Abstract
Several fungal laccases have been compared for the oxidation of a nonphenolic lignin dimer, 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)propan-1,3-diol (I), and a phenolic lignin model compound, phenol red, in the presence of the redox mediators 1-hydroxybenzotriazole (1-HBT) or violuric acid. The oxidation rates of dimer I by the laccases were in the following order: Trametes villosa laccase (TvL) > Pycnoporus cinnabarinus laccase (PcL) > Botrytis cinerea laccase (BcL) > Myceliophthora thermophila laccase (MtL) in the presence of either 1-HBT or violuric acid. The order is the same if the laccases are used at the same molar concentration or added to the same activity (with ABTS [2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)] as a substrate). During the oxidation of dimer I, both 1-HBT and violuric acid were to some extent consumed. Their consumption rates also follow the above order of laccases, i.e., TvL > PcL > BcL > MtL. Violuric acid allowed TvL and PcL to oxidize dimer I much faster than 1-HBT, while BcL and violuric acid oxidized dimer I more slowly than BcL and 1-HBT. The oxidation rate of dimer I is dependent upon both kcat and the stability of the laccase. Both 1-HBT and violuric acid inactivated the laccases, violuric acid to a greater extent than 1-HBT. The presence of dimer I or phenol red in the reaction mixture slowed down this inactivation. The inactivation is mainly due to the reaction of the redox mediator free radical with the laccases. We did not find any relationship between the carbohydrate content of the laccases and their inactivation. When the redox potential of the laccases is in the range of 750 to 800 mV, i.e., above that of the redox mediator, it does not affect kcat and the oxidation rate of dimer I.
Conventional pulp-bleaching techniques with chlorine or chlorine-based chemicals can, under certain conditions, generate chlorinated organic compounds that are toxic to the environment. The pulp and paper industry is facing an increasing pressure from environmentally concerned organizations to replace the conventional bleaching techniques with environmentally benign ones. Enzymatic bleaching methods have recently drawn much attention as being environmentally friendly. In addition to xylanase, laccase has been the most actively investigated enzyme for biobleaching of kraft pulp because laccase can be produced in large amounts at a reasonable price and use cheap oxygen as an electron acceptor. However, expensive redox mediators are still a hurdle in the implementation of laccase in pulp bleaching.
Laccase (EC 1.10.3.1) belongs to a family of multi-copper oxidases that are widespread in numerous fungi, in various plant species (18), in the bacterium Azospirillum lipoferum (10), and in a dozen of studied insects (25). Laccase has various functions, including participation in lignin biosynthesis (21), plant pathogenicity (22), the degradation of plant cell walls (12, 17), insect sclerotization (3), bacterial melanization (10), and melanin-related virulence for humans (26). Chemically, all of these functions of laccases are related to oxidation of a range of aromatic substances. However, the net effect of such oxidations could be very different and even work in opposite directions. Plant laccases, for example, oxidize monolignols to form polymeric lignins, whereas laccases from white-rot fungi degrade and depolymerize lignins.
In the degradation of lignin by white-rot fungi, the redox potential of the lignin-degrading enzymes has long been believed to play a crucial role because nonphenolic subunits, the most predominant lignin substructures in wood, have high redox potentials. The well-studied lignin peroxidase is able to oxidize nonphenolic aromatic compounds with very high ionization potentials such as 1,2-dimethoxybenzene (E1/2 = 1,500 mV) and veratryl alcohol (14, 20). Lignin peroxidase was thus once believed to be a key enzyme for fungal degradation of lignin, whereas laccase was believed to be less important because it could not oxidize veratryl alcohol (a typical model compound for nonphenolic lignin). The highest redox potential of a laccase reported so far does not exceed 800 mV, which is believed not to be high enough to oxidize a nonphenolic lignin structure. However, it has been demonstrated that laccase is able to oxidize some compounds (redox mediators) with a higher redox potential than laccase itself, although the mechanism by which this happens is not known (2, 7). In the presence of such redox mediators, laccase is also able to oxidize nonphenolic lignin model compounds and decrease pulp kappa number to a great extent (5, 8). Several effective redox mediators have been reported so far (2, 5, 6, 8, 13). The importance of the redox potential of laccases in the oxidation of lignin model compounds by laccase/mediator systems will be addressed here.
While much effort has been devoted to search for more effective redox mediators, the laccase parameters governing lignin degradation and pulp bleaching are still not fully elucidated. In an effort to determine these parameters, we compared the ability of different laccases for the oxidation of lignin model compounds in a laccase-mediator system. More specifically, four laccases from different fungal species were purified and used to oxidize the β-O-4 dimer I (the most predominant lignin substructure) and phenol red (a phenolic lignin model compound). Laccases from the different sources were found to oxidize dimer I and phenol red at different rates. Criteria for a better laccase and more effective laccase-mediator systems for pulp bleaching have been suggested.
MATERIALS AND METHODS
Chemicals.
1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)propan-1,3-diol (dimer I) and 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)-3-hydroxy-1-propanone (dimer II) were prepared according to established procedures (1). All other chemicals, such as 1-hydroxybenzotriazole (1-HBT), violuric acid, and phenol red were purchased from commercial sources.
Enzyme preparations.
Recombinant Trametes villosa laccase isoform-1 (TvL) and Myceliophthora thermophila laccase (MtL) were purified from crude laccases kindly provided by Novo Nordisk as reported previously (4, 29). Production and purification of Pycnoporus cinnabarinus laccase (PcL) and Botrytis cinerea laccase (BcL) were based on the reported procedures (9, 24).
Determination of the molar concentration of laccases.
The copper content of purified laccases was determined by the Chemical Analysis Laboratory of the University of Georgia by using an inductively coupled plasma mass spectrometer. The molar concentration of laccases was calculated as one-fourth of the copper concentration.
Enzyme assay.
Laccase activity was determined by using ABTS [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid] as a substrate (500 mM) at 420 nm (Emax = 3.6 × 104 M−1 cm−1) (9). The measurements were made in 50 mM sodium tartrate buffer (pH 4.5) at 30°C. One enzyme unit was defined as 1.0 μmol of product formed per min under the assay condition.
Oxidation of dimer I.
Dimer I, 1-HBT, and violuric acid were dissolved in dimethylformamide (DMF) as stock solutions (dimer I, 32.7 mM; 1-HBT, 1.00 M; violuric acid, 0.50 M). Phenol red stock solutions (0.50 mM) was prepared by dissolving phenol red in sodium acetate buffer solution (50 mM, pH 4.5). Each reaction mixture contained 1.0 mM β-O-4 dimer I; 10 mM 1-HBT or violuric acid; 2.0 U of purified laccases per ml or 0.252 μM purified laccases; and sodium acetate buffer (50 mM, pH 4.5) in a 7-ml vial. The total volume of the reaction mixture was 2.5 ml. The reaction was carried out in a stainless steel autoclave that holds four reaction vials and was pressurized with oxygen (400 kPa). The autoclave was shaken (150 rpm, Φ 18 mm) at room temperature. Samples (90 μl) taken at different reaction times were acidified with 5% trifluoroacetic acid (10 μl) and centrifuged for 5.0 min at 7,000 × g. The supernatant was analyzed by high-performance liquid chromatography (HPLC).
The oxidation of dimer I and consumption of redox mediators were monitored by reversed-phase HPLC by using a Microsorb-MV C-18 column (Rainin Instrument Co., Inc.). The mobile phase was acetonitrile-water (35:65) containing 0.1% trifluoroacetic acid. Compounds were detected by UV absorption at 280 nm. A standard mixture of dimer I, dimer II, 1-HBT, and violuric acid was used for identification and quantification of the eluted compounds by an external standard analysis. Values reported represent the means from duplicates of two independent experiments with a maximal sample mean deviation of ±3%.
Oxidation of phenol red.
Oxidation of phenol red was determined by the decrease in absorbance at 432.1 nm at 30°C. The reaction solution (1.0 ml) contained phenol red (75 μM), various concentrations of violuric acid, laccases (0.55 U; ABTS as a substrate), and sodium acetate buffer (50 mM, pH 4.5).
Laccase stability.
Laccases (4.0 U) (TvL, 12.6 μg; MtL, 40.4 μg; PcL, 38.6 μg; BcL, 82.8 μg) in 1,000 μl of sodium acetate buffer (50 mM, pH 4.5) were incubated in 30°C. Laccase activity was measured at different incubation times by ABTS assay. Violuric acid (10 mM), 1-HBT (10 mM), lignin dimer (I) (1.0 mM), and phenol red (0.25 mM) were used in the study of laccase stability. Values reported represent the means of values from three independent experiments, with a maximal sample mean deviation of ±5%.
Kinetic parameters of laccases with selected substrates.
The laccase-catalyzed oxidation of ABTS, syringaldazine, 1-HBT, and violuric acid, accompanied by the concomitant O2 reduction, was monitored by a Hansatech (Norfolk, United Kingdom) DW1/AD O2 cell at 20°C with air-saturated 0.3 ml of 10 mM morpholinethane sulfonic acid-NaOH (pH 5.5), 5 to 60 mM of substrates, and an adequate amount of laccase (2 to 4 μM). The stock solution of substrates (0.5 to 1.0 M) was made in DMF, and the 6% DMF in the assay solution added along with a substrate had no detectable effect on the measurement. After the voltage reading of the substrate and buffer solution in the O2 cell stabilized, laccase (a few microliters) was added through an airtight syringe into the solution to initiate the reaction and the initial output voltage changes were used to calculate the initial reaction rate (v). The [substrate] − v curves were of Michaelis type, and the apparent kinetic parameter Km was determined by fitting v and [substrate] to v = Vmax × [substrate]/(Km + [substrate]) with the Prizm program of GraphPad (San Diego, Calif.), and the apparent kcat was determined from kcat = Vmax/[laccase]. The [O2] in the air-saturated assay solution was assumed to be 0.28 mM, the same as in plain water. The standard deviation was used to estimate the range of the Km and kcat. ABTS was also used to calibrate the O2 cell.
Measurement of redox potential of laccases.
The redox potential of laccases was determined by spectrophotometric redox titration (27).
RESULTS
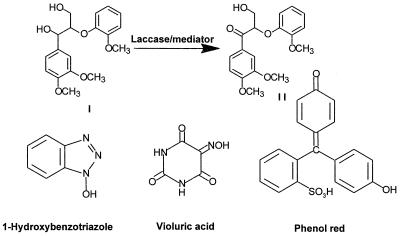
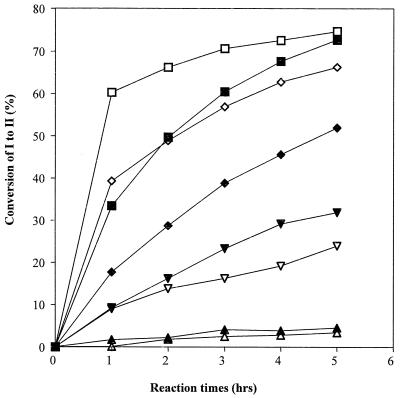
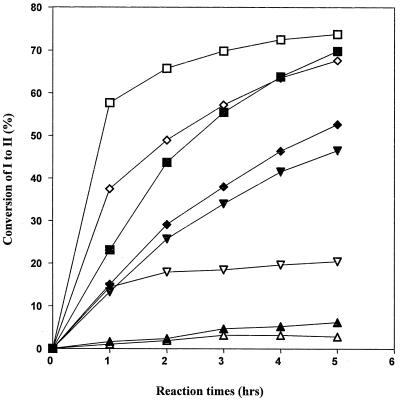
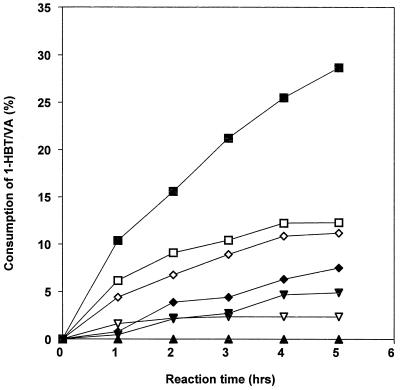
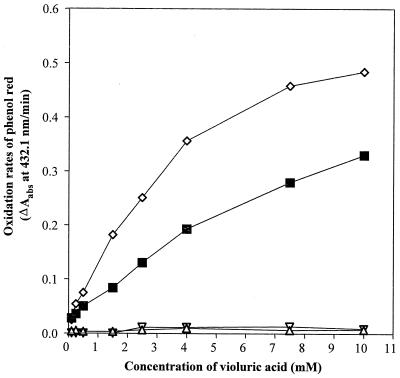
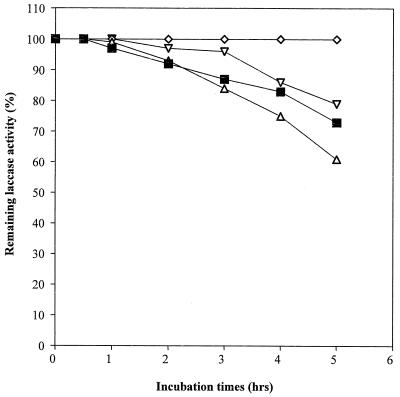
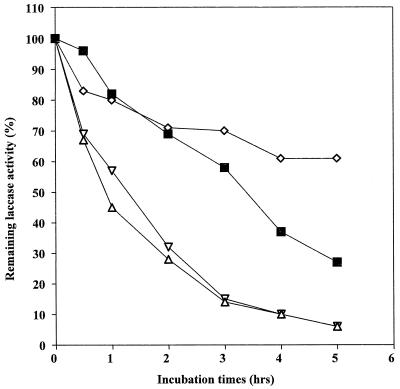
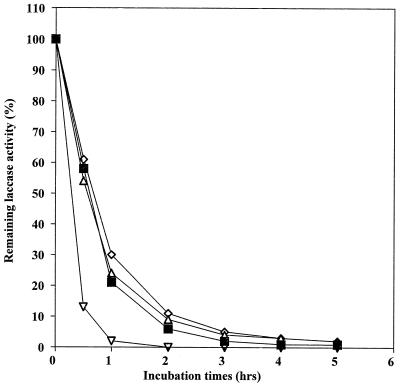
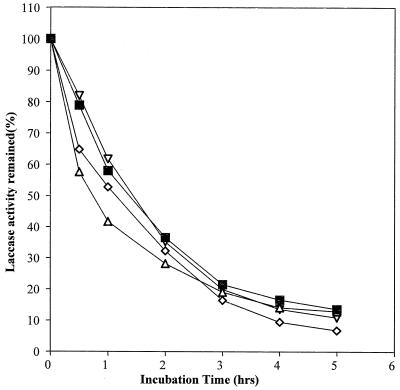
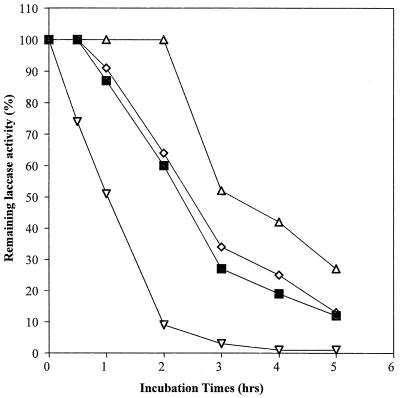
Laccases have been purified to apparent homogeneity (one band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels) from the following fungal sources: P. cinnabarinus, B. cinerea, T. villosa, and M. thermophila. The purified laccases were used to oxidize dimer I and phenol red in the presence of 1-HBT or violuric acid. The chemical structures of dimer I and its oxidation product and the structures of the redox mediators are shown in Fig. 1. Oxidation of dimer I, in the presence of either 1-HBT or violuric acid by laccases, used at the same molar concentration, is shown in Fig. 2. The oxidation rates of dimer I by the different laccases are as follows: TvL > PcL > BcL > MtL. With both TvL and PcL, violuric acid oxidizes dimer I to dimer II faster than 1-HBT, while 1-HBT allowed BcL to oxidize dimer I to dimer II faster than violuric acid. MtL oxidized dimer I very slowly in the presence of either 1-HBT or violuric acid. The same oxidation rate order of laccases remained when the same number of laccase units (ABTS as a substrate) were used (Fig. 3). Consumption of either 1-HBT or violuric acid also rank the laccases in the same order (Fig. 4). The consumption rate of 1-HBT by TvL is the highest. The oxidation of phenol red by laccase-violuric acid is shown in Fig. 5. The oxidation rate is in the following order: PcL > TvL > BcL ≈ MtL. The stability of laccases in a sodium acetate (NaOAc) buffer solution at 30°C is shown in Fig. 6. PcL is very stable in the NaOAc buffer solution. Both 1-HBT and violuric acid inactivated the laccases; violuric acid did so faster than 1-HBT (Fig. 7 and 8). With 1-HBT (Fig. 7), PcL was the most stable with TvL next. The stabilities of BcL and MtL were approximately the same. With violuric acid (Fig. 8), BcL was the least stable, while the other three laccases had almost the same stabilities. Addition of the dimer I to the solution of laccase and violuric acid stabilized the laccases to some extent (Fig. 9). Phenol red also slowed down the inactivation of laccases by violuric acid (Fig. 10). However, the stabilizing effect varied very much between the laccases and was in the following order: MtL > PcL > TvL > BcL.
FIG. 1.
Oxidation of dimer I and structures of laccase mediators and phenol red.
FIG. 2.
Oxidation of dimer I to dimer II by laccase with the same molar concentration plus violuric acid (VA) and 1-HBT. Symbols: TvL + VA (□), TvL + 1-HBT (■), PcL + VA (◊), PcL + 1-HBT (⧫), BcL + VA (▿), BcL + 1-HBT (▾), MtL + VA (▵), MtL + 1-HBT (▴).
FIG. 3.
Oxidation of dimer I to dimer II by laccase with the same activity plus violuric acid (VA) and 1-HBT. Symbols: TvL + VA (□), TvL + 1-HBT (■), PcL + VA (◊), PcL + 1-HBT (⧫), BcL + VA (▿), BcL + 1-HBT (▾), MtL + VA (▵), MtL + 1-HBT (▴).
FIG. 4.
Consumption of violuric acid (VA) and 1-HBT by laccases with the same activity. Symbols: TvL + 1-HBT (■), TvL + VA (□), PcL + 1-HBT (⧫), PcL + VA (◊), BcL + 1-HBT (▾), BcL + VA (▿), MtL + 1-HBT (▴), MtL + VA (▵).
FIG. 5.
Oxidation of phenol red by laccase plus violuric acid (VA). Symbols: PcL + VA (◊), TvL + VA (■), BcL + VA (▿), MtL + VA (▵). Data are the means of values from three replicates with a maximal sample mean deviation of ±3%.
FIG. 6.
Laccase stability in NaOAc buffer (pH 4.5, 50 mM) at 30°C. Symbols: PcL + VA (◊), TvL + VA (■), BcL + VA (▿), MtL + VA (▵).
FIG. 7.
Laccase stability in NaOAc buffer (pH 4.5, 50 mM) containing 1-HBT (10 mM) at 30°C. Symbols: PcL + VA (◊), TvL + VA (■), BcL + VA (▿), MtL + VA (▵).
FIG. 8.
Laccase stability in NaOAc buffer (pH 4.5, 50 mM) containing violuric acid (VA) (10 mM) at 30°C. Symbols: PcL + VA (◊), TvL + VA (■), BcL + VA (▿), MtL + VA (▵).
FIG. 9.
Laccase stability in NaOAc buffer (pH 4.5, 50 mM) containing violuric acid (VA) (10 mM) and dimer I (1.0 mM) at 30°C. Symbols: PcL + VA (◊), TvL + VA (■), BcL + VA (▿), MtL + VA (▵).
FIG. 10.
Laccase stability in NaOAc buffer (pH 4.5, 50 mM) containing violuric acid (VA) (10 mM) and phenol red (1.0 mM) at 30°C. Symbols: PcL + VA (◊), TvL + VA (■), BcL + VA (▿), MtL + VA (▵).
Some properties of the laccases are listed in Table 1. The molecular masses of the laccases ranged from 63 to 85 kDa, and the pIs of the laccases varied from 3.5 to 4.2 (4, 9, 24, 29). Although the molecular masses and pIs of the laccases are similar, the carbohydrate contents of the laccases are quite different (from 0.5 to 49%) (4, 9, 24, 29). The redox potential of the laccases could be divided into two groups. TvL, PcL and BcL have redox potentials of ca. 750 to 790 mV, while the redox potential of MtL is about 450 mV.
TABLE 1.
Some molecular properties of the studied laccases
A comparison of two kinetic parameters for the laccases with the four selected substrates is presented in Table 2. The value of Km of the different laccases is very much dependent upon the substrate. For example, with ABTS as substrate, the Km of the studied laccases is ranked in the order MtL > TvL > BcL > PcL, while the Km value order is PcL ≈ TvL > MtL > BcL with syringaldazine as the substrate. The kcat of the laccases is also dependent upon the substrate. The oxidation rate of ABTS by laccases is in the order TvL > PcL > MtL > BcL, whereas the oxidation rate of syringaldazine is in the order TvL > MtL > PcL > BcL. The oxidation rate of 1-HBT by the laccases is in the order TvL > PcL > BcL > MtL, whereas the oxidation rate with violuric acid is in the order PcL > TvL > BcL > MtL.
TABLE 2.
Comparison of kinetic parameters of laccases with selected substratesa
| Species |
Km (μM)
|
kcat (min−1)
|
||||||
|---|---|---|---|---|---|---|---|---|
| ABTS | Syringaldazine | 1-HBT | Violuric acid | ABTS | Syringaldazine | 1-HBT | Violuric acid | |
| T. villosa I | 58 ± 8 | 3.9 ± 0.5 | 15,000 ± 3,000 | 5,000 ± 1,000 | 2,700 ± 100 | 3,000 ± 100 | 84 ± 6 | 260 ± 20 |
| M. thermophila | 96 ± 5 | 2.9 ± 0.4 | 10,000 ± 6,000 | 18,000 ± 2,000 | 440 ± 300 | 1,100 ± 250 | 0.12 ± 0.02 | 27 ± 1 |
| P. cinnabarinus | 18 ± 5 | 4 ± 1 | 29,000 ± 7,000 | 9,000 ± 1,000 | 920 ± 50 | 180 ± 10 | 22 ± 2 | 370 ± 20 |
| B. cinerea | 28 ± 8 | 0.8 ± 0.2 | 12,000 ± 4,000 | 11,000 ± 1,000 | 23 ± 2 | 52 ± 3 | 10 ± 1 | 40 ± 2 |
The Km/kcat values are the results of nonlinear regression fitting of Michaelis-Menten equation on 6 to 10 experimental readings that covered the whole rate-[substrate] profile (from the initial, linear phase to the saturated phase).
DISCUSSION
Two methods have been used to compare the four different laccases for their ability to oxidize dimer I. The first was to use the laccases at the same molar concentration. This is an effort to directly correlate the active center of laccase to the oxidation rate of dimer I. Since it has been demonstrated that all these laccases contain four copper atoms per molecule (4, 9, 24, 29), a laccase solution with the same molar concentration of copper therefore contains the same amount of laccase molecules. Very different oxidation rates were found for the oxidation of dimer I by these laccases. However, using this comparison may raise a concern that laccases might be inactivated to a different extent. Even if such a concern seems unwarranted, laccases were also compared on their activity basis (ABTS as a substrate) for the oxidation of dimer I. The order of the oxidation rate was found to be the same: TvL > PcL > BcL > MtL in the presence of either 1-HBT or violuric acid.
Since BcL has a much lower kcat than the other laccases with ABTS as a substrate (Table 2), much more of BcL had to be added to reach the same activity with ABTS as the other laccases. This explains why the difference in the oxidation rate between BcL and TvL/PcL in the presence of 1-HBT was smaller when the laccases were used at the same activity level rather than at the same molar concentration.
The next step was to determine what caused the differences in oxidation rates. First of all, the stability of laccase will be one of the key parameters to affect the oxidation rates. It has been shown that the remaining laccase activity after pulp bleaching was related to the kappa number decrease of the pulp, i.e., the higher the laccase activity left the lower the remaining pulp kappa number (6). In our experiments, the laccases were inactivated by both 1-HBT and violuric acid. The addition of dimer I and phenol red decreased this inactivation. However, the stability of laccases does not rank in the same order as the oxidation rates. This implies that the difference in oxidation rates for the different laccases is dependent not only upon the stability of laccases but also upon other factors such as kcat for the redox mediator. As with 1-HBT, laccase-generated free radical of violuric acid would react with dimer I to form a catalytic cycle but also react with the laccase, thereby causing inactivation of the enzyme (16). A high concentration of the violuric acid free radical would accelerate both oxidation of dimer I and inactivation of the laccase. The inactivation of laccase would slow down the production of violuric acid free radical, which would subsequently affect the oxidation of dimer I. The oxidation rate of dimer I is thus dependent upon both kcat for the redox mediator and laccase stability to violuric acid free radical. With 1-HBT, laccases with higher kcat oxidize dimer I at a faster rate. With violuric acid, PcL has higher kcat than TvL and PcL is also inactivated faster than TvL. The net result is thus that PcL oxidizes dimer I more slowly than TvL. If VA free radical generated by laccase could be consumed by a substrate fast enough, inactivation of laccase would be slowed down and oxidation of the substrate would mainly depend upon kcat of violuric acid. This hypothesis could be verified from the comparison of laccase stability in the presence of dimer I and phenol red, respectively (Fig. 9 and 10), and from the oxidation of phenol red in a laccase-violuric acid system (Fig. 5). It is known that laccases alone are unable to oxidize phenol red, although phenol red is much easier to oxidize than dimer I. Nevertheless, in the presence of violuric acid, phenol red is rapidly oxidized by some laccases. The oxidation rate directly correlates with kcat of the laccases for violuric acid, i.e., PcL oxidizes phenol red faster than TvL (Table 2 and Fig. 5). PcL is also inactivated by violuric acid slower than TvL in the presence of phenol red. This is because the free radical of violuric acid generated by laccase is rapidly consumed by phenol red. BcL was somehow very vulnerable to the violuric acid free radical even in the presence of phenol red. The fast inactivation is in accord with the limited oxidation of phenol red by BcL-violuric acid (Fig. 5).
As described above, two crucial parameters to affect the oxidation rates of dimer I are the inactivation of laccase and the kcat of a laccase for a laccase mediator. These two parameters are discussed further here. Inactivation of laccases in a laccase-mediator system is largely dependent upon the mediator. For example, violuric acid inactivated the laccases much more quickly than 1-HBT. The mechanism of inactivation of laccases by different redox mediators is still not fully understood. It has been demonstrated that a significant part of the aromatic amino acids in laccase from T. versicolor, tyrosine and tryptophan, disappeared and that an increase in the molecular weight of the laccase was observed when the laccase was incubated with 1-HBT (2). Because dimer I and phenol red both slow down the inactivation of laccases by violuric acid, the free radical of violuric acid seems to be responsible for the inactivation of laccases. If the reaction of aromatic amino acids in the laccase molecule with a free radical of a redox mediator is indeed the reason for the inactivation of laccases, replacement of aromatic amino acid residues with a nonaromatic substitute by site-directed mutagenesis may help to make laccase less vulnerable to free-radical attack.
Glycosylation of laccase is believed to play a role in secretion, susceptibility to proteolytic degradation, copper retention, and thermal stability (11, 15, 19, 24). The four laccases described here show striking differences in their carbohydrate content. However, the high carbohydrate content of a laccase did not enhance the thermostability of the laccase, nor did the carbohydrate content of the laccase appear to prevent inactivation of the laccase by 1-HBT or violuric acid radicals.
The consumption of laccase mediators in a laccase-mediator system is an important issue for laccase-based pulp bleaching. The consumption rate of either 1-HBT or violuric acid is in the same order as the oxidation rate of dimer I, but no correlation between these consumption rates and the inactivation of the laccases could be seen. It is known that 1-HBT is degraded to benzotriazole that is no longer a redox mediator (23). Degradation of violuric acid seems to be more complex than degradation of 1-HBT because several small unidentified peaks can be seen from the HPLC spectrum after the oxidation by laccase.
The difference in the redox potentials between TvL, PcL, and BcL is minimal, while the oxidation rate of dimer I is quite different for these enzymes, i.e., there was no direct correlation between the redox potential of the laccases and the oxidation rate of dimer I and phenol red. There is also no correlation between redox potential, Km, and kcat. All of these results imply that the redox potential of a laccase is not the crucial factor for the oxidation rate of a laccase mediator (kcat). The kcat is dependent upon the electron transfer from a substrate to type 1 copper that is certainly affected by a redox potential difference between the laccase and a substrate. The kcat is also dependent upon other laccase parameters such as the affinity between the laccase and the substrate. The internal electron transfer step in a laccase could also be a rate-determining step besides electron transfer between laccase and a substrate. However, all of this does not imply that the redox potential of a laccase is not important. On the contrary, the inability of MtL to oxidize dimer I in the presence of 1-HBT or violuric acid is most likely because the redox potential of the enzyme (450 mV) is too low to effectively oxidize the redox mediators. Since an effective laccase mediator must have a redox potential high enough to oxidize nonphenolic lignin, laccase also must have high enough redox potential to make the oxidation of an effective laccase mediator kinetically possible.
From this investigation, we can conclude that an effective laccase-mediator system for lignin degradation should have the following features. Effective laccases must have a high kcat for an effective laccase mediator with high redox potential and must be resistant to inactivation by the free radical of the laccase mediator. The effective laccase mediator discussed here suggested that the free radical generated by laccase must be able to effectively oxidize nonphenolic lignin. Searching for a more effective laccase mediator for a laccase is certainly a way to enhance the effect of lignin degradation. For a specific laccase mediator, modification of laccase structure to increase its kcat and to improve its stability to the free-radical attack is another way to develop a better laccase-mediator system for pulp bleaching. Significant changes in Km and kcat of laccases modified by site-directed mutations have recently been demonstrated (28). That one laccase mediator is more effective than another with one laccase does not necessarily mean that the same order of effectiveness would remain with any other laccase since the effectiveness of a laccase-mediator system for lignin degradation depends on both the laccase and the laccase mediator and therefore the combination thereof.
ACKNOWLEDGMENT
This research was supported by a grant from the National Science Foundation (MCB-9507331) to K.-E. L. Eriksson.
REFERENCES
- 1.Adler E, Eriksson E. Guaiacylglycerol and its β-guaiacyl ether. Acta Chem Scand. 1955;9:341–345. [Google Scholar]
- 2.Amann M. 9th International Symposium on Wood and Pulping Chemistry, Montreal, Quebec, Canada. 1997. The lignozym® process coming closer to the mill; pp. F4:1–5. [Google Scholar]
- 3.Andersen S O. Sclerotization and tanning in cuticle. In: Kerkut G A, Gillert L I, editors. Comparative insect physiology, biochemistry and pharmacology. Vol. 3. Oxford, England: Pergamon Press; 1985. pp. 59–64. [Google Scholar]
- 4.Berka R M, Schneider P, Golightly E J, Brown S H, Madden M, Brown K M, Halkier T, Mondorf K, Xu F. Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol. 1997;63:3151–3157. doi: 10.1128/aem.63.8.3151-3157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourbonnais R, Paice M G. Enzymatic delignification of Kraft pulp using laccase and a mediator. TAPPI (Tech Assoc Pulp Pap Ind) J. 1996;79:199–204. [Google Scholar]
- 6.Bourbonnais R, Paice M G, Freiermuth B, Bodie E, Borneman S. Reactivities of various mediators and laccases with Kraft pulp and lignin model compounds. Appl Environ Microbiol. 1997;63:4627–4632. doi: 10.1128/aem.63.12.4627-4632.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourbonnais R, Leech D, Paice M G. Electrochemical analysis of the interactions of laccase mediators with lignin. Biochim Biophys Acta. 1998;1379:381–390. doi: 10.1016/s0304-4165(97)00117-7. [DOI] [PubMed] [Google Scholar]
- 8.Call, H. P. 1994. World patent application WO 94/29510.
- 9.Eggert C, Temp U, Eriksson K-E L. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faure D, Boullant M L, Bally R. Isolation of Azospirillum lipoferum 4T Tn5 mutants affected in melanization and laccase activity. Appl Environ Microbiol. 1994;60:3412–3415. doi: 10.1128/aem.60.9.3413-3415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatakka A. Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol Rev. 1994;13:125–135. [Google Scholar]
- 12.Katase T, Bollag J-M. Transformation of trans-4-hydroxycinnamic acid by a laccase of the fungus Trametes versicolor: its significance in humification. Soil Sci. 1991;151:291–296. [Google Scholar]
- 13.Kawai S, Umezawa T, Higuchi T. Oxidation of methoxylated benzyl alcohols by laccase of Coriolus versicolor in the presence of syringaldehyde. Wood Res. 1989;76:10–16. [Google Scholar]
- 14.Kersten P J, Kalyanaraman B, Hammel K E, Reinhammer B, Kirk T K. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J. 1990;268:475–480. doi: 10.1042/bj2680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz M B, Champe S P. Purification and characterization of the conidial laccase of Aspergillus nidulans. J Bacteriol. 1982;151:1338–1345. doi: 10.1128/jb.151.3.1338-1345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li K, Helm R F, Eriksson K-E L. Mechanistic studies of the oxidation of a nonphenolic lignin model compound by the laccase/1-hydroxybenzotriazole redox system. Biotechnol Appl Biochem. 1998;27:239–243. [Google Scholar]
- 17.Machuca A, Duran N. Phenol oxidases production and wood degradation by a thermophilic fungus Thermoascus aurantiacus. Appl Biochem Biotechnol. 1993;43:37–44. [Google Scholar]
- 18.Mayer A M. Polyphenol oxidases in plants—recent progress. Phytochemistry. 1987;26:11–20. [Google Scholar]
- 19.Minuth W, Klischies M, Esser K. The phenoloxidases of the ascomycete Podospora anserina, structural differences between laccases of high and low molecular weight. Eur J Biochem. 1978;90:73–82. doi: 10.1111/j.1432-1033.1978.tb12576.x. [DOI] [PubMed] [Google Scholar]
- 20.Muheim A, Fiechter A, Harvery P J, Schoemaker H E. On the mechanism of oxidation of non-phenolic lignin model compounds by the laccase-ABTS couple. Holzforschung. 1992;46:121–126. [Google Scholar]
- 21.O’Malley D M, Whetten R, Bao W, Chen C-L, Sederoff R. The role of laccase in lignification. Plant J. 1993;4:751–757. [Google Scholar]
- 22.Sbaghi M, Jeandet P, Bessis R, Leroux P. Degradation of stilbene-type phytoalexins in relation to the pathogenicity of Botrytis cinerea to grapevines. Plant Pathol. 1996;45:139–144. [Google Scholar]
- 23.Sealey J, Ragauskas A. 9th International Symposium on Wood and Pulping Chemistry, Montreal, Quebec, Canada. 1997. Fundamental investigations into the chemical mechanisms involved in laccase mediator biobleaching; pp. F1:1–4. [Google Scholar]
- 24.Slomczynski D, Nakas J P, Tanenbaum S W. Production and Characterization of laccase from Botrytis cinerea 61–34. Appl Environ Microbiol. 1995;61:907–912. doi: 10.1128/aem.61.3.907-912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas B R, Yonekura M, Morgan T D, Czapla T H, Hopkins T L, Kramer K J. A trypsin-solubilized laccase from pharate pupal integument of the tobacco hornworm, Manduca sexta. Insect Biochem. 1989;19:611–622. [Google Scholar]
- 26.Williamson P R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J bacterial. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu F, Shin W, Brown S H, Wahleithner J, Sundaram U M, Solomon E I. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta. 1996;1292:303–311. doi: 10.1016/0167-4838(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 28.Xu F, Berka R M, Wahleithner J A, Nelson B A, Shuster J R, Brown S H, Palmer A E, Solomon E I. Site-directed mutations in fungal laccase: effect on redox potential, activity and pH profile. Biochem J. 1998;334:63–70. doi: 10.1042/bj3340063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaver D S, Xu F, Golightly E J, Brown K M, Brown S H, Rey M W, Schneider P, Halkier T, Mondorf K, Dalboge H. Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Appl Environ Microbiol. 1996;62:834–841. doi: 10.1128/aem.62.3.834-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]