Abstract
Objective: Although some meta-analyses have shown a correlation between a high neutrophil-to-lymphocyte ratio (NLR) and low survival in patients with gliomas, their conclusions are controversial, and no study has specifically explored the relationship between a high pre-treatment and pre-operative NLR and low survival in patients with glioblastoma (GBM). Therefore, we further investigated this correlation through meta-analysis. Methods: We searched the PubMed, Metstr, and Cochrane databases in March 2022 for published literature related to high pre-treatment and pre-operative NLR and low survival in patients with GBM. The literature was rigorously searched according to inclusion and exclusion criteria to calculate the overall hazard ratio (HR) and 95% confidence interval (CI) corresponding to a high NLR using a random effects model. Results: The total HR for the pre-treatment and pre-operative NLR was 1.46 (95% CI: 1.17–1.75, p = 0.000, I2 = 76.5%), indicating a significant association between a high pre-treatment and pre-operative NLR, and low overall survival in patients with GBM. Sub-group analysis was performed because of the high heterogeneity. The results for the sub-group with a cut-off value of 4 showed an HR of 1.39 (95% CI: 1.12–1.65, p = 0.000, I2 = 22.2%), with significantly low heterogeneity, whereas those for the sub-group without a cut-off value of 4 showed an HR of 1.45 (95% CI: 1.01–1.89, p = 0.000, I2 = 83.3%). Conclusions: The results of this study demonstrate that a high pre-treatment and pre-operative NLR suggests low survival in patients with GBM based on data from a large sample. Furthermore, the meta-regression analysis results indicate that underlying data, such as age and extent of surgical resection, lead to a high degree of heterogeneity, providing a theoretical basis for further research.
Keywords: glioblastoma, GBM, glioblastoma multiforme, NLR, neutrophil-to-lymphocyte ratio, prognostic
1. Introduction
Glioblastoma (GBM) is a common, highly malignant glioma (grade IV) that is often accompanied by high morbidity and mortality, with a 5-year overall survival (OS) rate of <5% [1]. Despite a range of standardised treatments, such as surgery combined with radiotherapy (e.g., temozolomide) [2], patient factors and tumour heterogeneity have led to significant differences in prognostic outcomes [3]. In recent years, several studies have demonstrated the prognostic significance of the peripheral blood neutrophil-to-lymphocyte ratio (NLR) in glioblastoma [4,5] and other cancers [6,7,8]. The NLR is a convenient, low-cost, and easily measured marker that responds to the balance between inflammation and the immune system. Numerous studies have shown that increased lymphocytes around the tumour or in the peripheral blood often suggest a poor prognosis, whereas lymphocytosis indicates a better prognosis [9,10,11,12,13,14].
Several meta-analyses have shown that a high NLR is associated with poor survival in patients with glioma [15,16,17], and one [18] reported a correlation between high NLR and glioma grade. However, no meta-analysis has been performed on studies with a high NLR and poor overall survival (OS) prior to GBM treatment and surgery. In the studies by Lei et al. [15] and Yang et al. [17], sub-group analyses showed a correlation between a high NLR and poor OS in patients with GBM. However, these analyses only indicated a small number of studies. Furthermore, studies by He et al. [19] and Besiroglu et al. [20] demonstrated that a high NLR was not associated with poor OS in patients with GBM. Since only high-grade gliomas and patients who underwent standardised surgery were included in the study by He et al. [19], confounding factors for low-grade gliomas were excluded, restricting these findings to a smaller subset of GBM patients. Besiroglu et al. [20] reported that an increase in pre-treatment neutrophil count might have improved the treatment response to bevacizumab in patients with GBM, leading to an inability to demonstrate the prognostic significance of NLR. However, this needs to be validated by further studies. Given the differences in correlations between a high NLR and poor OS before GBM treatment, closer examination of this relationship is warranted.
2. Methods
2.1. Identification of Studies and Collection of Data
We conducted this meta-analysis based on the recommendations and criteria developed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [21]. A search was conducted in March 2022 to identify all literature on pre-treatment and pre-procedure NLR related to GBM survival. Searches were performed using the terms “glioblastoma” or “GBM” or “glioblastoma multiforme” and “NLR” or “neutrophil/lymphocyte ratio” and “prognosis” and their variants in the PubMed, Metstr (http://fmrs.metstr.com/index.aspx (accessed on 5 March 2022 )), and Cochrane databases. The final literature for inclusion was screened manually by two researchers (X.G. and H.J.) and reviewed by a third researcher (T.Z.) to avoid omissions. Finally, basic data were extracted from the included literature, including information on the first author, country, year of publication, type of study, age, sex, sample size, extent of surgical resection, number of patients undergoing secondary surgery, NLR thresholds, and treatment options (Table 1). In addition, we extracted corresponding survival rates and 95% confidence interval (CI) values. In cases where both univariate and multivariate analysis results were available, we prioritised extraction of outcome values from multivariate analysis. For the literature where hazard ratio (HR) values and 95% CIs for the corresponding OS were not mentioned, but the corresponding Kaplan–Meier curves were provided, survival data were extracted and converted to HR and 95% CIs using Engauge Digitiser version 12.1 (Xi’an, China) with the interrupted point-taking method.
Table 1.
Main characteristics of 19 studies included in meta-analysis.
| First Author and Year | Country | Patients (N) | Design | Age (mean ± SD) | Gender (M) | Sample Size (N) | Gross Total Resection (N) | Second Surgery (N) | NLR Cut-off | Therapy | Outcome | HR (95%CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bambury 2013 | USA | GBM multiforme | Retrospective | 56.6 ± 11.53 | 65 | 84 | 23 | NR | NLR = 4 | S + C+ T | MA | 1.81 (1.08–3.02) |
| Sheng Han 2015 | China | GBM | Retrospective | 50.4 ± 15.4 | 95 | 152 | 75 | NR | NLR = 4 | S + C | MA | 2.068 (1.304–3.277) |
| Peng-Fei Wang 2017 | China | GBM | Prospective | 52.1 ± 0.984 | 96 | 166 | 102 | NR | NLR = 4 | NR | MA | 1.712 (1.026–2.858) |
| Marta Lopes 2018 | Portugal | GBM multiforme | Retrospective | NR | NR | 126 | NR | NR | NLR = 5 | NR | MA | 1.56 (1.04–2.34) |
| Weiji Weng 2018 | China | GBM | Retrospective | NR | 53 | 105 | 57 | NR | NLR = 4 | S + C+ T | NR | 1.953 (1.255–3.039) |
| Peng-Fei Wang 2018 | China | GBM | Retrospective | NR | NR | 314 | NR | NR | NR | NR | UA | 0.57 (0.41–0.79) |
| ÖZLEM YERSAL 2018 | Turkey | GBM | Retrospective | 56.8 ± 13.1 | 39 | 80 | 42 | NR | NLR = 4 | S + C+ T | UA | 1.258 (0.727–2.179) |
| SybrenL.N. Maas 2019 | Netherlands | GBM | Retrospective | NR | NR | 479 | NR | NR | NLR = 4 | NR | MA | 1.11 (0.75–1.65) |
| Yajuan Lv 2019 | China | GBM | Retrospective | 53.25 ± 13.9 | 113 | 192 | NR | NR | NLR = 2.7 | NR | MA | 0.637 (0.454–0.894) |
| Yunfei Hao 2019 | China | GBM multiforme | Retrospective | 55 ± 13.55 | 116 | 187 | 112 | NR | NLR = 4.1 | S + C+ T | UA | 2.574 (1.849–3.581) |
| Agam Brenner 2019 | Israel | GBM multiforme | Retrospective | 57.73 ± 12.43 | 46 | 89 | 59 | 23 | NLR = 4 | S + C+ T | MA | 0.856 (0.49–1.496) |
| Ozlem MERMUT 2020 | Turkey | GBM multiforme | Retrospective | 58.0 ± 13.02 | 47 | 75 | 31 | NR | NLR = 4 | NR | MA | 2.14 (1.11–4.14) |
| Alessandra Marini 2020 | Italy | GBM | Retrospective | NR | 65 | 124 | 64 | NR | NLR = 4 | S + C | MA | 1.58 (1–2.49) |
| Mehmet Besiroglu 2021 | Turkey | GBM multiforme | Retrospective | 47.2 ± 12.1 | 58 | 107 | 73 | NR | NLR = 2.9 | S + C+ T | MA | 1.38 (0.87–2.17) |
| Anne Clavreul 2021 | France | GBM | Retrospective | 61.5 ± 8.8 | 65 | 85 | 46 | NR | NLR = 2.06 | S + C+ T | NR | 1.67 (1.03–2.71) |
| Vildan Kaya 2017 | Antalya | GBM | Retrospective | 55.7 ± 16.3 | NR | 90 | NR | NR | NLR = 5 | NR | MA | 2.41 (1.26–4.58) |
| Zhen-Yu Zhang 2019 | China | GBM | Retrospective | NR | NR | 170 | NR | NR | NLR = 7.25 | NR | MA | 2.228 (1.329–3.733) |
| Xing-Wang Zhou 2016 | China | GBM | Retrospective | 52.85 ± 4.03 | 50 | 84 | 59 | NR | NLR = 4 | C | UA | 1.264 (0.785–2.035) |
| Qian He 2021 | China | GBM | Retrospective | NR | NR | 62 | 46 | NR | NLR = 3.31 | S + C | UA | 1.766 (0.941–3.316) |
Note: UA: univariate analysis; MA: multivariate analysis; S + C + T: Stupp + chemoradiotherapy + temozolomide; NLR: neutrophil count/lymphocyte count; HR: hazard ratio; CI: confidence interval; NR: not reported; NOS: Newcastle-Ottawa quality assessment.
2.2. Inclusion and Exclusion Criteria
Adherence to the inclusion and exclusion criteria helped avoid the influence of surgery and radiotherapy on screening results. Literature addressing the prognostic value of the pre-treatment and pre-operative NLR in patients with GBM and including HR values for OS and their corresponding 95% CIs or Kaplan–Meier curves as well as the number of patients were included. Literature with unclear sources of the NLR, measuring NLR values extracted during radiotherapy, and including patients with chronic diseases or cachexia, which affect NLR values; basic experiments, similar meta-analyses, reviews, letters, and duplicates from different sources were excluded.
2.3. Quality Assessment
Two independent reviewers (X.G. and H.J.) used the Newcastle-Ottawa Quality Assessment Scale (NOS) [22] to assess the quality of the included studies. The scale consists of three main items—selection (0–4 points), comparability control for important factors (0–2 points), and outcome (0–3 points). Each main item also includes sub-items with a total score of 9 (Table 2). Based on the relevant literature, studies with an NOS score ≥ 5 were considered high-quality studies [22]. In addition, the senior author (Y.Z.) resolved any disputes between the two reviewers regarding results.
Table 2.
Results of quality assessment using the Newcastle-Ottawa Scale for case-control studies.
| Author and Year | Selection (0–4 points) | Comparability Control for Important Factor (0–2 points) | Outcome (0–3 points) | Scores (9 Points) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Nonexposed Cohort | Ascertainment of Exposure | Demonstration that Outcome of Interest was not Present at Start of Study | Assessment of Outcome | was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | |||
| Bambury 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sheng Han 2015 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 6 |
| Peng-Fei Wang 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Marta Lopes 2018 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 5 |
| Weiji Weng 2018 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 5 |
| Peng-Fei Wang 2018 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 6 |
| ÖZLEM YERSAL 2018 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 |
| SybrenL.N. Maas 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Yajuan Lv 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Yunfei Hao 2019 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Agam Brenner 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Ozlem MERMUT 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Alessandra Marini 2020 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Mehmet Besiroglu 2021 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| Anne Clavreul 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Vildan Kaya 2017 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 5 |
| Zhen-Yu Zhang 2019 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Xing-Wang Zhou 2016 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Qian He 2021 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
2.4. Statistical Analysis
All meta-data analyses were performed using Stata version 16.0 (Xi’an, China). The overall HRs and 95% CIs for the pre-treatment and pre-operative NLR values from the literature were calculated and presented as forest plots. Null was defined as the point when the value for the vertical axis of the plot exceeded 1, that is, when the combined HR and 95% CI surpassed 1, suggesting that a high NLR is not associated with low OS in patients with GBM (p < 0.05). Second, the Higgins I-squared (I2) statistic was used to assess the heterogeneity among studies. Following the Cochrane Handbook criteria, I2 > 50% indicated significant heterogeneity [23]. In these cases, a random effects model was chosen to analyse the final results. Otherwise, a fixed effects model was selected. In addition, when there was significant heterogeneity, we performed sub-group, meta-regression, and sensitivity analyses to search for sources of heterogeneity and increase result robustness. Finally, we used funnel plots to detect publication bias. If asymmetry was present in the funnel plots, Egger’s linear regression and Begg’s rank correlation were used to quantitatively evaluate whether there was a publication bias.
3. Results
3.1. Search Results
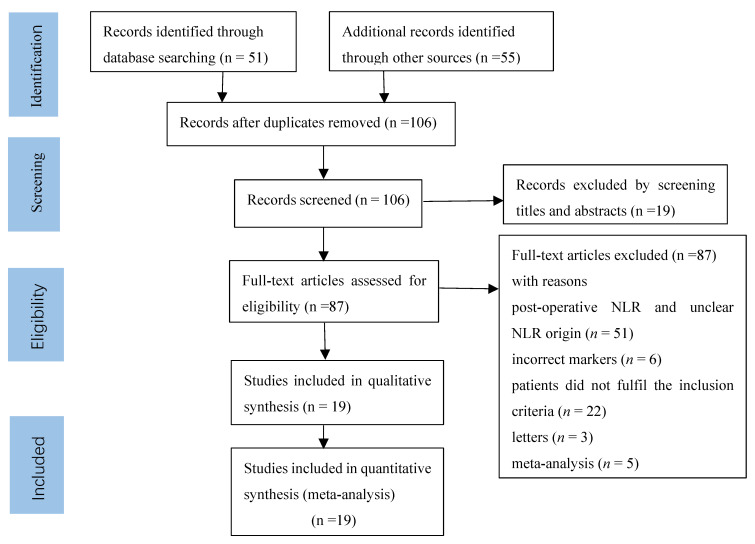
Among the 106 studies obtained based on the search criteria, 87 were excluded for the following reasons: post-operative NLR and unclear NLR origin (n = 51), incorrect markers (n = 6), patients did not fulfil the inclusion criteria (n = 22), letters (n = 3), and meta-analysis (n = 5) (Figure 1). For the final analysis, 19 studies were identified [4,9,19,20,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], and the main characteristics of the included studies are shown in Table 1. A total of 2762 patients (938 men and 1824 women) were enrolled in 19 studies. The studies were from China (n = 9), Turkey (n = 4), the USA (n = 1), Portugal (n = 1), the Netherlands (n = 1), Israel (n = 1), Italy (n = 1), and France (n = 1). The extent of surgical resection was also assessed, such as gross total resection (GTR) in 789 patients and other resection measures in 1973 patients. Notably, only one study reported 23 patients who underwent a second surgery, and one study reported the pre-treatment NLR. In addition, NLR thresholds varied in the included literature, with 10 studies having a threshold value of 4 and the remaining 9 studies with thresholds of 2.39, 7, 2.7, 4.1, 2.9, 2.42, 5, 7.25, and 3.31. Seven studies had a post-operative treatment regimen of Stupp + chemoradiotherapy + temozolomide, 3 studies had a post-operative treatment regimen of Stupp + chemoradiotherapy, and 9 studies did not report a post-operative treatment regimen. In terms of analytical methods, 5 studies were obtained by univariate analysis, 2 by unknown analytical methods, and 12 by multivariate analyses.
Figure 1.
Selection process for the including studies.
3.2. Correlation between OS and NLR in GBM
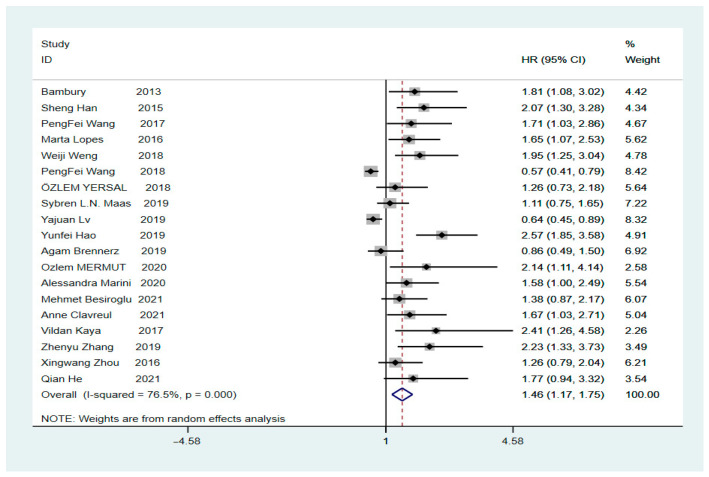
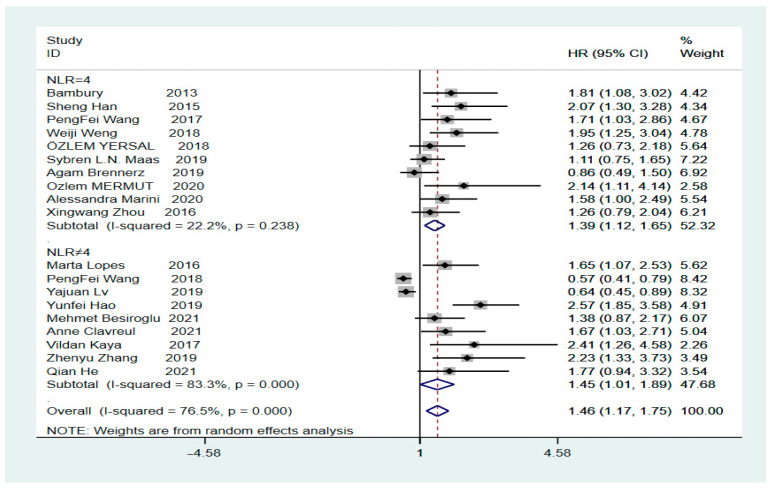
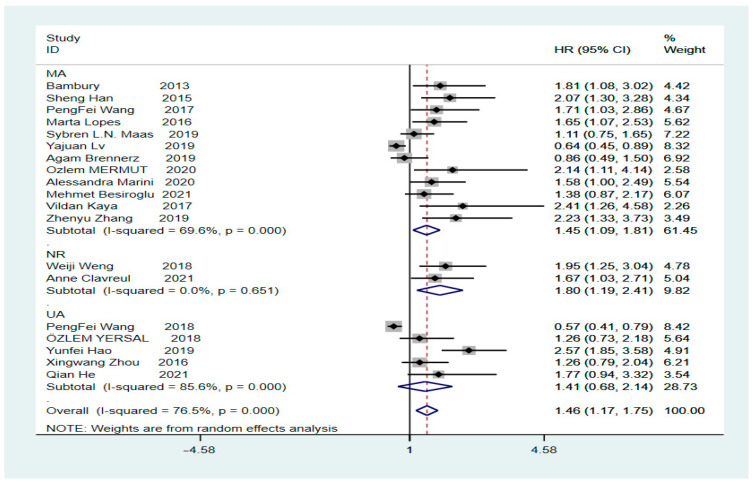
The final HR value and corresponding 95% CI was 1.46 (1.17–1.75, p = 0.000), calculated using Stata version 16.0, indicating a significant correlation between high values of the pre-treatment NLR and low OS in patients with GBM (Figure 2, Tau-squared = 0.2504). Heterogeneity analysis revealed an I2 value of 76.5% (p = 0.000), indicating significant heterogeneity. To clarify whether the heterogeneity was due to studies involving secondary surgery, the overall HR for the NLR was calculated; it was found to be 1.51 (95% CI: 1.2–1.8, p = 0.000, I2 = 77.8%) after removing a study on secondary surgery (Supplementary Figure S1). Subsequently, we performed sub-group analysis based on the presence or absence of a cut-off value of 4. The HR for the sub-group with a cut-off value of 4 was 1.39 (95% CI: 1.12–1.65, p = 0.000, I2 = 22.2%), showing a significant decrease in heterogeneity, indicating that a preoperative NLR of >4 suggests a low OS rate in patients with GBM. In contrast, the HR for the sub-group with a cut-off value other than 4 was 1.45 (95% CI: 1.01–1.89, p = 0.000, I2 = 83.3%) (Figure 3), showing a high level of heterogeneity. However, further sub-group analysis could not be performed to find the source of heterogeneity because of the different cut-off values for each sub-group. The subsequent sensitivity analysis for the sub-group with a cut-off value other than 4 showed that individual studies were not associated with heterogeneity (Supplementary Figure S2).
Figure 2.
Forest plot illustrating the relationship between the NLR and OS in glioblastoma patients [4,9,19,20,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
Figure 3.
Inclusion of subgroups with a literature cut-off value of 4 and subgroups without a cut-off value of 4 in the analysis [4,9,19,20,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
3.3. Heterogeneity and Sensitivity Analysis
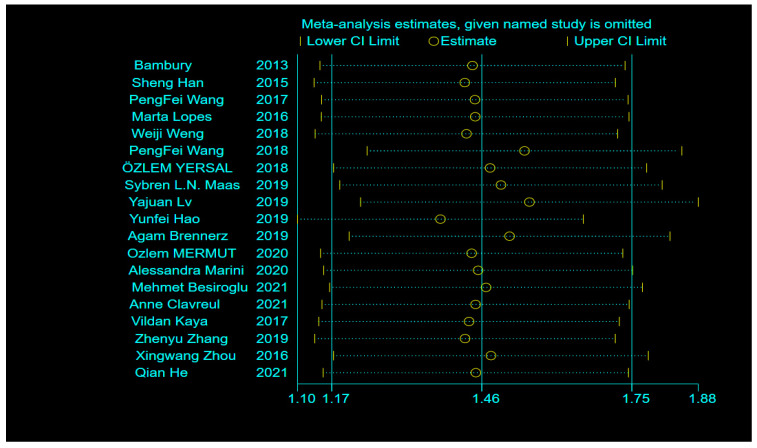
To further identify sources of heterogeneity, sensitivity analysis was performed, revealing that single-factor studies were not associated with high heterogeneity (Figure 4). Sub-group analysis was again performed for univariate, multivariate, and unknown sources, which showed HRs of 1.41 (95% CI: 0.68–2.14, p = 0.000, I2 = 86.5%), 1.45 (95% CI: 1.09–1.81, p = 0.000, I2 = 69.6%), and 1.80 (95% CI: 1.19–2.41, p = 0.000, I2 = 0.0%), respectively. Sub-group analysis indicated that the heterogeneity in both univariate and multivariate sources was not significantly reduced (Figure 5). Finally, we performed meta-regression analysis to evaluate whether country, year of publication, age, sex, extent of surgical resection, and treatment method affected the heterogeneity. The results revealed a heterogeneity of Tau-squared = 0 after inclusion of these six variables, which was 0.2504, less than the previous value of 0.2504 (Figure 2). These data imply that country, year of publication, age, sex, extent of surgical resection, and treatment method can be used to explain 76.5% heterogeneity among studies (Table 3).
Figure 4.
Sensitivity analysis of the relationship between single studies and heterogeneity in the included literature [4,9,19,20,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
Figure 5.
Analysis of subgroups with different sources of endings [4,9,19,20,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
Table 3.
Meta-regression of baseline data.
| Hr | Coef. | Std. Err. | t | p > |t| | [95% Conf.] | Interval |
|---|---|---|---|---|---|---|
| year | −0.0623897 | 0.2007026 | −0.31 | 0.761 | −0.4996832 | 0.3749038 |
| country | 0.0193201 | 0.1760801 | −0.11 | 0.914 | −0.4029657 | 0.3643255 |
| age | 0.1452009 | 0.2485765 | 0.58 | 0.57 | −0.3964008 | 0.6868027 |
| gender | 0.879301 | 0.1934452 | 0.45 | 0.658 | −0.3335507 | 0.5094109 |
| Extent of surgery |
−0.1954218 | 0.3527367 | −0.55 | 0.59 | −0.963969 | 0.5731255 |
| treatment | 0.1881377 | 0.4750795 | 0.4 | 0.699 | −0.8469717 | 1.223247 |
| cons | 1.393349 | 2.010993 | 0.69 | 0.502 | −2.988229 | 5.774926 |
Note: Meta-regression Number of obs = 19; REML estimate of between-study variance tau2 = 0; % residual variation due to heterogeneity I-squared_res = 0.00%; Proportion of between-study variance explained Adj R-squared = %; Joint test for all covariates Model F (6,12) = 0.25; With Knapp–Hartung modification Prob > F = 0.9513.
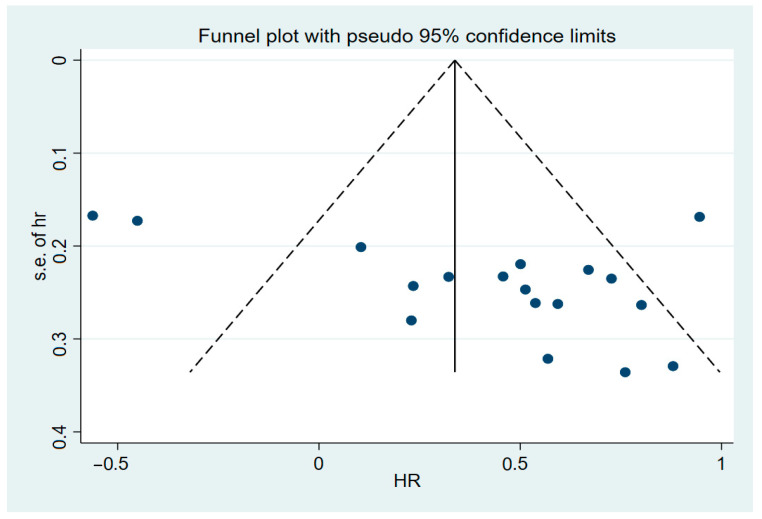
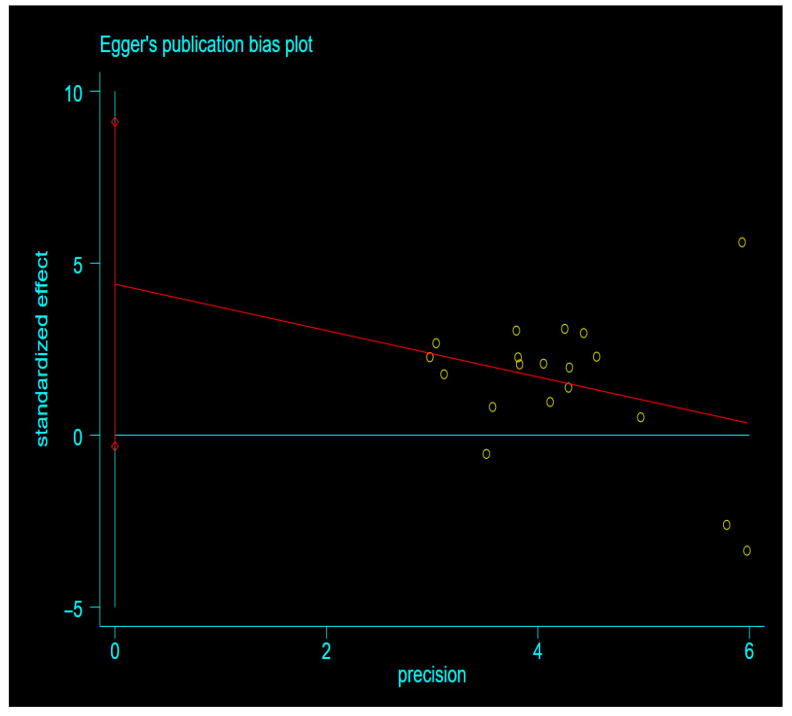
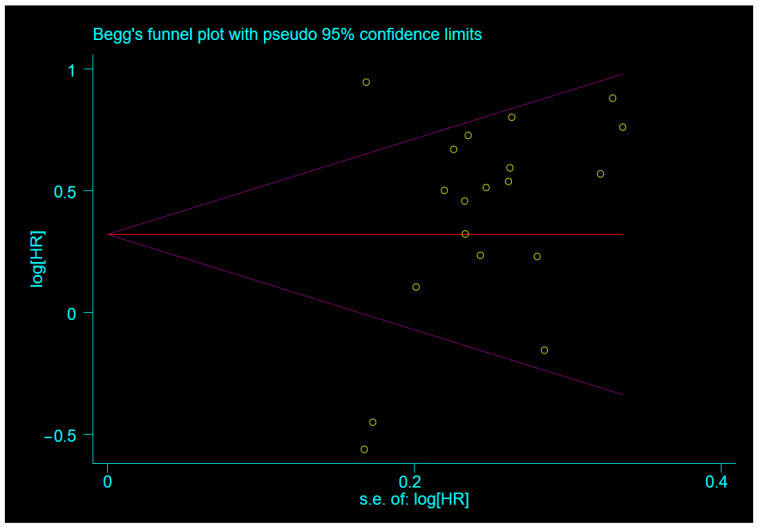
3.4. Publication Bias
Since 19 studies were included, we used funnel plots to check for the presence of publication bias. Egger’s and Begg’s tests were used to quantitatively assess the presence of publication bias after finding funnel plot asymmetry (Appendix A Figure A1, Figure A2 and Figure A3). As shown in Table 4, the p-value obtained was 0.066 > 0.05 (95% CI: −0.3302535–9.108586), indicating that there was no publication bias in the included studies.
Table 4.
Egger’s test.
| Std_Eff | Coef. | Std. Err | t | P > |t| | [95% Conf. Interval] | |
|---|---|---|---|---|---|---|
| Slope | −0.6745632 | 0.5180683 | −1.30 | 0.210 | −1.767592 | 0.4184653 |
| Bias | 4.389166 | 2.236887 | 1.96 | 0.066 | −0.3302535 | 9.108586 |
4. Discussions
The prognostic significance of a high NLR before GBM treatment and surgery was evaluated in 19 studies, including 2762 patients. The results of a parallel meta-analysis of data from published literature found that high pre-treatment and preoperative NLRs indicate poor and statistically significant survival in patients with GBM.
Numerous studies have shown that the NLR is associated with the prognosis of certain malignancies [39,40], but a significant prognostic value has not been found in intermediate diseases, such as breast cancer [41] and gastric cancer [42]. Although some studies have shown that the preoperative NLR is associated with poor OS in patients with GBM, Weng et al. [23] and Zhou et al. [36] reported conflicting results. The results reported by Brenner et al. [29] and Bambury et al. [4] also differed, although the NLR thresholds and treatments were identical and multivariate analyses were performed. To date, the mechanism underlying the prognostic role of the NLR in GBM is unclear [19], but one study has proposed that in the GBM microenvironment, tumour-infiltrating lymphocytes are the main regulatory T cells capable of suppressing the immune response [43]. Another study suggested that the NLR may drive cancer cell proliferation by increasing the availability of growth factors, angiogenic factors, and other pro-neoplastic signalling molecules, thus portending a worse prognosis [44,45].
Although previous meta-analyses have shown a correlation between high NLR values and poor OS in patients with GBM, the result was based on sub-group analysis that included not only a small body of literature but also different sources of the NLR. Furthermore, the meta-analysis by Wang et al. [15,16,17] included the study by Mason et al. [46]. The application of hormone therapy, radiotherapy, and chemotherapy can affect NLR values [47]. Additionally, in the meta-analysis of Wang et al. [16], the included studies by Weng et al. [25] and Lei et al. [15] contained data inclusion errors, suggesting publication bias. However, the quantitative results were not assessed using tests, such as Egger’s test. We believe that these issues ultimately affect the credibility of the results. Therefore, we expanded our sample size to avoid these problems and investigated the correlation between high pre-treatment NLR values and poor OS in patients with GBM. Using pre-treatment NLR values as the study index and excluding NLR literature obtained during the application of hormone therapy or radiotherapy, we showed that high pre-treatment NLR values suggested poor prognosis in patients with GBM (HR = 1.46; 95% CI: 1.17–1.75, p = 0.000, I2 = 76.5%). Owing to the presence of high heterogeneity, we performed sub-group analysis of an NLR with different cut-off values and found significantly lower heterogeneity in sub-group analysis of the NLR with a cut-off value of 4 (I2 = 22.2%) and without a cut-off value of 4 (I2 = 83.3%). However, sensitivity analysis failed to clarify the source of high heterogeneity. This result is consistent with the findings reported by Bambury et al. [4,5], who demonstrated that an NLR > 4 is an independent indicator of poor prognosis in patients with GBM.
Among the included studies, one study reported 23 patients who underwent a second surgery [31]. Secondary surgery can significantly prolong the survival of patients with recurrent GBM [48]. This may be attributed to the ability of secondary surgery to overcome the negative impact of the first incomplete resection on survival [49] and reduce the residual tumour volume [50]. However, studies have confirmed that the area of repeat craniectomy does not affect survival in patients who achieve the GTR value with the first surgery, whereas survival is significantly improved if the first surgery involves subtotal resection [49]. The effect of secondary surgery on patient survival is controversial and requires further investigation.
A high pre-operative NLR allows for glioma grading [23]; this may be related to the progressive features of gliomas, such as inflammation, angiogenesis, and invasion. Neutrophils are the first to reach the site of inflammation [51], and as the tumour grade increases, elevated neutrophil levels inhibit the cytolytic activity of quiescent lymphocytes, leading to a decrease in the number of lymphocytes or reduced function [52]. In addition, glioma-derived factors may affect the number of circulating and infiltrating neutrophils, whilst promoting GBM cell proliferation by upregulating S100A4 [13,53] and thereby affecting their infiltration into tumours [14]. However, studies have also shown that the specificity of neutrophils is low and increases in the presence of subclinical infections and acute stress [23]. Additional studies are required to confirm their role in tumours.
Furthermore, a high pre-operative NLR can distinguish between patients with non-lesional epilepsy, acoustic neuroma, and meningioma; healthy individuals; and patients with glioma. It also has the highest diagnostic accuracy for glioma not only as an indicator for determining glioma grading but also for identifying isocitrate dehydrogenase wild-type and mutant GBM [54,55]. Although the NLR cannot distinguish between GBM and metastases [56] as an inflammatory marker of the host, it can provide, to some extent, useful information for clinical treatment options [5]. For example, as the NLR increases, tumour aggressiveness and poor prognosis also increase, for which aggressive treatment modalities (such as targeted therapy or immunotherapy) are therapeutic options [34]. In addition, anti-neutrophil factor drugs can be used to treat an elevated NLR [54]. Moreover, an increase in the pre-operative NLR can guide the periodic review of patients for the assessment of tumour progression [57].
Although we included, for the first time, 19 studies exploring the relationship between the NLR and GBM prognosis with optimistic results, this study has some limitations. First, the critical values of the NLR in the included literature vary, and it is not possible to compare between the critical values and define a critical value for a high NLR, making the clinical judgement of a high pre-operative NLR controversial. Second, one of the included studies reported secondary surgery, which may prolong patient survival. Third, most included studies had a retrospective design, and although we performed meta-regression to identify sources of heterogeneity, we were unable to find prospective clinical studies. A large multicentre prospective randomised study to determine the correlation between high a pre-treatment and pre-operative NLR and poor OS in patients with GBM is warranted to increase the validity of our findings.
5. Conclusions
For the first time, this study demonstrated a correlation between a high pre-treatment and pre-operative NLR and poor OS in patients with GBM using a large sample size. The study results demonstrated that a high NLR suggests reduced survival in patients with GBM, providing a theoretical basis for prospective clinical studies. Although the study results showed a high degree of heterogeneity, underlying data on age, sex, and extent of surgical resection were identified as sources of heterogeneity after meta-regression analysis. A high NLR may be used to predict the survival of patients with GBM and can serve as a basis for adopting appropriate interventions to improve their prognosis.
Acknowledgments
Firstly: I would like to thank my supervisor, Yuelin Zhang, for his careful guidance from topic selection to writing; secondly, I would like to thank the other two authors (Z and J) who participated in this study for their selfless dedication. We would like to thank Editage (www.editage.cn (accessed on 12 May 2022)) for English language editing.
Abbreviations and Acronyms
| GBM | glioblastoma |
| OS | overall survival |
| UA | univariate |
| MA | multivariate |
| S + C + T | Stupp + chemoradiotherapy + temozolomide |
| NLR | neutrophil count/lymphocyte count |
| HR | hazard ratio |
| CI | confidence interval |
| NR | not reported |
| NOS | Newcastle-Ottawa quality assessment |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/brainsci12050675/s1, Figure S1: Cancellation of 1 secondary surgical study, Forest plot illustrating the relationship between NLR and OS in glioblastoma patients, Figure S2: Subgroup sensitivity analysis for truncation values other than4.
Appendix A
Figure A1.
Publication bias detection for included studies (funnel plot).
Figure A2.
Quantitative analysis of publication bias using Egger’s linear regression method.
Figure A3.
Quantitative analysis of publication bias using the Berger rank correlation method.
Author Contributions
All authors contributed equally to preparing the manuscript. Y.Z. proposed the direction of writing and guided the completion of writing; X.G. completed the literature screening, data extraction and article writing; H.J. completed a quality assessment of the included literature, the application software calculated the results; T.Z. completed the translation and final inspection. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares that the content of the article was composed without any commercial or financial relationship and without any conflict of interest.
Funding Statement
This study was supported by a grant (No. 2018ZDXM-SF-040) from the Key Project of Shaanxi Province-the field of Social Development.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lapointe S., Perry A., Butowski N.A. Primary brain tumours in adults. Lancet. 2018;392:432–446. doi: 10.1016/S0140-6736(18)30990-5. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R., Mason W.P., Van Den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Aum D.J., Kim D.H., Beaumont T.L., Leuthardt E.C., Dunn G.P., Kim A.H. Molecular and cellular heterogeneity: The hallmark of glioblastoma. Neurosurg. Focus. 2014;37:E11. doi: 10.3171/2014.9.FOCUS14521. [DOI] [PubMed] [Google Scholar]
- 4.Bambury R.M., Teo M.Y., Power D.G., Yusuf A., Murray S., Battley J.E., Drake C., O’Dea P., Bermingham N., Keohane C., et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J. Neuro Oncology. 2013;114:149–154. doi: 10.1007/s11060-013-1164-9. [DOI] [PubMed] [Google Scholar]
- 5.McNamara M.G., Lwin Z., Jiang H., Templeton A.J., Zadeh G., Bernstein M., Chung C., Millar B.-A., Laperriere N., Mason W.P. Factors impacting survival following second surgery in patients with glioblastoma in the temozolomide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J. Neurooncol. 2014;117:147–152. doi: 10.1007/s11060-014-1366-9. [DOI] [PubMed] [Google Scholar]
- 6.Szkandera J., Absenger G., Liegl-Atzwanger B., Pichler M., Stotz M., Samonigg H., Glehr M., Zacherl M., Stojakovic T., Gerger A., et al. Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients. Br. J. Cancer. 2013;108:1677–1683. doi: 10.1038/bjc.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng J.-F., Huang Y., Chen Q.-X. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J. Surg. Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y.-Y., Choi C.H., Kim H.-J., Kim T.-J., Lee J.-W., Lee J.-H., Bae D.-S., Kim B.-G. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res. 2012;32:1555–1561. [PubMed] [Google Scholar]
- 9.Han S., Liu Y., Li Q., Li Z., Hou H., Wu A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15:617. doi: 10.1186/s12885-015-1629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malietzis G., Giacometti M., Kennedy R.H., Athanasiou T., Aziz O., Jenkins J.T. The Emerging Role of Neutrophil to Lymphocyte Ratio in Determining Colorectal Cancer Treatment Outcomes: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2014;21:3938–3946. doi: 10.1245/s10434-014-3815-2. [DOI] [PubMed] [Google Scholar]
- 11.Rutledge W.C., Kong J., Gao J., Gutman D.A., Cooper L.A., Appin C., Park Y., Scarpace L., Mikkelsen T., Cohen M.L., et al. Tumor-Infiltrating Lymphocytes in Glioblastoma Are Associated with Specific Genomic Alterations and Related to Transcriptional Class. Clin. Cancer Res. 2013;19:4951–4960. doi: 10.1158/1078-0432.CCR-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohr J., Ratliff T., Huppertz A., Ge Y., Dictus C., Ahmadi R., Grau S., Hiraoka N., Eckstein V., Ecker C.R., et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin. Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 13.Liang J., Piao Y., Holmes L., Fuller G., Henry V., Tiao N., De Groot J.F. Neutrophils Promote the Malignant Glioma Phenotype through S100A4. Clin. Cancer Res. 2013;20:187–198. doi: 10.1158/1078-0432.CCR-13-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fossati G., Ricevuti G., Edwards S.W., Walker C., Dalton A., Rossi M.L. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98:349–354. doi: 10.1007/s004010051093. [DOI] [PubMed] [Google Scholar]
- 15.Lei Y.-Y., Li Y.-T., Hu Q.-L., Wang J., Sui A.-X. Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: A systematic review and meta-analysis. World J. Surg. Oncol. 2019;17:152. doi: 10.1186/s12957-019-1686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Kang K., Lin Q., Hai J. Prognostic Significance of Preoperative Systemic Cellular Inflammatory Markers in Gliomas: A Systematic Review and Meta-Analysis. Clin. Transl. Sci. 2020;13:179–188. doi: 10.1111/cts.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C., Wen H.B., Zhao Y.H., Huang W.H., Wang Z.F., Li Z.Q. Systemic Inflammatory Indicators as Prognosticators in Glioblastoma Patients: A Comprehensive Meta-Analysis. Front. Neurol. 2020;11:580101. doi: 10.3389/fneur.2020.580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dos Santos A.G., de Carvalho R.F., Morais A.N.L.R., Silva T.M., RibeiroBaylão V.M., Azevedo M., de Oliveira A.J.M. Role of neutrophil-lymphocyte ratio as a predictive factor of glioma tumor grade: A systematic review. Crit. Rev. Oncol. Hematol. 2021;163:103372. doi: 10.1016/j.critrevonc.2021.103372. [DOI] [PubMed] [Google Scholar]
- 19.He Q., Li L., Ren Q. The Prognostic Value of Preoperative Systemic Inflammatory Response Index (SIRI) in Patients with High-Grade Glioma and the Establishment of a Nomogram. Front. Oncol. 2021;11:671811. doi: 10.3389/fonc.2021.671811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besiroglu M., Shbair A.T., Yasin A.I., Topcu A., Turk H.M., Demir T. Systemic Inflammatory Markers for Prediction of Bevacizumab Benefit in Glioblastoma Multiforme. J. Coll. Physicians Surg. Pak. 2021;31:39–44. doi: 10.29271/jcpsp.2021.01.39. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Wang P., Song H.-W., Cai H.-Q., Kong L.-W., Yao K., Jiang T., Li S., Yan C.-X. Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival. Oncotarget. 2017;8:50117–50123. doi: 10.18632/oncotarget.15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopes M., Carvalho B., Vaz R., Linhares P. Influence of neutrophil–lymphocyte ratio in prognosis of glioblastoma multiforme. J. Neuro-Oncology. 2017;136:173–180. doi: 10.1007/s11060-017-2641-3. [DOI] [PubMed] [Google Scholar]
- 26.Weng W., Chen X., Gong S., Guo L., Zhang X. Preoperative neutrophil–lymphocyte ratio correlated with glioma grading and glioblastoma survival. Neurol. Res. 2018;40:917–922. doi: 10.1080/01616412.2018.1497271. [DOI] [PubMed] [Google Scholar]
- 27.Wang P.-F., Meng Z., Song H.-W., Yao K., Duan Z.-J., Yu C.-J., Li S., Yan C.-X. Preoperative Changes in Hematological Markers and Predictors of Glioma Grade and Survival. Front. Pharmacol. 2018;9:886. doi: 10.3389/fphar.2018.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odabaşi E., Kemal Y. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with glioblastoma. Mol. Clin. Oncol. 2018;9:453–458. doi: 10.3892/mco.2018.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maas S.L., Draaisma K., Snijders T.J., Senders J.T., Berendsen S., Seute T., Schiffelers R.M., Van Solinge W., Berg M.J.T., Robe P.A., et al. Routine Blood Tests Do Not Predict Survival in Patients with Glioblastoma—Multivariable Analysis of 497 Patients. World Neurosurg. 2019;126:e1081–e1091. doi: 10.1016/j.wneu.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 30.Lv Y., Zhang S., Liu Z., Tian Y., Liang N., Zhang J. Prognostic value of preoperative neutrophil to lymphocyte ratio is superior to systemic immune inflammation index for survival in patients with Glioblastoma. Clin. Neurol. Neurosurg. 2019;181:24–27. doi: 10.1016/j.clineuro.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Hao Y., Li X., Chen H., Huo H., Liu Z., Tian F., Chai E. A Cumulative Score Based on Preoperative Neutrophil-Lymphocyte Ratio and Fibrinogen in Predicting Overall Survival of Patients with Glioblastoma Multiforme. World Neurosurg. 2019;128:e427–e433. doi: 10.1016/j.wneu.2019.04.169. [DOI] [PubMed] [Google Scholar]
- 32.Brenner A., Friger M., Geffen D.B., Kaisman-Elbaz T., Lavrenkov K. The Prognostic Value of the Pretreatment Neutrophil/Lymphocyte Ratio in Patients with Glioblastoma Multiforme Brain Tumors: A Retrospective Cohort Study of Patients Treated with Combined Modality Surgery, Radiation Therapy, and Temozolomide Chemotherapy. Oncology. 2019;97:255–263. doi: 10.1159/000500926. [DOI] [PubMed] [Google Scholar]
- 33.Mermut O., Inanc B. The effect of indicators of systemic inflammatory response on survival in glioblastoma multiforme. Turk. Neurosurg. 2020;30:658–665. doi: 10.5137/1019-5149.JTN.27068-19.1. [DOI] [PubMed] [Google Scholar]
- 34.Marini A., Dobran M., Aiudi D., Pesaresi A., di Somma L.G.M., Iacoangeli M. Pre-operative hematological markers as predictive factors for overall survival and progression free survival in glioblastomas. Clin. Neurol. Neurosurg. 2020;197:106162. doi: 10.1016/j.clineuro.2020.106162. [DOI] [PubMed] [Google Scholar]
- 35.Clavreul A., Lemée J.-M., Soulard G., Rousseau A., Menei P. A Simple Preoperative Blood Count to Stratify Prognosis in Isocitrate Dehydrogenase-Wildtype Glioblastoma Patients Treated with Radiotherapy plus Concomitant and Adjuvant Temozolomide. Cancers. 2021;13:5778. doi: 10.3390/cancers13225778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaya V., Yıldırım M., Yazıcı G., Yalçın A.Y., Orhan N., Güzel A. Prognostic Significance of Indicators of Systemic Inflammatory Responses in Glioblastoma Patients. Asian Pac. J. Cancer Prev. 2017;18:3287–3291. doi: 10.22034/APJCP.2017.18.12.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z.-Y., Zhan Y.-B., Zhang F.-J., Yu B., Ji Y.-C., Zhou J.-Q., Bai Y.-H., Wang Y.-M., Wang L., Jing Y., et al. Prognostic value of preoperative hematological markers combined with molecular pathology in patients with diffuse gliomas. Aging. 2019;11:6252–6272. doi: 10.18632/aging.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X.-W., Dong H., Yang Y., Luo J.-W., Wang X., Liu Y.-H., Mao Q. Significance of the prognostic nutritional index in patients with glioblastoma: A retrospective study. Clin. Neurol. Neurosurg. 2016;151:86–91. doi: 10.1016/j.clineuro.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Templeton A.J., Mcnamara M.G., Šeruga B., Vera-Badillo F.E., Aneja P., Ocaña A., Leibowitz-Amit R., Sonpavde G., Knox J.J., Tran B., et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 40.Gu X., Gao X., Li X., Qi X., Ma M., Qin S., Yu H., Sun S., Zhou D., Wang W. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: Evidence from 16,266 patients. Sci. Rep. 2016;6:22089. doi: 10.1038/srep22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leitch E.F., Chakrabarti M., Crozier J.E.M., McKee R.F., Anderson J.H., Horgan P.G., McMillan D.C. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br. J. Cancer. 2007;97:1266–1270. doi: 10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutta S., Crumley A.B., Fullarton G.M., Horgan P.G., McMillan D.C. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. Am. J. Surg. 2012;204:294–299. doi: 10.1016/j.amjsurg.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Wainwright D.A., Sengupta S., Han Y., Lesniak M.S. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011;13:1308–1323. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske Lekarske Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 46.Mason M., Maurice C., McNamara M.G., Tieu M.T., Lwin Z., Millar B.-A., Menard C., Laperriere N., Milosevic M., Atenafu E.G., et al. Neutrophil–lymphocyte ratio dynamics during concurrent chemo-radiotherapy for glioblastoma is an independent predictor for overall survival. J. Neuro-Oncology. 2017;132:463–471. doi: 10.1007/s11060-017-2395-y. [DOI] [PubMed] [Google Scholar]
- 47.Yovino S., A Grossman S. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012;1:149–154. doi: 10.2217/cns.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montemurro N., Fanelli G.N., Scatena C., Ortenzi V., Pasqualetti F., Mazzanti C.M., Morganti R., Naccarato F.P.A.G., Perrini P. Surgical outcome and molecular pattern characterization of recurrent glio-blastoma multiforme: A single-center retrospective series. Clin. Neurol Neurosurg. 2021;207:106735. doi: 10.1016/j.clineuro.2021.106735. [DOI] [PubMed] [Google Scholar]
- 49.Bloch O., Hab S.J., Sun M.Z., Aghi M.K., McDermott M.W., Berger M.S., Parsa A.T. Impact of extent of resection for recurrent glioblastoma on overall survival: Clinical article. J. Neurosurg. 2012;117:1032–1038. doi: 10.3171/2012.9.JNS12504. [DOI] [PubMed] [Google Scholar]
- 50.Yong R.L., Wu T., Mihatov N., Shen M.J., Brown M., Zaghloul K.A., Park G.E., Park J.K. Residual tumor volume and patient survival following reoperation for recurrent glioblastoma. J. Neurosurg. 2014;121:802–809. doi: 10.3171/2014.6.JNS132038. [DOI] [PubMed] [Google Scholar]
- 51.Gandhi P., Khare R., Gulwani H., Kaur S. Circulatory YKL-40 & NLR: Underestimated Prognostic Indicators in Diffuse Glioma. Int. J. Mol. Cell. Med. 2018;7:111–118. doi: 10.22088/IJMCM.BUMS.7.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregory A.D., Houghton A.M. Tumor-Associated Neutrophils: New Targets for Cancer Therapy: Figure 1. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 53.Massara M., Persico P., Bonavita O., Poeta V.M., Locati M., Simonelli M., Bonecchi R. Neutrophils in Gliomas. Front. Immunol. 2017;8:1349. doi: 10.3389/fimmu.2017.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng S.-H., Huang J.-L., Chen M., Wang B.-L., Ou Q.-S., Huang S.-Y. Diagnostic value of preoperative inflammatory markers in patients with glioma: A multicenter cohort study. J. Neurosurg. 2018;129:583–592. doi: 10.3171/2017.3.JNS161648. [DOI] [PubMed] [Google Scholar]
- 55.Sharma G., Jain S.K., Sinha V.D. Peripheral Inflammatory Blood Markers in Diagnosis of Glioma and IDH Status. J. Neurosci Rural. Pract. 2021;12:88–94. doi: 10.1055/s-0040-1721166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baran O., Kemerdere R., Korkmaz T.S., Kayhan A., Tanriverdi T. Can preoperative neutrophil to lymphocyte, lymphocyte to monocyte, or platelet to lymphocyte ratios differentiate glioblastoma from brain metastasis? Medicine. 2019;98:e18306. doi: 10.1097/MD.0000000000018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao C., Li L., Guo X., Song D., Wang M., Zhai Y., Yang F., Xue Y., Wei X. Preoperative Predictors of Early Mortality Risk in Isocitrate Dehydrogenase-Wild-Type Glioblastoma Patients Treated with Standard Therapy. Cancer Manag. Res. 2021;ume 13:1159–1168. doi: 10.2147/CMAR.S290053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.