Abstract
Simple Summary
Males are much more susceptible to head and neck cancers than females regardless of whether they drink alcohol or smoke tobacco. Sex differences in the incidence of head and neck cancer are most evident in the lower part of the upper aerodigestive tract. Our results suggest a direction for future research on head and neck cancer epidemiology.
Abstract
Background: Descriptive epidemiologists have repeatedly reported that males are more susceptible to head and neck cancers. However, most published data are those of cross-sectional studies, and no population-based cohort study has yet been published. The aim of this study was to compare the prevalence of head and neck cancers in healthy males with females. Methods: A retrospective cohort study using the Korean National Health Insurance Service database on 9,598,085 individuals who underwent regular health checkups from 1 January to 31 December 2009. We sought head and neck cancers developed during the 10-year follow-up. Results: A total of 10,732 (incidence rate (IR) per 1000 person-years 0.25) individuals were newly diagnosed with head and neck cancer among the 9,598,085 individuals during the 10-year follow-up. The IR was 0.19 in males (8500 affected) and 0.06 in females (2232 affected). Notably, the male–female ratio increased with age below 70 years but decreased thereafter. The male–female difference was most apparent for laryngeal cancer; the male IR was 11-fold higher in the 40 s and 20-fold higher in the 60 s, followed by hypopharyngeal cancer (6.8- and 24.2-fold). Males smoked more and drank more alcohol than females (p < 0.0001 *, p < 0.0001 *). When never-smokers/-drinkers (only) were compared, males remained at a 2.9-fold higher risk of head and neck cancer than females. The hazard ratios for head and neck cancers in males tended to increase in the lower part of the upper aerodigestive tract: larynx (13.9) > hypopharynx (10.9) > oropharynx (4.4) > nasopharynx (2.9) > sinonasal region (1.8) > oral (1.6). Only the salivary gland cancer incidence did not differ between the sexes; the gland is not in the upper aerodigestive tract. Conclusion: Males are much more susceptible to head and neck cancers than females regardless of whether they drink alcohol or smoke tobacco. Sex differences in the incidence of head and neck cancer are most evident in the 60 s in the lower part of the upper aerodigestive tract, such as the larynx and hypopharynx.
Keywords: head and neck neoplasms, squamous cell carcinoma of head and neck, sex characteristics, gender difference, sex difference, alcohol drinking, smoking, cohort studies
1. Introduction
Descriptive epidemiologists have repeatedly reported differences in the cancer prevalence between males and females; males are more susceptible to most cancers [1,2]. The latest report (2019) also found that cancers were 1.2-fold more common in males [3,4]. This may reflect endogenous sex-specific biological differences or exogenous environmental variations that affect health care, cancer perceptions, and treatment compliance [5,6]. Head and neck cancer is the seventh most common cancer worldwide; there were more than 900,000 new cases in 2020 [7]. Most of such cancers are squamous cell carcinomas and include sinonasal, oral, nasopharyngeal, oropharyngeal, hypopharyngeal, laryngeal, and salivary gland cancers [7,8]. The incidence of head and neck cancers in males was higher than in females [3,4]. An analysis of data extracted from the November 2007 Surveillance, Epidemiology, and End Results (SEER) cancer registry revealed that laryngeal (male–female ratio 5.17), hypopharyngeal (4.13), tonsil (3.07), and oropharyngeal (3.06) cancers were among the top 10 cancers with the largest male–female ratios (the tonsils were distinguished from the oropharynx) [1]. The trends in head and neck cancers in South Korea (1999–2012) were similar: the male-to-female ratio exceeded 1.0 for all such cancers; the incidence of oral cancer was 2.8-fold higher in males than in females, and the incidence of pharyngeal and laryngeal cancers was 4.5-fold higher [9]. However, most published data are those of cross-sectional studies using information from cancer registries such as SEER [1,2,3,4,9,10]; no population-based cohort study that followed-up healthy individuals in terms of head and neck cancer incidence has yet been published in English. Thus, we performed a cohort study using the Korean National Health Insurance Service (KNHIS) database on 10 million healthy individuals who underwent regular health checkups in 2009 and who were then followed up for 10 years. We investigated the prevalence of, and risk factors for, head and neck cancers to suggest promising directions for future research.
2. Materials and Methods
2.1. Data Source and Study Population
The KNHIS is a mandatory public health insurance system administered by the Korean government; it covers about 97% of the entire Korean population (with the exception of the 3% of Medicaid beneficiaries) [11]. The KNHIS provides health care benefits and regular health checkup benefits every 2 years to all subscribers. The KNHIS database contains health information on most Koreans including: demographics; claims (general information on specifications; diagnoses as defined by the International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes); prescriptions and interventions; death information; and regular health check-up data [12].
2.2. Study Design and Patient Selection
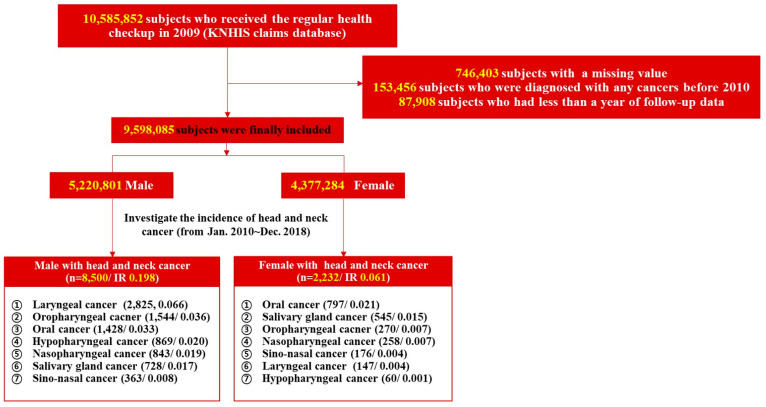
We performed a retrospective cohort study using the KNHIS database on 10,585,852 individuals who underwent regular health checkups from 1 January to 31 December 2009. Of these, data for 746,403 were unavailable, and we excluded 153,456 with a history of any type of cancer before and within 1 year after the index date, and 87,908 who underwent less than 1 year of follow-up. A total of 9,598,085 were finally included. We sought head and neck cancers developed during the 10-year follow-up by searching the relevant ICD-10-CM codes from 1 January 2010 to 31 December 2018 (Supplementary Table S1). The flow chart of subject selection from the KNHIS database is summarized in Figure 1. The following data were extracted from the database: age (years), smoking and alcohol consumption status, income, regular exercise status, height, weight, body mass index, waist circumference, abdominal obesity, glucose level, diabetes mellitus status, blood pressure, hypertension status, estimated glomerular filtration rate, chronic kidney disease status, cholesterol and triglyceride levels, and dyslipidemia status. The study protocol was approved by the Institutional Review Board of the Catholic University of Korea (no. PC21ZISI0235).
Figure 1.
Flow chart of the study. Abbreviations: KNHIS, Korean National Health Insurance Service; IR, incidence rate per 1000 person-years.
2.3. Potential Confounders
Variables that might possibly affect the head and neck cancer incidence were regarded as confounders: age; body mass index; smoking status; alcohol consumption level; low income; regular exercise, diabetes mellitus, and hypertension status [13,14,15,16,17,18,19]. Age was classified into <40, 40–64, and ≥65 years. The body mass index was the weight/height squared (kg/m2) and was divided into <25 and ≥25 kg/m2. Smoking status was classified as “never”, “past”, and “current” as recorded on the baseline survey. Alcohol consumption was based on that per week (“none”; “mild” < 30 g/day; or “heavy” ≥ 30 g/day). Income was determined by the monthly insurance premium (which reflects income). Regular exercise was defined as moderate to vigorous physical activity on at least 4 days a week.
2.4. Outcome Measurements
The incidence of head and neck cancers was determined by reference to the ICD-10-CM codes registered during the 10-year follow-up (from 1 year after baseline screening in 2009 until the end of follow-up or 31 December 2018); head and neck cancer was included in a co-payment reduction program for those with critical illnesses. In South Korea, virtually all people apply for such assistance if they are diagnosed with cancer because KNHIS covers 95% of all payments.
2.5. Statistical Analysis
All analyses were performed with the aid of SAS ver. 9.4 (SAS Institute, Cary, NC, USA); the p-values are two-sided. A p-value < 0.05 was considered significant. Descriptive statistics were used to record baseline characteristics. Males and females were compared using the χ2 test and one-way analysis of variance. Multivariate analyses employing a Cox’s proportional-hazards model were performed to determine the hazard ratios (HRs) for head and neck cancer incidence by sex. Covariates were not adjusted in regression model 1; age, body mass index, smoking status, alcohol consumption level, low income, and regular exercise status were adjusted in model 2; diabetes mellitus and hypertension status were additionally adjusted in model 3.
3. Results
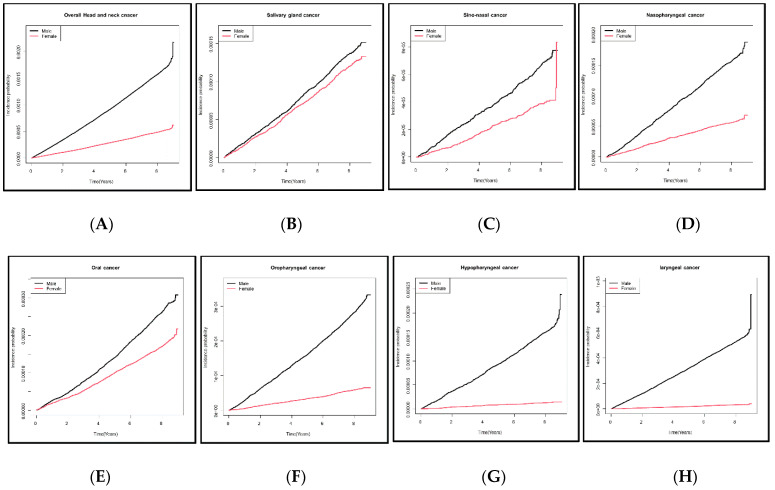
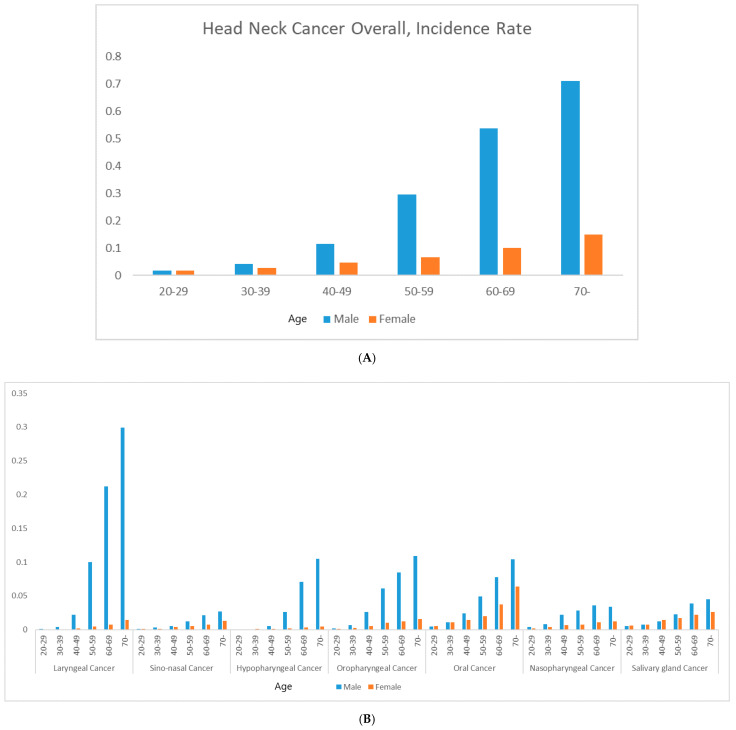
The baseline characteristics of the 9,598,085 subjects are summarized in Table 1. The mean age was 45.63 ± 13.43 years in males and 48.64 ± 14.5 years in females, thus significantly different (p < 0.0001 *). Of males, 30.88% stated that they were never-smokers, 24.06% were ex-smokers, and 45.06% were current smokers; among females, 95.04% were never-smokers. Of males, 13.4% were heavy alcohol consumers (≥30 g/day), 54.19% mild consumers (<30 g/day), and 32.07% were non-consumers. Of females, 74.9% were non-consumers, 24.3% were mild consumers, and 1.11% were heavy consumers. Significantly fewer males than females reported that they were never-smokers/-drinkers (both p < 0.0001 *). Males exhibited a significantly higher body mass index, a higher income, more regular exercise, and higher rates of diabetes mellitus and hypertension than females (all p < 0.0001 *). A total of 10,732 (incidence rate (IR) per 1000 person-years 0.25) individuals were newly diagnosed with head and neck cancer among the 9,598,085 individuals who underwent regular health checkups during the 10-year follow-up. The IR was 0.19 in males (8500 affected) and 0.06 in females (2232 affected). Laryngeal cancer was the most common male cancer and oral cancer the most common female cancer; the IRs for the various cancers are shown in Figure 1. Kaplan–Meier survival curves revealed that males had a higher incidence than females of all new head and neck cancers except salivary gland cancer (Figure 2). The adjusted HR for all head and neck cancers was significantly higher in males than females after adjusting for confounders (HR 2.816 [2.656, 2.985], p < 0.0001 *) in Model 3 (Table 2). The adjusted HRs for laryngeal cancer (10.981 [9.171, 13.148], p < 0.0001 *), sinonasal cancer (1.758 [1.388, 2.226], p < 0.0001 *), hypopharyngeal cancer (9.949 [7.476, 13.239], p < 0.0001 *), oropharyngeal cancer (4.589 [3.942, 5.343], p < 0.0001 *), oral cancer (1.472 [1.31, 1.654)], p < 0.0001 *), and nasopharyngeal cancer (2.805 [2.359, 3.336], p < 0.0001 *) were significantly higher in males, but the difference was not significant for salivary gland cancer (HR 1.139 [0.977, 1.327], p = 0.0966) as revealed by Model 3. The IRs of head and neck cancers in previously healthy males and females by age are shown in Figure 3 (the details are in Supplementary Table S2). The male–female ratios of cancer incidence were 1.0 in the 20 s, 1.5 in the 30 s, 2.5 in the 40 s, 4.4 in the 50 s, 5.4 in the 60 s, and 4.6 in the 70 s. The male–female ratio of head and neck cancers among never-smokers/-drinkers (only) were: hypopharyngeal (9.949), laryngeal (10.981), oral (1.472), sinonasal (1.758), nasopharyngeal (2.805), and oropharyngeal (4.589).
Table 1.
Baseline characteristics of the original cohort of subjects.
| Parameters | Male (n = 5,220,801) | Female (n = 4,377,284) | p-Value |
|---|---|---|---|
| Age (years) | 45.63 ± 13.43 | 48.64 ± 14.5 | <0.0001 * |
| Age group | <0.0001 * | ||
| <40 years | 1,944,807 (37.25%) | 1,085,391 (24.8%) | |
| 40–64 years | 2,723,688 (52.17%) | 2,619,380 (59.84%) | |
| ≥65 years | 552,306 (10.58%) | 672,513 (15.36%) | |
| Smoking status | <0.0001 * | ||
| Never-smoker | 1,612,028 (30.88%) | 4,160,334 (95.04%) | |
| Ex-smoker | 1,256,220 (24.06%) | 69,232 (1.58%) | |
| Current smoker | 2,352,553 (45.06%) | 147,718 (3.37%) | |
| Drinking status | <0.0001 * | ||
| None | 1,674,378 (32.07%) | 3,264,933 (74.59%) | |
| Mild (<30 g/day) | 2,828,959 (54.19%) | 1,063,614 (24.3%) | |
| Heavy (≥30 g/day) | 717,464 (13.74%) | 48,737 (1.11%) | |
| Low income | 785,104 (15.04%) | 1,092,812 (24.97%) | <0.0001 * |
| Regular exercise | 1,030,226 (19.73%) | 678,186 (15.49%) | <0.0001 * |
| Height (cm) | 170.01 ± 6.44 | 156.48 ± 6.22 | <0.0001 * |
| Weight (kg) | 69.87 ± 10.54 | 56.73 ± 8.42 | <0.0001 * |
| Body mass index (kg/m2) | 24.13 ± 3.33 | 23.19 ± 3.54 | <0.0001 * |
| Body mass index (kg/m2) | <0.0001 * | ||
| <25 | 3,276,855 (62.77%) | 3,189,643 (72.87%) | |
| ≥25 | 1,943,946 (37.23%) | 1,187,641 (27.13%) | |
| Waist circumference (cm) | 83.56 ± 8.26 | 76.2 ± 9.3 | <0.0001 * |
| Abdominal obesity(waist circumference, M ≥ 90 cm/F ≥ 85 cm) | 1,115,781 (21.37%) | 768,973 (17.57%) | <0.0001 * |
| Glucose (mM) | 99.06 ± 25.77 | 95.07 ± 21.14 | <0.0001 * |
| Diabetes mellitus | 508,895 (9.75%) | 321,744 (7.35%) | <0.0001 * |
| Systolic BP (mmHg) | 124.66 ± 14.13 | 119.76 ± 15.71 | <0.0001 * |
| Diastolic BP (mmHg) | 78.05 ± 9.72 | 74.25 ± 10.09 | <0.0001 * |
| Hypertension | 1,395,431 (26.73%) | 1,071,413 (24.48%) | <0.0001 * |
| eGFR (mL/min/1.73 m2) | 87.99 ± 51.5 | 87.21 ± 36.52 | <0.0001 * |
| Chronic kidney disease (eGFR < 60) | 314,306 (6.02%) | 344,244 (7.86%) | <0.0001 * |
| Total cholesterol (mM) | 194.67 ± 40.93 | 196.15 ± 42.08 | <0.0001 * |
| HDL cholesterol (mM) | 53.56 ± 31.54 | 60 ± 33.83 | <0.0001 * |
| LDL cholesterol (mM) | 118.42 ± 190.63 | 122.75 ± 214.43 | <0.0001 * |
| Triglyceride (mM) | 129.15 (129.09–129.21) | 95.75 (95.7–95.8) | <0.0001 * |
| Dyslipidemia | 866,675 (16.6%) | 869,691 (19.87%) | <0.0001 * |
* Significant at p < 0.05. Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure 2.
Kaplan–Meier curve of the cumulative incidence of various head and neck cancers in the original cohort of healthy males and females: (A) all head and neck cancers; (B) salivary gland cancer, (C) sinonasal cancer, (D) nasopharyngeal cancer, (E) oral cancer, (F) oropharyngeal cancer, (G) hypopharyngeal cancer, and (H) laryngeal cancer.
Table 2.
Multivariate Cox’s proportional hazard model for the incidence of head and neck cancer by sex. Model 1: unadjusted. Model 2: adjusted for age, body mass index (BMI), low income, smoking status, drinking status, and regular exercise. Model 3: adjusted for age, body mass index (BMI), low income, smoking status, drinking status, regular exercise, and diabetes mellitus and hypertension status.
| Per 1000 Person-Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Event | Duration | Rate | HR (95% CI) | ||||||
| Model 1 | p-Value | Model 2 | p-Value | Model 3 | p-Value | |||||
| All head and neck cancers | ||||||||||
| Male | 5,220,801 | 8500 | 42,731,332.51 | 0.19892 | 3.238 (3.09, 3.392) | <0.0001 | 2.83 (2.67, 3) | <0.0001 | 2.816 (2.656, 2.985) | <0.0001 |
| Female | 4,377,284 | 2232 | 36,231,562.63 | 0.0616 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Laryngeal cancer | ||||||||||
| Male | 5,220,801 | 2825 | 42,747,447.1 | 0.06609 | 16.306 (13.816, 19.244) | <0.0001 | 11.036 (9.218, 13.213) | <0.0001 | 10.981 (9.171, 13.148) | <0.0001 |
| Female | 4,377,284 | 147 | 36,238,096.36 | 0.00406 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Sinonasal cancer | ||||||||||
| Male | 5,220,801 | 363 | 42,755,919.66 | 0.00849 | 1.754 (1.465, 2.1) | <0.0001 | 1.77 (1.398, 2.242) | <0.0001 | 1.758 (1.388, 2.226) | <0.0001 |
| Female | 4,377,284 | 176 | 36,238,089.5 | 0.00486 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Hypopharyngeal cancer | ||||||||||
| Male | 5,220,801 | 869 | 42,754,938.24 | 0.02033 | 12.345 (9.504, 16.037) | <0.0001 | 9.987 (7.505, 13.289) | <0.0001 | 9.949 (7.476, 13.239) | <0.0001 |
| Female | 4,377,284 | 60 | 36,238,435.42 | 0.00166 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Oropharyngeal cancer | ||||||||||
| Male | 5,220,801 | 1544 | 42,752,316.34 | 0.03612 | 4.856 (4.267, 5.527) | <0.0001 | 4.613 (3.963, 5.371) | <0.0001 | 4.589 (3.942, 5.343) | <0.0001 |
| Female | 4,377,284 | 270 | 36,237,728.87 | 0.00745 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Oral cancer | ||||||||||
| Male | 5,220,801 | 1428 | 42,752,896.93 | 0.0334 | 1.523 (1.396, 1.661) | <0.0001 | 1.483 (1.32, 1.666) | <0.0001 | 1.472 (1.31, 1.654) | <0.0001 |
| Female | 4,377,284 | 797 | 36,236,352.3 | 0.02199 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Nasopharyngeal cancer | ||||||||||
| Male | 5,220,801 | 843 | 42,754,118.86 | 0.01972 | 2.778 (2.417, 3.194) | <0.0001 | 2.798 (2.353, 3.327) | <0.0001 | 2.805 (2.359, 3.336) | <0.0001 |
| Female | 4377284 | 258 | 36237707.17 | 0.00712 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Salivary gland cancer | ||||||||||
| Male | 5,220,801 | 728 | 42,754,522.91 | 0.01703 | 1.132 (1.013, 1.265) | 0.0285 | 1.145 (0.983, 1.335) | 0.0815 | 1.139 (0.977, 1.327) | 0.0966 |
| Female | 4,377,284 | 545 | 36,236,699.52 | 0.01504 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
Model 1: non-adjusted; Model 2: AGE HE_BMI INCOME_LOW SMOKING DRINKER_3LEVEL PA_REGULAR; Model 3: AGE HE_BMI INCOME_LOW SMOKING DRINKER_3LEVEL PA_REGULAR DMYN HPYN.
Figure 3.
The incidence of head and neck cancers in healthy men and women by age during the 10-year follow-up period. (A) All head and neck cancers and (B) cancers by the subsites.
4. Discussion
The incidence of cancers varies by sex/gender; these may influence the cancer risk in different ways. Sex is a biological concept determined by the sex chromosomes [20,21,22,23,24]; gender refers to social concepts that can vary by society and time [5,6]. The complex and dynamic interactions between sex and gender throughout the lifespan may affect health care; cancer susceptibility, perception, progression; and treatment compliance. Cancer-preventing lifestyles and minimal exposure to risk factors are critical. Men are more likely to exhibit behavioral risk factors such as alcohol consumption and smoking. However, even after adjusting for these factors, men still evidenced a higher incidence of cancer [2,25,26]. Between-sex differences in anatomy, physiology, body composition, and drug metabolism might affect cancer incidence [27]. Sex hormones may play critical roles in carcinogenesis and cancer susceptibility: androgens are significantly associated with a higher cancer prevalence (and poor outcomes) in men; estrogens seem to exert protective effects in females [20,21,22,23]. However, as the IRs of most cancers are higher even in male children and adolescents, the sex hormones do not fully explain the sex disparity [28]. It has been suggested that sexual differences in the humoral and cell-mediated immune responses to viral infection may explain the differences in cancer prevalence [29,30,31,32]. Generally, females mount stronger humoral and cell-mediated immune responses than men throughout life [33]. Differences in the regulation of the immune response and the infection burden may contribute to sex differences in cancer incidence including human papillomavirus (HPV)-associated cancer.
The head and neck cancer IR in Korean males was about three-fold that in females; we studied a cohort of 10 million. Notably, the male–female ratio increased with age below 70 years but decreased thereafter. The male–female difference was most apparent for laryngeal cancer; the male IR was 11-fold higher in the 40 s and 20-fold higher in the 60 s, followed by hypopharyngeal cancer (6.8- and 24.2-fold). A decreased sexual gap in terms of socioeconomic activity, less exposure to tobacco and alcohol, and reduced sex hormones at ages > 70 years may play roles, but much remains unclear [2,20,21,22,23,25,26,34,35].
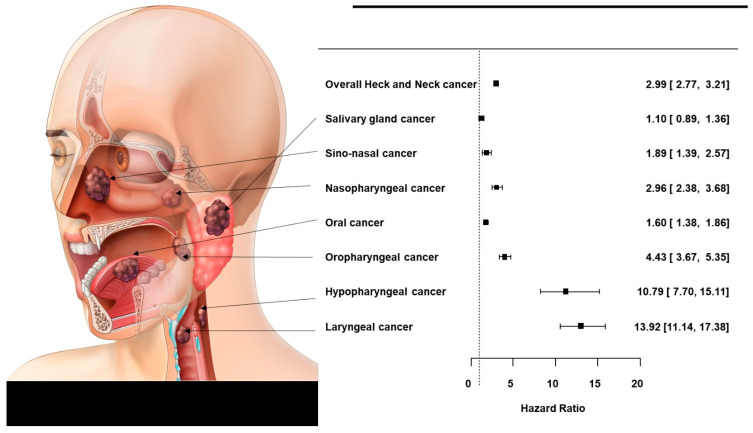
Males smoked more and drank more alcohol than females; both are major risk factors for head and neck cancers [36,37,38,39]. Lewin et al. reported that the relative risk (RR) of head and neck cancer among current smokers was 6.5-fold than that of non-smokers. Twenty years of smoking cessation is required before the risk declines to the level of the non-smoker. The RR associated with alcohol consumption ≥ 50 g/day was 5.5 compared to consumption of <10 g/day [36]. In terms of bodyweight management, interestingly, more males (19.7%) than females (15.4%) stated that they exercised regularly, but males were more likely to be overweight (males 37.2% vs. females 27.1%). Diabetes and hypertension rates were higher in males, and the chronic kidney disease and dyslipidemia rates were higher in females. More females than males had low incomes (males 15.0%, females 24.9%). Males remained at a 2.8-fold higher risk of head and neck cancer than females even after adjusting for these confounding factors [13,14,15,16,17,18,19]: the laryngeal cancer risk was 10.9-fold higher and that of hypopharyngeal cancer was 9.9-fold higher in males than females. When never-smokers/-drinkers (only) were compared, males remained at a 2.9-fold higher risk of head and neck cancer than females: a 13.9-fold higher risk of laryngeal cancer, a 10.9-fold higher risk of hypopharyngeal cancer, a 4.4-fold higher risk of oropharyngeal cancer, a 2.9-fold higher risk for nasopharyngeal cancer, a 1.8-fold higher risk for nasal sinus cancer, and a 1.6-fold higher risk for oral cancer. Interestingly, the HRs for head and neck cancers in males tended to increase in anatomical order closer to the esophagus in the upper aerodigestive tract: larynx > hypopharynx > oropharynx > nasopharynx > sinonasal region > mouth (Figure 4). Only the salivary gland cancer incidence did not differ between the sexes; the gland is not in the upper aerodigestive tract. Helicobacter pylori (H. pylori) infection causes duodenal and gastric ulcers [40] and can migrate to the upper aerodigestive tract [41]; it is found in dental plaque, saliva, tonsils, and even adenoidal tissue (associated with gastroesophageal reflux). These materials/tissues may serve as bacterial reservoirs [42]. In a comparative analysis of 109,360 patients with peptic ulcer disease and 218,720 controls detailed in the Taiwan National Health Insurance Research Database (1997–2013), Lu et al. [43] found that those with peptic ulcers were at a higher risk of laryngeal and hypopharyngeal cancers (adjusted HRs: 2.27 [95% CI: 1.16–4.44] and 2.00 [95% CI, 1.13–3.55]). In a systematic review of 244 studies, Ibrahim et al. found that the male sex was associated with a higher incidence of H. pylori infection. Although it remains unclear whether such infection is a significant risk factor for head and neck cancer, and how sex might affect the acquisition and persistence of the infection, our results suggest a direction for future research on head and neck cancer epidemiology.
Figure 4.
Forest plot of the hazard ratios for all subsites of head and neck cancers in males compared to females (never-smokers and never-drinkers only). A multivariate Cox’s proportional hazard model adjusted for age, body mass index, low income, regular exercise, and diabetes mellitus and hypertension status was employed.
The limitations of our study are that epidemiological data on human papillomavirus were not included and the effect of cervical cancer vaccination in females was not considered, although HPV infection increases the risk of head and neck cancers.
Laryngeal cancer is the most male-prevalent head-and-neck cancer. Concerning differences in the results of the carcinogenic effects of tobacco smoking and drinking, we investigated the prevalence of laryngeal cancer in both sexes lacking exposure to these carcinogens, but found that the incidence of laryngeal cancer remained 16.9-times higher in males. Hormone control of cancer progression might be a mechanism, as the larynx is a secondary sex organ that undergoes physiological changes during puberty, which suggests a relationship with sex hormone receptors, such as estrogen receptor. Estrogen receptors are found in head and neck subsites, especially in the larynx, and several authors have suggested that estrogens play a direct role in regulating laryngeal cancer progression [44,45,46]. Verma et al. reported that laryngeal cancers responded to 17β-estradiol via the estrogen receptor and that higher estrogen-receptor expression was correlated with a better survival [45,47]. Atef et al. found that estrogen, progesterone, and androgen receptors were positive in 56%, 50%, and 64% of 50 laryngeal cancer patients, respectively; also, the expressions of estrogen and progesterone receptors were significantly higher, whereas that of androgen receptor was lower, in patients with aggressive clinical and pathological manifestations [48]. With the possible susceptibility of laryngeal cancer to estrogens, further investigation is needed to demonstrate the effects of estrogen and estrogen receptors on laryngeal cancer progression and possible prognostic markers in the treatment of laryngeal cancer.
5. Conclusions
Males are much more susceptible to head and neck cancers than females regardless of whether they drink alcohol or smoke tobacco. Sex differences in the incidence of head and neck cancer are most evident in the 60 s in the lower part of the upper aerodigestive tract, such as the larynx and hypopharynx. Further research is needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14102521/s1, Table S1: The International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes used to diagnose head-and-neck cancers; Table S2: The incidence of head-and-neck cancers in healthy men and women by age during the 10-year follow-up period.
Author Contributions
Conception and design: Y.-H.J., J.-O.P. and K.-D.H.; collection and assembly of data: I.-C.N. and K.-D.H.; data analysis and interpretation: C.-S.K., K.-D.H. and S.-J.P.; manuscript writing: J.-O.P., D.-H.L. and H.-B.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All data were collected in accordance with the World Medical Association Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of the Catholic University of Korea (no. PC21ZISI0235).
Informed Consent Statement
Patient consent was waived for the retrospective data evaluation.
Data Availability Statement
Data available upon request due to data sharing restrictions. The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (no. NRF-2021R1C1C1004073) and by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 202011D15). This work was also supported by the Catholic University of Korea, Eunpyeong St. Mary’s Hospital, Research Institute of Medical Science.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cook M.B., Dawsey S.M., Freedman N.D., Inskip P.D., Wichner S.M., Quraishi S.M., Devesa S.S., McGlynn K.A. Sex disparities in cancer incidence by period and age. Cancer Epidemiol. Biomarkers Prev. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgren G., Liang L., Adami H.O., Chang E.T. Enigmatic sex disparities in cancer incidence. Eur. J. Epidemiol. 2012;27:187–196. doi: 10.1007/s10654-011-9647-5. [DOI] [PubMed] [Google Scholar]
- 3.Ward E.M., Sherman R.L., Henley S.J., Jemal A., Siegel D.A., Feuer E.J., Firth A.U., Kohler B.A., Scott S., Ma J., et al. Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20–49 Years. J. Natl. Cancer Inst. 2019;111:1279–1297. doi: 10.1093/jnci/djz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong M., Cioffi G., Wang J., Waite K.A., Ostrom Q.T., Kruchko C., Lathia J.D., Rubin J.B., Berens M.E., Connor J., et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer Epidemiol. Biomark. Prev. 2020;29:1389–1397. doi: 10.1158/1055-9965.EPI-20-0036. [DOI] [PubMed] [Google Scholar]
- 5.Clayton J.A., Tannenbaum C. Reporting Sex, Gender, or Both in Clinical Research? JAMA. 2016;316:1863–1864. doi: 10.1001/jama.2016.16405. [DOI] [PubMed] [Google Scholar]
- 6.Polderman T.J.C., Kreukels B.P.C., Irwig M.S., Beach L., Chan Y.M., Derks E.M., Esteva I., Ehrenfeld J., Heijer M.D., Posthuma D., et al. The Biological Contributions to Gender Identity and Gender Diversity: Bringing Data to the Table. Behav. Genet. 2018;48:95–108. doi: 10.1007/s10519-018-9889-z. [DOI] [PubMed] [Google Scholar]
- 7.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Suh J.D., Cho J.H. Trends in Head and Neck Cancer in South Korea Between 1999 and 2012. Clin. Exp. Otorhinolaryngol. 2016;9:263–269. doi: 10.21053/ceo.2015.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorak M.T., Karpuzoglu E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012;3:268. doi: 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D.S. Introduction: Health of the health care system in Korea. Soc. Work. Public Health. 2010;25:127–141. doi: 10.1080/19371910903070333. [DOI] [PubMed] [Google Scholar]
- 12.Lee J., Lee J.S., Park S.H., Shin S.A., Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 13.Hashibe M., Brennan P., Benhamou S., Castellsague X., Chen C., Curado M.P., Dal Maso L., Daudt A.W., Fabianova E., Fernandez L., et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 14.Anantharaman D., Marron M., Lagiou P., Samoli E., Ahrens W., Pohlabeln H., Slamova A., Schejbalova M., Merletti F., Richiardi L., et al. Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. Oral Oncol. 2011;47:725–731. doi: 10.1016/j.oraloncology.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Conway D.I., Brenner D.R., McMahon A.D., Macpherson L.M., Agudo A., Ahrens W., Bosetti C., Brenner H., Castellsague X., Chen C., et al. Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE consortium pooled analysis of 31 case-control studies from 27 countries. Int. J. Cancer. 2015;136:1125–1139. doi: 10.1002/ijc.29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conway D.I., Petticrew M., Marlborough H., Berthiller J., Hashibe M., Macpherson L.M. Socioeconomic inequalities and oral cancer risk: A systematic review and meta-analysis of case-control studies. Int. J. Cancer. 2008;122:2811–2819. doi: 10.1002/ijc.23430. [DOI] [PubMed] [Google Scholar]
- 17.Figueiredo R.A., Weiderpass E., Tajara E.H., Ström P., Carvalho A.L., de Carvalho M.B., Kanda J.L., Moyses R.A., Wünsch-Filho V. Diabetes mellitus, metformin and head and neck cancer. Oral Oncol. 2016;61:47–54. doi: 10.1016/j.oraloncology.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Khanna A., Sturgis E.M., Dahlstrom K.R., Xu L., Wei Q., Li G., Gross N.D. Association of pretreatment body mass index with risk of head and neck cancer: A large single-center study. Am. J. Cancer Res. 2021;11:2343–2350. [PMC free article] [PubMed] [Google Scholar]
- 19.Christakoudi S., Kakourou A., Markozannes G., Tzoulaki I., Weiderpass E., Brennan P., Gunter M., Dahm C.C., Overvad K., Olsen A., et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer. 2020;146:2680–2693. doi: 10.1002/ijc.32576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clocchiatti A., Cora E., Zhang Y., Dotto G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer. 2016;16:330–339. doi: 10.1038/nrc.2016.30. [DOI] [PubMed] [Google Scholar]
- 21.Chlebowski R.T., Wactawski-Wende J., Ritenbaugh C., Hubbell F.A., Ascensao J., Rodabough R.J., Rosenberg C.A., Taylor V.M., Harris R., Chen C., et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. New Engl. J. Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 22.Hsu J.W., Hsu I., Xu D., Miyamoto H., Liang L., Wu X.R., Shyr C.R., Chang C. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am. J. Pathol. 2013;182:1811–1820. doi: 10.1016/j.ajpath.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto H., Yang Z., Chen Y.T., Ishiguro H., Uemura H., Kubota Y., Nagashima Y., Chang Y.J., Hu Y.C., Tsai M.Y., et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl. Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 24.Giuliano A.R., Nyitray A.G., Kreimer A.R., Pierce Campbell C.M., Goodman M.T., Sudenga S.L., Monsonego J., Franceschi S. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int. J. Cancer. 2015;136:2752–2760. doi: 10.1002/ijc.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertin M., Thébaud-Mony A., Counil E. Do Women and Men Have the Same Patterns of Multiple Occupational Carcinogenic Exposures? Results from a Cohort of Cancer Patients. Ann. Work. Expo. Health. 2018;62:450–464. doi: 10.1093/annweh/wxx116. [DOI] [PubMed] [Google Scholar]
- 26.Pukkala E., Martinsen J.I., Lynge E., Gunnarsdottir H.K., Sparén P., Tryggvadottir L., Weiderpass E., Kjaerheim K. Occupation and cancer—follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- 27.Özdemir B.C., Csajka C., Dotto G.P., Wagner A.D. Sex Differences in Efficacy and Toxicity of Systemic Treatments: An Undervalued Issue in the Era of Precision Oncology. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;36:2680–2683. doi: 10.1200/JCO.2018.78.3290. [DOI] [PubMed] [Google Scholar]
- 28.Williams L.A., Richardson M., Marcotte E.L., Poynter J.N., Spector L.G. Sex ratio among childhood cancers by single year of age. Pediatric Blood Cancer. 2019;66:e27620. doi: 10.1002/pbc.27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fish E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillison M.L., Koch W.M., Capone R.B., Spafford M., Westra W.H., Wu L., Zahurak M.L., Daniel R.W., Viglione M., Symer D.E., et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 31.Henle G., Henle W. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int. J. Cancer. 1976;17:1–7. doi: 10.1002/ijc.2910170102. [DOI] [PubMed] [Google Scholar]
- 32.Lleo A., Battezzati P.M., Selmi C., Gershwin M.E., Podda M. Is autoimmunity a matter of sex? Autoimmun. Rev. 2008;7:626–630. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 34.Li W.-Z., Lv S.-H., Liu G.-Y., Liang H., Xia W.-X., Xiang Y.-Q. Age-dependent changes of gender disparities in nasopharyngeal carcinoma survival. Biol. Sex Differ. 2021;12:18. doi: 10.1186/s13293-021-00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.OuYang P.Y., Zhang L.N., Lan X.W., Xie C., Zhang W.W., Wang Q.X., Su Z., Tang J., Xie F.Y. The significant survival advantage of female sex in nasopharyngeal carcinoma: A propensity-matched analysis. Br. J. Cancer. 2015;112:1554–1561. doi: 10.1038/bjc.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewin F., Norell S.E., Johansson H., Gustavsson P., Wennerberg J., Biörklund A., Rutqvist L.E. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: A population-based case-referent study in Sweden. Cancer. 1998;82:1367–1375. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1367::AID-CNCR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Franceschi S., Bidoli E., Negri E., Barbone F., La Vecchia C. Alcohol and cancers of the upper aerodigestive tract in men and women. Cancer Epidemiol. Biomarkers Prev. 1994;3:299–304. [PubMed] [Google Scholar]
- 38.Kato I., Nomura A.M. Alcohol in the aetiology of upper aerodigestive tract cancer. Eur. J. Cancer B Oral Oncol. 1994;30b:75–81. doi: 10.1016/0964-1955(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 39.Choi S.Y., Kahyo H. Effect of cigarette smoking and alcohol consumption in the aetiology of cancer of the oral cavity, pharynx and larynx. Int. J. Epidemiol. 1991;20:878–885. doi: 10.1093/ije/20.4.878. [DOI] [PubMed] [Google Scholar]
- 40.Marshall B.J., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama A., Yokoyama T., Omori T., Matsushita S., Mizukami T., Takahashi H., Higuchi S., Maruyama K., Ishii H., Hibi T. Helicobacter pylori, chronic atrophic gastritis, inactive aldehyde dehydrogenase-2, macrocytosis and multiple upper aerodigestive tract cancers and the risk for gastric cancer in alcoholic Japanese men. J. Gastroenterol. Hepatol. 2007;22:210–217. doi: 10.1111/j.1440-1746.2006.04377.x. [DOI] [PubMed] [Google Scholar]
- 42.Kurtaran H., Uyar M.E., Kasapoglu B., Turkay C., Yilmaz T., Akcay A., Kanbay M. Role of Helicobacter pylori in pathogenesis of upper respiratory system diseases. J. Natl. Med. Assoc. 2008;100:1224–1230. doi: 10.1016/S0027-9684(15)31471-1. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y.T., Hsin C.H., Lu Y.C., Wu M.C., Huang J.Y., Huang C.C., Wang P.H., Yang S.F. Risk of head and neck cancer in patients with peptic ulcers and the effect of Helicobacter pylori treatment. Sci. Rep. 2021;11:6229. doi: 10.1038/s41598-021-85598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laffont S., Seillet C., Guéry J.-C. Estrogen Receptor-Dependent Regulation of Dendritic Cell Development and Function. Front. Immunol. 2017;8:108. doi: 10.3389/fimmu.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma A., Schwartz N., Cohen D.J., Boyan B.D., Schwartz Z. Estrogen signaling and estrogen receptors as prognostic indicators in laryngeal cancer. Steroids. 2019;152:108498. doi: 10.1016/j.steroids.2019.108498. [DOI] [PubMed] [Google Scholar]
- 46.Fei M., Zhang J., Zhou J., Xu Y., Wang J. Sex-related hormone receptor in laryngeal squamous cell carcinoma: Correlation with androgen estrogen-ɑ and prolactin receptor expression and influence of prognosis. Acta Otolaryngol. 2018;138:66–72. doi: 10.1080/00016489.2017.1373851. [DOI] [PubMed] [Google Scholar]
- 47.Verma A., Schwartz N., Cohen D.J., Patel V., Nageris B., Bachar G., Boyan B.D., Schwartz Z. Loss of Estrogen Receptors is Associated with Increased Tumor Aggression in Laryngeal Squamous Cell Carcinoma. Sci. Rep. 2020;10:4227. doi: 10.1038/s41598-020-60675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atef A., El-Rashidy M.A., Elzayat S., Kabel A.M. The prognostic value of sex hormone receptors expression in laryngeal carcinoma. Tissue Cell. 2019;57:84–89. doi: 10.1016/j.tice.2019.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request due to data sharing restrictions. The data presented in this study are available upon request from the corresponding author.