Abstract
Simple Summary
Standard treatment protocol for PIPAC consists of three procedures and completion of treatment has been shown to be prognostic of improved survival. The aim of this study was to identify predictors for completion of treatment. This retrospective multicentric cohort study included all patients with peritoneal metastases undergoing PIPAC at three PIPAC expert centers. Overall, 183 patients had 517 PIPACs. Bimodal treatment was found as an independent predictive factor for completing the three procedures (OR = 4.202, 95%CI [1.813, 10.630], p < 0.001), as well as prior bowel obstruction (OR = 0.389, 95%CI [0.153, 0.920], p = 0.037). In conclusion, the absence of ascites and prior bowel obstruction can help to select patients suitable for PIPAC. Best results seem to be achieved when PIPAC is combined with systemic chemotherapy.
Abstract
Background: The standard treatment protocol for PIPAC consists of three procedures. Completion of treatment has been shown to be prognostic of improved survival. The aim of this study was to identify predictors for completion of treatment. Methods: Retrospective multicentric cohort study of patients with peritoneal metastases undergoing PIPAC in three PIPAC expert centers. Per protocol (PP) treatment was defined as patients receiving ≥3 PIPACs and was compared to patients receiving <3. Results: Overall, 183 patients had 517 PIPACs. The main reasons for stopping PIPAC were disease progression in 50% patients, bowel obstruction in 15%, patient’s refusal to pursue in 10%, conversion to cytoreductive surgery in 7%, and medical reasons in 8%. Overall, 95 patients (52%) had PP treatment. The PP median OS was 17 vs. 7 months, p = 0.001. PP patients had r ascites (410 ± 100 mL vs. 960 ± 188 mL, p = 0.001), no prior history of bowel obstruction (12% vs. 24%, p = 0.028), and more bimodal treatment (39% vs. 13%, p < 0.001). After multiple regression, bimodal treatment was found as an independent predictive factor for completing PP (OR = 4.202, 95%CI [1.813, 10.630], p < 0.001), along with prior bowel obstruction (OR = 0.389, 95%CI [0.153, 0.920], p = 0.037). Conclusion: The absence of ascites and prior bowel obstruction can help to select patients suitable for PIPAC. Best results seem to be achieved when PIPAC is combined with systemic chemotherapy.
Keywords: PIPAC, peritoneal cancer, carcinomatosis, peritoneal surface malignancies, peritoneal metastases
1. Introduction
Peritoneal metastasis (PM) remains a therapeutic challenge with ominous prognosis mainly due to poor response to systemic chemotherapy [1,2,3]. Intraperitoneal chemotherapy delivery has been proposed as an alternative therapeutic option to enhance drug concentrations in tissue and to reduce systemic toxicity [4].
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) was introduced in 2011 as an innovative intraperitoneal drug delivery method in several experimental and clinical studies [5,6,7]. The actual standard treatment consists of three PIPAC procedures planned every 4–6 weeks in association with systemic chemotherapy and can be pursued depending on tolerance and treatment response [8,9]. Recent prospective and retrospective cohort studies show good tolerance of repeated PIPAC treatment and the rare occurrence of intraoperative and postoperative complications [6,10,11,12].
Preliminary oncological results show encouraging tumoral response to PIPAC after the completion of three applications [13,14]. However, many patients do not complete the full treatment course and, for various reasons, have to stop after only one or two procedures, hence receiving only limited benefit [15]. We aimed to investigate predictive factors of PIPAC treatment discontinuation in order to better select patients who are most likely to benefit from a complete PIPAC treatment course.
2. Materials and Methods
This is a retrospective multicentric cohort study including consecutive patients undergoing PIPAC treatment from January 2015 to January 2020 in Lausanne University Hospital (CHUV), Switzerland; Dupuytren Limoges University Hospital, France; and Montpellier Cancer Institute (ICM), France. Patients with peritoneal metatases (PM) form various origins (ovarian, colorectal, gastric, hepato-pancreato-biliary (HPB), and mesothelioma) were included and all indications for PIPAC treatment were decided in a multidisciplinary tumor board in line with current practice consensus [6]. The exclusion criteria were age <18 years old and patient’s refusal to participate.
Per protocol (PP) treatment was defined as patients receiving ≥3 PIPACs and was compared to patients with 1 or 2 PIPACs (<3 PIPAC group).
2.1. Outcomes
Reasons to stop PIPAC were stratified into 10 groups: intraperitoneal progression, extraperitoneal progression, bowel obstruction, patient’s refusal to pursue, conversion to curative cytoreductive surgery, non-access, absence of disease, PIPAC complication, death, or other medical reasons. Other medical reason included, for example, infections, pulmonary embolism, or myocardial infarction.
Baseline variables (demographics, previous systemic IV chemotherapy, symptoms before PIPAC, surgical details, and postoperative complications according to Clavien-Dindo [16]) were compared between the two groups to investigate factors which could predict discontinuation of treatment.
2.2. Data Management
Demographic, surgical, and oncological data were retrieved from prospectively maintained institutional databases. The following variables were extracted: gender, age, primary tumor origin, ASA score, ECOG Performance Status Scale [17], number of previous lines and cycles of systemic chemotherapy, presence of symptoms before PIPAC (abdominal pain, ascites, obstructive symptoms, nausea), Peritoneal Cancer Index (PCI) [18], Peritoneal Regression Grading Score (PRGS) [19], postoperative complications [16], and overall survival. Ascites volume was measured at PIPAC#1, based on the intraoperative aspirates. Bimodal treatment was defined as the patient being under systemic chemotherapy in the interval between PIPACs. Median PRGS was calculated from the scores of biopsies taken during each individual PIPAC.
2.3. PIPAC Procedure and Safety Considerations
The PIPAC procedure has been described previously and was applied according to current recommendations and safety protocols [9,20]. Oxaliplatin was applied at a dose of 92 mg/m2 for carcinomatosis of mostly colorectal origin and, selectively, for other digestive origins (gastric or pancreatic cancer). Cisplatin (7.5 mg/m2) in combination with doxorubicin (1.5 mg/m2), with dose adaptation (10.5 mg/m2 and 2.1 mg/m2) since 2019, was applied for the remaining cases [21].
2.4. Statistical Analysis
Continuous variables were presented as mean with standard deviation (SD) or median with interquartile range (IQR) according to their distribution. Categorical variables were reported as frequencies (%) and compared with chi-square test. Student’s t-test or Mann–Whitney test were used to compare continuous variables. Multivariable analyses were performed by using a multiple logistic regression integrating variables with univariate p-values ≤0.1. Kaplan–Meier survival curves were used to analyze time-to-event data and to compare two groups of subjects. All statistical tests were two-sided and a p-value of <0.05 was used to indicate statistical significance. Statistical analyses were performed with GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
In total, 517 procedures were performed for 183 patients; 53 patients had only 1 PIPAC (29%), 35 had 2 PIPACs (19%), 60 had 3 PIPACs (33%) and 35 had >3 PIPACs (19%). Ninety-five patients (52%) had completed PP treatment. PM origin was ovarian in 59 (32%) patients, colorectal in 55 (30%), gastric in 37 (20%), HPB in 18 (10%), and mesothelioma in 14 (8%). No difference was observed between the two groups regarding PM origin (p = 0.52). Overall median survival was longer in the PP group compared to the <3 PIPAC group (16 vs. 7.2 months, p = <0.001).
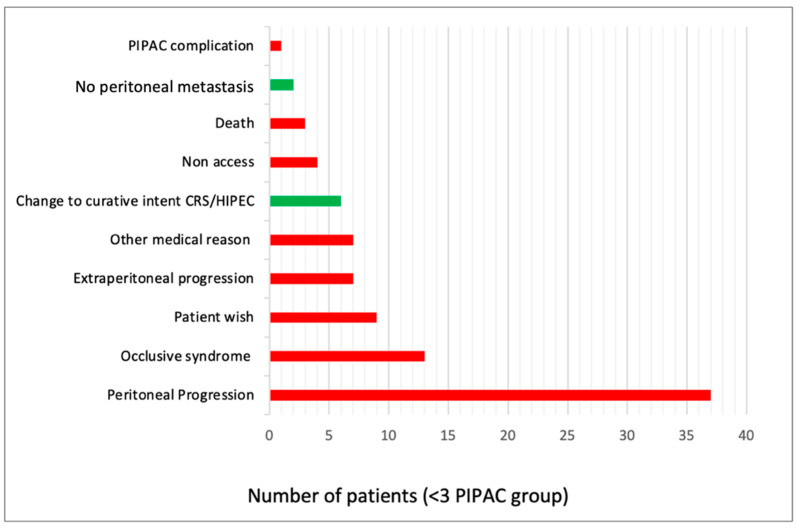
The main reason for interrupting PIPAC treatment was oncological progression, in 44 (50%) patients (42% intraperitoneal and 8% extraperitoneal). All reasons for PIPAC interruption are described in Figure 1.
Figure 1.
Reasons for PIPAC interruption before PIPAC#3. Data presented as number of patients. PIPAC—Pressurized IntraPeritoneal Aerosol Chemotherapy. CRS/HIPEC—Cytoreductive Surgery/Hyperthermic Intraperitoneal Chemotherapy.
The absence of prior history of bowel obstruction before PIPAC1, lower volume of ascites (<500 mL) retrieved during the first procedure (PIPAC1), and bimodal treatment were associated with completion of PIPAC treatment (p = 0.028, p = 0.001, and p < 0.001, respectively) (Table 1). After multiple regression, bimodal treatment was found as an independent predictive factor for completing PP treatment (OR = 4.202, 95%CI [1.813, 10.630], p < 0.001), along with prior bowel obstruction (OR = 0.389, 95%CI [0.153, 0.920], p = 0.037).
Table 1.
Potential predictors for completion of PIPAC treatment.
| ° | <3 PIPAC Procedures n = 88 Patients |
≥3 Procedures (PP) n = 95 Patients |
p-Value |
|---|---|---|---|
| Demographics | |||
| Gender male, n (%) | 28 (32) | 38 (40) | 0.632 |
| Mean age (IQR) | 65 (55–72) | 63 (53–71) | 0.638 |
| ASA III–IV, n (%) | 41 (47) | 38 (40) | 0.371 |
| ECOG | 0.189 | ||
| 0–1, n (%) | 53 (60) | 66 (70) | |
| ≥2, n (%) | 35 (40) | 29 (30) | |
| Previous systemic chemotherapy | |||
| ≥3 lines, n (%) | 29 (33) | 35 (37) | 0.644 |
| ≥12 cycles, n (%) | 27 (31) | 40 (42) | 0.134 |
| Symptoms before PIPAC procedures | |||
| Abdominal pain, n (%) | 22 (25) | 19 (20) | 0.472 |
| Ascites, n (%) | 24 (27) | 18 (19) | 0.219 |
| Prior bowel obstruction (ileus), n (%) | 21 (24) | 11 (12) | 0.028 |
| Nausea, n (%) | 14 (16) | 7 (7) | 0.150 |
| Surgical details | |||
| Median PCI (IQR) at PIPAC#1 | 19 (10–29) | 18 (9–25) | 0.213 |
| Mean ascites (mL) (SD), at PIPAC#1 | 960 (188) | 410 (100) | 0.001 |
| Intraperitoneal chemotherapy regimen | 0.640 | ||
| Oxaliplatin, n (%) | 25 (28) | 30 (32) | |
| Cisplatin + Doxorubicin, n (%) | 63 (72) | 65 (68) | |
| Bimodal treatment, n (%) | 11 (13) | 37 (39) | <0.001 |
| Postoperative complications | |||
| Overall, n (%) | 31 (35) | 42 (44) | 0.210 |
| Severe compilation III-IV, n (%) | 7 (8) | 5 (5) | 0.462 |
Median (IQR—interquartile range or range), mean (SD—standard deviation), or number (%) as appropriate. Statistical significance (p < 0.05) is highlighted in bold. ASA: American Association of Anesthesiologists physical status classification system. ECOG Performance Status Scale [17]. PCI—Peritoneal Cancer Index [18].
Prior history of bowel obstruction and bimodal treatment were found as independent predictive factors of the discontinuation of PIPAC after multivariable logistic regression (OR = 0.389, 95%CI [0.153, 0.920], p = 0.037) and (OR = 4.202, 95%CI [1.813, 10.630], p < 0.001), respectively (Table 2).
Table 2.
Multivariable logistic regression analysis correlating with completion of the per protocol treatment.
| Baseline Variable | OR | 95% CI for OR | p-Value |
|---|---|---|---|
| Prior bowel obstruction (yes vs. no) | 0.389 | 0.153 to 0.920 | 0.037 |
| Ascites ≥ 500 mL (yes vs. no) | 0.649 | 0.304 to 1.35 | 0.254 |
| Bimodal treatment (yes vs. no) | 4.281 | 1.851 to 10.79 | 0.001 |
After univariate analysis, p-values ≤ 0.1 were incorporated in the multivariable analysis. Statistical significance (p < 0.05) is highlighted in bold. OR: odds ratio.
Median PRGS at baseline was comparable in the two groups at PIPAC#1, 2 (IQR: 1.2–3.2) for the PP group and 2 (IQR: 2–2) for the <3 PIPACs group (p = 0.145). The mean PRGS score was lower in the PP group at PIPAC#3 compared to patients in the <3 PIPACs group at PIPAC#2, with 1 (IQR: 1–1.25) vs. 2 (IQR: 1–3), respectively (p = 0.009).
4. Discussion
The absence of ascites and prior bowel obstruction increases the chances of completing the three PIPAC procedures and best results seem to be achieved when PIPAC is combined with systemic chemotherapy. The association between better overall survival and complete PIPAC treatment is encouraging, but this finding should be taken with caution due to possible selection bias. Optimal patient selection appears mandatory, and caution is warranted in patients with obstructive symptoms, abundant ascites, and limited life expectancy.
This study confirmed the feasibility and safety of PIPAC treatment [6]. In fact, less than 3% of patients had to stop their treatment directly because of a technical problem during PIPAC (non-access, n = 4/183) or directly caused by the procedure (postoperative complications, n = 1/88). Of note, 76 (42%) patients in our series had prior major surgery (including 11 cytoreductive surgery + HIPEC). Although our study is modest in numbers, this point is important to note and can support the evaluation of PIPAC as a neoadjuvant or an adjuvant prophylactic treatment [22,23,24].
The early discontinuation of the planned protocol is a regularly reported problem for PIPAC as many retrospective series have a median number of 2.5 PIPAC/patient [25].
Oncologic progression remains the main reason for interruption of PIPAC treatment in half of the patients; this can be partially explained by a negative selection bias. Indeed, in the majority of published series [6,7], most patients receiving PIPAC are in palliative situations with advanced, aggressive, and refractory disease. However, we were not able to highlight a link between the oncological disease history and PIPAC treatment discontinuation. Neither PM origin, intraoperative tumor load (PCI), nor the amount of previous systemic chemotherapy (lines or cycles) seemed to play a role. Our findings suggest that an ascites-producing peritoneal disease might be possibly linked to early termination of PIPAC therapy. The presence of malignant ascites has already been reported to be associated with poorer prognosis [26]. Intraperitoneal and extraperitoneal progression underlined in our series supports the rigorous monitoring of the disease progression during the exploration phase of the PIPAC procedure, along with current recommendations [9,27]. In line with our results, the use of bimodal treatment, combining PIPAC with systemic chemotherapy might be a good option to limit tumoral progression. While bimodal or bidirectional treatment is regularly practiced and reported as feasible and safe [28,29], no clear data on a survival benefit could be confirmed up to date. Further evidence arising from large registry data is expected in the future [30,31].
Our results did not reveal any difference regarding general condition (ASA score) and performance status (ECOG scale) in patients with PP treatment or early termination. Furthermore, the proportion of patients with an ECOG ≥2 was similar between the two groups. This can be explained by the fact that PIPAC is mostly well-tolerated, with no negative impact on quality of life, and is thus applicable repeatedly even in patients with a certain degree of functional impairment [10]. In contrast, severe functional impairment (ECOG 3–4) is still currently a relative contra-indication to PIPAC treatment [32].
Nine patients (10%) decided to stop the treatment prematurely. No clear reason could be identified. Fear of surgery or general anesthesia and refusal of repeated hospitalizations were mentioned, but not systematically. The proportion of patients willing to withdraw PIPAC therapy found in our study is comparable to studies evaluating the discontinuation of systemic chemotherapy (10–20%) [33]. In a palliative setting, the patient’s decision to withdraw chemotherapy, whatever its administration route, is multifactorial and encompasses broader psychologic, physiologic, and personal aspects that are beyond the scope of the present study. PIPAC is probably similarly tolerated to systemic chemotherapy.
No correlation was found between pathological response to systemic chemotherapy at baseline and treatment discontinuation. Baseline PRGS was comparable in the two groups at PIPAC#1. Patients who completed PP treatment showed a better pathological response with a significantly lower PRGS and longer OS. The same observations were made regarding PIPAC for PM of appendicular origin in recent reports [14].
The current study has some limitations; these are mainly related to its retrospective nature and limited patient number. Small differences between the comparative groups might have passed undetected due to type II error. Although the two groups were comparable for ASA score, ECOG, and baseline symptoms, heterogeneity in previously administrated treatments and disease presentation are further limiting factors. Therefore, it would be suitable to extend this study to a larger group of patients with more selective inclusion criteria.
5. Conclusions
In conclusion, the reasons for stopping are multifold. Benefiting from a bimodal treatment and the absence of prior bowel obstruction before PIPAC1 increases the chances of completing the three procedures. Treatment completion is associated with better prognosis, although at this stage a direct relationship between PIPAC and overall survival cannot be established. The first published results of cohort studies on overall survival are still pending.
There is a need for further investigations in order to allow proper patient selection with good care and precise criteria. The elaboration of a predictive score for complete PIPAC treatment is part of the ongoing international PIPAC cohort study and could allow better patient selection criteria in the future.
Acknowledgments
This original work was presented as a poster at the 2nd Congress of the International Society for the Study of Pleura and Peritoneum (ISSPP) on 7–8 October 2021 in Roma, Italy. The abstract is published in Pleura Peritoneum (https://www.degruyter.com/document/doi/10.1515/pp-2021-0141/html (accessed on: 2 February 2022)).
Author Contributions
A.B., D.C., L.T., O.S., A.T., M.H. and H.T.F. meet all the criteria for the definition of authorship and contributed substantially to the manuscript; H.T.F. and M.H. conceived and designed the study; A.B., L.T. and H.T.F. managed the data and analyzed the data. H.T.F. is the corresponding author. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Informed written consent was obtained for all participants and the study was approved by the Ethics Committee of canton de Vaud (CER-VD), Lausanne, Switzerland: #2019-00747.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
MH: ENCARE: Consultant fee (institution); Nestlé: Research funding. Capnomed: Sponsoring of scientific meetings; MSD, Fresenius: Speaker honorary (institution); ERAS society: Board member, chair education; ISSPP: Board member, chair education. A.B., D.C., L.T., O.S., A.T. and H.T.F. declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lemmens V.E., Klaver Y.L., Verwaal V.J., Rutten H.J., Coebergh J.W., de Hingh I.H. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: A population-based study. Int. J. Cancer. 2011;128:2717–2725. doi: 10.1002/ijc.25596. [DOI] [PubMed] [Google Scholar]
- 2.Sasson A.R., Kim J. Many Challenges of Peritoneal Carcinomatosis. J. Oncol. Pract. 2017;13:435–436. doi: 10.1200/JOP.2017.024588. [DOI] [PubMed] [Google Scholar]
- 3.Segelman J., Granath F., Holm T., Machado M., Mahteme H., Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012;99:699–705. doi: 10.1002/bjs.8679. [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker P.H. Peritoneal carcinomatosis: Natural history and rational therapeutic interventions using intraperitoneal chemotherapy. Cancer Treat. Res. 1996;81:149–168. doi: 10.1007/978-1-4613-1245-1_13. [DOI] [PubMed] [Google Scholar]
- 5.Solass W., Kerb R., Murdter T., Giger-Pabst U., Strumberg D., Tempfer C., Zieren J., Schwab M., Reymond M.A. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: First evidence for efficacy. Ann. Surg. Oncol. 2014;21:553–559. doi: 10.1245/s10434-013-3213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alyami M., Hubner M., Grass F., Bakrin N., Villeneuve L., Laplace N., Passot G., Glehen O., Kepenekian V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019;20:e368–e377. doi: 10.1016/S1470-2045(19)30318-3. [DOI] [PubMed] [Google Scholar]
- 7.Grass F., Vuagniaux A., Teixeira-Farinha H., Lehmann K., Demartines N., Hubner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br. J. Surg. 2017;104:669–678. doi: 10.1002/bjs.10521. [DOI] [PubMed] [Google Scholar]
- 8.Sgarbura O., Villeneuve L., Alyami M., Bakrin N., Torrent J.J., Eveno C., Hubner M., ISSPP PIPAC Study Group Current practice of pressurized intraperitoneal aerosol chemotherapy (PIPAC): Still standardized or on the verge of diversification? Eur J. Surg. Oncol. 2021;47:149–156. doi: 10.1016/j.ejso.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Hubner M., Grass F., Teixeira-Farinha H., Pache B., Mathevet P., Demartines N. Pressurized IntraPeritoneal Aerosol Chemotherapy—Practical aspects. Eur. J. Surg. Oncol. 2017;43:1102–1109. doi: 10.1016/j.ejso.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira Farinha H., Grass F., Kefleyesus A., Achtari C., Romain B., Montemurro M., Demartines N., Hubner M. Impact of Pressurized Intraperitoneal Aerosol Chemotherapy on Quality of Life and Symptoms in Patients with Peritoneal Carcinomatosis: A Retrospective Cohort Study. Gastroenterol. Res. Pract. 2017;2017:4596176. doi: 10.1155/2017/4596176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taibi A., Sgarbura O., Hubner M., Bardet S.M., Alyami M., Bakrin N., Durand Fontanier S., Eveno C., Gagniere J., Pache B., et al. Feasibility and Safety of Oxaliplatin-Based Pressurized Intraperitoneal Aerosol Chemotherapy with or without Intraoperative Intravenous 5-Fluorouracil and Leucovorin for Colorectal Peritoneal Metastases: A Multicenter Comparative Cohort Study. Ann. Surg. Oncol. 2022:1–9. doi: 10.1245/s10434-022-11577-2. [DOI] [PubMed] [Google Scholar]
- 12.Taibi A., Teixeira Farinha H., Durand Fontanier S., Sayedalamin Z., Hubner M., Sgarbura O. Pressurized Intraperitoneal Aerosol Chemotherapy Enhanced by Electrostatic Precipitation (ePIPAC) for Patients with Peritoneal Metastases. Ann. Surg. Oncol. 2021;28:3852–3860. doi: 10.1245/s10434-020-09332-6. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira Farinha H., Hubner M., Somashekhar S.P., Abba J., Rao R.G., Willaert W. Treatment response after Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for peritoneal metastases of colorectal origin. Eur. J. Surg. Oncol. 2022;48:e114. doi: 10.1016/j.ejso.2021.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira Farinha H., Somashekhar S.P., Abba J., Sgarbura O., Alyami M., Rao R.G., Willaert W., Hubner M. Assessment of treatment response after Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for appendicular peritoneal metastases. Eur. J. Surg. Oncol. 2022;48:e161. doi: 10.1016/j.ejso.2021.12.324. [DOI] [Google Scholar]
- 15.Lurvink R.J., Rovers K.P., Nienhuijs S.W., Creemers G.J., Burger J.W.A., de Hingh I.H.J. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC-OX) in patients with colorectal peritoneal metastases-a systematic review. J. Gastrointest. Oncol. 2021;12:S242–S258. doi: 10.21037/jgo-20-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oken M.M., Creech R.H., Tormey D.C., Horton J., Davis T.E., McFadden E.T., Carbone P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Jacquet P., Sugarbaker P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 19.Solass W., Sempoux C., Detlefsen S., Carr N.J., Bibeau F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: Proposal of the Peritoneal Regression Grading Score (PRGS) Pleura Peritoneum. 2016;1:99–107. doi: 10.1515/pp-2016-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girardot-Miglierina A., Clerc D., Alyami M., Villeneuve L., Sgarbura O., Reymond M.A., Hubner M., the ISSPP PIPAC Study Group Consensus statement on safety measures for pressurized intraperitoneal aerosol chemotherapy. Pleura Peritoneum. 2021;6:139–149. doi: 10.1515/pp-2021-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tempfer C.B., Giger-Pabst U., Seebacher V., Petersen M., Dogan A., Rezniczek G.A. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol. Oncol. 2018;150:23–30. doi: 10.1016/j.ygyno.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Adjuvant Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in Resected High Risk Colon Cancer Patients: NCT03280511. [(accessed on 12 February 2022)]; Available online: https://clinicaltrials.gov.
- 23.Adjuvant PIPAC in Gastric Cancer Patients (PIPAC-OPC4): NCT04047004. [(accessed on 15 February 2022)]; Available online: https://clinicaltrials.gov.
- 24.Benefits of Pressured Intraperitoneal Aerosol Chemotherapy (PIPAC) in Patients With T3-4 Gastric Cancer Cyt—(GASPACCO): NCT04595929. [(accessed on 15 February 2022)]; Available online: https://clinicaltrials.gov.
- 25.Nowacki M., Alyami M., Villeneuve L., Mercier F., Hubner M., Willaert W., Ceelen W., Reymond M., Pezet D., Arvieux C., et al. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: An international survey study. Eur. J. Surg. Oncol. 2018;44:991–996. doi: 10.1016/j.ejso.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Sangisetty S.L., Miner T.J. Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J. Gastrointest. Surg. 2012;4:87–95. doi: 10.4240/wjgs.v4.i4.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubner M., Alyami M., Villeneuve L., Cortes-Guiral D., Nowacki M., So J., Sgarbura O., the ISSPP PIPAC Study Group Consensus guidelines for pressurized intraperitoneal aerosol chemotherapy: Technical aspects and treatment protocols. Eur. J. Surg. Oncol. 2021;48:789–794. doi: 10.1016/j.ejso.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Ploug M., Graversen M., Pfeiffer P., Mortensen M.B. Bidirectional treatment of peritoneal metastasis with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) and systemic chemotherapy: A systematic review. BMC Cancer. 2020;20:105. doi: 10.1186/s12885-020-6572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Giorgio A., Schena C.A., El Halabieh M.A., Abatini C., Vita E., Strippoli A., Inzani F., Rodolfino E., Romano B., Pacelli F., et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): A bidirectional approach for gastric cancer peritoneal metastasis. Surg. Oncol. 2020;34:270–275. doi: 10.1016/j.suronc.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Mortensen M.B., Glehen O., Horvath P., Hubner M., Hyung-Ho K., Konigsrainer A., Pocard M., Reymond M.A., So J., Fristrup C.W. The ISSPP PIPAC database: Design, process, access, and first interim analysis. Pleura Peritoneum. 2021;6:91–97. doi: 10.1515/pp-2021-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graversen M., Lundell L., Fristrup C., Pfeiffer P., Mortensen M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as an outpatient procedure. Pleura Peritoneum. 2018;3:20180128. doi: 10.1515/pp-2018-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadiradze G., Giger-Pabst U., Zieren J., Strumberg D., Solass W., Reymond M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J. Gastrointest. Surg. 2016;20:367–373. doi: 10.1007/s11605-015-2995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmieri L.J., Dubreuil O., Bachet J.B., Trouilloud I., Locher C., Coriat R., Moryoussef F., Landi B., Perkins G., Hautefeuille V., et al. Reasons for chemotherapy discontinuation and end-of-life in patients with gastrointestinal cancer: A multicenter prospective AGEO study. Clin. Res. Hepatol. Gastroenterol. 2021;45:101431. doi: 10.1016/j.clinre.2020.03.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.