Abstract
Matrix vesicles are key players in the development of the growth plate during endochondral bone formation. They are involved in the turnover of the extracellular matrix and its mineralization, as well as being a vehicle for chondrocyte communication and regulation. These extracellular organelles are released by the cells and are anchored to the matrix via integrin binding to collagen. The exact function and makeup of the vesicles are dependent on the zone of the growth plate in which they are produced. Early studies defined their role as sites of initial calcium phosphate deposition based on the presence of crystals on the inner leaflet of the membrane and subsequent identification of enzymes, ion transporters, and phospholipid complexes involved in mineral formation. More recent studies have shown that they contain small RNAs, including microRNAs, that are distinct from the parent cell, raising the hypothesis that they are a distinct subset of exosomes. Matrix vesicles are produced under complex regulatory pathways, which include the action of steroid hormones. Once in the matrix, their maturation is mediated by the action of secreted hormones. How they convey information to cells, either through autocrine or paracrine actions, is now being elucidated.
Keywords: exosomes; matrix vesicles; microRNA; 1α,25(OH)2D3; 24R,25(OH)2D3
1. Introduction
Matrix vesicles (MVs) are extracellular organelles ranging in size from 50 to 150 nm in diameter that are anchored to the extracellular matrix (ECM) of mineralized tissues via integrin binding to collagen. They were first identified in the 1960s by transmission electron microscopy of calcifying neoplasms, as well as in calcifying tissues, including the mammalian growth plate, the osteoid synthesized during primary bone formation, in dentine during tooth formation, and in the intima of blood vessels undergoing calcification [1,2]. In all cases, they shared a circular morphology and a bilaminar membrane [3,4,5]. Because they were originally identified at sites where the first calcium phosphate crystals were deposited in the matrix [6], the early publications describing them focused on their role in mineralization [7,8,9].
Methods for isolating MVs led to the observation that they were enriched with tissue non-specific alkaline phosphatase (TNAP), an ecto-enzyme that is anchored to the outer leaflet of the MV membrane via glycosylphosphatidylinositol [10] and is also present in the plasma membranes of the parent cells [1]. This observation led to the use of alkaline phosphatase-specific activity as the defining characteristic of matrix vesicles isolated by enzymic digestion of the tissue, followed by differential ultracentrifugation of the ECM digest. Biochemical analysis of the isolated MVs provided additional information that explained how they contribute to calcification, first by providing sites for initial calcium phosphate crystal formation and via enzymes that increase local phosphate ion concentration. Subsequent studies demonstrated that MVs also contained enzymes that could process the ECM, facilitating crystal growth, and that MV composition and function were regulated by systemic hormones, including 1α,25-dyhydroxyvitamin D3 [1α,25(OH)2D3] [8,11,12,13,14,15].
The ability to isolate MVs from cultured chondrocytes and osteoblasts has enabled investigators to gain a more complete understanding of their composition and the role that they play in regulating the cells that produce them. The goal of this review is to provide a synopsis of these studies, using the mammalian growth plate as the primary model system, given the depth of information that exists in the literature. As our understanding of extracellular vesicles has increased, the question of whether MVs are a subspecies of exosomes is important to address, particularly in light of the finding that MVs contain microRNAs that are also reported to be in exosomes. The mammalian growth plate as a model system is no less valuable in this regard, as it provides us with a well-defined linear system for tracking how MVs change as a function of space and time in vivo, and these relationships are retained in culture.
2. The Mammalian Growth Plate
The model system that has yielded the most information about matrix vesicles is the mammalian growth plate. Mammalian bone growth is driven by two types of ossification. Endochondral ossification is responsible for long bone growth, while intramembranous ossification primarily produces the flat bones found in the skull [16]. Both processes stem from mesenchymal tissue.
Embryologically, the skeletal bone begins as a cartilage template. This template forms a region of transition in which the chondrocytes align in columns, termed the growth plate. This process is regulated by a number of factors, including parathyroid-hormone-related peptide (PTHrP) and Indian hedgehog (Ihh) [17,18,19]. As the chondrocytes undergo terminal differentiation, they mineralize their ECM and secrete proteins that recruit osteoclasts to resorb the calcified cartilage as well as vascular endothelial cells, resulting in capillary invasion of the tissue [20]. Osteoprogenitor cells then migrate to the sites via the newly formed vasculature, differentiate into osteoblasts, and form bone [21].
A similar process occurs during post-fetal long bone growth, where the growth plate is located between epiphysis and metaphysis and at the chondro-occipital junction at the base of the skull, as well as at the interface of bone and the costochondral cartilage. All of these sites are characterized by a region containing chondrocytes in a glycosaminoglycan-rich, type II collagen matrix [22,23]. These cells respond to regulatory signals to align in columns and undergo terminal differentiation [20]. Growth plates can be divided into different zones populated by chondrocytes with distinct phenotypical characteristics. Chondrocytes mature as they move through the growth plate, passing from the proliferating cell zone through the prehypertrophic and hypertrophic cell zones and finally into the calcified cartilage zone [20]. The rate and extent of this process are under hormonal control, including thyroid hormone [24], estrogen and testosterone [25,26,27], and the vitamin D3 metabolites, 1α,25(OH)2D3 and 24R,25-dyhydroxyvitamin D3 [24R,25(OH)2D3] [28,29,30,31,32].
3. Matrix Vesicles: Extracellular Matrix Microsomes
Matrix vesicles are a distinctive feature of the growth plate ECM. They are produced by the growth plate chondrocytes and appear to be bud off laterally from the chondrocyte parent cell, although the exact process by which this occurs remains unclear [5]. Recent work using osteoblasts implicates Stx4a, a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein, in the release of matrix vesicles into the matrix as Stx4a conditional knockout mice were observed to contain fewer matrix vesicles [33]. Two other types of SNARE proteins (Snap23 and Vamp2) were also found to be differentially expressed though their role in vesicle release was not investigated.
MVs are anchored within the ECM via integrin binding to type II collagen [3]. They can be isolated from growth plate cartilage by digesting the ECM with collagenase and hyaluronidase followed by differential ultracentrifugation and have been shown to be heterogeneous in content based on electrophoretic mobility and biochemically [34,35]. Their payload consists of minerals, enzymes, factors, and microRNAs with distinct packaging dependent on where in the growth plate they are produced [36,37,38]. While much is known about the morphology and composition of these extracellular organelles in their role as initial sites of calcium phosphate formation, we are only now beginning to understand how they contribute to the overall physiology of the growth plate.
Matrix vesicles possess a unique phospholipid composition, with high levels of cardiolipin and sphingomyelin compared to the plasma membrane [39]. Phosphatidylserine and phosphatidylinositol are enriched in the inner leaflet of the phospholipid bilayer [4,40]. Calcium–phosphatidylserine–phosphate annexin complexes, which are found at hydroxyapatite nucleation sites, are present in matrix vesicles [41,42] together with a proteolipid phosphatidylinositol complex capable of mineral deposition in vitro [43]. This suggested that the primary role of the extracellular organelle was to provide sites for initial crystal formation in tissues, such as the hypertrophic cell zone of the growth plate, primary bone, or fracture callus, where there was no pre-existing mineral [1].
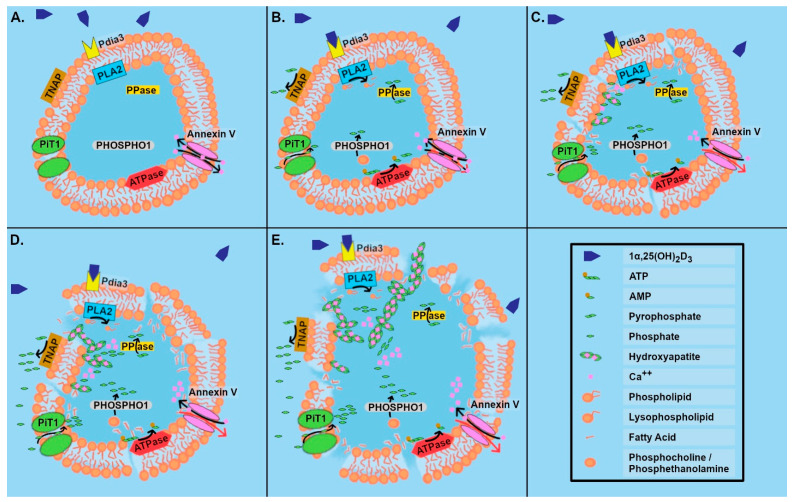
Other data supported this hypothesis. When first formed, matrix vesicles contain high levels of magnesium and adenosine trisphosphate (ATP), known inhibitors of calcification [44,45], as well as carbonic anhydrase [46], and ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) [42,47]. High levels of alkaline phosphatase activity are present along with the mineral octacalcium phosphate. As MVs mature in the matrix, they become leaky due to the action of phospholipases, which are regulated via 1α,25(OH)2D3 that is secreted by the chondrocytes [48]. Calcium ions are able to diffuse in, and at the same time, the active transport of Ca++ out of the vesicles is reduced by the action of ATPases on ATP, generating the calcification inhibitor pyrophosphate and adenosine monophosphate (AMP) [49]. The Ca++ eventually dilutes the inhibitory effects of Mg on apatite formation. Ultimately, pyrophosphatase (PPase) acts on pyrophosphate to form free phosphate, which can then interact with Ca++ to form apatite crystals on the inner leaflet of the membrane [50]. In addition, MVs possess ion transporters, particularly for phosphate, enabling its enrichment within the microsomes during crystal formation [1,47,51]. Another phosphatase present in matrix vesicles is phosphoethanolamine/phosphocholine phosphatase 1 (PHOSPHO1), which acts on phosphocholine and phosphoethanolamine to initiate crystal formation [47]. As the MV breaks down, these initial crystals can provide sites for epitaxial growth of apatite crystals within the collagen matrix. Matrix vesicle TNAP participates in this process by modulating the phosphorylation status of osteopontin in the matrix, thereby facilitating crystal growth [52] (Figure 1A–E).
Figure 1.
Growth zone matrix vesicle breakdown and hydroxyapatite crystal formation in response to 1α,25(OH)2D3. (A) Annexin V mediated transport of Ca++ maintains homeostasis inside vesicle. (B) 1α,25(OH)2D3 binding with PDIA3 on vesicle membrane activates phospholipase A2 (PLA2). This results in release of arachidonic acid and the production of lysophospholipid. ATPase activity is reduced due to lack of an energy source, so active transport of Ca++ out of the vesicle via annexin V is reduced. The action of ATPase produces AMP and pyrophosphate, which is a calcification inhibitor. PPase breaks pyrophosphate down into phosphates, and on the outer leaflet of the membrane, TNAP generates free phosphate that is available for transport into the vesicle by PiT1. Inside the vesicle, PHOSPHO1 releases phosphate from phosphocholine or phosphoethanolamine. (C) The matrix vesicle membrane becomes leaky. The first hydroxyapatite crystals have formed on the inside of the matrix vesicle following nucleation via Ca-phosphatidylserine-phosphate complexes; active transport of Ca++ via annexin V no longer occurs. (D) There is increased accumulation of calcium and phosphate along the surface of the membrane. (E) Membrane integrity is lost, and hydroxyapatite crystals grow out into the ECM.
A similar process has been studied in osteoblast-derived matrix vesicles, where a group of membrane transporters and enzymes has been demonstrated to be involved in the regulation of hydroxyapatite crystal nucleation and growth. ENPP1 found on the outer leaflet of osteoblast matrix vesicles is involved in regulating hydroxyapatite crystal growth by generating the inhibitor pyrophosphate from ATP, while annexin and ankylin protein (ANK) provide a transmembrane channel for pyrophosphate to enter the extracellular organelles [53]. TNAP, also located on the outer leaflet, is able to degrade the pyrophosphate into monophosphate ions required for crystal growth, while two matrix vesicle membrane transporters, sodium/phosphate co-transporter type III (PiT1) and annexin V, are able to transport monophosphate ions and Ca++, respectively, into the vesicles. By impacting the makeup and location of specific ions, these enzymes and transporters can regulate crystal growth.
Matrix vesicles can be isolated from tissues such as growth plate cartilage through a one-step purification process. Their isolation requires initial digestion of the ECM via collagenase and/or hyaluronidase, followed by careful removal of the intact cells via centrifugation and differential centrifugation of the supernatant to pellet large organelles such as mitochondria, nuclei, endoplasmic reticulum, endosomes, and plasma membrane (PM). The remaining supernatant is centrifuged at high speed to pellet the matrix vesicles. Because the MVs are not lysed during isolation, their native conformation remains intact; thus, they remain right side out, with the ectoenzyme TNAP facing outward. In contrast, PMs are isolated from lysed cells and may form liposomes with the outer leaflet of the membrane facing the interior, away from the TNAP substrate in the media. Scientists have taken advantage of this to define MV purity as having alkaline phosphatase specific activity that is a minimum of two-fold greater than that of the PM fraction [54]. In addition, their relative purity can be readily determined using marker enzymes and proteins, including validation that they are not mitochondrial membrane fragments based on their high cardiolipin content [15,39,55].
Using these criteria to identify MVs, investigators have been able to address questions related to MV production, composition, and function. Studies using somatic cell hybrids to monitor alkaline phosphatase enrichment demonstrated that matrix vesicles are produced as specific organelles and are not simply membrane debris left in the matrix during ECM synthesis [56].
This same approach can be used to isolate matrix vesicles from cell cultures, which has enabled careful cataloging of their contents and how they are regulated. Studies from a variety of laboratories have shown that they possess annexins 2, 5, and 6 [51], as well as various enzymes that process the ECM, including matrix metalloproteinases (MMP2, MMP3) [36,48], a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), and tissue inhibitor of metalloproteinases (TIMP1, TIMP2). Given their size, 50–150 nm diameter, and the complexity of their composition, it is likely that there is more than one kind of matrix vesicle. Indeed, differential centrifugation of isolated matrix vesicles shows that there are different populations that vary in density as well as alkaline phosphatase content [57].
Matrix vesicles have been isolated from soft tissues as well as mineralizing tissues. They are present in vascular smooth muscle [58], and their content changes at vascular sites that are becoming mineralized [42]. Similarly, there are distinct differences in matrix vesicles isolated from the upper growth plate, which does not mineralize, and those isolated from hypertrophic cartilage, which is becoming calcified during endochondral bone formation. Matrix vesicles are also present in tissues that do not become calcified, including some cancers [1,2]. In general, they all contain enzymes associated with matrix modification.
Studies comparing matrix vesicles produced by resting zone growth plate (RC) cartilage and those produced by prehypertrophic/upper hypertrophic growth zone (GC) cartilage show that they differ markedly [59]. They possess a different membrane phospholipid composition; their alkaline phosphatase activity differs, and their MMP content differs. RC matrix vesicles are enriched in neutral MMPs, whereas GC matrix vesicles are enriched in acid MMPs [36]. This implies that the production of the microsomes is under genomic regulation, but once in the matrix, control of their function must involve non-genomic mechanisms, as they lack the machinery necessary for gene transcription and protein synthesis.
4. Regulation of Matrix Vesicle Function
Production of matrix vesicles is under genomic regulation. Not surprisingly, knockout mice lacking expression of key components such as TNAP, PPase, or PHOSPHO1 [50], produce matrix vesicles that lack these proteins and whose function is altered as a result. Direct evidence of genomic regulation by steroid hormones supports this. The vitamin D metabolites 1α,25(OH)2D3 and 24R,25(OH)2D3 regulate costochondral growth plate chondrocytes via mechanisms that are specific to the cell maturation zone from which the cells were isolated. 1α,25(OH)2D3 regulates GC chondrocytes both through the nuclear vitamin D receptor (VDR) and through a membrane-associated receptor, protein disulfide isomerase A3 (PDIA3), which mediates its effects via a protein kinase C (PKC) signal transduction pathway. PKC-alpha (PKCα) is increased via a phosphatidylinositol-specific phospholipase C (PLC)-dependent mechanism, as well as through the stimulation of phospholipase A2 (PLA2) activity. Arachidonic acid and its downstream metabolite prostaglandin E2 (PGE2) also modulate cell response to 1α,25(OH)2D3. In contrast, 24R,25(OH)2D3 exerts its effects on RC chondrocytes through a separate, membrane-associated receptor that also involves PKC pathways. However, PKCα is increased via a phospholipase D (PLD)-mediated mechanism, as well as through inhibition of the PLA2 pathway [60,61].
Matrix vesicle production is also regulated in a zone-specific manner. Studies using matrix vesicles produced by RC and GC chondrocytes in culture show that 24R,25(OH)2D3 stimulates the production of RC matrix vesicles by RC cells, including the incorporation of neutral MMPs and increased TNAP. However, it does not affect matrix vesicle production by GC cells. In contrast, 1α,25(OH)2D3 increases the activity of acid MMP and TNAP in matrix vesicles produced by GC chondrocytes, but it does not modify the RC matrix vesicle composition. These observations have been confirmed in vivo using vitamin D deficient/phosphate deficient rats as a model. The rachitic animals were treated with 25(OH)D3, 1α,25(OH)2D3, or 24R,25(OH)2D3; at harvest, matrix vesicles isolated from the growth plate were examined, and their MMP activities were compared. The results showed that the composition of the extracellular microsomes was comparable to those obtained using matrix vesicles isolated from cell culture [62].
Additional evidence that matrix vesicle composition is controlled genomically involves the incorporation of PKCα and PKC-zeta (PKCζ) following treatment with the vitamin D metabolites. After 24 h of exposure to the hormones, plasma membranes isolated from cell lysates contained primarily PKCα, but matrix vesicles contained primarily PKCζ. This differential enrichment of PKCζ is regulated by 24R,25(OH)2D3 in RC matrix vesicles and by 1α,25(OH)2D3 in GC matrix vesicles [48].
Matrix vesicles are also regulated once in the matrix via non-genomic mechanisms. Chondrocytes possess the ability to generate vitamin D metabolites locally [63]. Moreover, they secrete them at high levels and under regulation by hormones and growth factors [63]. In vitro, treatment of isolated naïve matrix vesicles with 1α,25(OH)2D3 or 24R,25(OH)2D3 produced an intriguing finding. Whereas 24R,25(OH)2D3 stimulated TNAP and PKCα, it inhibited PLA2 and PKCζ activity in matrix vesicles produced by RC cells [13,64,65]. In contrast, 1α,25(OH)2D3 stimulated TNAP, PKCα, and PLA2 and inhibited PKCζ in matrix vesicles from GC cells. Moreover, 1α,25(OH)2D3 activated PKCα and PLA2 in RC matrix vesicles, but its effect was much less robust than in GC matrix vesicles.
While the mechanisms by which the vitamin D metabolites act on matrix vesicles once in the matrix are not yet known, the biological consequences are becoming understood. It is evident that the primary function of 24R,25(OH)2D3 is to ensure that matrix vesicles remain structurally stable in the matrix, at least with respect to the integrity of the membrane. 24R,25(OH)2D3 treatment of RC cells results in the production of matrix vesicles with higher levels of phosphatidylcholine than GC matrix vesicles [55]. Moreover, 24R,25(OH)2D3 inhibits PLA2, thereby limiting the production of lysophospholipids, which has the effect of stabilizing the phospholipid bilayer. 1α,25(OH)2D3 treatment of GC cells results in matrix vesicles with a higher percentage of acidic phospholipids, and when GC matrix vesicles are treated directly with the hormone, PLA2 is stimulated, and production of lysphophospholipids is increased. The lysophospholipids stimulate PKCα activity [66], and they destabilize the membrane, contributing to the leakiness associated with uptake of Ca++ and initial mineral formation.
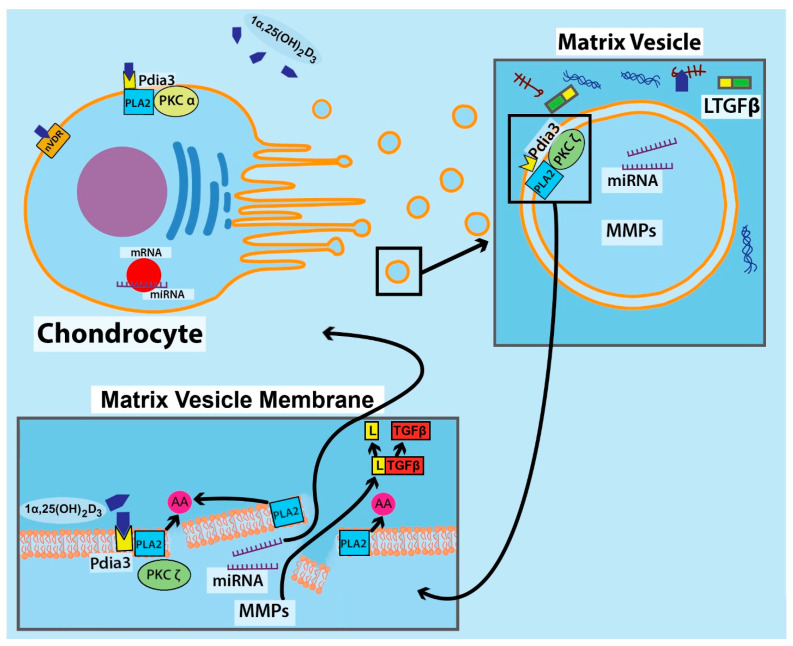
The destabilized membrane also provides a pathway for the release of MMPs and aggrecanases into the ECM (Figure 2). One outcome of this is activation of latent transforming growth factor-beta (TGFβ), which is stored in the ECM via latent TGFβ-binding protein [67]. The matrix vesicles lack collagenase [36], so the release of the enzymes contributes to the degradation of the proteoglycan content, thereby removing a major inhibitor of crystal growth while retaining the collagen matrix to support the calcification process [68,69]. The importance of these enzymes was demonstrated clearly when matrix vesicles were incubated in a proteoglycan-rich gel containing Ca++ and free phosphate [45]. Only when they were pretreated with 1α,25(OH)2D3 did they contribute to apatite formation in the gel, indicating that the release of the enzymes and subsequent degradation of glycosaminoglycan was necessary.
Figure 2.
Growth zone chondrocyte with plasma membrane-associated receptors (VDR and PDIA3) for 1α,25(OH)2D3 release matrix vesicles into the ECM that contain microRNA and matrix metalloproteinases (MMPs). Chondrocyte plasma membrane PDIA3 acts via PKCα. Matrix vesicles contain the PDIA3 receptor complexed with PKCζ. 1α,25(OH)2D3 binds to matrix vesicle PDIA3, activating PLA2 to release arachidonic acid and destabilize the vesicle membrane; this releases the contents into the ECM. MMP released from MVs activates latent TGFβ (LTGFβ) in the extracellular matrix removing the latent binding protein. MicroRNAs act back on the chondrocytes, but whether the microRNAs are released into the ECM or are transported to the cells via the matrix vesicles or as membrane liposomes is not known.
5. Matrix Vesicles as ECM-Linked Exosomes
Are MVs a species of exosome? Growth plate chondrocytes do produce vesicles typical of exosomes that they release into the culture media [9]. However, they differ significantly from matrix vesicles. They are not enriched in alkaline phosphatase activity, their phospholipid content mimics that of the plasma membrane, and they possess different enzymes than are found in matrix vesicles. Whether they are exosomes is not yet resolved, although we have reported the presence of microRNA’s [70], as have others [71,72,73].
Matrix vesicles share physical characteristics with exosomes in terms of size, but unlike exosomes, they are anchored in the ECM. As noted above, they are structurally and compositionally distinct from the media vesicles, but are they a unique species of exosomes that possess small RNAs capable of modulating cell behavior? This is an attractive idea for a number of reasons. The growth plate is not vascularized, and the chondrocytes are surrounded by a large ECM, limiting communication among cells. The arrangement of cells in the growth plate is linear, with RC cells at the top destined to undergo maturation as GC cells. Studies show that treatment of RC chondrocytes with 24R,25(OH)2D3 ultimately induces a transition to a GC phenotype, complete with a shift in response from 24R,25(OH)2D3 to 1α,25(OH)2D3 [74]. Under normal bone growth, RC cells undergo proliferation, and post-proliferative prehypterophic chondrocytes become hypertrophic, producing a new set of matrix vesicles and undertaking a massive reorganization of the ECM to accommodate their size as well as facilitate neovascularization and calcification. This occurs under mechanical load, and cells communicate with each other via the release of a variety of factors, including cytokines, growth factors, hormones, and other small molecules. Recent data suggest that matrix vesicles may contribute to this communication in a manner that is similar to exosomes.
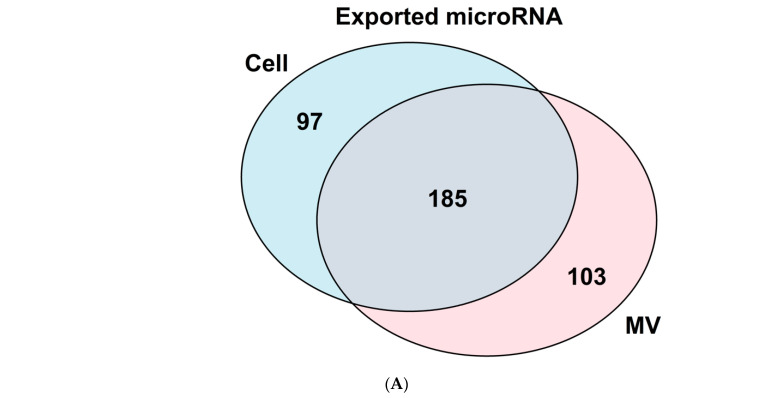
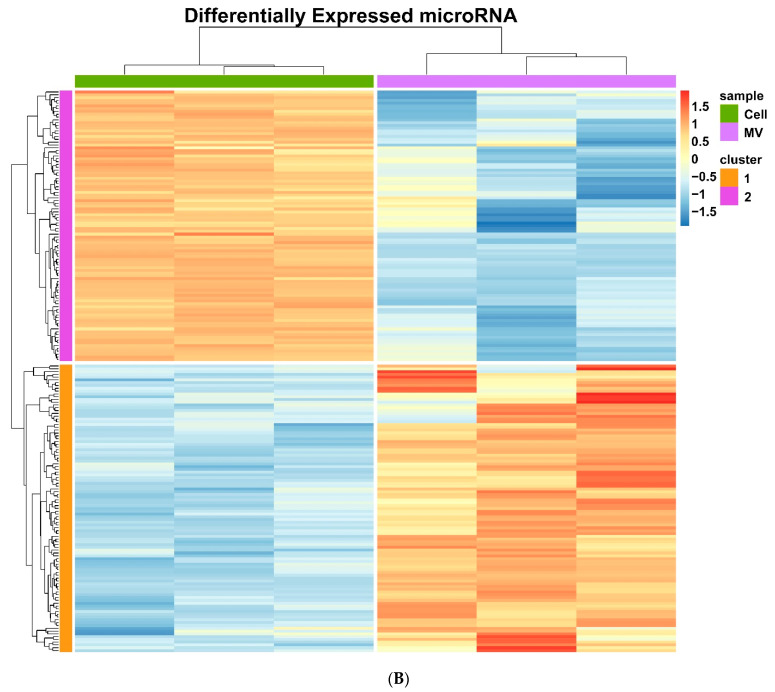
To test this hypothesis, we undertook a careful analysis of matrix vesicle RNA content. Initial studies demonstrated clearly that a subset of microRNAs was differentially concentrated in the MVs isolated from GC chondrocyte cultures compared to the parent cells [70]. (Figure 3A,B) Similarly, four microRNA were unique to RC matrix vesicles compared to their parent cells: miR-451-5p, miR-223-3p, miR-142-3p, and miR-122-5p; and miR-22-3p was among the most abundant miRNA. Moreover, when we compared RNAs in MVs isolated from GC cultures to those isolated from RC cultures, we found distinct cell-specific differences in microRNA content [59], suggesting that they may play a differential role in chondrocyte regulation.
Figure 3.
Examination of chondrocyte and matrix vesicle microRNA populations. (A) Venn diagram comparing differentially expressed (p-value < 0.05 and absolute log 2-fold change > 1) microRNA found in growth zone chondrocytes and matrix vesicles with 97 microRNA found only in the chondrocytes, 103 only in vesicles, and 185 shared between cells and vesicles. (B) Heatmap of the differentially expressed (p-value < 0.05 and absolute log 2-fold change > 1) microRNA from growth zone chondrocytes with cells on the left and matrix vesicles on the right (n = 3).
Experiments assessing the chondrocyte response to specific microRNAs that are selectively exported in matrix vesicles by GC chondrocytes indicate that they do have direct effects on the cells [38]. To test this, RC and GC chondrocytes were transfected with synthetic mimics of MV microRNAs. miR-122 drove both RC and GC cells toward a proliferative phenotype, stabilized the matrix, and inhibited terminal differentiation. Other MV microRNA also had a regulatory effect on the growth plate chondrocytes. miR-22 increased PTHrP and Ihh production in RC and GC chondrocytes. miR-451 decreased alkaline phosphatase activity (an important factor in tissue mineralization) in both RC and GC cells though to a lesser extent than miR-122. In addition, miR-451 increased OPG production in GC cells [38].
The MV microRNA has been reported to be present in exosomes produced by other cell types. As noted for cancer cells, miR-122 stimulates proliferation [75]. In growth plate cells, this has the effect of increasing cell proliferation, decreasing the production of maturation markers, and producing matrix components characteristic of proliferating cartilage [38]. Transfecting miR-122 into rat articular chondrocyte cells in an in vitro model of osteoarthritis results in increased proliferation and inhibition of inflammatory markers. In contrast, miR-451 stimulates the production of matrix processing enzymes, such as MMP13, which leads to remodeling of the ECM in preparation for calcification [76,77]. Together with the observation that rapid signaling at the MV membrane by vitamin D metabolites can modulate the rate and extent of release of factors from matrix vesicles as well as activate factors such as TGFβ1 and TGFβ2 in the ECM, these new data strongly support the idea that matrix vesicles act as a method to control events in the ECM as well as how cells in the growth plate undergo terminal differentiation [48,65,78,79].
6. Conclusions
Lessons learned from the study of MVs over the last fifty years have shown that they perform multiple functions in ECM-rich tissues such as cartilage and bone. Our early understanding that they provide sites of initial mineral formation was first based on microscopic studies, and biochemical analysis supported the concept that this was their primary role. As technology has progressed, it has become possible to examine other aspects of MV biology, showing us that they can function in an exosome-like manner, carrying microRNAs into the ECM and facilitating the dissemination of information. While this is similar to exosomes, it is distinctly different, underscoring the need to examine the role of these extracellular organelles in the context of the cells and tissues that produce them.
Acknowledgments
The authors thank their students, colleagues, and staff who contributed to this work. The underlying research was funded by the National Institutes of Health, the Department of Defense, and the Price Gilbert, Jr. Foundation.
Abbreviations
| 1α,25(OH)2D3 | 1α,25-dyhydroxyvitamin D3 |
| 24R,25(OH)2D3 | 24R,25-dyhydroxyvitamin D3 |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| AMP | adenosine monophosphate |
| ANK | annexin and ankylin protein |
| ATP | adenosine triphosphate |
| ECM | extracellular matrix |
| ENPP1 | ectonucleotide pyrophosphatase/phosphodiesterase 1 |
| GC | growth zone |
| Ihh | Indian hedgehog |
| MMP | matrix metalloproteinases |
| MV | matrix vesicles |
| PDIA3 | protein disulfide isomerase A3 |
| PGE2 | prostaglandin E2 |
| PHOSPHO1 | phosphoethanolamine/phosphocholine phosphatase 1 |
| PiT1 | sodium/phosphate co-transporter type III |
| PKC | protein kinase C |
| PKCα | PKC-alpha |
| PKCζ | PKC-zeta |
| PLA2 | phospholipase A2 |
| PLC | phospholipase C |
| PLD | phospholipase D |
| PM | plasma membrane |
| PPase | pyrophosphatase |
| PTHrP | parathyroid hormone-related peptide |
| RC | resting zone |
| SNARE | soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| TGFβ | transforming growth factor-beta |
| TIMP | tissue inhibitor of metalloproteinases |
| TNAP | tissue non-specific alkaline phosphatase |
| VDR | vitamin D receptor |
Author Contributions
Conceptualization, B.D.B. and Z.S.; formal analysis, N.C.A.; writing–original draft preparation, B.D.B. and N.C.A.; writing–review and editing, B.D.B.; visualization, N.C.A.; supervision, Z.S.; funding acquisition, B.D.B., Z.L. and Z.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Joan and Morgan Massey Foundation; National Institute of Arthritis and Musculoskeletal and Skin Diseases 1 R01 AR072500; National Institute of Dental and Craniofacial Research R03DE027146.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anderson H.C., Garimella R., Tague S.E. The Role of Matrix Vesicles in Growth Plate Development and Biomineralization. Front. Biosci. 2005;10:822–837. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 2.Becker A., Thakur B.K., Weiss J.M., Kim H.S., Peinado H., Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson H.C. Matrix Vesicles and Calcification. Curr. Rheumatol. Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 4.Bommanavar S., Hosmani J., Togoo R.A., Baeshen H.A., Raj A.T., Patil S., Bhandi S., Birkhed D. Role of Matrix Vesicles and Crystal Ghosts in Bio-Mineralization. J. Bone Miner. Metab. 2020;36:759–764. doi: 10.1007/s00774-020-01125-x. [DOI] [PubMed] [Google Scholar]
- 5.Cecil R.N.A., Clarke Anderson H. Freeze-Fracture Studies of Matrix Vesicle Calcification in Epiphyseal Growth Plate. Metab. Bone Dis. Relat. Res. 1978;1:89–95. doi: 10.1016/0221-8747(78)90043-7. [DOI] [Google Scholar]
- 6.Anderson H.C. Electron Microscopic Studies of Induced Cartilage Development and Calcification. J. Cell Biol. 1967;35:81–101. doi: 10.1083/jcb.35.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson H.C. Vesicles Associated with Calcification in the Matrix of Epiphyseal Cartilage. J. Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boskey A.L. Current Concepts of the Physiology and Biochemistry of Calcification. Clin. Orthop. Relat. Res. 1981;12:225–257. doi: 10.1097/00003086-198106000-00036. [DOI] [PubMed] [Google Scholar]
- 9.Wuthier R.E., Chin J.E., Hale J.E., Register T.C., Hale L.V., Ishikawa Y. Isolation and Characterization of Calcium-Accumulating Matrix Vesicles from Chondrocytes of Chicken Epiphyseal Growth Plate Cartilage in Primary Culture. J. Biol. Chem. 1985;260:15972–15979. doi: 10.1016/S0021-9258(17)36354-8. [DOI] [PubMed] [Google Scholar]
- 10.Miao D., Scutt A. Histochemical Localization of Alkaline Phosphatase Activity in Decalcified Bone and Cartilage. J. Histochem. Cytochem. 2002;50:333–340. doi: 10.1177/002215540205000305. [DOI] [PubMed] [Google Scholar]
- 11.McLean F.M., Keller P.J., Genge B.R., Walters S.A., Wuthier R.E. Disposition of Preformed Mineral in Matrix Vesicles. Internal Localization and Association with Alkaline Phosphatase. J. Biol. Chem. 1987;262:10481–10488. doi: 10.1016/S0021-9258(18)60986-X. [DOI] [PubMed] [Google Scholar]
- 12.Kirsch T., Wuthier R.E. Stimulation of Calcification of Growth Plate Cartilage Matrix Vesicles by Binding to Type II and X Collagens. J. Biol. Chem. 1994;269:11462–11469. doi: 10.1016/S0021-9258(19)78146-0. [DOI] [PubMed] [Google Scholar]
- 13.Dean D.D., Boyan B.D., Muniz O.E., Howell D.S., Schwartz Z. Vitamin D Metabolites Regulate Matrix Vesicle Metalloproteinase Content in a Cell Maturation-Dependent Manner. Calcif. Tissue Int. 1996;59:109–116. doi: 10.1007/s002239900096. [DOI] [PubMed] [Google Scholar]
- 14.Boskey A.L. Mineral-Matrix Interactions in Bone and Cartilage. Clin. Orthop. Relat. Res. 1992:244–274. doi: 10.1097/00003086-199208000-00041. [DOI] [PubMed] [Google Scholar]
- 15.Boyan B.D., Schwartz Z., Carnes D.L., Ramirez V. The Effects of Vitamin D Metabolites on the Plasma and Matrix Vesicle Membranes of Growth and Resting Cartilage Cells in Vitro. Endocrinology. 1988;122:2851–2860. doi: 10.1210/endo-122-6-2851. [DOI] [PubMed] [Google Scholar]
- 16.Purcell P., Trainor P.A. The Mighty Chondrocyte: No Bones about It. J. Dent. Res. 2015;94:1625–1627. doi: 10.1177/0022034515605457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi T., Chung U., Schipani E., Starbuck M., Karsenty G., Katagiri T., Goad D.L., Lanske B., Kronenberg H.M. PTHrP and Indian Hedgehog Control Differentiation of Growth Plate Chondrocytes at Multiple Steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 18.Mak K.K., Kronenberg H.M., Chuang P.-T., Mackem S., Yang Y. Indian Hedgehog Signals Independently of PTHrP to Promote Chondrocyte Hypertrophy. Development. 2008;135:1947–1956. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Eerden B.C.J., Karperien M., Wit J.M. Systemic and Local Regulation of the Growth Plate. Endocr. Rev. 2003;24:782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- 20.Emons J., Chagin A.S., Sävendahl L., Karperien M., Wit J.M. Mechanisms of Growth Plate Maturation and Epiphyseal Fusion. Horm. Res. Paediatr. 2011;75:383–391. doi: 10.1159/000327788. [DOI] [PubMed] [Google Scholar]
- 21.Kronenberg H.M. PTHrP and Skeletal Development. Ann. N. Y. Acad. Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 22.Inada M., Yasui T., Nomura S., Miyake S., Deguchi K., Himeno M., Sato M., Yamagiwa H., Kimura T., Yasui N., et al. Maturational Disturbance of Chondrocytes in Cbfa1-Deficient Mice. Dev. Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.de Frutos C.A., Vega S., Manzanares M., Flores J.M., Huertas H., Martínez-Frías M.L., Nieto M.A. Snail1 Is a Transcriptional Effector of FGFR3 Signaling during Chondrogenesis and Achondroplasias. Dev. Cell. 2007;13:872–883. doi: 10.1016/j.devcel.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Robson H., Siebler T., Stevens D.A., Shalet S.M., Williams G.R. Thyroid Hormone Acts Directly on Growth Plate Chondrocytes to Promote Hypertrophic Differentiation and Inhibit Clonal Expansion and Cell Proliferation. Endocrinology. 2000;141:3887–3897. doi: 10.1210/endo.141.10.7733. [DOI] [PubMed] [Google Scholar]
- 25.Allen M.R., Burr D.B. Basic and Applied Bone Biology. Academic Press; Cambridge, MA, USA: 2014. 4-Bone Modeling and Remodeling; pp. 75–90. [Google Scholar]
- 26.Schwartz Z., Nasatzky E., Ornoy A., Brooks B.P., Soskolne W.A., Boyan B.D. Gender-Specific, Maturation-Dependent Effects of Testosterone on Chondrocytes in Culture. Endocrinology. 1994;134:1640–1647. doi: 10.1210/endo.134.4.8137726. [DOI] [PubMed] [Google Scholar]
- 27.Jonason J.H., Xiao G., Zhang M., Xing L., Chen D. Post-Translational Regulation of Runx2 in Bone and Cartilage. J. Dent. Res. 2009;88:693–703. doi: 10.1177/0022034509341629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grumbles R.M., Shao L., Jeffrey J.J., Howell D.S. Regulation of Rat Interstitial Collagenase Gene Expression in Growth Cartilage and Chondrocytes by Vitamin D3, Interleukin-1 Beta, and Okadaic Acid. J. Cell. Biochem. 1996;63:395–409. doi: 10.1002/(SICI)1097-4644(19961215)63:4<395::AID-JCB2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Sylvia V.L., del Toro F., Dean D.D., Hardin R.R., Schwartz Z., Boyan B.D. Effects of 1α,25-(OH)(2)D(3) on Rat Growth Zone Chondrocytes Are Mediated via Cyclooxygenase-1 and Phospholipase A(2) J. Cell. Biochem. 2001;81((Suppl. 36)):32–45. doi: 10.1002/jcb.1072. [DOI] [PubMed] [Google Scholar]
- 30.Boyan B.D., Sylvia V.L., Dean D.D., Pedrozo H., del Toro F., Nemere I., Posner G.H., Schwartz Z. 1,25-(OH)2D3 Modulates Growth Plate Chondrocytes via Membrane Receptor-Mediated Protein Kinase C by a Mechanism That Involves Changes in Phospholipid Metabolism and the Action of Arachidonic Acid and PGE2. Steroids. 1999;64:129–136. doi: 10.1016/S0039-128X(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz Z., Schlader D.L., Swain L.D., Boyan B.D. Direct Effects of 1,25-Dihydroxyvitamin D3 and 24,25-Dihydroxyvitamin D3 on Growth Zone and Resting Zone Chondrocyte Membrane Alkaline Phosphatase and Phospholipase-A2 Specific Activities. Endocrinology. 1988;123:2878–2884. doi: 10.1210/endo-123-6-2878. [DOI] [PubMed] [Google Scholar]
- 32.Gerstenfeld L.C., Kelly C.M., von Deck M., Lian J.B. Comparative Morphological and Biochemical Analysis of Hypertrophic, Non-Hypertrophic and 1,25(OH)2D3 Treated Non-Hypertrophic Chondrocytes. Connect. Tissue Res. 1990;24:29–39. doi: 10.3109/03008209009152420. [DOI] [PubMed] [Google Scholar]
- 33.Kawai S., Michikami I., Kitagaki J., Hata K., Kiyonari H., Abe T., Amano A., Wakisaka S. Syntaxin 4a Regulates Matrix Vesicle-Mediated Bone Matrix Production by Osteoblasts. J. Bone Miner. Res. 2017;32:440–448. doi: 10.1002/jbmr.3056. [DOI] [PubMed] [Google Scholar]
- 34.Wu L.N., Genge B.R., Wuthier R.E. Association between Proteoglycans and Matrix Vesicles in the Extracellular Matrix of Growth Plate Cartilage. J. Biol. Chem. 1991;266:1187–1194. doi: 10.1016/S0021-9258(17)35300-0. [DOI] [PubMed] [Google Scholar]
- 35.Dean D.D., Schwartz Z., Muniz O.E., Gomez R., Swain L.D., Howell D.S., Boyan B.D. Matrix Vesicles Are Enriched in Metalloproteinases That Degrade Proteoglycans. Calcif. Tissue Int. 1992;50:342–349. doi: 10.1007/BF00301632. [DOI] [PubMed] [Google Scholar]
- 36.Dean D.D., Schwartz Z.V., Muniz O.E., Gomez R., Swain L.D., Howell D.S., Boyan B.D. Matrix Vesicles Contain Metalloproteinases That Degrade Proteoglycans. Bone Miner. 1992;17:172–176. doi: 10.1016/0169-6009(92)90731-R. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura R., Wakabayashi M., Hata K., Matsubara T., Honma S., Wakisaka S., Kiyonari H., Shioi G., Yamaguchi A., Tsumaki N., et al. Osterix Regulates Calcification and Degradation of Chondrogenic Matrices through Matrix Metalloproteinase 13 (MMP13) Expression in Association with Transcription Factor Runx2 during Endochondral Ossification. J. Biol. Chem. 2012;287:33179–33190. doi: 10.1074/jbc.M111.337063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asmussen N.C., Cohen D.J., Lin Z., McClure M.J., Boyan B.D., Schwartz Z. Specific MicroRNAs Found in Extracellular Matrix Vesicles Regulate Proliferation and Differentiation in Growth Plate Chondrocytes. Calcif. Tissue Int. 2021;109:455–468. doi: 10.1007/s00223-021-00855-y. [DOI] [PubMed] [Google Scholar]
- 39.Wuthier R.E. Lipid Composition of Isolated Epiphyseal Cartilage Cells, Membranes and Matrix Vesicles. BBA-Lipids Lipid Metab. 1975;409:128–143. doi: 10.1016/0005-2760(75)90087-9. [DOI] [PubMed] [Google Scholar]
- 40.Majeska R.J., Holwerda D.L., Wuthier R.E. Localization of Phosphatidylserine in Isolated Chick Epiphyseal Cartilage Matrix Vesicles with Trinitrobenzenesulfonate. Calcif. Tissue Int. 1979;27:41–46. doi: 10.1007/BF02441159. [DOI] [PubMed] [Google Scholar]
- 41.Wuthier R.E., Lipscomb G.F. Matrix Vesicles: Structure, Composition, Formation and Function in Calcification. Front. Biosci. 2011;16:2812–2902. doi: 10.2741/3887. [DOI] [PubMed] [Google Scholar]
- 42.Cui L., Houston D.A., Farquharson C., MacRae V.E. Characterisation of Matrix Vesicles in Skeletal and Soft Tissue Mineralisation. Bone. 2016;87:147–158. doi: 10.1016/j.bone.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Boyan B.D., Schwartz Z., Swain L.D., Khare A. Role of Lipids in Calcification of Cartilage. Anat. Rec. 1989;224:211–219. doi: 10.1002/ar.1092240210. [DOI] [PubMed] [Google Scholar]
- 44.Majeska R.J., Wuthier R.E. Studies on Matrix Vesicles Isolated from Chick Epiphyseal Cartilage. Association of Pyrophosphatase and ATPase Activities with Alkaline Phosphatase. BBA-Enzymol. 1975;391:51–60. doi: 10.1016/0005-2744(75)90151-5. [DOI] [PubMed] [Google Scholar]
- 45.Boskey A.L., Boyan B.D., Doty S.B., Feliciano A., Greer K., Weiland D., Swain L.D., Schwartz Z. Studies of Matrix Vesicle-Induced Mineralization in a Gelatin Gel. Bone Miner. 1992;17:257–262. doi: 10.1016/0169-6009(92)90747-2. [DOI] [PubMed] [Google Scholar]
- 46.Gay C.V., Anderson R.E., Leach R.M. Activities and Distribution of Alkaline Phosphatase and Carbonic Anhydrase in the Tibial Dyschondroplastic Lesion and Associated Growth Plate of Chicks. Avian Dis. 1985;29:812–821. doi: 10.2307/1590672. [DOI] [PubMed] [Google Scholar]
- 47.Millán J.L. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif. Tissue Int. 2013;93:299–306. doi: 10.1007/s00223-012-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyan B.D., Wong K.L., Fang M., Schwartz Z. 1α,25(OH)2D3 Is an Autocrine Regulator of Extracellular Matrix Turnover and Growth Factor Release via ERp60 Activated Matrix Vesicle Metalloproteinases. J. Steroid Biochem. Mol. Biol. 2007;103:467–472. doi: 10.1016/j.jsbmb.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golub E.E. Role of Matrix Vesicles in Biomineralization. Biochim. Biophys. Acta. 2009;1790:1592–1598. doi: 10.1016/j.bbagen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav M.C., Simão A.M.S., Narisawa S., Huesa C., McKee M.D., Farquharson C., Millán J.L. Loss of Skeletal Mineralization by the Simultaneous Ablation of PHOSPHO1 and Alkaline Phosphatase Function: A Unified Model of the Mechanisms of Initiation of Skeletal Calcification. J. Bone Miner. Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balcerzak M., Malinowska A., Thouverey C., Sekrecka A., Dadlez M., Buchet R., Pikula S. Proteome Analysis of Matrix Vesicles Isolated from Femurs of Chicken Embryo. Proteomics. 2008;8:192–205. doi: 10.1002/pmic.200700612. [DOI] [PubMed] [Google Scholar]
- 52.Narisawa S., Yadav M.C., Millán J.L. In Vivo Overexpression of Tissue-Nonspecific Alkaline Phosphatase Increases Skeletal Mineralization and Affects the Phosphorylation Status of Osteopontin. J. Bone Miner. Res. 2013;28:1587–1598. doi: 10.1002/jbmr.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasegawa T., Yamamoto T., Tsuchiya E., Hongo H., Tsuboi K., Kudo A., Abe M., Yoshida T., Nagai T., Khadiza N., et al. Ultrastructural and Biochemical Aspects of Matrix Vesicle-Mediated Mineralization. Jpn. Dent. Sci. Rev. 2017;53:34–45. doi: 10.1016/j.jdsr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz Z., Knight G., Swain L.D., Boyan B.D. Localization of Vitamin D3-Responsive Alkaline Phosphatase in Cultured Chondrocytes. J. Biol. Chem. 1988;263:6023–6026. doi: 10.1016/S0021-9258(18)68743-5. [DOI] [PubMed] [Google Scholar]
- 55.Boyan B.D., Schwartz Z., Swain L.D., Carnes D.L., Zislis T. Differential Expression of Phenotype by Resting Zone and Growth Region Costochondral Chondrocytes in Vitro. Bone. 1988;9:185–194. doi: 10.1016/8756-3282(88)90008-7. [DOI] [PubMed] [Google Scholar]
- 56.Leach R.J., Schwartz Z., Johnson-Pais T.L., Dean D.D., Luna M., Boyan B.D. Osteosarcoma Hybrids Can Preferentially Target Alkaline Phosphatase Activity to Matrix Vesicles: Evidence for Independent Membrane Biogenesis. J. Bone Miner. Res. 1995;10:1614–1624. doi: 10.1002/jbmr.5650101103. [DOI] [PubMed] [Google Scholar]
- 57.Shapiro I.M., Landis W.J., Risbud M.V. Matrix Vesicles: Are They Anchored Exosomes? Bone. 2015;79:29–36. doi: 10.1016/j.bone.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds J.L., Joannides A.J., Skepper J.N., McNair R., Schurgers L.J., Proudfoot D., Jahnen-Dechent W., Weissberg P.L., Shanahan C.M. Human Vascular Smooth Muscle Cells Undergo Vesicle-Mediated Calcification in Response to Changes in Extracellular Calcium and Phosphate Concentrations: A Potential Mechanism for Accelerated Vascular Calcification in ESRD. J. Am. Soc. Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 59.Lin Z., McClure M.J., Zhao J., Ramey A.N., Asmussen N., Hyzy S.L., Schwartz Z., Boyan B.D. MicroRNA Contents in Matrix Vesicles Produced by Growth Plate Chondrocytes Are Cell Maturation Dependent. Sci. Rep. 2018;8:3609. doi: 10.1038/s41598-018-21517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz Z., Shaked D., Hardin R.R., Gruwell S., Dean D.D., Sylvia V.L., Boyan B.D. 1α,25(OH)2D3 Causes a Rapid Increase in Phosphatidylinositol-Specific PLC-Beta Activity via Phospholipase A2-Dependent Production of Lysophospholipid. Steroids. 2003;68:423–437. doi: 10.1016/S0039-128X(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz N., Verma A., Bivens C.B., Schwartz Z., Boyan B.D. Rapid Steroid Hormone Actions via Membrane Receptors. BBA-Mol. Cell Res. 2016;1863:2289–2298. doi: 10.1016/j.bbamcr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Dean D.D., Boyan B.D., Schwart Z., Muniz O.E., Carreno M.R., Maeda S., Howell D.S. Effect of 1α,25-Dihydroxyvitamin D3 and 24R,25-Dihydroxyvitamin D3 on Metalloproteinase Activity and Cell Maturation in Growth Plate Cartilage in Vivo. Endocrine. 2001;14:311–323. doi: 10.1385/ENDO:14:3:311. [DOI] [PubMed] [Google Scholar]
- 63.Boyan B.D., Sylvia V.L., Dean D.D., Schwartz Z. Encyclopedia of Hormones. Academic Press; Cambridge, MA, USA: 2003. Vitamin D and Cartilage; pp. 592–598. [Google Scholar]
- 64.Boyan B.D., Sylvia V.L., McKinney N., Schwartz Z. Membrane Actions of Vitamin D Metabolites 1α,25(OH)2D3 and 24R,25(OH)2D3 Are Retained in Growth Plate Cartilage Cells from Vitamin D Receptor Knockout Mice. J. Cell. Biochem. 2003;90:1207–1223. doi: 10.1002/jcb.10716. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz Z., Sylvia V.L., Larsson D., Nemere I., Casasola D., Dean D.D., Boyan B.D. 1α,25(OH)2D3 Regulates Chondrocyte Matrix Vesicle Protein Kinase C (PKC) Directly via G-Protein-Dependent Mechanisms and Indirectly via Incorporation of PKC during Matrix Vesicle Biogenesis. J. Biol. Chem. 2002;277:11828–11837. doi: 10.1074/jbc.M110398200. [DOI] [PubMed] [Google Scholar]
- 66.Boyan B.D., Wang L., Wong K.L., Jo H., Schwartz Z. Plasma Membrane Requirements for 1α,25(OH)2D3 Dependent PKC Signaling in Chondrocytes and Osteoblasts. Steroids. 2006;71:286–290. doi: 10.1016/j.steroids.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 67.Boyan B.D., Schwartz Z., Park-Snyder S., Dean D.D., Yang F., Twardzik D., Bonewald L.F. Latent Transforming Growth Factor-Beta Is Produced by Chondrocytes and Activated by Extracellular Matrix Vesicles upon Exposure to 1,25-(OH)2D3. J. Biol. Chem. 1994;269:28374–28381. doi: 10.1016/S0021-9258(18)46938-4. [DOI] [PubMed] [Google Scholar]
- 68.Ortega N., Behonick D.J., Werb Z. Matrix Remodeling during Endochondral Ossification. Trends Cell Biol. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Angelo M., Billings P.C., Pacifici M., Leboy P.S., Kirsch T. Authentic Matrix Vesicles Contain Active Metalloproteases (MMP): A Role for Matrix Vesicle-Associated MMP-13 in Activation of Transforming Growth Factor-B. J. Biol. Chem. 2001;276:11347–11353. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- 70.Lin Z., Rodriguez N.E., Zhao J., Ramey A.N., Hyzy S.L., Boyan B.D., Schwartz Z. Selective Enrichment of MicroRNAs in Extracellular Matrix Vesicles Produced by Growth Plate Chondrocytes. Bone. 2016;88:47–55. doi: 10.1016/j.bone.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Minamizaki T., Nakao Y., Irie Y., Ahmed F., Itoh S., Sarmin N., Yoshioka H., Nobukiyo A., Fujimoto C., Niida S., et al. The Matrix Vesicle Cargo MiR-125b Accumulates in the Bone Matrix, Inhibiting Bone Resorption in Mice. Commun. Biol. 2020;3:30. doi: 10.1038/s42003-020-0754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slavkin H.C., Bringas P., Bavetta L.A. Ribonucleic Acid within the Extracellular Matrix during Embryonic Tooth Formation. J. Cell. Physiol. 1969;73:179–190. doi: 10.1002/jcp.1040730304. [DOI] [PubMed] [Google Scholar]
- 73.Collino F., Deregibus M.C., Bruno S., Sterpone L., Aghemo G., Viltono L., Tetta C., Camussi G. Microvesicles Derived from Adult Human Bone Marrow and Tissue Specific Mesenchymal Stem Cells Shuttle Selected Pattern of MiRNAs. PLoS ONE. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boyan B.D., Sylvia V.L., Dean D.D., del Toro F., Schwartz Z. Differential Regulation of Growth Plate Chondrocytes by 1,25-(OH)2D3 and 24R,25-(OH)2D3 Involves Cell-Maturation-Specific Membrane-Receptor-Activated Phospholipid Metabolism. Crit. Rev. Oral Biol. Media. 2002;13:143–154. doi: 10.1177/154411130201300205. [DOI] [PubMed] [Google Scholar]
- 75.Lian J.-H., Wang W.-H., Wang J.-Q., Zhang Y.-H., Li Y. MicroRNA-122 Promotes Proliferation, Invasion and Migration of Renal Cell Carcinoma Cells through the PI3K/Akt Signaling Pathway. Asian Pac. J. Cancer Prev. 2013;14:5017–5021. doi: 10.7314/APJCP.2013.14.9.5017. [DOI] [PubMed] [Google Scholar]
- 76.Scott K.M., Cohen D.J., Hays M., Nielson D.W., Grinstaff M.W., Lawson T.B., Snyder B.D., Boyan B.D., Schwartz Z. Regulation of Inflammatory and Catabolic Responses to IL-1β in Rat Articular Chondrocytes by MicroRNAs MiR-122 and MiR-451. Osteoarthr. Cartil. 2021;29:113–123. doi: 10.1016/j.joca.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Scott K.M., Cohen D.J., Boyan B.D., Schwartz Z. MiR-122 and the WNT/β-Catenin Pathway Inhibit Effects of Both Interleukin-1β and Tumor Necrosis Factor-α in Articular Chondrocytes in Vitro. J. Cell Biochem. 2022:1–11. doi: 10.1002/jcb.30244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maeda S., Dean D.D., Gay I., Schwartz Z., Boyan B.D. Activation of Latent Transforming Growth Factor Beta1 by Stromelysin 1 in Extracts of Growth Plate Chondrocyte-Derived Matrix Vesicles. J. Bone Miner. Res. 2001;16:1281–1290. doi: 10.1359/jbmr.2001.16.7.1281. [DOI] [PubMed] [Google Scholar]
- 79.Gay I., Schwartz Z., Sylvia V.L., Boyan B.D. Lysophospholipid Regulates Release and Activation of Latent TGF-Beta1 from Chondrocyte Extracellular Matrix. BBA-Mol. Cell Biol. Lipids. 2004;1684:18–28. doi: 10.1016/j.bbalip.2004.04.006. [DOI] [PubMed] [Google Scholar]