Abstract
The squamous cell carcinoma antigen (SCCA) is a tumor marker that has gained increasing attention for its biological functions and significance in normal physiological and pathological processes. Not only SCCA but also circulating immune complexes of SCCA and immunoglobulin M (IgM) are involved in normal physiological and pathological processes, providing a background for numerous clinical studies aimed at assessing the potential role of SCCA, SCCA–IgM, and SCCA isoform complexes in clinical practice. Previous studies support the clinical value of SCCA as a tumor marker for either diagnosing squamous cancers or monitoring the response to radiotherapy or chemotherapy, tumor relapse, and treatment failure. However, these studies show contrasting results, making the diagnostic or prognostic value of SCCA controversial. To reduce clinical heterogeneity across studies and achieve a more accurate and reliable comparison of results, a standardized detection method, scoring system, and cutoff level need to be established. Moreover, despite the fact that performances of different methods are comparable, the dynamic observation of tumor marker kinetics should be conducted under the same method.
Keywords: squamous cell carcinoma antigen, tumor marker, serine protease inhibitors, diagnosis, prognosis
1. Introduction
The squamous cell carcinoma antigen (SCCA), a tumor-specific antigen, was first isolated by Kato and Torigoe from squamous cell carcinoma (SCC) tissues of the uterine cervix in the 1970s [1]. SCCA consists of two highly homologous isoforms, SCCA1 and SCCA2, encoded by SERPINB3 and SERPINB4 genes, respectively, and is located on the long arm of chromosome 18 (18q21.3). SCCA1 and SCCA2, also termed SERPINB3 and SERPINB4, respectively, belong to the serine protease inhibitor family (SERPINBs) and consist of an ovalbumin-like domain with nine α-helices and three antiparallel β-sheets, and a reactive center loop that is essential for binding and inhibiting the target protease [2].
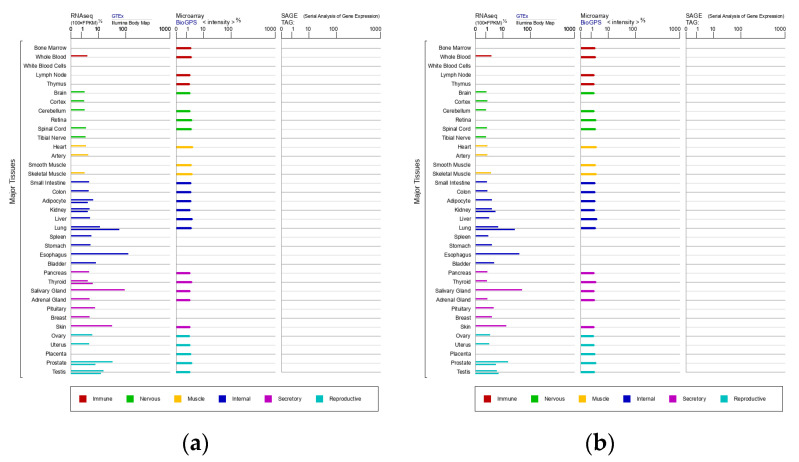
SCCA1 and SCCA2 share a 98% degree of homology and are 92% identical at the amino acid level. SCCA1 is the neutral form of SCCA (pI = 6.4), while SCCA2 is the acidic form (pI = 5.9) [3]. Normal squamous epithelium cells can express SCCA, and SCCA1 and SCCA2 are usually co-expressed in the same tissues of the immune, nervous, muscular, secretory, and reproductive systems and internal organs, as shown in Figure 1 from GeneCards, a database of human genes (www.genecards.org, 13 January 2022) [4]. A genetic variant of SCCA has been reported in a minority of hepatocellular carcinoma (HCC) cases [5,6]. SCCA1 mainly inhibits papain-like cysteine proteases, while SCCA2 inhibits chymo-trypsin-like serine proteases [7]. These isoforms may be differently expressed in tumors and skin diseases [8].
Figure 1.
Expression of SCCA1 (a) and SCCA2 (b) in major tissues.
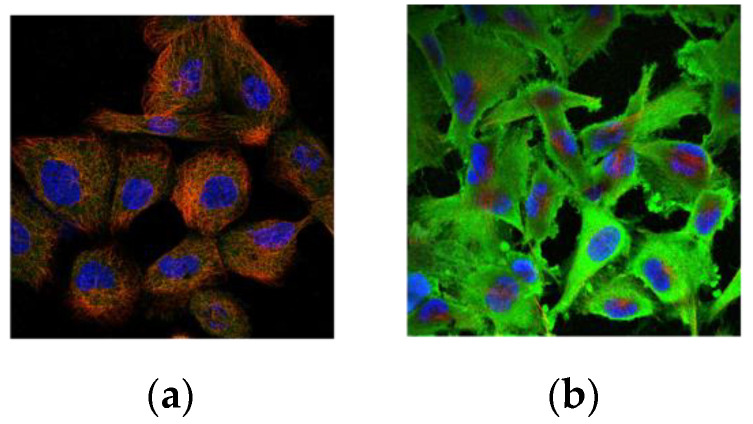
SCCA1 and SCCA2 are mainly located in the cytoplasm. However, this precise subcellular localization may change under various conditions, as these isoforms can also be detected in the cytosol, nucleus, plasma membrane, or lysosomes or extracellularly, which suggests their precise biological functions [9,10,11]. Figure 2 shows typical images of the subcellular localization of SCCA1 in human cells from the Human Protein Atlas (www.proteinatlas.org, 13 January 2022).
Figure 2.
Subcellular localization of SCCA1 in human cells: (a) cytosol (CAB036006:A-431); (b) membrane and cytosol (CAB036006:U-251 MG).
Factors affecting the detectability of SCCA in serum have been determined by researchers. These include:
Tumor size and volume (more tumor cells result in a larger amount of SCCA);
Invasiveness of the primary tumor or recurrence;
Lymph node metastasis (LNM) (secreted SCCA from tumor cells in lymph nodes is easily detected in the bloodstream [12]);
Distant metastasis (circulating tumor cells enable easy detection of secreted SCCA in blood tests [13]);
Impairment of immunosurveillance.
The expression of SCCA in peripheral T-lymphocytes indicates that both tumor cells and T-lymphocytes may cause the production of serum SCCA [14]. Additionally, the existence of immunoglobulin M (IgM) contributes to the formation of SCCA–IgM complexes.
Even though SCCA testing is routinely performed to help clinicians diagnose and manage diseases, studies have shown conflicting results regarding its usefulness. Our work aims to review studies to review studies of SCCA in recent years, discuss the potential causes of consistent and inconsistent results, and explore the possible solutions.
2. SCCA Measurement
The demand for routine clinical SCCA testing has rapidly increased because of its vital role in the diagnosis and prognosis of SCCs and other diseases. Detection methods have also improved with the development of biotechnology. The low degree of biochemical diversity of biotechnological products has enabled the generation of monoclonal antibodies specific for either SCCA1 or SCCA2, allowing separate measurements of each instead of total SCCA. Common methods used to detect SCCA levels in serum and tissue include radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), Western blot (WB), immunohistochemistry (IHC), and immunoluminometric assay.
One of the first methods to detect SCCA was radioimmunoassay [1,15]. Although radioimmunoassay is sensitive and reliable, it has numerous disadvantages including radiation exposure and poor reagent stability, gradually making its use less widespread.
ELISA, a test based on antigen–antibody reaction and color change to identify protein levels, used to be the most common method for detecting SCCA. However, ELISA also came with disadvantages, including poor repeatability, a narrow linear range, poor detection efficiency, and tedious experimental procedures; thus, it is not sufficient to meet the clinical demand.
The basic principle of WB consists of using specific antibodies to target proteins in cells or biological tissues during gel electrophoresis and analyzing the molecular size of the proteins in relation to the reference ladder to measure protein expression. The complex experimental procedures and high requirements for WB have also limited its use for large-batch testing in the clinic. However, because of its high specificity and accuracy, WB remains a method of choice in research studies. Meanwhile, IHC is a method applying the principles of immunology and histochemistry to qualitatively or quantitatively identify components in cells or tissues in situ. Both WB and IHC are only suitable for small samples.
Recently, automated non-radioimmunoassay methods have been performed to measure SCCA levels. Electrochemiluminescence immunoassay (ECLIA) with the Roche Cobas e602 system and chemiluminescent microparticle immunoassay (CLIA) with the Abbott ARCHITECT i2000 system are two common automated systems used in the clinical laboratory. Although there is a strong positive correlation (r = 0.9658) between these two systems, the tested SCCA levels are reportedly higher with the Roche system than with the Abbott system [8]. These differences might be attributed to the different serum proteins and heterophilic antibodies used [16] and different reactivities against antigens among specific antibodies produced by various manufacturers [17]. Reportedly, the Roche system can detect SCCA1 and SCCA2 in an equimolar manner [18], while the Abbott system cannot determine the proportions of SCCA1 and SCCA2 [8] or can only detect SCCA1 but not SCCA2 [19]. Manual ELISA and automated ECLIA have shown comparable performances in SCCA detection [20]. However, despite comparable performances between different methods, the dynamic observation of tumor marker kinetics should be performed under the same method.
3. SCCA Levels in Cancer and Inflammation
The biological functions and significance of SCCA in normal physiological and pathological processes have been widely studied. Besides SCCA, circulating immune complexes of SCCA and IgM have also been reported in normal physiological and pathological processes, providing a background for studies investigating the potential role of SCCA, SCCA isoforms, and SCCA–IgM complexes in clinical practice [21] (Table 1).
A recent study on the relationship between SCCA and 39 different clinically defined diseases shows that SCCA is a clinically used biomarker not only for SCC but also for other human diseases [7].
3.1. Cervical Cancer
Imaging techniques, including computed tomography (CT) and positron emission tomography (PET)/CT, have been increasingly applied in the clinical diagnosis and treatment of cervical cancer; however, such methods have limitations, including false-negative and false-positive results [22,23]. Nevertheless, the diagnostic efficacy of combined PET/CT with serum SCCA in the diagnosis of early recurrent cervical cancer is higher than that of either PET/CT or serum SCCA methods alone [24,25]. Thus, this combined method is clinically valuable in the diagnosis of early postoperative metastases and recurrence in cervical cancer.
Ryu HK et al. [26] reported that SCCA levels > 1.86 and >0.9 ng/mL were the optimal pretreatment and posttreatment cutoff values for predicting recurrence, respectively, and were significantly associated with poor disease-free survival (DFS). With a high risk of cancer recurrence, closer surveillance is thus needed after complete remission therapy [26]. Another study reported 4, 1.5, and 4 ng/mL as the best cutoff values for SCCA at pretreatment, treatment, and recurrence, and that the predicting value of SCCA after treatment and at recurrence for recurrence and survival is significant only when pretreatment SCCA levels ≥ 4 ng/mL [27].
Multivariate analysis, particularly of surgical treatment for early-stage cervical SCC (stages IB–IIA), demonstrated that SCCA levels ≥ 2.75 ng/mL can be used as a potential marker to predict LNM in early stage cervical cancer preoperatively [28]. Another study investigating the utility and cutoff level of serum SCCA to predict LNM in locally advanced cervical cancer showed that the SCCA level significantly correlated with paraaortic lymph node status, but not with pelvic lymph node status and parametrial involvement [29]. In another study, the positive lymph node rate of patients with pretreatment SCCA levels ≥ 3.9 ng/mL significantly decreased after neoadjuvant chemotherapy (NACT). The overall survival (OS) was significantly longer in the NACT group than in the conventional group (with radical surgery alone) when the pretreatment SCCA levels were ≥4.55 ng/mL [30].
Not only are elevated pretreatment SCCA levels associated with radiotherapy resistance, extensive tumors, and poor survival in cervical cancer patients treated with definitive concurrent chemoradiotherapy (CCRT) but also the rate of SCCA reduction during CCRT can predict tumor response after treatment [31]. SCCA also shows high sensitivity for the early detection of cervical cancer relapse during follow-up after CCRT, and it is cost-effective [31]. Considering the optimal posttreatment SCCA level cutoff of 1.8 ng/mL for predicting treatment failure, patients with posttreatment SCCA levels ≥ 1.8 ng/mL are more likely to come to treatment failure and poor survival [32].
Even with the potential benefit of monitoring SCCA during follow-up of early cervical cancer patients after treatment, the cure rate of patients with elevated SCCA levels does not seem to improve, partly owing to the lack of curative salvage treatments [33].
A novel model of measuring kinetic change in SCCA levels before surgery, and on days, weeks and months after surgery [34], and their associations with clinicopathologic characteristics show that SCCA levels decreased dramatically after surgery and postoperative SCCA could keep stable or fluctuate to some extent within a half-year [34]. The SCCA levels preoperatively and at earlier time points postoperatively were higher in patients with risk factors. The kinetic trend of SCCA might be mainly affected by postoperative adjuvant therapy, as the SCCA levels reached the same level between the low-risk, intermediate-risk, and high-risk group after completion of treatment [34]. A new model of SCCA, such as SCCRR (pre-RT SCCA − mid-RT SCCA/pre-RT SCCA × 100) [35] was identified as an independent and strong prognostic parameter for patients with cervical cancer receiving radiation therapy (RT). Chang et al. [36] established that the hazard at time t is dependent on SCCA value at the same time and that patients should be followed with routine SCCA assessments, and the time-dependent SCCA is the best predictor of both relapse and death.
The failure of posttreatment SCCA levels to normalize may predict tumor relapse, and adjuvant therapies should be considered for these patients [31]. There is a much higher percentage of early stage (FIGO stage IB/IIA) cervical cancer patients with elevated preoperative serum SCCA (>1.9 ng/mL) who had postoperative indications for adjuvant radiotherapy than of patients with normal preoperative SCCA. For IBI patients with no indications for adjuvant radiotherapy, the percentage of patients with elevated preoperative serum SCCA was much higher than that of patients with normal serum SCCA levels relapse within 2 years [37].
On the one hand, surgery is the standard treatment for early-stage cervical cancer patients. On the other hand, postoperative concurrent chemoradiotherapy is recommended for patients with any high-risk features, such as LNM, parametrial involvement, and positive surgical margins. However, for tumor patients with a combination of intermediate-risk factors such as large size, lymphovascular involvement, and deep stromal invasion, there is no consensus on whether adjuvant chemotherapy should be administered to those with two intermediate-risk factors, as this may lead to over or under treatment. A study indicated that preoperative SCCA can be a predictive marker for the use of adjuvant chemotherapy in cervical SCC with intermediate-risk factors [38]. It indicated that for patients with a high pretreatment SCCA level, those who received adjuvant chemotherapy have a better prognosis and a lower rate of distant metastasis than do patients who did not receive chemotherapy; this is not observed in patients with a low pretreatment SCCA level [38].
Another study found that cervical cancer patients with elevated pretreatment SCCA did not benefit from adjuvant chemotherapy and a considerable proportion of these patients had postoperative indications for adjuvant radiotherapy [39]. For these cervical cancer patients with elevated pretreatment SCCA, the choice of radical hysterectomy and adjuvant chemotherapy should be prudent [39].
During follow-up of cervical cancer patients treated with radiotherapy, renal dysfunction was significantly associated with a greater incidence of SCCA elevation [40].
3.2. Lung Cancer
SCCA has also been widely used as one of the tumor markers for monitoring non-small cell lung cancer (NSCLC), although recent reports challenge its value in routine test because of low sensitivity [41].
As the incidence of peripheral SCCs of the lung (p-SqCCs) has increased over recent years, multivariate analysis of p-SqCCs patients revealed that serum SCCA is also an independent prognostic factor for completely resected p-SCCs; stage T1 p-SCC with a high serum SCCA level or vascular invasion should be upgraded to T2a, which accurately reflects survival status of patients with p-SqCCs [42].
SCCA1 was identified as a predictive biomarker for response to platinum combination chemotherapy (PtC) and also as an independent prognostic value for untreated patients with resected NSCLC. SCCA1 expression strongly correlates with clinical response in PtC-treated NSCLC patients [11]. On the one hand, a targeted therapy is not currently available for most patients with advanced-stage disease and PtC remains a key part of the systemic treatment for them. On the other hand, a SCCA1 score of ≥2 [43] identifies a subgroup of patients with stage IV NSCLC who have a poor survival when treated with PtC, similar to that estimated of untreated or chemorefractory stage IV NSCLC, indicating SCCA1 as a useful biomarker that identifies a highly resistant subgroup for whom PtC should be avoided [43].
SCCA has not only been found in patients with SCC but also in patients with non-squamous cell lung cancer (NSCC) and nonmalignant pulmonary disease (NMPD). However, SCCA may serve a contradictory role in different types of NSCLC. SCCA1 is significantly decreased in metastatic lesions when compared with that in paired primary SCC, supporting an inhibition role of SCCA1 in tumor invasion and metastasis; this phenomenon does not exist in AC. High SCCA1 expression indicates poor prognosis in AC, and SCCA1 may potentially function primarily as a negative regulator of cell death in AC. The contrasting value of SCCA1 in AC and SCC, indicates a dual pathogenic role of SCCA1 in different histologic types of NSCLC [11]. Although SCCA is still a widely used tumor marker for monitoring NSCLC, a study reevaluated the efficacy of SCCA and CYFRA21-1 in diagnosing NSCLC. The sensitivity of SCCA is significantly lower than that of CYFRA21-1 for patients with both NSCLC and metastasis, and the combination of SCCA with CYFRA21-1 can only induce a minimal increase in CYFRA21-1 sensitivity [41]. This indicates that SCCA should be considered as an inefficient factor for NSCLC diagnosis.
Elevated SCCA levels (>1.5 ng/mL) have been reported in SCC (52.7%), NSCC (14.2%), and NMPD (28.4%) patients. However, serum SCC levels of ≥40.0 ng/mL in NSCC patients and ≥20.0 ng/mL in NMPD patients have not been observed [44]. Interstitial lung disease (ILD) is the major determinant of prognosis of patients with systemic sclerosis (SSc). With a cutoff value for serum SCCA–IgM at >200 AU/mL, SCCA–IgM was significantly higher in SSc patients with ILD, which is a major determinant of the prognosis in SSc patients, than in those without ILD [45].
3.3. Neck and Head Cancer
Even though studies have recently been conducted to identify the correlation between SCCA and clinicopathologic features in head and neck SCC (HNSCC) patients to evaluate the clinical usefulness of serum SCCA in the management of patients with HNSCC, the role of SCCA in these patients remains controversial.
The pretreatment serum SCCA2, not SCCA1, was significantly higher in HNSCC patients than in controls [46]. Another study indicated that SCCA1 is highly active in oral SCC but nearly undetectable or expressed at a low level in normal tissues [47].
SCCA levels have a significant correlation with male sex and TNM stage, and SCCA is significantly higher in patients with advanced T and N classification tumors. One analysis indicated that it may not be used as a predictive marker for OS and DFS in HNSCC patients [48], while another revealed that SCCA was a significant risk factor against OS in cancers of the oral cavity, hypopharynx, and larynx, but not in oropharyngeal cancer [13]. SCCA was higher in lymph nodes of HNSCC patients than in those of other patients, and fine needle aspiration of cervical lymph node samples containing SCCA is a reliable test for detecting HNSCC and precedes an even more accurate detection of HNSCC LNM when used in addition to fine needle aspiration cytology [49].
Inverted papilloma (IP) is a benign neoplasm, although it has a high tendency to recur or develop malignancy. It is vital to identify it from inflammatory diseases. Elevated serum SCCA levels were observed in 83.3% of patients with IP regardless of whether this being new or recurrent and in 5.3% of inflammatory patients, and the sensitivity and specificity of diagnosis IP from IP and inflammatory groups, based on SCCA levels (1.5 ng/mL), were 83.3% and 94.7%, respectively [50]. Another study indicated that the sensitivity and specificity were 80.0% and 93.3% for IP diagnosis, respectively, based on the SCCA level (>1.5 ng/mL) in IP and nasal polyp groups [51]. Although serum SCCA levels were elevated in both IP and sinonasal SCC groups, the distribution and proportion of SCCA1 and SCCA2 were distinct in these patients. Patients with sinonasal IP predominantly express SCCA1, whereas those with sinonasal SCC predominantly express SCCA2 [52].
Yamashita et al. [50] also found that smoking and tumor volume significantly correlated with SCCA levels in IP. Several studies have shown that postoperative rather than preoperative SCCA levels significantly decreased in the IP group [50,51,53]. Even though postoperative SCCA levels were positively associated with future recurrence, with a good ability to identify recurrence, no correlation between SCCA levels and recurrence during follow-up was found [53]. The high postoperative SCCA level might be induced by residual disease, and most recurrence events develop at the site of the original tumor, indicating that residual disease is the main cause of recurrence [53].
SCCA1 is not only associated with prognosis and chemoresistance but also with the microenvironment in esophageal adenocarcinoma (EAC) [54]. SCCA1 and SCCA1–IgM serum levels were significantly higher and lower, respectively, in EAC patients than in healthy controls. Even though SCCA–IgM and free SCCA1 were inversely correlated with immune activation markers, only free SCCA1 was significantly associated with worse OS. In vitro, EAC cell lines overexpressing SCCA1 showed significantly more resistance to cell death induced by different chemotherapeutic drugs [54], and human recombinant SCCA1 was shown to induce a significant increase in the inhibitory molecule PD-L1 levels in monocytes in vitro. Aside from tumor cells, SCCA was also expressed in peripheral T-lymphocytes, indicating that both tumor cells and T-lymphocytes may contribute to increased serum SCCA [14]. Therefore, the reduced tumor chemosensitivity and intra-tumoral immunity impairment induced by SCCA might a contributing factor to poor prognosis in EACs overexpressing SCCA1 [54].
3.4. Liver Cancer
SCCA has also been found to be elevated in liver cancer and has been detected as SCCA–IgM complexes in serum of hepatocellular carcinoma (HCC) patients [21].
Reliable evidence shows that SCCA expression progressively increases during hepatocarcinogenesis, i.e., from chronic liver disease to dysplastic nodules to HCC [55]. Tumoral SCCA, but not peritumoral or serum SCCA, is dependent on nodule size, and there was an inverse correlation between nodule sizes and SCCA levels [56]. The significantly stronger expression of SCCA in smaller than in larger HCC could be important for early HCC detection [56,57]. While a positive correlation was observed between tissue and serum levels of SCCA in liver cirrhosis patients, there was no such correlation in HCC [56]. Interestingly, Li et al. [6] reported the rate of SCCA2 expression was much higher than SCCA1. Altogether, SCCA only shows moderate diagnostic accuracy for HCC screening, possibly because SCCA is also increased in liver cirrhosis and chronic liver disease [58].
An increasing number of studies have shown that both pretreatment serum AFP and SCCA–IgM levels were significantly higher in HCC patients than in patients with cirrhosis [59]. The combination of SCCA–IgM complexes and AFP has greater sensitivity than either biomarker alone, particularly for patients with AFP values between 20–200 ng/mL [57]. The serum SCCA–IgM level was significantly lower in HCC patients who underwent surgical resection than in those who received other therapy (TACE, RFA, or palliative care) [59]. This decrease in SCCA–IgM levels might be caused by the removal of cancer tissues or the reduction in liver damage and associated immune response [59]. Serum SCCA–IgM levels at baseline and 1 month after treatment were significantly lower in patients who responded to therapy than in those who did not respond [60], suggesting that SCCA–IgM level determination could help predict the response to therapy in HCC patients.
Circulating SCCA–IgM was more frequently detected in patients with chronic hepatitis C (CHC) than in those with negative hepatitis C virus (HCV) [61]. In HCV-positive patients, there is a significant correlation between the SCCA1 level in the liver and the serum SCCA–IgM level. The kinetics of SCCA–IgM is related to antiviral therapy. In patients with sustained virologic response, the SCCA–IgM level decreased significantly after treatment for a half-year and remained persistently low even a half-year after the last therapy [61]. In contrast, although non-responders showed a significant decrease after a half-year of treatment, SCCA–IgM reached almost initial values after a half-year of therapy withdrawal. As previous studies have shown that nonalcoholic steatohepatitis (NASH) occurs frequently in HCV infection and is recognized as an increasing risk factor for liver disease and HCC development, SCCA–IgM may be useful for the identification of HCV-infected patients with a high risk of disease progression and HCC development [61,62]; SCCA–IgM and HCV genotype 3 have a significant association with the presence of NASH. In contrast, SCCA–IgM does not seem to have a role in the identification and prognosis of nonalcoholic fatty liver disease in patients with obesity, prediabetes, and diabetes undergoing sleeve gastrectomy [63].
SCCA–IgM baseline of patients who developed HCC were significantly higher than patients who did not during the same follow-up period, especially in HCV-infected patients, and positivity of SCCA–IgM at baseline was associated with a significantly shorter HCC-free survival [64].
SCCA–IgM is a dynamic biomarker, alternating as the disease progresses [21] and over therapy process [60]. Accordingly, monitoring of SCCA–IgM variations before and after therapy or at different time points might be more useful than monitoring at a single time point, and it can provide a better understanding of disease progression [21,60].
3.5. Inflammation
Recent studies have indicated the usefulness of monitoring SCCA levels in pediatric and adult atopic dermatitis (AD). Serum SCCA levels in the psoriasis group was significantly higher than those in controls and significantly decreased after treatment [65]. Both SCCA1 and SCCA2 were higher in AD patients than in volunteers and positively correlated with the clinical severity of AD [19,65]. However, with the predominant expression of SCCA1 and SCCA2 in cervical cancer and AD patients, respectively, detection and discrimination between SCCA1 and SCCA2 are critical in estimating the severity of AD and distinguishing AD from other cancers [19].
Serum SCCA2 levels were significantly higher in patients with lichen planus (LP) than in healthy controls, as well as in female patients than in male patients [66]. The mean serum SCCA2 levels were significantly higher in patients with eruptive LP than in those with localized and hypertrophic forms. Further studies are needed to assess the therapeutic effect of SCCA2 blockade, which could improve outcomes of LP patients [66].
Watanabe et al. [67] have stated that even though SCCA1 and SCCA2 highly correlated, SCCA2 may have much better clinical usage. Consistent with previous studies, their study showed that serum SCCA2 levels were significantly higher in psoriasis patients than in healthy controls and correlated well with disease severity and reflected treatment efficacy. Only serum SCCA2 showed a significant increase in AD when assessed in each age group or in subgroup analysis [67,68]. Increased SCCA2 staining was observed in the lesional skin of psoriasis patients, and skin SCCA2 levels correlated with serum SCCA2 levels. The underlying mechanism for the significant relation between SCCA2 level and disease severity remains unclear yet. An earlier study showed that keratinocytes produced mainly SCCA2 with stimulation of IL-4 or IL-13, IL-17, and IL-22 [19,67].
Table 1.
Summary of investigation of SCCA in studies.
| Ref. | Marker | Disease | Sample Type | Sample Collection Time | Method | Cut-Off | Conclusion |
|---|---|---|---|---|---|---|---|
| Pontisso et al., 2004 [5] | SCCA1 variant | HCC | Tissues | At surgery | IHC | - | Diagnose HCC |
| Li et al., 2014 [6] | SCCA1/2, SCCA1 variant |
HCC | Tissues | At surgery | RT-PCR Sequencing | - | Diagnose HCC |
| Lin et al., 2011 [12] | SCCA | OSCC | Serum | Preoperative | CLIA | 2.0 ng/mL | Predict metastasis, DFS and OS |
| Imai et al., 2015 [13] | SCCA | HNSCC | Serum | Pretreatment | CLIA | 1.1 ng/mL | Predict survival |
| Beneduce et al., 2005 [21] | SCCA-IgM | HCC | Serum | Pretreatment | EIA/WB | 120 AU/mL | Diagnose HCC |
| Ryu et al., 2015 [26] | SCCA | CSCC | Serum | Pretreatment Posttreatment | - | 1.86 ng/mL 0.9 ng/mL |
Predict recurrence |
| Choi et al., 2020 [27] | SCCA | CSCC (stage IB-IVA | Serum | Pretreatment Treatment Recurrence |
IRA | 4 ng/mL 1.5 ng/mL 4 ng/mL |
Predict recurrence and survival |
| Zhu et al., 2021 [28] | SCCA | Early CSCC | Serum | Preoperative | CLIA | 1.5 ng/mL | Predict LNM and survival |
| Lekskul et al., 2015 [29] | SCCA | CSCC (stage IB2-IVA | Serum | Pretreatment | CLIA | 1.5 ng/mL | Predict pelvic and paraaortic LNM |
| Chen et al., 2020 [30] | SCCA | CSCC | Serum | Pretreatment posttreatment | ECLIA | 3.9 ng/mL 2.7 ng/mL |
Evaluate the LNM and prognosis of CSCC who received neoadjuvant chemotherapy |
| Wang et al., 2019 [32] | SCCA | CSCC | Serum | Posttreatment | CLIA | 1.8 ng/mL | Predict treatment failure and poor survival of CSCC who received concurrent chemoradiotherapy |
| Salvatici et al., 2016 [33] | SCCA | CSCC (stage I-II) | Serum | Posttreatment | CLIA | 1.5 ng/mL | Early diagnosis of recurrence |
| Ye et al., 2020 [34] | SCCA | CSCC (stage IB1-IIA2) | Serum | day 0 (the day before surgery)/postoperative day 4, weeks 2–4, months 2–4 and months 5–7 | Single molecule assay (Simoa) prototype immunoassay | 2.49/0.66, 0.61, 0.72, and 0.71 ng/mL | Predict disease aggressiveness and treatment response |
| Chang et al., 2020 [36] | SCCA | CSCC | Serum | Pretreatment Posttreatment | - | - | Predict relapse and death |
| Reesink-Peters et al., 2005 [37] | SCCA | Early CSCC | Serum | CLIA | 1.9 ng/mL | Predict tumor relapse, and guide adjuvant therapies | |
| Guo et al., 2020 [38] | SCCA | CSCC with intermediate-risk factor | Serum | Preoperative | ELISA | 6.09 ng/mL | Predict the use of adjuvant chemotherapy |
| Yuan et al., 2021 [39] | SCCA | CSCC (stage IB-IIA) | Serum | Pretreatment | CLIA | 6.09 ng/mL | Guide adjuvant therapies |
| Oike et al., 2021 [40] | SCCA | CSCC (stage IB-IVA) | Serum | During follow-up | CLIA | 1.5 ng/mL | Improve the quality of follow-up and monitor renal dysfunction |
| Kinoshita et al., 2014 [42] | SCCA | SqCC | Serum | Presurgery | - | 1.5 ng/mL | Predict prognosis of resected peripheral-SqCC |
| Urquhart et al., 2013 [43] | SCCA1 | NSCLC (stage IV) | Tissues | Pretherapy | IHC | IHC score ≥ 2 | Predict resistance to PtC |
| Yasumatsu et al., 2019 [46] | SCCA2 | HNSCC | Serum | Pretreatment | CLIA | 1.5 ng/mL | Predict progression and guide management of HNSCC |
| Wu et al., 2020 [47] | SCCA1 | OSCC | Cell Tissues |
- | WB | - | Provide target for OSCC gene therapy. |
| van Schaik et al., 2019 [49] | SCCA | HNSCC | FNA sample | Pretreatment | CLIA | 0.3 μg/mL | Diagnose HNSCC in cervical lymph nodes. |
| Yamashita et al., 2016 [50] | SCCA | Nasal IP | Serum | Presurgery/postsurgery | CLIA | 1.5 ng/mL | Distinguish new and recurrent IP from inflammatory diseases. |
| Promsopa et al., 2021 [51] | SCCA | IP | Serum | Presurgery/postsurgery | CLIA | 1.5 ng/mL | Distinguish IP from patients with nasal polyps and rhinitis. |
| Yasumatsu et al., 2018 [52] | SCCA1/2 | Sinonasal SCC and IP | Serum Tissue |
Pretherapy | CLIA | 1.5 ng/mL | Distinguish sinonasal IP from squamous cell carcinoma |
| van Zijl et al., 2017 [53] | SCCA | Sinonasal IP | Serum | Pretreatment/posttreatment | microparticle enhanced immuno assay | 2.6/0.8 | Predict IP recurrence |
| Turato et al., 2019 [54] | SCCA1 SCCA-IgM |
EAC | Serum Tissue |
At surgery | ELISA IHC |
156 AU/mL | Predict immune surveillance impairment and reduced chemosensitivity |
| Trerotoli et al., 2009 [56] | SCCA | HCC | Tissue | Pretherapy | ELISA IHC |
- | Early diagnosis of HCC |
| Bui et al., 2018 [59] | SCCA-IgM | Liver diseases | Serum | Presurgery | ELISA | - | Monitor cirrhosis in an Asian cohort of patients. |
| Giannelli et al., 2007 [57] | SCCA-IgM | HCC | Serum | Pretherapy Posttherapy |
ELISA | - | Increase the accuracy of HCC diagnosis, especially when AFP values in 20–200 ng/mL. |
| Guarino et al., 2017 [60] | SCCA-IgM | HCC | Serum | at baseline (T0) and one month after treatment (T1) | ELISA | 120 AU/mL | Predict the outcome of therapy |
| Martini et al., 2015 [61] | SCCA-IgM | HCV-infected patients | Serum | Pretherapy Posttherapy |
ELISA | - | Identify HCV-infected with a high risk of disease progression and HCC |
| Biasiolo et al., 2016 [64] | SCCA-IgM | Liver cirrhosis | Serum | During follow-up | ELISA | 156 AU/mL | Predict and manage cirrhotic patients at higher risk of HCC development. |
| Khattab et al., 2020 [66] | SCCA2 | Lichen planus | Serum | Pretherapy | ELISA | - | Diagnose lichen planus and predict disease severity |
| Watanabe et al., 2016 [67] | SCCA2 | Psoriasis | Serum Biopsy sample Cellular lysates |
serial examinations | ELISA IHC WB |
- | Associate with disease severity and reflects treatment efficacy |
| Takeuchi et al., 2019 [68] | SCCA1/2 | Pediatric atopic dermatitis | Serum | Pretherapy | - | - | Diagnose pediatric atopic dermatitis in the Ishigaki cohort. |
Abbreviations: CSCC—cervical squamous cell carcinoma; HCC—hepatocellular carcinoma; OSCC—oral-cavity squamous cell carcinoma; HNSCC—head and neck squamous cell carcinoma; SqCC—squamous cell carcinomas (p-SqCCs) of the lung; NSCLC—non-small-cell lung cancer; IP—inverted papilloma; EAC—esophageal adenocarcinoma; IHC—immunohistochemistry; RT-PCR—reverse transcription-polymerase chain reaction; CLIA—chemiluminescent microparticle immunoassay; EIA—enzyme immunoassay; WB—Western blot; IRA—immunoradiometric assay; PtC—platinum doublet chemotherapy; LNM—lymph node metastasis.
3.6. Others
Diagnosis of penile cancer, a genitourinary system cancer, is mainly based on self or clinical examination, and confirmatory biopsy [69]. SCCA is the first serum biomarker with clinical use in managing penile cancer [70]. Monitoring SCCA might not only indicate metastases before imaging or clinical examination [70,71], but also be useful in predicting treatment response and DFS of penile cancer patients [72,73]. Serum SCCA can also help to diagnosis bladder cancer, another genitourinary system cancer [8]. Cytoplasmic SCCA, not nuclear SCCA, in bladder cancer cells is associated with bladder carcinoma cells squamous metaplasia [74]. Even a case report of poorly differentiated bladder carcinoma described that SCCA is positive [75]. It has also been reported that SERPINB4 gene was deleted in all patients of malignant peripheral nerve sheath tumor-like melanoma, a rare malignancy melanoma, and may have a tumor suppressor activity [76].
4. Combination of SCCA with Other Markers in Clinical Practice
Apart from the combination of SCCA and AFP in HCC, many studies have also evaluated the value of combining the detection of SCCA and other indicators including CRP, albumin, and noncoding RNAs for clinical use. One study indicated that serum SCCA, hypersensitive C-reactive protein (hs-CRP), and CA125 in the recurrence group of cervical cancer patients were significantly higher than those in the non-recurrence group, with a significant positive correlation between SCCA and hs-CRP, and SCCA and CA125, in the recurrence group patients [77]. Elevated preoperative CRP and SCCA levels adversely influence DFS and OS of oral SCC patients [78]. Preoperative CRP and SCCA levels were also identified as independent biomarkers for LNM, advanced tumor stage, and disease-specific survival in patients with penile SCC [79].
Preoperative serum SCCA and albumin levels can predict survival of esophageal SCC patients with stage T13N0M0, and patients with high SCCA and low albumin levels may have a poor survival outcome [80]. There is a superiority of both miR-215 and SCCA–IgM over AFP in HCC diagnosis, especially in distinguishing HCC with AFP levels < 200 ng/mL and with small-sized focal lesions from cirrhotic patients [81]. In addition, combined pretreatment serum circulating tumor cells (CTC) and pretreatment SCCA demonstrates better predictive accuracy than do the FIGO stage and serum CTC or SCCA alone [82].
5. Related Mechanisms
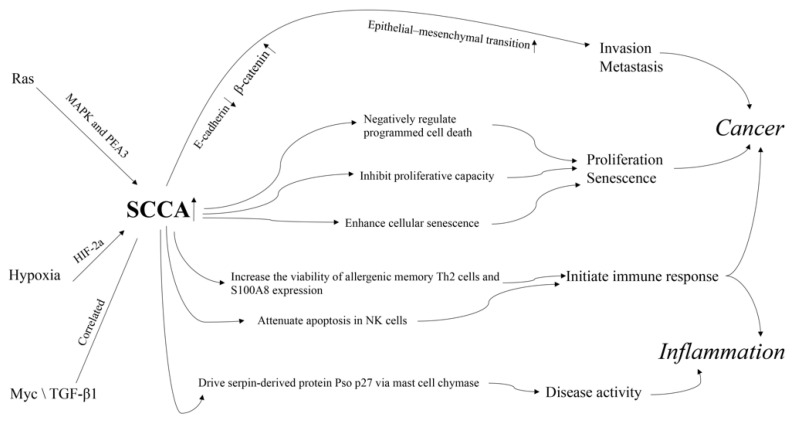
Both in vitro and in vivo studies have documented the important roles of SCCA in inflammatory processes and cancer (Figure 3). However, their exact mechanisms remain largely unclear.
Figure 3.
The mechanism of action of SCCA in cancer and inflammation.
First, the abnormal expression of SCCAs is reportedly related to cancer hallmarks and classic tumor related pathways. SERPINB3 can lead to changes in clusters of loosely connected elongated cells, decrease in desmosomal junctions, and widening of intercellular spaces, at both the autocrine and paracrine levels; these are associated with a reduction in E-cadherin and an increase in β-catenin [10]. These results show that SERPINB3 can trigger epithelial–mesenchymal transition, contributing to invasion and metastasis of cancer cells [10]. SERPINB3 has also been shown as a negative regulator of programmed cell death by protecting against leaked lysosomal proteases [11] and by reducing cytochrome C-dependent caspase 3 and 9 activity [83]. Yuan et al. [84] observed that upregulated SERPINB4 in IMR-90 and NHBE cells can markedly inhibit proliferative capacity and induction of the cyclin-dependent kinase inhibitor p16Ink4a and enhance the appearance of cellular senescence features including enlarged cell size and strong SA-ß-gal staining. SERPINB4 inhibition could suppress its effect on the appearance of several important cellular senescence hallmarks. Together, these results suggest that there is a necessity of SERPINB4 induction for the initiation of senescence programming.
SCCA1 and SCCA2 are upregulated by oncogenic Ras via MAPK and the ETS transcription factor PEA3 [85], which can lead to inhibition of protein turnover and an unfolded protein response and is essential for Ras-mediated NF-κB activation, cytokine production, and tumor growth [85,86]. Additionally, SERPINB3 expression has recently been found to be upregulated during hypoxia through HIF-2a-dependent mechanisms [64]. Upregulation of SCCA1 in hepatoblastoma positively correlated with Myc expression [87] and is associated with TGF-β1 and cytoplasmic β-catenin in HCC with poor prognosis [88].
Second, abnormal expression of SCCAs is reportedly related to immune cells and responses. SERPINB3 and SERPINB4 are upregulated in memory Th2 and innate helper 2 cells of allergy patients. Silencing of SERPINB3 and SERPINB4 can decrease the viability of allergenic memory Th2 cells [89] and the expression of pro-inflammatory marker, S100A8, in human keratinocytes. These reports support a role of SERPINB3 and SERPINB4 in the initiation of the acute inflammatory response [90] and provide a therapeutic approach for allergic diseases by ablation of allergic memory TH2 cells through SERPINB3 and SERPINB4. SERPINB3 can attenuate apoptosis by contrasting cytochrome c release from the mitochondria and via an antichemotactic effect in NK cells [91]. SERPINB3 and SERPINB4 mutations may exert immunogenic effect and help initiate immune response and can later be reinvigorated through checkpoint blockade [92]. The involvement of SERPINB3 in defective programmed cell death, a critical feature of autoimmunity, warrants further studies.
Moreover, SERPINB3 and SERPINB4 can drive the serpin-derived protein Pso p27, an autoantigen correlated to disease activity that contributes to inflammation in skin lesions, through non-canonical cleavage via mast cell chymase [93].
6. Conclusions
Previous studies have affirmed the clinical value of the tumor marker SCCA either for diagnosis or monitoring of the response to radiotherapy or chemotherapy, tumor relapse, and failure of treatment, albeit with some contrasting results because of:
Differences in the primary site or stage distribution;
Different measurement methods;
Different cutoff values for SCCA;
Different distributions of SCCA isoforms, including SCCA1, SCCA2, and SCCA–IgM;
Ethnic biases.
Thus, the diagnostic and prognostic roles of SCCA remain controversial. To reduce the clinical heterogeneity across studies and provide more accurate and reliable comparability, a standardized detection method, scoring system, and cutoff level are warranted. Despite comparable performances between different methods, the same method should always be employed for the dynamic observation of marker kinetics.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kato H., Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621–1628. doi: 10.1002/1097-0142(197710)40:4<1621::AID-CNCR2820400435>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y., Sheshadri N., Zong W.X. SERPINB3 and B4: From biochemistry to biology. Semin. Cell Dev. Biol. 2017;62:170–177. doi: 10.1016/j.semcdb.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato H., Nagaya T., Torigoe T. Heterogeneity of a tumor antigen TA-4 of squamous cell carcinoma in relation to its appearance in the circulation. Gan. 1984;75:433–435. [PubMed] [Google Scholar]
- 4.Stelzer G., Rosen N., Plaschkes I., Zimmerman S., Twik M., Fishilevich S., Stein T.I., Nudel R., Lieder I., Mazor Y., et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016;54:1.30.1–1.30.33. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 5.Pontisso P., Calabrese F., Benvegnu L., Lise M., Belluco C., Ruvoletto M.G., Marino M., Valente M., Nitti D., Gatta A., et al. Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. Br. J. Cancer. 2004;90:833–837. doi: 10.1038/sj.bjc.6601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S., Gao Y., Yang B., Liang Z., Wang Y., Zhai D., Jing L., Liu T., Wang F., Du Z., et al. Squamous cell carcinoma antigen 1 and 2 mRNA and a new variant expressed in hepatocellular carcinoma. Neoplasma. 2014;61:718–723. doi: 10.4149/neo_2014_087. [DOI] [PubMed] [Google Scholar]
- 7.Yang D., Wang J., Zhang L. Progress in Molecular Biology and Translational Science. Elsevier; Amsterdam, The Netherlands: 2019. Serum SCCA levels in patients suffering cancers or other diseases; pp. 165–175. [DOI] [PubMed] [Google Scholar]
- 8.Chen H., Tian L., Chen J., Sun P., Han R., Wu X., Dai S. Evaluation of 2 commercially systems for detection of serum squamous cell carcinoma antigen in pan squamous cell carcinoma. Cancer Control. 2020;27:1–9. doi: 10.1177/1073274820983025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesimer M., Scull M., Brighton B., DeMaria G., Burns K., O’Neal W., Pickles R.J., Sheehan J.K. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: A possible role in innate defense. FASEB J. 2009;23:1858–1868. doi: 10.1096/fj.08-119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quarta S., Vidalino L., Turato C., Ruvoletto M., Calabrese F., Valente M., Cannito S., Fassina G., Parola M., Gatta A., et al. SERPINB3 induces epithelial-mesenchymal transition. J. Pathol. 2010;221:343–356. doi: 10.1002/path.2708. [DOI] [PubMed] [Google Scholar]
- 11.Petty R.D., Kerr K.M., Murray G.I., Nicolson M.C., Rooney P.H., Bissett D., Collie-Duguid E.S. Tumor transcriptome reveals the predictive and prognostic impact of lysosomal protease inhibitors in non-small-cell lung cancer. J. Clin. Oncol. 2006;24:1729–1744. doi: 10.1200/JCO.2005.03.3399. [DOI] [PubMed] [Google Scholar]
- 12.Lin W.H., Chen I.H., Wei F.C., Huang J.J., Kang C.J., Hsieh L.L., Wang H.M., Huang S.F. Clinical significance of preoperative squamous cell carcinoma antigen in oral-cavity squamous cell carcinoma. Laryngoscope. 2011;121:971–977. doi: 10.1002/lary.21721. [DOI] [PubMed] [Google Scholar]
- 13.Imai R., Takenaka Y., Yasui T., Nakahara S., Yamamoto Y., Hanamoto A., Takemoto N., Fukusumi T., Cho H., Yamamoto M., et al. Prognostic significance of serum squamous cell carcinoma antigen in patients with head and neck cancer. Acta Otolaryngol. 2015;135:295–301. doi: 10.3109/00016489.2014.951454. [DOI] [PubMed] [Google Scholar]
- 14.Derakhshan S., Poosti A., Razavi A.E., Moosavi M.A., Mahdavi N., Naieni F.B., Hesari K.K., Rahpeima A. Evaluation of squamous cell carcinoma antigen 1 expression in oral squamous cell carcinoma (tumor cells and peritumoral T-lymphocytes) and verrucous carcinoma and comparison with normal oral mucosa. J. Appl. Oral Sci. 2021;29:e20210374. doi: 10.1590/1678-7757-2021-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crombach G., Scharl A., Vierbuchen M., Wurz H., Bolte A. Detection of squamous cell carcinoma antigen in normal squamous epithelia and in squamous cell carcinomas of the uterine cervix. Cancer. 1989;63:1337–1342. doi: 10.1002/1097-0142(19890401)63:7<1337::AID-CNCR2820630719>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Herman D.S., Ranjitkar P., Yamaguchi D., Grenache D.G., Greene D.N. Endogenous alkaline phosphatase interference in cardiac troponin I and other sensitive chemiluminescence immunoassays that use alkaline phosphatase activity for signal amplification. Clin. Biochem. 2016;49:1118–1121. doi: 10.1016/j.clinbiochem.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Mori E., Kurano M., Tobita A., Shimosaka H., Yatomi Y. Existence of a squamous cell carcinoma antigen-immunoglobulin complex causes a deviation between squamous cell carcinoma antigen concentrations determined using two different immunoassays: First report of squamous cell carcinoma antigen coupling with immunoglobulin A. Ann. Clin. Biochem. 2017;54:655–663. doi: 10.1177/0004563216677584. [DOI] [PubMed] [Google Scholar]
- 18.Holdenrieder S., Molina R., Qiu L., Zhi X., Rutz S., Engel C., Kasper-Sauer P., Dayyani F., Korse C.M. Technical and clinical performance of a new assay to detect squamous cell carcinoma antigen levels for the differential diagnosis of cervical, lung, and head and neck cancer. Tumor Biol. 2018;40:1–13. doi: 10.1177/1010428318772202. [DOI] [PubMed] [Google Scholar]
- 19.Ohta S., Shibata R., Nakao Y., Azuma Y., Taniguchi K., Arima K., Suzuki S., Shiraishi H., Iwasaka T., Izuhara K. The usefulness of combined measurements of squamous cell carcinoma antigens 1 and 2 in diagnosing atopic dermatitis. Ann. Clin. Biochem. 2012;49:277–284. doi: 10.1258/acb.2011.011065. [DOI] [PubMed] [Google Scholar]
- 20.Lee S., Kim J., Uh Y., Kim H.S., Lee J.H. Comparison Between a Manual Squamous Cell Carcinoma Antigen Assay and an Automated Assay in a Clinical Setting. Lab. Med. 2018;49:254–258. doi: 10.1093/labmed/lmy003. [DOI] [PubMed] [Google Scholar]
- 21.Beneduce L., Castaldi F., Marino M., Quarta S., Ruvoletto M., Benvegnu L., Calabrese F., Gatta A., Pontisso P., Fassina G. Squamous cell carcinoma antigen-immunoglobulin M complexes as novel biomarkers for hepatocellular carcinoma. Cancer. 2005;103:2558–2565. doi: 10.1002/cncr.21106. [DOI] [PubMed] [Google Scholar]
- 22.Mirpour S., Mhlanga J.C., Logeswaran P., Russo G., Mercier G., Subramaniam R.M. The role of PET/CT in the management of cervical cancer. AJR Am. J. Roentgenol. 2013;201:W192–W205. doi: 10.2214/AJR.12.9830. [DOI] [PubMed] [Google Scholar]
- 23.Mittra E., El-Maghraby T., Rodriguez C.A., Quon A., McDougall I.R., Gambhir S.S., Iagaru A. Efficacy of 18F-FDG PET/CT in the evaluation of patients with recurrent cervical carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:1952–1959. doi: 10.1007/s00259-009-1206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y.Y., Fan W., Zhang X., Liang P.Y., Lin X.P., Zhang Y.R., Li Y.H. Complementary roles of squamous cell carcinoma antigen and (18)F-FDG PET/CT in suspected recurrence of cervical squamous cell cancer. J. Cancer. 2015;6:287–291. doi: 10.7150/jca.10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi C., He S., Cai L., Zhang L., Ding H., Chen Y. A study on the clinical value of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography combined with serum squamous cell carcinoma antigen in diagnosing recurrence/metastases in patients with early metaphase cervical cancer. Oncol. Lett. 2021;22:746. doi: 10.3892/ol.2021.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu H.K., Baek J.S., Kang W.D., Kim S.M. The prognostic value of squamous cell carcinoma antigen for predicting tumor recurrence in cervical squamous cell carcinoma patients. Obstet. Gynecol. Sci. 2015;58:368–376. doi: 10.5468/ogs.2015.58.5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi K.H., Yu M., Jeong S., Lee J.H. Can serial evaluation of serum SCC-Ag-level predict tumor recurrence and patient survival in squamous-cell carcinoma of uterine cervix treated with definitive chemoradiotherapy? A multi-institutional analysis. Int. J. Clin. Oncol. 2020;25:1405–1411. doi: 10.1007/s10147-020-01664-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhu C., Zhang W., Wang X., Jiao L., Chen L., Jiang J. Predictive value of preoperative serum squamous cell carcinoma antigen level for lymph node metastasis in early-stage cervical squamous cell carcinoma. Medicine. 2021;100:e26960. doi: 10.1097/MD.0000000000026960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lekskul N., Charakorn C., Lertkhachonsuk A.A., Rattanasiri S., Israngura Na Ayudhya N. The level of squamous cell carcinoma antigen and lymph node metastasis in locally advanced cervical cancer. Asian Pac. J. Cancer Prev. 2015;16:4719–4722. doi: 10.7314/APJCP.2015.16.11.4719. [DOI] [PubMed] [Google Scholar]
- 30.Chen P., Jiao L., Ren F., Wang D.B. Clinical value of serum squamous cell carcinoma antigen levels in predicting chemosensitivity, lymph node metastasis, and prognosis in patients with cervical squamous cell carcinoma. BMC Cancer. 2020;20:423. doi: 10.1186/s12885-020-06934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu J., Wang W., Wang Y., Liu C., Wang P. The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiat. Oncol. 2019;14:146. doi: 10.1186/s13014-019-1355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W., Liu X., Hou X., Lian X., Liu Z., Shen J., Sun S., Yan J., Miao Z., Wang D., et al. Posttreatment squamous cell carcinoma antigen predicts treatment failure in patients with cervical squamous cell carcinoma treated with concurrent chemoradiotherapy. Gynecol. Oncol. 2019;155:224–228. doi: 10.1016/j.ygyno.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Salvatici M., Achilarre M.T., Sandri M.T., Boveri S., Vanna Z., Landoni F. Squamous cell carcinoma antigen (SCC-Ag) during follow-up of cervical cancer patients: Role in the early diagnosis of recurrence. Gynecol. Oncol. 2016;142:115–119. doi: 10.1016/j.ygyno.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Ye S., Sun X., Kang B., Wu F., Zheng Z., Xiang L., Lesenechal M., Heskia F., Liang J., Yang H. The kinetic profile and clinical implication of SCC-Ag in squamous cervical cancer patients undergoing radical hysterectomy using the Simoa assay: A prospective observational study. BMC Cancer. 2020;20:138. doi: 10.1186/s12885-020-6630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.H., Lee S.W., Kim J.R., Kim Y.S., Yoon M.S., Jeong S., Kim J.H., Lee J.Y., Eom K.Y., Jeong B.K., et al. Tumour size, volume, and marker expression during radiation therapy can predict survival of cervical cancer patients: A multi-institutional retrospective analysis of KROG 16-01. Gynecol. Oncol. 2017;147:577–584. doi: 10.1016/j.ygyno.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 36.Chang C., Chen J., Huang C.H., Lee W.Y., Hsu L.C., Chiang A.J. Time-dependent squamous cell carcinoma antigen in prediction of relapse and death of patients with cervical cancer. J. Low. Genit. Tract Dis. 2020;24:38–42. doi: 10.1097/LGT.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 37.Reesink-Peters N., van der Velden J., Ten Hoor K.A., Boezen H.M., de Vries E.G., Schilthuis M.S., Mourits M.J., Nijman H.W., Aalders J.G., Hollema H., et al. Preoperative serum squamous cell carcinoma antigen levels in clinical decision making for patients with early-stage cervical cancer. J. Clin. Oncol. 2005;23:1455–1462. doi: 10.1200/JCO.2005.02.123. [DOI] [PubMed] [Google Scholar]
- 38.Guo H.T., Bi X.H., Lei T., Lv X., Yao G., Chen Y., Liu C. Preoperative SCC-Ag as a predictive marker for the use of adjuvant chemotherapy in cervical squamous cell carcinoma with intermediate-risk factors. BMC Cancer. 2020;20:441. doi: 10.1186/s12885-020-06928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Z., Cao D., Zhang Y., Shen K., Yang J., Yu M., Zhou H. Could adjuvant chemotherapy improve prognosis for cervical cancer patients with elevated pretreatment serum squamous-cell carcinoma antigen? Risk Manag. Healthc Policy. 2021;14:109–116. doi: 10.2147/RMHP.S273848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oike T., Oike T., Ando K., Iwase A., Ohno T. The Non-Cancer Specific Elevation of the Serum Squamous Cell Carcinoma Antigen during the Post-Radiotherapy Follow-Up of Cervical Cancer Patients. Diagnostics. 2021;11:1585. doi: 10.3390/diagnostics11091585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kagohashi K., Satoh H., Ishikawa H., Ohtsuka M., Sekizawa K. A re-evaluation of squamous cell carcinoma antigen (SCC) as a serum marker for non-small cell lung cancer. Med. Oncol. 2008;25:187–189. doi: 10.1007/s12032-007-9021-3. [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita T., Ohtsuka T., Hato T., Goto T., Kamiyama I., Tajima A., Emoto K., Hayashi Y., Kohno M. Prognostic factors based on clinicopathological data among the patients with resected peripheral squamous cell carcinomas of the lung. J. Thorac. Oncol. 2014;9:1779–1787. doi: 10.1097/JTO.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 43.Urquhart G., Kerr K.M., Nicolson M., Loo P.S., Sharma R., Shrimali R., Petty R.D. Serpin b3 is associated with poor survival after chemotherapy and is a potential novel predictive biomarker in advanced non-small-cell lung cancer. J. Thorac. Oncol. 2013;8:1502–1509. doi: 10.1097/JTO.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 44.Kagohashi K., Satoh H., Kurishima K., Kadono K., Ishikawa H., Ohtsuka M., Sekizawa K. Squamous cell carcinoma antigen in lung cancer and nonmalignant respiratory diseases. Lung. 2008;186:323–326. doi: 10.1007/s00408-008-9108-4. [DOI] [PubMed] [Google Scholar]
- 45.Zanatta E., Martini A., Scarpieri E., Biasiolo A., Ortolan A., Benvenuti F., Cozzi F., Pontisso P., Doria A. Squamous cell carcinoma antigen-IgM (SCCA-IgM) is associated with interstitial lung disease in systemic sclerosis. Jt. Bone Spine. 2020;87:331–335. doi: 10.1016/j.jbspin.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Yasumatsu R., Nakano T., Hashimoto K., Kogo R., Wakasaki T., Nakagawa T. The clinical value of serum squamous cell carcinoma antigens 1 and 2 in head and neck squamous cell carcinoma. Auris Nasus Larynx. 2019;46:135–140. doi: 10.1016/j.anl.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Wu J., Guo Q., Zhang G., Zhao L., Lv Y., Wang J., Liu J., Shi W. Study on the targeted therapy of oral squamous cell carcinoma with a plasmid expressing PE38KDEL toxin under control of the SERPINB3 promoter. Cancer Med. 2020;9:2213–2222. doi: 10.1002/cam4.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Travassos D.C., Fernandes D., Massucato E.M.S., Navarro C.M., Bufalino A. Squamous cell carcinoma antigen as a prognostic marker and its correlation with clinicopathological features in head and neck squamous cell carcinoma: Systematic review and meta-analysis. J. Oral Pathol. Med. 2018;47:3–10. doi: 10.1111/jop.12600. [DOI] [PubMed] [Google Scholar]
- 49.van Schaik J.E., Muller Kobold A.C., van der Laan B., van der Vegt B., van Hemel B.M., Plaat B.E.C. Squamous cell carcinoma antigen concentration in fine needle aspiration samples: A new method to detect cervical lymph node metastases of head and neck squamous cell carcinoma. Head Neck. 2019;41:2561–2565. doi: 10.1002/hed.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita Y., Uehara T., Hasegawa M., Deng Z., Matayoshi S., Kiyuna A., Kondo S., Maeda H., Ganaha A., Suzuki M. Squamous cell carcinoma antigen as a diagnostic marker of nasal inverted papilloma. Am. J. Rhinol. Allergy. 2016;30:122–127. doi: 10.2500/ajra.2016.30.4287. [DOI] [PubMed] [Google Scholar]
- 51.Promsopa C., Suwansri S., Khuntikij P. The serum squamous cell carcinoma antigen level in inverted sinonasal papilloma and nasal polyps patients. World J. Otorhinolaryngol. Head Neck Surg. 2021;7:23–27. doi: 10.1016/j.wjorl.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasumatsu R., Nakano T., Sato M., Jiroumaru R., Hashimoto K., Kogo R., Wakasaki T., Nakashima T., Nakagawa T. Combination of serum squamous cell carcinoma antigens 1 and 2 as potential diagnostic marker for sinonasal squamous cell carcinoma and inverted papilloma. Head Neck. 2018;40:2583–2589. doi: 10.1002/hed.25351. [DOI] [PubMed] [Google Scholar]
- 53.van Zijl F., Monserez D.A., Korevaar T.I.M., Bugter O., Wieringa M.H., Baatenburg de Jong R.J., Hardillo J.A.U. Postoperative value of serum squamous cell carcinoma antigen as a predictor of recurrence in sinonasal inverted papilloma. Clin. Otolaryngol. 2017;42:528–535. doi: 10.1111/coa.12757. [DOI] [PubMed] [Google Scholar]
- 54.Turato C., Scarpa M., Kotsafti A., Cappon A., Quarta S., Biasiolo A., Cavallin F., Trevellin E., Guzzardo V., Fassan M., et al. Squamous cell carcinoma antigen 1 is associated to poor prognosis in esophageal cancer through immune surveillance impairment and reduced chemosensitivity. Cancer Sci. 2019;110:1552–1563. doi: 10.1111/cas.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montagnana M., Danese E., Lippi G. Squamous cell carcinoma antigen in hepatocellular carcinoma: Ready for the prime time? Clin. Chim. Acta. 2015;445:161–166. doi: 10.1016/j.cca.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 56.Trerotoli P., Fransvea E., Angelotti U., Antonaci G., Lupo L., Mazzocca A., Mangia A., Antonaci S., Giannelli G. Tissue expression of Squamous Cellular Carcinoma Antigen (SCCA) is inversely correlated to tumor size in HCC. Mol. Cancer. 2009;8:29. doi: 10.1186/1476-4598-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giannelli G., Fransvea E., Trerotoli P., Beaugrand M., Marinosci F., Lupo L., Nkontchou G., Dentico P., Antonaci S. Clinical validation of combined serological biomarkers for improved hepatocellular carcinoma diagnosis in 961 patients. Clin. Chim. Acta. 2007;383:147–152. doi: 10.1016/j.cca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Yu J., Wang Z.J., Chen L.H., Dong W.Z. Diagnostic value of serum squamous cell carcinoma antigen for hepatocellular carcinoma: A systematic review and meta-analysis. Scand. J. Clin. Lab. Investig. 2017;77:8–14. doi: 10.1080/00365513.2016.1238504. [DOI] [PubMed] [Google Scholar]
- 59.Bui Huu H., Ha Thuc N., Thi Le H.P., Thi Thanh T.D., Luong Bac A., Tiribelli C., Pontisso P., Gallotta A., Paneghetti L., Fassina G. Characterization of SCCA-IgM as a biomarker of liver disease in an Asian cohort of patients. Scand. J. Clin. Lab. Investig. 2018;78:204–210. doi: 10.1080/00365513.2018.1432072. [DOI] [PubMed] [Google Scholar]
- 60.Guarino M., Di Costanzo G.G., Gallotta A., Tortora R., Paneghetti L., Auriemma F., Tuccillo C., Fassina G., Caporaso N., Morisco F. Circulating SCCA-IgM complex is a useful biomarker to predict the outcome of therapy in hepatocellular carcinoma patients. Scand. J. Clin. Lab. Investig. 2017;77:448–453. doi: 10.1080/00365513.2017.1336569. [DOI] [PubMed] [Google Scholar]
- 61.Martini A., Fattovich G., Guido M., Bugianesi E., Biasiolo A., Ieluzzi D., Gallotta A., Fassina G., Merkel C., Gatta A., et al. HCV genotype 3 and squamous cell carcinoma antigen (SCCA)-IgM are independently associated with histological features of NASH in HCV-infected patients. J. Viral Hepat. 2015;22:800–808. doi: 10.1111/jvh.12394. [DOI] [PubMed] [Google Scholar]
- 62.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 63.Bettini S., Bordigato E., Milan G., Dal Pra C., Favaretto F., Belligoli A., Sanna M., Serra R., Foletto M., Prevedello L., et al. SCCA-IgM as a potential biomarker of non-alcoholic fatty liver disease in patients with obesity, prediabetes and diabetes undergoing sleeve gastrectomy. Obes. Facts. 2019;12:291–306. doi: 10.1159/000499717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biasiolo A., Trotta E., Fasolato S., Ruvoletto M., Martini A., Gallotta A., Fassina G., Angeli P., Gatta A., Pontisso P. Squamous cell carcinoma antigen-IgM is associated with hepatocellular carcinoma in patients with cirrhosis: A prospective study. Dig. Liver Dis. 2016;48:197–202. doi: 10.1016/j.dld.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 65.Sun Z., Shi X., Wang Y., Zhao Y. Serum squamous cell carcinoma antigen in psoriasis: A potential quantitative biomarker for disease severity. Dermatology. 2018;234:120–126. doi: 10.1159/000488672. [DOI] [PubMed] [Google Scholar]
- 66.Khattab F.M., Samir M.A. Measurement of squamous cell carcinoma antigen 2 in lichen planus patients. J. Cosmet. Dermatol. 2020;19:1780–1784. doi: 10.1111/jocd.13216. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe Y., Yamaguchi Y., Komitsu N., Ohta S., Azuma Y., Izuhara K., Aihara M. Elevation of serum squamous cell carcinoma antigen 2 in patients with psoriasis: Associations with disease severity and response to the treatment. Br. J. Dermatol. 2016;174:1327–1336. doi: 10.1111/bjd.14426. [DOI] [PubMed] [Google Scholar]
- 68.Takeuchi S., Furusyo N., Ono J., Azuma Y., Takemura M., Esaki H., Yamamura K., Mitamura Y., Tsuji G., Kiyomatsu-Oda M., et al. Serum squamous cell carcinoma antigen (SCCA)-2 correlates with clinical severity of pediatric atopic dermatitis in Ishigaki cohort. J. Dermatol. Sci. 2019;95:70–75. doi: 10.1016/j.jdermsci.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Marchioni M., Berardinelli F., De Nunzio C., Spiess P., Porpiglia F., Schips L., Cindolo L. New insight in penile cancer. Minerva Urol. Nefrol. 2018;70:559–569. doi: 10.23736/S0393-2249.18.03215-0. [DOI] [PubMed] [Google Scholar]
- 70.Kommu S., Hadway P., Watkin N. Squamous cell carcinoma antigen as a biomarker for penile cancer. BJU Int. 2005;95:478–479. doi: 10.1111/j.1464-410X.2005.05378.x. [DOI] [PubMed] [Google Scholar]
- 71.Touloupidis S., Zisimopoulos A., Giannakopoulos S., Papatsoris A.G., Kalaitzis C., Thanos A. Clinical usage of the squamous cell carcinoma antigen in patients with penile cancer. Int. J. Urol. 2007;14:174–176. doi: 10.1111/j.1442-2042.2007.01694.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Y., Ye D.W., Yao X.D., Zhang S.L., Dai B., Zhang H.L., Shen Y.J. The value of squamous cell carcinoma antigen in the prognostic evaluation, treatment monitoring and followup of patients with penile cancer. J. Urol. 2008;180:2019–2023. doi: 10.1016/j.juro.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 73.Zargar-Shoshtari K., Sharma P., Spiess P.E. Insight into novel biomarkers in penile cancer: Redefining the present and future treatment paradigm? Urol. Oncol. 2018;36:433–439. doi: 10.1016/j.urolonc.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Yamazaki K., Kumamoto Y., Tsukamoto T. Expression of squamous cell carcinoma-associated antigen in bladder cancer cells—A comparative study with the expression of proliferating cell nuclear antigen (PCNA) Nihon Hinyokika Gakkai Zasshi. 1994;85:589–598. doi: 10.5980/jpnjurol1989.85.589. [DOI] [PubMed] [Google Scholar]
- 75.Hoshi S., Numahata K., Morozumi K., Katumata Y., Kuromoto A., Takai Y., Hoshi K., Bilim V., Sasagawa I. Bladder cancer metastasis producing beta-human chorionic gonadotropin, squamous cell carcinoma antigen, granulocyte-colony stimulating factor, and parathyroid hormone-related protein. IJU Case Rep. 2018;14:47–50. doi: 10.1002/iju5.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muniz T.P., Sorotsky H., Kanjanapan Y., Rose A.A.N., Araujo D.V., Fortuna A., Ghazarian D., Kamil Z.S., Pugh T., Mah M., et al. Genomic landscape of malignant peripheral nerve sheath tumor–like melanoma. J. Investig. Dermatol. 2021;141:2470–2479. doi: 10.1016/j.jid.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Guo S., Yang B., Liu H., Li Y., Li S., Ma L., Liu J., Guo W. Serum expression level of squamous cell carcinoma antigen, highly sensitive C-reactive protein, and CA-125 as potential biomarkers for recurrence of cervical cancer. J. Cancer Res. Ther. 2017;13:689–692. doi: 10.4103/jcrt.JCRT_414_17. [DOI] [PubMed] [Google Scholar]
- 78.Adel M., Tsao C.K., Wei F.C., Chien H.T., Lai C.H., Liao C.T., Wang H.M., Fan K.H., Kang C.J., Chang J.T., et al. Preoperative SCC antigen, CRP serum levels, and lymph node density in oral squamous cell carcinoma. Medicine. 2016;95:e3149. doi: 10.1097/MD.0000000000003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Z.S., Yao K., Li Y.H., Chen J.P., Deng C.Z., Zhao Q., Chen P., Wang B., Mi Q.W., Liu Z.W., et al. Clinical significance of preoperative C-reactive protein and squamous cell carcinoma antigen levels in patients with penile squamous cell carcinoma. BJU Int. 2016;118:272–278. doi: 10.1111/bju.13379. [DOI] [PubMed] [Google Scholar]
- 80.Wu L.L., Liu X., Huang W., Lin P., Long H., Zhang L.J., Ma G.W. Preoperative squamous cell carcinoma antigen and albumin serum levels predict the survival of patients with stage T1-3N0M0 esophageal squamous cell carcinoma: A retrospective observational study. J. Cardiothorac. Surg. 2020;15:115. doi: 10.1186/s13019-020-01163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ali L.H., Higazi A.M., Moness H.M., Farag N.M., Saad Z.M., Moukareb H.A., Soliman W., El Sagheer G., Abd El Hamid S.R., Abdl Hamid H. Clinical significances and diagnostic utilities of both miR-215 and squamous cell carcinoma antigen-IgM versus alpha-fetoprotein in Egyptian patients with hepatitis C virus-induced hepatocellular carcinoma. Clin. Exp. Gastroenterol. 2019;12:51–66. doi: 10.2147/CEG.S179832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wen Y.F., Cheng T.T., Chen X.L., Huang W.J., Peng H.H., Zhou T.C., Lin X.D., Zeng L.S. Elevated circulating tumor cells and squamous cell carcinoma antigen levels predict poor survival for patients with locally advanced cervical cancer treated with radiotherapy. PLoS ONE. 2018;13:e0204334. doi: 10.1371/journal.pone.0204334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hashimoto K., Kiyoshima T., Matsuo K., Ozeki S., Sakai H. Effect of SCCA1 and SCCA2 on the suppression of TNF-alpha-induced cell death by impeding the release of mitochondrial cytochrome c in an oral squamous cell carcinoma cell line. Tumor Biol. 2005;26:165–172. doi: 10.1159/000086949. [DOI] [PubMed] [Google Scholar]
- 84.Yuan L., Zhai L., Qian L., Huang D., Ding Y., Xiang H., Liu X., Thompson J.W., Liu J., He Y.H., et al. Switching off IMMP2L signaling drives senescence via simultaneous metabolic alteration and blockage of cell death. Cell Res. 2018;28:625–643. doi: 10.1038/s41422-018-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Catanzaro J.M., Sheshadri N., Pan J.A., Sun Y., Shi C., Li J., Powers R.S., Crawford H.C., Zong W.X. Oncogenic Ras induces inflammatory cytokine production by upregulating the squamous cell carcinoma antigens SerpinB3/B4. Nat. Commun. 2014;5:3729. doi: 10.1038/ncomms4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sheshadri N., Catanzaro J.M., Bott A.J., Sun Y., Ullman E., Chen E.I., Pan J.A., Wu S., Crawford H.C., Zhang J., et al. SCCA1/SERPINB3 promotes oncogenesis and epithelial-mesenchymal transition via the unfolded protein response and IL6 signaling. Cancer Res. 2014;74:6318–6329. doi: 10.1158/0008-5472.CAN-14-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turato C., Buendia M.A., Fabre M., Redon M.J., Branchereau S., Quarta S., Ruvoletto M., Perilongo G., Grotzer M.A., Gatta A., et al. Over-expression of SERPINB3 in hepatoblastoma: A possible insight into the genesis of this tumour? Eur. J. Cancer. 2012;48:1219–1226. doi: 10.1016/j.ejca.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Turato C., Vitale A., Fasolato S., Ruvoletto M., Terrin L., Quarta S., Ramirez Morales R., Biasiolo A., Zanus G., Zali N., et al. SERPINB3 is associated with TGF-beta1 and cytoplasmic beta-catenin expression in hepatocellular carcinomas with poor prognosis. Br. J. Cancer. 2014;110:2708–2715. doi: 10.1038/bjc.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shamji M.H., Temblay J.N., Cheng W., Byrne S.M., Macfarlane E., Switzer A.R., Francisco N.D.C., Olexandra F., Jacubczik F., Durham S.R., et al. Antiapoptotic serine protease inhibitors contribute to survival of allergenic TH2 cells. J. Allergy Clin. Immunol. 2018;142:569–581.e565. doi: 10.1016/j.jaci.2017.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sivaprasad U., Kinker K.G., Ericksen M.B., Lindsey M., Gibson A.M., Bass S.A., Hershey N.S., Deng J., Medvedovic M., Khurana Hershey G.K. SERPINB3/B4 contributes to early inflammation and barrier dysfunction in an experimental murine model of atopic dermatitis. J. Investig. Dermatol. 2015;135:160–169. doi: 10.1038/jid.2014.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vidalino L., Doria A., Quarta S., Zen M., Gatta A., Pontisso P. SERPINB3, apoptosis and autoimmunity. Autoimmun. Rev. 2009;9:108–112. doi: 10.1016/j.autrev.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 92.Riaz N., Havel J.J., Kendall S.M., Makarov V., Walsh L.A., Desrichard A., Weinhold N., Chan T.A. Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat. Genet. 2016;48:1327–1329. doi: 10.1038/ng.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iversen O.J., Lysvand H., Slupphaug G. Pso p27, a SERPINB3/B4-derived protein, is most likely a common autoantigen in chronic inflammatory diseases. Clin. Immunol. 2017;174:10–17. doi: 10.1016/j.clim.2016.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.