Abstract
Ammonia-oxidizing bacteria (AOB) are thought to contribute significantly to N2O production and methane oxidation in soils. Most of our knowledge derives from experiments with Nitrosomonas europaea, which appears to be of minor importance in most soils compared to Nitrosospira spp. We have conducted a comparative study of levels of aerobic N2O production in six phylogenetically different Nitrosospira strains newly isolated from soils and in two N. europaea and Nitrosospira multiformis type strains. The fraction of oxidized ammonium released as N2O during aerobic growth was remarkably constant (0.07 to 0.1%) for all the Nitrosospira strains, irrespective of the substrate supply (urea versus ammonium), the pH, or substrate limitation. N. europaea and Nitrosospira multiformis released similar fractions of N2O when they were supplied with ample amounts of substrates, but the fractions rose sharply (to 1 to 5%) when they were restricted by a low pH or substrate limitation. Phosphate buffer (versus HEPES) doubled the N2O release for all types of AOB. No detectable oxidation of atmospheric methane was detected. Calculations based on detection limits as well as data in the literature on CH4 oxidation by AOB bacteria prove that none of the tested strains contribute significantly to the oxidation of atmospheric CH4 in soils.
Nitrogen transformation in soil and water is considered one of the main reasons for the increasing N2O levels in the atmosphere (6), and ammonia-oxidizing bacteria (AOB) appear to be important in this process (7, 8, 10, 27–29). The immediate (but partial) inhibiting effects of specific nitrification inhibitors on N2O production in soils (2, 5, 7, 11) demonstrate the direct role of nitrifier metabolism in producing N2O. AOB are also thought to be involved in the oxidation of atmospheric methane by aerobic soils, since methane is an alternative substrate for ammonia monooxygenase (3, 19). Methane oxidation in soil and its inhibition patterns may indicate that ammonia oxidizers are involved directly or indirectly (1, 15, 37). The kinetics of methane oxidation observed in type strains of AOB so far suggest a minor role, however (1, 13, 20). Thus, unless significantly higher oxidation rates are demonstrated in new isolates, their direct participation appears insignificant.
Nitrosomonas europaea appears to produce N2O by more than one mechanism. Moderate amounts are released under full aeration, but the release increases sharply in response to oxygen limitation (12, 24). Poth and Focht (23) showed that N. europaea denitrified with NO2− as the electron acceptor and that the labelling pattern observed (with either 15NH4+ or 15NO2−) indicated that N2O was primarily a product of NO2− reduction, rather than a by-product of NH3 oxidation. The presence of nitrite reductase in N. europaea has been demonstrated in several investigations (9, 22, 25, 26), and it is probably involved in the production of N2O by this organism under oxygen-limiting conditions (17). Under full aeration, however, the production of small amounts of N2O by N. europaea seems to be a direct by-product of NH2OH oxidation to NO2−, as judged from the pattern of inhibition and substrate dependency of the process (17). This possibility implies that the enzyme hydroxylamine oxidoreductase is directly involved in N2O production, since it catalyzes complete NH2OH oxidation to NO2− without any free intermediates (38). The possible involvement of hydroxylamine oxidoreductase in the release of N2O hypothetically implies that any external factor of importance for this periplasmic enzyme may affect the N2O/NO2− product ratio. Experiments with whole soil communities have suggested that acidity as such results in a high product ratio (21).
Nitrosomonas is the most commonly used genus in laboratory studies of AOB, but Nitrosospira seems to be the most dominant species in natural environments (14, 32). On this basis, we decided to do a comparative investigation of levels of N2O production in AOB, using two N. europaea and Nitrosospira multiformis (see below) type strains and six Nitrosospira strains which have recently been isolated from various terrestrial environments (18, 33, 34). The experiment allowed CH4 concentrations to be measured along with N2O, thus allowing us to check whether any of the cultures were able to oxidize significant amounts of methane at atmospheric concentrations. This approach is insensitive compared to tracer (14C) methods commonly used for measuring CH4 oxidation by AOB (3). However, calculations showed that if a culture had anywhere near the oxidation rates necessary for AOB to play a role in soil, we would easily detect and measure its oxidation in our experiments.
MATERIALS AND METHODS
Cultures.
Six strains of AOB isolated from acidic forest soils (strains III2, III7, and L115), acid sandy loam from Zambia (strain AF), neutral clay loam soil (strain 40K1), and wastewater from a treatment facility (strain B6) were used. These strains were identified as Nitrosospira spp. based on morphology and their 16S ribosomal DNA (rDNA) sequences (18, 33, 34). One N. europaea culture (provided by J. Prosser, University of Aberdeen, Aberdeen, Scotland) and the Nitrosolobus multiformis type culture ATCC 25196 (American Type Culture Collection, Rockville, Md.) were also used. We assume that a new name for the ATCC 25196 strain will be Nitrosospira multiformis because of the 16S rDNA-based consensus to merge the genera Nitrosospira, Nitrosolobus, and Nitrosovibrio into one genus, Nitrosospira (33, 34). Herein we use the name Nitrosospira multiformis.
Medium.
The basic medium used was a liquid mineral (LM) medium containing (per liter) KH2PO4 (0.2 g), CaCl2 · 2H2O (0.02 g), MgSO4 · 7H2O (0.04 g), FeNaEDTA (3.8 mg), phenol red (0.1 mg), NaMoO4 · 2H2O (0.1 mg), MnCl2 (0.2 mg), CoCl2 · 6H2O (0.002 mg), ZnSO4 · 7H2O (0.1 mg), and CuSO4 · 5H2O (0.02 mg). The N source and the buffering of the LM medium for each experiment are reported below. The N source was added from stock solutions containing either 1.0 M (NH4)2SO4 or 1.0 M urea (analysis grade; Merck). The medium and the stock solutions were sterilized by autoclaving at 121°C for 20 min.
Culturing and harvesting of cells.
The cell inoculum for the experiment was grown in 150-ml batches of medium in 500-ml E flasks. The medium was the LM medium with 3.8 mM (NH4)2SO4 and 15 mM HEPES sodium salt (art. 15231; Merck); its pH was adjusted to 7.5. The flasks were incubated on a reciprocal shaker (100 rpm) at 21°C in the dark, and the cells were harvested by filtration with a 0.2-μm-pore-size polycarbonate membrane (Nuclepore, Costar Europe Ltd., Badhoevedorp, The Netherlands). For harvesting of about 300 ml of culture, three to four membranes had to be used due to clogging. The cells were washed off the filters and dispersed in about 15 ml of substrate-free LM medium. The dispersion was obtained by pumping the cells through a 0.5-mm-diameter needle a dozen times. The cell density was determined by fluorescence microscopy after staining the cells with acridine orange. The inoculum was kept at 21°C in the dark and used within 3 h.
Incubation experiments for determination of N2O production and CH4 oxidation.
The incubation experiments were designed to investigate N2O production as well as CH4 consumption during growth on NH4+ or urea. In the first experiment, the medium [LM medium supplemented with 3.8 mM (NH4)2SO4 or urea] was buffered with 15 mM HEPES. Since the cultures were to be prevented from being exposed to atmospheric CO2, the medium contained 0.84 mM sodium carbonate to avoid CO2 limitation of growth (assuming that 0.11 mol of C is assimilated per mol of NH4+ oxidized [4], this quantity should be more than sufficient). The pH was regulated to 7.5. Batches of 20 ml of medium were placed in 120-ml serum flasks and inoculated to reach an initial cell density of 1 × 107 to 2 × 107 cells ml−1. The flasks were then sealed with Teflon-faced silicone septa (20-ST3; Chromacol Ltd., Herts, United Kingdom) and incubated at 25°C in the dark (no methane was added). The cultures were stirred with a 2-cm-long magnetic bar in each flask, operated with pulsed stirring (60 s of stirring at 300 rpm and 15-s pauses) with a Telemodul 20P (Variomag, Munich, Germany) equipped with a 15-flask-position magnetic plate (Variomag model no. HP15) submersed in a thermostated water bath. The samplings were done periodically during the incubation. Gas samples were taken directly from the serum flasks into the injection loop of the gas chromatograph via a peristaltic pump system equipped with a side port needle to penetrate the silicone septa. Liquid samples for NH4+ and NO2− analysis were taken with sterile syringes (through the silicone septa) and frozen to −20°C until analysis.
The results of the first experiment suggested that some of the cultures increased the relative production of N2O as the culture reached a critically low pH. The second experiment was designed to test if this pattern was reproducible and to increase the resolution of the experiment by changing the buffer and monitoring the pH along with the production of NO2− and N2O. To prolong the period of activity in the critical pH range (pH 5.3 to 6.4), 20 mM phosphate buffer was used instead of HEPES (based on the titration curves for the two buffers for this pH range). The buffer consisted of NaH2PO4 and Na2HPO4 in a 31:9 ratio, giving an initial pH of 6.4 in the LM medium (at 20 mM phosphate). The cells for the inoculum were grown and harvested as in the previous experiment and resuspended in substrate-free 20 mM phosphate-buffered LM medium. The serum flasks were filled with 40 ml of 20 mM phosphate-buffered LM medium containing 7.6 mM NH4+ and sealed with Teflon-faced silicone septa (type 20-ST3; Chromacol Ltd.), and 0.5 ml of CO2 was added to ensure the presence of C for growth (the medium contained no carbonate). The amount of oxygen in the remaining 80 ml of gas headspace was about 0.7 mmol per flask, which is about twice the molar amount of NH4+ per flask. Thus, if complete oxidation of all the NH4+ occurs (1.5 mol of O2 per mol of NH3), the cultures would approach oxygen-limiting conditions. However, the buffering capacity of the medium was deliberately made so weak that acid inhibition of growth would occur before 50% of the NH3 was oxidized.
Chemical analyses.
The gas concentrations were determined with a gas chromatograph equipped with three detectors (thermal conductivity, flame ionization, and electron capture detectors) allowing sensitive and rapid measurement of N2O, CH4, and CO2 with a single injection (30). The sampling system extracts 3 ml for each determination. The pressure reduction resulting from repeated samplings from the same bottle was accounted for when we calculated the amounts of N2O and CH4 (reported amounts are the values that would be reached if sampling did not take place).
A flow injection system (FIA star; Tecator AB, Höganäs, Sweden) was used to analyze NH4+ and NO2−. Nitrite was determined by color reaction from Griess-Ilosvay reagents I and II and was measured at a wavelength of 540 nm. Ammonium was determined by merging a sample with NaOH to form gaseous NH3, which passed through a Teflon membrane to a pH indicator mixture (Application Sub Note ASN 151-01/92; Tecator). pH was measured with a 6-mm-diameter pH electrode (ROSS Semi-micro Combination pH electrode, model 8103; ATI Orion, Boston, Mass.), which allows measurement in small sample volumes.
RESULTS
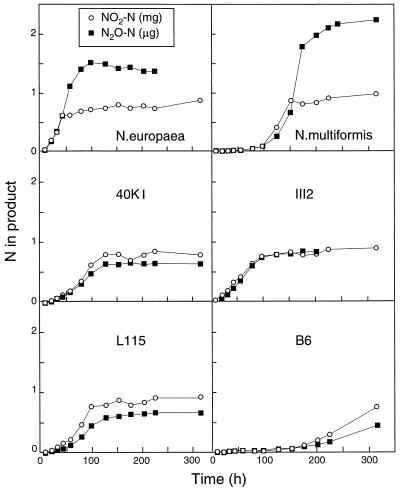
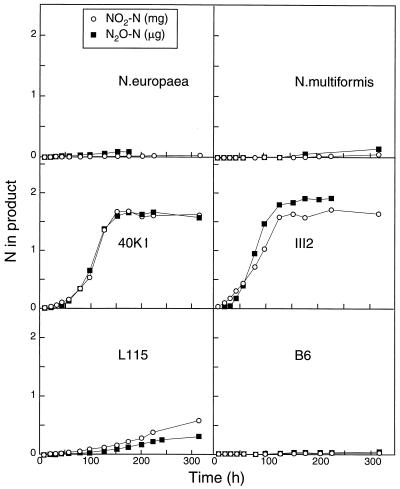
Figures 1 and 2 show the accumulations of N2O (in micrograms of N per flask) and NO2− (in milligrams of N per flask) when cells were grown in HEPES-buffered medium supplemented with NH4+ and urea, respectively. The III7 and AF cultures failed to grow in this experiment (data not shown). For some unknown reason, strain B6 did not show significant activity in the urea medium (Fig. 2), despite its proven urease activity in a previous experiment (unpublished). Slight production of NO2− and N2O was found in N. europaea and Nitrosospira multiformis cultures. The NO2− concentrations in the stationary phase were between 0.5 and 1 mg of NO2-N per flask, which is 25 to 50% of the total NH4+-N in the medium (i.e., 2.1 mg of N per flask). In contrast, the urea-grown urease-positive strains (40K1 and III2) accumulated around 1.5 mg of NO2−-N, i.e., around 75% of the added urea N (Fig. 2). The final pH values for each culture are shown in Table 1.
FIG. 1.
Accumulation of N2O (in micrograms of N per flask) and NO2− (in milligrams of N per flask) during growth with ammonium as the substrate in the HEPES-buffered medium (initial pH, 7.5).
FIG. 2.
Accumulation of N2O (in micrograms of N per flask) and NO2− (in milligrams of N per flask) during growth with urea as the substrate in the HEPES-buffered medium (initial pH, 7.5).
TABLE 1.
Final pH values measured in the cultures grown with either urea or ammonium
| Culture | pH with following substrate:
|
|
|---|---|---|
| Ammonium | Urea | |
| Nitrosomonas europaea | 5.99 | 7.45 |
| Nitrosospira multiformis | 5.38 | 7.17 |
| Nitrosospira sp. strain 40K1 | 6.20 | 6.56 |
| Nitrosospira sp. strain III2 | 5.99 | 6.45 |
| Nitrosospira sp. strain L115 | 5.71 | 6.07 |
| Nitrosospira sp. strain B6 | 5.50 | 7.41 |
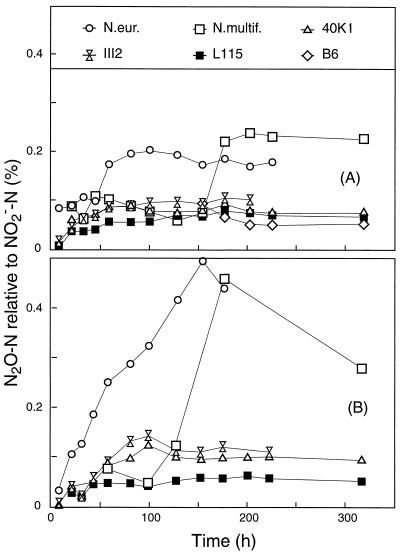
The accumulation of N2O-N was very close to 0.1% of the NO2−-N accumulated for all the strains of Nitrosospira through the whole growth cycle when the strains were grown on both NH4+ and urea (Fig. 3). The same was true for the early phase of NH4+-grown N. europaea and Nitrosospira multiformis, but in these cultures the accumulation of N2O continued after the NO2− concentrations reached a stable level.
FIG. 3.
Accumulated N2O-N as percentages of accumulated NO2−-N in cells grown in the HEPES-buffered medium with ammonium (A) and urea (B). N.eur., N. europaea; N.multif., Nitrosospira multiformis.
Methane concentrations were measured through the whole incubation experiment, but the level (2.1 ml liter−1 [ppmv]) remained practically constant (within 2.10 ± 0.02 ppmv [mean ± standard deviation]) throughout the whole incubation (data not shown). Approximate upper confidence limits for possible CH4 oxidation in the cultures was thus around 0.1 nmol of CH4 per flask.
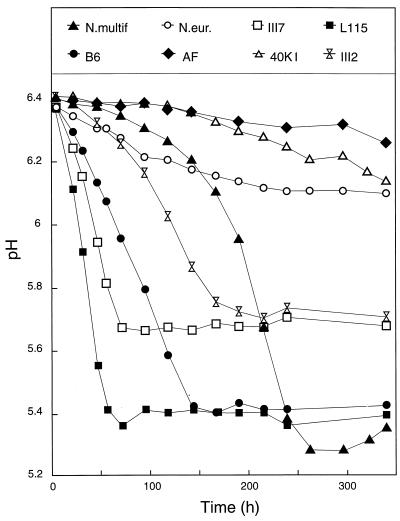
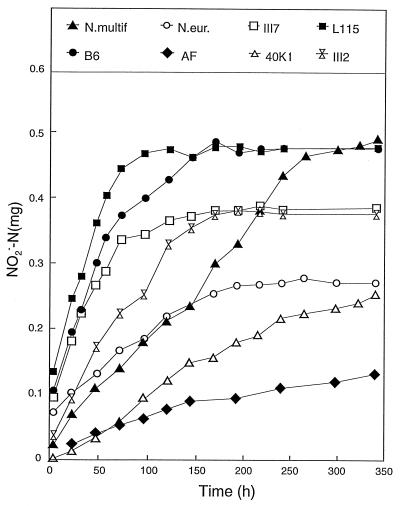
The results of the second experiment are shown in Fig. 4 to 7. In this experiment, the medium (NH4+ only) was buffered with 20 mM phosphate to enhance the resolution of the experiment in the desired pH range and pH was measured in every sample (Fig. 4). The NO2− concentration stabilized at different levels, reflecting the pH tolerance of each strain. The stable levels reached for NO2− accumulation did not exceed 0.5 mg of N per flask, which is only 12% of the NH4+-N in the medium.
FIG. 4.
Measured pH in the cultures growing in the phosphate-buffered medium. N.multif., Nitrosospira multiformis; N.eur., N. europaea.
FIG. 7.
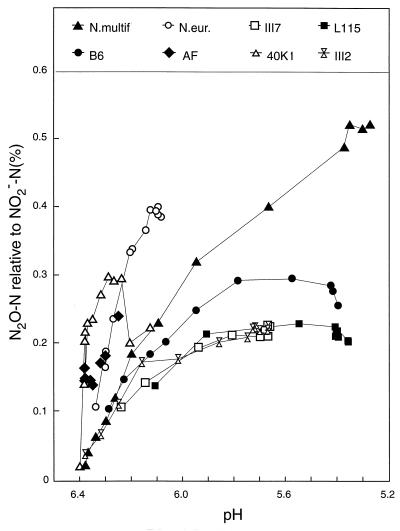
Yields of N2O-N as percentages of NO2−-N in the phosphate-buffered medium, plotted against the measured pH. N.multif., Nitrosospira multiformis; N.eur., N. europaea.
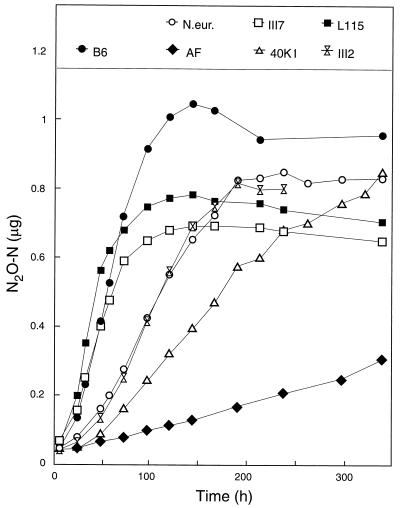
The accumulation of N2O (Fig. 6) resembled that of NO2− (Fig. 5), as was found in the first experiment with HEPES-buffered media (Fig. 1 and 2). The N2O production by Nitrosospira multiformis culture (data not shown in Fig. 6) showed a pattern similar to those of the other cultures, but the production was much higher (final amount, 3 μg per flask). The relative production of N2O (as a percentage of NO2− produced) and its relationship with pH in the medium are illustrated in Fig. 7, where accumulated N2O as a percentage of NO2− is plotted against the measured pH. For the Nitrosospira cultures, the level of N2O increased gradually through the first part of the growth period but reached more or less stable levels between 0.2 and 0.3%. In contrast, N. europaea and Nitrosospira multiformis continued to higher levels (0.4 and 0.5%, respectively). Thus, the experiment with phosphate buffer reproduced the pattern of the previous experiment as far as the contrast between the Nitrosospira strains and the two type strains is concerned, but the relative levels of N2O production of the Nitrosospira strains were higher and more variable in phosphate-buffered medium than in HEPES-buffered medium.
FIG. 6.
Accumulation of N2O (in micrograms of N per flask) during growth in the phosphate-buffered medium. N.eur., N. europaea.
FIG. 5.
Accumulation of NO2− (in milligrams of N per flask) during growth in the phosphate-buffered medium. N.multif., Nitrosospira multiformis; N.eur., N. europaea.
DISCUSSION
Production of N2O.
The first experiment with cells grown in HEPES-buffered medium with an initial pH of 7.5 (Fig. 1 to 3) demonstrated that all cultures, including N. europaea and Nitrosospira multiformis, had surprisingly similar N2O/NO2− product ratios during unrestricted growth. For the urease-positive Nitrosospira strains, the product ratios with urea as the substrate were practically identical to those with NH4+ as the substrate, and the rankings of the cultures (III2 > 40K1 > L115) were identical for the two substrates (Fig. 3). The product ratios were in the lower range of those observed in other ammonia-oxidizing cultures during aerobic unrestricted growth (12, 17).
In response to unfavorable conditions, however, the two type strains (N. europaea and Nitrosospira multiformis) differed significantly from the Nitrosospira strains. When the oxidation of NH3 to NO2− became acid limited (Fig. 1), the two type strains continued to produce N2O for some time, which resulted in significantly higher product ratios towards the end of the incubation (Fig. 3A). The relative levels of production of N2O over NO2−, if calculated for single time increments in the transition between active phase and stationary phase (50 to 100 h for N. europaea and 150 to 200 h for Nitrosospira multiformis), were in the range of 1 to 5% (N2O-N production as a percentage of NO2−-N production). The type strains also showed high product ratios when they were grown in the urea medium (Fig. 3B). The production of NO2− by the urease-negative type strains in the urea medium was due to traces of NH4+ present in the medium (some hydrolysis of urea occurred during the autoclaving). This result implies that the cultures were active but severely starved in this medium. Hence, limitation of the supply of NH4+ seems to affect the product ratio in the same way that acidity did. We may be reporting the same phenomenon (starvation), however, since the pH controls the concentration of NH3, which is the substrate for ammonia monooxygenase (18, 38). The half-saturation constant (Km) for some of the Nitrosospira strains (AF, L115, B6, and 40K1) ranged from 6 to 11 μM NH3, which is comparable to the levels of affinity in the type strains (18). Thus, differences in affinity hardly explain the differences in the levels of N2O production between the type strains and the Nitrosospira strains. The apparent Kms of indigenous AOB in forest soils have been found to be 2 to 3 orders of magnitude lower (10 to 15 nM NH3 [31]) than those of all cultured organisms, suggesting that certain soils harbor AOB essentially different from those cultivated so far, including the Nitrosospira strains used in this study.
The second experiment was designed to improve determination of the regulating effects of pH and substrate supply, by using an initial pH of 6.4 in a weakly (20 mM) phosphate-buffered medium. The measured pH demonstrated that this aim was largely reached, in terms of several measurements of both NO2− and N2O production as the cultures approached their characteristic critical pHs. These limits agree reasonably well with direct observations of each strain’s pH tolerance (strains were exposed to preset pH values of 5 to 8.5; cf. with the work of Jiang and Bakken [18]). Strain AF, however, is unlikely to be near its critical pH level in the present experiment, since it was found to be at least as acid tolerant as L115 and B6 (18). The explanation may be low viability in the inoculum.
The production of N2O as a percentage of NO2− in the phosphate-buffered medium (Fig. 7) contrasts with the results obtained with the HEPES-buffered medium in several ways. The product ratio increased gradually through the first 50 to 100 h of the incubation, both for the Nitrosospira strains and for the two type strains. The Nitrosospira strains which reached a stationary-phase level of production (L115, B6, III7, and III2) also stabilized the product ratios at levels around 0.2% (Fig. 7), which is at least twice the level found in the HEPES-buffered medium (Fig. 3). The ranking of relevant Nitrosospira strains (III2, 40K1, and L115) in the phosphate buffer (40K1 > III2 = L115, final ratios) (Fig. 7) was different from that in the HEPES buffer (III2 > 40K1 > L115). The only pattern in experiment 1 (HEPES-buffered medium) which was really reproduced in experiment 2 was the ability of N. europaea and Nitrosospira multiformis to continue the production of N2O longer than the production of NO2− when it was confronted with acid limitation of activity, resulting in higher final relative N2O values than those of the Nitrosospira strains (Fig. 7).
The effect of buffer systems on N2O production by N. europaea was investigated by Hynes and Knowles (17), and they found that a HEPES-buffered medium (pH 7.5) resulted in much lower relative N2O production than a phosphate-buffered medium at the same pH, which is in agreement with our results. In contrast, they observed that two other organic buffer systems [N-tris(hydroxymethyl)methylglycine (pH 8) and tris(hydroxymethyl)aminomethane (pH 8.5)] resulted in relative N2O production much higher than that of the phosphate buffer systems. It seems clear that the production of N2O, which probably occurs in the periplasm (due to the location of hydroxylamine oxidoreductase in this compartment), can be strongly affected by a number of external factors other than pH and oxygen concentrations. This conclusion implies that one should not extrapolate the present values for N2O production to populations in natural environments without reservations.
The purpose of the present investigation was primarily to investigate whether consistent differences exist between AOB in their abilities to produce N2O. It seems reasonable to conclude that no such consistent differences exist among the recently isolated strains of Nitrosospira. On the other hand, all the Nitrosospira strains produced consistently less relative amounts of N2O than the two type strains, N. europaea and Nitrosospira multiformis. The relatively high N2O production by the two type strains occurred only during restricted activity, either due to acidity (Fig. 3A and 7) or due to low substrate supplies (Fig. 3B). A correlation between acidity and the relative N2O production rate was found for the Nitrosospira strains as well, but it was weaker than for the two type strains and dependent on the buffer system used.
Populations of AOB in the natural environment are likely to be frequently confronted with starvation as well as critically low pH values (35). The present investigation strongly suggests that the ammonia oxidized by such starving and/or acid-limited populations is likely to yield more N2O than that released by an exponentially growing culture under optimal conditions. This effect apparently depends on the composition of the AOB community.
Oxidation of CH4.
In this study, no significant oxidation of atmospheric CH4 was observed. This result may be attributed primarily to the insensitivity of our approach. The available data on methane oxidation by AOB have been obtained by using radioactively labelled methane, which allows extremely low rates to be determined. Hyman and Wood (16) found that CH4 oxidation by N. europaea had a Vmax of 0.065 mmol of CH4 g (dry weight) of cells−1 h−1 and a Ks of 6.6 μM CH4. In the present experiment, the CH4 concentration in the gas phase was around 2 ppmv, which means that the CH4 concentration in the liquid medium was around 2.5 nM (assuming equilibrium and with the solubility of CH4 being calculated according to the method of Wilhelm et al. [36]). If we use the Vmax and Ks values reported above, we find that the oxidation rate in our flasks should be 25 nmol g (dry weight) of cells−1 h−1. Assuming a cell dry weight of 0.06 × 10−12 g and an average cell density of 6 × 107 cells ml−1 in the 40-ml medium per flask in the second experiment, we obtain an oxidation rate of approximately 5 pmol flask−1 h−1, which is equivalent to 0.1% h−1 (total methane in the flask was 6 nmol). Thus, if the organisms were actively oxidizing methane through the entire incubation period of 320 h, the methane concentration in the flasks should have been reduced by approximately 30%.
If ammonia oxidizers behaved according to the enzyme kinetics referred to above, we would need 2 × 109 cells g (dry weight) of soil−1 to account for the observed CH4 oxidation in forest soils (1 to 3 ng of CH4 g−1 day−1 [30]). This cell density is at least 3 orders of magnitude higher than the density of AOB found in soils. Thus, if AOB were to contribute to the observed CH4 oxidation in aerobic soils, there would have to exist species that oxidize atmospheric CH4 at a rate orders of magnitudes faster than that observed to date. If the Nitrosospira strains had anywhere near such high rates of methane oxidation, we would be able to measure their activities with the present experimental design (i.e., without radioactive tracers). We did not and conclude that none of our strains are likely to have contributed significantly to the oxidation of atmospheric CH4 in the soil from which they were isolated.
ACKNOWLEDGMENTS
This work was financed by the Norwegian Research Council, project no. 114038/111, which is a part of the Nordic (NKJ) research program Ecological Roles of Ammonia-Oxidizing Bacteria in Soils.
REFERENCES
- 1.Adamsen A P S, King G M. Methane oxidation in temperate and subarctic forest soils: rates, vertical zonation, and responses to water and nitrogen. Appl Environ Microbiol. 1993;59:485–490. doi: 10.1128/aem.59.2.485-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aulakh M S, Rennie D A, Paul E A. Acetylene and N-serve have effects upon N2O emissions from NH4+ and NO3− treated soils under aerobic and anaerobic conditions. Soil Biol Biochem. 1984;16:351–356. [Google Scholar]
- 3.Bedard C, Knowles R. Physiology, biochemistry and specific inhibitors of CH4, NH4, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belser L W. Bicarbonate uptake by nitrifiers: effects of growth rate, pH, substrate concentration, and metabolic inhibitors. Appl Environ Microbiol. 1984;48:1100–1104. doi: 10.1128/aem.48.6.1100-1104.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackmer A M, Bremner J M, Schmidt E L. Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil. Appl Environ Microbiol. 1980;40:1060–1066. doi: 10.1128/aem.40.6.1060-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouwman A F. Exchange of greenhouse gases between terrestrial ecosystems and the atmosphere. In: Bouwman A F, editor. Soils and the greenhouse effect. Chichester, United Kingdom: Wiley and Sons; 1990. pp. 61–127. [Google Scholar]
- 7.Bremner J M, Blackmer A M. Effect of acetylene and soil water content on emission of nitrous oxide from soils. Nature. 1979;280:380–381. [Google Scholar]
- 8.Bremner J M, Blackmer A M. Terrestrial nitrification as a source of atmospheric nitrous oxide. In: Delwiche C C, editor. Denitrification, nitrification and atmospheric nitrous oxide. New York, N.Y: John Wiley & Sons Inc.; 1981. pp. 151–170. [Google Scholar]
- 9.Dispirito A A, Taaffe L R, Lipscomb J D, Hooper A B. A ’blue’ copper oxidase from Nitrosomonas europaea. Biochim Biophys Acta. 1985;827:320–326. [Google Scholar]
- 10.Duxbury J M, Bouldin D R, Terry R E, Tate R L., III Emissions of nitrous oxide from soils. Nature. 1982;298:462–464. [Google Scholar]
- 11.Goodroad L L, Keeney D R. Nitrous oxide production in aerobic soils under varying pH, temperature, and water content. Soil Biol Biochem. 1984;16:39–44. [Google Scholar]
- 12.Goreau T J, Kaplan W A, Wofsy S C, McElroy M B, Valois F W, Watson S W. Production of NO2− and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl Environ Microbiol. 1980;40:526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson R S, Hanson T E. Mathanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiorns W D, Hastings R C, Head I M, McGarthy A J, Saunders J R, Pickup R W, Hall G H. Amplification of 16S ribosomal RNA genes of autotrophic ammonia-oxidizing bacteria demonstrates the ubiquity of Nitrosospira in the environment. Microbiology. 1995;141:2793–2800. doi: 10.1099/13500872-141-11-2793. [DOI] [PubMed] [Google Scholar]
- 15.Hutsch B, Webster C P, Powlson D. Long term effects of nitrogen fertilization on methane oxidation in soil of the Broadbalk experiment. Soil Biol Biochem. 1993;25:1307–1315. [Google Scholar]
- 16.Hyman M R, Wood P M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983;212:31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynes R K, Knowles R. Production of nitrous oxide by Nitrosomonas europaea: effects of acetylene, pH, and oxygen. Can J Microbiol. 1984;30:1397–1404. [Google Scholar]
- 18.Jiang, Q. Q., and L. R. Bakken. Comparison of Nitrosospira strains isolated from terrestrial environments. FEMS Microbiol. Ecol., in press. [DOI] [PubMed]
- 19.Jones R D, Morita R Y. Methane oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl Environ Microbiol. 1983;45:401–410. doi: 10.1128/aem.45.2.401-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemedtsson, L., Q. Jiang, Å. K. Klemedtsson, and L. R. Bakken. Autotrophic ammonium oxidizing bacteria in Swedish mor humus. Soil Biol. Biochem., in press.
- 21.Martikainen P J. Nitrous oxide emission associated with autotrophic ammonium oxidation in acid coniferous forest soil. Appl Environ Microbiol. 1985;50:1519–1525. doi: 10.1128/aem.50.6.1519-1525.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller D J, Nicolas D J D. Characterization of a soluble cytochrome oxidase/nitrite reductase of Nitrosomonas europaea. J Gen Microbiol. 1985;131:2851–2854. [Google Scholar]
- 23.Poth M, Focht D D. 15N kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl Environ Microbiol. 1985;49:1134–1141. doi: 10.1128/aem.49.5.1134-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remde A, Conrad R. Production of nitric oxide in Nitrosomonas europaea by reduction of nitrite. Arch Microbiol. 1990;154:187–191. [Google Scholar]
- 25.Ritchie G A F, Nicolas D J D. Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J. 1972;126:1181–1191. doi: 10.1042/bj1261181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie G A F, Nicolas D J D. The partial characterization of purified nitrite reductase and hydroxylamine oxidase from Nitrosomonas europaea. Biochem J. 1974;138:471–480. doi: 10.1042/bj1380471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson G P, Tiedje J M. Nitrous oxide sources in aerobic soils. Nitrification, denitrification and other biological processes. Soil Biol Biochem. 1987;19:187–193. [Google Scholar]
- 28.Seitzinger S P, Pilson M E O, Watson S W. Nitrous oxide production in nearshore marine sediments. Science. 1983;222:1244–1245. doi: 10.1126/science.222.4629.1244. [DOI] [PubMed] [Google Scholar]
- 29.Sitaula B K, Bakken L R. Nitrous oxide release from spruce forest soil: relationships with nitrification, methane uptake, temperature, moisture and fertilization. Soil Biol Biochem. 1993;25:1415–1421. [Google Scholar]
- 30.Sitaula B K, Luo J, Bakken L R. Rapid analysis of climate gases by wide bore capillary gas chromatography. J Environ Qual. 1992;21:493–496. [Google Scholar]
- 31.Stark J M, Firestone M K. Kinetic characteristics of ammonium oxidizer communities in a California oak woodland-annual grassland. Soil Biol Biochem. 1996;28:1307–1317. [Google Scholar]
- 32.Stephen J R, McCaig A E, Smith Z, Prosser J I, Embley T M. Molecular diversity of soil and marine 16S rRNA gene sequences related to β-subgroup ammonia-oxidizing bacteria. Appl Environ Microbiol. 1996;62:4147–4154. doi: 10.1128/aem.62.11.4147-4154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Utåker J B, Bakken L R, Jiang Q Q, Nes I F. Phylogenetic analysis of seven new isolates of ammonia-oxidizing bacteria based on 16S rRNA gene sequences. Syst Appl Microbiol. 1995;18:549–559. [Google Scholar]
- 34.Utåker J B, Nes I F. A qualitative evaluation of the published oligonucleotides specific for the 16S rRNA gene sequences of the ammonia-oxidizing bacteria. Syst Appl Microbiol. 1998;21:72–88. doi: 10.1016/S0723-2020(98)80010-6. [DOI] [PubMed] [Google Scholar]
- 35.Verstraete W. Nitrification. Ecol Bull. 1981;33:303–314. [Google Scholar]
- 36.Wilhelm E, Battino R, Wilcock R J. Low pressure solubility of gases in liquid water. Chem Rev. 1977;77:219–262. [Google Scholar]
- 37.Willison T W, Cook R, Müller A, Powlson D. CH4 oxidation in soils fertilized with organic and inorganic N, differential effects. Soil Biol Biochem. 1996;28:1135–1136. [Google Scholar]
- 38.Wood P M. Nitrification as a bacterial energy source. In: Prosser J I, editor. Nitrification. Oxford, United Kingdom: IRL Press; 1986. pp. 39–62. [Google Scholar]