Abstract
The dynamics of the microbial food sources for Aedes triseriatus larvae in microcosms were found to be strongly influenced by larval presence. The total abundance of bacteria in water samples generally increased in response to larvae, including populations of cultivable, facultatively anaerobic bacteria. Additionally, a portion of the community shifted from Pseudomonaceae to Enterobacteriaceae. Bacterial abundance on leaf material was significantly reduced in the presence of actively feeding larvae. Principle-component analysis of whole community fatty acid methyl ester (FAME) profiles showed that larvae changed the microbial community structure in both the water column and the leaf material. Cyclopropyl FAMEs, typically associated with bacteria, were reduced in microcosms containing larvae; however, other bacterial fatty acids showed no consistent response. Long-chain polyunsaturated fatty acids characteristic of microeukaryotes (protozoans and meiofauna) declined in abundance when larvae were present, indicating that larval feeding reduced the densities of these microorganisms. However, presumed fungal lipid markers either increased or were unchanged in response to larvae. Larval presence also affected microbial nitrogen metabolism through modification of the physiochemical conditions or by grazing on populations of bacteria involved in nitrification-denitrification. Stemflow primarily influenced inorganic ion and organic compound concentrations in the microcosms and had less-pronounced effects on microbial community parameters than did larval presence. Stemflow treatments diluted concentrations of all inorganic ions (chloride, sulfate, and ammonium) and organic compounds (total dissolved organic carbon, soluble carbohydrates, and total protein) measured, with the exceptions of nitrite and nitrate. Stemflow addition did not measurably affect larval biomass in the microcosms but did enhance development rates and early emergence patterns of adults.
Larvae of the La Crosse encephalitis vector, Aedes triseriatus, develop in water-filled treeholes and artificial containers (e.g., tires) in the eastern United States. These small aquatic habitats are detritus-based ecosystems with mosquito larvae often being the top-level consumer (30). Treehole mosquito production is thought to be closely linked to the quantity and quality of deciduous leaf litter input (51, 76, 85), the input of nutrients contained in stemflow and rainwater (14, 84), and the density of larvae (15, 29). The consistent demonstration of competitive, density-dependent growth of treehole mosquitoes (see, for example, references 8, 15, 24, 37, 51, 52, and 53) raises the question of what specifically defines the limiting resource in these habitats. Observations of feeding behavior and gut analysis indicate that A. triseriatus larvae actively filter the water column and graze on surfaces (59, 81, 82). Bacteria appear to be the primary food item for A. triseriatus and related species (59, 83), although many types of protozoans, fungi, and other microeukaryotes are also consumed (50, 59). Since A. triseriatus larvae ingest little of the large particulate matter (i.e., leaves) directly, the quality and availability of heterotrophic microbial biomass are likely the most important factors in larval growth and intraspecific resource limitation.
Although A. triseriatus larvae meet the bulk of their nutritional needs from microbial cells and/or metabolites, there is a conspicuous lack of understanding beyond these general observations. The eventual transformation of leaf litter and stemflow nutrients into mosquito biomass is clearly mediated by microorganisms and their activities, but the term “microorganism” and its synonyms have often been used in ecological studies with the implication that they represent a static, nutritionally homogeneous group of decomposers. This oversimplification ignores the dynamics of the system potentially induced by stemflow events, temporal decomposition processes, and larval activity. For example, bacterial abundance in treehole systems has been shown to fluctuate considerably during the larval development period and differs in the presence versus the absence of larvae (84). Cochran-Stafira and von Ende (18) have recently shown that pitcher plant mosquito larvae, Wyeomyia smithii, can strongly influence the abundance and composition of bacterial and protozoan communities in simulated larval habitats. In addition to fluctuations in overall microbial biomass and composition, microbial taxa may also vary widely in their biochemical and nutritional content and in their susceptibility to invertebrate digestive processes (6, 58, 64). Understanding this nutritional variability is an important prerequisite toward understanding larval development in these habitats. The proposed use of transgenic microorganisms as mosquito larvicides (65) further necessitates a prior knowledge of microbial community dynamics and the factors controlling them.
In this study, we examined some of the potential interactions between A. triseriatus larvae, stemflow flushing events, and microbial community function and composition through the use of microcosms. We concentrated on examining changes in the bacterial community because previous work suggested the numerical importance of bacteria to A. triseriatus feeding ecology (83, 84). Our overall goal was to relate larval activity to microbial community characteristics and to reveal microbial community attributes which need to be addressed in future field and nutritional studies. A key aspect of the research was to examine microbial changes with or without mosquito larvae because larval feeding and activity likely select for different microbial populations. Our approach was to examine changes in the microbial community from several levels: (i) total bacterial numbers (direct microscopic counts [DMCs]), (ii) cultivable bacteria (CFU on general media), (iii) bacterial isolate identification, (iv) whole community fatty acid methyl ester (FAME) patterns, and (v) inorganic ion and organic nutrient concentrations as indicators of microbial metabolism.
MATERIALS AND METHODS
Microcosm experimental setup and sampling.
Microcosms were similar to those illustrated in Walker et al. (84) and were constructed from 2-liter plastic food containers. An overflow spout (closed with 70-μm [pore size] nylon mesh) was inserted at the 1-liter level. Each microcosm contained 3 g (dry weight) of beech leaf litter and 1 liter of stemflow collected from a single beech tree at a study site near East Lansing, Mich., and stored at 4°C. Leaf material was added without prior leaching. A 2-ml aliquot of homogenized contents from several beech treeholes was used to inoculate the microcosms. Microcosms were then conditioned at 15°C for 10 days. Four days prior to the addition of A. triseriatus larvae, the temperature was slowly increased and then maintained at the experimental temperature of 20°C under 12 h of light and 12 h of dark indirect fluorescent lighting. A. triseriatus eggs were collected from tires placed at East Lansing field sites and from laboratory stocks. First-instar larvae (24 to 36 h old) were added to one-half of the microcosms in batches of 100/microcosm. Henceforth, the date of larval addition will be referred to as day zero.
Initially, 52 microcosms (44 used in the experiment plus 2 extra per treatment) were set up and randomly assigned to four treatment groups of unequal size as follows: (i) to receive no larvae or stemflow (n = 18 microcosms), (ii) to receive larvae but no stemflow (n = 14), (iii) to receive stemflow only (n = 10), and (iv) to receive larvae and stemflow (n = 10). Four randomly selected microcosms from each group were destructively sampled on days 0, 7, 14, and 21. The group sizes were unequal because microcosms did not receive larvae until after day 0 sampling or receive stemflow until after day 7 sampling. Therefore, only group 1 was sampled on each of the 4 days. Group 2 was sampled on days 7, 14, and 21, and groups 3 and 4 were sampled on days 14 and 21.
Stemflow was added to the appropriate treatments in 1-liter aliquots by pouring it through a funnel equipped with tubing and a syringe needle (16 gauge) to control the rate of flow and to simulate a natural flushing rate. Microcosms were visually inspected every 2 days from day 7 to day 21 for the presence of emergent adults. If present, adults were collected with an aspirator and dried (45°C) for 48 h before being weighed.
Microcosms were sampled and dismantled as follows. The pH of the water column at mid depth was measured with a standard pH electrode (Orion Research, Inc., Cambridge, Mass.), and the contents of each container were then gently poured through 100-μm (pore-size) mesh nylon screen to separate the water column from large detritus particles. The sieved water was then immediately subsampled for bacterial isolations and direct microscopic counts. Two 10-ml subsamples were frozen (−70°C) for later chemical analyses. The remainder of the water was refrigerated until the lipid extraction step. Detritus particles and larvae retained on the screen or in the original container were immediately refrigerated until sorted. Leaf material (individual leaves and large fragments) was gently rinsed in sterile, chilled potassium phosphate buffer (0.1 mM, pH 7) and visually inspected for larvae or purpae. Leaf material was then stacked and subsampled by slicing vertically through the center of the stack with a razor blade. The subsamples, 1 g (wet weight) for DMCs and 2 g (wet weight) for lipid analysis, included fragments from both near the midvein and the leaf margins. Sections of each major leaf fragment and horizontal zone on each leaf were thus included in the subsamples.
DMCs.
The DAPI (4′,6-diamidino-2-phenylindole) fluorescent staining procedure (66, 83) was used to count bacteria in water and detritus samples. A 1-ml portion of water column was preserved with formalin (3.4% final formaldehyde concentration), and a 1 g of leaf material subsample was preserved in 10 ml of buffered formalin (3.7% final formaldehyde concentration). Leaf material was homogenized on ice with a Tissuemizer (Tekmar Corp., Cincinnati, Ohio). Appropriate dilutions of both water column and detritus were filtered onto 0.2-μm-pore-size black Nucleopore filters (Costar, Cambridge, Mass.) and stained with DAPI at a final concentration of 2 μg/ml for 5 min. Two filters and at least 10 fields per filter (200 cells per filter) were counted for each subsample.
Bacterial isolates.
Cultivable bacteria in microcosm water samples were enumerated from general media after incubation under aerobic and anaerobic conditions. Then, 1 ml of the water column was added to 9 ml of sterile, deoxygenated potassium phosphate buffer (0.1 mM, pH 7) under a stream of N2 and transferred to an anaerobic glove box (atmosphere of 10% H2, 5% CO2, and 85% N2) for serial dilution and subsequent plating on anaerobic Trypticase soy agar (TSA; Difco, Inc.). Anaerobic TSA was prepared according to strict anaerobic procedures (41) and differed compositionally from aerobic TSA by the presence of hemin, cysteine, vitamin K, and resazurin (redox indicator). Plates were incubated in the glove box (25 ± 2°C), and colonies were counted daily for 1 week. Plate counts on aerobic TSA and R2A media were obtained from serial dilutions of 1 ml of water column in sterile potassium phosphate buffer (0.1 mM, pH 7). TSA is a rich, nonselective medium for heterotrophic bacteria, and R2A is a more-defined, nutrient-dilute, and nonselective medium found to give good counting efficiency for aquatic bacteria (68).
Countable aerobic TSA plates from the above study were used as a source of isolates for bacterial community composition analysis. All colonies from a randomly chosen section (one-quarter of the total plate surface area) of each replicate were picked, streaked for isolation, and identified with the microbial identification system (MIS [described below]). Day 14 isolates were excluded because of low numbers of total colonies (<20) on readable plates in some replicates and to keep the total number of isolates manageable. Therefore, bacterial taxon compositional data was collected only for days 0, 7, and 21.
FAMEs.
Whole-community and individual-isolate fatty acid profiles were characterized by using MIS (MIDI, Inc., Newark, Del. [61]). The principle of the system relies upon the unique patterns of cellular fatty acids and other lipids associated with microbial taxa (61, 70) and has been used previously to distinguish microbial communities (13, 16, 35). We employed standard nomenclature in referring to individual compounds. Fatty acids are listed in the form C:X, where “C” is total number of compounds and “X” refers to the degree of unsaturation (number of double bonds present). With unsaturated acids, the designation may include the location of the double bonds in the format wZc or wZt, where Z indicates the number of carbons from the methyl end of the molecule (w), and c or t refers to a cis or trans orientation. Branched-chain fatty acids are prefixed with an i for iso or an a for anteiso methyl group positions. Additionally, locations of other methyl or hydroxyl groups may be noted as a suffix in the form AMe or AOH, where “A” refers to the number of carbons from the carboxyl end of the molecule. A cy prefix refers to cyclopropyl fatty acids.
Identification of isolates.
Isolates were treated according to standard MIS protocol (35, 61). Briefly, this procedure calls for growing the cells on TSBA for 24 to 48 h, saponification of whole cells in methanolic NaOH, esterification of fatty acids in acidic methanol, and extraction of FAMEs with methyl-tert-butyl ether/hexane. Analysis of FAMEs by capillary gas chromatography was done according to standard MIS procedure (61). Because several bacterial groups identified in this study (e.g., Enterobacteriaceae and Pseudomonaceae) are ill defined by the MIDI system at the genus or species level and because isolates were grown on TSA instead of TSBA, we chose to analyze and present isolate data in the form of higher taxonomic levels (family) or Gram stain groups.
Whole-community FAME profiles.
Whole-community lipids were extracted as follows. The entire water column remaining after subsampling (ca. 900 ml) was filtered through glass fiber filters (nominal pore size of 1.0 μm, type A/E; Gelman Sciences, Ann Arbor, Mich.), and the microbial biomass in the filtrate was concentrated by centrifugation. The water column pellet and filters from each sample were then combined. Water column and leaf material samples were then extracted similarly by a modification of the standard Bligh and Dyer (9) procedure in which dichloromethane replaces chloroform and a supersaturated sodium bromide solution aids in the biphasic separation (63). A known aliquot of the organic phase was dried under N2, and fatty acids were saponified and derivatized according to the procedures of Dowling et al. (22) as described by Peterson and Klug (63). FAMEs were concentrated under N2 and analyzed by capillary gas chromatography as described above (63). Compounds were identified with MIS software, which relies on the relative retention times of peaks. As an internal control, we also added standard mixes from MIDI, Inc. (24 compounds, 10:0 to 30:0 saturated), to representative samples after extraction. Additionally, standard methyl esters of the long-chain polyunsaturated fatty acids arachidonate (20:4 w6) and docosahexanoate (22:6 w3) obtained from Sigma Chemical (St. Louis, Mo.) were added to leaf material FAME samples after extraction in order to verify the positions of peaks in complex samples.
The above-described technique does not partition the phospholipid fatty acid (PLFA) fraction and therefore yields a greater quantity and diversity of FAMEs. However, resultant FAME profiles require more extensive interpretation because of the presence of nonmicrobial lipids (19). Additionally, limitations of the standard MIDI injection system restrict the sensitivity of the analysis (63). We chose this more-direct lipid extraction–“whole-cell” analysis for several reasons. First, non-PLFA lipids are potentially valuable as microbial group markers (61, 80, 91) and as nutrients for mosquito larvae (75). Second, our preliminary trials with PLFA analysis on 1-liter microcosm water samples indicated low and variable recovery. Treatment effects were distinguishable with principal component analysis (PCA [see below]); however, PCA scores from PLFAs were significantly correlated with total peak area extracted, suggesting that component fatty acid patterns were partially determined by the amount of phospholipid present. The whole-cell FAME technique yielded higher and more-consistent levels of total peak area which showed no correlation with PCA score. PLFA techniques would likely have been more appropriate for the higher levels and increased diversity of lipids in leaf extracts, but we chose to keep the water column and leaf material lipid analyses comparable.
In order to manage the analyses and to examine lipids which came primarily from microbial sources, we eliminated peaks which (i) eluted later than 24:0, (ii) were present in less than 10% of all samples, and (iii) were likely derived from the original beech leaf material. In the latter category, we eliminated all primary alcohols and long-chain (>20 carbons) hydrocarbons which likely were derived from leaf cuticular compounds (19, 21, 38). We also eliminated palmitic acid (16:0) and stearic acid (18:0) peaks from analysis because they are ubiquitous in organisms (including higher plants) and therefore have limited value in distinguishing microbial groups. Finally, in order to meet criteria that the number of variables not exceed the number of samples for PCA and similar multivariate analyses (90), we eliminated minor FAMES (those whose average mole percent value was ≤0.1) from the leaf material data set. The final subsets of FAMEs were derived from 70 and 84 different MIDI-recognized peaks in water and leaf samples, respectively. Most of the reduction from the original datasets resulted from the removal of rare (found in <10% of samples) peaks or very-long-chain hydrocarbons. Total peak areas in the original data sets were 1.5 and 2 times higher than the reduced data sets for water and leaf samples, respectively. Preliminary exploration of the data with PCA (see below) revealed that none of the eliminated peaks would have contributed substantially to component loadings.
Inorganic anions and ammonium.
Concentrations of inorganic anions (nitrite, nitrate, and sulfate) previously implicated as potentially important in microbial metabolism in treeholes (84) were measured with chemical suppression ion chromatography and conductivity detection (Dionex model 4500i; Dionex Corp., Sunnyvale, Calif. [40]). Chloride was also monitored by using the same analysis in a role as a conservative (nonmetabolizable) tracer. Water samples were first filtered through glass fiber filters (Gelman type A/E). Ammonium concentrations were determined colorimetrically (indophenol blue reaction [17]) with the aid of an Alpkem automated flow analyzer (Alpkem Corp., Wilsonville, Oreg.) on nonfiltered samples.
Organic compounds.
After filtration (Gelman type A/E), dissolved organic carbon (DOC) in water column samples was analyzed with an Ionics TOC Laboratory Carbon Analyzer (model 1505; Ionics, Inc., Watertown, Mass.). Briefly, inorganic carbon (carbonates, CO2) was purged from the samples with acidification (1 N H2SO4) and sparging with N2. Subsequent oxidation of the remaining organic carbon to CO2 was accomplished with a platinum catalyst in a high-temperature (850°C) chamber. CO2 released upon oxidation was quantified with an infrared gas analyzer. Soluble carbohydrates were determined by the phenol-sulfuric acid method (23) with glucose as a standard. Total protein in nonfiltered samples was quantified by the Folin-Ciocalteu reagent method (55) with modifications suggested for water high in organics (79). Bovine serum albumin (Sigma Chemical) was used for standard-curve preparations.
Statistics.
Because the stemflow was not added as a treatment until after the second sampling date, all data except for fatty acid profiles and individual FAME concentrations were analyzed with t tests for day 7 comparisons (effect of larvae only) and full-factorial (time, larvae, and stemflow effects) analysis of variance (ANOVA) for the final two sampling dates. Day zero data is included for illustration in all figures but was not included in the analyses. Data were transformed appropriately (log x+1, square root, or arcsine-square root for percentage data), if necessary, based upon Bartlett’s test for homogeneity of variance (72). The statistical package used was SYSTAT 5.2.1 (89) with the general linear model ANOVA (MGLH subprogram).
FAMEs from whole-community lipid extracts were expressed as mole percentages of total peaks used in the analysis (16, 35, 63). For PCA, these percentages were transformed by the log-ratio method of Aitchison (2) to overcome the problems associated with constrained data sets, e.g., correlations among individual variables which sum to a constant are not free to vary between −1 and 1 (42). The transformation is given by the formula y = log(a/ü), where a is the FAME percentage value and ü is the geometric mean of percentage values within the sample. PCA was performed on the transformed data set by using a covariance matrix in the SYSTAT 5.2.1-FACTOR subprogram (89). Prior to the calculation of percentages for use in PCA, all zero (nondetectable) values were replaced with a value corresponding to 50% of the GC detection limit used in the assay.
Because fatty acids represent nutritionally valuable compounds in addition to serving as indicators of microbial community structure, we compared the treatment effects on individual FAMEs and/or groups of related FAMEs by using total amounts (in micrograms) in each sample rather than the percentages. A conversion factor of 0.73 ng/U of peak area based on a C19:0 FAME standard was used to calculate FAME quantity (35). Because of the presence of all zero values (nondetectable quantities) and the resultant lack of variance in some FAME groups, we used the Kruskal-Wallis nonparametric test (SYSTAT 5.2.1, NPAR subprogram [89]) to test the main effects of larvae and stemflow on each of the three sampling dates. FAMEs and/or FAME groups analyzed were chosen based upon their prevalence in contributing to PC component loadings. Groups were formed from FAMEs with presumed common origins and/or functions; however, similar FAMEs with different PC loading signs were analyzed individually.
RESULTS
Bacterial abundance.
DMCs of bacteria in the microcosms’ water columns fluctuated slightly across treatments and during the course of the experiment (Fig. 1). Cell numbers ranged from 3.85 × 106 to 2.97 × 107 per ml and were increased by the larval presence, although ANOVA interaction terms indicated this effect was significant only on the final sampling date in the no-stemflow treatments (Table 1). DMCs of bacteria from leaf material (Fig. 1) varied less overall (range of 2.11 × 109 to 7.68 × 109) than water column DMCs and were significantly depressed by larval presence throughout the study (Table 1 and Fig. 1). Stemflow treatments had no main effect on bacterial abundance in the water column or in leaf material at the time points measured in this experiment.
FIG. 1.
Bacterial abundance as DMCs in microcosm leaf material and water columns and CFU in the water column only. Values are means ± 1 standard error (SE); n = 3 or 4 for each point. Arrows denote the times of stemflow additions.
TABLE 1.
Probability values from t tests (day 7 only) and factorial ANOVA of bacterial abundance in mosquito microcosms
| Variable |
Pa at day(s):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 7
|
14 and 21
|
|||||||
| Larvae | Time | Larvae | Stemflow | Time × larvae | Time × stemflow | Larvae × stemflow | Time × larvae × stemflow | |
| CFU (aerobic TSA) | 0.326 | 0.356 | 0.018 | 0.961 | 0.621 | 0.963 | 0.315 | 0.304 |
| CFU (anaerobic TSA) | 0.309 | 0.816 | 0.000 | 0.969 | 0.000 | 0.138 | 0.860 | 0.278 |
| CFU (aerobic R2A) | 0.949 | 0.953 | 0.046 | 0.500 | 0.121 | 0.210 | 0.321 | 0.672 |
| DMC (water) | 0.459 | 0.059 | 0.022 | 0.255 | 0.046 | 0.294 | 0.504 | 0.040 |
| DMC (leaf) | 0.014 | 0.652 | 0.020 | 0.584 | 0.781 | 0.267 | 0.728 | 0.765 |
Total n = 8 for each variable on day 7, and n = 30 to 32 for each variable in the ANOVA.
Numbers of cultivable bacteria (CFU) from the water column were lower than DMCs and showed different trends depending upon the medium type (Fig. 1). There were larva effects on the CFU from all medium types, since larval presence apparently enhanced cultivable bacterial numbers (Table 1). The most pronounced larval-presence effect was observed in the relatively higher numbers of bacteria capable of growing anaerobically on TSA. The decline in anaerobic-facultative populations seen over the course of the experiment in the larva-absent treatments did not occur in microcosms with larvae (Fig. 1).
Bacterial isolates.
Both larvae and stemflow influenced isolates obtained on aerobic TSA (Fig. 2). The presence of larvae significantly reduced the percentages of pseudomonads on day 7, although this group declined over time in the experiment overall (Fig. 2 and Table 2). Larval presence also significantly increased the percentages of enteric bacteria, while stemflow enhanced non-Bacillus gram-positive organisms (Fig. 2 and Table 2). Unidentified isolates comprised a greater proportion of cultivable bacteria in the later stages of the experiment (Fig. 2). Bacillus spp. and gram-negative organisms other than pseudomonads or enteric bacteria were always minor proportions of the isolates and did not vary with any treatment (Fig. 2 and Table 2).
FIG. 2.
Composition of cultivable (TSA medium) bacteria from microcosm water columns. Values are mean percentages of total identifications ± 1 SE; n = 3 or 4 for each point.
TABLE 2.
Probability values from t tests (day 7 only) and factorial ANOVA of cultivable bacterial taxonomic groups (analyzed as percentages of total isolates) in mosquito microcosms
| Group |
Pa at day:
|
|||
|---|---|---|---|---|
| 7
|
21
|
|||
| Larvae | Larvae | Stemflow | Larvae × stemflow | |
| % Pseudomonads | 0.016 | 0.072 | 0.610 | 0.955 |
| % Enteric bacteria | 0.006 | 0.041 | 0.161 | 0.110 |
| % Bacillus spp. | 0.536b | 0.720 | 0.996 | 0.789 |
| % Other gram-negative bacteria | 0.101 | 0.436 | 0.120 | 0.048 |
| % Other gram-positive bacteria | 0.322 | 0.171 | 0.006 | 0.890 |
| % Unidentified | 0.903 | 0.463 | 0.560 | 0.374 |
Total n = 8 for each variable on day 7, and n = 16 for each variable in the ANOVA.
The Kruskal-Wallis nonparametric analysis was used for Bacillus spp. (day 7 only) due to all the zero values being in one group.
Whole-community FAME profiles.
We utilized subsets of 31 and 44 individual FAMEs for profiles of the water column (Table 3) and leaf material (Table 4), respectively. Of these, monounsaturated fatty acids of 16 and 18 carbons, as well as longer-chain (>20 carbons) acids, tended to account for the greatest relative percentage in water column samples (Table 3), while a 16-carbon monounsaturated acid; a 19-carbon, dimethyl acetyl cyclopropyl acid; unresolved 18 carbon compounds (18:2w6c and a-18:0); and longer-chain (>20 carbons) fatty acids dominated the subset of leaf material FAMEs (Table 4). Branched-chain and monounsaturated fatty acids were also common in both water and leaf samples.
TABLE 3.
Moles percent values for individual FAMEs extracted from microcosm water columns and used in PCAa
| FAME | Mean mol% (SE)
|
||||
|---|---|---|---|---|---|
| Initial (time zero [n = 4]) | Larvae absent
|
Larvae present
|
|||
| No stemflow (n = 12) | Stemflow (n = 7) | No stemflow (n = 12) | Stemflow (n = 8) | ||
| 12:0 | 0.00 (0.00) | 0.06 (0.06) | 0.00 (0.00) | 0.22 (0.09) | 0.07 (0.07) |
| i-14:0 | 6.48 (1.36) | 1.43 (0.38) | 0.44 (0.28) | 0.81 (0.21) | 0.37 (0.19) |
| a-14:0 | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.45 (0.17) | 0.73 (0.30) |
| 14:0 | 3.29 (0.60) | 3.53 (0.38) | 4.12 (0.41) | 2.81 (0.16) | 3.09 (0.29) |
| i-15:1 | 0.26 (0.26) | 0.04 (0.04) | 0.00 (0.00) | 1.05 (0.28) | 0.45 (0.22) |
| i-15:0 | 3.18 (0.24) | 2.92 (0.19) | 3.54 (0.29) | 4.30 (0.21) | 3.31 (0.24) |
| a-15:0 | 2.19 (0.25) | 1.89 (0.14) | 1.68 (0.47) | 3.41 (0.18) | 2.64 (0.20) |
| 15:0 | 1.32 (0.10) | 1.03 (0.24) | 1.19 (0.42) | 1.52 (0.23) | 1.46 (0.39) |
| i-16:0 | 1.19 (0.08) | 1.00 (0.25) | 0.56 (0.26) | 1.15 (0.22) | 0.81 (0.25) |
| 16:1 w11c | 5.90 (0.92) | 1.36 (0.51) | 0.00 (0.00) | 0.31 (0.16) | 0.00 (0.00) |
| 16:1 w7c | 23.10 (2.07) | 26.20 (2.33) | 26.52 (1.81) | 20.66 (0.96) | 17.40 (1.50) |
| 16:1 w5c | 1.91 (0.17) | 1.58 (0.32) | 1.33 (0.49) | 2.42 (0.30) | 2.68 (0.37) |
| i-17:1 w8 | 2.08 (0.27) | 0.80 (0.30) | 0.00 (0.00) | 0.60 (0.22) | 0.13 (0.13) |
| i-17:0 | 0.75 (0.44) | 0.76 (0.25) | 0.40 (0.40) | 0.80 (0.22) | 0.63 (0.36) |
| 17:1 w8c | 0.00 (0.00) | 0.40 (0.21) | 0.00 (0.00) | 0.65 (0.24) | 0.15 (0.15) |
| cy-17:0 | 3.26 (0.46) | 1.66 (0.28) | 0.79 (0.38) | 0.60 (0.27) | 0.00 (0.00) |
| 17:0 | 0.00 (0.00) | 0.18 (0.12) | 0.00 (0.00) | 0.37 (0.16) | 0.37 (0.27) |
| 18:1 w3c | 1.80 (0.28) | 2.18 (0.35) | 1.59 (0.68) | 1.95 (0.41) | 1.61 (0.58) |
| 18:1 w9c | 4.56 (0.53) | 6.71 (0.48) | 9.69 (1.72) | 10.37 (0.66) | 8.83 (0.87) |
| Σ 18:1 w7c, 18:1 w9t, 18:1 w12t | 12.94 (1.62) | 18.19 (1.29) | 14.48 (1.83) | 15.33 (0.79) | 11.81 (1.37) |
| cy-19:0 | 0.80 (0.40) | 2.51 (0.22) | 1.62 (0.60) | 1.62 (0.36) | 0.64 (0.31) |
| 18:0 2OH | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.45 (0.16) | 0.32 (0.21) |
| 20:4 w6c | 6.03 (0.51) | 4.69 (0.42) | 5.64 (1.08) | 2.81 (0.90) | 1.50 (0.60) |
| 20:5 w3c | 4.73 (0.79) | 3.05 (0.56) | 2.30 (0.65) | 1.47 (0.50) | 0.87 (0.33) |
| 20:2 w6c | 0.00 (0.00) | 0.09 (0.09) | 0.00 (0.00) | 0.21 (0.11) | 0.00 (0.00) |
| 20:0 | 0.00 (0.00) | 0.06 (0.06) | 0.00 (0.00) | 2.70 (0.28) | 2.56 (0.18) |
| 21:0 | 0.00 (0.00) | 0.14 (0.12) | 0.00 (0.00) | 1.19 (0.48) | 1.58 (0.64) |
| 22:6 w3c | 2.98 (0.79) | 2.38 (0.66) | 0.51 (0.34) | 0.33 (0.23) | 0.00 (0.00) |
| 22:0 | 5.34 (4.45) | 11.97 (5.77) | 5.65 (1.82) | 11.89 (1.71) | 20.37 (4.11) |
| 23:0 | 0.00 (0.00) | 0.05 (0.05) | 4.47 (4.47) | 0.07 (0.07) | 0.37 (0.37) |
| 24:0 | 0.20 (0.20) | 3.14 (1.17) | 11.31 (5.71) | 7.47 (1.68) | 14.84 (3.27) |
Σ denotes unresolved peaks. Values are mean mole percentages with one SE indicated in parentheses.
TABLE 4.
Moles percent values for individual FAMEs extracted from microcosm leaf material and used in PCAa
| FAME | Mean mol% (SE)
|
|||||
|---|---|---|---|---|---|---|
| Senescent beech leaf (n = 4) | Initial (time zero [n = 4]) | Larvae absent
|
Larvae present
|
|||
| No stemflow (n = 12) | Stemflow (n = 8) | No stemflow (n = 12) | Stemflow (n = 8) | |||
| 12:0 | 0.66 (0.03) | 0.66 (0.02) | 0.64 (0.05) | 0.59 (0.03) | 0.51 (0.06) | 0.45 (0.08) |
| i-14:0 | 0.00 (0.00) | 0.68 (0.04) | 0.52 (0.03) | 0.55 (0.02) | 0.40 (0.03) | 0.34 (0.05) |
| 14:0 | 2.41 (0.11) | 2.82 (0.03) | 2.96 (0.10) | 2.82 (0.10) | 2.79 (0.07) | 2.54 (0.14) |
| i-15:1 | 0.00 (0.00) | 0.79 (0.05) | 0.59 (0.05) | 0.57 (0.03) | 0.54 (0.06) | 0.52 (0.09) |
| i-15:0 | 0.41 (0.04) | 2.81 (0.62) | 2.94 (0.29) | 3.50 (0.28) | 3.58 (0.30) | 3.93 (0.74) |
| a-15:0 | 0.44 (0.06) | 1.86 (0.22) | 1.92 (0.14) | 2.00 (0.04) | 1.66 (0.12) | 1.62 (0.08) |
| 15:1 w6c | 0.41 (0.05) | 0.44 (0.03) | 0.53 (0.06) | 0.37 (0.02) | 0.47 (0.08) | 0.42 (0.07) |
| 15:0 | 1.24 (0.08) | 1.22 (0.08) | 1.26 (0.06) | 1.16 (0.05) | 1.46 (0.38) | 1.16 (0.07) |
| i-16:1 | 0.00 (0.00) | 0.80 (0.10) | 0.54 (0.07) | 0.58 (0.03) | 0.44 (0.08) | 0.40 (0.12) |
| i-16:0 | 0.08 (0.08) | 0.59 (0.02) | 0.51 (0.06) | 0.65 (0.03) | 0.53 (0.02) | 0.48 (0.08) |
| 16:1 w7c | 3.87 (0.46) | 11.06 (0.72) | 8.77 (0.49) | 7.22 (0.24) | 6.61 (0.35) | 6.50 (0.39) |
| 16:1 w5c | 0.45 (0.05) | 0.54 (0.06) | 0.50 (0.04) | 1.37 (0.27) | 0.86 (0.19) | 0.86 (0.26) |
| 16:1 w3c | 0.84 (0.09) | 3.78 (0.39) | 2.92 (0.33) | 3.27 (0.20) | 3.74 (0.22) | 3.14 (0.45) |
| i-17:1 w8 | 0.00 (0.00) | 1.17 (0.16) | 0.60 (0.08) | 0.51 (0.03) | 0.39 (0.06) | 0.34 (0.11) |
| i-17:0 | 0.00 (0.00) | 0.30 (0.05) | 0.38 (0.06) | 0.46 (0.03) | 0.26 (0.07) | 0.32 (0.11) |
| a-17:0 | 0.14 (0.14) | 0.29 (0.03) | 0.34 (0.03) | 0.34 (0.01) | 0.25 (0.04) | 0.16 (0.06) |
| 17:1 w8c | 1.69 (0.13) | 1.58 (0.07) | 1.70 (0.05) | 1.73 (0.04) | 1.86 (0.05) | 1.80 (0.09) |
| cy-17:0 | 0.75 (0.07) | 1.92 (0.14) | 1.87 (0.12) | 1.90 (0.04) | 1.70 (0.08) | 1.63 (0.09) |
| 17:0 | 1.15 (0.09) | 1.89 (0.10) | 1.76 (0.04) | 1.81 (0.04) | 1.92 (0.04) | 1.78 (0.11) |
| 18:3 w6c | 0.20 (0.12) | 0.47 (0.20) | 0.27 (0.09) | 0.56 (0.10) | 0.69 (0.43) | 0.79 (0.35) |
| i-18:0 | 0.00 (0.00) | 0.18 (0.11) | 0.22 (0.06) | 0.37 (0.04) | 0.26 (0.06) | 0.28 (0.09) |
| Σ 18:2 w6c, a-18:0 | 29.68 (0.69) | 7.14 (1.23) | 8.98 (0.96) | 10.80 (1.23) | 10.36 (1.00) | 11.34 (2.00) |
| 18:1 w9c | 31.61 (3.29) | 6.87 (0.65) | 9.18 (1.37) | 7.66 (0.44) | 7.71 (0.34) | 8.87 (1.19) |
| Σ 18:1 w7c, 18:1 w9t, 18:1 w12t | 0.00 (0.00) | 6.09 (0.29) | 4.80 (0.18) | 4.58 (0.13) | 4.19 (0.15) | 4.41 (0.25) |
| 17:0 3OH | 0.09 (0.09) | 0.27 (0.02) | 0.30 (0.07) | 0.22 (0.01) | 0.16 (0.04) | 0.19 (0.06) |
| 19:1 w11 | 0.00 (0.00) | 0.44 (0.04) | 0.30 (0.05) | 0.36 (0.01) | 0.32 (0.05) | 0.25 (0.08) |
| 19:1 w8 | 2.14 (0.24) | 0.00 (0.00) | 0.81 (0.47) | 0.09 (0.09) | 0.10 (0.05) | 0.32 (0.24) |
| cy-19:0 | 0.85 (0.14) | 0.53 (0.04) | 0.55 (0.08) | 0.60 (0.05) | 0.08 (0.05) | 0.04 (0.04) |
| 19:0 | 0.48 (0.06) | 0.67 (0.02) | 0.69 (0.05) | 0.58 (0.09) | 0.63 (0.07) | 0.59 (0.14) |
| 18:0 2OH | 0.69 (0.04) | 0.64 (0.04) | 0.65 (0.02) | 0.57 (0.03) | 0.60 (0.02) | 0.46 (0.11) |
| cy-19:0 DMAb | 2.88 (0.13) | 13.93 (0.47) | 12.50 (1.24) | 12.42 (1.87) | 14.35 (0.41) | 13.07 (1.95) |
| 20:5 w3c | 0.00 (0.00) | 0.71 (0.09) | 0.52 (0.07) | 0.47 (0.08) | 0.08 (0.04) | 0.00 (0.00) |
| 18:0 3OH | 0.85 (0.29) | 0.18 (0.18) | 0.35 (0.16) | 0.00 (0.00) | 0.12 (0.06) | 0.05 (0.05) |
| 20:1 w11 | 0.00 (0.00) | 5.63 (0.49) | 4.17 (0.64) | 5.71 (0.20) | 5.64 (0.23) | 4.88 (0.74) |
| 20:0 | 2.20 (0.23) | 2.76 (0.13) | 2.83 (0.10) | 2.47 (0.10) | 2.68 (0.10) | 2.02 (0.32) |
| 19:0 2OH | 0.00 (0.00) | 0.86 (0.16) | 0.45 (0.08) | 0.64 (0.06) | 0.54 (0.08) | 0.76 (0.15) |
| a-21:0 | 0.00 (0.00) | 2.24 (0.76) | 2.07 (0.29) | 2.62 (0.05) | 2.48 (0.24) | 2.27 (0.36) |
| 21:0 | 1.01 (0.61) | 1.95 (0.11) | 2.14 (0.09) | 2.04 (0.07) | 2.17 (0.08) | 1.96 (0.15) |
| 22:6 w3c | 0.00 (0.00) | 0.48 (0.05) | 0.24 (0.08) | 0.26 (0.04) | 0.21 (0.07) | 0.34 (0.24) |
| 22:1 w7c | 0.00 (0.00) | 0.02 (0.02) | 0.21 (0.10) | 0.27 (0.11) | 0.29 (0.15) | 0.17 (0.11) |
| 22:0 | 5.08 (0.54) | 6.74 (1.06) | 7.88 (0.23) | 7.54 (0.25) | 8.35 (0.42) | 10.44 (2.74) |
| 23:1 w7c | 0.82 (0.12) | 0.00 (0.00) | 0.35 (0.13) | 0.05 (0.05) | 0.04 (0.04) | 0.00 (0.00) |
| 23:0 | 1.71 (0.13) | 1.18 (0.17) | 1.57 (0.16) | 1.50 (0.07) | 1.60 (0.05) | 1.54 (0.12) |
| 24:0 | 5.17 (0.56) | 4.82 (0.98) | 6.74 (0.28) | 6.22 (0.17) | 6.39 (0.62) | 6.57 (0.48) |
Σ denotes unresolved peaks. Values are mean mole percentages with one SE indicated in parentheses. Senescent beech leaf refers to leaf material analyzed for FAME prior to submersion in the microcosm.
DMA, dimethyl acetyl.
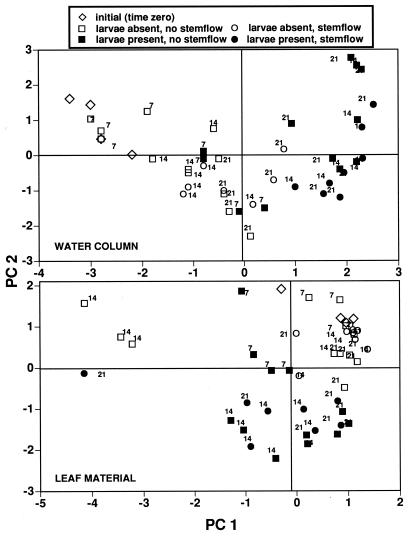
PCA of lipid profiles in the microcosms from the water column and leaf samples (Fig. 3) indicated a larval effect but also high variability among samples. The percentages of total variance explained by the first two components were 51.1 and 36.5% for water column and leaf material, respectively. Nonetheless, there was still a clear trend for separation of larva-present versus larva-absent treatments in the principal component 1 (PC 1) scores for water column FAMEs and the PC 2 scores for leaf material FAMEs. These trends were highly significant (Table 5) for the main effect of larvae on days 14 and 21. For both water column and leaf samples, day 7 samples were grouped more closely with initial (time zero) replicates than other time points, and larvae significantly affected PC 1 scores. Additionally, larvae and stemflow had significant main effects on water column PC 2 scores, and stemflow had a significant effect on leaf PC 2 scores. Leaf and water PC 1 scores changed significantly between days 14 and 21, but significant interaction terms (Table 5) indicated this occurred only within particular larva-stemflow treatment combinations.
FIG. 3.
Principle component analysis of whole-community FAME profiles extracted from microcosm leaf material and water columns. Numbers near symbols indicate the sampling time (in days) of each point. The total variances for water column PC 1 and PC 2 were 35.7 and 15.4%, respectively, and for leaf material PC 1 and PC 2 were 21.6 and 14.9%, respectively.
TABLE 5.
Probability values from t tests (day 7 only) and factorial ANOVA of PC scores derived from whole-community FAME profiles in microcosms
| Variable |
Pa at day(s):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 7
|
14 and 21
|
|||||||
| Larvae | Time | Larvae | Stemflow | Time × larvae | Time × stemflow | Larvae × stemflow | Time × larvae × stemflow | |
| Water PC 1 | 0.001 | 0.025 | 0.000 | 0.213 | 0.007 | 0.354 | 0.143 | 0.644 |
| Water PC 2 | 0.029 | 0.929 | 0.005 | 0.050 | 0.310 | 0.588 | 0.066 | 0.093 |
| Leaf PC 1 | 0.002 | 0.024 | 0.493 | 0.240 | 0.128 | 0.004 | 0.016 | 0.389 |
| Leaf PC 2 | 0.179 | 0.432 | 0.000 | 0.026 | 0.042 | 0.029 | 0.376 | 0.194 |
Total n = 8 for day 7, and n = 31 or 32 for each variable in the ANOVA.
Among FAME groups which contributed most to separation along PC axes (i.e., those with the highest loading values [89]) (Table 6), concentrations of polyunsaturated long-chain acids (20:4 w6, 20:5 w3, and 22:6 w3) declined with larval presence in both the water column (day 14 only [Table 7]) and the leaf material samples (days 14 and 21 [Table 8]). Cyclopropyl fatty acids showed similar trends, since cy-19:0 FAMEs were significantly lower in leaf material and cy-17:0 fatty acids were lower in the water column when larvae were present (Tables 7 and 8). The monounsaturated 16:1 w11c fatty acids declined significantly in the water column on day 7 in the presence of larvae, and a monounsaturated 19-carbon FAME increased significantly in the presence of larvae. Branched 14:0 FAMEs decreased significantly in larval treatments on day 7 but increased significantly in the presence of larvae on day 21 (Table 7), while branched 17-carbon FAMEs decreased significantly in leaf material on day 14. Long-chain saturated acids in the water column were significantly increased by larvae on all dates (Table 7). Stemflow did not significantly affect the concentrations of high-loading FAME groups in the water column, although the monounsaturated and longer-chain, odd-carbon-number, saturated FAMEs showed a strong decreasing trend with the treatment (Table 7). Stemflow did significantly increase the concentrations of high-loading branched FAME groups and an 18-carbon polyunsaturated fatty acid in leaf material (Table 8).
TABLE 6.
Individual FAMEs with highest loading values for the PC 1 and PC 2 scores of microcosm water column and leaf materiala
| Water column
|
Leaf material
|
|||||||
|---|---|---|---|---|---|---|---|---|
| FAME | PC 1 loading value | FAME | PC 2 loading value | FAME | PC 1 loading value | FAME | PC 2 loading value | |
| 20:0 | 0.621 | i-17:1 | 0.424 | 19:1 w8 | −0.695 | 20:5 w3c | 0.630 | |
| 24:0 | 0.613 | i-14:0 | 0.396 | 18:0 3OH | −0.494 | cy-19:0 | 0.590 | |
| 22:6 w3c | −0.590 | i-17:0 | 0.371 | 19:0 2OH | 0.434 | 22:6 w3c | 0.400 | |
| 20:4 w6c | −0.560 | 20:4 w6c | −0.350 | 20:1 w11 | 0.429 | i-17:0 | 0.290 | |
| 20:5 w3c | −0.520 | 16:1 w11c | 0.347 | a-21:0 | 0.419 | i-17:1 | 0.270 | |
| cy-17:0 | −0.520 | 17:1 w8c | 0.302 | 19:1 w11 | 0.402 | 17:0 3OH | 0.250 | |
| 22:0 | 0.448 | i-15:1 | 0.294 | cy-19:0 | 0.306 | a-17:0 | 0.230 | |
| 16:1 w11c | −0.440 | 24:0 | −0.270 | i-16:1 | 0.283 | i-16:1 | 0.220 | |
| 21:0 | 0.442 | 17:0 | 0.249 | cy-19:0 DMA | 0.253 | a-21:0 | −0.200 | |
| a-14:0 | 0.347 | 20:5 w3c | −0.220 | i-18:0 | 0.232 | 18:3 w6c | 0.140 | |
See Fig. 4 and the text for an illustration of PCA and an explanation of fatty acid nomenclature.
TABLE 7.
Probability values from Kruskal-Wallis nonparametric analysis of FAME groups showing highest loading values on PC 1 and PC 2 scores for microcosm water columns
| FAME |
Pa for:
|
||||
|---|---|---|---|---|---|
| Larvae
|
Stemflow
|
||||
| Day 7 | Day 14 | Day 21 | Day 14 | Day 21 | |
| Σ i-14:0, a-14:0 | 0.042 (↓) | 0.749 | 0.006 (↑) | 0.286 | 0.695 |
| Σ i-15:1, i-17:1, i-17:0 | 0.375 | 0.207 | 0.098 (↑) | 0.249 | 0.449 |
| 16:1 w11c | 0.014 (↓) | 0.538 | 0.171 | 0.064 (↓) | 0.171 |
| 17:1 w8c | 0.321 | 0.644 | 0.082 (↑) | 0.064 (↓) | 0.508 |
| cy-17:0 | 0.245 | 0.004 (↓) | 0.783 | 0.277 | 0.130 |
| Σ 17:0, 21:0 | 0.317 | 0.012 (↑) | 0.006 (↑) | 0.062 (↓) | 0.896 |
| Σ 20:0, 22:0, 24:0 | 0.021 (↑) | 0.027 (↑) | 0.049 (↑) | 0.600 | 0.563 |
| Σ 20:4 w6c, 20:5 w3c, 22:6 w3c | 0.149 | 0.003 (↓) | 0.157 | 0.674 | 0.814 |
See Fig. 4 and Table 6 for PCA results and the text for an explanation of the fatty acid nomenclature. Larva and stemflow main effects are compared on the sample days indicated. Arrows in parentheses next to significant or marginally significant probability values indicate an increase (↑) or a decrease (↓) in the FAME group due to the main effect. Total n = 8 for each variable on day 7, and n = 15 or 16 for each variable on days 14 and 21.
TABLE 8.
Probability values from Kruskal-Wallis nonparametric analysis of FAME groups showing highest loading values on PC 1 and PC 2 for microcosm leaf material
| FAME |
Pa for:
|
||||
|---|---|---|---|---|---|
| Larvae
|
Stemflow
|
||||
| Day 7 | Day 14 | Day 21 | Day 14 | Day 21 | |
| Σ i-16:1, i-18:0 | 0.773 | 0.461 | 0.674 | 0.045 (↑) | 0.314 |
| Σ a-17:0, i-17:0, i-17:1 | 0.149 | 0.035 (↓) | 0.093 (↓) | 0.045 (↑) | 0.874 |
| a-21:0 | 1.000 | 0.916 | 1.000 | 0.046 (↑) | 0.985 |
| Σ 19:1 w8, | 0.047 (↑) | 0.190 | 0.927 | 0.190 | 0.098 (↑) |
| Σ 19:1 w11, 20:1 w8 | 0.248 | 0.834 | 0.834 | 0.093 (↑) | 0.491 |
| Σ 17:0 3 OH, 18:0 3OH, 19:0 2OH | 0.248 | 0.172 | 1.000 | 0.248 | 0.916 |
| cy-19:0 | 0.146 | 0.004 (↓) | 0.000 (↓) | 0.277 | 0.699 |
| cy-19:0 DMA | 0.773 | 0.753 | 0.529 | 0.093 (↑) | 0.958 |
| 18:3 w6c | 0.375 | 0.913 | 0.305 | 0.062 (↑) | 0.006 (↑) |
| Σ 20:5 w3c, 22:6 w3c | 0.083 (↓) | 0.002 (↓) | 0.025 (↓) | 0.695 | 0.420 |
See Fig. 4 and Table 7 for PCA results and the text for an explanation of the fatty acid nomenclature. Larva and stemflow main effects are compared on the sample days indicated. Arrows in parentheses next to significant or marginally significant probability values indicate an increase (↑) or a decrease (↓) in the FAME group due to the main effect. Total n = 8 for each variable on day 7, and n = 15 or 16 for each variable on days 14 and 21.
Inorganic ions.
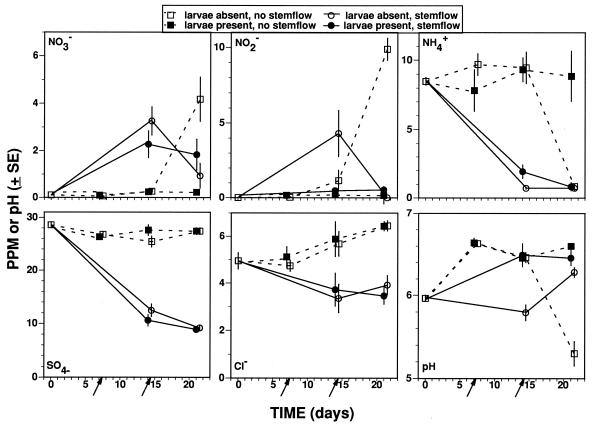
The presence of larva and stemflow events strongly influenced inorganic ion concentrations in the microcosm water column (Fig. 4), although significant ANOVA interaction terms indicated these effects were largely time dependent (Table 9). The most pronounced larval effect among the ions that we measured was the apparent suppression of nitrite and nitrate production in the larva-present/no-stemflow treatments (Fig. 4). In microcosms without larvae and stemflow, decreases in ammonium concentration and pH on day 21 samples were associated with an elevation in nitrite and nitrate levels. Additionally, day 14 larva-absent/stemflow microcosms showed a similar but less-pronounced elevation of nitrite and nitrate and accompanying depression of ammonium concentrations and pH compared to larva-present/stemflow treatments. Stemflow additions significantly raised nitrate levels and diluted ammonium, chloride, and sulfate concentrations (Fig. 4 and Table 9). Chloride levels tended to rise with time in microcosms not receiving stemflow (Fig. 4).
FIG. 4.
Concentration of major inorganic ions and pH in microcosm water columns. Values are means ± 1 SE; n = 3 or 4 for each point. Arrows denote times of stemflow additions.
TABLE 9.
Probability values from t tests (day 7 only) and factorial ANOVA of inorganic ions and pH in mosquito microcosms
| Variable |
Pa at day(s):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 7
|
14 and 21
|
|||||||
| Larvae | Time | Larvae | Stemflow | Time × larvae | Time × stemflow | Larvae × stemflow | Time × larvae × stemflow | |
| Nitrite | 0.454 | 0.621 | 0.000 | 0.031 | 0.771 | 0.000 | 0.000 | 0.000 |
| Nitrate | 0.423 | 0.489 | 0.013 | 0.002 | 0.142 | 0.000 | 0.010 | 0.001 |
| Ammonium | 0.305 | 0.000 | 0.000 | 0.000 | 0.014 | 0.013 | 0.028 | 0.000 |
| Sulfate | 0.395 | 0.200 | 0.986 | 0.000 | 0.814 | 0.011 | 0.089 | 0.131 |
| Chloride | 0.475 | 0.256 | 0.940 | 0.000 | 0.464 | 0.469 | 0.843 | 0.665 |
| pH | 0.874 | 0.045 | 0.000 | 0.435 | 0.007 | 0.000 | 0.132 | 0.000 |
Total n = 8 for each variable on day 7, and n = 29 to 32 for each variable in the ANOVA.
Organic compounds.
Concentrations of DOC, soluble carbohydrates, and protein were also strongly influenced by larval presence and stemflow flushing (Fig. 5). Measured organic compounds were significantly increased by the presence of mosquito larvae and decreased by stemflow flushing (Table 10). Stemflow and larval presence interacted significantly in affecting concentrations of soluble carbohydrates (Table 10), with the presence of larvae increasing carbohydrate levels only in microcosms receiving stemflow.
FIG. 5.
Concentrations of organic compounds in microcosm water columns. Values are means ± 1 SE; n = 3 or 4 for each point. Arrows denote times of stemflow additions.
TABLE 10.
Probability values from t tests (day 7 only) and factorial ANOVA of water column organic compounds in mosquito microcosms
| Organic compound |
Pa at day(s):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 7
|
14 and 21
|
|||||||
| Larvae | Time | Larvae | Stemflow | Time × larvae | Time × stemflow | Larvae × stemflow | Time × larvae × stemflow | |
| Total DOC | 0.185 | 0.150 | 0.043 | 0.000 | 0.082 | 0.847 | 0.236 | 0.236 |
| Soluble carbohydrate | 0.257 | 0.783 | 0.017 | 0.000 | 0.030 | 0.398 | 0.024 | 0.515 |
| Total protein | 0.824 | 0.989 | 0.000 | 0.000 | 0.258 | 0.644 | 0.957 | 0.999 |
Total n = 8 for each variable on day 7, and n = 32 for each variable in the ANOVA.
Mosquito production.
Stemflow flushing of microcosms had no demonstrable effect on average larval dry mass, total larval dry mass, or survival during the 21 days of the experiment. The only significant effect on any of the above parameters was that total biomass per microcosm of larval and pupal stages decreased between day 14 and day 21, independent of stemflow (ANOVA F value = 10.652, P = 0.007, n = 16). However, stemflow treatments did significantly enhance pupation and emergence rates (ANOVA F value = 4.934, P = 0.046, n = 16). Adult production during the course of the experiment was almost entirely attributed to male emergence (1 female of 74 adults collected).
DISCUSSION
Mosquito larvae affected almost every level of microbial community structure or bacterial abundance measured and also influenced microbially mediated nitrogen dynamics. While stemflow simulation events tended to strongly affect ion and nutrient concentrations, stemflow had only minor influences on microbial population and community dynamics in this study. These results imply that A. triseriatus larvae are key modifiers of microbial dynamics in container habitats and exert substantial direct or indirect control of their microbial food resources.
In contrast to previous reports of bacteria in treeholes (62, 84), this study indicates that total bacterial numbers and CFU in the water column can be increased by the larval presence. Methodological differences may account for some of the conflicting results. Our technique of decanting the water column before subsampling likely destroyed any stratification of bacterial numbers with depth, whereas Walker et al. (84) collected water samples within a few centimeters of the surface in a zone heavily filtered by larvae (82). Other studies in small-container habitats (e.g., references 18 and 39) have noted increases in water column bacterial abundance due to the release of particulates by feeding activities of larval insects. Conceivably, water column bacteria are both grazed and regenerated by the varied feeding habits of A. triseriatus larvae, resulting in little overall change in water column bacterial abundance or the slight increases observed.
Total leaf-associated bacterial numbers in each microcosm were estimated to be at least an order of magnitude greater than total water column numbers (1010 versus 109), and the reduction of bacterial abundance in the leaf detritus was most likely due directly to grazing by larvae (29). Mosquito larval grazing on leaf surface microorganisms has been reported only from qualitative observations (29, 59), although the overall importance of leaf surfaces to A. triseriatus growth has been demonstrated (51). Our measured reductions of nearly 50% of leaf-associated bacteria by larvae are particularly noteworthy in that many leaf-associated bacteria were presumably unavailable for larval grazing because they resided within the leaf matrix or were associated with the bottom surfaces of leaves. Significant reductions of bacterial biomass by macroinvertebrate surface feeders has only been observed in instances of very high grazing pressure (87). Results here also show that larval feeding effects on leaf bacterial abundance can occur even when larvae are in the early stages of development (Fig. 1). The importance of leaf-associated microorganisms to early larval development was suggested by the study of Walker et al. (85).
Although overall abundances of water column bacteria did not change dramatically in response to stemflow or larva treatments, the taxonomic and functional composition of cultivable bacteria was strongly altered. The increase of cultivable forms on aerobic and anaerobic media in the presence of larvae suggests that larvae contributed to enriched and more anoxic conditions favorable to the growth of facultative anaerobes. Further, the proportion of the facultatively anaerobic group, Enterobacteriaceae, increased in the presence of larvae (Fig. 2). The temporal decline in anaerobic populations in the treatments without larvae suggests that enriched and oxygen-consuming conditions in the water column of these microcosms were waning over the course of the experiment. This may have been related to a decrease in the rate of release of labile compounds during the initial leaf decomposition period and a corresponding decline in microbial oxygen demand. The overall faster rate of decline in concentrations of organic compounds (Fig. 5) in microcosms without larvae and the overall temporal decline in abundance of Enterobacteriaceae (Fig. 2) are consistent with this view.
Larval feeding may also have directly affected bacterial community composition through differential digestibility of bacterial taxa (see, for example, references 3, 12, 47, and 57). Community composition shifts seen here (Fig. 2) could be explained if pseudomonad bacteria were generally more susceptible to digestion by larvae than enteric bacteria. King et al. (47) have shown that bacterioplankton communities may be shaped by zooplankton grazing and that pseudomonads declined while enteric bacteria and some gram-positive organisms increased in relative proportion within zooplankton guts. Enteric bacteria are presumably more resistant to digestion in animal alimentary tracts since many forms are known to proliferate there (10). Pseudomonad bacteria have been reported to be readily digestible by Aedes larvae (73) and other aquatic invertebrates (47, 49), but little information is available about this topic in general.
Compositional changes in bacterial groups may also have resulted through trophic interactions with protozoa. It is well established that protozoa impact bacterial abundances in aquatic systems and are in turn affected by larger invertebrate predators (4, 18, 44, 45, 78). Bacterial taxonomic and metabolic groups are also influenced by protozoan grazing (31, 36, 45). Since mosquito larvae graze upon protozoans in container habitats (1, 48, 56, 62), bacterial community compositional changes observed here may have been the result of the release from protozoan predation. Cochran-Stafira and von Ende (18) have demonstrated that the response of individual bacterial taxa to the presence of pitcher plant mosquito larvae is at least partially mediated via the intermediate trophic level of flagellates and ciliates in the system.
In contrast to the effects of larvae, the only demonstrable effect stemflow had on isolate composition was that of increasing the proportions of non-Bacillus, gram-positive organisms. This group is a diverse collection of non-spore-forming rods, cocci, and actinomycetes found in a variety of habitats. The reasons for their appearance and increased abundance with the stemflow treatment are unclear; however, some forms are listed as common inhabitants of plant surfaces (43, 74) and are known to use plant surface hydrocarbons (e.g., waxes and cuticle components) as energy sources (32). Stemflow would presumably carry epiphytic bacteria and plant surface compounds into treehole habitats.
Among the groups of fatty acids associated with bacteria and which discriminated communities in PCA were the cyclopropyl acids. Cyclopropyl acids are common among many gram-negative bacteria and have been considered markers of anaerobic bacteria (80). Larval activity clearly decreased the proportions of these FAMEs relative to controls at various time points in the study. The decline in cy-17:0 and cy-19:0 fatty acids in the larva-present treatments is consistent with a decline in pseudomonad bacteria in the water column (Fig. 2) and bacteria in general on leaf material, but it is inconsistent with larval enhancement of enteric and anaerobic bacteria (Fig. 1). Higher proportions of cyclopropyl fatty acids are also considered a sign of physiological stress and slowed growth in eubacterial populations (5, 34, 38); therefore, decreased proportions may indicate that larval cropping of bacterial cells and/or recirculation of nutrients was stimulating bacterial growth (26, 54). However, because precursors to cy-17:0 and cy-19:0 fatty acids, 16:1 w7c and 18:1 w7c, respectively, also declined in the presence of larvae, an overall decline in some eubacterial groups rather than a physiological shift in existing populations is suggested (5, 63).
Few other presumed bacterial marker FAMEs showed any consistent trends with treatment, reflecting both the limits of “signature” lipid methodology and the dynamic nature of bacterial populations in response to grazing and environmental changes. Fatty acids presumed to be markers for bacterial groups include 14- to 17-carbon, branched-chain (iso and anteiso) fatty acids as gram-positive markers (46), odd-number-saturated (e.g., 15:0) fatty acids and hydroxylated fatty acids as gram-negative markers (38, 61, 91), and 16:1 w7c and 18:1 w11c monounsaturated acids as general eubacterial markers (80, 92). The branched 14:0 FAMEs in the water column and branched 17-carbon FAMEs in leaf material responded to larval presence and increased in stemflow treatments. The latter trend is consistent with increasing proportions of gram-positive organisms on day 21 (Fig. 2). However, branched-chain fatty acids are also found in some gram-negative bacteria, including Cytophaga spp. (35, 46), a common component of detrital bacterial communities. A hydroxylated 18:0 FAME was detectable only in larva-present treatments in water column samples (Table 3) and may reflect the relatively high proportions of enteric bacteria observed, but mid- and long-chain hydroxylated fatty acids are also widely distributed among other microorganisms (92). Some monounsaturated FAMEs in the water column declined in the presence of larvae or with stemflow (Tables 3 and 7); however, this trend in general markers for eubacteria was inconsistent with the observed larval enhancement of total bacterial numbers (Fig. 1). Most presumed bacterial fatty acid biomarkers in leaf material did not decline in association with the observed decrease in overall bacterial abundance attributed to larval presence. This may again reflect limits in the methodology applied here or else a grazing effect on lipid metabolism in leaf-associated microorganisms (60).
The depression in levels of long-chain polyunsaturated fatty acids (PUFAs) in the presence of larvae likely resulted from larval grazing on protozoa and meiofauna (e.g., rotifers, nematodes, etc.). PUFAs can also be characteristic of microeukaryotes such as fungi and algae (38, 80, 87), but other presumed fungal markers did not decline with larval presence (see below), and we have rarely observed algae in treeholes or simulated treehole habitats. PUFAs are not only microeukaryote markers but are essential nutrients for most invertebrates (11, 64). Mosquitoes in particular show a dietary requirement for C20 and longer PUFAs in order for adult development (20). The fact that these lipids were often nondetectable when larvae were present in both leaf and water column samples suggests that larvae could have been “overgrazing” this microbial resource. Although clearly a key element of container habitat food webs, the nutritional importance of protozoans and meiofauna to larvae has not been quantified.
Potential fungal FAME markers showed no clear response to larvae but suggest that some fungal groups were stimulated by larvae and stemflow. If the increase in long-chain unsaturated acids reflected fungal tissue growth (88) and not leaf material degradation products or larval excretion and exuvia, then larval presence appeared to increase populations of at least some fungal taxa. Selective feeding on and alteration of fungal community components have been demonstrated for other aquatic invertebrates (6, 69), and it is very likely that some fungal strains or growth forms are more readily ingested by larvae than others. Although Fish and Carpenter (29) reported A. triseriatus larvae feeding on fungal hyphae, size classes in excess of 50 μM would be increasingly difficult for even mature larvae to ingest (59). Increases in another potential fungal marker in leaf material, 18:3 w6c (Table 8), in the stemflow treatments suggest leaf-associated fungi responded more to nutrient input (e.g., nitrate) than to larval grazing on the leaf surface.
FAME groups from leaf material were consistent with the expected trends of microbial succession when dry leaves undergo submersed decay. Compared to the original beech leaf material (prior to introduction into microcosms), incubated leaves showed detectable quantities of polyunsaturated and most branched-chain fatty acids, increases in the cy-19:0 dimethyl acetyl (an anaerobic marker [61]), and decreases in the presumed terrestrial fungal markers 18:2 w6c and 18:1 w9c (80, 91) (Table 4). These trends are consistent with increases in protozoans, eubacteria, and anaerobic bacteria, and overall decreases in fungi normally associated with submersed decay of senescent leaves in stagnant fresh waters (7). Of the FAMEs deemed important to PCA, we could detect only the hydroxylated and cyclo fatty acids (Table 4) in the original leaf material, and these presumably were from “terrestrial” bacterial epiphytes.
Few studies have examined the effects of invertebrate feeding on microbial community lipid patterns, and these studies have dealt mainly with marine systems (25, 27, 28, 60, 71, 87). As in this study, a common feature appears to be demonstrable declines in microeukaryote markers after invertebrate feeding and various (positive, negative, or none) responses from presumed bacterial markers. As other investigators have pointed out (35, 92), the lack of unique biomarkers in many instances and the potential inequality in extraction of lipids from different taxa present restrict conclusions about changes in specific microbial groups. In our study, significant changes in pseudomonad versus enteric groups in response to larval presence would not have been observed with whole-community lipid profiles.
A. triseriatus larvae also directly or indirectly influenced microbial communities involved in nitrogen transformations within the system. Truncated nitrification processes, as evidenced by the accumulation of NO2 in the latter stages of the experiment, were evident primarily in the larva-absent/no-stemflow treatment. Nitrite buildup and pH decline in these microcosms suggest that conditions were stratifying in these relatively undisturbed treatments to the point that inhibition occurred through acid accumulation and lowered O2 (67). Stemflow treatments also showed less tendency for nitrite accumulation, suggesting that disturbance and aeration were important factors. Vertical movement and feeding behavior of larvae might be expected to limit the extent of anaerobic zones; however, anaerobic bacterial numbers were higher in microcosms with larvae (Fig. 1). This apparent contradiction is explained if the larvae had stimulated populations of nitrite and/or nitrate consumers (e.g., nitrate respiring or denitrifying bacteria). It is assumed that denitrification or other consumptive processes prevent the accumulation of nitrate and/or nitrite in natural treeholes (84).
Surprisingly, there was little evidence to indicate that larvae increased ammonium levels as would be expected due to their excretory processes. The lack of ammonium buildup in the presence of larvae suggests that larval excretion of NH4 was being balanced by its removal through microbial metabolism, part of which could have been the activity of nitrifiers. Larval grazing may have stimulated nitrifiers, as has been shown for protozoan grazing (33, 77). However, without measurement of specific rates of ammonia oxidation, nitrification, and product consumption (e.g., nitrate respiration and/or denitrification), it is difficult to assess the mechanism of larval impact on the process.
The major measurable effect of stemflow treatments in this study was the dilution of most inorganic and organic solutes. Although a dilution of some solutes would be expected, previous studies have generally concluded that stemflow is a net source of nutrients (14, 84). Nitrate, sulfate, and nitrite levels in the stemflow used as a treatment during the course of the experiment were 3.8, 6.6, and 0.4 ppm, respectively, but solute concentrations in the initial batch of stemflow were not measured. The values were lower than previously reported for stemflow collected at the same site in an earlier study (84), and the effects of these ions as nutrients or electron acceptors may subsequently have been muted in this study.
Sulfate, reportedly a positive stimulant for mosquito growth in microcosms (14), behaved much like chloride, a conservative tracer, and stemflow simply diluted the initial pool sizes. The trend toward increasing concentrations of chloride with time in the stemflow-absent treatments reflects evaporation over the course of the experiment, and because sulfate values don’t exactly mimic chloride trends, some minor microbial metabolism of sulfate likely occurred. However, the consistent presence of oxidized nitrogen compounds in the microcosms suggests sulfate metabolism (i.e., sulfate reduction) was quantitatively unimportant in this study.
Stemflow dilution of organic compounds contrasts with their increase in the presence of larvae, suggesting that larval activity in the form of excretion, physical disturbance, stimulation of microbial decomposer activity, or some combination thereof, was more important than stemflow in the supply of substrate for water column microorganisms. This idea is consistent with the increase in total protein and general trend of higher bacterial numbers in microcosms containing larvae. Invertebrate presence and feeding activity have often been shown to enhance microbial activity and/or abundance (39, 54). The mechanism is usually presumed to be the release and resuspension of inorganic nutrients. However, in small-container systems characterized by allocthonous input and high inorganic nutrient levels (84), invertebrate feeding may be more important as a source of labile carbon. Larval grazing on leaf surfaces may also link water column microbial communities more directly with leaf-associated microorganisms through stimulation of decomposition activity (26) and subsequent release of organic substrates.
Although this study concentrated on microbial dynamics in the microcosms, ultimately we hope to understand how these dynamics may influence mosquito production. Early adult emergence patterns were affected by stemflow treatment, but it was difficult to discern a stemflow-microbial community-mosquito link in the parameters we measured. Previous studies (14, 84) have also shown that stemflow additions generally enhance emergence rates, but the mechanism for this enhancement is unclear. Apart from the potential benefits of stirring and homogenizing the water column, stemflow events are thought to add nutrients and flush inhibitory metabolites from the system (14, 84). Stemflow treatments in this study generally decreased the levels of soluble carbon sources and protein, a result that is likely to have negative impact on microbial growth in carbon-limited habitats (86). However, our stemflow treatment may have supplied a small pulse of very labile carbon sources, providing favorable conditions for short-term microbial growth responses not measured in this study. A previous study (85) suggests that the input of readily available, soluble organics (carbohydrates and protein) from leaf material leachate is important in determining larval developmental rates, presumably through stimulation of microbial growth, and stemflow may have acted similarly here.
The study presented here would also seem to support the idea that the microbial component of these container habitats is more strongly influenced by top-down predator effects than by nutrient changes brought about by stemflow events. This seems to be characteristic of many freshwater systems, including relatively simple ones (4, 18, 78). At present, however, we cannot distinguish between the direct effects of larval grazing and the indirect effects of nutrient regeneration or modification of the physiochemical environment by larvae. Our results indicate that larval activity can alter the environment of container habitats such that system inputs in the form of stemflow and in situ microbial processes are modified. The consequences for specific microbial populations remain uncertain, but they are ultimately important in our understanding of mosquito growth and microbial dynamics in these habitats.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI21884. We also gratefully acknowledge the support and facilities associated with the Center for Microbial Ecology at Michigan State University.
We thank Bill Morgan, Sandy Marsh, Jon Ervin, Helen Corlew, Amber Wujek, and Tracy Craig for technical assistance; Soren Peterson for advice on lipid analyses; and Wendy Goodfriend for review of the manuscript. Lab strain mosquito eggs were kindly provided by Mark Blackmore through the lab of the late George Craig at Notre Dame University.
Footnotes
This paper is contribution 890 of the W. K. Kellogg Biological Station.
REFERENCES
- 1.Addicot J F. Predation and prey community structure: an experimental study of the effect of mosquito larvae on the protozoan communities of pitcher plants. Ecology. 1974;55:475–492. [Google Scholar]
- 2.Aitchison J. The statistical analysis of compositional data. London, England: Chapman and Hall; 1986. [Google Scholar]
- 3.Austin D A, Baker J H. Fate of bacteria ingested by larvae of the freshwater mayfly, Ephermera danica. Microb Ecol. 1988;15:323–332. doi: 10.1007/BF02012645. [DOI] [PubMed] [Google Scholar]
- 4.Balciunas D, Lawler S P. Effects of basal resources, predation, and alternative prey in microcosm food chains. Ecology. 1995;76:1327–1336. [Google Scholar]
- 5.Balkwill D L, Murphy E M, Fair D M, Ringelberg D B, White D C. Microbial communities in high and low recharge environments: implications for microbial transport in the Vadose zone. Microb Ecol. 1998;35:156–171. doi: 10.1007/s002489900070. [DOI] [PubMed] [Google Scholar]
- 6.Barlocher F. The role of fungi in the nutrition of stream invertebrates. Bot J Linn Soc. 1985;91:83–94. [Google Scholar]
- 7.Barlocher F, Mackay R J, Wiggins G B. Detritus processing in a temporary vernal pool in southern Ontario. Arch Hydrobiol. 1978;81:269–295. [Google Scholar]
- 8.Barrerra R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:117–127. [Google Scholar]
- 9.Bligh E L G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 10.Brenner D J. Family 1: enterobacteriaceae. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 408–516. [Google Scholar]
- 11.Brett M T, Müller-Navarra D C. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biol. 1997;38:483–499. [Google Scholar]
- 12.Brinkhurst R O, Chua K E. Preliminary investigations of the exploitation of some potential nutritional resources by three sympatric tubificid oligochaetes. J Fish Res Bd Can. 1969;26:2659–2668. [Google Scholar]
- 13.Buyer J S, Drinkwater L E. Comparison of substrate utilization assay and fatty acid analysis of soil microbial communities. J Microbiol Methods. 1997;30:3–11. [Google Scholar]
- 14.Carpenter S R. Stemflow chemistry: effects on population dynamics of detritivorous mosquitoes in tree-hole ecosystems. Oecologia. 1982;53:1–6. doi: 10.1007/BF00377128. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter S R. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- 16.Cavigelli M A, Robertson G P, Klug M J. Fatty acid methyl ester (FAME) profiles as measures of soil microbial community structure. In: Collins H P, Robertson G P, Klug M J, editors. The significance and regulation of soil biodiversity. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 99–113. [Google Scholar]
- 17.Clesceri L S, Greenberg A E, Trussell R R, editors. Standard methods for the examination of water and wastewater. Washington, D.C: American Public Health Association; 1989. pp. 111–143. [Google Scholar]
- 18.Cochran-Stafira D L, von Ende C N. Integrating bacteria into food webs: studies with Sarracenia purpurea inquilines. Ecology. 1998;79:880–898. [Google Scholar]
- 19.Currie B R, Johns R B. Lipids as indicators of the origin of organic matter in fine marine particulate matter. Aust J Mar Freshwater Res. 1988;39:371–383. [Google Scholar]
- 20.Dadd R H. Essential fatty acids for mosquitoes, other insects and vertebrates. In: Ghaskaran G, Friedman S, Rodriguez J G, editors. Current topics in insect endocrinology and nutrition. New York, N.Y: Plenum Publishing Corp.; 1981. pp. 189–214. [Google Scholar]
- 21.Dove H, Mayes R W. Plant wax components: a new approach to estimating intake and diet composition in herbivores. J Nutr. 1996;126:13–26. doi: 10.1093/jn/126.1.13. [DOI] [PubMed] [Google Scholar]
- 22.Dowling N J E, Widdel F, White D C. Phospholipid ester-linked fatty acid biomarkers of acetate-oxidizing sulfate reducers and other sulphide-forming bacteria. J Gen Microbiol. 1986;132:1815–1825. [Google Scholar]
- 23.Dubois M, Giles K A, Hamilton J, Rebers K P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1965;28:350–356. [Google Scholar]
- 24.Edgerly J S, Livdahl T P. Density-dependent interactions within a complex life cycle: the roles of cohort structure and mode of recruitment. J Anim Ecol. 1992;61:139–150. [Google Scholar]
- 25.Federle T L W, Livingston R J, Meeter D A, White D C. Modifications of estuarine sedimentary microbiota by exclusion of epibenthic predators. J Exp Mar Biol Ecol. 1983;73:81–94. [Google Scholar]
- 26.Fenchel T, Harrison P. The significance of bacterial grazing and mineral cycling for the decomposition of particulate detritus. In: Anderson J M, Macfadyen A, editors. The role of terrestrial and aquatic organisms in decomposition processes. Oxford, England: Blackwell Scientific Publications; 1976. pp. 285–299. [Google Scholar]
- 27.Findlay R H, White D C. The effects of feeding by the sand dollar Mellita quinquiesperforata (Leske) on the benthic microbial community. J Exp Mar Biol Ecol. 1983;72:25–41. [Google Scholar]
- 28.Findlay R H, Trexler M B, White D C. Response of a benthic microbial community to biotic disturbance. Mar Ecol Prog Ser. 1990;62:135–148. [Google Scholar]
- 29.Fish D, Carpenter S R. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology. 1982;63:283–288. [Google Scholar]
- 30.Frank J H, Lounibos L P, editors. Phytotelmata: terrestrial plants as hosts for aquatic insect communities. Medford, N.J: Plexus; 1983. [Google Scholar]
- 31.Gonzales J M, Sherr E B, Sherr B F. Differential feeding by marine flagellates on growing versus starving, and on motile versus nonmotile, bacterial prey. Mar Ecol Prog Ser. 1993;102:257–267. [Google Scholar]
- 32.Goodfellow M. The family Nocardiaceae. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes: a handbook on the biology of bacteria. Ecophysiology, isolation, identification, applications. Vol. 2. New York, N.Y: Springer-Verlag; 1992. pp. 1188–1213. [Google Scholar]
- 33.Griffiths B S. Enhanced nitrification in the presence of bacteriophagous protozoa. Soil Biol Biochem. 1989;21:1045–1051. [Google Scholar]
- 34.Guckert J B, Hood M A, White D C. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol. 1986;52:794–801. doi: 10.1128/aem.52.4.794-801.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haack S K, Garchow H, Odelson D A, Forney L J, Klug M J. Accuracy, reproducibility, and interpretation of fatty acid methyl ester profiles of model bacterial communities. Appl Environ Microbiol. 1994;60:2483–2493. doi: 10.1128/aem.60.7.2483-2493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn M W, Hofle M G. Grazing pressure by a bacterivorous flagellate reverses the relative abundance of Comamonas acidovorans PX54 and Vibrio strain CB5 in chemostat cocultures. Appl Environ Microbiol. 1998;64:1910–1918. doi: 10.1128/aem.64.5.1910-1918.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hard J J, Bradshaw W E, Malarkey D J. Resource- and density-dependent development in tree-hole mosquitoes. Oikos. 1989;54:137–144. [Google Scholar]
- 38.Harwood J L, Russell N J. Lipids in plants and microbes. London, England: George Allen & Unwin; 1984. [Google Scholar]
- 39.Heard S B. Pitcher-plant midges and mosquitoes: a processing chain commensalism. Ecology. 1994;75:1647–1660. [Google Scholar]
- 40.Hedin L O, Armesto J J, Johnson A H. Patterns of nutrient loss from unpolluted, old-growth temperate forests: evaluation of biogeochemical theory. Ecology. 1995;76:493–509. [Google Scholar]
- 41.Holdeman L V, Cato E P, Moore W E C, editors. Anaerobic laboratory manual. 4th ed. Blacksburg, Va: Virginia Polytechnic Institute and State University; 1975. [Google Scholar]
- 42.Joliffe I T. Principal component analysis. New York, N.Y: Springer-Verlag; 1986. [Google Scholar]
- 43.Jones D, Keddie R M. The genus Arthrobacter. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes: a handbook on the biology of bacteria. Ecophysiology, isolation, identification, applications. Vol. 2. New York, N.Y: Springer-Verlag; 1992. pp. 1283–1299. [Google Scholar]
- 44.Jürgens K. Impact of Daphnia on planktonic microbial food webs: a review. Mar Microb Food Webs. 1994;8:295–324. [Google Scholar]
- 45.Jürgens K, Gude H. The potential importance of grazing-resistant bacteria in planktonic systems. Mar Ecol Prog Ser. 1994;112:169–188. [Google Scholar]
- 46.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King C H, Sanders R W, Shotts E B, Jr, Porter K G. Differential survival of bacteria ingested by zooplankton from a stratified eutrophic lake. Limnol Oceanogr. 1991;36:829–845. [Google Scholar]
- 48.Kurihara Y. The succession of aquatic dipterous larvae inhabiting bamboo phytotelmata. In: Frank J H, Lounibos L P, editors. 1983. Phytotelmata: terrestrial plants as hosts for aquatic insect communities. Medford, N.J: Plexus; 1983. pp. 55–77. [Google Scholar]
- 49.Ladle M, Hansford R G. The feeding of the larvae of Simulium austeni Edwards and Simulium (Wilhelmia) spp. Hydrobiologia. 1981;78:17–24. [Google Scholar]
- 50.Laird M. The natural history of larval mosquito habitats. San Diego, Calif: Academic Press, Inc.; 1988. [Google Scholar]
- 51.Leonard P M, Juliano S A. Effect of leaf litter and density on fitness and population performance of the hole mosquito Aedes triseriatus. Ecol Entomol. 1995;20:125–136. [Google Scholar]
- 52.Livdahl T P. Competition within and between hatching cohorts of a tree hole mosquito. Ecology. 1982;63:1751–1760. [Google Scholar]
- 53.Livdahl T P, Willey M S. Prospects for invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- 54.Lopez G R, Levinton J S, Slobodkin L B. The effect of grazing by the detritivore Orchestia grillus on Spartina litter and its associated microbial community. Oecologia. 1977;30:111–127. doi: 10.1007/BF00345415. [DOI] [PubMed] [Google Scholar]
- 55.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 56.Maguire B, Jr, Belk D, Wells G. Control of community structure by mosquito larvae. Ecology. 1968;49:207–210. [Google Scholar]
- 57.Malone K M, Nolan R A. Aerobic bacterial flora of the larval gut of the black fly Prosimulium mixtum (Diptera: Simuliidae) from Newfoundland, Canada. J Med Entomol. 1978;14:641–645. [Google Scholar]
- 58.Martin M M, Kukor J J. Role of mycophagy and bacteriophagy invertebrate nutrition. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C: ASM Press; 1984. pp. 257–263. [Google Scholar]
- 59.Merritt R W, Dadd R H, Walker E D. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- 60.Morrison S J, White D C. Effects of grazing by estuarine gammardien amphipods on the microbiota of allocthonous detritus. Appl Environ Microbiol. 1980;40:659–671. doi: 10.1128/aem.40.3.659-671.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paisley R, editor. MIS whole cell fatty acid analysis by gas chromatography training manual. Newark, Del: MIDI, Inc.; 1996. [Google Scholar]
- 62.Paradise C J, Dunson W A. Effects of sodium concentration on Aedes triseriatus (Diptera: Culicidae) and microorganisms in treeholes. J Med Entomol. 1998;35:839–844. doi: 10.1093/jmedent/35.5.839. [DOI] [PubMed] [Google Scholar]
- 63.Peterson S O, Klug M J. Effects of sieving, storage, and incubation temperature on the phospholipid fatty acid profile of a soil microbial community. Appl Environ Microbiol. 1994;60:2421–2430. doi: 10.1128/aem.60.7.2421-2430.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips N W. Role of different microbes and substrates as potential suppliers of specific, essential nutrients to marine detritivores. Bull Mar Sci. 1984;35:283–289. [Google Scholar]
- 65.Porter A G, Davidson E W, Liu J W. Mosquitocidal toxins of bacilli and their genetic manipulation for effective biological control of mosquitoes. Microbiol Rev. 1993;57:838–861. doi: 10.1128/mr.57.4.838-861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 67.Princic A, Mahne I, Megusar F, Paul E A, Tiedje J M. Effects of pH and oxygen and ammonium concentrations on the community structure of nitrifying bacteria from wastewater. Appl Environ Microbiol. 1998;64:3584–3590. doi: 10.1128/aem.64.10.3584-3590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reasoner D J, Geldreich E E. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossi L. Interactions between invertebrates and microfungi in freshwater ecosystems. Oikos. 1985;44:175–184. [Google Scholar]
- 70.Sasser M. Identification of bacteria through fatty acid analysis. In: Klement Z, Rudolph K, Sands D C, editors. Methods in phytobacteriology. Budapest, Hungary: Akademiai Kiado; 1990. pp. 199–204. [Google Scholar]
- 71.Smith G A, Nickels J S, Davis W M, Martz R F, Findlay R H, White D C. Perturbations in the biomass, metabolic activity, and community structure of the estuarine detrital microbiota: resource partitioning in amphipod grazing. J Exp Mar Biol Ecol. 1982;64:125–143. [Google Scholar]
- 72.Sokal R R, Rohlf F J. Biometry. W. H. San Francisco, Calif: Freeman & Co.; 1981. [Google Scholar]
- 73.Sota T, Kato K. Bacteria as diet for the mosquito Aedes (Stegomyia) (Diptera: Culicidae): preliminary experiments with Pseudomonas fluorescens. Appl Entomol Zool. 1994;29:589–600. [Google Scholar]
- 74.Stackebrandt E, Prauser H. The family Cellulomonadaceae. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes: a handbook on the biology of bacteria. Ecophysiology, isolation, identification, applications. Vol. 2. New York, N.Y: Springer-Verlag; 1993. pp. 1323–1345. [Google Scholar]
- 75.Stanley-Samuelson D W, Jurenka R A, Cripps C, Blomquist G, de Renobales M. Fatty acids in insects: composition, metabolism, and biological significance. Arch Insect Biochem Physiol. 1988;9:1–33. [Google Scholar]
- 76.Strand M, Herms D A, Ayers M P, Kubiske M E, Kaufman M G, Walker E D, Pregitzer K S, Merritt R W. Effects of atmospheric CO2, light availability, and tree species on the quality of leaf detritus as a resource for treehole mosquitoes. Oikos. 1999;84:277–283. [Google Scholar]
- 77.Strauss E A, Dodds W K. Influence of protozoa and nutrient availability on nitrification rates in subsurface sediments. Microb Ecol. 1997;34:155–165. doi: 10.1007/s002489900045. [DOI] [PubMed] [Google Scholar]
- 78.Tranvik L J, Hansson L-A. Predator regulation of aquatic microbial abundance in simple food webs of sub-Antarctic lakes. Oikos. 1997;79:347–356. [Google Scholar]
- 79.Van Handel E. Determination and significance of suspended protein in wastewater. J Am Mosq Control Assoc. 1986;2:146–149. [PubMed] [Google Scholar]
- 80.Vestal J R, White D C. Lipid analysis in microbial ecology. Bioscience. 1989;39:535–541. [PubMed] [Google Scholar]
- 81.Walker E D, Merritt R W. The significance of leaf detritus to mosquito (Diptera: Culicidae) productivity from treeholes. Environ Entomol. 1988;17:199–206. [Google Scholar]
- 82.Walker E D, Merritt R W. Behavior of larval Aedes triseriatus (Diptera: Culicidae) J Med Entomol. 1991;28:581–589. doi: 10.1093/jmedent/28.5.581. [DOI] [PubMed] [Google Scholar]
- 83.Walker E D, Olds E J, Merritt R W. Gut content analysis of mosquito larvae (Diptera: Culicidae) using DAPI stain and epifluorescence microscopy. J Med Entomol. 1988;25:551–554. doi: 10.1093/jmedent/25.6.551. [DOI] [PubMed] [Google Scholar]
- 84.Walker E D, Lawson D L, Morgan W T, Klug M J. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems. Ecology. 1991;72:1529–1546. [Google Scholar]
- 85.Walker E D, Kaufman M G, Ayres M P, Riedel M H, Merritt R W. Effect of variation in quality of leaf detritus on growth of the eastern tree hole mosquito, Aedes triseriatus (Diptera: Culicidae) Can J Zool. 1997;75:706–718. [Google Scholar]
- 86.Wetzel R G. Death, detritus, and energy flow in aquatic ecosystems. Freshwater Biol. 1995;33:83–89. [Google Scholar]
- 87.White D C, Findlay R H. Biochemical markers for measurement of predation effects on the biomass, community structure, nutritional status, and metabolic activity of microbial biofilms. Hydrobiology. 1988;159:119–132. [Google Scholar]
- 88.White D C, Bobbie R J, Nickels J S, Fazio S D, Davis W M. Nonselective biochemical methods for the determination of fungal mass and community structure in estuarine detrital microflora. Bot Marina. 1980;23:239–250. [Google Scholar]
- 89.Wilkinson L. SYSTAT: the system for statistics. Evanston, Ill: SYSTAT, Inc.; 1989. [Google Scholar]
- 90.Williams B K, Titus K. Assessment of sampling stability in ecological applications of discriminant analysis. Ecology. 1988;69:1275–1285. [Google Scholar]
- 91.Zelles L, Bai Q Y, Beck T, Beese F. Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biol Biochem. 1992;24:317–323. [Google Scholar]
- 92.Zelles L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere. 1997;35:275–294. doi: 10.1016/s0045-6535(97)00155-0. [DOI] [PubMed] [Google Scholar]