Abstract
Simple Summary
HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer (BC) is an emerging subtype of BC with promising results with antibody drug conjugate (ADC) in the metastatic setting. In the early setting, few data have been reported regarding the predictive and prognostic impact of HER2-low status in triple-negative BC (TNBC).
Abstract
Purpose: Investigates the link between HER2 status and histological response after neoadjuvant chemotherapy in patients with early TNBC. Methods: We retrieved clinical and anatomopathological data retrospectively from 449 patients treated for the first time with standard neoadjuvant chemotherapy for early unilateral BC between 2005 and 2020. The primary endpoint was pathological complete response (pCR, i.e., ypT0 ypN0), according to HER2 status. Secondary endpoints included invasive disease-free survival (I-DFS) and overall survival (OS). Results: 437 patients were included, and 121 (27.7%) patients had HER2-low tumours. The pCR rate was not significantly different between the HER2-low group vs. the HER2-0 group (35.7% versus 41.8%, p = 0.284) in either univariate analysis or multivariate analysis adjusted for TNM classification and grade (odds ratio [OR] = 0.70, confidence interval [CI] 95% 0.45–1.08). With a median follow-up of 72.9 months, no significant survival differences were observed between patients with HER2-low tumours vs. patients with HER2-0 tumours in terms of I-DFS (p = 0.487) and OS (p = 0.329). Conclusions: In our cohort, HER2 status was not significantly associated with pCR in a manner consistent with data published recently on TNBC. However, the prognostic impact of HER2-low expression among TNBC patients warrants further evaluation.
Keywords: triple-negative breast cancer, complete histologic response, HER2-low, prognosis
1. Introduction
Triple-negative breast cancer (TNBC), defined by the lack of expression of oestrogen and progesterone receptors (ER and PR, respectively) and associated with the absence of HER2 overexpression/amplification, represents approximately 15% of cases of early stage invasive breast cancer [1]. Chemotherapy is the cornerstone of treatment in this phenotype, with no biomarkers that can identify the patients most likely to respond to cytotoxics. Targeted therapies have shown contrasting results, notably for anti-angiogenic [2] and anti-EGFR [3] therapies. More recently, PARP inhibitors in patients with a germline mutation of BRCA1/2 [4,5] and immunotherapy [6] have shown efficacy in early settings. TNBC is a very heterogeneous group of tumours, at the phenotypical, molecular, prognosis, and predictive levels. TNBC includes a large proportion of basal-like tumours identified by Perou and Sorlie and characterized by extensive proliferation, and poor prognosis with a risk of early relapse [7,8,9,10]. Lehmann et al. [11] initially described six molecular subtypes, subsequently refined into four subtypes including basal-like 1 and 2 (BL1, BL2), mesenchymal-like (M), and luminal androgen receptor (LAR) with differential responses to chemotherapy (Lehmann et al., 2016). Farmer et al. identified, by analysing 49 breast tumours, three subgroups: luminal, basal and molecular apocrine. The HER2 amplified tumours were distributed in the luminal and molecular apocrine subgroups [12], representing between 22 and 33% of TNBCs with strong expression of androgen receptors. Jézéquel et al. [13] distinguished three clusters by analysing the transcriptional profile of 107 cases of TNBC: C2 and C3 were distinguished by immune responses, and C1 was enriched in the luminal androgen receptor (LAR). In this last cluster (C1), molecular apocrine, correlation with PAM50 luminal B, and HER2-enriched subtypes were close, with high expression of the ERBB2 pathway. According to the 2018 ASCO and College of American Pathologists (CAP), HER2 protein overexpression assessed by immunohistochemistry (IHC) score 3+ or HER2 gene amplification, assessed by in situ hybridization (ISH) assay, is the predictive biomarker of HER2-targeted therapies in breast cancer, such as trastuzumab. A subpopulation of TNBC does not overexpress HER2 or show HER2 amplification but is molecularly enriched in genes of the “HER2” group, as previously seen. Recently, a new entity has emerged named HER2-low BC, defined by IHC 1+, or IHC 2+ without amplification assessed by ISH. It represents between 45 and 60% of HER2-negative BC tumours according to ASCO 4 CAP [14,15], which includes 37% of TNBC. The oncogenic role of HER2 in HER2-low BC is still unclear. Preclinical studies suggest that the activity of certain antibody drug conjugates (ADC) may be dependent on the expression levels of HER2 protein rather than on HER2 amplification [16], and some data seem to show promising results with trastuzumab deruxtecan in advanced HER2-low BC [17], rather than a bystander effect. These newer HER2 ADC have the potential to overcome HER2 expression heterogeneity [18]. In the neoadjuvant setting, pCR is associated with better relapse-free survival (RFS), especially in the TNBC phenotype [19,20,21]. Masuda et al. [22] reported the profile of response to anthracycline/taxane-based neoadjuvant chemotherapy (NACT) in 146 TNBCs, classified according to Lehmann’s subtypes, with the highest pCR rate presented by the BL1 subtype (52%) and the lowest in the BL2 and LAR subtypes (0% and 10%, respectively). Previous studies suggest that pCR rates are significantly higher for patients with HER2 overexpression, compared with patients with low HER2 expression [15], but there is not much data on the impact of HER2 low expression in response to chemotherapy, and what there is has rather contradictory results [23]. Finally, further studies have identified an elevated platelet to lymphocyte ratio (PLR), a marker of inflammation, as a very poor prognosis factor in terms of OS in TNBC (cut-off > 190) [24,25,26,27,28]. A high PLR is also associated with lower response to neoadjuvant chemotherapy [29,30,31]. The aim of the present study was thus to investigate the impact of HER2 status (HER2-low versus HER2-0) on the histological response after NACT in a cohort of patients with early TNBC. The secondary objectives were to evaluate the prognostic impact of HER2 status and baseline PLR in early TNBC.
2. Materials and Methods
2.1. Study Design
This retrospective study includes all patients treated with NACT for early TNBC between 2005 and 2020 at the ICO. Patients were included if they fulfilled the following criteria: (a) unilateral TNBC (defined by ER and PR < 10%, HER2 0 or 1+ in IHC or HER2++ in IHC with ISH negative); (b) T1-2, N0-3, M0 staging according to UICC criteria; (c) treated with NACT and surgery; (d) over the age of 18 years. Exclusion criteria were (a) metastatic or relapsed disease, (b) male patients, (c) not amenable to surgery, (d) radiotherapy performed before surgery, (e) previous or concomitant malignancies, (f) previously received adjuvant chemotherapy. The following information was recorded for each patient: (a) age at diagnosis; (b) clinical stage; (c) TNM stage and ultrasound tumour size; (d) histological type and axillary lymph node involvement; (e) Elston and Ellis grade; (f) hormone receptor status and HER2 status; (g) presence of tumour infiltrating lymphocytes (TILS); (h) mitotic account and Ki67; (i) type of surgery (conservative or mastectomy; sentinel node or axillary dissection); (j) biological data for calculating the PLR; (k) details of systemic neoadjuvant treatment; (l) adjuvant radiotherapy and (m) outcomes. pCR was defined as the absence of residual invasive cancer regarding breast and axillary lymph node after NACT, using Sataloff and the residual disease index known as the residual cancer burden (RCB) classification. The pCR was thus defined as classification RCB-0 and/or Sataloff’s TA-NA or TA-NB, i.e., ypT0 ypN0 [32]. We recorded data on androgen receptor or CK5/6 positivity, but this information was only available for 129 and 143 of the 449 patients, respectively, and was therefore not used in the data analysis.
2.2. Definition of the End Points
The main objective was to study the association between HER2 immunohistochemical status (HER2-low vs. HER2-0) and pCR after NACT in early TNBC. The primary endpoint was pCR, according to HER2 status. The secondary objectives were to compare pCR rates in HER2 1+ versus 2+ subgroups, compare early response rates, and estimate invasive disease-free survival (I-DFS), distant disease-free survival (D-DFS), OS, breast cancer specific survival and impact of the baseline PLR rate (high versus low with a cut-off equal to 190). Follow-up data were collected for each patient, including I-DFS (time from diagnosis to the earliest locoregional or distant disease recurrence, invasive controlateral cancer, second primary cancer, or death), D-DFS (defined as the time until metastasis or death), and OS (time from diagnosis to death from any cause) [33]. Patients who did not experience the event of interest were censored at their last follow-up.
2.3. Statistical Analysis
Categorical variables were described by the number and percentage of each modality of the variable. Continuous data were described by the median, minimum, and maximum. Comparisons between groups were made using Fisher’s exact test for categorical variables and the Kruskall–Wallis test for continuous variables. The Wilcoxon signed rank test was used to examine changes over time (pre- and post-chemotherapy). The analyses evaluating the associations between pCR and clinically relevant parameters were performed with univariate and multivariate logistic regression models; the ORs and corresponding confidence intervals (CIs) were reported. Survival data were estimated using the Kaplan–Meier method and presented with their 95% confidence intervals for the overall population and by group. Univariate analyses were performed using the log-rank test for categorical variables or the Cox proportional hazards model for continuous variables [34]. All analyses were performed using Stata® version 16 and R software version 4.0.2. All tests used were two-sided with an α threshold at 5%. An independent ethics committee (CHU Angers) approved the study protocol (N° 2020-133).
3. Results
3.1. Patient and Tumour Characteristics
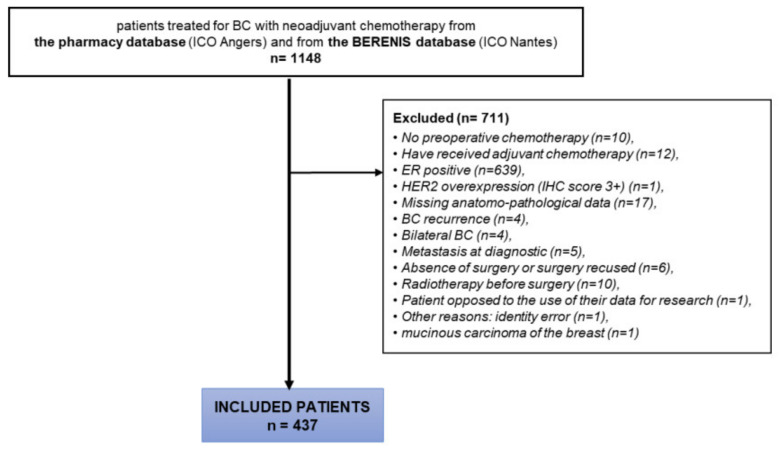
A total of 437 out of 1148 patients were included into the study from 2005 to 2020 at ICO (Figure 1). Details of patient characteristics are found in Table 1. Of the 437 TNBC patients included, 121 (27.7%) had an HER2-low tumour (90 HER2 1+, 28 HER2 2+). Five patients were classified according to IHC status on postoperative histology in the absence of HER2 IHC status available on biopsies. The median age was 51 years (range: 42–62). Non-specific invasive carcinomas were predominant (95.2%). Most of the tumours (93.1%) were classified as ≥ T2 (5.8% T2; 24.5% T3; 12.8% T4) and 60.6% had node axillary involvement (49.1% N1, 10% N2, 1.4% N3). There was no significant association between HER2 status and stages T and N. mSBR grade was not significant between the two groups, with 73.3% of grade III in HER2-low vs. 77% in HER2-0 groups, while Ki67 was significantly higher in the HER2-low group with a mean of 60.9% (sd: 22.7) versus 49.0% (22.8) in the HER2- 0 group, however with missing data for 199 and 85 patients from these two groups, respectively (p = 0.008). Systemic NACT included a sequential of anthracyclin followed by taxanes in 95% of patients. The dose-dense regimen in this sequential was administered to 57 patients (13%), 9 patients in the HER2-low group (7.4%) and 48 patients in the HER2-0 group (15.2%). A total of 38 patients (8.7%) received associated platinum salts. The time from diagnosis to the beginning of chemotherapy and the number of postponements of treatment were well balanced in both groups.
Figure 1.
CONSORT diagram of the study.
Table 1.
Baseline patient characteristics.
| HER2-0 (n = 316) |
HER2-Low (n = 121) |
p-Value | Total (n = 437) |
|
|---|---|---|---|---|
| Age at diagnostic | ||||
| Median (range) | 51.0 (25–89) | 52.0 (22–89) | 51.0 (22–89) | |
| Histological type | ||||
| Non-specific | 304 (96.2%) | 112 (92.6%) | 416 (95.2%) | |
| Invasive lobular | 4 (1.3%) | 3 (2.5%) | 7 (1.6%) | |
| Apocrine | 1 (0.3%) | 2 (1.7%) | 3 (0.7%) | |
| Other histological type | 7 (2.2%) | 4 (3.2%) | 11 (2.5%) | |
| Stage T | p = 0.053 | |||
| T0-T1 | 15 (4.7%) | 15 (12.4%) | 30 (6.7%) | |
| T2 | 178 (56.3%) | 66 (54.5%) | 252 (56.1%) | |
| T3 | 81 (25.6%) | 26 (21.5%) | 110 (24.5%) | |
| T4 | 42 (13.3%) | 14 (11.6%) | 57 (12.7%) | |
| Stage N | p = 0.662 | |||
| N0 | 122/315 (38.7%) | 50/121 (41.3%) | 172 (39.4%) | |
| N+ | 193/315 (61.3%) | 71/121 (58.7%) | 264 (60.6%) | |
| RE | ||||
| 0 | 295/315 (93.7%) | 116/120 (96.7%) | 423/447 (94.5%) | |
| 1–10% | 20/315 (6.3%) | 4/120 (3.3%) | 24/447 (5.5%) | |
| HER2 status | ||||
| 1+ | 0 | 90/119 (76.3%) | ||
| 2+ | 0 | 28/119 (23.7%) | ||
| mSBR grade | p = 0.452 | |||
| Grade II | 72/313 (23%) | 32/120 (26.7%) | 104/433 (24%) | |
| Grade III | 241/313 (77%) | 88/120 (73.3%) | 329/433 (76%) | |
| Mitotic index (/mm2) | ||||
| Median (range) | 8.1 (1.1–31.5) | 8.8 (0.0, 30.0) | 8.3 (0.0, 31.5) | |
| Missing | 254 | 99 | 353 | |
| Ki67 (%) | p = 0.011 | |||
| Median (range) | 50.0 (10.0, 90.0) | 64.0 (10.0, 95.0) | 52.0 (10.0, 95.0) | |
| Missing | 199 | 85 | 284 | |
| Neoadjuvant chemotherapy | p = 0.013 | |||
| Anthracycline-taxane (A-T) | 301/316 (95,3%) | 114/121 (94,2%) | 415/437 (95%) | |
| A-T dose dense regimen | 48/316 (15.2%) | 9/121 (7.4%) | 57/437 (13%) | |
| Platinum salts | 33/316 (10.4%) | 5/121(4.1%) | 38/448 (8.7%) | |
| Mammary surgery | p = 0.226 | |||
| Mastectomy | 107 (33.1%) | 50 (39.7%) | 157 (35.0%) | |
| Conservative | 216 (66.9%) | 76 (60.3%) | 292 (65.0%) | |
| Germline mutation | ||||
| Number of screened patients | 125 | 48 | 173 | |
| BRCA1 | 18 (14.4%) | 8 (16.7%) | 26 (15.0%) | |
| BRCA2 | 9 (7.2%) | 1 (2.1%) | 10 (5.8%) | |
| PALB2 | 1 (0.8%) | 0 (0.0%) | 1 (0.6%) | |
| No identified mutation | 97 (77.6%) | 39 (81.3%) | 136 (78.6%) | |
3.2. Predictive Value of HER2 Status on pCR
The pCR rate was not significantly different between the HER2-low versus HER2-0 groups (35.5% versus 42.7%, p = 0.284) in univariate and multivariate analysis (Table 2). Only stages T3–T4 vs. T0 were significant in multivariate analysis. There was also no significant difference in pCR rate in the HER2 1+ and 2+ subgroups (Supplementary Table S1). The clinical and pathological characteristics of the patients after surgery are listed in Supplementary Table S2. Achieving a partial or complete response on early imaging during chemotherapy was not significantly associated with attaining a pCR (pCR 44.6% in HER-low vs. 50.0% in HER2-0, p = 0.522).
Table 2.
pCR according to HER2 status (HER2-low versus HER2-0); univariate and multivariate analysis according to HER2 status.
| HER2-0 | HER2-Low | Total | ||||
|---|---|---|---|---|---|---|
| (n = 316) | (n = 121) | (n = 449) | ||||
| pCR (i.e., ypT0 ypN0) | p = 0.192 | |||||
| No | 181 (57.3%) | 78 (64.5%) | 259 (59.3%) | |||
| Yes | 135 (42.7%) | 43 (35.5%) | 178 (40.7%) | |||
| Univariate |
Multivariate (n = 432) |
|||||
| OR | IC 95% | p-value | OR | IC 95% | p-value | |
| HER2 | ||||||
| HER2 0 | 1.00 | 1.00 | ||||
| HER 1+ or 2+ | 0.74 | [0.48;1.14] | 0.172 | 0.66 | [0.42; 1.03] | 0.066 |
| Stade N | ||||||
| N0 | 1.00 | 1.00 | ||||
| N+ | 1.01 | [0.68; 1.49] | 0.965 | 1.08 | [0.42; 1.03] | 0.700 |
| Stade T | ||||||
| T0-T1 | 1.00 | 1.00 | ||||
| T2 | 0.61 | [0.28; 1.31] | 0.201 | 0.60 | [0.27; 1.32] | 0.207 |
| T3-T4 | 0.37 | [0.17; 0.81] | 0.014 | 0.35 | [0.16; 0.80] | 0.012 |
| mSBR grade (biopsy) | ||||||
| Grade II | 1.00 | 1.00 | ||||
| Grade III | 1.08 | [0.69; 1.70] | 0.729 | 1.07 | [0.72; 1.78] | 0.773 |
3.3. Prognostic Value of HER2 Status
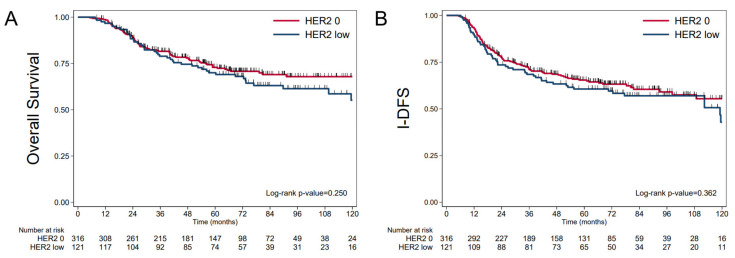
Median follow-up was 72.9 months (95% CI 69.9; 77.5) and median survival was not achieved. OS at 5 years was 72.02% (95%CI 67.22; 76.23). No association with OS was found (p = 0.329) between groups: 5-year OS was 70.00% (95%CI 67.22; 76.23) in the HER2-low group versus 72.9% (95%CI 67.1; 77.8) in the HER2-0 group (Figure 2). Five-year I-DFS was 63.99% (95%CI 59.08; 68.48) with median I-DFS at 124.3 months (95%CI 112.3; NR). The most frequent first event was metastatic relapse, which occurred in 115 patients (26.3%). No significant association with I-DFS was found between the two groups (p = 0.487): 5-year I-DFS was 60.6% (95%CI: 51.2; 68.8) in the HER2-low group, versus 65.4% (95%CI: 59.5; 70.6) in the HER2-0 group (Figure 2). The five-year D-DFS was 66.94% (95%CI: 62.07; 71.33), without reaching a significant difference between the two groups (p = 0.210): 63.1% in the HER2-low group vs. 68.5% in the HER2-0 group (Supplementary Table S3). Five-year specific survival was 73.19% (95%CI 68.44; 77.35) for the entire cohort.
Figure 2.
Outcomes data according to HER2 status (HER2-low versus HER-0). (A) OS, (B) I-DFS.
3.4. Prognostic Value of PLR
A baseline high PLR rate (≥190) differed significantly depending on HER2 status: 37/113 (32.7%) and 60/284 (21.1%) in the HER2-low and HER2-0 groups, respectively (p = 0.02). At the end of chemotherapy, the rate of high PLR was considerably increased, with a rate of 75.5% (<0.001) in the entire cohort (Supplementary Table S4), 65.3% in the HER2-low, and 80.5% in the HER2-0 groups, with no significant difference between the two groupes (p = 0.376).
A baseline high PLR using a cut-off of 190 had lower OS in the HER2-0 versus HER2-low groups, without reaching significant difference, with 5-year OS rates of 58.7% and 74.7%, respectively, versus 76.5% and 67.1% in these same groups when associated with baseline PLR of less than 190 (Supplementary Table S5 and Figure S1). A baseline high PLR also had a lower 5-year I-DFS rate in the HER2-0 group compared to the HER2-low group with no statistical significance: 52.6% and 70.2%, respectively, versus 70.2% and 58.3% in these same groups when associated with baseline PLR of less than 190 (p = 0.010). Finally, baseline high PLR was associated with poor prognosis in terms of OS and I-DFS in the HER2-0 group, while the opposite trend was observed in the HER2 low group (Supplementary Figure S1).
4. Discussion
A total of 121 patients (27.7%) from our TNBC cohort were HER2-low. A lower rate of pCR was observed in the HER2-low versus HER2-0 groups, with no statistical significance (35.5% versus 42.7%, p = 192). No association with OS was found between groups (p = 0.329). Our results complete insights into the clinical characteristics of HER2-low BC. The proportion of HER2-low group patients was coherent with the literature data, according to Scott M. et al. at ASCO 2021, with 38% HER2-low patients in their cohort of 389 TNBC [35], and 36.5% (26.8% 1+ and 9.8% 2+) in the study by Schettini et al. [14]. In this study, basal-like tumours were mostly concentrated within the IHC 0 (43.7%) and TNBC (84.7%) groups compared to IHC 2+ (9.8%), IHC 1+ (15.2%), HER2-low (13.4%) and HR-positive tumours (3.9%). Braso Maristany et al. observed no significant difference regarding subtypes identified by PAM50 distribution between HER2-0 and HER2-low in a cohort of 80 patients (p = 0.091) treated for BC (28.8% TNBC and 54.4% HR+) [36]. HER2- low tumours were more frequently found within HR-positive disease compared to TNBC (65.4% vs. 36.5%, p < 0.001, also reflecting the proportion of these phenotypes in BC [14]. Lehmann’s classification identified most TNBC as basal-like (80.6%). Interestingly, the LAR subtype was predominantly identified as either HER2-enriched (74%) or luminal B (14%) [13,37,38].
The pCR rate in the present cohort of TNBC was 42.7% of the 437 TNBC, which is not so different from the pCR observed in the GeparTrio study, of 509 cases of TNBC treated in the neoadjuvant setting with anthracyclines and taxanes, with a pCR rate of 39% [39,40]. In our cohort, the pCR rate was not significantly different between the HER2-low versus HER2-0 groups (35.5 % versus 42.7 %, respectively, p = 0.284) in univariate analysis, and even in multivariate analysis adjusted for grade, only stage T3-T4 was significantly associated with a poor response. There are few data in the literature regarding the histological response after NACT of HER2-low and HER2-0 tumours. In a study published in 2012 [41], Wang et al. showed that the pCR rate in a cohort of HER2-low patients treated with anthracycline-based NACT was 9.6% (out of 229 patients). Santonja et al. looked at the correlation between Lehmann’s classification and the achievement of pCR in neoadjuvant therapy in a cohort of 125 TNBCs (non-basal-like included 5 HER2-enriched and 1 luminal A). Despite small patient numbers, it was shown that LAR patients presented a lower rate of pCR (14.3% pCR in LAR versus 41.9% in other subtypes combined, p = 0.07) [42]. In another retrospective study of 146 TNBC patients who received a sequential of anthracycline and taxanes, the pCR rate was 28%, with significant differences between subtypes: the pCR rate in the BL1 subtype was the highest (52%), while those in the BL2, LAR, and MSL subtypes were 0%, 10%, and 23%, respectively [18]. Similarly, Echavarria et al. observed that among patients pretreated with NACT, the BL1 subtype had the highest pCR rate (65.6%), followed by BL2 (47.4%), while the LAR subtype had a significantly lower pCR rate (21.4%) [43]. Recently, a pooled analysis of 2310 patients with HER2 non-amplified early BC from four prospective neoadjuvant clinical trials, published by Denkert et al., included 1162 patients with TNBC (395 patients HER2-low and 767 HER2-0). They observed no difference in terms of pCR rate in the TNBC subgroup: 45.6 % in the HER2-low group vs 44.9 % in the HER-0 group (p = 0.51), while pCR was significantly lower in the HER2-low patients (17.5% versus 23.6%; p = 0.024) in the hormone-receptor-positive subgroup [44]. Similarly, Moura Leite et al. recently reported a cohort of 855 HER2 non-amplified patients with 313 cases of TNBC treated with NACT, included 49 HER2-low, with no difference in terms of pCR rate in relation to HER2 status: 51% versus 47% in HER2-low versus HER2-0, respectively (p = 0.64) [45]. To note, in this last cohort, almost half of the patients had received a dose dense regimen, and half of the patients had also received carboplatin in both the HER2-low and HER2-0 groups. In our cohort, in HER2-0 and HER2-low, only 15.2% and 7.4% received a dose dense regimen and 10.4% and 4.1% received platinum salts. At the last San Antonio symposium, Reinert et al. reported in a cohort of 122 TNBC patients, a higher, although non-statistically significant, pCR rate in HER2-0 versus HER2-low tumours (56% vs. 39%, p = 0.09) [46].
In our cohort, with median follow-up of 72.9 months, a trend with lower outcomes were observed in the HER2-low group but with no significant association with I-DFS or OS found between the HER2-low and HER2-0 groups. Rossi et al. studied the prognostic differences between HER2 0/1+/2+ tumours [47] in a cohort that included 15% of TNBC, and observed at diagnosis larger tumours, frequently more proliferative tumours (higher-grade, higher Ki-67 rate), and more extensive axillary lymph node involvement in patients with tumours with HER2 scores of 0 and 1+, compared to HER2 score 2, regardless of HR (hormone receptor) status. The 5-year DFS rates were 86%, 84%, 62% and 63% for patients with tumours categorized as HER2 0, 1+, 2+; and HER2 amplified (2+, ISH+ or 3+), respectively. HER2-low status was associated in the cohort published by Denkert et al. with better outcomes with a 3-year DFS of 84.5% and a 3-year OS of 90.2% vs 74.4% and 84.3% in the HER2-0 group respectively, with median follow-up of 46.6 months (p = 0.0076 and p = 0.016) [44]. In contrast, Moura Leite et al. observed no significant prognostic value of HER2-low status, with 5-year RFS rates of 75.6% versus 70.8% (p = 0.23) for TNBC with HER2-low versus HER2-0, and 5-year OS of 79.1% versus 80.3% (p = 0.71), respectively after a median of 59 months [45]. Surprisingly, the HER2-low group in our cohort showed outcomes slightly lower than in the HER2-0 group, without reaching a significant difference between the two groups. Only higher proliferation, known to be associated with relapse, in the HER2-low group, especially regarding the Ki67 rate, differed from the HER2-0 group (median 64% vs 50% in the HER2-0 group, p = 0.008). Ki67 was lower on average in the Rossi et al. cohort [47] at 20% for HER-0 and 1+ and 26% for 2+, but more than 80% of these patients were RH+ in this study. At the molecular level, Shettini et al. reported a downregulation of proliferation-related genes in the HER2-low group. Ki-67 has been assayed in many studies as a predictive marker of response in early BC, however conflicting results have been published [48,49,50,51]. Some authors have investigated whether its level correlates with the achievement of pCR: in one study, the three groups of Ki67 ≤15% versus 15.1%-35% versus >35% had pCR-rates of 10%, 22.4%, and 39.0% in TNBC, respectively [51]. Nevertheless, in the study by Moura Leite et al., median Ki67 was 70% in HER2-low groups vs. 60% in HER2-0 (p = 0.80), and the proportion of grade III tumours did not differ between the two groups (67.3% in HER2-low vs. 67.8%, p = 0.91). Too much data was missing on proliferation markers (65%) to make a hypothesis in our cohort. Regarding other biological parameters, a high PLR rate at baseline seems to have a prognostic value. It has been previously shown that tumour cells induce the synthesis of the platelet stimulating factors that promote tumour growth [52] through systemic inflammation. Thisis associated with the release of several pro-inflammatory mediators such as interleukin, known to stimulate megakaryocyte proliferation leading to thrombocytosis [53]. Chemotherapy influences these parameters through its bone marrow toxicity. Similarly, the number of circulating lymphocytes reflects the systemic inflammation, tumour suppressive activity, and immunomodulation induced by chemotherapy. In light of this, patients with low PLR might have a better prognosis, thanks to better antitumour activity. A high PLR (≥190) at baseline in our cohort was associated with lower OS without statistical significance in the HER2-0 group, while the opposite trend was observed in the HER2-low group, with no difference in the PLR rate at the end of treatment. It would be interesting to conduct further studies on these subjects to see if there is an association between evolution in PLR levels during chemotherapy and survival, reflecting the immunomodulation induced by the treatments. Surprisingly, rates of high and low PLR increased with the same ratio in the HER2-low and HER2-0 groups. Kim et al. studied dynamic changes in PLR ratio and observed that a low PLR value at pre- and post-systemic treatment was significantly associated with better prognosis [54]. Further research is needed to determine the timing of evaluation of the PLR ratio and its prognostic value. TNBC with HER2-low status is probably a heterogeneous entity; HER2 expression can in fact present a variable profile, with considerable intra-tumoral heterogeneity [55,56]. This heterogeneity has been explored in TNBC by single cell analysis and may be associated with a decreased likelihood of achieving a pCR [57,58]. Trastuzumab deruxtecan seems to show better efficacy in score 2+ than score 1+ [17]. Current clinical trials involving HER2-ADCs suggest promising results in the treatment of HER2-low expressing BC, especially for ADCs responsible for the by-stander effect, with more efficacy in case of intra-tumoral heterogeneity. The study included a large cohort of patients with TNBC, bi-centrically. Some limitations need to be notified, including the retrospective nature of this study with outcomes affected by differences in terms of patient characteristics. On the other hand, 2/3 of the patients were HER2-0, which does not represent the characteristics of the HER2 negative population but is consistent regarding the TNBC phenotype. Finally, a major strength of the present work is that it is reported with significant hindsight regarding OS, with median of follow-up of 72.9 months.
5. Conclusions
To our knowledge, our study is the third recent cohort with response data in HER2-low TNBC after NACT. In our cohort, HER2-low status had no significant prognostic value on survival and no predictive effect on pCR after NACT. In addition, the trend towards poorer survival contrasts with published data in the HER2-low group, although without reaching significance and with significant follow-up. The prognostic value of HER2-low expression warrants further evaluation. We hypothesize that HER2-low TNBC is a heterogeneous entity with the interest of studying the dynamic changes on the residual disease in this entity after chemotherapy, and this needs to be characterized at the molecular level.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers14102509/s1, Table S1. Association between HER2-low (HER2 1+ versus HER2 1+) and pCR, Table S2. Clinicopathological characteristics of the tumours after surgery, Table S3. D-DFS according to HER2 status, Table S4. Evolution of PLR and NLR at baseline and after treatment, Table S5. Outcomes according to baseline PLR et HER2 status, Figure S1. Outcomes according to baseline PLR. (A) OS and (B) I-DFS.
Author Contributions
C.D. and A.P. designed the study and contributed to the analysis and interpretation of the data. Data collection was performed by C.D. and E.M. supervised all the statistical analysis. C.D., E.M., C.L., P.J., J.-S.F., P.A., M.C. and A.P. approved the final manuscript and contributed to critical revisions of its intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved 3 November 2020 by the Institutional Review Board (or Ethics Committee) of CHU Angers (N° 2020-133).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The datasets supporting the conclusions of this article are included within the article (and its additional files).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freres P., Collignon J., Gennigens C., Scagnol I., Rorive A., Barbeaux A., Coucke P.A. Jérusalem Gle cancer du sein «triple né-gatif». Rev. Med. Liège. 2010;65:120–126. [PubMed] [Google Scholar]
- 2.Miller K., Wang M., Gralow J., Dickler M., Cobleigh M., Perez E.A., Shenkier T., Cella D., Davidson N.E. Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 3.Nabholtz J.M., Abrial C., Mouret-Reynier M.-A., Dauplat M.M., Weber B., Gligorov J., Forest A.M., Tredan O., Vanlemmens L., Petit T., et al. Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: Identification of biologically defined signatures predicting treatment impact. Ann. Oncol. 2014;25:1570–1577. doi: 10.1093/annonc/mdu183. [DOI] [PubMed] [Google Scholar]
- 4.Mateo J., Lord C.J., Serra V., Tutt A., Balmaña J., Castroviejo-Bermejo M., Cruz C., Oaknin A., Kaye S.B., de Bono J.S. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019;30:1437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tutt A.N., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., Gelber R.D., de Azambuja E., Fielding A., Balmaña J., et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid P., Cortes J., Pusztai L., McArthur H., Kümmel S., Bergh J., Denkert C., Park Y.H., Hui R., Harbeck N., et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 7.Perou C.M., Sørlie T., Eisen M.B., Van De Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 8.Sorlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 10.Deconstructing the Molecular Portraits of Breast Cancer—Prat A and Perou CM—2011—Molecular Oncology—Wiley Online Library. [(accessed on 9 May 2021)]. Available online: https://febs.onlinelibrary.wiley.com/doi/full/10.1016/j.molonc.2010.11.
- 11.Lehmann B.D., Bauer J.A., Chen X., Sanders M.E., Chakravarthy A.B., Shyr Y., Pietenpol J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farmer P., Bonnefoi H., Becette V., Tubiana-Hulin M., Fumoleau P., Larsimont D., MacGrogan G., Bergh J., Cameron D., Goldstein D., et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 13.Jézéquel P., Kerdraon O., Hondermarck H., Guérin-Charbonnel C., Lasla H., Gouraud W., Canon J.-L., Gombos A., Dalenc F., Delaloge S., et al. Identification of three subtypes of triple-negative breast cancer with potential therapeutic implications. Breast Cancer Res. 2019;21:65. doi: 10.1186/s13058-019-1148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schettini F., Chic N., Brasó-Maristany F., Paré L., Pascual T., Conte B., Martínez-Sáez O., Adamo B., Vidal M., Barnadas E., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7:1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarantino P., Hamilton E., Tolaney S.M., Cortes J., Morganti S., Ferraro E., Marra A., Viale G., Trapani D., Cardoso F., et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020;38:1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 16.Takegawa N., Tsurutani J., Kawakami H., Yonesaka K., Kato R., Haratani K., Hayashi H., Takeda M., Nonagase Y., Maenishi O., et al. [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int. J. Cancer. 2019;145:3414–3424. doi: 10.1002/ijc.32408. [DOI] [PubMed] [Google Scholar]
- 17.Modi S., Park H., Murthy R.K., Iwata H., Tamura K., Tsurutani J., Moreno-Aspitia A., Doi T., Sagara Y., Redfern C., et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low–Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020;38:1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pernas S., Tolaney S.M. Targeting HER2 heterogeneity in early-stage breast cancer. Curr. Opin. Oncol. 2020;32:545–554. doi: 10.1097/CCO.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 19.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 20.Esserman L.J., Berry D.A., Cheang M.C.U., Yau C., Perou C.M., Carey L., DeMichele A., Gray J.W., Conway-Dorsey K., Lenburg M.E., et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: Results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res. Treat. 2011;132:1049–1062. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liedtke C., Mazouni C., Hess K.R., André F., Tordai A., Mejia J.A., Symmans W.F., Gonzalez-Angulo A.M., Hennessy B., Green M., et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J. Clin. Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 22.Masuda H., Baggerly K.A., Wang Y., Zhang Y., Gonzalez-Angulo A.M., Meric-Bernstam F., Valero V., Lehmann B.D., Pietenpol J.A., Hortobagyi G.N., et al. Differential Response to Neoadjuvant Chemotherapy Among 7 Triple-Negative Breast Cancer Molecular Subtypes. Clin. Cancer Res. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Xu B., Yuan P., Li Q., Zhang P., Cai R., Ma F., Fan Y., Luo Y. HER2 as a Predictive Factor for Successful Neoadjuvant Anthracycline Chemotherapy of Locally Advanced and Early Breast Cancer. Int. J. Biol. Markers. 2014;29:187–192. doi: 10.5301/jbm.5000094. [DOI] [PubMed] [Google Scholar]
- 24.Templeton A.J., Ace O., McNamara M.G., Al-Mubarak M., Vera-Badillo F.E., Hermanns T., Seruga B., Ocana A., Tannock I.F., Amir E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Prev. Biomark. 2014;23:1204–1212. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 25.Asano Y., Kashiwagi S., Onoda N., Noda S., Kawajiri H., Takashima T., Ohsawa M., Kitagawa S., Hirakawa K. Predictive Value of Neutrophil/Lymphocyte Ratio for Efficacy of Preoperative Chemotherapy in Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2015;23:1104–1110. doi: 10.1245/s10434-015-4934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huszno J., Kolosza Z. Prognostic value of the neutrophil-lymphocyte, platelet-lymphocyte and monocyte-lymphocyte ratio in breast cancer patients. Oncol. Lett. 2019;18:6275–6283. doi: 10.3892/ol.2019.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Huang Z., Wang Q., Sun B., Ding L., Meng X., Wu S. Usefulness of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in hormone-receptor-negative breast cancer. OncoTargets Ther. 2016;9:4653–4660. doi: 10.2147/OTT.S106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Losada B., Guerra J.A., Malón D., Jara C., Rodriguez L., Del Barco S. Pretreatment neutrophil/lymphocyte, platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/monocyte ratios and outcome in elderly breast cancer patients. Clin. Transl. Oncol. 2018;21:855–863. doi: 10.1007/s12094-018-1999-9. [DOI] [PubMed] [Google Scholar]
- 29.Cuello-López J., Fidalgo-Zapata A., López-Agudelo L., Vasquez-Trespalacios E.M. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS ONE. 2018;13:e0207224. doi: 10.1371/journal.pone.0207224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano Y., Kashiwagi S., Onoda N., Noda S., Kawajiri H., Takashima T., Ohsawa M., Kitagawa S., Hirakawa K. Platelet–Lymphocyte Ratio as a Useful Predictor of the Therapeutic Effect of Neoadjuvant Chemotherapy in Breast Cancer. PLoS ONE. 2016;11:e0153459. doi: 10.1371/journal.pone.0153459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiranata S., Anjani I.A.W., Saputra I.P.G.S., Sadvika I.G.A.S., Prabawa I.P.Y., Supadmanaba I.G., Wihandani D.M., Adiputra P.A.T., Sudarsa I.W., Lestari A.A.W. Pretreatment Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as a Stage Determination in Breast Cancer. Open Access Maced. J. Med Sci. 2020;8:1058–1063. doi: 10.3889/oamjms.2020.5336. [DOI] [Google Scholar]
- 32.Research C for DE and Pathological Complete Response in Neoadjuvant Treatment of High-Risk EarlyStage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. In U.S. Food and Drug Administration. [(accessed on 10 May 2022)]; Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pathologicalcomplete-response-neoadjuvant-treatment-high-risk-early-stage-breast-cancer-use.
- 33.Gourgou-Bourgade S., Cameron D., Poortmans P., Asselain B., Azria D., Cardoso F., A’Hern R., Bliss J., Bogaerts J., Bonnefoi H., et al. Guidelines for time-to-event end point definitions in breast cancer trials: Results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials) Ann. Oncol. 2015;26:873–879. doi: 10.1093/annonc/mdv106. [DOI] [PubMed] [Google Scholar]
- 34.Anderson J.R., Cain K.C., Gelber R.D. Analysis of Survival by Tumor Response and Other Comparisons of Time-to-Event by Outcome Variables. J. Clin. Oncol. 2008;26:3913–3915. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 35.Scott M., Vandenberghe M.E., Scorer P., Boothman A.-M., Barker C. Prevalence of HER2 low in breast cancer subtypes using the VENTANA anti-HER2/neu (4B5) assay. J. Clin. Oncol. 2021;39:1021. doi: 10.1200/JCO.2021.39.15_suppl.1021. [DOI] [Google Scholar]
- 36.Brasó-Maristany F., Paré L., Chic N., Martínez-Sáez O., Pascual T., Mallafré-Larrosa M., Schettini F., González-Farré B., Sanfeliu E., Martínez D., et al. Gene expression profiles of breast cancer metastasis according to organ site. Mol. Oncol. 2021;16:69–87. doi: 10.1002/1878-0261.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prat A., Adamo B., Cheang M.C.U., Anders C.K., Carey L.A., Perou C.M. Molecular Characterization of Basal-Like and Non-Basal-Like Triple-Negative Breast Cancer. Oncologist. 2013;18:123–133. doi: 10.1634/theoncologist.2012-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehmann B.D., Pietenpol J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014;232:142–150. doi: 10.1002/path.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huober J., Von Minckwitz G., Denkert C., Tesch H., Weiss E., Zahm D.M., Belau A., Khandan F., Hauschild M., Thomssen C., et al. Effect of neoadjuvant anthracycline–taxane-based chemotherapy in different biological breast cancer phenotypes: Overall results from the GeparTrio study. Breast Cancer Res. Treat. 2010;124:133–140. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- 40.Gonçalves A. Chimiothérapie néo-adjuvante des cancers du sein HER2-positifs et triple-négatifs. Bull. Cancer. 2016;103:S76–S89. doi: 10.1016/S0007-4551(16)30149-7. [DOI] [PubMed] [Google Scholar]
- 41.Wang J., Xu B., Yuan P., Zhang P., Li Q., Ma F., Fan Y. TOP2A amplification in breast cancer is a predictive marker of anthracycline-based neoadjuvant chemotherapy efficacy. Breast Cancer Res. Treat. 2012;135:531–537. doi: 10.1007/s10549-012-2167-5. [DOI] [PubMed] [Google Scholar]
- 42.Santonja A., Sánchez-Muñoz A., Lluch A., Chica-Parrado M.R., Albanell J., Chacón J.I., Antolín S., Jerez J.M., De La Haba J., De Luque V., et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget. 2018;9:26406–26416. doi: 10.18632/oncotarget.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Echavarria I., López-Tarruella S., Picornell A.C., García-Saenz J., Jerez-Gilarranz Y., Hoadley K.A., Gómez H.L., Moreno F., Del Monte-Millan M., Márquez-Rodas I., et al. Pathological Response in a Triple-Negative Breast Cancer Cohort Treated with Neoadjuvant Carboplatin and Docetaxel According to Lehmann’s Refined Classification. Clin. Cancer Res. 2018;24:1845–1852. doi: 10.1158/1078-0432.CCR-17-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denkert C., Seither F., Schneeweiss A., Link T., Blohmer J.-U., Just M., Wimberger P., Forberger A., Tesch H., Jackisch C., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22:1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 45.Leite L.D.M., Cesca M.G., Tavares M.C., Santana D.M., Saldanha E.F., Guimarães P.T., Sá D.D.S., Simões M.F.E., Viana R.L., Rocha F.G., et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res. Treat. 2021;190:155–163. doi: 10.1007/s10549-021-06365-7. [DOI] [PubMed] [Google Scholar]
- 46.Reinert T., Sartori G.P., AB Souza A., Pellegrini R., Rosa M.L., Rossatto N., Coelho G.P., Litvin I.E., Zerwes F., Millen E., et al. Abstract PS4-22: Prevalence of HER2-low and HER2-zero subgroups and correlation with response to neoadjuvant chemotherapy (NACT) in patients with HER2-negative breast cancer. Cancer Res. 2021;81:PS4-22. doi: 10.1158/1538-7445.sabcs20-ps4-22. [DOI] [Google Scholar]
- 47.Rossi V., Sarotto I., Maggiorotto F., Berchialla P., Kubatzki F., Tomasi N., Redana S., Martinello R., Valabrega G., Aglietta M., et al. Moderate Immunohistochemical Expression of HER-2 (2+) Without HER-2 Gene Amplification Is a Negative Prognostic Factor in Early Breast Cancer. Oncologist. 2012;17:1418–1425. doi: 10.1634/theoncologist.2012-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Azambuja E., Cardoso F., De Castro G., Colozza M., Mano M.S., Durbecq V., Sotiriou C., Larsimont D., Piccart-Gebhart M., Paesmans M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12 155 patients. Br. J. Cancer. 2007;96:1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colozza M., Azambuja E., Cardoso F., Sotiriou C., Larsimont D., Piccart M.J. Proliferative markers as prognostic and predictive tools in early breast cancer: Where are we now? Ann. Oncol. 2005;16:1723–1739. doi: 10.1093/annonc/mdi352. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X., Chen L., Huang B., Wang Y., Ji L., Wu J., Di G., Liu G., Yu K., Shao Z., et al. The prognostic and predictive potential of Ki-67 in triple-negative breast cancer. Sci. Rep. 2020;10:225. doi: 10.1038/s41598-019-57094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denkert C., Loibl S., Müller B.M., Eidtmann H., Schmitt W., Eiermann W., Gerber B., Tesch H., Hilfrich J., Huober J., et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: A translational investigation in the neoadjuvant GeparTrio trial. Ann. Oncol. 2013;24:2786–2793. doi: 10.1093/annonc/mdt350. [DOI] [PubMed] [Google Scholar]
- 52.Seretis C., Seretis F., Lagoudianakis E., Politou M., Gemenetzis G., Salemis N.S. Enhancing the Accuracy of Platelet to Lymphocyte Ratio after Adjustment for Large Platelet Count: A Pilot Study in Breast Cancer Patients. Int. J. Surg. Oncol. 2012;2012:653608. doi: 10.1155/2012/653608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krenn-Pilko S., Langsenlehner T., Thurner E.-M., Stojakovic T., Pichler M., Gerger A., Kapp K.S. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br. J. Cancer. 2014;110:2524–2530. doi: 10.1038/bjc.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J.-Y., Jung E.J., Kim J.-M., Lee H.S., Kwag S.-J., Park J.-H., Park T., Jeong S.-H., Jeong C.-Y., Ju Y.-T. Dynamic changes of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predicts breast cancer prognosis. BMC Cancer. 2020;20:1206. doi: 10.1186/s12885-020-07700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marchiò C., Annaratone L., Marques A., Casorzo L., Berrino E., Sapino A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2020;72:123–135. doi: 10.1016/j.semcancer.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Sapino A., Goia M., Recupero D., Marchiò C. Current Challenges for HER2 Testing in Diagnostic Pathology: State of the Art and Controversial Issues. Front. Oncol. 2013;3:129. doi: 10.3389/fonc.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim C., Gao R., Sei E., Brandt R., Hartman J., Hatschek T., Crosetto N., Foukakis T., Navin N.E. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell. 2018;173:879–893.e13. doi: 10.1016/j.cell.2018.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garrido-Castro A.C., Lin N.U., Polyak K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019;9:176–198. doi: 10.1158/2159-8290.CD-18-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The datasets supporting the conclusions of this article are included within the article (and its additional files).