12.Munoz-Gonzalez J.I., Alvarez-Twose I., Jara-Acevedo M., Henriques A., Vinas E., Prieto C., Sanchez-Munoz L., Caldas C., Mayado A., Matito A., et al. Frequency and prognostic impact of KIT and other genetic variants in indolent systemic mastocytosis. Blood. 2019;134:456–468. doi: 10.1182/blood.2018886507. [DOI] [PubMed] [Google Scholar]
- 13.Munoz-Gonzalez J.I., Jara-Acevedo M., Alvarez-Twose I., Merker J.D., Teodosio C., Hou Y., Henriques A., Roskin K.M., Sanchez-Munoz L., Tsai A.G., et al. Impact of somatic and germline mutations on the outcome of systemic mastocytosis. Blood Adv. 2018;2:2814–2828. doi: 10.1182/bloodadvances.2018020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarden Y., Kuang W.J., Yang-Feng T., Coussens L., Munemitsu S., Dull T.J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: A new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orfao A., Garcia-Montero A.C., Sanchez L., Escribano L. Recent advances in the understanding of mastocytosis: The role of KIT mutations. Br. J. Haematol. 2007;138:12–30. doi: 10.1111/j.1365-2141.2007.06619.x. [DOI] [PubMed] [Google Scholar]
- 16.Li C.L., Johnson G.R. Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic long-term repopulating cells. Blood. 1994;84:408–414. doi: 10.1182/blood.V84.2.408.408. [DOI] [PubMed] [Google Scholar]
- 17.Majumder S., Brown K., Qiu F.H., Besmer P. c-kit protein, a transmembrane kinase: Identification in tissues and characterization. Mol. Cell. Biol. 1988;8:4896–4903. doi: 10.1128/mcb.8.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orfao A., Matarraz S., Perez-Andres M., Almeida J., Teodosio C., Berkowska M.A., van Dongen J.J.M., EuroFlow Immunophenotypic dissection of normal hematopoiesis. J. Immunol. Methods. 2019;475:112684. doi: 10.1016/j.jim.2019.112684. [DOI] [PubMed] [Google Scholar]
- 19.Teodosio C., Mayado A., Sanchez-Munoz L., Morgado J.M., Jara-Acevedo M., Alvarez-Twose I., Garcia-Montero A.C., Matito A., Caldas C., Escribano L., et al. The immunophenotype of mast cells and its utility in the diagnostic work-up of systemic mastocytosis. J. Leukoc. Biol. 2015;97:49–59. doi: 10.1189/jlb.5RU0614-296R. [DOI] [PubMed] [Google Scholar]
- 20.Okayama Y., Kawakami T. Development, migration, and survival of mast cells. Immunol. Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura Y., Hirotab S. Kit as a human oncogenic tyrosine kinase. Cell. Mol. Life Sci. CMLS. 2004;61:2924–2931. doi: 10.1007/s00018-004-4273-y. [DOI] [PubMed] [Google Scholar]
- 22.Kitayama H., Tsujimura T., Matsumura I., Oritani K., Ikeda H., Ishikawa J., Okabe M., Suzuki M., Yamamura K., Matsuzawa Y., et al. Neoplastic transformation of normal hematopoietic cells by constitutively activating mutations of c-kit receptor tyrosine kinase. Blood. 1996;88:995–1004. doi: 10.1182/blood.V88.3.995.995. [DOI] [PubMed] [Google Scholar]
- 23.Munoz-Gonzalez J.I., Garcia-Montero A.C., Orfao A., Alvarez-Twose I. Pathogenic and diagnostic relevance of KIT in primary mast cell activation disorders. Ann. Allergy Asthma Immunol. 2021;127:427–434. doi: 10.1016/j.anai.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Akin C., Metcalfe D.D. Systemic Mastocytosis. Annu. Rev. Med. 2004;55:419–432. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 25.Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A., et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pullarkat S.T., Pullarkat V., Kroft S.H., Wilson C.S., Ahsanuddin A.N., Mann K.P., Thein M., Grody W.W., Brynes R.K. Systemic mastocytosis associated with t(8;21)(q22;q22) acute myeloid leukemia. J. Hematop. 2009;2:27–33. doi: 10.1007/s12308-009-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotlar K., Horny H.P., Simonitsch I., Krokowski M., Aichberger K.J., Mayerhofer M., Printz D., Fritsch G., Valent P. CD25 indicates the neoplastic phenotype of mast cells: A novel immunohistochemical marker for the diagnosis of systemic mastocytosis (SM) in routinely processed bone marrow biopsy specimens. Am. J. Surg. Pathol. 2004;28:1319–1325. doi: 10.1097/01.pas.0000138181.89743.7b. [DOI] [PubMed] [Google Scholar]
- 28.Pignon J.M., Giraudier S., Duquesnoy P., Jouault H., Imbert M., Vainchenker W., Vernant J.P., Tulliez M. A new c-kit mutation in a case of aggressive mast cell disease. Br. J. Haematol. 1997;96:374–376. doi: 10.1046/j.1365-2141.1997.d01-2042.x. [DOI] [PubMed] [Google Scholar]
- 29.Pardanani A., Lasho T., Elala Y., Wassie E., Finke C., Reichard K.K., Chen D., Hanson C.A., Ketterling R.P., Tefferi A. Next-generation sequencing in systemic mastocytosis: Derivation of a mutation-augmented clinical prognostic model for survival. Am. J. Hematol. 2016;91:888–893. doi: 10.1002/ajh.24426. [DOI] [PubMed] [Google Scholar]
- 30.Baek J.O., Kang H.K., Na S.Y., Lee J.R., Roh J.Y., Lee J.H., Kim H.J., Park S. N822K c-kit mutation in CD30-positive cutaneous pleomorphic mastocytosis after germ cell tumour of the ovary. Br. J. Dermatol. 2012;166:1370–1373. doi: 10.1111/j.1365-2133.2012.10816.x. [DOI] [PubMed] [Google Scholar]
- 31.Arredondo A.R., Gotlib J., Shier L., Medeiros B., Wong K., Cherry A., Corless C., Arber D.A., Valent P., George T.I. Myelomastocytic leukemia versus mast cell leukemia versus systemic mastocytosis associated with acute myeloid leukemia: A diagnostic challenge. Am. J. Hematol. 2010;85:600–606. doi: 10.1002/ajh.21713. [DOI] [PubMed] [Google Scholar]
- 32.Jawhar M., Schwaab J., Alvarez-Twose I., Shoumariyeh K., Naumann N., Lubke J., Perkins C., Munoz-Gonzalez J.I., Meggendorfer M., Kennedy V., et al. MARS: Mutation-Adjusted Risk Score for Advanced Systemic Mastocytosis. J. Clin. Oncol. 2019;37:2846–2856. doi: 10.1200/JCO.19.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwaab J., Cabral do O.H.N., Naumann N., Jawhar M., Weiss C., Metzgeroth G., Schmid A., Lubke J., Reiter L., Fabarius A., et al. Importance of Adequate Diagnostic Workup for Correct Diagnosis of Advanced Systemic Mastocytosis. J. Allergy Clin. Immunol. Pract. 2020;8:3121–3127.e3121. doi: 10.1016/j.jaip.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Pullarkat V.A., Pullarkat S.T., Calverley D.C., Brynes R.K. Mast cell disease associated with acute myeloid leukemia: Detection of a new c-kit mutation Asp816His. Am. J. Hematol. 2000;65:307–309. doi: 10.1002/1096-8652(200012)65:4<307::AID-AJH10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Pullarkat V.A., Bueso-Ramos C., Lai R., Kroft S., Wilson C.S., Pullarkat S.T., Bu X., Thein M., Lee M., Brynes R.K. Systemic mastocytosis with associated clonal hematological non-mast-cell lineage disease: Analysis of clinicopathologic features and activating c-kit mutations. Am. J. Hematol. 2003;73:12–17. doi: 10.1002/ajh.10322. [DOI] [PubMed] [Google Scholar]
- 36.Sotlar K., Colak S., Bache A., Berezowska S., Krokowski M., Bultmann B., Valent P., Horny H.P. Variable presence of KITD816V in clonal haematological non-mast cell lineage diseases associated with systemic mastocytosis (SM-AHNMD) J. Pathol. 2010;220:586–595. doi: 10.1002/path.2677. [DOI] [PubMed] [Google Scholar]
- 37.Longley B.J., Jr., Metcalfe D.D., Tharp M., Wang X., Tyrrell L., Lu S.Z., Heitjan D., Ma Y. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc. Natl. Acad. Sci. USA. 1999;96:1609–1614. doi: 10.1073/pnas.96.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horny H.P., Sotlar K., Sperr W.R., Valent P. Systemic mastocytosis with associated clonal haematological non-mast cell lineage diseases: A histopathological challenge. J. Clin. Pathol. 2004;57:604–608. doi: 10.1136/jcp.2003.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai S., Ichikawa M., Takahashi T., Sato H., Yokota H., Oshima K., Izutsu K., Hangaishi A., Kanda Y., Motokura T., et al. The origin of neoplastic mast cells in systemic mastocytosis with AML1/ETO-positive acute myeloid leukemia. Exp. Hematol. 2007;35:1747–1752. doi: 10.1016/j.exphem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Lasho T., Finke C., Zblewski D., Hanson C.A., Ketterling R.P., Butterfield J.H., Tefferi A., Pardanani A. Concurrent activating KIT mutations in systemic mastocytosis. Br. J. Haematol. 2016;173:153–156. doi: 10.1111/bjh.13560. [DOI] [PubMed] [Google Scholar]
- 41.Yabe M., Masukawa A., Kato S., Yabe H., Nakamura N., Matsushita H. Systemic mastocytosis associated with t(8;21) acute myeloid leukemia in a child: Detection of the D816A mutation of KIT. Pediatric Blood Cancer. 2012;59:1313–1316. doi: 10.1002/pbc.24250. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsumi M., Miura H., Inagaki H., Shinkai Y., Kato A., Kato T., Hamada-Tsutsumi S., Tanaka M., Kudo K., Yoshikawa T., et al. An aggressive systemic mastocytosis preceded by ovarian dysgerminoma. BMC Cancer. 2020;20:1162. doi: 10.1186/s12885-020-07653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura R., Chakrabarti S., Akin C., Robyn J., Bahceci E., Greene A., Childs R., Dunbar C.E., Metcalfe D.D., Barrett A.J. A pilot study of nonmyeloablative allogeneic hematopoietic stem cell transplant for advanced systemic mastocytosis. Bone Marrow Transpl. 2006;37:353–358. doi: 10.1038/sj.bmt.1705245. [DOI] [PubMed] [Google Scholar]
- 44.Heinrich M.C., Joensuu H., Demetri G.D., Corless C.L., Apperley J., Fletcher J.A., Soulieres D., Dirnhofer S., Harlow A., Town A., et al. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib-sensitive tyrosine kinases. Clin. Cancer Res. 2008;14:2717–2725. doi: 10.1158/1078-0432.CCR-07-4575. [DOI] [PubMed] [Google Scholar]
- 45.Frederiksen J.K., Shao L., Bixby D.L., Ross C.W. Shared clonal cytogenetic abnormalities in aberrant mast cells and leukemic myeloid blasts detected by single nucleotide polymorphism microarray-based whole-genome scanning. Genes Chromosomes Cancer. 2016;55:389–396. doi: 10.1002/gcc.22342. [DOI] [PubMed] [Google Scholar]
- 46.Jawhar M., Schwaab J., Meggendorfer M., Naumann N., Horny H.P., Sotlar K., Haferlach T., Schmitt K., Fabarius A., Valent P., et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica. 2017;102:1035–1043. doi: 10.3324/haematol.2017.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanternier F., Cohen-Akenine A., Palmerini F., Feger F., Yang Y., Zermati Y., Barète S., Sans B., Baude C., Ghez D., et al. Phenotypic and Genotypic Characteristics of Mastocytosis According to the Age of Onset. PLoS ONE. 2008;3:e1906. doi: 10.1371/journal.pone.0001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valent P., Berger J., Cerny-Reiterer S., Peter B., Eisenwort G., Hoermann G., Mullauer L., Mannhalter C., Steurer M., Bettelheim P., et al. Chronic mast cell leukemia (MCL) with KIT S476I: A rare entity defined by leukemic expansion of mature mast cells and absence of organ damage. Ann. Hematol. 2015;94:223–231. doi: 10.1007/s00277-014-2207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Georgin-Lavialle S., Lhermitte L., Suarez F., Yang Y., Letard S., Hanssens K., Feger F., Renand A., Brouze C., Canioni D., et al. Mast cell leukemia: Identification of a new c-Kit mutation, dup(501-502), and response to masitinib, a c-Kit tyrosine kinase inhibitor. Eur. J. Haematol. 2012;89:47–52. doi: 10.1111/j.1600-0609.2012.01761.x. [DOI] [PubMed] [Google Scholar]
- 50.Rouet A., Aouba A., Damaj G., Soucie E., Hanssens K., Chandesris M.O., Livideanu C.B., Dutertre M., Durieu I., Grandpeix-Guyodo C., et al. Mastocytosis among elderly patients: A multicenter retrospective French study on 53 patients. Medicine. 2016;95:e3901. doi: 10.1097/MD.0000000000003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soucie E., Hanssens K., Mercher T., Georgin-Lavialle S., Damaj G., Livideanu C., Chandesris M.O., Acin Y., Létard S., de Sepulveda P., et al. In aggressive forms of mastocytosis, TET2 loss cooperates with c-KITD816V to transform mast cells. Blood. 2012;120:4846–4849. doi: 10.1182/blood-2011-12-397588. [DOI] [PubMed] [Google Scholar]
- 52.Mital A., Piskorz A., Lewandowski K., Wasag B., Limon J., Hellmann A. A case of mast cell leukaemia with exon 9 KIT mutation and good response to imatinib. Eur. J. Haematol. 2011;86:531–535. doi: 10.1111/j.1600-0609.2011.01598.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L.Y., Smith M.L., Schultheis B., Fitzgibbon J., Lister T.A., Melo J.V., Cross N.C., Cavenagh J.D. A novel K509I mutation of KIT identified in familial mastocytosis-in vitro and in vivo responsiveness to imatinib therapy. Leuk. Res. 2006;30:373–378. doi: 10.1016/j.leukres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 54.Akin C., Fumo G., Yavuz A.S., Lipsky P.E., Neckers L., Metcalfe D.D. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103:3222–3225. doi: 10.1182/blood-2003-11-3816. [DOI] [PubMed] [Google Scholar]
- 55.Broderick V., Waghorn K., Langabeer S.E., Jeffers M., Cross N.C.P., Hayden P.J. Molecular response to imatinib in KIT F522C-mutated systemic mastocytosis. Leuk. Res. 2019;77:28–29. doi: 10.1016/j.leukres.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 56.Nakagomi N., Hirota S. Juxtamembrane-type c-kit gene mutation found in aggressive systemic mastocytosis induces imatinib-resistant constitutive KIT activation. Lab. Investig. 2007;87:365–371. doi: 10.1038/labinvest.3700524. [DOI] [PubMed] [Google Scholar]
- 57.Büttner C., Henz B.M., Welker P., Sepp N.T., Grabbe J. Identification of Activating c-kit Mutations in Adult-, but not in Childhood-Onset Indolent Mastocytosis: A Possible Explanation for Divergent Clinical Behavior. J. Investig. Dermatol. 1998;111:1227–1231. doi: 10.1046/j.1523-1747.1998.00414.x. [DOI] [PubMed] [Google Scholar]
- 58.Furitsu T., Tsujimura T., Tono T., Ikeda H., Kitayama H., Koshimizu U., Sugahara H., Butterfield J.H., Ashman L.K., Kanayama Y., et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J. Clin. Investig. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spector M.S., Iossifov I., Kritharis A., He C., Kolitz J.E., Lowe S.W., Allen S.L. Mast-cell leukemia exome sequencing reveals a mutation in the IgE mast-cell receptor beta chain and KIT V654A. Leukemia. 2012;26:1422–1425. doi: 10.1038/leu.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartmann K., Wardelmann E., Ma Y., Merkelbach-Bruse S., Preussner L.M., Woolery C., Baldus S.E., Heinicke T., Thiele J., Buettner R., et al. Novel germline mutation of KIT associated with familial gastrointestinal stromal tumors and mastocytosis. Gastroenterology. 2005;129:1042–1046. doi: 10.1053/j.gastro.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 61.Wang H.J., Lin Z.M., Zhang J., Yin J.H., Yang Y. A new germline mutation in KIT associated with diffuse cutaneous mastocytosis in a Chinese family. Clin. Exp. Dermatol. 2014;39:146–149. doi: 10.1111/ced.12225. [DOI] [PubMed] [Google Scholar]
- 62.de Melo Campos P., Machado-Neto J.A., Scopim-Ribeiro R., Visconte V., Tabarroki A., Duarte A.S., Barra F.F., Vassalo J., Rogers H.J., Lorand-Metze I., et al. Familial systemic mastocytosis with germline KIT K509I mutation is sensitive to treatment with imatinib, dasatinib and PKC412. Leuk. Res. 2014;38:1245–1251. doi: 10.1016/j.leukres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Alvarez-Twose I., Matito A., Morgado J.M., Sanchez-Munoz L., Jara-Acevedo M., Garcia-Montero A., Mayado A., Caldas C., Teodosio C., Munoz-Gonzalez J.I., et al. Imatinib in systemic mastocytosis: A phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. 2017;8:68950–68963. doi: 10.18632/oncotarget.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frost M.J., Ferrao P.T., Hughes T.P., Ashman L.K. Juxtamembrane mutant V560GKit is more sensitive to Imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816VKit is resistant. Mol. Cancer Ther. 2002;1:1115–1124. [PubMed] [Google Scholar]
- 65.Akin C., Brockow K., D’Ambrosio C., Kirshenbaum A.S., Ma Y., Longley B.J., Metcalfe D.D. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp. Hematol. 2003;31:686–692. doi: 10.1016/S0301-472X(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 66.Ma Y., Zeng S., Metcalfe D.D., Akin C., Dimitrijevic S., Butterfield J.H., McMahon G., Longley B.J. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–1744. doi: 10.1182/blood.V99.5.1741. [DOI] [PubMed] [Google Scholar]
- 67.Valent P., Blatt K., Eisenwort G., Herrmann H., Cerny-Reiterer S., Thalhammer R., Müllauer L., Hoermann G., Sadovnik I., Schwarzinger I., et al. FLAG-induced remission in a patient with acute mast cell leukemia (MCL) exhibiting t(7;10)(q22;q26) and KIT D816H. Leuk. Res. Rep. 2014;3:8–13. doi: 10.1016/j.lrr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naumann N., Jawhar M., Schwaab J., Kluger S., Lubke J., Metzgeroth G., Popp H.D., Khaled N., Horny H.P., Sotlar K., et al. Incidence and prognostic impact of cytogenetic aberrations in patients with systemic mastocytosis. Genes Chromosomes Cancer. 2018;57:252–259. doi: 10.1002/gcc.22526. [DOI] [PubMed] [Google Scholar]
- 69.Valent P., Horny H.P., Escribano L., Longley B.J., Li C.Y., Schwartz L.B., Marone G., Nunez R., Akin C., Sotlar K., et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk. Res. 2001;25:603–625. doi: 10.1016/S0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 70.Yavuz A.S., Lipsky P.E., Yavuz S., Metcalfe D.D., Akin C. Evidence for the involvement of a hematopoietic progenitor cell in systemic mastocytosis from single-cell analysis of mutations in the c-kit gene. Blood. 2002;100:661–665. doi: 10.1182/blood-2002-01-0203. [DOI] [PubMed] [Google Scholar]
- 71.Akin C. Clonality and molecular pathogenesis of mastocytosis. Acta Haematol. 2005;114:61–69. doi: 10.1159/000085563. [DOI] [PubMed] [Google Scholar]
- 72.Kocabas C.N., Yavuz A.S., Lipsky P.E., Metcalfe D.D., Akin C. Analysis of the lineage relationship between mast cells and basophils using the c-kit D816V mutation as a biologic signature. J. Allergy Clin. Immunol. 2005;115:1155–1161. doi: 10.1016/j.jaci.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 73.Akin C., Kirshenbaum A.S., Semere T., Worobec A.S., Scott L.M., Metcalfe D.D. Analysis of the surface expression of c-kit and occurrence of the c-kit Asp816Val activating mutation in T cells, B cells, and myelomonocytic cells in patients with mastocytosis. Exp. Hematol. 2000;28:140–147. doi: 10.1016/S0301-472X(99)00145-9. [DOI] [PubMed] [Google Scholar]
- 74.Mayado A., Teodosio C., Dasilva-Freire N., Jara-Acevedo M., Garcia-Montero A.C., Alvarez-Twose I., Sanchez-Munoz L., Matito A., Caldas C., Munoz-Gonzalez J.I., et al. Characterization of CD34(+) hematopoietic cells in systemic mastocytosis: Potential role in disease dissemination. Allergy. 2018;73:1294–1304. doi: 10.1111/all.13413. [DOI] [PubMed] [Google Scholar]
- 75.Valent P., Akin C., Arock M., Bock C., George T.I., Galli S.J., Gotlib J., Haferlach T., Hoermann G., Hermine O., et al. Proposed Terminology and Classification of Pre-Malignant Neoplastic Conditions: A Consensus Proposal. EBioMedicine. 2017;26:17–24. doi: 10.1016/j.ebiom.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor M.L., Sehgal D., Raffeld M., Obiakor H., Akin C., Mage R.G., Metcalfe D.D. Demonstration that mast cells, T cells, and B cells bearing the activating kit mutation D816V occur in clusters within the marrow of patients with mastocytosis. J. Mol. Diagn. JMD. 2004;6:335–342. doi: 10.1016/S1525-1578(10)60529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Montero A.C., Jara-Acevedo M., Alvarez-Twose I., Teodosio C., Sanchez-Munoz L., Muniz C., Munoz-Gonzalez J.I., Mayado A., Matito A., Caldas C., et al. KIT D816V-mutated bone marrow mesenchymal stem cells in indolent systemic mastocytosis are associated with disease progression. Blood. 2016;127:761–768. doi: 10.1182/blood-2015-07-655100. [DOI] [PubMed] [Google Scholar]
- 78.Jara-Acevedo M., Teodosio C., Sanchez-Munoz L., Alvarez-Twose I., Mayado A., Caldas C., Matito A., Morgado J.M., Munoz-Gonzalez J.I., Escribano L., et al. Detection of the KIT D816V mutation in peripheral blood of systemic mastocytosis: Diagnostic implications. Mod. Pathol. 2015;28:1138–1149. doi: 10.1038/modpathol.2015.72. [DOI] [PubMed] [Google Scholar]
- 79.Teodosio C., Garcia-Montero A.C., Jara-Acevedo M., Sanchez-Munoz L., Pedreira C.E., Alvarez-Twose I., Matarraz S., Morgado J.M., Barcena P., Matito A., et al. Gene expression profile of highly purified bone marrow mast cells in systemic mastocytosis. J. Allergy Clin. Immunol. 2013;131:1213–1224. doi: 10.1016/j.jaci.2012.12.674. [DOI] [PubMed] [Google Scholar]
- 80.Traina F., Visconte V., Jankowska A.M., Makishima H., O’Keefe C.L., Elson P., Han Y., Hsieh F.H., Sekeres M.A., Mali R.S., et al. Single nucleotide polymorphism array lesions, TET2, DNMT3A, ASXL1 and CBL mutations are present in systemic mastocytosis. PLoS ONE. 2012;7:e43090. doi: 10.1371/journal.pone.0043090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Damaj G., Joris M., Chandesris O., Hanssens K., Soucie E., Canioni D., Kolb B., Durieu I., Gyan E., Livideanu C., et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS ONE. 2014;9:85362. doi: 10.1371/journal.pone.0085362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen W., Szankasi P., Sederberg M., Schumacher J., Frizzell K.A., Gee E.P., Patel J.L., South S.T., Xu X., Kelley T.W. Concurrent detection of targeted copy number variants and mutations using a myeloid malignancy next generation sequencing panel allows comprehensive genetic analysis using a single testing strategy. Br. J. Haematol. 2016;173:49–58. doi: 10.1111/bjh.13921. [DOI] [PubMed] [Google Scholar]
- 83.Cross N.C.P., Hoade Y., Tapper W.J., Carreno-Tarragona G., Fanelli T., Jawhar M., Naumann N., Pieniak I., Lubke J., Ali S., et al. Recurrent activating STAT5B N642H mutation in myeloid neoplasms with eosinophilia. Leukemia. 2019;33:415–425. doi: 10.1038/s41375-018-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson T.M., Maric I., Simakova O., Bai Y., Chan E.C., Olivares N., Carter M., Maric D., Robyn J., Metcalfe D.D. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–463. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jawhar M., Schwaab J., Schnittger S., Sotlar K., Horny H.P., Metzgeroth G., Muller N., Schneider S., Naumann N., Walz C., et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia. 2015;29:1115–1122. doi: 10.1038/leu.2015.4. [DOI] [PubMed] [Google Scholar]
- 86.Visconte V., Makishima H., Maciejewski J.P., Tiu R.V. Emerging roles of the spliceosomal machinery in myelodysplastic syndromes and other hematological disorders. Leukemia. 2012;26:2447–2454. doi: 10.1038/leu.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hanssens K., Brenet F., Agopian J., Georgin-Lavialle S., Damaj G., Cabaret L., Chandesris M.O., de Sepulveda P., Hermine O., Dubreuil P., et al. SRSF2-p95 hotspot mutation is highly associated with advanced forms of mastocytosis and mutations in epigenetic regulator genes. Haematologica. 2014;99:830–835. doi: 10.3324/haematol.2013.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tefferi A., Levine R.L., Lim K.H., Abdel-Wahab O., Lasho T.L., Patel J., Finke C.M., Mullally A., Li C.Y., Pardanani A., et al. Frequent TET2 mutations in systemic mastocytosis: Clinical, KITD816V and FIP1L1-PDGFRA correlates. Leukemia. 2009;23:900–904. doi: 10.1038/leu.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grinfeld J., Nangalia J., Baxter E.J., Wedge D.C., Angelopoulos N., Cantrill R., Godfrey A.L., Papaemmanuil E., Gundem G., MacLean C., et al. Classification and Personalized Prognosis in Myeloproliferative Neoplasms. N. Engl. J. Med. 2018;379:1416–1430. doi: 10.1056/NEJMoa1716614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pardanani A.D., Lasho T.L., Finke C., Zblewski D.L., Abdelrahman R.A., Wassie E.A., Gangat N., Hanson C.A., Ketterling R.P., Tefferi A. ASXL1 and CBL mutations are independently predictive of inferior survival in advanced systemic mastocytosis. Br. J. Haematol. 2016;175:534–536. doi: 10.1111/bjh.13865. [DOI] [PubMed] [Google Scholar]
- 91.Papaemmanuil E., Gerstung M., Malcovati L., Tauro S., Gundem G., Van Loo P., Yoon C.J., Ellis P., Wedge D.C., Pellagatti A., et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3699. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bejar R. CHIP, ICUS, CCUS and other four-letter words. Leukemia. 2017;31:1869–1871. doi: 10.1038/leu.2017.181. [DOI] [PubMed] [Google Scholar]
- 93.Haenisch B., Frohlich H., Herms S., Molderings G.J. Evidence for contribution of epigenetic mechanisms in the pathogenesis of systemic mast cell activation disease. Immunogenetics. 2014;66:287–297. doi: 10.1007/s00251-014-0768-3. [DOI] [PubMed] [Google Scholar]
- 94.Itzykson R., Fenaux P. Epigenetics of myelodysplastic syndromes. Leukemia. 2014;28:497–506. doi: 10.1038/leu.2013.343. [DOI] [PubMed] [Google Scholar]
- 95.Shih A.H., Abdel-Wahab O., Patel J.P., Levine R.L. The role of mutations in epigenetic regulators in myeloid malignancies. Nature reviews. Cancer. 2012;12:599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- 96.Jawhar M., Schwaab J., Hausmann D., Clemens J., Naumann N., Henzler T., Horny H.P., Sotlar K., Schoenberg S.O., Cross N.C., et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30:2342–2350. doi: 10.1038/leu.2016.190. [DOI] [PubMed] [Google Scholar]
- 97.Munoz-Gonzalez J.I., Alvarez-Twose I., Jara-Acevedo M., Zanotti R., Perkins C., Jawhar M., Sperr W.R., Shoumariyeh K., Schwaab J., Greiner G., et al. Proposed global prognostic score for systemic mastocytosis: A retrospective prognostic modelling study. Lancet Haematol. 2021;8:e194–e204. doi: 10.1016/S2352-3026(20)30400-2. [DOI] [PubMed] [Google Scholar]
- 98.Villarino A.V., Kanno Y., O’Shea J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leardini D., Messelodi D., Muratore E., Baccelli F., Bertuccio S.N., Anselmi L., Pession A., Masetti R. Role of CBL Mutations in Cancer and Non-Malignant Phenotype. Cancers. 2022;14:839. doi: 10.3390/cancers14030839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chung Y.R., Schatoff E., Abdel-Wahab O. Epigenetic alterations in hematopoietic malignancies. Int. J. Hematol. 2012;96:413–427. doi: 10.1007/s12185-012-1181-z. [DOI] [PubMed] [Google Scholar]
- 101.Weinberg D.N., Papillon-Cavanagh S., Chen H., Yue Y., Chen X., Rajagopalan K.N., Horth C., McGuire J.T., Xu X., Nikbakht H., et al. The histone mark H3K36me2 recruits DNMT3A and shapes the intergenic DNA methylation landscape. Nature. 2019;573:281–286. doi: 10.1038/s41586-019-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vainchenker W., Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129:667–679. doi: 10.1182/blood-2016-10-695940. [DOI] [PubMed] [Google Scholar]
- 103.Sperling A.S., Gibson C.J., Ebert B.L. The genetics of myelodysplastic syndrome: From clonal haematopoiesis to secondary leukaemia. Nature reviews. Cancer. 2017;17:5–19. doi: 10.1038/nrc.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Latchman D.S. Transcription-Factor Mutations and Disease. N. Engl. J. Med. 1996;334:28–33. doi: 10.1056/NEJM199601043340108. [DOI] [PubMed] [Google Scholar]
- 105.Vainchenker W., Delhommeau F., Constantinescu S.N., Bernard O.A. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118:1723–1735. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- 106.Kales S.C., Ryan P.E., Nau M.M., Lipkowitz S. Cbl and human myeloid neoplasms: The Cbl oncogene comes of age. Cancer Res. 2010;70:4789–4794. doi: 10.1158/0008-5472.CAN-10-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sargin B., Choudhary C., Crosetto N., Schmidt M.H., Grundler R., Rensinghoff M., Thiessen C., Tickenbrock L., Schwable J., Brandts C., et al. Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood. 2007;110:1004–1012. doi: 10.1182/blood-2007-01-066076. [DOI] [PubMed] [Google Scholar]
- 108.Caligiuri M.A., Briesewitz R., Yu J., Wang L., Wei M., Arnoczky K.J., Marburger T.B., Wen J., Perrotti D., Bloomfield C.D., et al. Novel c-CBL and CBL-b ubiquitin ligase mutations in human acute myeloid leukemia. Blood. 2007;110:1022–1024. doi: 10.1182/blood-2006-12-061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reindl C., Quentmeier H., Petropoulos K., Greif P.A., Benthaus T., Argiropoulos B., Mellert G., Vempati S., Duyster J., Buske C., et al. CBL exon 8/9 mutants activate the FLT3 pathway and cluster in core binding factor/11q deletion acute myeloid leukemia/myelodysplastic syndrome subtypes. Clin. Cancer Res. 2009;15:2238–2247. doi: 10.1158/1078-0432.CCR-08-1325. [DOI] [PubMed] [Google Scholar]
- 110.Grand F.H., Hidalgo-Curtis C.E., Ernst T., Zoi K., Zoi C., McGuire C., Kreil S., Jones A., Score J., Metzgeroth G., et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009;113:6182–6192. doi: 10.1182/blood-2008-12-194548. [DOI] [PubMed] [Google Scholar]
- 111.Beer P.A., Delhommeau F., LeCouedic J.P., Dawson M.A., Chen E., Bareford D., Kusec R., McMullin M.F., Harrison C.N., Vannucchi A.M., et al. Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood. 2010;115:2891–2900. doi: 10.1182/blood-2009-08-236596. [DOI] [PubMed] [Google Scholar]
- 112.Makishima H., Cazzolli H., Szpurka H., Dunbar A., Tiu R., Huh J., Muramatsu H., O’Keefe C., Hsi E., Paquette R.L., et al. Mutations of e3 ubiquitin ligase cbl family members constitute a novel common pathogenic lesion in myeloid malignancies. J. Clin. Oncol. 2009;27:6109–6116. doi: 10.1200/JCO.2009.23.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bader M.S., Meyer S.C. JAK2 in Myeloproliferative Neoplasms: Still a Protagonist. Pharmaceuticals. 2022;15:160. doi: 10.3390/ph15020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo Y.J., Pan W.W., Liu S.B., Shen Z.F., Xu Y., Hu L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Szybinski J., Meyer S.C. Genetics of Myeloproliferative Neoplasms. Hematol. Oncol. Clin. N Am. 2021;35:217–236. doi: 10.1016/j.hoc.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 116.Naumann N., Lubke J., Shomali W., Reiter L., Horny H.P., Jawhar M., Dangelo V., Fabarius A., Metzgeroth G., Kreil S., et al. Clinical and histopathological features of myeloid neoplasms with concurrent Janus kinase 2 (JAK2) V617F and KIT proto-oncogene, receptor tyrosine kinase (KIT) D816V mutations. Br. J. Haematol. 2021;194:344–354. doi: 10.1111/bjh.17567. [DOI] [PubMed] [Google Scholar]
- 117.Chiosea S.I., Sherer C.K., Jelic T., Dacic S. KRAS mutant allele-specific imbalance in lung adenocarcinoma. Mod. Pathol. 2011;24:1571–1577. doi: 10.1038/modpathol.2011.109. [DOI] [PubMed] [Google Scholar]
- 118.Krasinskas A.M., Moser A.J., Saka B., Adsay N.V., Chiosea S.I. KRAS mutant allele-specific imbalance is associated with worse prognosis in pancreatic cancer and progression to undifferentiated carcinoma of the pancreas. Mod. Pathol. 2013;26:1346–1354. doi: 10.1038/modpathol.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chang Y.Y., Lin J.K., Lin T.C., Chen W.S., Jeng K.J., Yang S.H., Wang H.S., Lan Y.T., Lin C.C., Liang W.Y., et al. Impact of KRAS mutation on outcome of patients with metastatic colorectal cancer. Hepato Gastroenterol. 2014;61:1946–1953. [PubMed] [Google Scholar]
- 120.Pardanani A., Shah S., Mannelli F., Elala Y.C., Guglielmelli P., Lasho T.L., Patnaik M.M., Gangat N., Ketterling R.P., Reichard K.K., et al. Mayo alliance prognostic system for mastocytosis: Clinical and hybrid clinical-molecular models. Blood Adv. 2018;2:2964–2972. doi: 10.1182/bloodadvances.2018026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ichikawa M., Goyama S., Asai T., Kawazu M., Nakagawa M., Takeshita M., Chiba S., Ogawa S., Kurokawa M. AML1/Runx1 Negatively Regulates Quiescent Hematopoietic Stem Cells in Adult Hematopoiesis. J. Immunol. 2008;180:4402–4408. doi: 10.4049/jimmunol.180.7.4402. [DOI] [PubMed] [Google Scholar]
- 122.Ding Y., Harada Y., Imagawa J., Kimura A., Harada H. AML1/RUNX1 point mutation possibly promotes leukemic transformation in myeloproliferative neoplasms. Blood. 2009;114:5201–5205. doi: 10.1182/blood-2009-06-223982. [DOI] [PubMed] [Google Scholar]
- 123.Gaidzik V.I., Bullinger L., Schlenk R.F., Zimmermann A.S., Rock J., Paschka P., Corbacioglu A., Krauter J., Schlegelberger B., Ganser A., et al. RUNX1 mutations in acute myeloid leukemia: Results from a comprehensive genetic and clinical analysis from the AML study group. J. Clin. Oncol. 2011;29:1364–1372. doi: 10.1200/JCO.2010.30.7926. [DOI] [PubMed] [Google Scholar]
- 124.Bejar R., Stevenson K.E., Caughey B.A., Abdel-Wahab O., Steensma D.P., Galili N., Raza A., Kantarjian H., Levine R.L., Neuberg D., et al. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J. Clin. Oncol. 2012;30:3376–3382. doi: 10.1200/JCO.2011.40.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jawhar M., Schwaab J., Naumann N., Horny H.-P., Sotlar K., Haferlach T., Metzgeroth G., Fabarius A., Valent P., Hofmann W.-K., et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood. 2017;130:137–145. doi: 10.1182/blood-2017-01-764423. [DOI] [PubMed] [Google Scholar]
- 126.Pardanani A., Lasho T., Barraco D., Patnaik M., Elala Y., Tefferi A. Next generation sequencing of myeloid neoplasms with eosinophilia harboring the FIP1L1-PDGFRA mutation. Am. J. Hematol. 2016;91:10–11. doi: 10.1002/ajh.24273. [DOI] [PubMed] [Google Scholar]
- 127.Sharma S., Kelly T.K., Jones P.A. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Figueroa M.E., Lugthart S., Li Y., Erpelinck-Verschueren C., Deng X., Christos P.J., Schifano E., Booth J., van Putten W., Skrabanek L., et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell. 2010;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reszka E., Jablonska E., Wieczorek E., Valent P., Arock M., Nilsson G., Nedoszytko B., Niedoszytko M. Epigenetic Changes in Neoplastic Mast Cells and Potential Impact in Mastocytosis. Int. J. Mol. Sci. 2021;22:2964. doi: 10.3390/ijms22062964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gorska A., Jablonska E., Reszka E., Niedoszytko M., Lange M., Gruchala-Niedoszytko M., Jarczak J., Strapagiel D., Gorska-Ponikowska M., Bastian P., et al. DNA methylation profile in patients with indolent systemic mastocytosis. Clin. Transl. Allergy. 2021;11:e12074. doi: 10.1002/clt2.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Katoh M. Functional and cancer genomics of ASXL family members. Br. J. Cancer. 2013;109:299–306. doi: 10.1038/bjc.2013.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gelsi-Boyer V., Trouplin V., Adelaide J., Bonansea J., Cervera N., Carbuccia N., Lagarde A., Prebet T., Nezri M., Sainty D., et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br. J. Haematol. 2009;145:788–800. doi: 10.1111/j.1365-2141.2009.07697.x. [DOI] [PubMed] [Google Scholar]
- 133.Carbuccia N., Murati A., Trouplin V., Brecqueville M., Adelaide J., Rey J., Vainchenker W., Bernard O.A., Chaffanet M., Vey N., et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia. 2009;23:2183–2186. doi: 10.1038/leu.2009.141. [DOI] [PubMed] [Google Scholar]
- 134.Kar S.A., Jankowska A., Makishima H., Visconte V., Jerez A., Sugimoto Y., Muramatsu H., Traina F., Afable M., Guinta K., et al. Spliceosomal gene mutations are frequent events in the diverse mutational spectrum of chronic myelomonocytic leukemia but largely absent in juvenile myelomonocytic leukemia. Haematologica. 2013;98:107–113. doi: 10.3324/haematol.2012.064048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jia Y., Li P., Fang L., Zhu H., Xu L., Cheng H., Zhang J., Li F., Feng Y., Li Y., et al. Negative regulation of DNMT3A de novo DNA methylation by frequently overexpressed UHRF family proteins as a mechanism for widespread DNA hypomethylation in cancer. Cell Discov. 2016;2:16007. doi: 10.1038/celldisc.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walter M.J., Ding L., Shen D., Shao J., Grillot M., McLellan M., Fulton R., Schmidt H., Kalicki-Veizer J., O’Laughlin M., et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia. 2011;25:1153–1158. doi: 10.1038/leu.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tie R., Zhang T., Fu H., Wang L., Wang Y., He Y., Wang B., Zhu N., Fu S., Lai X., et al. Association between DNMT3A mutations and prognosis of adults with de novo acute myeloid leukemia: A systematic review and meta-analysis. PLoS ONE. 2014;9:e93353. doi: 10.1371/journal.pone.0093353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jankowska A.M., Makishima H., Tiu R.V., Szpurka H., Huang Y., Traina F., Visconte V., Sugimoto Y., Prince C., O’Keefe C., et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood. 2011;118:3932–3941. doi: 10.1182/blood-2010-10-311019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chase A., Cross N.C.P. Aberrations of EZH2 in Cancer. Clinical Cancer Res. 2011;17:2613–2618. doi: 10.1158/1078-0432.CCR-10-2156. [DOI] [PubMed] [Google Scholar]
- 141.Ernst T., Chase A.J., Score J., Hidalgo-Curtis C.E., Bryant C., Jones A.V., Waghorn K., Zoi K., Ross F.M., Reiter A., et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat. Genet. 2010;42:722–726. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- 142.Nikoloski G., Langemeijer S.M.C., Kuiper R.P., Knops R., Massop M., Tonnissen E.R.L.T.M., van der Heijden A., Scheele T.N., Vandenberghe P., de Witte T., et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat. Genet. 2010;42:665–667. doi: 10.1038/ng.620. [DOI] [PubMed] [Google Scholar]
- 143.Ito S., D’Alessio A.C., Taranova O.V., Hong K., Sowers L.C., Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tan L., Shi Y.G. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012;139:1895–1902. doi: 10.1242/dev.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Holmfeldt L., Mullighan C.G. The role of TET2 in hematologic neoplasms. Cancer Cell. 2011;20:1–2. doi: 10.1016/j.ccr.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 146.Delhommeau F., Dupont S., Della Valle V., James C., Trannoy S., Masse A., Kosmider O., Le Couedic J.P., Robert F., Alberdi A., et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 147.Bejar R. Splicing Factor Mutations in Cancer. Adv. Exp. Med. Biol. 2016;907:215–228. doi: 10.1007/978-3-319-29073-7_9. [DOI] [PubMed] [Google Scholar]
- 148.Fu X.D. Specific commitment of different pre-mRNAs to splicing by single SR proteins. Nature. 1993;365:82–85. doi: 10.1038/365082a0. [DOI] [PubMed] [Google Scholar]
- 149.Edmond V., Moysan E., Khochbin S., Matthias P., Brambilla C., Brambilla E., Gazzeri S., Eymin B. Acetylation and phosphorylation of SRSF2 control cell fate decision in response to cisplatin. EMBO J. 2011;30:510–523. doi: 10.1038/emboj.2010.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yoshida K., Sanada M., Shiraishi Y., Nowak D., Nagata Y., Yamamoto R., Sato Y., Sato-Otsubo A., Kon A., Nagasaki M., et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 151.Meggendorfer M., Roller A., Haferlach T., Eder C., Dicker F., Grossmann V., Kohlmann A., Alpermann T., Yoshida K., Ogawa S., et al. SRSF2 mutations in 275 cases with chronic myelomonocytic leukemia (CMML) Blood. 2012;120:3080–3088. doi: 10.1182/blood-2012-01-404863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thol F., Kade S., Schlarmann C., Loffeld P., Morgan M., Krauter J., Wlodarski M.W., Kolking B., Wichmann M., Gorlich K., et al. Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood. 2012;119:3578–3584. doi: 10.1182/blood-2011-12-399337. [DOI] [PubMed] [Google Scholar]
- 153.Mian S.A., Smith A.E., Kulasekararaj A.G., Kizilors A., Mohamedali A.M., Lea N.C., Mitsopoulos K., Ford K., Nasser E., Seidl T., et al. Spliceosome mutations exhibit specific associations with epigenetic modifiers and proto-oncogenes mutated in myelodysplastic syndrome. Haematologica. 2013;98:1058–1066. doi: 10.3324/haematol.2012.075325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Will C.L., Luhrmann R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 2011;3:a003707. doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pellagatti A., Armstrong R.N., Steeples V., Sharma E., Repapi E., Singh S., Sanchi A., Radujkovic A., Horn P., Dolatshad H., et al. Impact of spliceosome mutations on RNA splicing in myelodysplasia: Dysregulated genes/pathways and clinical associations. Blood. 2018;132:1225–1240. doi: 10.1182/blood-2018-04-843771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Malcovati L., Papaemmanuil E., Bowen D.T., Boultwood J., Della Porta M.G., Pascutto C., Travaglino E., Groves M.J., Godfrey A.L., Ambaglio I., et al. Clinical significance of SF3B1 mutations in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Blood. 2011;118:6239–6246. doi: 10.1182/blood-2011-09-377275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Graubert T.A., Shen D., Ding L., Okeyo-Owuor T., Lunn C.L., Shao J., Krysiak K., Harris C.C., Koboldt D.C., Larson D.E., et al. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat. Genet. 2011;44:53–57. doi: 10.1038/ng.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hirabayashi S., Flotho C., Moetter J., Heuser M., Hasle H., Gruhn B., Klingebiel T., Thol F., Schlegelberger B., Baumann I., et al. Spliceosomal gene aberrations are rare, coexist with oncogenic mutations, and are unlikely to exert a driver effect in childhood MDS and JMML. Blood. 2012;119:e96–e99. doi: 10.1182/blood-2011-12-395087. [DOI] [PubMed] [Google Scholar]
- 159.Broesby-Olsen S., Kristensen T.K., Møller M.B., Bindslev-Jensen C., Vestergaard H. Adult-onset systemic mastocytosis in monozygotic twins with KIT D816V and JAK2 V617F mutations. J. Allergy Clin. Immunol. 2012;130:806–808. doi: 10.1016/j.jaci.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 160.Lim K.H., Tefferi A., Lasho T.L., Finke C., Patnaik M., Butterfield J.H., McClure R.F., Li C.Y., Pardanani A. Systemic mastocytosis in 342 consecutive adults: Survival studies and prognostic factors. Blood. 2009;113:5727–5736. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 161.Galata G., Garcia-Montero A.C., Kristensen T., Dawoud A.A.Z., Munoz-Gonzalez J.I., Meggendorfer M., Guglielmelli P., Hoade Y., Alvarez-Twose I., Gieger C., et al. Genome-wide association study identifies novel susceptibility loci for KIT D816V positive mastocytosis. Am. J. Hum. Genet. 2021;108:284–294. doi: 10.1016/j.ajhg.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.De Vita S., Schneider R.K., Garcia M., Wood J., Gavillet M., Ebert B.L., Gerbaulet A., Roers A., Levine R.L., Mullally A., et al. Loss of Function of TET2 Cooperates with Constitutively Active KIT in Murine and Human Models of Mastocytosis. PLoS ONE. 2014;9:96209. doi: 10.1371/journal.pone.0096209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bejar R., Stevenson K., Abdel-Wahab O., Galili N., Nilsson B., Garcia-Manero G., Kantarjian H., Raza A., Levine R.L., Neuberg D., et al. Clinical Effect of Point Mutations in Myelodysplastic Syndromes. N. Engl. J. Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kristensen T., Vestergaard H., Moller M.B. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J. Mol. Diagn. JMD. 2011;13:180–188. doi: 10.1016/j.jmoldx.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kristensen T., Vestergaard H., Bindslev-Jensen C., Moller M.B., Broesby-Olsen S. Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am. J. Hematol. 2014;89:493–498. doi: 10.1002/ajh.23672. [DOI] [PubMed] [Google Scholar]
- 166.Erben P., Schwaab J., Metzgeroth G., Horny H.P., Jawhar M., Sotlar K., Fabarius A., Teichmann M., Schneider S., Ernst T., et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann. Hematol. 2014;93:81–88. doi: 10.1007/s00277-013-1964-1. [DOI] [PubMed] [Google Scholar]
- 167.Hoermann G., Gleixner K.V., Dinu G.E., Kundi M., Greiner G., Wimazal F., Hadzijusufovic E., Mitterbauer G., Mannhalter C., Valent P., et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014;69:810–813. doi: 10.1111/all.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoermann G., Sotlar K., Jawhar M., Kristensen T., Bachelot G., Nedoszytko B., Carter M.C., Horny H.P., Bonadonna P., Sperr W.R., et al. Standards of Genetic Testing in the Diagnosis and Prognostication of Systemic Mastocytosis in 2022: Recommendations of the EU-US Cooperative Group. J. Allergy Clin. Immunol. Pract. 2022 doi: 10.1016/j.jaip.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 169.Steensma D.P., Bejar R., Jaiswal S., Lindsley R.C., Sekeres M.A., Hasserjian R.P., Ebert B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zink F., Stacey S.N., Norddahl G.L., Frigge M.L., Magnusson O.T., Jonsdottir I., Thorgeirsson T.E., Sigurdsson A., Gudjonsson S.A., Gudmundsson J., et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–752. doi: 10.1182/blood-2017-02-769869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jacobs K.B., Yeager M., Zhou W., Wacholder S., Wang Z., Rodriguez-Santiago B., Hutchinson A., Deng X., Liu C., Horner M.J., et al. Detectable clonal mosaicism and its relationship to aging and cancer. Nat. Genet. 2012;44:651–658. doi: 10.1038/ng.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Laurie C.C., Laurie C.A., Rice K., Doheny K.F., Zelnick L.R., McHugh C.P., Ling H., Hetrick K.N., Pugh E.W., Amos C., et al. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat. Genet. 2012;44:642–650. doi: 10.1038/ng.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hall J., Al Hafidh J., Balmert E., Dabbas B., Vaupel C., El Hader C., McGinniss M., Beruti S., Bejar R. Somatic Mutations Indicative of Clonal Hematopoiesis Are Present in a Large Fraction of Cytopenic Patients Who Lack Diagnostic Evidence of MDS. Blood. 2014;124:3272. doi: 10.1182/blood.V124.21.3272.3272. [DOI] [Google Scholar]
- 174.Shlush L.I. Age-related clonal hematopoiesis. Blood. 2018;131:496–504. doi: 10.1182/blood-2017-07-746453. [DOI] [PubMed] [Google Scholar]
- 175.Valent P., Kern W., Hoermann G., Milosevic Feenstra J.D., Sotlar K., Pfeilstöcker M., Germing U., Sperr W.R., Reiter A., Wolf D., et al. Clonal Hematopoiesis with Oncogenic Potential (CHOP): Separation from CHIP and Roads to AML. Int. J. Mol. Sci. 2019;20:789. doi: 10.3390/ijms20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shah S., Pardanani A., Elala Y.C., Lasho T.L., Patnaik M.M., Reichard K.K., Hanson C.A., Ketterling R.P., Tefferi A. Cytogenetic abnormalities in systemic mastocytosis: WHO subcategory-specific incidence and prognostic impact among 348 informative cases. Am. J. Hematol. 2018;93:1461–1466. doi: 10.1002/ajh.25265. [DOI] [PubMed] [Google Scholar]
- 177.Youk J., Koh Y., Kim J.W., Kim D.Y., Park H., Jung W.J., Ahn K.S., Yun H., Park I., Sun C.H., et al. A scientific treatment approach for acute mast cell leukemia: Using a strategy based on next-generation sequencing data. Blood Res. 2016;51:17–22. doi: 10.5045/br.2016.51.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li P., Biancon G., Patel T., Pan Z., Kothari S., Halene S., Prebet T., Xu M.L. Comprehensive Clinicopathologic and Molecular Analysis of Mast Cell Leukemia With Associated Hematologic Neoplasm: A Report and In-Depth Study of 5 Cases. Front. Oncol. 2021;11:730503. doi: 10.3389/fonc.2021.730503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lasho T.L., Finke C.M., Zblewski D., Patnaik M., Ketterling R.P., Chen D., Hanson C.A., Tefferi A., Pardanani A. Novel recurrent mutations in ethanolamine kinase 1 (ETNK1) gene in systemic mastocytosis with eosinophilia and chronic myelomonocytic leukemia. Blood Cancer J. 2015;5:e275. doi: 10.1038/bcj.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Toledo M.A.S., Gatz M., Sontag S., Gleixner K.V., Eisenwort G., Feldberg K., Hamouda A.E.I., Kluge F., Guareschi R., Rossetti G., et al. Nintedanib targets KIT D816V neoplastic cells derived from induced pluripotent stem cells of systemic mastocytosis. Blood. 2021;137:2070–2084. doi: 10.1182/blood.2019004509. [DOI] [PubMed] [Google Scholar]
- 181.Dorrance A. “Mast”ering drug discovery with iPSCs. Blood. 2021;137:1993–1994. doi: 10.1182/blood.2020010456. [DOI] [PubMed] [Google Scholar]
- 182.Shomali W., Gotlib J. Response Criteria in Advanced Systemic Mastocytosis: Evolution in the Era of KIT Inhibitors. Int. J. Mol. Sci. 2021;22:2983. doi: 10.3390/ijms22062983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rowe R.G., Daley G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019;20:377–388. doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Andersohn F., Konzen C., Garbe E. Systematic review: Agranulocytosis induced by nonchemotherapy drugs. Ann. Intern. Med. 2007;146:657–665. doi: 10.7326/0003-4819-146-9-200705010-00009. [DOI] [PubMed] [Google Scholar]
- 185.Schwartz L.B., Metcalfe D.D., Miller J.S., Earl H., Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N. Engl. J. Med. 1987;316:1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 186.Schwartz L.B. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol. Allergy Clin. N. Am. 2006;26:451–463. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.