Abstract
Resting cells of Desulfovibrio desulfuricans coupled the oxidation of a range of electron donors to Tc(VII) reduction. The reduced technetium was precipitated as an insoluble low-valence oxide. The optimum electron donor for the biotransformation was hydrogen, although rapid rates of reduction were also supported when formate or pyruvate was supplied to the cells. Technetium reduction was less efficient when the growth substrates lactate and ethanol were supplied as electron donors, while glycerol, succinate, acetate, and methanol supported negligible reduction. Enzyme activity was stable for several weeks and was insensitive to oxygen. Transmission electron microscopy showed that the radionuclide was precipitated at the periphery of the cell. Cells poisoned with Cu(II), which is selective for periplasmic but not cytoplasmic hydrogenases, were unable to reduce Tc(VII), a result consistent with the involvement of a periplasmic hydrogenase in Tc(VII) reduction. Resting cells, immobilized in a flowthrough membrane bioreactor and supplied with Tc(VII)-supplemented solution, accumulated substantial quantities of the radionuclide when formate was supplied as the electron donor, indicating the potential of this organism as a biocatalyst to treat Tc-contaminated wastewaters.
Technetium (99Tc; half-life, 2.1 × 105 years), a fission product of 235U, is a problematic component of some wastes from the nuclear fuel cycle (19). In its most stable form, the highly soluble pertechnetate ion (TcO4− [9]), the element is very mobile in the environment (25, 27) but can enter the food chain; the anion is actively assimilated by plants using sulfate transport mechanisms (3). For these reasons treatment at its source is highly desirable.
Due to the low solubility of reduced Tc, e.g., Tc(IV) and Tc(V) oxides (9), microbial reduction of the pertechnetate ion has been proposed as the basis of a biotechnological method for treating Tc(VII)-contaminated effluents (16, 19). Lloyd and Macaskie (13) subsequently developed a novel PhosphorImager-based technique to monitor the microbial reduction of Tc(VII). Using this technique, they demonstrated the direct enzymatic reduction of Tc(VII) by resting cells of Shewanella putrefaciens and Geobacter metallireducens. The reduced Tc products detected in culture fluids were species specific. Only soluble reduced Tc species were detected in cultures of S. putrefaciens. In contrast, only trace amounts of the soluble species were detected in the cultures of G. metallireducens, and appreciable quantities of the radionuclide were precipitated, probably as an insoluble low-valence oxide of Tc. Recent studies have also shown that anaerobically grown cells of Escherichia coli are able to couple the oxidation of formate or hydrogen to Tc(VII) reduction and precipitation (11, 12). The enzyme responsible for Tc(VII) reduction was identified, by using physiological and genetic approaches, as hydrogenase 3, a component of the formate hydrogenlyase (FHL) complex (11). Resting cells of this organism, immobilized in a membrane bioreactor and supplied with formate or hydrogen as electron donors for Tc(VII) reduction, have also been used successfully to remove the radionuclide from a challenge solution, supplied to the flowthrough bioreactor (12).
Several studies have suggested that sulfate-reducing bacteria (SRB) may be involved in Tc(VII) reduction, with the radionuclide precipitated as an insoluble sulfide (1, 8, 22). Lovley (16) subsequently proposed, on the basis of broad-metal reductase activity against other high-valence metals [Cr(VI), Fe(III), Mn(IV), and U(VI)], that SRB may be able to reduce Tc(VII) enzymatically. This hypothesis is also supported by the following observations: (i) the pertechnetate anion is bioavailable as a sulfate analogue and may therefore be a surrogate electron acceptor for anoxic growth, (ii) SRB are the closest known relatives to G. metallireducens [subsequently shown to reduce Tc(VII)] by the criterion of 16S rRNA analysis (17), and (iii) SRB have high uptake-hydrogenase activities in the periplasm (20); hydrogenase-mediated reduction of Tc(VII) by E. coli has already been demonstrated (11).
Indeed, we have recently confirmed that SRB are able to reduce and precipitate heptavalent Tc enzymatically (15). Resting cells of Desulfovibrio desulfuricans, supplied with hydrogen as an electron donor, reduced the pertechnetate anion as an electron acceptor in lieu of sulfate. Previous studies have suggested that in the presence of sulfate, H2S is produced by SRB, resulting in the formation of insoluble Tc(VII) and Tc(IV) sulfides (21). In our recent studies, however, proton-induced X-ray emission (PIXE) showed Tc to be the major element detected in a black precipitate from sulfate-free (nonsulfidogenic) resting cultures (15). Minimal sulfur was found in association with the enzymatically reduced Tc by energy-dispersive X-ray microanalysis and PIXE, while reduction of the radionuclide was confirmed by X-ray absorption spectroscopy (XAS). Electron microscopy studies showed the reduced, precipitated Tc to be cell associated. The goals of the present study were therefore to further characterize the enzyme system responsible for Tc(VII) reduction, in the absence of sulfate, by a model SRB (D. desulfuricans) and to determine whether immobilized cells of this organism could be used to treat Tc(VII)-contaminated water in a flowthrough bioreactor.
MATERIALS AND METHODS
Organism and cultivation conditions.
D. desulfuricans ATCC 29577 was obtained from D. R. Lovley, University of Massachusetts. Stocks of the organism were maintained in 100-ml aliquots of Postgate’s medium B (23) in 110-ml serum bottles sealed with a butyl rubber stopper under an atmosphere of N2 at 30°C. The N2 was passed through an oxygen trap (Phase Separations, Ltd., Deeside, Clwyd, United Kingdom). Stocks of the organism were resubcultured every 3 to 4 weeks (inoculum added to 10% of final volume). Cultures for metal reduction experiments were grown in Postgate’s medium C (23), in sealed bottles, also under N2. This growth medium contained a lower concentration of iron in addition to citrate: the latter prevents precipitation of the ferrous sulfide formed in the cultures (23). Inocula (10% [vol/vol]) were added to medium C (from the medium B stocks), and the cells were grown for 48 h at 30°C. Cells were then repeatedly subcultured until negligible iron sulfide precipitate, carried over from medium B, was noted at the end of the 48-h growth phase (typically two subcultures in medium C). Cells were then collected by centrifugation at 5,000 rpm for 20 min (in an MSE benchtop centrifuge) and washed four times in 20 mM morpholinepropanesulfonic acid (MOPS)-NaOH buffer (pH 7.0; preequilibrated with N2 to displace O2). The cells were resuspended in MOPS buffer at a biomass density of about 0.5 g (dry weight) liter−1, measured as described by Lloyd and Macaskie (13). All manipulations of the cells were made under an atmosphere of nitrogen.
Tc(VII) reduction by resting cell suspensions.
Aliquots (2 to 8 ml) of the washed cell suspension resuspended in MOPS buffer were transferred under nitrogen to 12-ml serum bottles sealed with butyl rubber stoppers. Ammonium pertechnetate (NH4TcO4; Amersham International, Amersham, Buckinghamshire, United Kingdom) was added to a final concentration of 250 μM to 1.0 mM from a concentrated stock solution through the rubber stopper by using a hypodermic syringe fitted with a needle. Potential electron donors (ethanol, glycerol, methanol, or sodium salts of formate, pyruvate, lactate, succinate, or acetate) were added from concentrated stocks to a final concentration of 10 mM as appropriate. All solutions were de-aerated with nitrogen before use. When hydrogen was supplied as an electron donor for metal reduction, the gas was introduced into the headspace of the bottles displacing the nitrogen. The cultures were incubated at 30°C without agitation.
Electron microscopy.
Bacterial pellets were harvested with a Heraeus Sepatech Biofuge 13 microfuge (13,000 rpm, 10 min), rinsed twice in distilled water, and sectioned for viewing. Bacterial pellets were fixed for 60 min in 2.5% (wt/vol) aqueous glutaraldehyde, washed once in distilled water, and then fixed for a further 60 min in 1% (wt/vol) aqueous osmium tetroxide. The cells were then dehydrated by using an ethanol series (70, 90, 100, 100, and 100% ethanol in water; 15 min each step). Two 15-min washes in propylene preceded embedding in epoxy resin under vacuum for 20 min. The resin was left to polymerize (24 h, 60°C). Sections (100 to 150 nm thick) were cut from the resin block with a microtome and placed onto a carbon-coated copper grid prior to analyses. Sections were viewed with a JEOL 120CX2 transmission electron microscope fitted with a Link ISI EDAX system (Jeol Ltd., Welwyn Garden City, Hertfordshire, United Kingdom). The limit of resolution of the EDAX microprobe was approximately 0.1 μm.
Hollow-fiber bioreactor.
A 17-ml glass reactor which contained a fiber bundle composed of 12 XM50 acrylic hollow-fiber membranes (1.1 mm, internal diameter; Romicon) was used throughout this study (see reference 12). The fibers were embedded in PTFE end plugs by using Rapid Araldite adhesive (Ciba-Geigy Plastics, Cambridge, United Kingdom), with the fiber bundle held in place by a silicone O-ring which fitted over the PTFE end plugs. The molecular mass cutoff of the membranes was 50 kDa, and the fiber bundle occupied about 3 ml (total volume) of the reactor. The fiber bundles were washed sequentially in 0.05 M H3PO4, 0.125 M NaOH, and distilled water (30 min each wash) prior to use as described by Devereux and Hoare (5).
All components of the reactor, except the fiber bundle, were autoclaved at 121°C for 15 min prior to use. Sterilization of the fibers was done in situ by pumping sodium hypochlorite (200 ppm of available chloride ion) through the reactors at 10 ml h−1 for 1 h. Residual hypochlorite was rinsed out before use by pumping sterile phosphate-buffered saline (20 mM, pH 7.0, supplemented with 8.5 mM NaCl) through the reactors at 40 ml h−1 for a minimum of 4 h.
Cells were inoculated into the reactor by pumping about 200 ml of washed cells in 20 mM MOPS buffer (pH 7.0) into the reactor at a flow rate of 50 ml h−1. The biomass concentration in the 17-ml reactor was equivalent to 5 g (dry weight) of cells per liter of reactor volume. The reactor was operated in “transverse mode” (10) with MOPS buffer supplemented with 50 μM Tc(VII) and 25 mM formate as an electron donor pumped into the reactor via the shell-side port at a flow rate of 5 ml h−1. The effluent exiting the reactor was collected from the lumen side of the hollow fibers.
Measurement of Tc.
Tc in solution was assayed by autoradiography by using a PhosphorImager (Molecular Dynamics, Sevenoaks, Kent, United Kingdom) as described by Lloyd and Macaskie (13). Samples (150 μl) were removed from the cultures, and a 10-μl aliquot was placed on 3MM cellulose chromatography paper. The remaining sample was centrifuged in a Heraeus Sepatech Biofuge 13 microfuge (13,000 rpm, 10 min). Then, 10 μl of the culture supernatant was placed on the chromatography paper adjacent to the noncentrifuged sample, and the paper was wrapped in cling film. The radionuclide-impregnated paper was then exposed to a storage phosphor screen (Molecular Dynamics). After 16 h of exposure, the spots of radioactivity were visualized with a PhosphorImager, and a densitometer scan of the resulting image was made by using the ImageQuant software package (Molecular Dynamics). Tc uptake was calculated by dividing the peak height corresponding to the culture supernatant by the peak height obtained from the sample (prior to centrifugation) and was expressed as the percentage removed from solution.
Reproducibility of data.
Protein determinations and PhosphorImager analyses were done in triplicate; the experimental error was within 5% of the mean throughout.
RESULTS
Effect of electron donor.
To determine the optimal electron donor for Tc(VII) reduction and precipitation by D. desulfuricans, washed resting cells were challenged with 250 μM Tc and supplied with a range of organic acids, alcohols, or hydrogen. The organic acids (formate, pyruvate, lactate, succinate, or acetate) and alcohols (ethanol, glycerol, and methanol) were supplied at a concentration of 10 mM; hydrogen was supplied in the headspace of the cultures. Tc removal from solution was measured after 24 h of incubation at 30°C, and the uptake of the radionuclide by the biomass was calculated and expressed as a concentration ratio (CR; ratio of becquerels of Tc per gram [dry weight] of cells to becquerels of Tc per milliliter of spent medium). Hydrogen and formate were the best electron donors, with CR of 12,850 and 11,648, respectively (Table 1). These cultures contained a black precipitate indicative of reduced insoluble Tc (15). Other substrates, including pyruvate (CR = 6,393) and lactate (CR = 2,940) (the latter being the electron donor for sulfate reduction in the growth medium), supported less-efficient reduction of the radionuclide. Some Tc removal was also noted with ethanol (CR = 1,093), which is also utilized for growth (4). Very little removal was recorded when succinate, glycerol, acetate, or methanol were supplied as the electron donors. Very low amounts of Tc were removed by dead autoclaved cells supplied with lactate, formate, or hydrogen (CR = 20 to 28) or by control cultures containing live cells incubated under nitrogen in the absence of the electron donor (CR = 68). This bioaccumulation probably represents nonspecific biosorption onto the biomass.
TABLE 1.
Effect of electron donor on Tc reduction and removal by resting cells of D. desulfuricansa
| Electron donor | CR |
|---|---|
| Hydrogen | 12,850 |
| Formate | 11,648 |
| Pyruvate | 6,393 |
| Lactate | 2,940 |
| Ethanol | 1,093 |
| Succinate | 363 |
| Glycerol | 336 |
| Acetate | 197 |
| Methanol | 101 |
Results are expressed as the CR, calculated after 24 h of incubation at 30°C.
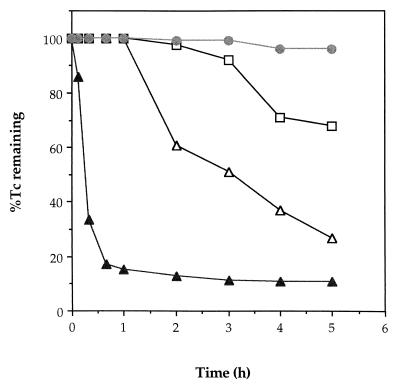
The rates of Tc(VII) reduction by resting cells supplied with hydrogen, formate or lactate as the electron donor were also measured (Fig. 1). Tc(VII) reduction was most rapid with hydrogen; approximately 85% of the 250 μM Tc challenge was removed in less than 1 h. Analysis of the supernatant from these cultures by chromatographic separation followed by visualization of the different oxidation states of Tc with a PhosphorImager (13) showed that only 5% (12.5 μM) of the Tc(VII) added remained in the heptavalent state, with the remaining 10% detected as a nonmigrating spot, probably Tc(V) previously reported as a product of hydrogen-dependent Tc(VII) reduction by D. desulfuricans (15). In a previous study, similar levels of formate-dependent Tc reduction and removal were only achieved after several days of incubation with resting cells of E. coli at a similar biomass concentration (11). Rapid rates of reduction were also noted when formate was supplied as the electron donor, with a substantial loss in reaction rate when lactate was utilized. The enzyme activity was stable with hydrogen; washed cells remained active for several weeks when stored at 4°C under nitrogen. In addition, sparging of the culture with air for 15 min had no discernible effect on enzyme activity (if assayed immediately), and freeze-dried cells, kept in air, also maintained most of their radionuclide reducing activity. Complete loss in activity was noted, however, if lactate or formate was used as the electron donor, suggesting that the enzyme systems required to generate reducing equivalents from the organic compounds were oxygen sensitive.
FIG. 1.
Tc(VII) reduction by resting cells of D. desulfuricans supplied with hydrogen (▴), formate (▵), and lactate (□) as an electron donor. Control cultures (●) contained no added electron donor.
Localization of the site of reduced Tc deposition.
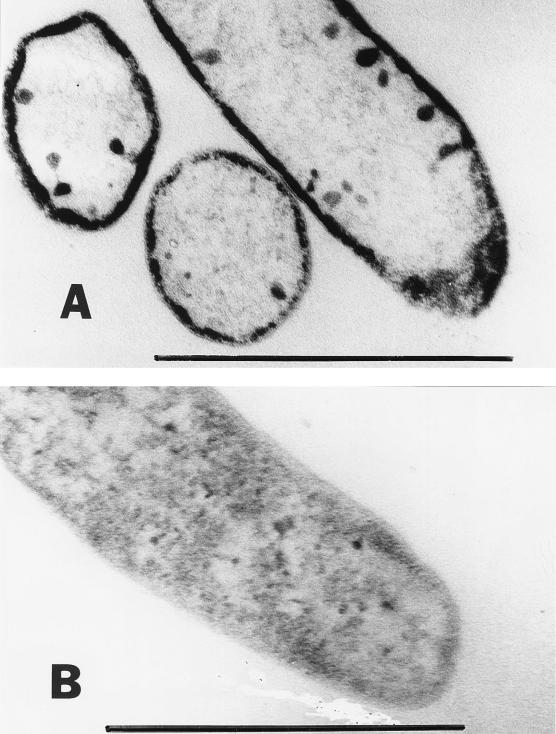
In a previous study, XAS and PIXE analysis confirmed that Tc(VII) was reduced and precipitated as a low-valence oxide by D. desulfuricans (15). Preliminary observations of air-dried preparations of cells by transmission electron microscopy also suggested that the reduced Tc precipitate was cell associated. In this study we looked at thin sections of cells by transmission electron microscopy to determine more accurately the site of reduced Tc precipitation. Cells challenged with 1 mM Tc (89% removed from solution over a period of 24 h) contained an electron-opaque precipitate at the periphery of the cell (Fig. 2A). Analysis of the sections by energy-dispersive X-ray analysis confirmed that the Tc was confined to this area of the cells (data not shown). Cells which were not challenged with the radionuclide remained unstained (Fig. 2B). A similar pattern of elemental palladium deposition has also been noted in resting cultures of D. desulfuricans supplied with hydrogen and Pd(II) (14), suggesting a common mode of enzymatic metal reduction. These results suggest a role for a periplasmic enzyme in Tc(VII) reduction. Given the recently identified role of hydrogenase 3 as the Tc(VII) reductase in E. coli (11), it is possible that a periplasmic hydrogenase acts as the radionuclide reductase in D. desulfuricans. Indeed, Tc(VII) reductase activity was abolished by preincubation of the cells in 0.5 mM Cu(II) for 10 min prior to challenge with Tc(VII). Cu(II) is an inhibitor of periplasmic but not cytoplasmic hydrogenases (7).
FIG. 2.
Transmission electron micrographs of thin sections of D. desulfuricans incubated in the presence (A) or absence (B) of 1 mM Tc(VII). Hydrogen was supplied as the electron donor for metal reduction. Electron-dense reduced Tc was precipitated at the periphery of the cell in culture A. Bar = 1 μm.
Tc reduction and accumulation by immobilized cells.
The following experiment was done to determine whether resting cells of D. desulfuricans immobilized in a flowthrough bioreactor could be used to treat a Tc(VII)-contaminated solution. Resting cells were immobilized in a hollow-fiber membrane bioreactor at a concentration of 5 g (dry weight) of cells per liter of reactor volume. MOPS buffer supplemented with 50 μM Tc(VII) and 25 mM formate was pumped into the reactor at a flow rate of 5 ml h−1, with the reactor operated in “transverse” or “ultrafiltration” mode (10). Tc(VII)-supplemented buffer was delivered from the shell-side of the reactor, and treated solution was collected from the lumina of the fibers. A control reactor was also constructed and supplied with buffer containing Tc(VII) but not formate.
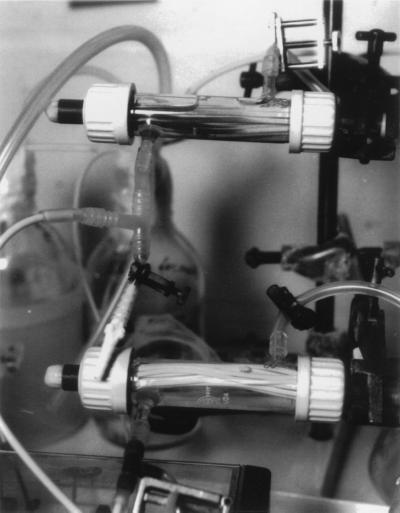
Within a few hours of operation, the fibers in the reactor supplied with Tc(VII) and formate began to darken. After 72 h, a heavy black precipitate (reduced insoluble Tc [15]) was noted (Fig. 3). The precipitate was most apparent around the inlet into the reactor, indicating that Tc removal was efficient and localized in the first few milliliters of reactor space. No precipitate was noted on the fibers in the control reactor supplied with Tc(VII) but not formate. At the end of the experiment (94 h of continuous operation), the fibers were removed from the reactor and exposed to a PhosphorImager screen for 6 h. Comparatively high concentrations of Tc were detected on the fibers from the reactor supplied with formate (Fig. 4). Image analysis of Fig. 4 showed that only 1% of this high loading was accumulated on the fibers in the control reactor.
FIG. 3.
Deposition of reduced Tc (black precipitate) in a hollow-fiber membrane bioreactor containing resting cells of D. desulfuricans and supplied with MOPS buffer supplemented with 50 μM Tc(VII) and 25 mM formate (top bioreactor). No black precipitate was deposited in a control bioreactor supplied with Tc(VII) but no electron donor (formate [bottom bioreactor]). The bioreactors were operated for 94 h at 30°C.
FIG. 4.
Tc accumulation by membrane-entrapped cells of D. desulfuricans. (A) Fiber bundle from a bioreactor supplied with MOPS buffer supplemented with 50 μM Tc(VII) and 25 mM formate was heavily stained by the radionuclide. (B) Fiber bundle from a control bioreactor supplied with Tc(VII) but no electron donor (formate), lightly stained by the radionuclide. Tc was visualized by using a PhosphorImager (exposure time to storage phosphor screen of 6 h). Hollow-fiber membrane bioreactors were operated for 94 h.
DISCUSSION
The physiological factors underlying Tc(VII) reduction and precipitation by D. desulfuricans were investigated. Hydrogen was the preferred substrate for Tc(VII) reduction, a finding consistent with the involvement of a hydrogenase. Hydrogenases have recently been identified as oxyanion metal reductases in Clostridium pasteurianum (28) and E. coli (11). Indeed, the periplasm is a major site of hydrogenase activity in SRB (20), and our observation of Tc precipitation at the periphery of the cell is entirely consistent with direct enzymatic reduction of Tc(VII) catalyzed by a periplasmic hydrogenase. In support of this hypothesis, cells treated with Cu(II), a selective poison for periplasmic but not cytoplasmic hydrogenases, had no Tc(VII) reductase activity. Lovley has also shown that hydrogenase activity plays a pivotal role in metal reduction by Desulfovibrio vulgaris (16). Hydrogen-dependent U(VI) and Cr(VI) reduction is catalyzed by cytochrome c3 coupled to a hydrogenase, the latter enzyme being required to abstract electrons from the gas for metal reduction (16). As the periplasm contains appreciable quantities of cytochrome c3 (20), this enzyme could also be the biocatalyst responsible for Tc(VII) reduction in SRB.
The oxidation of several organic electron donors was also coupled to Tc(VII) reduction but did not support the rapid rate of reduction noted with hydrogen. Of these electron donors, Tc(VII) reduction was most rapid with formate. It is interesting that SRB of the genus Desulfovibrio contain a rudimentary FHL complex consisting of a formate dehydrogenase coupled to a hydrogenase via a cytochrome (20). This complex is also located in the periplasm. Thus, formate oxidation may also be coupled to Tc(VII) reduction by a multienzyme complex analogous to the FHL complex of E. coli as described by Lloyd et al. (11).
The rate of Tc(VII) reduction recorded in resting cultures of D. desulfuricans supplied with hydrogen (800 μmol of Tc g of biomass−1 h−1) was far higher than those recorded in other bacteria studied to date (64, 28, and 36 times the rate measured in E. coli, S. putrefaciens, and G. metallireducens cultures respectively [11, 13]). This factor, in combination with the demonstrated oxygen- and radio-tolerance of the enzyme catalyzing Tc(VII) reduction, suggests that SRB show considerable potential to treat Tc(VII)-contaminated wastewater. Indeed, cells of D. desulfuricans, immobilized a membrane bioreactor, reduced and accumulated substantial quantities of Tc over a period of 94 h. In light of the very high CRs noted in this study (e.g., 12,850 for resting cells supplied with hydrogen), these results also highlight the role of SRB in Tc(VII) mobility in the environment as a subject that warrants further investigation. As a comparison, much lower levels of uptake have been recorded in other aquatic organisms, including phytoplankton (CR = 17 [6]), brown algae (CR = 250 to 2,500 [26]), green algae (CR = 1 to 20 [6, 24]), and lobster (CR = 1,000 to 1,400 [2]).
In an earlier study we showed that resting cells of D. desulfuricans, incubated in the presence of both lactate and sulfate, were able to reduce sulfate to H2S, with Tc subsequently precipitated as an insoluble sulfide (15). The CR in sulfidogenic cultures (CR = 5,748) was considerably lower than that achieved in this study with direct enzymatic reduction with formate or hydrogen supplied as electron donors. Other advantages of enzymatic precipitation over biogenic sulfide precipitation include the following: (i) there is no need to supplement low-sulfide wastewater streams with added sulfate; (ii) nongrowing cells can be used, leading to the generation of a low-biomass waste for disposal; (iii) potentially hazardous toxic H2S is not produced as a byproduct of the process, and indeed other workers have noted technical difficulties in using resting SRB cells to reduce sulfate to H2S (18); (iv) the process is potentially environmentally benign if hydrogen is used as a feedstock because there is no additional carbon substrate added to the wastewater; and (v) the reduced Tc is held within the outer compartments of the cell, potentially spatially separated from oxidizing and chelating agents that may be present in the effluent. In order to develop further an efficient bioprocess to treat Tc-contaminated solution, we are currently assessing the ability of SRB to reduce and precipitate Tc in a continuously operating bioreactor under more realistic operating conditions. Results from these studies will be reported elsewhere.
ACKNOWLEDGMENTS
We thank D. R. Lovley, University of Massachusetts, for the bacterial strain used in this study. Useful discussions with H. Eccles (BNFL) are also acknowledged.
This work was supported by grants from BNFL and the BBSRC (grant JO6276). The PhosphorImager used for autoradiography was purchased with shared equipment grants from The Wellcome Trust (037160/Z/92) and the United Kingdom Medical Research Council (G9216078MB). A storage phosphor screen was provided for use in this work by Molecular Dynamics.
REFERENCES
- 1.Beasley T M, Lorz H V. A review of the biological and geochemical behaviour of Tc in the marine environment. In: Desmet G, Myttenaere C, editors. Technetium in the environment. London, England: Elsevier; 1986. pp. 197–216. [Google Scholar]
- 2.Beasley T M, Lorz H V. A review of the biological and geochemical behaviour of Tc in the marine environment. J Environ Radioactivity. 1986;3:1–22. [Google Scholar]
- 3.Cataldo D A, Garland T R, Wildung R E, Fellows R J. Comparative metabolic behaviour and interrelationships of Tc and S in soybean plants. Health Phys. 1989;57:281–288. doi: 10.1097/00004032-198908000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Devereux R, Delaney M, Widdel F, Stahl D A. Natural relationships among sulfate-reducing eubacteria. J Bacteriol. 1989;171:6689–6695. doi: 10.1128/jb.171.12.6689-6695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux N, Hoare M. Membrane separation of protein precipitates: studies with cross-flow in hollow fibres. Biotechnol Bioeng. 1986;18:422–431. doi: 10.1002/bit.260280316. [DOI] [PubMed] [Google Scholar]
- 6.Fisher N S. Bioaccumulation of technetium by marine phytoplankton. Environ Sci Technol. 1982;16:579–581. doi: 10.1021/es00103a008. [DOI] [PubMed] [Google Scholar]
- 7.Fitz R M, Cypionka H. Generation of a proton gradient in Desulfovibrio vulgaris. Arch Microbiol. 1991;155:444–448. [Google Scholar]
- 8.Henrot J. Bioaccumulation and chemical modification of Tc by soil bacteria. Health Phys. 1989;57:239–245. doi: 10.1097/00004032-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Kotegov K V, Pavlov O N, Shvendov V P. Technetium. In: Emelius H J, Sharpe A G, editors. Advances in inorganic chemistry and radiochemistry. New York, N.Y: Academic Press, Inc.; 1968. pp. 1–90. [Google Scholar]
- 10.Lloyd J R, Bunch A W. Ethylenogenesis by Escherichia coli B SPAO immobilised in a hollow-fibre bioreactor. Enzyme Microb Technol. 1996;18:113–120. [Google Scholar]
- 11.Lloyd J R, Cole J A, Macaskie L E. Reduction and removal of heptavalent technetium from solution by Escherichia coli. J Bacteriol. 1997;179:2014–2021. doi: 10.1128/jb.179.6.2014-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd J R, Harding C L, Macaskie L E. Tc(VII) reduction and accumulation by immobilized cells of Escherichia coli. Biotechnol Bioeng. 1997;55:505–510. doi: 10.1002/(SICI)1097-0290(19970805)55:3<505::AID-BIT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd J R, Macaskie L E. A novel PhosphorImager-based technique for monitoring the microbial reduction of technetium. Appl Environ Microbiol. 1996;62:578–582. doi: 10.1128/aem.62.2.578-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd J R, Yong P, Macaskie L E. Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Appl Environ Microbiol. 1998;64:4607–4609. doi: 10.1128/aem.64.11.4607-4609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd J R, Nolting H-F, Solé V A, Bosecker K, Macaskie L E. Technetium reduction and precipitation by sulphate-reducing bacteria. Geomicrobiol J. 1998;15:43–56. [Google Scholar]
- 16.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 17.Lovley D R, Giovannoni S J, White D C, Champine J E, Phillips E J P, Gorby Y A, Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159:336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- 18.Lovley D R, Phillips E J P. Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol. 1992;54:1472–1480. doi: 10.1128/aem.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macaskie L E. The application of biotechnology to the treatment of wastes produced from the nuclear fuel cycle: biodegradation and bioaccumulation as a means of treating radionuclide-containing streams. Crit Rev Biotechnol. 1991;11:41–112. doi: 10.3109/07388559109069183. [DOI] [PubMed] [Google Scholar]
- 20.Peck H D. Bioenergetic strategies of the sulfate-reducing bacteria. In: Odom J M, Singleton R, editors. Sulfate-reducing bacteria: contemporary perspectives. New York, N.Y: Springer-Verlag; 1993. pp. 41–87. [Google Scholar]
- 21.Peretrukhin V F, Khizhnyak T V, Lyalikova N N, German K E. Biosorption of technetium-99 and some actinides by bottom sediments of Lake Beloe Kosino of the Moscow region. Radiochemistry. 1996;38:440–443. [Google Scholar]
- 22.Pignolet L, Fonsny K, Capot F, Moureau Z. Role of various microorganisms on Tc behaviour in sediments. Health Phys. 1989;57:791–800. doi: 10.1097/00004032-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Postgate J R. The sulphate-reducing bacteria. Cambridge, United Kingdom: Cambridge University Press; 1979. [Google Scholar]
- 24.Sombre L, Carraro S, Myttenaere C. Contamination of a freshwater green alga (Scenedesmus obliquus) by radionuclides typical in central PWR effluents: culture in a turbidostat. Ann Belg Ver Stralingsbescherming. 1987;12:157–170. [Google Scholar]
- 25.Trabalka J R, Garten C T., Jr . Behaviour of the long-lived synthetic elements and their natural analogues in food chains. In: Lett J T, Ehman U K, Cox A B, editors. Advances in radiation biology. Vol. 10. London, United Kingdom: Academic Press, Inc.; 1983. pp. 68–73. [Google Scholar]
- 26.Van der Ben D, Cognean M, Robberecht V. Factors influencing the uptake of Tc by the brown algae Fucus serratus. Mar Pollut Bull. 1990;21:84–86. [Google Scholar]
- 27.Wildung R E, McFadden K M, Garland T R. Technetium sources and behaviour in the environment. J Environ Qual. 1979;8:156–161. [Google Scholar]
- 28.Yanke L J, Bryant R D, Laishley E J. Hydrogenase I of Clostridium pasteurianum functions as a novel selenite reductase. Anaerobe. 1995;1:61–67. doi: 10.1016/s1075-9964(95)80457-9. [DOI] [PubMed] [Google Scholar]